User login
Aortic Stenosis and Surgery
Aortic stenosis (AS) is a common problem among aging patients,1 who often require surgical procedures. The medical consultant must determine whether the presence of a systolic murmur suggesting AS needs additional evaluation before the patient proceeds to surgery. This decision requires interpretation of cardiac murmurs, and understanding the natural history, pathophysiology, and risks of AS.
PATHOPHYSIOLOGY
Aortic stenosis is a progressive disease that leads to predictable impairment of cardiac responses to physiologic stresses of surgery. AS typically results from degenerative calcification or from a bicuspid aortic valve, both of which cause progressive constriction of left ventricular outflow.24 The heart compensates by left ventricular hypertrophy. Systolic ejection of blood across the stenotic valve requires more time than normal, leaving less time for diastolic refilling. Left ventricular hypertrophy creates a less compliant left ventricle that becomes dependent on left atrial contraction for optimal filling. Atrial fibrillation with loss of the atrial kick is particularly problematic for patients with AS and left ventricular hypertrophy. Thickened myocardium increases myocardial oxygen consumption and impairs myocardial perfusion. Myocardial oxygen demand in the hypertrophied ventricle results from increased systolic pressure on the ventricle, increased systolic contraction time, and increased muscle mass. Reduced capillary density in hypertrophied muscle, and diminished perfusion pressure because of a reduced aortic‐coronary pressure differential, impair myocardial perfusion. Shortened diastole allows less blood flow to the myocardium.
At rest, with a controlled heart rate and sinus rhythm to allow for left atrial contraction to enhance left ventricular filling, patients may tolerate significant AS. However, increased heart rate in response to physiologic stress reduces diastolic filling time, diminishes somewhat tenuous myocardial perfusion, and increases afterload.
Additionally, the left ventricle depends on adequate filling pressures; the hypertrophied ventricle is prone to reduced cardiac output because of reductions of preload caused by hypovolemia or venodilation. Venodilation has been a particular concern with epidural anesthesia, although recent studies suggest that this modality can be used safely.5 Many anesthetic agents reduce systemic blood pressure and thereby reduce the aortic‐coronary perfusion pressure gradient leading to reduced coronary blood flow. For surgical patients with significant AS, anesthetic management requires appropriate intravascular volume to optimize preload, heart rate control to allow adequate left ventricular filling along with time for coronary artery flow, and sufficient systemic blood pressure to maintain coronary artery blood flow.
IDENTIFYING AORTIC STENOSIS IN PREOPERATIVE PATIENTS AND JUDGING ITS SEVERITY
Many older patients are found to have a systolic murmur consistent with AS prior to surgery. The first step in evaluation is a detailed history to determine exercise capacity and to elicit any history of chest pain, heart failure symptoms, or syncope. A key question for the medical consultant is whether or not patients should have further evaluation of the murmur prior to surgery, typically starting with transthoracic echocardiography. Table 1 outlines echocardiographic criteria for grading AS severity. The history and physical exam inform the decision of whether to pursue echocardiography. Although it is not clear from the literature whether identification of AS by echocardiography improves outcomes (this question is unlikely to be addressed by randomized trials), anesthesiologists generally want to know if significant AS is present, as it impacts intraoperative monitoring and management. So the question then becomes the following: Can clinicians reliably exclude moderatesevere AS based on history and a careful cardiovascular exam?
| Aortic Stenosis | |||
|---|---|---|---|
| Indicator | Mild | Moderate | Severe |
| |||
| Jet velocity (m/s) | <3.0 | 3.04.0 | >4.0 |
| Mean gradient (mmHg) | <25 | 2540 | >40 |
| Valve area (cm2) | >1.5 | 1.01.5 | <1.0 |
| Valve area index (cm2/m2) | <0.6 | ||
For ruling in severe AS, effort syncope provides the highest positive predictive value; stenosis was found to be severe in all patients with a history of effort syncope in a sample of 67 patients with AS.6 The presence of a loud, late‐peaking systolic murmur or significant delay and decrease in the carotid upstroke, argue for severe AS.7 Etchells et al developed a simple decision rule for detecting moderatesevere AS (defined as an aortic valve area of 1.2 cm2 or less, or a peak transvalvular gradient of 25 mmHg or more), based on a study of 162 inpatients who were examined by a senior medical resident and a general internist.8 If no murmur was heard over the right clavicle, AS was rare (1/69 [1.4%]; likelihood ratio (LR) 0.10 [95% confidence interval (CI) 0.020.44]). If there was a murmur radiating to the right clavicle with 3 to 4 associated findings (reduced second heart sound, reduced carotid volume, slow carotid upstroke, and murmur loudest in the second right intercostal space), moderatesevere AS was common (6/7 [86%]; LR 40 [95% CI 6.6239]).
Absence of radiation of a systolic murmur to the right carotid artery is a useful finding to exclude AS, with a negative likelihood ratio of 0.05 to 0.10.9 Although no single physical exam finding or combination of findings can reliably exclude hemodynamically significant AS when a systolic murmur radiates to the right neck, the combination of an early‐peaking, soft (grade 2 or less) systolic murmur, normal timing and upstroke of the carotids, and an audible aortic second sound substantially lessen the likelihood of severe AS. A recent study of 376 inpatients who underwent meticulous cardiac examination by a single investigator (blinded to the diagnosis in >96% of cases), followed by echocardiography, provides additional information about the operating characteristics of physical examination in determining the etiology of systolic murmurs.10 Murmurs heard diagonally across the chest from the right upper sternal border to the apex (broad apical‐base pattern) predicted increased aortic velocity that would be consistent with AS. Other findings that increased the likelihood of aortic valve disease included delayed carotid upstroke, absent second heart sound (S2), radiation to the clavicles and neck on both sides, and a humming quality to the murmur. This study concluded that the physical examination is not reliable in determining the severity of AS. While generally true, this study actually reveals that any pattern of murmur radiation other than the broad apical‐base pattern excluded severe AS entirely among 221 patients with murmurs, and excluded moderate AS in all but 3 of these patients.
A retrospective study of 3997 hip fracture patients evaluated 908 echocardiograms done to investigate cardiac murmurs detected during preoperative assessment.11 These echocardiograms detected 272 patients with AS that had not been previously diagnosed. Thirty patients had severe AS. Detection of AS prompted changes in anesthesia management. The authors argued for preoperative echocardiograms for all hip fracture patients in whom a murmur is detected.
In summary, no finding by history can exclude AS. However, if the murmur is not heard across the precordium and does not radiate to the clavicle or right neck, severe AS is very unlikely.10 For patients in whom the murmur suggests the possibility of severe AS, echocardiography is prudent.
PROGNOSIS OF ADVANCED AS
Symptomatic AS portends poor prognosis in the absence of aortic valve replacement. In a cohort of patients with severe AS who refused aortic valve replacement (AVR), patients survived a mean of 45 months after onset of angina, 27 months following onset of syncope, and only 11 months after the beginning of left heart failure.12 Recent studies further define the natural history of severe asymptomatic AS. A study of 128 consecutive patients with asymptomatic severe AS identified by echocardiography found 93% survival at 1 year, 91% at 2 years, and 87% at 4 years, suggesting a relatively benign prognosis.13 However, many patients developed symptoms during follow‐up and required aortic valve replacement. A larger study of 622 asymptomatic AS patients with aortic‐jet velocity greater than 4 m/s found that 82% of patients were free of cardiac symptoms after 1 year, but only 33% were free of cardiac symptoms or intervention at 5 years.14 Patients with asymptomatic, very severe AS, defined as peak aortic‐jet velocity of 5.0 m/s or greater have an even worse prognosis with an event‐free survival of 12% at 4 years and only 3% at 6 years.15
Although short‐term (1 to 5 years) prognosis for severe symptomatic AS is poor, and asymptomatic but severe AS also carries substantial risk, the major issue for the medical consultant evaluating patients prior to noncardiac surgery is the very short‐term perioperative risk imposed by AS. Put simply, will the patient survive surgery and the postoperative period of rehabilitation?
NONCARDIAC SURGERY AND AS
The evidence that AS increases risk of cardiac complications and cardiac death for patients undergoing noncardiac surgery is limited to retrospective studies. In the early 1960s, a retrospective study of cardiac risk among 766 patients found 10% mortality among 59 patients with an aortic valve abnormality.16 The 15 patients who underwent either intrathoracic or intra‐abdominal procedures did particularly poorly, with a mortality of 20%. As part of a large cohort study used to develop the first widely employed cardiac risk index for noncardiac surgery, Goldman et al found 13% (3/23 patients) cardiac mortality among patients with important valvular AS.17 In comparison, cardiac mortality among 978 patients without identified AS was 1.6% (16/978 patients).
More recent studies demonstrate lower perioperative mortality for AS patients. These studies are summarized in Table 2. A retrospective chart audit of all patients with AS who underwent noncardiac surgery, in Hamilton, Ontario, Canada between 1992 and 1994, identified 55 patients with a mean aortic valve area of 0.9 cm2 and compared outcome to that of 55 randomly selected control patients.18 The investigators defined cardiac complications as onset of congestive heart failure, myocardial infarction within 7 postoperative days, dysrhythmias requiring cardioversion, unplanned or prolonged intensive care unit stay resulting from cardiac complications, and cardiac death. Cardiac complications occurred in 5 (9%) patients with AS and 6 (11%) control patients. There was 1 cardiac death among patients with AS.
| Study (Year) | Study Type | No. of Patients | Summary of Patients | Outcomes | Other Comments |
|---|---|---|---|---|---|
| |||||
| McBrien et al11 (2009) | Database study of all patients with hip fracture admitted to a single hospital in Belfast, UK, 20012005 | 272 | Hip fracture, mild (AVA 1.52.0, peak velocity 1.72.9 m/sec): 168 patients; moderate (AVA 1.01.4, peak velocity 3.04.0): 64 patients; severe (AVA <1.0, peak velocity >4.0): 30 patients. Control group without AS: 3481 patients | 30‐day mortality: mild AS, 3.9%; moderate AS, 6.2%; severe AS, 5.1%. Controls, 7.4% | Invasive blood pressure monitoring used more frequently for patients with AS |
| Calleja et al23 (2010) | Retrospective chart review of patients with AS who underwent noncardiac surgery, 19982007; compared patients with severe AS to age‐ and gender‐matched controls with lesser AS | 30 patients with severe AS | Severe AS defined as AVA <1.0, peak velocity >40 m/sec. Most surgeries considered intermediate risk | Intraoperative hypotension more common in patients with severe AS (30% vs 17%). Perioperative MI 3% in severe AS and controls; no deaths in patients with severe AS | 80% of cases involved general anesthesia; 80% were elective |
| Raymer and Yang18 (1998) | Retrospective chart audit of patients with AS who underwent noncardiac surgery compared to matching controls | 55 patients | Mild (AVA 1.01.6 cm2): 18 patients; moderate (AVA 0.80.99 cm2): 13; severe (AVA <0.8 cm2): 24 | 5/55 (9%) AS patients experienced postoperative complications (2 heart failure; 1 ventricular fibrillation; 1 MI and CHF; 1 MI, CHF, and death); 6/55 control patients had cardiac complications | Controls and cases not well‐matched. Death occurred in 84‐year‐old patient, with AVA 0.7 cm2, undergoing an abdominal aortic aneurysm repair |
| Torsher et al21 (1998) | Retrospective record review of all patients with severe AS (AVA <0.5 cm2/m2 body surface area or mean gradient >50 mmHg), undergoing noncardiac surgery at Mayo Clinic, Rochester, MN, 19881992 | 19 patients (28 surgical procedures) | 84% of patients were symptomatic, most with dyspnea. Mean AVA for the group was 0.67 cm2 with AVA index 0.37 cm2/m2 | 2/19 (11%) postoperative cardiac events (both deaths) | Intraoperative hypotension requiring vasopressors occurred in 16 procedures among 14 patients |
| Kertai et al19 (2004) | Retrospective study at Erasmus Medical Center, Rotterdam, the Netherlands, of all patients with moderate (mean gradient 2529 mmHg) or severe (mean gradient >50 mmHg) AS undergoing noncardiac surgery, 19912000; compared to controls from the same database | 108 patients | 92 patients with moderate AS, 16 with severe AS: 38% vascular, 21% orthopedic, 12% abdominal procedures | 15 deaths or nonfatal MI among patients with AS (14% event rate); 4 events among 216 controls (1.8%) | Patients had higher cardiac risk indicators prior to surgery and were much older than controls. RCRI was predictive of events among patients with AS; RCRI 0 points = 0% rate, 1 point = 10%, 2 points = 16%, 3 points or more = 29% |
| Zahid et al22 (2005) | National Hospital Discharge Survey Database patients diagnosed with AS who underwent noncardiac surgery compared 1:2 to matched controls without AS, 19962002 | 5149 patients with diagnosis of AS | 59.7% low‐risk, 35.4% moderate‐risk, 4.9% high‐risk surgery; 29.6% patients known to have heart failure, 15.0% coronary artery disease | Acute MI 3.9% patients with AS; 2.0% controls. Death 5.4% AS patients vs 5.7% controls | Large database study that does not afford assessment of severity of AS or even echocardiographic confirmation of the diagnosis |
A retrospective analysis of 108 patients with AS who underwent noncardiac surgery, at Erasmus Medical Center in The Netherlands between 1991 and 2000, provides insight regarding severity of stenosis and perioperative outcomes.19 Cardiac complications (cardiac death or nonfatal myocardial infarction within 30 days of surgery) occurred in 15/108 (14%) patients with AS, with the majority of these complications being cardiac deaths. A control group of 216 patients suffered a cardiac complication rate of 1.8%. Multivariate adjustment for other risk factors demonstrated an odds ratio of 5.2 (95% CI 1.617.0) for cardiovascular complication in patients with AS. Moderate AS was associated with 11% complication rate (10/92 patients), while severe stenosis was associated with 31% cardiac complications (5/16 patients). Table 3 summarizes cardiac risk among the patients in this study using the Revised Cardiac Risk Index.20
| RCRI* Risk Indicators | Patients With Aortic Stenosis | Patients Without Aortic Stenosis |
|---|---|---|
| ||
| 0 | 0/18 (0%) | 0/108 (0%) |
| 1 | 3/31 (10%) | 2/64 (3%) |
| 2 | 6/38 (16%) | 1/33 (3%) |
| 3 or more | 6/21 (29%) | 1/18 (6%) |
In contrast, the Mayo Clinic experience with severe AS (defined as an aortic valve area index <0.5 cm2/m2 or mean transvalvular gradient >50 mmHg) suggested substantially lower complication rates among patients undergoing noncardiac surgery.21 In this series of 19 patients undergoing a variety of surgical procedures between 1988 and 1992, there were no intraoperative events, but 2 (11%) major postoperative events (1 myocardial infarction and 1 death related to multiorgan failure). The authors concluded that selected patients with severe AS could undergo noncardiac surgery with acceptable risk, and speculated that their experience of better outcomes was due to more aggressive intraoperative and postoperative monitoring and therapy, specifically prompt recognition and therapy of intraoperative hypotension.
A large database study identified 5149 patients undergoing noncardiac surgery, between 1996 and 2002, with a coexistent AS based on International Classification of Diseases, Ninth Revision (ICD‐9) discharge codes, and compared these patients to 10,284 controls.22 Acute myocardial infarction occurred more frequently among patients with AS (3.9% vs 2.0%, P < 0.001), but in‐hospital mortality was not more frequent (5.4% vs 5.7%). The association of perioperative nonfatal myocardial infarction persisted after adjustment for comorbidities. While the results of this study might be interpreted as showing no increase in perioperative mortality for patients with AS who are undergoing noncardiac surgery, there is no way to determine the severity of AS among study patients and endpoints were not uniformly sought, but rather, obtained by ICD‐9 reporting. A recent study of 30 patients with asymptomatic but severe AS, who underwent low‐ or intermediate‐risk noncardiac surgery, found that 30% of patients required intraoperative vasopressor use for hypotension, but there were no deaths, arrhythmias, or heart failure events.23
Summarizing evidence on noncardiac surgery for patients with AS, symptomatic AS is associated with an increased risk of adverse cardiac events in patients undergoing noncardiac surgery. Severe, asymptomatic AS increases risk of intraoperative hemodynamic instability and adverse perioperative cardiac outcomes, although mortality appears to be less than that associated with symptomatic AS.
ECHOCARDIOGRAPHY PRIOR TO NONCARDIAC SURGERY
There are no studies showing that preoperative echocardiograms lessen the perioperative risk for patients with AS. However, as noted earlier, physical examination alone is not adequate to determine the valvular abnormality causing a systolic murmur in many patients, nor is the exam accurate in determining severity of AS in many patients. Echocardiography clarifies both of these issues. Preoperative echocardiography should inform the approach to anesthesia and, for elective surgical procedures, should allow more accurate assessment of operative risk. Because aortic stenosis typically progresses in a relatively slow and steady fashion, demonstration of mild aortic stenosis by echocardiogram within the preceding few years is considered reassuring.
Emergent surgery (for example, exploratory laparotomy for a ruptured viscus) typically does not allow time for echocardiography prior to the procedure. If a previous echocardiogram is available, this may be useful in deciding the intensity of intraoperative monitoring. However, the presence of a suspicious systolic murmur should prompt careful hemodynamic monitoring and the anesthesiologist should be made aware of the suspicion of AS.
For patients with AS facing urgent surgery (for example, repair of a hip fracture), there is typically time to review previous echocardiograms and, if there has been no recent echocardiogram, it is reasonable to obtain one. The presence of severe AS by echocardiogram should prompt careful hemodynamic monitoring. Some anesthesiologists advocate the use of intraoperative transesophageal echocardiography (TEE) to monitor ventricular filling in patients with severe AS.2426 Intraoperative TEE provides real‐time assessment of the cause of left ventricular dysfunction and allows the anesthesiologist to manipulate hemodynamics to address the dysfunction. Intraoperative TEE prompted significant changes in therapy for 4 of 7 patients with AS in a larger cohort of noncardiac surgical patients monitored with TEE.27 A retrospective study of 123 intraoperative TEE examinations found an impact on management in 81% of patients undergoing noncardiac surgery, although only a small number of these patients had cardiac valvular abnormalities.28 Recent anesthesiology practice guidelines recommend that TEE be considered in patients who have cardiovascular pathology that might result in severe hemodynamic, pulmonary, or neurologic compromise.29 The anesthesiologist should decide potential utility of intraoperative TEE, but it is important that the consulting hospitalist be aware of this possible approach to hemodynamic monitoring. Intraoperative TEE requires specialized expertise and may not available in many hospitals.
For elective surgery, presence of a murmur suggestive of significant AS mandates echocardiography, unless there are study results available from the preceding year.30 Optimally, symptomatic AS should be addressed by aortic valve replacement prior to noncardiac surgery. For patients requiring semi‐urgent surgery but are deteriorating because of severe AS, temporizing percutaneous balloon valvuloplasty can be considered, but there are limited data and serious complication rates can be high.3133 Among 15 AS patients requiring noncardiac surgery but with a contraindication to valve replacement, 3 experienced ventricular perforation during percutaneous balloon valvuloplasty, with 1 death.31 In another series of 7 patients, there were no complications of the valvuloplasties, and all 7 patients underwent uncomplicated noncardiac surgery under general anesthesia thereafter.33
In the absence of interventions to improve cardiac hemodynamics, patients could proceed to necessary noncardiac surgery, understanding the high risk of mortality and morbidity (Table 2). These patients should have careful perioperative hemodynamic monitoring and could be considered for intraoperative TEE if available.
Patients with asymptomatic but severe AS can proceed to low‐ or moderate‐risk surgical procedures without further intervention, but with appropriate hemodynamic monitoring. Those patients with asymptomatic but severe AS needing high‐risk surgery should consider valve replacement prior to surgery. In addition, we believe most patients with severe AS should have a cardiologist involved in their perioperative care.
CONCLUSIONS
In summary, patients with suspected AS who require noncardiac surgery need thoughtful consideration by the medical consultant. Careful cardiac examination should be performed on all patients prior to noncardiac surgery. If there is no precordial murmur radiating to the right carotid artery or right clavicle, and if there are no other signs (eg, delayed or reduced carotid upstroke, or absent or distant second heart sound) or symptoms (eg, history of angina, congestive heart failure, or exertional syncope or presyncope), then echocardiography performed for the purpose of discovering AS is not necessary. The majority of patients with a suggestive systolic murmur should be evaluated with echocardiography to provide more accurate prognostic estimates and to guide hemodynamic management during the operation. Patients with severe symptomatic AS are at particularly high risk of cardiac complications, and aortic valve replacement should take priority if the noncardiac surgery can be delayed.
Acknowledgements
The authors would like to acknowledge Dr. Jason Qu for his advice on intraope rative TEE.
Note Added in Proof
Disclosure: Nothing to report.
- , , , , , . Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011.
- . Valvular aortic stenosis in the elderly. Cardiol Rev. 2007;15:217–225.
- , . Aortic stenosis. Lancet. 2009;373:956–966.
- , . Aortic valve stenosis. Anesthesiol Clin. 2009;27:519–532.
- , , . Hypotensive epidural anesthesia in patients with aortic stenosis undergoing total hip replacement. Reg Anesth Pain Med. 2008;33:129–133.
- , , . Identifying severe aortic valvular stenosis by bedside examination. Acta Med Scand. 1985;218:397–400.
- , , , , , . Physical examination in valvular aortic stenosis: correlation with stenosis severity and prediction of clinical outcome. Am Heart J. 1999;137:298–306.
- , , , , . A bedside clinical prediction rule for detecting moderate or severe aortic stenosis. J Gen Intern Med. 1998;13:699–704.
- , , . Does this patient have an abnormal systolic murmur? JAMA. 1997;277:564–571.
- . Etiology and diagnosis of systolic murmurs in adults. Am J Med. 2010;123:913–921.
- , , , et al. Previously undiagnosed aortic stenosis revealed by auscultation in the hip fracture population—echocardiographic findings, management and outcome. Anaesthesia. 2009;64:863–870.
- , . The natural history of aortic valve stenosis. Eur Heart J. 1988;9(suppl E):57–64.
- , , , et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617.
- , , , et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;111:3290–3295.
- , , , et al. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156.
- , . Surgical risk in the cardiac patient. J Chronic Dis. 1964;17:57–72.
- , , , et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850.
- , . Patients with aortic stenosis: cardiac complications in non‐cardiac surgery. Can J Anaesth. 1998;45:855–859.
- , , , et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med. 2004;116:8–13.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , . Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 1998;81:448–452.
- , , , . Perioperative risk of noncardiac surgery associated with aortic stenosis. Am J Cardiol. 2005;96:436–438.
- , , , , , . Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159–1163.
- , . Role of intraoperative transesophageal echocardiography in patients undergoing noncardiac surgery. J Cardiovasc Med (Hagerstown). 2008;9:993–1003.
- , , , . Preoperative and perioperative care for patients with suspected or established aortic stenosis facing noncardiac surgery. Chest. 2005;128:2944–2953.
- , . Impact of TEE in noncardiac surgery. Int Anesthesiol Clin. 2008;46:121–136.
- , , , . Impact of intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:768–771.
- , , , . Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274–280.
- Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096.
- , , , et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–1996.
- , , , . Palliative percutaneous aortic balloon valvuloplasty before noncardiac operations and invasive diagnostic procedures. Mayo Clin Proc. 1989;64:753–757.
- , , , , . Palliation of valvular aortic stenosis by balloon valvuloplasty as preoperative preparation for noncardiac surgery. Am J Cardiol. 1988;62:1309–1310.
- , , . Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039–1041.
- , , , et al. 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142.
Aortic stenosis (AS) is a common problem among aging patients,1 who often require surgical procedures. The medical consultant must determine whether the presence of a systolic murmur suggesting AS needs additional evaluation before the patient proceeds to surgery. This decision requires interpretation of cardiac murmurs, and understanding the natural history, pathophysiology, and risks of AS.
PATHOPHYSIOLOGY
Aortic stenosis is a progressive disease that leads to predictable impairment of cardiac responses to physiologic stresses of surgery. AS typically results from degenerative calcification or from a bicuspid aortic valve, both of which cause progressive constriction of left ventricular outflow.24 The heart compensates by left ventricular hypertrophy. Systolic ejection of blood across the stenotic valve requires more time than normal, leaving less time for diastolic refilling. Left ventricular hypertrophy creates a less compliant left ventricle that becomes dependent on left atrial contraction for optimal filling. Atrial fibrillation with loss of the atrial kick is particularly problematic for patients with AS and left ventricular hypertrophy. Thickened myocardium increases myocardial oxygen consumption and impairs myocardial perfusion. Myocardial oxygen demand in the hypertrophied ventricle results from increased systolic pressure on the ventricle, increased systolic contraction time, and increased muscle mass. Reduced capillary density in hypertrophied muscle, and diminished perfusion pressure because of a reduced aortic‐coronary pressure differential, impair myocardial perfusion. Shortened diastole allows less blood flow to the myocardium.
At rest, with a controlled heart rate and sinus rhythm to allow for left atrial contraction to enhance left ventricular filling, patients may tolerate significant AS. However, increased heart rate in response to physiologic stress reduces diastolic filling time, diminishes somewhat tenuous myocardial perfusion, and increases afterload.
Additionally, the left ventricle depends on adequate filling pressures; the hypertrophied ventricle is prone to reduced cardiac output because of reductions of preload caused by hypovolemia or venodilation. Venodilation has been a particular concern with epidural anesthesia, although recent studies suggest that this modality can be used safely.5 Many anesthetic agents reduce systemic blood pressure and thereby reduce the aortic‐coronary perfusion pressure gradient leading to reduced coronary blood flow. For surgical patients with significant AS, anesthetic management requires appropriate intravascular volume to optimize preload, heart rate control to allow adequate left ventricular filling along with time for coronary artery flow, and sufficient systemic blood pressure to maintain coronary artery blood flow.
IDENTIFYING AORTIC STENOSIS IN PREOPERATIVE PATIENTS AND JUDGING ITS SEVERITY
Many older patients are found to have a systolic murmur consistent with AS prior to surgery. The first step in evaluation is a detailed history to determine exercise capacity and to elicit any history of chest pain, heart failure symptoms, or syncope. A key question for the medical consultant is whether or not patients should have further evaluation of the murmur prior to surgery, typically starting with transthoracic echocardiography. Table 1 outlines echocardiographic criteria for grading AS severity. The history and physical exam inform the decision of whether to pursue echocardiography. Although it is not clear from the literature whether identification of AS by echocardiography improves outcomes (this question is unlikely to be addressed by randomized trials), anesthesiologists generally want to know if significant AS is present, as it impacts intraoperative monitoring and management. So the question then becomes the following: Can clinicians reliably exclude moderatesevere AS based on history and a careful cardiovascular exam?
| Aortic Stenosis | |||
|---|---|---|---|
| Indicator | Mild | Moderate | Severe |
| |||
| Jet velocity (m/s) | <3.0 | 3.04.0 | >4.0 |
| Mean gradient (mmHg) | <25 | 2540 | >40 |
| Valve area (cm2) | >1.5 | 1.01.5 | <1.0 |
| Valve area index (cm2/m2) | <0.6 | ||
For ruling in severe AS, effort syncope provides the highest positive predictive value; stenosis was found to be severe in all patients with a history of effort syncope in a sample of 67 patients with AS.6 The presence of a loud, late‐peaking systolic murmur or significant delay and decrease in the carotid upstroke, argue for severe AS.7 Etchells et al developed a simple decision rule for detecting moderatesevere AS (defined as an aortic valve area of 1.2 cm2 or less, or a peak transvalvular gradient of 25 mmHg or more), based on a study of 162 inpatients who were examined by a senior medical resident and a general internist.8 If no murmur was heard over the right clavicle, AS was rare (1/69 [1.4%]; likelihood ratio (LR) 0.10 [95% confidence interval (CI) 0.020.44]). If there was a murmur radiating to the right clavicle with 3 to 4 associated findings (reduced second heart sound, reduced carotid volume, slow carotid upstroke, and murmur loudest in the second right intercostal space), moderatesevere AS was common (6/7 [86%]; LR 40 [95% CI 6.6239]).
Absence of radiation of a systolic murmur to the right carotid artery is a useful finding to exclude AS, with a negative likelihood ratio of 0.05 to 0.10.9 Although no single physical exam finding or combination of findings can reliably exclude hemodynamically significant AS when a systolic murmur radiates to the right neck, the combination of an early‐peaking, soft (grade 2 or less) systolic murmur, normal timing and upstroke of the carotids, and an audible aortic second sound substantially lessen the likelihood of severe AS. A recent study of 376 inpatients who underwent meticulous cardiac examination by a single investigator (blinded to the diagnosis in >96% of cases), followed by echocardiography, provides additional information about the operating characteristics of physical examination in determining the etiology of systolic murmurs.10 Murmurs heard diagonally across the chest from the right upper sternal border to the apex (broad apical‐base pattern) predicted increased aortic velocity that would be consistent with AS. Other findings that increased the likelihood of aortic valve disease included delayed carotid upstroke, absent second heart sound (S2), radiation to the clavicles and neck on both sides, and a humming quality to the murmur. This study concluded that the physical examination is not reliable in determining the severity of AS. While generally true, this study actually reveals that any pattern of murmur radiation other than the broad apical‐base pattern excluded severe AS entirely among 221 patients with murmurs, and excluded moderate AS in all but 3 of these patients.
A retrospective study of 3997 hip fracture patients evaluated 908 echocardiograms done to investigate cardiac murmurs detected during preoperative assessment.11 These echocardiograms detected 272 patients with AS that had not been previously diagnosed. Thirty patients had severe AS. Detection of AS prompted changes in anesthesia management. The authors argued for preoperative echocardiograms for all hip fracture patients in whom a murmur is detected.
In summary, no finding by history can exclude AS. However, if the murmur is not heard across the precordium and does not radiate to the clavicle or right neck, severe AS is very unlikely.10 For patients in whom the murmur suggests the possibility of severe AS, echocardiography is prudent.
PROGNOSIS OF ADVANCED AS
Symptomatic AS portends poor prognosis in the absence of aortic valve replacement. In a cohort of patients with severe AS who refused aortic valve replacement (AVR), patients survived a mean of 45 months after onset of angina, 27 months following onset of syncope, and only 11 months after the beginning of left heart failure.12 Recent studies further define the natural history of severe asymptomatic AS. A study of 128 consecutive patients with asymptomatic severe AS identified by echocardiography found 93% survival at 1 year, 91% at 2 years, and 87% at 4 years, suggesting a relatively benign prognosis.13 However, many patients developed symptoms during follow‐up and required aortic valve replacement. A larger study of 622 asymptomatic AS patients with aortic‐jet velocity greater than 4 m/s found that 82% of patients were free of cardiac symptoms after 1 year, but only 33% were free of cardiac symptoms or intervention at 5 years.14 Patients with asymptomatic, very severe AS, defined as peak aortic‐jet velocity of 5.0 m/s or greater have an even worse prognosis with an event‐free survival of 12% at 4 years and only 3% at 6 years.15
Although short‐term (1 to 5 years) prognosis for severe symptomatic AS is poor, and asymptomatic but severe AS also carries substantial risk, the major issue for the medical consultant evaluating patients prior to noncardiac surgery is the very short‐term perioperative risk imposed by AS. Put simply, will the patient survive surgery and the postoperative period of rehabilitation?
NONCARDIAC SURGERY AND AS
The evidence that AS increases risk of cardiac complications and cardiac death for patients undergoing noncardiac surgery is limited to retrospective studies. In the early 1960s, a retrospective study of cardiac risk among 766 patients found 10% mortality among 59 patients with an aortic valve abnormality.16 The 15 patients who underwent either intrathoracic or intra‐abdominal procedures did particularly poorly, with a mortality of 20%. As part of a large cohort study used to develop the first widely employed cardiac risk index for noncardiac surgery, Goldman et al found 13% (3/23 patients) cardiac mortality among patients with important valvular AS.17 In comparison, cardiac mortality among 978 patients without identified AS was 1.6% (16/978 patients).
More recent studies demonstrate lower perioperative mortality for AS patients. These studies are summarized in Table 2. A retrospective chart audit of all patients with AS who underwent noncardiac surgery, in Hamilton, Ontario, Canada between 1992 and 1994, identified 55 patients with a mean aortic valve area of 0.9 cm2 and compared outcome to that of 55 randomly selected control patients.18 The investigators defined cardiac complications as onset of congestive heart failure, myocardial infarction within 7 postoperative days, dysrhythmias requiring cardioversion, unplanned or prolonged intensive care unit stay resulting from cardiac complications, and cardiac death. Cardiac complications occurred in 5 (9%) patients with AS and 6 (11%) control patients. There was 1 cardiac death among patients with AS.
| Study (Year) | Study Type | No. of Patients | Summary of Patients | Outcomes | Other Comments |
|---|---|---|---|---|---|
| |||||
| McBrien et al11 (2009) | Database study of all patients with hip fracture admitted to a single hospital in Belfast, UK, 20012005 | 272 | Hip fracture, mild (AVA 1.52.0, peak velocity 1.72.9 m/sec): 168 patients; moderate (AVA 1.01.4, peak velocity 3.04.0): 64 patients; severe (AVA <1.0, peak velocity >4.0): 30 patients. Control group without AS: 3481 patients | 30‐day mortality: mild AS, 3.9%; moderate AS, 6.2%; severe AS, 5.1%. Controls, 7.4% | Invasive blood pressure monitoring used more frequently for patients with AS |
| Calleja et al23 (2010) | Retrospective chart review of patients with AS who underwent noncardiac surgery, 19982007; compared patients with severe AS to age‐ and gender‐matched controls with lesser AS | 30 patients with severe AS | Severe AS defined as AVA <1.0, peak velocity >40 m/sec. Most surgeries considered intermediate risk | Intraoperative hypotension more common in patients with severe AS (30% vs 17%). Perioperative MI 3% in severe AS and controls; no deaths in patients with severe AS | 80% of cases involved general anesthesia; 80% were elective |
| Raymer and Yang18 (1998) | Retrospective chart audit of patients with AS who underwent noncardiac surgery compared to matching controls | 55 patients | Mild (AVA 1.01.6 cm2): 18 patients; moderate (AVA 0.80.99 cm2): 13; severe (AVA <0.8 cm2): 24 | 5/55 (9%) AS patients experienced postoperative complications (2 heart failure; 1 ventricular fibrillation; 1 MI and CHF; 1 MI, CHF, and death); 6/55 control patients had cardiac complications | Controls and cases not well‐matched. Death occurred in 84‐year‐old patient, with AVA 0.7 cm2, undergoing an abdominal aortic aneurysm repair |
| Torsher et al21 (1998) | Retrospective record review of all patients with severe AS (AVA <0.5 cm2/m2 body surface area or mean gradient >50 mmHg), undergoing noncardiac surgery at Mayo Clinic, Rochester, MN, 19881992 | 19 patients (28 surgical procedures) | 84% of patients were symptomatic, most with dyspnea. Mean AVA for the group was 0.67 cm2 with AVA index 0.37 cm2/m2 | 2/19 (11%) postoperative cardiac events (both deaths) | Intraoperative hypotension requiring vasopressors occurred in 16 procedures among 14 patients |
| Kertai et al19 (2004) | Retrospective study at Erasmus Medical Center, Rotterdam, the Netherlands, of all patients with moderate (mean gradient 2529 mmHg) or severe (mean gradient >50 mmHg) AS undergoing noncardiac surgery, 19912000; compared to controls from the same database | 108 patients | 92 patients with moderate AS, 16 with severe AS: 38% vascular, 21% orthopedic, 12% abdominal procedures | 15 deaths or nonfatal MI among patients with AS (14% event rate); 4 events among 216 controls (1.8%) | Patients had higher cardiac risk indicators prior to surgery and were much older than controls. RCRI was predictive of events among patients with AS; RCRI 0 points = 0% rate, 1 point = 10%, 2 points = 16%, 3 points or more = 29% |
| Zahid et al22 (2005) | National Hospital Discharge Survey Database patients diagnosed with AS who underwent noncardiac surgery compared 1:2 to matched controls without AS, 19962002 | 5149 patients with diagnosis of AS | 59.7% low‐risk, 35.4% moderate‐risk, 4.9% high‐risk surgery; 29.6% patients known to have heart failure, 15.0% coronary artery disease | Acute MI 3.9% patients with AS; 2.0% controls. Death 5.4% AS patients vs 5.7% controls | Large database study that does not afford assessment of severity of AS or even echocardiographic confirmation of the diagnosis |
A retrospective analysis of 108 patients with AS who underwent noncardiac surgery, at Erasmus Medical Center in The Netherlands between 1991 and 2000, provides insight regarding severity of stenosis and perioperative outcomes.19 Cardiac complications (cardiac death or nonfatal myocardial infarction within 30 days of surgery) occurred in 15/108 (14%) patients with AS, with the majority of these complications being cardiac deaths. A control group of 216 patients suffered a cardiac complication rate of 1.8%. Multivariate adjustment for other risk factors demonstrated an odds ratio of 5.2 (95% CI 1.617.0) for cardiovascular complication in patients with AS. Moderate AS was associated with 11% complication rate (10/92 patients), while severe stenosis was associated with 31% cardiac complications (5/16 patients). Table 3 summarizes cardiac risk among the patients in this study using the Revised Cardiac Risk Index.20
| RCRI* Risk Indicators | Patients With Aortic Stenosis | Patients Without Aortic Stenosis |
|---|---|---|
| ||
| 0 | 0/18 (0%) | 0/108 (0%) |
| 1 | 3/31 (10%) | 2/64 (3%) |
| 2 | 6/38 (16%) | 1/33 (3%) |
| 3 or more | 6/21 (29%) | 1/18 (6%) |
In contrast, the Mayo Clinic experience with severe AS (defined as an aortic valve area index <0.5 cm2/m2 or mean transvalvular gradient >50 mmHg) suggested substantially lower complication rates among patients undergoing noncardiac surgery.21 In this series of 19 patients undergoing a variety of surgical procedures between 1988 and 1992, there were no intraoperative events, but 2 (11%) major postoperative events (1 myocardial infarction and 1 death related to multiorgan failure). The authors concluded that selected patients with severe AS could undergo noncardiac surgery with acceptable risk, and speculated that their experience of better outcomes was due to more aggressive intraoperative and postoperative monitoring and therapy, specifically prompt recognition and therapy of intraoperative hypotension.
A large database study identified 5149 patients undergoing noncardiac surgery, between 1996 and 2002, with a coexistent AS based on International Classification of Diseases, Ninth Revision (ICD‐9) discharge codes, and compared these patients to 10,284 controls.22 Acute myocardial infarction occurred more frequently among patients with AS (3.9% vs 2.0%, P < 0.001), but in‐hospital mortality was not more frequent (5.4% vs 5.7%). The association of perioperative nonfatal myocardial infarction persisted after adjustment for comorbidities. While the results of this study might be interpreted as showing no increase in perioperative mortality for patients with AS who are undergoing noncardiac surgery, there is no way to determine the severity of AS among study patients and endpoints were not uniformly sought, but rather, obtained by ICD‐9 reporting. A recent study of 30 patients with asymptomatic but severe AS, who underwent low‐ or intermediate‐risk noncardiac surgery, found that 30% of patients required intraoperative vasopressor use for hypotension, but there were no deaths, arrhythmias, or heart failure events.23
Summarizing evidence on noncardiac surgery for patients with AS, symptomatic AS is associated with an increased risk of adverse cardiac events in patients undergoing noncardiac surgery. Severe, asymptomatic AS increases risk of intraoperative hemodynamic instability and adverse perioperative cardiac outcomes, although mortality appears to be less than that associated with symptomatic AS.
ECHOCARDIOGRAPHY PRIOR TO NONCARDIAC SURGERY
There are no studies showing that preoperative echocardiograms lessen the perioperative risk for patients with AS. However, as noted earlier, physical examination alone is not adequate to determine the valvular abnormality causing a systolic murmur in many patients, nor is the exam accurate in determining severity of AS in many patients. Echocardiography clarifies both of these issues. Preoperative echocardiography should inform the approach to anesthesia and, for elective surgical procedures, should allow more accurate assessment of operative risk. Because aortic stenosis typically progresses in a relatively slow and steady fashion, demonstration of mild aortic stenosis by echocardiogram within the preceding few years is considered reassuring.
Emergent surgery (for example, exploratory laparotomy for a ruptured viscus) typically does not allow time for echocardiography prior to the procedure. If a previous echocardiogram is available, this may be useful in deciding the intensity of intraoperative monitoring. However, the presence of a suspicious systolic murmur should prompt careful hemodynamic monitoring and the anesthesiologist should be made aware of the suspicion of AS.
For patients with AS facing urgent surgery (for example, repair of a hip fracture), there is typically time to review previous echocardiograms and, if there has been no recent echocardiogram, it is reasonable to obtain one. The presence of severe AS by echocardiogram should prompt careful hemodynamic monitoring. Some anesthesiologists advocate the use of intraoperative transesophageal echocardiography (TEE) to monitor ventricular filling in patients with severe AS.2426 Intraoperative TEE provides real‐time assessment of the cause of left ventricular dysfunction and allows the anesthesiologist to manipulate hemodynamics to address the dysfunction. Intraoperative TEE prompted significant changes in therapy for 4 of 7 patients with AS in a larger cohort of noncardiac surgical patients monitored with TEE.27 A retrospective study of 123 intraoperative TEE examinations found an impact on management in 81% of patients undergoing noncardiac surgery, although only a small number of these patients had cardiac valvular abnormalities.28 Recent anesthesiology practice guidelines recommend that TEE be considered in patients who have cardiovascular pathology that might result in severe hemodynamic, pulmonary, or neurologic compromise.29 The anesthesiologist should decide potential utility of intraoperative TEE, but it is important that the consulting hospitalist be aware of this possible approach to hemodynamic monitoring. Intraoperative TEE requires specialized expertise and may not available in many hospitals.
For elective surgery, presence of a murmur suggestive of significant AS mandates echocardiography, unless there are study results available from the preceding year.30 Optimally, symptomatic AS should be addressed by aortic valve replacement prior to noncardiac surgery. For patients requiring semi‐urgent surgery but are deteriorating because of severe AS, temporizing percutaneous balloon valvuloplasty can be considered, but there are limited data and serious complication rates can be high.3133 Among 15 AS patients requiring noncardiac surgery but with a contraindication to valve replacement, 3 experienced ventricular perforation during percutaneous balloon valvuloplasty, with 1 death.31 In another series of 7 patients, there were no complications of the valvuloplasties, and all 7 patients underwent uncomplicated noncardiac surgery under general anesthesia thereafter.33
In the absence of interventions to improve cardiac hemodynamics, patients could proceed to necessary noncardiac surgery, understanding the high risk of mortality and morbidity (Table 2). These patients should have careful perioperative hemodynamic monitoring and could be considered for intraoperative TEE if available.
Patients with asymptomatic but severe AS can proceed to low‐ or moderate‐risk surgical procedures without further intervention, but with appropriate hemodynamic monitoring. Those patients with asymptomatic but severe AS needing high‐risk surgery should consider valve replacement prior to surgery. In addition, we believe most patients with severe AS should have a cardiologist involved in their perioperative care.
CONCLUSIONS
In summary, patients with suspected AS who require noncardiac surgery need thoughtful consideration by the medical consultant. Careful cardiac examination should be performed on all patients prior to noncardiac surgery. If there is no precordial murmur radiating to the right carotid artery or right clavicle, and if there are no other signs (eg, delayed or reduced carotid upstroke, or absent or distant second heart sound) or symptoms (eg, history of angina, congestive heart failure, or exertional syncope or presyncope), then echocardiography performed for the purpose of discovering AS is not necessary. The majority of patients with a suggestive systolic murmur should be evaluated with echocardiography to provide more accurate prognostic estimates and to guide hemodynamic management during the operation. Patients with severe symptomatic AS are at particularly high risk of cardiac complications, and aortic valve replacement should take priority if the noncardiac surgery can be delayed.
Acknowledgements
The authors would like to acknowledge Dr. Jason Qu for his advice on intraope rative TEE.
Note Added in Proof
Disclosure: Nothing to report.
Aortic stenosis (AS) is a common problem among aging patients,1 who often require surgical procedures. The medical consultant must determine whether the presence of a systolic murmur suggesting AS needs additional evaluation before the patient proceeds to surgery. This decision requires interpretation of cardiac murmurs, and understanding the natural history, pathophysiology, and risks of AS.
PATHOPHYSIOLOGY
Aortic stenosis is a progressive disease that leads to predictable impairment of cardiac responses to physiologic stresses of surgery. AS typically results from degenerative calcification or from a bicuspid aortic valve, both of which cause progressive constriction of left ventricular outflow.24 The heart compensates by left ventricular hypertrophy. Systolic ejection of blood across the stenotic valve requires more time than normal, leaving less time for diastolic refilling. Left ventricular hypertrophy creates a less compliant left ventricle that becomes dependent on left atrial contraction for optimal filling. Atrial fibrillation with loss of the atrial kick is particularly problematic for patients with AS and left ventricular hypertrophy. Thickened myocardium increases myocardial oxygen consumption and impairs myocardial perfusion. Myocardial oxygen demand in the hypertrophied ventricle results from increased systolic pressure on the ventricle, increased systolic contraction time, and increased muscle mass. Reduced capillary density in hypertrophied muscle, and diminished perfusion pressure because of a reduced aortic‐coronary pressure differential, impair myocardial perfusion. Shortened diastole allows less blood flow to the myocardium.
At rest, with a controlled heart rate and sinus rhythm to allow for left atrial contraction to enhance left ventricular filling, patients may tolerate significant AS. However, increased heart rate in response to physiologic stress reduces diastolic filling time, diminishes somewhat tenuous myocardial perfusion, and increases afterload.
Additionally, the left ventricle depends on adequate filling pressures; the hypertrophied ventricle is prone to reduced cardiac output because of reductions of preload caused by hypovolemia or venodilation. Venodilation has been a particular concern with epidural anesthesia, although recent studies suggest that this modality can be used safely.5 Many anesthetic agents reduce systemic blood pressure and thereby reduce the aortic‐coronary perfusion pressure gradient leading to reduced coronary blood flow. For surgical patients with significant AS, anesthetic management requires appropriate intravascular volume to optimize preload, heart rate control to allow adequate left ventricular filling along with time for coronary artery flow, and sufficient systemic blood pressure to maintain coronary artery blood flow.
IDENTIFYING AORTIC STENOSIS IN PREOPERATIVE PATIENTS AND JUDGING ITS SEVERITY
Many older patients are found to have a systolic murmur consistent with AS prior to surgery. The first step in evaluation is a detailed history to determine exercise capacity and to elicit any history of chest pain, heart failure symptoms, or syncope. A key question for the medical consultant is whether or not patients should have further evaluation of the murmur prior to surgery, typically starting with transthoracic echocardiography. Table 1 outlines echocardiographic criteria for grading AS severity. The history and physical exam inform the decision of whether to pursue echocardiography. Although it is not clear from the literature whether identification of AS by echocardiography improves outcomes (this question is unlikely to be addressed by randomized trials), anesthesiologists generally want to know if significant AS is present, as it impacts intraoperative monitoring and management. So the question then becomes the following: Can clinicians reliably exclude moderatesevere AS based on history and a careful cardiovascular exam?
| Aortic Stenosis | |||
|---|---|---|---|
| Indicator | Mild | Moderate | Severe |
| |||
| Jet velocity (m/s) | <3.0 | 3.04.0 | >4.0 |
| Mean gradient (mmHg) | <25 | 2540 | >40 |
| Valve area (cm2) | >1.5 | 1.01.5 | <1.0 |
| Valve area index (cm2/m2) | <0.6 | ||
For ruling in severe AS, effort syncope provides the highest positive predictive value; stenosis was found to be severe in all patients with a history of effort syncope in a sample of 67 patients with AS.6 The presence of a loud, late‐peaking systolic murmur or significant delay and decrease in the carotid upstroke, argue for severe AS.7 Etchells et al developed a simple decision rule for detecting moderatesevere AS (defined as an aortic valve area of 1.2 cm2 or less, or a peak transvalvular gradient of 25 mmHg or more), based on a study of 162 inpatients who were examined by a senior medical resident and a general internist.8 If no murmur was heard over the right clavicle, AS was rare (1/69 [1.4%]; likelihood ratio (LR) 0.10 [95% confidence interval (CI) 0.020.44]). If there was a murmur radiating to the right clavicle with 3 to 4 associated findings (reduced second heart sound, reduced carotid volume, slow carotid upstroke, and murmur loudest in the second right intercostal space), moderatesevere AS was common (6/7 [86%]; LR 40 [95% CI 6.6239]).
Absence of radiation of a systolic murmur to the right carotid artery is a useful finding to exclude AS, with a negative likelihood ratio of 0.05 to 0.10.9 Although no single physical exam finding or combination of findings can reliably exclude hemodynamically significant AS when a systolic murmur radiates to the right neck, the combination of an early‐peaking, soft (grade 2 or less) systolic murmur, normal timing and upstroke of the carotids, and an audible aortic second sound substantially lessen the likelihood of severe AS. A recent study of 376 inpatients who underwent meticulous cardiac examination by a single investigator (blinded to the diagnosis in >96% of cases), followed by echocardiography, provides additional information about the operating characteristics of physical examination in determining the etiology of systolic murmurs.10 Murmurs heard diagonally across the chest from the right upper sternal border to the apex (broad apical‐base pattern) predicted increased aortic velocity that would be consistent with AS. Other findings that increased the likelihood of aortic valve disease included delayed carotid upstroke, absent second heart sound (S2), radiation to the clavicles and neck on both sides, and a humming quality to the murmur. This study concluded that the physical examination is not reliable in determining the severity of AS. While generally true, this study actually reveals that any pattern of murmur radiation other than the broad apical‐base pattern excluded severe AS entirely among 221 patients with murmurs, and excluded moderate AS in all but 3 of these patients.
A retrospective study of 3997 hip fracture patients evaluated 908 echocardiograms done to investigate cardiac murmurs detected during preoperative assessment.11 These echocardiograms detected 272 patients with AS that had not been previously diagnosed. Thirty patients had severe AS. Detection of AS prompted changes in anesthesia management. The authors argued for preoperative echocardiograms for all hip fracture patients in whom a murmur is detected.
In summary, no finding by history can exclude AS. However, if the murmur is not heard across the precordium and does not radiate to the clavicle or right neck, severe AS is very unlikely.10 For patients in whom the murmur suggests the possibility of severe AS, echocardiography is prudent.
PROGNOSIS OF ADVANCED AS
Symptomatic AS portends poor prognosis in the absence of aortic valve replacement. In a cohort of patients with severe AS who refused aortic valve replacement (AVR), patients survived a mean of 45 months after onset of angina, 27 months following onset of syncope, and only 11 months after the beginning of left heart failure.12 Recent studies further define the natural history of severe asymptomatic AS. A study of 128 consecutive patients with asymptomatic severe AS identified by echocardiography found 93% survival at 1 year, 91% at 2 years, and 87% at 4 years, suggesting a relatively benign prognosis.13 However, many patients developed symptoms during follow‐up and required aortic valve replacement. A larger study of 622 asymptomatic AS patients with aortic‐jet velocity greater than 4 m/s found that 82% of patients were free of cardiac symptoms after 1 year, but only 33% were free of cardiac symptoms or intervention at 5 years.14 Patients with asymptomatic, very severe AS, defined as peak aortic‐jet velocity of 5.0 m/s or greater have an even worse prognosis with an event‐free survival of 12% at 4 years and only 3% at 6 years.15
Although short‐term (1 to 5 years) prognosis for severe symptomatic AS is poor, and asymptomatic but severe AS also carries substantial risk, the major issue for the medical consultant evaluating patients prior to noncardiac surgery is the very short‐term perioperative risk imposed by AS. Put simply, will the patient survive surgery and the postoperative period of rehabilitation?
NONCARDIAC SURGERY AND AS
The evidence that AS increases risk of cardiac complications and cardiac death for patients undergoing noncardiac surgery is limited to retrospective studies. In the early 1960s, a retrospective study of cardiac risk among 766 patients found 10% mortality among 59 patients with an aortic valve abnormality.16 The 15 patients who underwent either intrathoracic or intra‐abdominal procedures did particularly poorly, with a mortality of 20%. As part of a large cohort study used to develop the first widely employed cardiac risk index for noncardiac surgery, Goldman et al found 13% (3/23 patients) cardiac mortality among patients with important valvular AS.17 In comparison, cardiac mortality among 978 patients without identified AS was 1.6% (16/978 patients).
More recent studies demonstrate lower perioperative mortality for AS patients. These studies are summarized in Table 2. A retrospective chart audit of all patients with AS who underwent noncardiac surgery, in Hamilton, Ontario, Canada between 1992 and 1994, identified 55 patients with a mean aortic valve area of 0.9 cm2 and compared outcome to that of 55 randomly selected control patients.18 The investigators defined cardiac complications as onset of congestive heart failure, myocardial infarction within 7 postoperative days, dysrhythmias requiring cardioversion, unplanned or prolonged intensive care unit stay resulting from cardiac complications, and cardiac death. Cardiac complications occurred in 5 (9%) patients with AS and 6 (11%) control patients. There was 1 cardiac death among patients with AS.
| Study (Year) | Study Type | No. of Patients | Summary of Patients | Outcomes | Other Comments |
|---|---|---|---|---|---|
| |||||
| McBrien et al11 (2009) | Database study of all patients with hip fracture admitted to a single hospital in Belfast, UK, 20012005 | 272 | Hip fracture, mild (AVA 1.52.0, peak velocity 1.72.9 m/sec): 168 patients; moderate (AVA 1.01.4, peak velocity 3.04.0): 64 patients; severe (AVA <1.0, peak velocity >4.0): 30 patients. Control group without AS: 3481 patients | 30‐day mortality: mild AS, 3.9%; moderate AS, 6.2%; severe AS, 5.1%. Controls, 7.4% | Invasive blood pressure monitoring used more frequently for patients with AS |
| Calleja et al23 (2010) | Retrospective chart review of patients with AS who underwent noncardiac surgery, 19982007; compared patients with severe AS to age‐ and gender‐matched controls with lesser AS | 30 patients with severe AS | Severe AS defined as AVA <1.0, peak velocity >40 m/sec. Most surgeries considered intermediate risk | Intraoperative hypotension more common in patients with severe AS (30% vs 17%). Perioperative MI 3% in severe AS and controls; no deaths in patients with severe AS | 80% of cases involved general anesthesia; 80% were elective |
| Raymer and Yang18 (1998) | Retrospective chart audit of patients with AS who underwent noncardiac surgery compared to matching controls | 55 patients | Mild (AVA 1.01.6 cm2): 18 patients; moderate (AVA 0.80.99 cm2): 13; severe (AVA <0.8 cm2): 24 | 5/55 (9%) AS patients experienced postoperative complications (2 heart failure; 1 ventricular fibrillation; 1 MI and CHF; 1 MI, CHF, and death); 6/55 control patients had cardiac complications | Controls and cases not well‐matched. Death occurred in 84‐year‐old patient, with AVA 0.7 cm2, undergoing an abdominal aortic aneurysm repair |
| Torsher et al21 (1998) | Retrospective record review of all patients with severe AS (AVA <0.5 cm2/m2 body surface area or mean gradient >50 mmHg), undergoing noncardiac surgery at Mayo Clinic, Rochester, MN, 19881992 | 19 patients (28 surgical procedures) | 84% of patients were symptomatic, most with dyspnea. Mean AVA for the group was 0.67 cm2 with AVA index 0.37 cm2/m2 | 2/19 (11%) postoperative cardiac events (both deaths) | Intraoperative hypotension requiring vasopressors occurred in 16 procedures among 14 patients |
| Kertai et al19 (2004) | Retrospective study at Erasmus Medical Center, Rotterdam, the Netherlands, of all patients with moderate (mean gradient 2529 mmHg) or severe (mean gradient >50 mmHg) AS undergoing noncardiac surgery, 19912000; compared to controls from the same database | 108 patients | 92 patients with moderate AS, 16 with severe AS: 38% vascular, 21% orthopedic, 12% abdominal procedures | 15 deaths or nonfatal MI among patients with AS (14% event rate); 4 events among 216 controls (1.8%) | Patients had higher cardiac risk indicators prior to surgery and were much older than controls. RCRI was predictive of events among patients with AS; RCRI 0 points = 0% rate, 1 point = 10%, 2 points = 16%, 3 points or more = 29% |
| Zahid et al22 (2005) | National Hospital Discharge Survey Database patients diagnosed with AS who underwent noncardiac surgery compared 1:2 to matched controls without AS, 19962002 | 5149 patients with diagnosis of AS | 59.7% low‐risk, 35.4% moderate‐risk, 4.9% high‐risk surgery; 29.6% patients known to have heart failure, 15.0% coronary artery disease | Acute MI 3.9% patients with AS; 2.0% controls. Death 5.4% AS patients vs 5.7% controls | Large database study that does not afford assessment of severity of AS or even echocardiographic confirmation of the diagnosis |
A retrospective analysis of 108 patients with AS who underwent noncardiac surgery, at Erasmus Medical Center in The Netherlands between 1991 and 2000, provides insight regarding severity of stenosis and perioperative outcomes.19 Cardiac complications (cardiac death or nonfatal myocardial infarction within 30 days of surgery) occurred in 15/108 (14%) patients with AS, with the majority of these complications being cardiac deaths. A control group of 216 patients suffered a cardiac complication rate of 1.8%. Multivariate adjustment for other risk factors demonstrated an odds ratio of 5.2 (95% CI 1.617.0) for cardiovascular complication in patients with AS. Moderate AS was associated with 11% complication rate (10/92 patients), while severe stenosis was associated with 31% cardiac complications (5/16 patients). Table 3 summarizes cardiac risk among the patients in this study using the Revised Cardiac Risk Index.20
| RCRI* Risk Indicators | Patients With Aortic Stenosis | Patients Without Aortic Stenosis |
|---|---|---|
| ||
| 0 | 0/18 (0%) | 0/108 (0%) |
| 1 | 3/31 (10%) | 2/64 (3%) |
| 2 | 6/38 (16%) | 1/33 (3%) |
| 3 or more | 6/21 (29%) | 1/18 (6%) |
In contrast, the Mayo Clinic experience with severe AS (defined as an aortic valve area index <0.5 cm2/m2 or mean transvalvular gradient >50 mmHg) suggested substantially lower complication rates among patients undergoing noncardiac surgery.21 In this series of 19 patients undergoing a variety of surgical procedures between 1988 and 1992, there were no intraoperative events, but 2 (11%) major postoperative events (1 myocardial infarction and 1 death related to multiorgan failure). The authors concluded that selected patients with severe AS could undergo noncardiac surgery with acceptable risk, and speculated that their experience of better outcomes was due to more aggressive intraoperative and postoperative monitoring and therapy, specifically prompt recognition and therapy of intraoperative hypotension.
A large database study identified 5149 patients undergoing noncardiac surgery, between 1996 and 2002, with a coexistent AS based on International Classification of Diseases, Ninth Revision (ICD‐9) discharge codes, and compared these patients to 10,284 controls.22 Acute myocardial infarction occurred more frequently among patients with AS (3.9% vs 2.0%, P < 0.001), but in‐hospital mortality was not more frequent (5.4% vs 5.7%). The association of perioperative nonfatal myocardial infarction persisted after adjustment for comorbidities. While the results of this study might be interpreted as showing no increase in perioperative mortality for patients with AS who are undergoing noncardiac surgery, there is no way to determine the severity of AS among study patients and endpoints were not uniformly sought, but rather, obtained by ICD‐9 reporting. A recent study of 30 patients with asymptomatic but severe AS, who underwent low‐ or intermediate‐risk noncardiac surgery, found that 30% of patients required intraoperative vasopressor use for hypotension, but there were no deaths, arrhythmias, or heart failure events.23
Summarizing evidence on noncardiac surgery for patients with AS, symptomatic AS is associated with an increased risk of adverse cardiac events in patients undergoing noncardiac surgery. Severe, asymptomatic AS increases risk of intraoperative hemodynamic instability and adverse perioperative cardiac outcomes, although mortality appears to be less than that associated with symptomatic AS.
ECHOCARDIOGRAPHY PRIOR TO NONCARDIAC SURGERY
There are no studies showing that preoperative echocardiograms lessen the perioperative risk for patients with AS. However, as noted earlier, physical examination alone is not adequate to determine the valvular abnormality causing a systolic murmur in many patients, nor is the exam accurate in determining severity of AS in many patients. Echocardiography clarifies both of these issues. Preoperative echocardiography should inform the approach to anesthesia and, for elective surgical procedures, should allow more accurate assessment of operative risk. Because aortic stenosis typically progresses in a relatively slow and steady fashion, demonstration of mild aortic stenosis by echocardiogram within the preceding few years is considered reassuring.
Emergent surgery (for example, exploratory laparotomy for a ruptured viscus) typically does not allow time for echocardiography prior to the procedure. If a previous echocardiogram is available, this may be useful in deciding the intensity of intraoperative monitoring. However, the presence of a suspicious systolic murmur should prompt careful hemodynamic monitoring and the anesthesiologist should be made aware of the suspicion of AS.
For patients with AS facing urgent surgery (for example, repair of a hip fracture), there is typically time to review previous echocardiograms and, if there has been no recent echocardiogram, it is reasonable to obtain one. The presence of severe AS by echocardiogram should prompt careful hemodynamic monitoring. Some anesthesiologists advocate the use of intraoperative transesophageal echocardiography (TEE) to monitor ventricular filling in patients with severe AS.2426 Intraoperative TEE provides real‐time assessment of the cause of left ventricular dysfunction and allows the anesthesiologist to manipulate hemodynamics to address the dysfunction. Intraoperative TEE prompted significant changes in therapy for 4 of 7 patients with AS in a larger cohort of noncardiac surgical patients monitored with TEE.27 A retrospective study of 123 intraoperative TEE examinations found an impact on management in 81% of patients undergoing noncardiac surgery, although only a small number of these patients had cardiac valvular abnormalities.28 Recent anesthesiology practice guidelines recommend that TEE be considered in patients who have cardiovascular pathology that might result in severe hemodynamic, pulmonary, or neurologic compromise.29 The anesthesiologist should decide potential utility of intraoperative TEE, but it is important that the consulting hospitalist be aware of this possible approach to hemodynamic monitoring. Intraoperative TEE requires specialized expertise and may not available in many hospitals.
For elective surgery, presence of a murmur suggestive of significant AS mandates echocardiography, unless there are study results available from the preceding year.30 Optimally, symptomatic AS should be addressed by aortic valve replacement prior to noncardiac surgery. For patients requiring semi‐urgent surgery but are deteriorating because of severe AS, temporizing percutaneous balloon valvuloplasty can be considered, but there are limited data and serious complication rates can be high.3133 Among 15 AS patients requiring noncardiac surgery but with a contraindication to valve replacement, 3 experienced ventricular perforation during percutaneous balloon valvuloplasty, with 1 death.31 In another series of 7 patients, there were no complications of the valvuloplasties, and all 7 patients underwent uncomplicated noncardiac surgery under general anesthesia thereafter.33
In the absence of interventions to improve cardiac hemodynamics, patients could proceed to necessary noncardiac surgery, understanding the high risk of mortality and morbidity (Table 2). These patients should have careful perioperative hemodynamic monitoring and could be considered for intraoperative TEE if available.
Patients with asymptomatic but severe AS can proceed to low‐ or moderate‐risk surgical procedures without further intervention, but with appropriate hemodynamic monitoring. Those patients with asymptomatic but severe AS needing high‐risk surgery should consider valve replacement prior to surgery. In addition, we believe most patients with severe AS should have a cardiologist involved in their perioperative care.
CONCLUSIONS
In summary, patients with suspected AS who require noncardiac surgery need thoughtful consideration by the medical consultant. Careful cardiac examination should be performed on all patients prior to noncardiac surgery. If there is no precordial murmur radiating to the right carotid artery or right clavicle, and if there are no other signs (eg, delayed or reduced carotid upstroke, or absent or distant second heart sound) or symptoms (eg, history of angina, congestive heart failure, or exertional syncope or presyncope), then echocardiography performed for the purpose of discovering AS is not necessary. The majority of patients with a suggestive systolic murmur should be evaluated with echocardiography to provide more accurate prognostic estimates and to guide hemodynamic management during the operation. Patients with severe symptomatic AS are at particularly high risk of cardiac complications, and aortic valve replacement should take priority if the noncardiac surgery can be delayed.
Acknowledgements
The authors would like to acknowledge Dr. Jason Qu for his advice on intraope rative TEE.
Note Added in Proof
Disclosure: Nothing to report.
- , , , , , . Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011.
- . Valvular aortic stenosis in the elderly. Cardiol Rev. 2007;15:217–225.
- , . Aortic stenosis. Lancet. 2009;373:956–966.
- , . Aortic valve stenosis. Anesthesiol Clin. 2009;27:519–532.
- , , . Hypotensive epidural anesthesia in patients with aortic stenosis undergoing total hip replacement. Reg Anesth Pain Med. 2008;33:129–133.
- , , . Identifying severe aortic valvular stenosis by bedside examination. Acta Med Scand. 1985;218:397–400.
- , , , , , . Physical examination in valvular aortic stenosis: correlation with stenosis severity and prediction of clinical outcome. Am Heart J. 1999;137:298–306.
- , , , , . A bedside clinical prediction rule for detecting moderate or severe aortic stenosis. J Gen Intern Med. 1998;13:699–704.
- , , . Does this patient have an abnormal systolic murmur? JAMA. 1997;277:564–571.
- . Etiology and diagnosis of systolic murmurs in adults. Am J Med. 2010;123:913–921.
- , , , et al. Previously undiagnosed aortic stenosis revealed by auscultation in the hip fracture population—echocardiographic findings, management and outcome. Anaesthesia. 2009;64:863–870.
- , . The natural history of aortic valve stenosis. Eur Heart J. 1988;9(suppl E):57–64.
- , , , et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617.
- , , , et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;111:3290–3295.
- , , , et al. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156.
- , . Surgical risk in the cardiac patient. J Chronic Dis. 1964;17:57–72.
- , , , et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850.
- , . Patients with aortic stenosis: cardiac complications in non‐cardiac surgery. Can J Anaesth. 1998;45:855–859.
- , , , et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med. 2004;116:8–13.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , . Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 1998;81:448–452.
- , , , . Perioperative risk of noncardiac surgery associated with aortic stenosis. Am J Cardiol. 2005;96:436–438.
- , , , , , . Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159–1163.
- , . Role of intraoperative transesophageal echocardiography in patients undergoing noncardiac surgery. J Cardiovasc Med (Hagerstown). 2008;9:993–1003.
- , , , . Preoperative and perioperative care for patients with suspected or established aortic stenosis facing noncardiac surgery. Chest. 2005;128:2944–2953.
- , . Impact of TEE in noncardiac surgery. Int Anesthesiol Clin. 2008;46:121–136.
- , , , . Impact of intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:768–771.
- , , , . Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274–280.
- Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096.
- , , , et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–1996.
- , , , . Palliative percutaneous aortic balloon valvuloplasty before noncardiac operations and invasive diagnostic procedures. Mayo Clin Proc. 1989;64:753–757.
- , , , , . Palliation of valvular aortic stenosis by balloon valvuloplasty as preoperative preparation for noncardiac surgery. Am J Cardiol. 1988;62:1309–1310.
- , , . Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039–1041.
- , , , et al. 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142.
- , , , , , . Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011.
- . Valvular aortic stenosis in the elderly. Cardiol Rev. 2007;15:217–225.
- , . Aortic stenosis. Lancet. 2009;373:956–966.
- , . Aortic valve stenosis. Anesthesiol Clin. 2009;27:519–532.
- , , . Hypotensive epidural anesthesia in patients with aortic stenosis undergoing total hip replacement. Reg Anesth Pain Med. 2008;33:129–133.
- , , . Identifying severe aortic valvular stenosis by bedside examination. Acta Med Scand. 1985;218:397–400.
- , , , , , . Physical examination in valvular aortic stenosis: correlation with stenosis severity and prediction of clinical outcome. Am Heart J. 1999;137:298–306.
- , , , , . A bedside clinical prediction rule for detecting moderate or severe aortic stenosis. J Gen Intern Med. 1998;13:699–704.
- , , . Does this patient have an abnormal systolic murmur? JAMA. 1997;277:564–571.
- . Etiology and diagnosis of systolic murmurs in adults. Am J Med. 2010;123:913–921.
- , , , et al. Previously undiagnosed aortic stenosis revealed by auscultation in the hip fracture population—echocardiographic findings, management and outcome. Anaesthesia. 2009;64:863–870.
- , . The natural history of aortic valve stenosis. Eur Heart J. 1988;9(suppl E):57–64.
- , , , et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617.
- , , , et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;111:3290–3295.
- , , , et al. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156.
- , . Surgical risk in the cardiac patient. J Chronic Dis. 1964;17:57–72.
- , , , et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850.
- , . Patients with aortic stenosis: cardiac complications in non‐cardiac surgery. Can J Anaesth. 1998;45:855–859.
- , , , et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med. 2004;116:8–13.
- , , , et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , . Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 1998;81:448–452.
- , , , . Perioperative risk of noncardiac surgery associated with aortic stenosis. Am J Cardiol. 2005;96:436–438.
- , , , , , . Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159–1163.
- , . Role of intraoperative transesophageal echocardiography in patients undergoing noncardiac surgery. J Cardiovasc Med (Hagerstown). 2008;9:993–1003.
- , , , . Preoperative and perioperative care for patients with suspected or established aortic stenosis facing noncardiac surgery. Chest. 2005;128:2944–2953.
- , . Impact of TEE in noncardiac surgery. Int Anesthesiol Clin. 2008;46:121–136.
- , , , . Impact of intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:768–771.
- , , , . Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:274–280.
- Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096.
- , , , et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–1996.
- , , , . Palliative percutaneous aortic balloon valvuloplasty before noncardiac operations and invasive diagnostic procedures. Mayo Clin Proc. 1989;64:753–757.
- , , , , . Palliation of valvular aortic stenosis by balloon valvuloplasty as preoperative preparation for noncardiac surgery. Am J Cardiol. 1988;62:1309–1310.
- , , . Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039–1041.
- , , , et al. 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142.
Delirium in Hospitalized Patients
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.
| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.
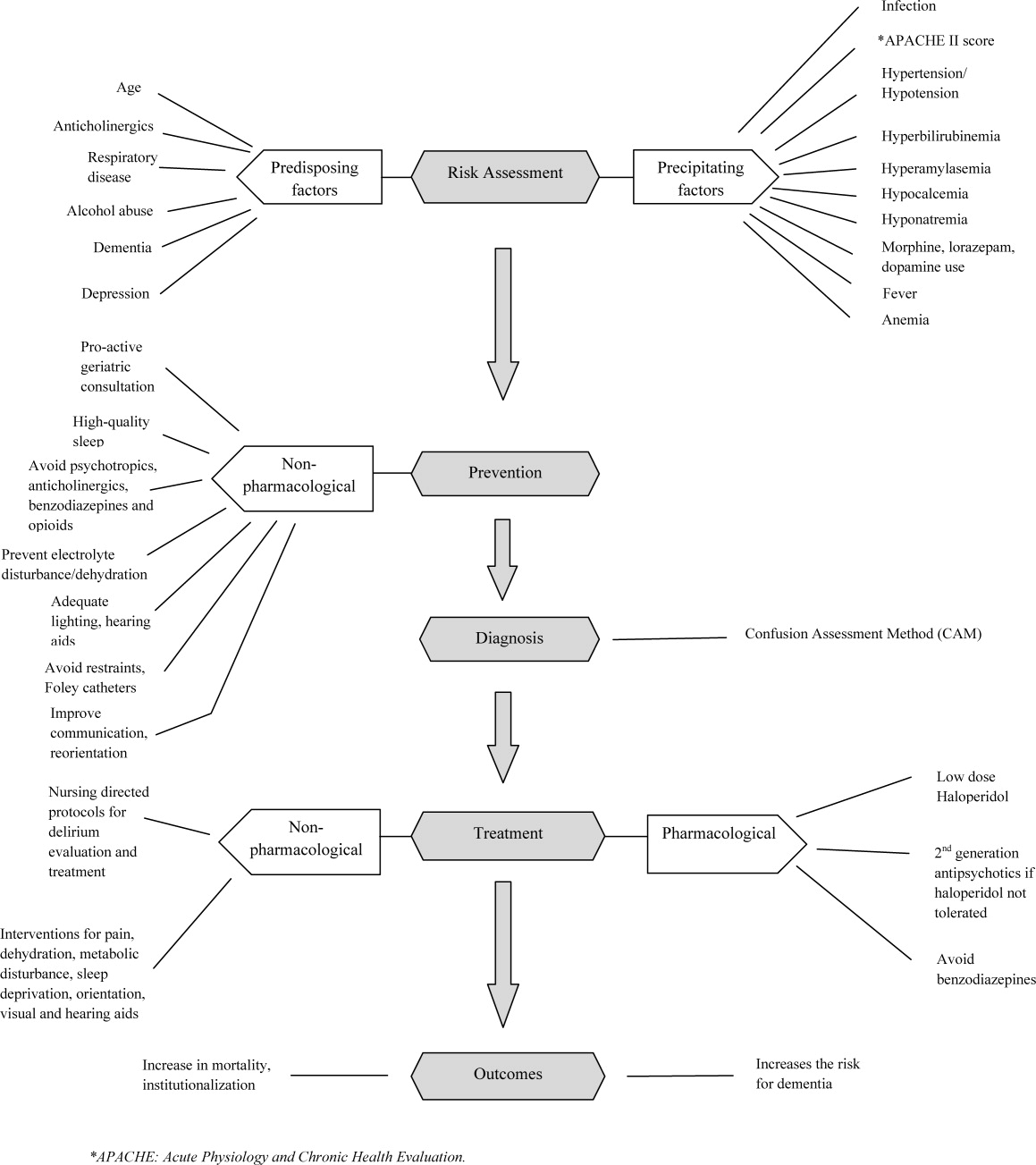
FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.
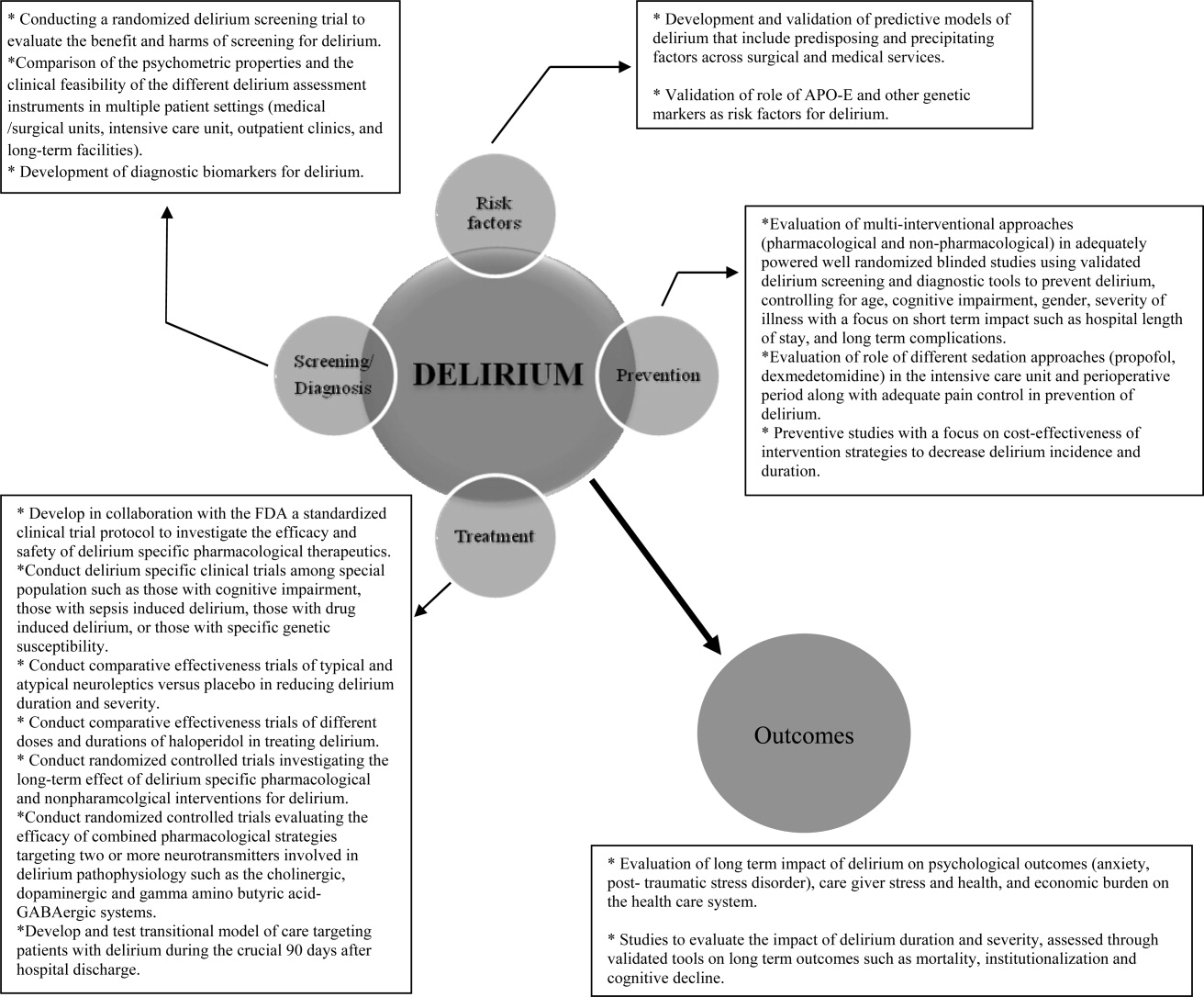
To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
Biosimilars and their use in hematology and oncology
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
Medullary thyroid cancer: advances in treatment and management of common adverse events associated with therapy
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
UV protection and sunscreens: What to tell patients
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
KEY POINTS
- Despite the known risks, nearly 28 million Americans use a sunbed or a sunlamp every year, and 70% of those are white women ages 16 to 29.
- Sunscreens have been a source of confusion in their labeling and their sun protection factor ratings. Revised FDA labeling requirements may help clinicians provide useful guidance to patients.
- The American Academy of Dermatology supports a ban on the nonmedical production and sale of indoor tanning devices.
- Recommendations to prevent UV damage include minimizing sun exposure during peak daylight hours, wearing clothing such as long-sleeve shirts, wide-brimmed hats, and sunglasses, and application of a broad-spectrum sunscreen with UV-A protection. Infants less than 6 months of age require additional protective measures.
Psoriasis: Evolving treatment for a complex disease
Much has changed in our understanding of psoriasis over the past decade, which is having a major effect on its treatment.
Although topical corticosteroids and phototherapy remain mainstays of treatment, a variety of biologic agents have given new hope to those with the most severe forms of the disease. We are also beginning to understand that patients with psoriasis are at greater risk of cardiovascular disease, though the exact nature of that risk and how we should respond remains unclear. Finally, genome-wide association studies are just beginning to unravel the genetic basis of psoriasis.
In this paper, we review the epidemiology and impact of psoriasis, current views of its pathogenesis, its varied clinical forms, and its treatment.
PSORIASIS IMPOSES A GREAT BURDEN
Psoriasis is common, with a reported prevalence ranging from approximately 2%1 to 4.7%.2 It can manifest at any age, but it is most common in two age groups, ie, 20 to 30 years and 50 to 60 years.
For the patient, the burden is great, affecting physical, psychological, and occupational well-being. In fact, patients with psoriasis report quality-of-life impairment equal to or worse than that in patients with cancer or heart disease.3,4 Notably, functional disability secondary to psoriatic arthritis has been reported in up to 19% of psoriatic arthritis patients, and this negatively affects quality of life.5
In 2004, the annual direct medical costs of psoriasis in the United States were estimated to exceed $1 billion. Its indirect costs, measured as missed days and loss of productivity at work, are estimated to exceed the direct costs by $15 billion annually.6,7
Linked to cardiovascular and other diseases
Studies in the past 10 years have uncovered a link between psoriasis, metabolic syndrome, and cardiovascular disease.8–13 Specifically, patients with severe psoriasis are at higher risk of myocardial infarction and cardiovascular death than control patients. Interestingly, the risk decreases with age; patients at greatest risk are young men with severe psoriasis.8–10
In a large cohort study in the United Kingdom7 comparing patients with and without psoriasis, the hazard ratio for cardiovascular death in patients with severe psoriasis was 1.57 (95% confidence interval 1.26–1.96). This translated to 3.5 excess deaths per 1,000 patient-years. These patients were also at higher risk of death from malignancies, chronic lower respiratory disease, diabetes, dementia, infection, kidney disease, and unknown causes.
How much of the risk is due to psoriasis itself, its treatments, associated behaviors, or other factors requires more study. However, some evidence points to the dysregulation of the immune system, notably chronic elevation of pro-inflammatory cytokines.
Psoriasis and its comorbid conditions are thought to arise from chronically elevated levels of cytokines such as tumor necrosis factor alpha (TNF-alpha), interleukin 1 beta (IL-1 beta), and IL-17. These cytokines impair insulin signaling, deregulate lipid metabolism, and increase atherosclerotic changes in the coronary, cerebral, and peripheral arteries. In addition, several other diseases that involve the immune system occur more frequently with psoriasis, including Crohn disease, ulcerative colitis, lymphoma, obesity, and type 2 diabetes.1,8,14–18
In view of the prevalence of these comorbid conditions and the risks they pose, primary care physicians should consider screening patients with severe psoriasis for metabolic disorders and cardiovascular risk factors and promptly begin preventive therapies.19 Unfortunately, to date, there are no consensus guidelines as to the appropriate screening tests or secondary cardiovascular preventive measures for patients with severe psoriasis.
A VICIOUS CIRCLE OF INFLAMMATION AND KERATINOCYTE PROLIFERATION
The hallmark of plaque psoriasis is chronic inflammation in the skin, leading to keratinocyte proliferation.
External and internal triggers that have been identified include cutaneous injury (eg, sunburn, drug rash, viral exanthems), infections (eg, streptococcal), hypocalcemia, pregnancy, psychogenic stress, drugs (eg, lithium, interferon, beta-blockers, and antimalarials), alcohol, smoking, and obesity.20–23
As reviewed by Nestle et al,24 the initiation of lesion formation is still poorly understood but is thought to occur when a trigger (physical trauma, bacterial product, cellular stress) causes DNA to be released from keratinocytes. DNA forms a complex with the antimicrobial protein LL-37 and activates plasmacytoid dendritic cells (PDCs) via toll-like receptor 9. Activated PDCs release type I interferons, which in turn activate myeloid dendritic cells. Myeloid dendritic cells release IL-20 locally, which speeds keratinocyte proliferation.
A subset of myeloid dendritic cells leaves the dermis and migrates to local lymph nodes, where they release IL-23 and activate naive T cells. T helper 1 (Th1) and Th17 cells are recruited to the lesions and begin producing numerous cytokines, including interferon gamma, IL-17, and IL-22. This cytokine milieu increases keratinocyte proliferation and causes the keratinocytes to secrete antimicrobial proteins (LL-37, beta defensins), chemokines, and S100 proteins. These soluble factors have three main functions: stimulation of dendritic cells to release more IL-23, recruitment of neutrophils to the epidermis, and activation of dermal fibroblasts.
This cycle of keratinocytes activating dendritic cells, dendritic cells activating T cells, and T cells activating keratinocytes appears to be the main force maintaining the disease.24 It is unclear, however, whether this applies to all forms of psoriasis or only to plaque psoriasis.
Genetic factors discovered
In recent years, genome-wide association studies have identified at least 10 psoriasis-susceptibility loci that involve functioning of the immune system.25 These genes include those of the major histocompatibility complex, cytokines, receptors, and beta-defensins.
Of specific interest, polymorphisms in the IL-12/IL-13 receptor, the p40 subunit of IL-12 and IL-23, and the p19 subunit of IL-23 strongly associate with psoriasis, supporting their critical role in the disease process and providing targets for medical therapy.26
PSORIASIS HAS SEVERAL CLINICAL PHENOTYPES
Psoriasis has several clinical variants, each with a distinct clinical course and response to treatment.27 Moreover, many patients present with more than one variant.
Plaque psoriasis
Plaques can persist for several months to years, even in the same location, and only about 5% of patients report complete remission for up to 5 years.
Inverse psoriasis
Guttate psoriasis
Erythrodermic psoriasis
Approximately 1% to 2.25% of all patients with psoriasis develop this severe form, affecting more than 75% of the body surface area. It presents as generalized erythema, which is the most prominent feature, and it is often associated with superficial desquamation, hair loss, nail dystrophy, and systemic symptoms such as fever, chills, malaise, or high-output cardiac failure. There may be a history of preceding characteristic psoriatic plaques, recent withdrawal of treatment (usually corticosteroids), phototoxicity, or infection.
Conversely, approximately 25% of all patients with erythroderma have underlying psoriasis.28
Pustular psoriasis
There are several forms of pustular psoriasis, including generalized pustular psoriasis, annular pustular psoriasis, impetigo herpetiformis (pustular psoriasis of pregnancy), and palmoplantar pustulosis. However, there is some evidence to suggest that palmoplantar pustulosis may be distinct from psoriasis.29
Several triggers have been identified, including pregnancy, rapid tapering of medications, hypocalcemia, infection, or topical irritants.
Generalized pustular psoriasis, annular pustular psoriasis, and impetigo herpetiformis often present in association with fever and other systemic symptoms and, if left untreated, can result in life-threatening complications including bacterial superinfection, sepsis, dehydration, and, in rare cases, acute respiratory distress secondary to aseptic pneumonitis.30
Placental insufficiency resulting in stillbirth or neonatal death and other fetal abnormalities can occur in severe pustular psoriasis of pregnancy.31
Psoriatic arthritis
Psoriatic arthritis is a seronegative inflammatory spondyloarthropathy that can result in erosive arthritis in up to 57% of cases and functional disability in up to 19%.32 Although rare in the general population, it affects approximately 6% to 10% of psoriasis patients and up to 40% of patients with severe psoriasis.33 In 70% of cases, psoriasis precedes the onset of arthritis by about 10 years, and approximately 10% to 15% of patients simultaneously present with psoriasis and arthritis or develop arthritis before skin involvement.5,34
Patients complain of joint discomfort that is most prominent after periods of prolonged rest. Patterns of involvement are extremely variable but have been reported as an asymmetric oligoarthritis (involving four or fewer joints) or polyarthritis (involving more than four joints) in most patients. A rheumatoid arthritis-like presentation with a symmetric polyarthropathy involving the small and medium-sized joints has also been reported, making it difficult to clinically distinguish this from rheumatoid arthritis.
A distal interphalangeal-predominant pattern is reported in 5% to 10% of patients. Axial disease resembling ankylosing spondylitis occurs only in 5% of patients. Arthritis mutilans, characterized by severe, rapidly progressive joint inflammation, joint destruction, and deformity, occurs rarely. Enthesitis, ie, inflammation at the point of attachment of tendons or ligaments to bone, is present in up to 42% of patients.5,35
Nail disease
Disease severity also varies
Disease severity also differs among patients. An estimated 80% of patients have mild to moderate disease and 20% have moderate to severe disease, which includes disease involving more than 5% of the body surface or involvement of the face, hands, feet, or genitalia.1
The Psoriasis Area and Severity Index (PASI) is an objective measure used in clinical trials. It incorporates the amount of redness, scaling, and induration of each psoriatic lesion over the body surface involved. A 75% improvement in the PASI score (PASI-75) is regarded as clinically significant.37
PSORIASIS IS DIAGNOSED CLINICALLY
In most cases, the diagnosis of psoriasis is made clinically and is straightforward. However, in more difficult cases, biopsy may be needed. In particular:
- The plaques of psoriasis may be confused with squamous cell carcinoma in situ, dermatophyte infection, or cutaneous T-cell lymphoma, especially if they are treatment-resistant.
- Guttate psoriasis may be difficult to distinguish from pityriasis rosea.
- Erythrodermic psoriasis must be distinguished from other causes of erythroderma, including Sézary syndrome, pityriasis rubra pilaris, and drug reactions.
- Intertrigo, candidiasis, extramammary Paget disease, squamous cell carcinoma, and contact dermatitis all may mimic inverse psoriasis.
- Palmoplantar pustulosis may be difficult to differentiate from dyshidrotic eczema.
- Generalized pustular psoriasis should be distinguished from a pustular drug eruption (acute generalized exanthematous pustular drug eruption or acute generalized exanthematous pustulosis), impetigo, candidiasis, or an autoimmune blistering disorder such as pemphigus.
TREATMENT OF LIMITED DISEASE
Topical corticosteroids
A topical corticosteroid, either by itself or combined with a steroid-sparing agent, is the first-line therapy for patients with limited disease. The potency required for treatment should be based on the extent of disease and on the location, the choice of vehicle, and the patient’s preference and age.
Several double-blind studies have assessed the efficacy of various topical corticosteroids in treating psoriasis. In general, super-potent (class I) and potent (class II) topical corticosteroids are more efficacious than mild or moderate corticosteroids.38 Class I and class II steroids include agents such as clobetasol propionate 0.05% (Temovate), betamethasone dipropionate 0.05% (Diprolene), fluocinonide 0.05% (Lidex), and desoximetasone 0.25% (Topicort).
Use of class I steroids should be limited to an initial treatment course of twice-daily application for 2 to 4 weeks in an effort to avoid some of the local toxicities such as skin atrophy, telangiectasia, and striae. Decreasing class I topical steroid use to 1 to 2 times per week with the gradual introduction of a steroid-sparing agent following the initial 2 to 4 weeks of treatment is advised.
Steroid-sparing agents
Steroid-sparing agents include vitamin D analogues, retinoids, and tacrolimus ointment (Protopic).
Vitamin D analogues and retinoids are thought to decrease keratinocyte proliferation and enhance keratinocyte differentiation.39 The vitamin D analogues are also considered first-line topical agents and include calcipotriol (Dovonex), calcipotriene (Dovonex), and calcitriol (Vectical). To prevent hypercalcemia, use of more than 100 g of vitamin D analogues per week should be avoided.39
Treatment of inverse psoriasis and scalp psoriasis may be challenging
The areas affected in inverse psoriasis, such as the genitalia and axillae, are more prone to side effects when potent topical steroids are used because of increased absorption and occlusion in these areas. Agents that minimize irritation and toxicity in sensitive areas, such as topical tacrolimus, less-potent topical steroids, or calcitriol, can be used.39
For scalp psoriasis, alternative vehicles such as shampoos, gels, solutions, oils, sprays, and foams have improved patient compliance and efficacy of treatment.40
PHOTOTHERAPY FOR SEVERE DISEASE
Narrow-band ultraviolet B
Narrow-band ultraviolet B, ie, light confined to wavelengths of 311 to 313 nm, is a first-line treatment for moderate to severe psoriasis, either as monotherapy or in combination with other treatments. It is an especially attractive option in patients who are on medications or who have comorbidities that may preclude treatment with other systemic agents.
The mechanism of action may be via immunosuppressive effects on Langerhans cells, alteration of cytokines and adhesion molecules that lead to an increase in Th2 cells, and induction of apoptosis of T lymphocytes. Additionally, ultraviolet light affects the proliferation and differentiation of keratinocytes.41
Dosing is based on skin type, and treatment usually involves two or three visits per week for a total of 15 to 20 treatments, with additional therapy for maintenance. Adding acitretin (Soriatane), with close monitoring of aspartate aminotransferase and alanine aminotransferase levels and the patient’s lipid panel, can be considered in treatment-resistant cases.42
Psoralen combined with ultraviolet A
Psoralen combined with ultraviolet A is another option. It can be considered if narrow-band ultraviolet B treatment fails. It is also useful for dark-skinned patients and those with thicker plaques because ultraviolet A penetrates deeper than ultraviolet B. Oral or topical treatment with psoralen is followed by ultraviolet A treatment.
The duration of remission is much longer with psoralen plus ultraviolet A than with narrow-band ultraviolet B. However, this treatment caries a significant risk of cutaneous squamous cell carcinoma and melanoma, especially in light-skinned people and those who receive high doses of ultraviolet A (200 or more treatments) or cyclosporine.40,41,43–46 Long-term effects include photoaging, lentigines, and telangiectasias. As a consequence of these well-established side effects, this treatment is used less frequently.
Cautions with phototherapy
Careful screening and caution should be used in patients who have:
- Fair skin that tends to burn easily
- A history of arsenic intake or treatment with ionizing radiation
- A history of use of photosensitizing medications (fluoroquinolone antibiotics, doxycycline, hydrochlorothiazide)
- A history of melanoma or atypical nevi
- Multiple risk factors for melanoma
- A history of nonmelanoma skin cancer
- Immunosuppression due to organ transplantation.
ORAL THERAPIES FOR SEVERE PSORIASIS
Patients who have severe psoriasis—ie, affecting more than 5% of the body surface or debilitating disease affecting the palms, soles, or genitalia—are best managed with systemic medications, especially if they do not have access to phototherapy.20
Methotrexate
In 1972, the US Food and Drug Administration (FDA) approved methotrexate for treating severe psoriasis.42 In studies of methotrexate at doses of 15 to 20 mg weekly, 36% to 68% of patients with severe plaque psoriasis achieved a PASI-75 score.40,42,47
Dosages of methotrexate for treating severe psoriasis range from 7.5 to 25 mg once a week. Patients should also receive a folate supplement of 1 to 5 mg every day except the day they take methotrexate. The folate is to protect against gastrointestinal side effects, bone marrow suppression, and hepatic toxicity associated with methotrexate.
Other side effects of methotrexate include pulmonary fibrosis and stomatitis. Pregnancy, nursing, alcoholism, chronic liver disease, immunodeficiency syndromes, bone-marrow hypoplasia, leukopenia, thrombocytopenia, anemia, and hypersensitivity to methotrexate are all contraindications to methotrexate use.
The National Psoriasis Foundation, in its 2009 guidelines for the use of methotrexate in treating psoriasis,48 recommends obtaining a complete blood cell count with platelets, blood urea nitrogen, creatinine, and liver function tests at baseline and at 1- to 3-month intervals thereafter.
Liver biopsies were previously recommended for patients receiving methotrexate long-term when the cumulative dose of therapy reached 1.5 g. However, given the invasive nature of the liver biopsy procedure and the low incidence of methotrexate-induced hepatotoxicity, this recommendation has been revised.
For patients with no significant risk factors for hepatic toxicity (eg, obesity, diabetes, hyperlipidemia, hepatitis, or history of or current alcohol consumption) and normal liver function tests, liver biopsy should be considered when a cumulative methotrexate dose of 3.5 to 4.0 g is reached. Alternatively, one may choose to continue to monitor the patient without liver biopsy or to switch to another medication, if possible.42,48
Patients at high risk should be monitored more carefully, and liver biopsy should be considered soon after starting methotrexate and repeated after every 1.0 to 1.5 g.48
No reliable noninvasive measures to evaluate for liver fibrosis are routinely available in the United States. Serial measurements of serum type III procollagen aminopeptide have been reported to correlate with the risk of developing liver fibrosis; however, this test is readily available only in Europe.49
Cyclosporine
Cyclosporine (Gengraf, Neoral, Sandimmune) is very effective for treating psoriasis, especially erythrodermic psoriasis. It is often used only short-term or as a bridge to other maintenance therapies because it has a rapid onset and because long-term therapy (3 to 5 years) is associated with a risk of glomerulosclerosis.50
Cyclosporine works by decreasing T-cell activation by binding cyclophilin, which leads to inhibition of transcription of calcineurin and nuclear factor of activated T cells.51 Given at doses of 2.5 to 5 mg/kg/day, cyclosporine has been shown to result in rapid improvement in up to 80% to 90% of psoriatic patients.52,53
The initial recommended dose of cyclosporine is usually 2.5 to 3 mg/kg/day in two divided doses, which is maintained for 4 weeks and then increased by 0.5 mg/kg/day until the disease is stable.42
Nephrotoxicity and hypertension are cyclosporine’s most serious side effects. Blood urea nitrogen, creatinine, and blood pressure should be monitored at baseline and then twice a month for the first 3 months and once monthly thereafter. Liver function tests, complete blood cell count, lipid profile, magnesium, uric acid, and potassium should also be checked every month.
Cyclosporine also increases the risk of cutaneous squamous cell carcinoma, especially in patients who have received psoralen plus ultraviolet A treatment.42
Patients with hypersensitivity to cyclosporine, a history of chronic infection (eg, tuberculosis, hepatitis B, hepatitis C), renal insufficiency, or a history of systemic malignancy should not receive cyclosporine.
Acitretin
Acitretin, an oral retinoid, has been used for several years to treat psoriasis. Its onset is slow, typically ranging from 3 to 6 months, and its effects are dose-dependent. It is most effective as a maintenance therapy, usually after the disease has been stabilized by agents such as cyclosporine, or in combination with other treatments such as phototherapy.42 Acitretin has been shown to be effective in patients with pustular psoriasis.54
Acitretin does not alter the immune system and has not been shown to have significant cumulative toxicities. Serum triglycerides are monitored closely, since acitretin can lead to hypertriglyceridemia.
All retinoids, including acitretin, are in pregnancy category X and should therefore be avoided during pregnancy. Although its half-life is only 49 hours, acitretin may be transformed to etretinate either spontaneously or as a result of alcohol ingestion. Etretinate has a half-life of 168 days and can take up to 3 years to be eliminated from the body. Therefore, acitretin is contraindicated in women who plan to become pregnant or who do not agree to use adequate contraception for 3 years after the drug is discontinued.42
Biologic agents
Advances in our understanding of the pathogenesis of psoriasis have resulted in more specific, targeted therapy.
Alefacept (Amevive) is a human Fc IgG1 receptor fused to the alpha subunit of LFA3. It binds to CD2, blocks costimulatory signaling, and induces apoptosis in activated memory T cells.
Alefacept was the first biologic agent approved by the FDA for the treatment of psoriasis and one of the few biologic agents to induce long-term remission.55 However, its use has declined because few patients achieved significant clearance of their psoriasis and its onset of action was much slower than that of other medications.56
The currently approved biologic therapies commonly used for moderate to severe psoriasis include the TNF-alpha inhibitors and ustekinumab (Stelara).
The TNF-alpha inhibitors include infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). They are generally well tolerated and highly effective. However, TNF-alpha inhibitors and other biologic agents are contraindicated in patients with serious infection, a personal history or a family history in a first-degree relative of demyelinating disease, or class III or IV congestive heart failure. Patients should be screened for active infection, including tuberculosis and hepatitis B, since reactivation has been reported following initiation of TNF-alpha inhibitors.1
Adalimumab is a human monoclonal antibody against TNF-alpha. It binds to soluble and membrane-bound TNF-alpha and prevents it from binding to p55 and p75 cell-surface TNF receptors.
The dosing schedule for adalimumab is 80 mg subcutaneously for the first week, followed by 40 mg subcutaneously the next week, and then 40 mg subcutaneously every 2 weeks thereafter.1
Etanercept is a recombinant human TNF-alpha receptor (p75) protein fused with the Fc portion of IgG1, which binds to soluble TNF-alpha.57 Dosing for etanercept is 50 mg subcutaneously twice weekly for the first 12 weeks, followed by 50 mg weekly thereafter.
Infliximab is a chimeric antibody composed of a human IgG1 constant region fused to a mouse variable region that binds to both soluble and membrane-bound TNF-alpha.58 Infliximab is given as an infusion at a dose of 5 mg/kg over 2 to 3 hours at weeks 0, 2, and 6, and then every 8 weeks thereafter.
Efficacy of TNF inhibitors. There are no specific guidelines for the sequence of initiation of TNF inhibitors because no studies have directly compared the efficacy of these medications. However, response to infliximab is relatively rapid compared with adalimumab and etanercept.
In a phase III clinical trial,59 as many as 80% of patients achieved PASI-75 clearance of their psoriasis after three doses of infliximab. Interestingly, only 61% of patients maintained PASI-75 clearance by week 50. This loss of efficacy of infliximab is also reported with other TNF-alpha inhibitors and is thought to be secondary to the development of antibodies to the drugs. For infliximab, this loss of efficacy is less when infliximab is given continuously rather than on an as-needed basis. Simultaneous treatment with methotrexate is also thought to decrease the development of antibodies to infliximab.60
Ustekinumab is an monoclonal antibody directed against the common p40 subunit of IL-12 and IL-23, which have been shown to be at increased levels in psoriatic lesions and important for the pathogenesis of psoriasis.
Between 66% and 76% of patients treated with ustekinumab achieved significant clearance of their disease after 12 weeks of treatment in two large phase III multicenter, randomized, double-blind, placebo-controlled trials.61,62
Dosing of ustekinumab is weight-based. For those weighing less than 100 kg, ustekinumab is given at 45 mg subcutaneously at baseline, at 4 weeks, and every 12 weeks thereafter. The same dosing schedule is used for those weighing more than 100 kg, but the dose is increased to 90 mg.
Guidelines for monitoring patients while on ustekinumab are similar to those for other biologic agents. Information on long-term toxicities is still being collected. However, injection-site reactions, serious infections, malignancies, and a single case of reversible posterior leukoencephalopathy have been reported.20
While biologic agents are significantly more expensive than the conventional therapies discussed above and insurance coverage for these agents varies, they have demonstrated superior efficacy and may be indicated for patients with recalcitrant moderate to severe psoriasis for whom multiple types of treatment have failed.
FOR PSORIATIC ARTHRITIS: SYSTEMIC MEDICATIONS
For patients with known or questionable psoriatic arthritis, evaluation by a rheumatologist is highly recommended.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are usually first-line in the treatment of mild psoriatic arthritis. If after 2 to 3 months of therapy with NSAIDs no benefit is achieved, treatment with methotrexate as monotherapy is a practical consideration because of its low cost. However, methotrexate as a monotherapy has not been shown to prevent radiologic progression of disease.5,32
The TNF-alpha inhibitors have been shown to have similar efficacy when compared among each other in the treatment of psoriatic arthritis.32,63 Based on radiologic evidence, ustekinumab has not shown to be as efficacious as the TNF-alpha inhibitors for treating psoriatic arthritis. Therefore, TNF inhibitors should be considered first-line in the treatment of psoriatic arthritis.21,64
Few studies have been done on the efficacy or sequence of therapies that should be used in the treatment of psoriatic arthritis. The American Academy of Dermatology’s Psoriasis Guidelines of Care recommend adding a TNF-alpha inhibitor or switching to a TNF-alpha inhibitor if no significant improvement is achieved after 12 to 16 weeks of treatment with oral methotrexate.20
FOR ERYTHRODERMIC PSORIASIS: MEDICATIONS THAT ACT PROMPTLY
The care of erythrodermic psoriatic patients is distinct from that of other psoriatic patients because of their associated systemic symptoms. Care should be taken to rule out sepsis, as this is a reported trigger of erythrodermic psoriasis.28
Systemic medications with a quick onset, such as oral cyclosporine, are recommended. Infliximab has also been reported to be beneficial because of its rapid onset.28
TREATMENT BASED ON THE TYPE AND THE SEVERITY OF PSORIASIS
The treatment of psoriasis can be as complex as the disease it itself and should be based on the type and the severity of psoriasis. Recognition of the various manifestations of psoriasis is important for effective treatment. However, in patients with moderate to severe psoriasis, atypical presentations, or recalcitrant disease, referral to a specialist is recommended.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58:826–850.
- Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26:314–320.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol 2002; 47:512–518.
- Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol 2010; 63:733–748.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012; 66:e67–e76.
- Schmitt JM, Ford DE. Work limitations and productivity loss are associated with health-related quality of life but not with clinical severity in patients with psoriasis. Dermatology 2006; 213:102–110.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol 2010; 163:586–592.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011; 270:147–157.
- Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol 2011; 64:495–501.
- Bremmer S, Van Voorhees AS, Hsu S, et al; National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 63:1058–1069.
- Tobin AM, Veale DJ, Fitzgerald O, et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol 2010; 37:1386–1394.
- Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol 2003; 48:805–821.
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006; 55:829–835.
- Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol 2012; 66:252–258.
- Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006; 126:2194–2201.
- Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol 2011; 65:84–91.
- Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor’s consensus: psoriasis and coronary artery disease. Am J Cardiol 2008; 102:1631–1643.
- American Academy of Dermatology Work Group; Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol 2011; 65:137–174.
- Mallbris L, Larsson P, Bergqvist S, Vingård E, Granath F, Ståhle M. Psoriasis phenotype at disease onset: clinical characterization of 400 adult cases. J Invest Dermatol 2005; 124:499–504.
- Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol 2011; 165:1162–1168.
- Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in US women: a prospective study. Arch Dermatol 2010; 146:1364–1369.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010; 42:985–990.
- Nair RP, Duffin KC, Helms C, et al; Collaborative Association Study of Psoriasis. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 2009; 41:199–204.
- Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156:258–262.
- Rosenbach M, Hsu S, Korman NJ, et al; National Psoriasis Foundation Medical Board. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 62:655–662.
- Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol 2011; 164:942–946.
- Kluger N, Bessis D, Guillot B, Girard C. Acute respiratory distress syndrome complicating generalized pustular psoriasis (psoriasis-associated aseptic pneumonitis). J Am Acad Dermatol 2011; 64:1154–1158.
- Roth MM. Pregnancy dermatoses: diagnosis, management, and controversies. Am J Clin Dermatol 2011; 12:25–41.
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008; 58:851–864.
- Ogdie A, Gelfand JM. Identification of risk factors for psoriatic arthritis: scientific opportunity meets clinical need. Arch Dermatol 2010; 146:785–788.
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53:573.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973; 3:55–78.
- McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol 2009; 23(suppl 1):9–13.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005; 64(suppl 2):ii65–ii68.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol 2002; 146:351–364.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60:643–659.
- Zivkovich AH, Feldman SR. Are ointments better than other vehicles for corticosteroid treatment of psoriasis? J Drugs Dermatol 2009; 8:570–572.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 2010; 62:114–135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009; 61:451–485.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol 2005; 44:1016–1021.
- Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol 1998; 134:1582–1585.
- Stern RS, Väkevä LH. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J Invest Dermatol 1997; 108:897–900.
- Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol 2009; 60:1001–1017.
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol 2008; 158:116–121.
- Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol 2009; 60:824–837.
- Zachariae H, Heickendorff L, Søgaard H. The value of aminoterminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol 2001; 144:100–103.
- Lowe NJ, Wieder JM, Rosenbach A, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 1996; 35:710–719.
- Gottlieb AB, Grossman RM, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol 1992; 98:302–309.
- Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med 1991; 324:277–284.
- Faerber L, Braeutigam M, Weidinger G, et al. Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients. Am J Clin Dermatol 2001; 2:41–47.
- Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized pustular psoriasis: a multicenter study in Japan. J Dermatol 1999; 26:141–149.
- Krueger GG, Ellis CN. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol 2003; 148:784–788.
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE; Alefacept Clinical Study Group. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139:719–727.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139:1627–1632.
- Gottlieb AB, Masud S, Ramamurthi R, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J Am Acad Dermatol 2003; 48:68–75.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007; 56:31.e1–31.e15.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Kimball AB, Papp KA, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Griffiths CE, Strober BE, van de Kerkhof P, et al; ACCEPT Study Group. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–128.
- Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009; 373:633–640.
Much has changed in our understanding of psoriasis over the past decade, which is having a major effect on its treatment.
Although topical corticosteroids and phototherapy remain mainstays of treatment, a variety of biologic agents have given new hope to those with the most severe forms of the disease. We are also beginning to understand that patients with psoriasis are at greater risk of cardiovascular disease, though the exact nature of that risk and how we should respond remains unclear. Finally, genome-wide association studies are just beginning to unravel the genetic basis of psoriasis.
In this paper, we review the epidemiology and impact of psoriasis, current views of its pathogenesis, its varied clinical forms, and its treatment.
PSORIASIS IMPOSES A GREAT BURDEN
Psoriasis is common, with a reported prevalence ranging from approximately 2%1 to 4.7%.2 It can manifest at any age, but it is most common in two age groups, ie, 20 to 30 years and 50 to 60 years.
For the patient, the burden is great, affecting physical, psychological, and occupational well-being. In fact, patients with psoriasis report quality-of-life impairment equal to or worse than that in patients with cancer or heart disease.3,4 Notably, functional disability secondary to psoriatic arthritis has been reported in up to 19% of psoriatic arthritis patients, and this negatively affects quality of life.5
In 2004, the annual direct medical costs of psoriasis in the United States were estimated to exceed $1 billion. Its indirect costs, measured as missed days and loss of productivity at work, are estimated to exceed the direct costs by $15 billion annually.6,7
Linked to cardiovascular and other diseases
Studies in the past 10 years have uncovered a link between psoriasis, metabolic syndrome, and cardiovascular disease.8–13 Specifically, patients with severe psoriasis are at higher risk of myocardial infarction and cardiovascular death than control patients. Interestingly, the risk decreases with age; patients at greatest risk are young men with severe psoriasis.8–10
In a large cohort study in the United Kingdom7 comparing patients with and without psoriasis, the hazard ratio for cardiovascular death in patients with severe psoriasis was 1.57 (95% confidence interval 1.26–1.96). This translated to 3.5 excess deaths per 1,000 patient-years. These patients were also at higher risk of death from malignancies, chronic lower respiratory disease, diabetes, dementia, infection, kidney disease, and unknown causes.
How much of the risk is due to psoriasis itself, its treatments, associated behaviors, or other factors requires more study. However, some evidence points to the dysregulation of the immune system, notably chronic elevation of pro-inflammatory cytokines.
Psoriasis and its comorbid conditions are thought to arise from chronically elevated levels of cytokines such as tumor necrosis factor alpha (TNF-alpha), interleukin 1 beta (IL-1 beta), and IL-17. These cytokines impair insulin signaling, deregulate lipid metabolism, and increase atherosclerotic changes in the coronary, cerebral, and peripheral arteries. In addition, several other diseases that involve the immune system occur more frequently with psoriasis, including Crohn disease, ulcerative colitis, lymphoma, obesity, and type 2 diabetes.1,8,14–18
In view of the prevalence of these comorbid conditions and the risks they pose, primary care physicians should consider screening patients with severe psoriasis for metabolic disorders and cardiovascular risk factors and promptly begin preventive therapies.19 Unfortunately, to date, there are no consensus guidelines as to the appropriate screening tests or secondary cardiovascular preventive measures for patients with severe psoriasis.
A VICIOUS CIRCLE OF INFLAMMATION AND KERATINOCYTE PROLIFERATION
The hallmark of plaque psoriasis is chronic inflammation in the skin, leading to keratinocyte proliferation.
External and internal triggers that have been identified include cutaneous injury (eg, sunburn, drug rash, viral exanthems), infections (eg, streptococcal), hypocalcemia, pregnancy, psychogenic stress, drugs (eg, lithium, interferon, beta-blockers, and antimalarials), alcohol, smoking, and obesity.20–23
As reviewed by Nestle et al,24 the initiation of lesion formation is still poorly understood but is thought to occur when a trigger (physical trauma, bacterial product, cellular stress) causes DNA to be released from keratinocytes. DNA forms a complex with the antimicrobial protein LL-37 and activates plasmacytoid dendritic cells (PDCs) via toll-like receptor 9. Activated PDCs release type I interferons, which in turn activate myeloid dendritic cells. Myeloid dendritic cells release IL-20 locally, which speeds keratinocyte proliferation.
A subset of myeloid dendritic cells leaves the dermis and migrates to local lymph nodes, where they release IL-23 and activate naive T cells. T helper 1 (Th1) and Th17 cells are recruited to the lesions and begin producing numerous cytokines, including interferon gamma, IL-17, and IL-22. This cytokine milieu increases keratinocyte proliferation and causes the keratinocytes to secrete antimicrobial proteins (LL-37, beta defensins), chemokines, and S100 proteins. These soluble factors have three main functions: stimulation of dendritic cells to release more IL-23, recruitment of neutrophils to the epidermis, and activation of dermal fibroblasts.
This cycle of keratinocytes activating dendritic cells, dendritic cells activating T cells, and T cells activating keratinocytes appears to be the main force maintaining the disease.24 It is unclear, however, whether this applies to all forms of psoriasis or only to plaque psoriasis.
Genetic factors discovered
In recent years, genome-wide association studies have identified at least 10 psoriasis-susceptibility loci that involve functioning of the immune system.25 These genes include those of the major histocompatibility complex, cytokines, receptors, and beta-defensins.
Of specific interest, polymorphisms in the IL-12/IL-13 receptor, the p40 subunit of IL-12 and IL-23, and the p19 subunit of IL-23 strongly associate with psoriasis, supporting their critical role in the disease process and providing targets for medical therapy.26
PSORIASIS HAS SEVERAL CLINICAL PHENOTYPES
Psoriasis has several clinical variants, each with a distinct clinical course and response to treatment.27 Moreover, many patients present with more than one variant.
Plaque psoriasis
Plaques can persist for several months to years, even in the same location, and only about 5% of patients report complete remission for up to 5 years.
Inverse psoriasis
Guttate psoriasis
Erythrodermic psoriasis
Approximately 1% to 2.25% of all patients with psoriasis develop this severe form, affecting more than 75% of the body surface area. It presents as generalized erythema, which is the most prominent feature, and it is often associated with superficial desquamation, hair loss, nail dystrophy, and systemic symptoms such as fever, chills, malaise, or high-output cardiac failure. There may be a history of preceding characteristic psoriatic plaques, recent withdrawal of treatment (usually corticosteroids), phototoxicity, or infection.
Conversely, approximately 25% of all patients with erythroderma have underlying psoriasis.28
Pustular psoriasis
There are several forms of pustular psoriasis, including generalized pustular psoriasis, annular pustular psoriasis, impetigo herpetiformis (pustular psoriasis of pregnancy), and palmoplantar pustulosis. However, there is some evidence to suggest that palmoplantar pustulosis may be distinct from psoriasis.29
Several triggers have been identified, including pregnancy, rapid tapering of medications, hypocalcemia, infection, or topical irritants.
Generalized pustular psoriasis, annular pustular psoriasis, and impetigo herpetiformis often present in association with fever and other systemic symptoms and, if left untreated, can result in life-threatening complications including bacterial superinfection, sepsis, dehydration, and, in rare cases, acute respiratory distress secondary to aseptic pneumonitis.30
Placental insufficiency resulting in stillbirth or neonatal death and other fetal abnormalities can occur in severe pustular psoriasis of pregnancy.31
Psoriatic arthritis
Psoriatic arthritis is a seronegative inflammatory spondyloarthropathy that can result in erosive arthritis in up to 57% of cases and functional disability in up to 19%.32 Although rare in the general population, it affects approximately 6% to 10% of psoriasis patients and up to 40% of patients with severe psoriasis.33 In 70% of cases, psoriasis precedes the onset of arthritis by about 10 years, and approximately 10% to 15% of patients simultaneously present with psoriasis and arthritis or develop arthritis before skin involvement.5,34
Patients complain of joint discomfort that is most prominent after periods of prolonged rest. Patterns of involvement are extremely variable but have been reported as an asymmetric oligoarthritis (involving four or fewer joints) or polyarthritis (involving more than four joints) in most patients. A rheumatoid arthritis-like presentation with a symmetric polyarthropathy involving the small and medium-sized joints has also been reported, making it difficult to clinically distinguish this from rheumatoid arthritis.
A distal interphalangeal-predominant pattern is reported in 5% to 10% of patients. Axial disease resembling ankylosing spondylitis occurs only in 5% of patients. Arthritis mutilans, characterized by severe, rapidly progressive joint inflammation, joint destruction, and deformity, occurs rarely. Enthesitis, ie, inflammation at the point of attachment of tendons or ligaments to bone, is present in up to 42% of patients.5,35
Nail disease
Disease severity also varies
Disease severity also differs among patients. An estimated 80% of patients have mild to moderate disease and 20% have moderate to severe disease, which includes disease involving more than 5% of the body surface or involvement of the face, hands, feet, or genitalia.1
The Psoriasis Area and Severity Index (PASI) is an objective measure used in clinical trials. It incorporates the amount of redness, scaling, and induration of each psoriatic lesion over the body surface involved. A 75% improvement in the PASI score (PASI-75) is regarded as clinically significant.37
PSORIASIS IS DIAGNOSED CLINICALLY
In most cases, the diagnosis of psoriasis is made clinically and is straightforward. However, in more difficult cases, biopsy may be needed. In particular:
- The plaques of psoriasis may be confused with squamous cell carcinoma in situ, dermatophyte infection, or cutaneous T-cell lymphoma, especially if they are treatment-resistant.
- Guttate psoriasis may be difficult to distinguish from pityriasis rosea.
- Erythrodermic psoriasis must be distinguished from other causes of erythroderma, including Sézary syndrome, pityriasis rubra pilaris, and drug reactions.
- Intertrigo, candidiasis, extramammary Paget disease, squamous cell carcinoma, and contact dermatitis all may mimic inverse psoriasis.
- Palmoplantar pustulosis may be difficult to differentiate from dyshidrotic eczema.
- Generalized pustular psoriasis should be distinguished from a pustular drug eruption (acute generalized exanthematous pustular drug eruption or acute generalized exanthematous pustulosis), impetigo, candidiasis, or an autoimmune blistering disorder such as pemphigus.
TREATMENT OF LIMITED DISEASE
Topical corticosteroids
A topical corticosteroid, either by itself or combined with a steroid-sparing agent, is the first-line therapy for patients with limited disease. The potency required for treatment should be based on the extent of disease and on the location, the choice of vehicle, and the patient’s preference and age.
Several double-blind studies have assessed the efficacy of various topical corticosteroids in treating psoriasis. In general, super-potent (class I) and potent (class II) topical corticosteroids are more efficacious than mild or moderate corticosteroids.38 Class I and class II steroids include agents such as clobetasol propionate 0.05% (Temovate), betamethasone dipropionate 0.05% (Diprolene), fluocinonide 0.05% (Lidex), and desoximetasone 0.25% (Topicort).
Use of class I steroids should be limited to an initial treatment course of twice-daily application for 2 to 4 weeks in an effort to avoid some of the local toxicities such as skin atrophy, telangiectasia, and striae. Decreasing class I topical steroid use to 1 to 2 times per week with the gradual introduction of a steroid-sparing agent following the initial 2 to 4 weeks of treatment is advised.
Steroid-sparing agents
Steroid-sparing agents include vitamin D analogues, retinoids, and tacrolimus ointment (Protopic).
Vitamin D analogues and retinoids are thought to decrease keratinocyte proliferation and enhance keratinocyte differentiation.39 The vitamin D analogues are also considered first-line topical agents and include calcipotriol (Dovonex), calcipotriene (Dovonex), and calcitriol (Vectical). To prevent hypercalcemia, use of more than 100 g of vitamin D analogues per week should be avoided.39
Treatment of inverse psoriasis and scalp psoriasis may be challenging
The areas affected in inverse psoriasis, such as the genitalia and axillae, are more prone to side effects when potent topical steroids are used because of increased absorption and occlusion in these areas. Agents that minimize irritation and toxicity in sensitive areas, such as topical tacrolimus, less-potent topical steroids, or calcitriol, can be used.39
For scalp psoriasis, alternative vehicles such as shampoos, gels, solutions, oils, sprays, and foams have improved patient compliance and efficacy of treatment.40
PHOTOTHERAPY FOR SEVERE DISEASE
Narrow-band ultraviolet B
Narrow-band ultraviolet B, ie, light confined to wavelengths of 311 to 313 nm, is a first-line treatment for moderate to severe psoriasis, either as monotherapy or in combination with other treatments. It is an especially attractive option in patients who are on medications or who have comorbidities that may preclude treatment with other systemic agents.
The mechanism of action may be via immunosuppressive effects on Langerhans cells, alteration of cytokines and adhesion molecules that lead to an increase in Th2 cells, and induction of apoptosis of T lymphocytes. Additionally, ultraviolet light affects the proliferation and differentiation of keratinocytes.41
Dosing is based on skin type, and treatment usually involves two or three visits per week for a total of 15 to 20 treatments, with additional therapy for maintenance. Adding acitretin (Soriatane), with close monitoring of aspartate aminotransferase and alanine aminotransferase levels and the patient’s lipid panel, can be considered in treatment-resistant cases.42
Psoralen combined with ultraviolet A
Psoralen combined with ultraviolet A is another option. It can be considered if narrow-band ultraviolet B treatment fails. It is also useful for dark-skinned patients and those with thicker plaques because ultraviolet A penetrates deeper than ultraviolet B. Oral or topical treatment with psoralen is followed by ultraviolet A treatment.
The duration of remission is much longer with psoralen plus ultraviolet A than with narrow-band ultraviolet B. However, this treatment caries a significant risk of cutaneous squamous cell carcinoma and melanoma, especially in light-skinned people and those who receive high doses of ultraviolet A (200 or more treatments) or cyclosporine.40,41,43–46 Long-term effects include photoaging, lentigines, and telangiectasias. As a consequence of these well-established side effects, this treatment is used less frequently.
Cautions with phototherapy
Careful screening and caution should be used in patients who have:
- Fair skin that tends to burn easily
- A history of arsenic intake or treatment with ionizing radiation
- A history of use of photosensitizing medications (fluoroquinolone antibiotics, doxycycline, hydrochlorothiazide)
- A history of melanoma or atypical nevi
- Multiple risk factors for melanoma
- A history of nonmelanoma skin cancer
- Immunosuppression due to organ transplantation.
ORAL THERAPIES FOR SEVERE PSORIASIS
Patients who have severe psoriasis—ie, affecting more than 5% of the body surface or debilitating disease affecting the palms, soles, or genitalia—are best managed with systemic medications, especially if they do not have access to phototherapy.20
Methotrexate
In 1972, the US Food and Drug Administration (FDA) approved methotrexate for treating severe psoriasis.42 In studies of methotrexate at doses of 15 to 20 mg weekly, 36% to 68% of patients with severe plaque psoriasis achieved a PASI-75 score.40,42,47
Dosages of methotrexate for treating severe psoriasis range from 7.5 to 25 mg once a week. Patients should also receive a folate supplement of 1 to 5 mg every day except the day they take methotrexate. The folate is to protect against gastrointestinal side effects, bone marrow suppression, and hepatic toxicity associated with methotrexate.
Other side effects of methotrexate include pulmonary fibrosis and stomatitis. Pregnancy, nursing, alcoholism, chronic liver disease, immunodeficiency syndromes, bone-marrow hypoplasia, leukopenia, thrombocytopenia, anemia, and hypersensitivity to methotrexate are all contraindications to methotrexate use.
The National Psoriasis Foundation, in its 2009 guidelines for the use of methotrexate in treating psoriasis,48 recommends obtaining a complete blood cell count with platelets, blood urea nitrogen, creatinine, and liver function tests at baseline and at 1- to 3-month intervals thereafter.
Liver biopsies were previously recommended for patients receiving methotrexate long-term when the cumulative dose of therapy reached 1.5 g. However, given the invasive nature of the liver biopsy procedure and the low incidence of methotrexate-induced hepatotoxicity, this recommendation has been revised.
For patients with no significant risk factors for hepatic toxicity (eg, obesity, diabetes, hyperlipidemia, hepatitis, or history of or current alcohol consumption) and normal liver function tests, liver biopsy should be considered when a cumulative methotrexate dose of 3.5 to 4.0 g is reached. Alternatively, one may choose to continue to monitor the patient without liver biopsy or to switch to another medication, if possible.42,48
Patients at high risk should be monitored more carefully, and liver biopsy should be considered soon after starting methotrexate and repeated after every 1.0 to 1.5 g.48
No reliable noninvasive measures to evaluate for liver fibrosis are routinely available in the United States. Serial measurements of serum type III procollagen aminopeptide have been reported to correlate with the risk of developing liver fibrosis; however, this test is readily available only in Europe.49
Cyclosporine
Cyclosporine (Gengraf, Neoral, Sandimmune) is very effective for treating psoriasis, especially erythrodermic psoriasis. It is often used only short-term or as a bridge to other maintenance therapies because it has a rapid onset and because long-term therapy (3 to 5 years) is associated with a risk of glomerulosclerosis.50
Cyclosporine works by decreasing T-cell activation by binding cyclophilin, which leads to inhibition of transcription of calcineurin and nuclear factor of activated T cells.51 Given at doses of 2.5 to 5 mg/kg/day, cyclosporine has been shown to result in rapid improvement in up to 80% to 90% of psoriatic patients.52,53
The initial recommended dose of cyclosporine is usually 2.5 to 3 mg/kg/day in two divided doses, which is maintained for 4 weeks and then increased by 0.5 mg/kg/day until the disease is stable.42
Nephrotoxicity and hypertension are cyclosporine’s most serious side effects. Blood urea nitrogen, creatinine, and blood pressure should be monitored at baseline and then twice a month for the first 3 months and once monthly thereafter. Liver function tests, complete blood cell count, lipid profile, magnesium, uric acid, and potassium should also be checked every month.
Cyclosporine also increases the risk of cutaneous squamous cell carcinoma, especially in patients who have received psoralen plus ultraviolet A treatment.42
Patients with hypersensitivity to cyclosporine, a history of chronic infection (eg, tuberculosis, hepatitis B, hepatitis C), renal insufficiency, or a history of systemic malignancy should not receive cyclosporine.
Acitretin
Acitretin, an oral retinoid, has been used for several years to treat psoriasis. Its onset is slow, typically ranging from 3 to 6 months, and its effects are dose-dependent. It is most effective as a maintenance therapy, usually after the disease has been stabilized by agents such as cyclosporine, or in combination with other treatments such as phototherapy.42 Acitretin has been shown to be effective in patients with pustular psoriasis.54
Acitretin does not alter the immune system and has not been shown to have significant cumulative toxicities. Serum triglycerides are monitored closely, since acitretin can lead to hypertriglyceridemia.
All retinoids, including acitretin, are in pregnancy category X and should therefore be avoided during pregnancy. Although its half-life is only 49 hours, acitretin may be transformed to etretinate either spontaneously or as a result of alcohol ingestion. Etretinate has a half-life of 168 days and can take up to 3 years to be eliminated from the body. Therefore, acitretin is contraindicated in women who plan to become pregnant or who do not agree to use adequate contraception for 3 years after the drug is discontinued.42
Biologic agents
Advances in our understanding of the pathogenesis of psoriasis have resulted in more specific, targeted therapy.
Alefacept (Amevive) is a human Fc IgG1 receptor fused to the alpha subunit of LFA3. It binds to CD2, blocks costimulatory signaling, and induces apoptosis in activated memory T cells.
Alefacept was the first biologic agent approved by the FDA for the treatment of psoriasis and one of the few biologic agents to induce long-term remission.55 However, its use has declined because few patients achieved significant clearance of their psoriasis and its onset of action was much slower than that of other medications.56
The currently approved biologic therapies commonly used for moderate to severe psoriasis include the TNF-alpha inhibitors and ustekinumab (Stelara).
The TNF-alpha inhibitors include infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). They are generally well tolerated and highly effective. However, TNF-alpha inhibitors and other biologic agents are contraindicated in patients with serious infection, a personal history or a family history in a first-degree relative of demyelinating disease, or class III or IV congestive heart failure. Patients should be screened for active infection, including tuberculosis and hepatitis B, since reactivation has been reported following initiation of TNF-alpha inhibitors.1
Adalimumab is a human monoclonal antibody against TNF-alpha. It binds to soluble and membrane-bound TNF-alpha and prevents it from binding to p55 and p75 cell-surface TNF receptors.
The dosing schedule for adalimumab is 80 mg subcutaneously for the first week, followed by 40 mg subcutaneously the next week, and then 40 mg subcutaneously every 2 weeks thereafter.1
Etanercept is a recombinant human TNF-alpha receptor (p75) protein fused with the Fc portion of IgG1, which binds to soluble TNF-alpha.57 Dosing for etanercept is 50 mg subcutaneously twice weekly for the first 12 weeks, followed by 50 mg weekly thereafter.
Infliximab is a chimeric antibody composed of a human IgG1 constant region fused to a mouse variable region that binds to both soluble and membrane-bound TNF-alpha.58 Infliximab is given as an infusion at a dose of 5 mg/kg over 2 to 3 hours at weeks 0, 2, and 6, and then every 8 weeks thereafter.
Efficacy of TNF inhibitors. There are no specific guidelines for the sequence of initiation of TNF inhibitors because no studies have directly compared the efficacy of these medications. However, response to infliximab is relatively rapid compared with adalimumab and etanercept.
In a phase III clinical trial,59 as many as 80% of patients achieved PASI-75 clearance of their psoriasis after three doses of infliximab. Interestingly, only 61% of patients maintained PASI-75 clearance by week 50. This loss of efficacy of infliximab is also reported with other TNF-alpha inhibitors and is thought to be secondary to the development of antibodies to the drugs. For infliximab, this loss of efficacy is less when infliximab is given continuously rather than on an as-needed basis. Simultaneous treatment with methotrexate is also thought to decrease the development of antibodies to infliximab.60
Ustekinumab is an monoclonal antibody directed against the common p40 subunit of IL-12 and IL-23, which have been shown to be at increased levels in psoriatic lesions and important for the pathogenesis of psoriasis.
Between 66% and 76% of patients treated with ustekinumab achieved significant clearance of their disease after 12 weeks of treatment in two large phase III multicenter, randomized, double-blind, placebo-controlled trials.61,62
Dosing of ustekinumab is weight-based. For those weighing less than 100 kg, ustekinumab is given at 45 mg subcutaneously at baseline, at 4 weeks, and every 12 weeks thereafter. The same dosing schedule is used for those weighing more than 100 kg, but the dose is increased to 90 mg.
Guidelines for monitoring patients while on ustekinumab are similar to those for other biologic agents. Information on long-term toxicities is still being collected. However, injection-site reactions, serious infections, malignancies, and a single case of reversible posterior leukoencephalopathy have been reported.20
While biologic agents are significantly more expensive than the conventional therapies discussed above and insurance coverage for these agents varies, they have demonstrated superior efficacy and may be indicated for patients with recalcitrant moderate to severe psoriasis for whom multiple types of treatment have failed.
FOR PSORIATIC ARTHRITIS: SYSTEMIC MEDICATIONS
For patients with known or questionable psoriatic arthritis, evaluation by a rheumatologist is highly recommended.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are usually first-line in the treatment of mild psoriatic arthritis. If after 2 to 3 months of therapy with NSAIDs no benefit is achieved, treatment with methotrexate as monotherapy is a practical consideration because of its low cost. However, methotrexate as a monotherapy has not been shown to prevent radiologic progression of disease.5,32
The TNF-alpha inhibitors have been shown to have similar efficacy when compared among each other in the treatment of psoriatic arthritis.32,63 Based on radiologic evidence, ustekinumab has not shown to be as efficacious as the TNF-alpha inhibitors for treating psoriatic arthritis. Therefore, TNF inhibitors should be considered first-line in the treatment of psoriatic arthritis.21,64
Few studies have been done on the efficacy or sequence of therapies that should be used in the treatment of psoriatic arthritis. The American Academy of Dermatology’s Psoriasis Guidelines of Care recommend adding a TNF-alpha inhibitor or switching to a TNF-alpha inhibitor if no significant improvement is achieved after 12 to 16 weeks of treatment with oral methotrexate.20
FOR ERYTHRODERMIC PSORIASIS: MEDICATIONS THAT ACT PROMPTLY
The care of erythrodermic psoriatic patients is distinct from that of other psoriatic patients because of their associated systemic symptoms. Care should be taken to rule out sepsis, as this is a reported trigger of erythrodermic psoriasis.28
Systemic medications with a quick onset, such as oral cyclosporine, are recommended. Infliximab has also been reported to be beneficial because of its rapid onset.28
TREATMENT BASED ON THE TYPE AND THE SEVERITY OF PSORIASIS
The treatment of psoriasis can be as complex as the disease it itself and should be based on the type and the severity of psoriasis. Recognition of the various manifestations of psoriasis is important for effective treatment. However, in patients with moderate to severe psoriasis, atypical presentations, or recalcitrant disease, referral to a specialist is recommended.
Much has changed in our understanding of psoriasis over the past decade, which is having a major effect on its treatment.
Although topical corticosteroids and phototherapy remain mainstays of treatment, a variety of biologic agents have given new hope to those with the most severe forms of the disease. We are also beginning to understand that patients with psoriasis are at greater risk of cardiovascular disease, though the exact nature of that risk and how we should respond remains unclear. Finally, genome-wide association studies are just beginning to unravel the genetic basis of psoriasis.
In this paper, we review the epidemiology and impact of psoriasis, current views of its pathogenesis, its varied clinical forms, and its treatment.
PSORIASIS IMPOSES A GREAT BURDEN
Psoriasis is common, with a reported prevalence ranging from approximately 2%1 to 4.7%.2 It can manifest at any age, but it is most common in two age groups, ie, 20 to 30 years and 50 to 60 years.
For the patient, the burden is great, affecting physical, psychological, and occupational well-being. In fact, patients with psoriasis report quality-of-life impairment equal to or worse than that in patients with cancer or heart disease.3,4 Notably, functional disability secondary to psoriatic arthritis has been reported in up to 19% of psoriatic arthritis patients, and this negatively affects quality of life.5
In 2004, the annual direct medical costs of psoriasis in the United States were estimated to exceed $1 billion. Its indirect costs, measured as missed days and loss of productivity at work, are estimated to exceed the direct costs by $15 billion annually.6,7
Linked to cardiovascular and other diseases
Studies in the past 10 years have uncovered a link between psoriasis, metabolic syndrome, and cardiovascular disease.8–13 Specifically, patients with severe psoriasis are at higher risk of myocardial infarction and cardiovascular death than control patients. Interestingly, the risk decreases with age; patients at greatest risk are young men with severe psoriasis.8–10
In a large cohort study in the United Kingdom7 comparing patients with and without psoriasis, the hazard ratio for cardiovascular death in patients with severe psoriasis was 1.57 (95% confidence interval 1.26–1.96). This translated to 3.5 excess deaths per 1,000 patient-years. These patients were also at higher risk of death from malignancies, chronic lower respiratory disease, diabetes, dementia, infection, kidney disease, and unknown causes.
How much of the risk is due to psoriasis itself, its treatments, associated behaviors, or other factors requires more study. However, some evidence points to the dysregulation of the immune system, notably chronic elevation of pro-inflammatory cytokines.
Psoriasis and its comorbid conditions are thought to arise from chronically elevated levels of cytokines such as tumor necrosis factor alpha (TNF-alpha), interleukin 1 beta (IL-1 beta), and IL-17. These cytokines impair insulin signaling, deregulate lipid metabolism, and increase atherosclerotic changes in the coronary, cerebral, and peripheral arteries. In addition, several other diseases that involve the immune system occur more frequently with psoriasis, including Crohn disease, ulcerative colitis, lymphoma, obesity, and type 2 diabetes.1,8,14–18
In view of the prevalence of these comorbid conditions and the risks they pose, primary care physicians should consider screening patients with severe psoriasis for metabolic disorders and cardiovascular risk factors and promptly begin preventive therapies.19 Unfortunately, to date, there are no consensus guidelines as to the appropriate screening tests or secondary cardiovascular preventive measures for patients with severe psoriasis.
A VICIOUS CIRCLE OF INFLAMMATION AND KERATINOCYTE PROLIFERATION
The hallmark of plaque psoriasis is chronic inflammation in the skin, leading to keratinocyte proliferation.
External and internal triggers that have been identified include cutaneous injury (eg, sunburn, drug rash, viral exanthems), infections (eg, streptococcal), hypocalcemia, pregnancy, psychogenic stress, drugs (eg, lithium, interferon, beta-blockers, and antimalarials), alcohol, smoking, and obesity.20–23
As reviewed by Nestle et al,24 the initiation of lesion formation is still poorly understood but is thought to occur when a trigger (physical trauma, bacterial product, cellular stress) causes DNA to be released from keratinocytes. DNA forms a complex with the antimicrobial protein LL-37 and activates plasmacytoid dendritic cells (PDCs) via toll-like receptor 9. Activated PDCs release type I interferons, which in turn activate myeloid dendritic cells. Myeloid dendritic cells release IL-20 locally, which speeds keratinocyte proliferation.
A subset of myeloid dendritic cells leaves the dermis and migrates to local lymph nodes, where they release IL-23 and activate naive T cells. T helper 1 (Th1) and Th17 cells are recruited to the lesions and begin producing numerous cytokines, including interferon gamma, IL-17, and IL-22. This cytokine milieu increases keratinocyte proliferation and causes the keratinocytes to secrete antimicrobial proteins (LL-37, beta defensins), chemokines, and S100 proteins. These soluble factors have three main functions: stimulation of dendritic cells to release more IL-23, recruitment of neutrophils to the epidermis, and activation of dermal fibroblasts.
This cycle of keratinocytes activating dendritic cells, dendritic cells activating T cells, and T cells activating keratinocytes appears to be the main force maintaining the disease.24 It is unclear, however, whether this applies to all forms of psoriasis or only to plaque psoriasis.
Genetic factors discovered
In recent years, genome-wide association studies have identified at least 10 psoriasis-susceptibility loci that involve functioning of the immune system.25 These genes include those of the major histocompatibility complex, cytokines, receptors, and beta-defensins.
Of specific interest, polymorphisms in the IL-12/IL-13 receptor, the p40 subunit of IL-12 and IL-23, and the p19 subunit of IL-23 strongly associate with psoriasis, supporting their critical role in the disease process and providing targets for medical therapy.26
PSORIASIS HAS SEVERAL CLINICAL PHENOTYPES
Psoriasis has several clinical variants, each with a distinct clinical course and response to treatment.27 Moreover, many patients present with more than one variant.
Plaque psoriasis
Plaques can persist for several months to years, even in the same location, and only about 5% of patients report complete remission for up to 5 years.
Inverse psoriasis
Guttate psoriasis
Erythrodermic psoriasis
Approximately 1% to 2.25% of all patients with psoriasis develop this severe form, affecting more than 75% of the body surface area. It presents as generalized erythema, which is the most prominent feature, and it is often associated with superficial desquamation, hair loss, nail dystrophy, and systemic symptoms such as fever, chills, malaise, or high-output cardiac failure. There may be a history of preceding characteristic psoriatic plaques, recent withdrawal of treatment (usually corticosteroids), phototoxicity, or infection.
Conversely, approximately 25% of all patients with erythroderma have underlying psoriasis.28
Pustular psoriasis
There are several forms of pustular psoriasis, including generalized pustular psoriasis, annular pustular psoriasis, impetigo herpetiformis (pustular psoriasis of pregnancy), and palmoplantar pustulosis. However, there is some evidence to suggest that palmoplantar pustulosis may be distinct from psoriasis.29
Several triggers have been identified, including pregnancy, rapid tapering of medications, hypocalcemia, infection, or topical irritants.
Generalized pustular psoriasis, annular pustular psoriasis, and impetigo herpetiformis often present in association with fever and other systemic symptoms and, if left untreated, can result in life-threatening complications including bacterial superinfection, sepsis, dehydration, and, in rare cases, acute respiratory distress secondary to aseptic pneumonitis.30
Placental insufficiency resulting in stillbirth or neonatal death and other fetal abnormalities can occur in severe pustular psoriasis of pregnancy.31
Psoriatic arthritis
Psoriatic arthritis is a seronegative inflammatory spondyloarthropathy that can result in erosive arthritis in up to 57% of cases and functional disability in up to 19%.32 Although rare in the general population, it affects approximately 6% to 10% of psoriasis patients and up to 40% of patients with severe psoriasis.33 In 70% of cases, psoriasis precedes the onset of arthritis by about 10 years, and approximately 10% to 15% of patients simultaneously present with psoriasis and arthritis or develop arthritis before skin involvement.5,34
Patients complain of joint discomfort that is most prominent after periods of prolonged rest. Patterns of involvement are extremely variable but have been reported as an asymmetric oligoarthritis (involving four or fewer joints) or polyarthritis (involving more than four joints) in most patients. A rheumatoid arthritis-like presentation with a symmetric polyarthropathy involving the small and medium-sized joints has also been reported, making it difficult to clinically distinguish this from rheumatoid arthritis.
A distal interphalangeal-predominant pattern is reported in 5% to 10% of patients. Axial disease resembling ankylosing spondylitis occurs only in 5% of patients. Arthritis mutilans, characterized by severe, rapidly progressive joint inflammation, joint destruction, and deformity, occurs rarely. Enthesitis, ie, inflammation at the point of attachment of tendons or ligaments to bone, is present in up to 42% of patients.5,35
Nail disease
Disease severity also varies
Disease severity also differs among patients. An estimated 80% of patients have mild to moderate disease and 20% have moderate to severe disease, which includes disease involving more than 5% of the body surface or involvement of the face, hands, feet, or genitalia.1
The Psoriasis Area and Severity Index (PASI) is an objective measure used in clinical trials. It incorporates the amount of redness, scaling, and induration of each psoriatic lesion over the body surface involved. A 75% improvement in the PASI score (PASI-75) is regarded as clinically significant.37
PSORIASIS IS DIAGNOSED CLINICALLY
In most cases, the diagnosis of psoriasis is made clinically and is straightforward. However, in more difficult cases, biopsy may be needed. In particular:
- The plaques of psoriasis may be confused with squamous cell carcinoma in situ, dermatophyte infection, or cutaneous T-cell lymphoma, especially if they are treatment-resistant.
- Guttate psoriasis may be difficult to distinguish from pityriasis rosea.
- Erythrodermic psoriasis must be distinguished from other causes of erythroderma, including Sézary syndrome, pityriasis rubra pilaris, and drug reactions.
- Intertrigo, candidiasis, extramammary Paget disease, squamous cell carcinoma, and contact dermatitis all may mimic inverse psoriasis.
- Palmoplantar pustulosis may be difficult to differentiate from dyshidrotic eczema.
- Generalized pustular psoriasis should be distinguished from a pustular drug eruption (acute generalized exanthematous pustular drug eruption or acute generalized exanthematous pustulosis), impetigo, candidiasis, or an autoimmune blistering disorder such as pemphigus.
TREATMENT OF LIMITED DISEASE
Topical corticosteroids
A topical corticosteroid, either by itself or combined with a steroid-sparing agent, is the first-line therapy for patients with limited disease. The potency required for treatment should be based on the extent of disease and on the location, the choice of vehicle, and the patient’s preference and age.
Several double-blind studies have assessed the efficacy of various topical corticosteroids in treating psoriasis. In general, super-potent (class I) and potent (class II) topical corticosteroids are more efficacious than mild or moderate corticosteroids.38 Class I and class II steroids include agents such as clobetasol propionate 0.05% (Temovate), betamethasone dipropionate 0.05% (Diprolene), fluocinonide 0.05% (Lidex), and desoximetasone 0.25% (Topicort).
Use of class I steroids should be limited to an initial treatment course of twice-daily application for 2 to 4 weeks in an effort to avoid some of the local toxicities such as skin atrophy, telangiectasia, and striae. Decreasing class I topical steroid use to 1 to 2 times per week with the gradual introduction of a steroid-sparing agent following the initial 2 to 4 weeks of treatment is advised.
Steroid-sparing agents
Steroid-sparing agents include vitamin D analogues, retinoids, and tacrolimus ointment (Protopic).
Vitamin D analogues and retinoids are thought to decrease keratinocyte proliferation and enhance keratinocyte differentiation.39 The vitamin D analogues are also considered first-line topical agents and include calcipotriol (Dovonex), calcipotriene (Dovonex), and calcitriol (Vectical). To prevent hypercalcemia, use of more than 100 g of vitamin D analogues per week should be avoided.39
Treatment of inverse psoriasis and scalp psoriasis may be challenging
The areas affected in inverse psoriasis, such as the genitalia and axillae, are more prone to side effects when potent topical steroids are used because of increased absorption and occlusion in these areas. Agents that minimize irritation and toxicity in sensitive areas, such as topical tacrolimus, less-potent topical steroids, or calcitriol, can be used.39
For scalp psoriasis, alternative vehicles such as shampoos, gels, solutions, oils, sprays, and foams have improved patient compliance and efficacy of treatment.40
PHOTOTHERAPY FOR SEVERE DISEASE
Narrow-band ultraviolet B
Narrow-band ultraviolet B, ie, light confined to wavelengths of 311 to 313 nm, is a first-line treatment for moderate to severe psoriasis, either as monotherapy or in combination with other treatments. It is an especially attractive option in patients who are on medications or who have comorbidities that may preclude treatment with other systemic agents.
The mechanism of action may be via immunosuppressive effects on Langerhans cells, alteration of cytokines and adhesion molecules that lead to an increase in Th2 cells, and induction of apoptosis of T lymphocytes. Additionally, ultraviolet light affects the proliferation and differentiation of keratinocytes.41
Dosing is based on skin type, and treatment usually involves two or three visits per week for a total of 15 to 20 treatments, with additional therapy for maintenance. Adding acitretin (Soriatane), with close monitoring of aspartate aminotransferase and alanine aminotransferase levels and the patient’s lipid panel, can be considered in treatment-resistant cases.42
Psoralen combined with ultraviolet A
Psoralen combined with ultraviolet A is another option. It can be considered if narrow-band ultraviolet B treatment fails. It is also useful for dark-skinned patients and those with thicker plaques because ultraviolet A penetrates deeper than ultraviolet B. Oral or topical treatment with psoralen is followed by ultraviolet A treatment.
The duration of remission is much longer with psoralen plus ultraviolet A than with narrow-band ultraviolet B. However, this treatment caries a significant risk of cutaneous squamous cell carcinoma and melanoma, especially in light-skinned people and those who receive high doses of ultraviolet A (200 or more treatments) or cyclosporine.40,41,43–46 Long-term effects include photoaging, lentigines, and telangiectasias. As a consequence of these well-established side effects, this treatment is used less frequently.
Cautions with phototherapy
Careful screening and caution should be used in patients who have:
- Fair skin that tends to burn easily
- A history of arsenic intake or treatment with ionizing radiation
- A history of use of photosensitizing medications (fluoroquinolone antibiotics, doxycycline, hydrochlorothiazide)
- A history of melanoma or atypical nevi
- Multiple risk factors for melanoma
- A history of nonmelanoma skin cancer
- Immunosuppression due to organ transplantation.
ORAL THERAPIES FOR SEVERE PSORIASIS
Patients who have severe psoriasis—ie, affecting more than 5% of the body surface or debilitating disease affecting the palms, soles, or genitalia—are best managed with systemic medications, especially if they do not have access to phototherapy.20
Methotrexate
In 1972, the US Food and Drug Administration (FDA) approved methotrexate for treating severe psoriasis.42 In studies of methotrexate at doses of 15 to 20 mg weekly, 36% to 68% of patients with severe plaque psoriasis achieved a PASI-75 score.40,42,47
Dosages of methotrexate for treating severe psoriasis range from 7.5 to 25 mg once a week. Patients should also receive a folate supplement of 1 to 5 mg every day except the day they take methotrexate. The folate is to protect against gastrointestinal side effects, bone marrow suppression, and hepatic toxicity associated with methotrexate.
Other side effects of methotrexate include pulmonary fibrosis and stomatitis. Pregnancy, nursing, alcoholism, chronic liver disease, immunodeficiency syndromes, bone-marrow hypoplasia, leukopenia, thrombocytopenia, anemia, and hypersensitivity to methotrexate are all contraindications to methotrexate use.
The National Psoriasis Foundation, in its 2009 guidelines for the use of methotrexate in treating psoriasis,48 recommends obtaining a complete blood cell count with platelets, blood urea nitrogen, creatinine, and liver function tests at baseline and at 1- to 3-month intervals thereafter.
Liver biopsies were previously recommended for patients receiving methotrexate long-term when the cumulative dose of therapy reached 1.5 g. However, given the invasive nature of the liver biopsy procedure and the low incidence of methotrexate-induced hepatotoxicity, this recommendation has been revised.
For patients with no significant risk factors for hepatic toxicity (eg, obesity, diabetes, hyperlipidemia, hepatitis, or history of or current alcohol consumption) and normal liver function tests, liver biopsy should be considered when a cumulative methotrexate dose of 3.5 to 4.0 g is reached. Alternatively, one may choose to continue to monitor the patient without liver biopsy or to switch to another medication, if possible.42,48
Patients at high risk should be monitored more carefully, and liver biopsy should be considered soon after starting methotrexate and repeated after every 1.0 to 1.5 g.48
No reliable noninvasive measures to evaluate for liver fibrosis are routinely available in the United States. Serial measurements of serum type III procollagen aminopeptide have been reported to correlate with the risk of developing liver fibrosis; however, this test is readily available only in Europe.49
Cyclosporine
Cyclosporine (Gengraf, Neoral, Sandimmune) is very effective for treating psoriasis, especially erythrodermic psoriasis. It is often used only short-term or as a bridge to other maintenance therapies because it has a rapid onset and because long-term therapy (3 to 5 years) is associated with a risk of glomerulosclerosis.50
Cyclosporine works by decreasing T-cell activation by binding cyclophilin, which leads to inhibition of transcription of calcineurin and nuclear factor of activated T cells.51 Given at doses of 2.5 to 5 mg/kg/day, cyclosporine has been shown to result in rapid improvement in up to 80% to 90% of psoriatic patients.52,53
The initial recommended dose of cyclosporine is usually 2.5 to 3 mg/kg/day in two divided doses, which is maintained for 4 weeks and then increased by 0.5 mg/kg/day until the disease is stable.42
Nephrotoxicity and hypertension are cyclosporine’s most serious side effects. Blood urea nitrogen, creatinine, and blood pressure should be monitored at baseline and then twice a month for the first 3 months and once monthly thereafter. Liver function tests, complete blood cell count, lipid profile, magnesium, uric acid, and potassium should also be checked every month.
Cyclosporine also increases the risk of cutaneous squamous cell carcinoma, especially in patients who have received psoralen plus ultraviolet A treatment.42
Patients with hypersensitivity to cyclosporine, a history of chronic infection (eg, tuberculosis, hepatitis B, hepatitis C), renal insufficiency, or a history of systemic malignancy should not receive cyclosporine.
Acitretin
Acitretin, an oral retinoid, has been used for several years to treat psoriasis. Its onset is slow, typically ranging from 3 to 6 months, and its effects are dose-dependent. It is most effective as a maintenance therapy, usually after the disease has been stabilized by agents such as cyclosporine, or in combination with other treatments such as phototherapy.42 Acitretin has been shown to be effective in patients with pustular psoriasis.54
Acitretin does not alter the immune system and has not been shown to have significant cumulative toxicities. Serum triglycerides are monitored closely, since acitretin can lead to hypertriglyceridemia.
All retinoids, including acitretin, are in pregnancy category X and should therefore be avoided during pregnancy. Although its half-life is only 49 hours, acitretin may be transformed to etretinate either spontaneously or as a result of alcohol ingestion. Etretinate has a half-life of 168 days and can take up to 3 years to be eliminated from the body. Therefore, acitretin is contraindicated in women who plan to become pregnant or who do not agree to use adequate contraception for 3 years after the drug is discontinued.42
Biologic agents
Advances in our understanding of the pathogenesis of psoriasis have resulted in more specific, targeted therapy.
Alefacept (Amevive) is a human Fc IgG1 receptor fused to the alpha subunit of LFA3. It binds to CD2, blocks costimulatory signaling, and induces apoptosis in activated memory T cells.
Alefacept was the first biologic agent approved by the FDA for the treatment of psoriasis and one of the few biologic agents to induce long-term remission.55 However, its use has declined because few patients achieved significant clearance of their psoriasis and its onset of action was much slower than that of other medications.56
The currently approved biologic therapies commonly used for moderate to severe psoriasis include the TNF-alpha inhibitors and ustekinumab (Stelara).
The TNF-alpha inhibitors include infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). They are generally well tolerated and highly effective. However, TNF-alpha inhibitors and other biologic agents are contraindicated in patients with serious infection, a personal history or a family history in a first-degree relative of demyelinating disease, or class III or IV congestive heart failure. Patients should be screened for active infection, including tuberculosis and hepatitis B, since reactivation has been reported following initiation of TNF-alpha inhibitors.1
Adalimumab is a human monoclonal antibody against TNF-alpha. It binds to soluble and membrane-bound TNF-alpha and prevents it from binding to p55 and p75 cell-surface TNF receptors.
The dosing schedule for adalimumab is 80 mg subcutaneously for the first week, followed by 40 mg subcutaneously the next week, and then 40 mg subcutaneously every 2 weeks thereafter.1
Etanercept is a recombinant human TNF-alpha receptor (p75) protein fused with the Fc portion of IgG1, which binds to soluble TNF-alpha.57 Dosing for etanercept is 50 mg subcutaneously twice weekly for the first 12 weeks, followed by 50 mg weekly thereafter.
Infliximab is a chimeric antibody composed of a human IgG1 constant region fused to a mouse variable region that binds to both soluble and membrane-bound TNF-alpha.58 Infliximab is given as an infusion at a dose of 5 mg/kg over 2 to 3 hours at weeks 0, 2, and 6, and then every 8 weeks thereafter.
Efficacy of TNF inhibitors. There are no specific guidelines for the sequence of initiation of TNF inhibitors because no studies have directly compared the efficacy of these medications. However, response to infliximab is relatively rapid compared with adalimumab and etanercept.
In a phase III clinical trial,59 as many as 80% of patients achieved PASI-75 clearance of their psoriasis after three doses of infliximab. Interestingly, only 61% of patients maintained PASI-75 clearance by week 50. This loss of efficacy of infliximab is also reported with other TNF-alpha inhibitors and is thought to be secondary to the development of antibodies to the drugs. For infliximab, this loss of efficacy is less when infliximab is given continuously rather than on an as-needed basis. Simultaneous treatment with methotrexate is also thought to decrease the development of antibodies to infliximab.60
Ustekinumab is an monoclonal antibody directed against the common p40 subunit of IL-12 and IL-23, which have been shown to be at increased levels in psoriatic lesions and important for the pathogenesis of psoriasis.
Between 66% and 76% of patients treated with ustekinumab achieved significant clearance of their disease after 12 weeks of treatment in two large phase III multicenter, randomized, double-blind, placebo-controlled trials.61,62
Dosing of ustekinumab is weight-based. For those weighing less than 100 kg, ustekinumab is given at 45 mg subcutaneously at baseline, at 4 weeks, and every 12 weeks thereafter. The same dosing schedule is used for those weighing more than 100 kg, but the dose is increased to 90 mg.
Guidelines for monitoring patients while on ustekinumab are similar to those for other biologic agents. Information on long-term toxicities is still being collected. However, injection-site reactions, serious infections, malignancies, and a single case of reversible posterior leukoencephalopathy have been reported.20
While biologic agents are significantly more expensive than the conventional therapies discussed above and insurance coverage for these agents varies, they have demonstrated superior efficacy and may be indicated for patients with recalcitrant moderate to severe psoriasis for whom multiple types of treatment have failed.
FOR PSORIATIC ARTHRITIS: SYSTEMIC MEDICATIONS
For patients with known or questionable psoriatic arthritis, evaluation by a rheumatologist is highly recommended.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are usually first-line in the treatment of mild psoriatic arthritis. If after 2 to 3 months of therapy with NSAIDs no benefit is achieved, treatment with methotrexate as monotherapy is a practical consideration because of its low cost. However, methotrexate as a monotherapy has not been shown to prevent radiologic progression of disease.5,32
The TNF-alpha inhibitors have been shown to have similar efficacy when compared among each other in the treatment of psoriatic arthritis.32,63 Based on radiologic evidence, ustekinumab has not shown to be as efficacious as the TNF-alpha inhibitors for treating psoriatic arthritis. Therefore, TNF inhibitors should be considered first-line in the treatment of psoriatic arthritis.21,64
Few studies have been done on the efficacy or sequence of therapies that should be used in the treatment of psoriatic arthritis. The American Academy of Dermatology’s Psoriasis Guidelines of Care recommend adding a TNF-alpha inhibitor or switching to a TNF-alpha inhibitor if no significant improvement is achieved after 12 to 16 weeks of treatment with oral methotrexate.20
FOR ERYTHRODERMIC PSORIASIS: MEDICATIONS THAT ACT PROMPTLY
The care of erythrodermic psoriatic patients is distinct from that of other psoriatic patients because of their associated systemic symptoms. Care should be taken to rule out sepsis, as this is a reported trigger of erythrodermic psoriasis.28
Systemic medications with a quick onset, such as oral cyclosporine, are recommended. Infliximab has also been reported to be beneficial because of its rapid onset.28
TREATMENT BASED ON THE TYPE AND THE SEVERITY OF PSORIASIS
The treatment of psoriasis can be as complex as the disease it itself and should be based on the type and the severity of psoriasis. Recognition of the various manifestations of psoriasis is important for effective treatment. However, in patients with moderate to severe psoriasis, atypical presentations, or recalcitrant disease, referral to a specialist is recommended.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58:826–850.
- Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26:314–320.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol 2002; 47:512–518.
- Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol 2010; 63:733–748.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012; 66:e67–e76.
- Schmitt JM, Ford DE. Work limitations and productivity loss are associated with health-related quality of life but not with clinical severity in patients with psoriasis. Dermatology 2006; 213:102–110.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol 2010; 163:586–592.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011; 270:147–157.
- Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol 2011; 64:495–501.
- Bremmer S, Van Voorhees AS, Hsu S, et al; National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 63:1058–1069.
- Tobin AM, Veale DJ, Fitzgerald O, et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol 2010; 37:1386–1394.
- Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol 2003; 48:805–821.
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006; 55:829–835.
- Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol 2012; 66:252–258.
- Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006; 126:2194–2201.
- Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol 2011; 65:84–91.
- Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor’s consensus: psoriasis and coronary artery disease. Am J Cardiol 2008; 102:1631–1643.
- American Academy of Dermatology Work Group; Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol 2011; 65:137–174.
- Mallbris L, Larsson P, Bergqvist S, Vingård E, Granath F, Ståhle M. Psoriasis phenotype at disease onset: clinical characterization of 400 adult cases. J Invest Dermatol 2005; 124:499–504.
- Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol 2011; 165:1162–1168.
- Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in US women: a prospective study. Arch Dermatol 2010; 146:1364–1369.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010; 42:985–990.
- Nair RP, Duffin KC, Helms C, et al; Collaborative Association Study of Psoriasis. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 2009; 41:199–204.
- Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156:258–262.
- Rosenbach M, Hsu S, Korman NJ, et al; National Psoriasis Foundation Medical Board. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 62:655–662.
- Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol 2011; 164:942–946.
- Kluger N, Bessis D, Guillot B, Girard C. Acute respiratory distress syndrome complicating generalized pustular psoriasis (psoriasis-associated aseptic pneumonitis). J Am Acad Dermatol 2011; 64:1154–1158.
- Roth MM. Pregnancy dermatoses: diagnosis, management, and controversies. Am J Clin Dermatol 2011; 12:25–41.
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008; 58:851–864.
- Ogdie A, Gelfand JM. Identification of risk factors for psoriatic arthritis: scientific opportunity meets clinical need. Arch Dermatol 2010; 146:785–788.
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53:573.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973; 3:55–78.
- McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol 2009; 23(suppl 1):9–13.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005; 64(suppl 2):ii65–ii68.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol 2002; 146:351–364.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60:643–659.
- Zivkovich AH, Feldman SR. Are ointments better than other vehicles for corticosteroid treatment of psoriasis? J Drugs Dermatol 2009; 8:570–572.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 2010; 62:114–135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009; 61:451–485.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol 2005; 44:1016–1021.
- Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol 1998; 134:1582–1585.
- Stern RS, Väkevä LH. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J Invest Dermatol 1997; 108:897–900.
- Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol 2009; 60:1001–1017.
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol 2008; 158:116–121.
- Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol 2009; 60:824–837.
- Zachariae H, Heickendorff L, Søgaard H. The value of aminoterminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol 2001; 144:100–103.
- Lowe NJ, Wieder JM, Rosenbach A, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 1996; 35:710–719.
- Gottlieb AB, Grossman RM, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol 1992; 98:302–309.
- Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med 1991; 324:277–284.
- Faerber L, Braeutigam M, Weidinger G, et al. Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients. Am J Clin Dermatol 2001; 2:41–47.
- Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized pustular psoriasis: a multicenter study in Japan. J Dermatol 1999; 26:141–149.
- Krueger GG, Ellis CN. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol 2003; 148:784–788.
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE; Alefacept Clinical Study Group. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139:719–727.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139:1627–1632.
- Gottlieb AB, Masud S, Ramamurthi R, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J Am Acad Dermatol 2003; 48:68–75.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007; 56:31.e1–31.e15.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Kimball AB, Papp KA, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Griffiths CE, Strober BE, van de Kerkhof P, et al; ACCEPT Study Group. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–128.
- Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009; 373:633–640.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58:826–850.
- Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26:314–320.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–407.
- Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol 2002; 47:512–518.
- Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol 2010; 63:733–748.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012; 66:e67–e76.
- Schmitt JM, Ford DE. Work limitations and productivity loss are associated with health-related quality of life but not with clinical severity in patients with psoriasis. Dermatology 2006; 213:102–110.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–1741.
- Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol 2010; 163:586–592.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011; 270:147–157.
- Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol 2011; 64:495–501.
- Bremmer S, Van Voorhees AS, Hsu S, et al; National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 63:1058–1069.
- Tobin AM, Veale DJ, Fitzgerald O, et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol 2010; 37:1386–1394.
- Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol 2003; 48:805–821.
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006; 55:829–835.
- Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol 2012; 66:252–258.
- Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006; 126:2194–2201.
- Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol 2011; 65:84–91.
- Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor’s consensus: psoriasis and coronary artery disease. Am J Cardiol 2008; 102:1631–1643.
- American Academy of Dermatology Work Group; Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol 2011; 65:137–174.
- Mallbris L, Larsson P, Bergqvist S, Vingård E, Granath F, Ståhle M. Psoriasis phenotype at disease onset: clinical characterization of 400 adult cases. J Invest Dermatol 2005; 124:499–504.
- Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol 2011; 165:1162–1168.
- Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in US women: a prospective study. Arch Dermatol 2010; 146:1364–1369.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509.
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010; 42:985–990.
- Nair RP, Duffin KC, Helms C, et al; Collaborative Association Study of Psoriasis. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 2009; 41:199–204.
- Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156:258–262.
- Rosenbach M, Hsu S, Korman NJ, et al; National Psoriasis Foundation Medical Board. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 62:655–662.
- Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol 2011; 164:942–946.
- Kluger N, Bessis D, Guillot B, Girard C. Acute respiratory distress syndrome complicating generalized pustular psoriasis (psoriasis-associated aseptic pneumonitis). J Am Acad Dermatol 2011; 64:1154–1158.
- Roth MM. Pregnancy dermatoses: diagnosis, management, and controversies. Am J Clin Dermatol 2011; 12:25–41.
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008; 58:851–864.
- Ogdie A, Gelfand JM. Identification of risk factors for psoriatic arthritis: scientific opportunity meets clinical need. Arch Dermatol 2010; 146:785–788.
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53:573.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973; 3:55–78.
- McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol 2009; 23(suppl 1):9–13.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005; 64(suppl 2):ii65–ii68.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol 2002; 146:351–364.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60:643–659.
- Zivkovich AH, Feldman SR. Are ointments better than other vehicles for corticosteroid treatment of psoriasis? J Drugs Dermatol 2009; 8:570–572.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 2010; 62:114–135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009; 61:451–485.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol 2005; 44:1016–1021.
- Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol 1998; 134:1582–1585.
- Stern RS, Väkevä LH. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J Invest Dermatol 1997; 108:897–900.
- Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol 2009; 60:1001–1017.
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol 2008; 158:116–121.
- Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol 2009; 60:824–837.
- Zachariae H, Heickendorff L, Søgaard H. The value of aminoterminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol 2001; 144:100–103.
- Lowe NJ, Wieder JM, Rosenbach A, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 1996; 35:710–719.
- Gottlieb AB, Grossman RM, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol 1992; 98:302–309.
- Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med 1991; 324:277–284.
- Faerber L, Braeutigam M, Weidinger G, et al. Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients. Am J Clin Dermatol 2001; 2:41–47.
- Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized pustular psoriasis: a multicenter study in Japan. J Dermatol 1999; 26:141–149.
- Krueger GG, Ellis CN. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol 2003; 148:784–788.
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE; Alefacept Clinical Study Group. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139:719–727.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139:1627–1632.
- Gottlieb AB, Masud S, Ramamurthi R, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J Am Acad Dermatol 2003; 48:68–75.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367–1374.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007; 56:31.e1–31.e15.
- Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684.
- Leonardi CL, Kimball AB, Papp KA, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674.
- Griffiths CE, Strober BE, van de Kerkhof P, et al; ACCEPT Study Group. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–128.
- Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009; 373:633–640.
KEY POINTS
- Studies in the past 10 years have uncovered a link between psoriasis, metabolic syndrome, and cardiovascular disease. Interestingly, the risk grows less with age; patients at greatest risk are young men with severe psoriasis.
- The most common presentation of psoriasis is plaque psoriasis. However, there are several other clinical variations of psoriasis, each of which has a distinct response to treatment and may be associated with significant systemic symptoms.
- Tumor necrosis factor inhibitors should be considered first-line in the treatment of psoriatic arthritis.
- Phototherapy and systemic medications including methotrexate, acitretin (Soriatane), cyclosporine (Gengraf, Neoral, Sandimmune), and biologic agents are the most effective treatments for moderate-to-severe psoriasis.
Undiluted acid used for vulvar surgery … and more
WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.

WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.

WIDE LOCAL EXCISION was performed on a 42-year-old woman with vulvar intraepithelial neoplasm, VIN II, with moderate dysplasia. Her ObGyn performed the surgery.
Instead of applying a diluted solution of acetic acid wash to delineate the borders of the dysplastic area, a highly concentrated acetic acid or trichloroacetic acid was used. The patient suffered severe chemical burns of the vulva that took several months to heal. She has permanent scarring of the vulvar area, severe tenderness, discoloration, and atrophy of the vaginal opening, with a band of thick scar tissue at the posterior fourchette. The perineum, extending to the anal area, is scarred, including a 2-mm plaque layer.
PATIENT’S CLAIM Sexual intercourse is extremely painful, and therefore impossible. She suffers discomfort at all times. Additional surgery has been recommended to alleviate her condition.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $600,000 Ohio settlement was reached.
Large baby with cervical spine injury
A WOMAN WAS IN LABOR with her third child. Her first baby was born by cesarean delivery. During the vaginal birth of her second child, shoulder dystocia was encountered; this child weighed 8 lb 4 oz at birth.
Using ultrasonography, the ObGyn determined vaginal birth was appropriate. Shoulder dystocia was encountered and the infant suffered injuries to the cervical spine and right arm. The newborn weighed 9 lb 13 oz.
PATIENT’S CLAIM The baby’s weight was grossly underestimated prior to delivery; ultrasonography was not properly performed or evaluated. The mother’s history, large fundal height, estimated fetal weight, and the mother’s request for a cesarean delivery should have resulted in the performance of a cesarean delivery.
PHYSICIAN’S DEFENSE Shoulder dystocia was not reasonably foreseeable. Injuries to the baby were due to the forces of labor.
VERDICT A confidential Texas settlement was reached.
Suture causes nerve damage
PELVIC PROLAPSE RECONSTRUCTION was performed; surgery included a pubovaginal sling procedure with graft, and repairs of Grade 2 cystocele and Grade 3 rectocele. The gynecologist used transvaginal sutures to attach the mesh to the sacrospinous ligament.
The patient immediately reported pain, tingling, and weakness in her buttocks and legs. The gynecologist diagnosed a hematoma and continued conservative treatment while waiting for the hematoma to resorb.
After 10 days, the patient terminated the gynecologist’s services and left the hospital. She saw a neurologist, who diagnosed proximal sciatic nerve irritation secondary to suturing. When a suture was removed from the sacral spinous ligament plexus, many of the patient’s neurologic symptoms immediately resolved. She still has pain and walks with a noticeable limp using a cane.
PATIENT’S CLAIM The gynecologist failed to determine that a suture was causing nerve damage. Removal of the suture within the first 3 days would have avoided neurologic injury.
PHYSICIAN’S DEFENSE Postsurgical care was proper. A neurologist was consulted, and a sonogram had ruled out deep vein thrombosis.
VERDICT A $1.58 million Illinois verdict was returned.
Colon damage after embolization
UTERINE FIBROID EMBOLIZATION was performed on a 51-year-old woman. The next day, she reported severe abdominal pain and was readmitted. A uterine infection was suspected, and she underwent a hysterectomy. Necrosis of the colon was found; a surgeon removed one-third of the colon and performed a colostomy. She underwent several operations, including rectal-vaginal fistula repair, before the colostomy was corrected.
PATIENT’S CLAIM Misdirected embolization injured an artery supplying the colon. She continues to suffer ongoing fecal urgency and frequency.
PHYSICIAN’S DEFENSE An anomalous connection between the patient’s uterine artery and mesenteric artery was impossible for the physician to have known prior to the embolization procedure.
VERDICT A California defense verdict was returned.

SEVERAL HOURS AFTER A WOMAN’S LABOR BEGAN, fetal bradycardia developed precipitously. The on-call ObGyn arrived after 10 minutes and ordered an immediate cesarean delivery, which occurred 22 minutes later. The child suffered a catastrophic, irreversible brain injury. He lived for 39 days before life support was removed and he died.
ESTATE’S CLAIM The nurses did not report decelerations to the ObGyn, and they were slow to notify him of the fetal bradycardia. The child would not have been injured if the nursing staff had reacted appropriately.
DEFENDANTS’ DEFENSE Isolated heart-rate decelerations during labor are not troubling. A cord accident occurred, which could not be predicted nor avoided. The ObGyn was called promptly; the emergency cesarean delivery was performed quickly. However, the injury already had occurred and was irreparable.
VERDICT A $1.18 million Kentucky verdict was returned. The hospital sought a mistrial because Facebook postings by a juror proved the case had been discussed and prejudged. The court found in favor of the hospital on its post-trial motion.
Bilateral mastectomy: nipples not spared
A 46-YEAR-OLD WOMAN UNDERWENT prophylactic bilateral mastectomy. A plastic surgeon drew presurgical markings on the day of surgery; the breast surgeon removed the nipples.
PATIENT’S CLAIM All parties had agreed the nipples would be spared. The plastic surgeon drew improper markings and failed to remind the breast surgeon prior to surgery that the nipples would be preserved.
PHYSICIAN’S DEFENSE The breast surgeon was at fault for misinterpreting the markings.
VERDICT The patient reached a pretrial settlement with the breast surgeon. The case proceeded against the plastic surgeon. A Maryland defense verdict was returned for the plastic surgeon.
Signs of intrauterine growth restriction; stillborn child
AT 24 WEEKS’ GESTATION, a 17-year-old woman who smoked reported spotting. An ultrasound demonstrated significant fetal growth restriction. The mother was hospitalized to assess the spotting; no testing was ordered to assess fetal growth. When blood was not found in the birth canal, she was discharged. During the next month, she saw the ObGyn three times; testing indicated that the fetus was at least 3 weeks behind the stage of pregnancy. The ObGyn did not order additional testing nor consult a specialist. At 31 weeks’ gestation, ultrasonography found no fetal heart tones. The stillborn was delivered by cesarean section.
ESTATE’S CLAIM A wrongful death suit was filed by the parents, who also claimed lack of informed consent concerning the risk of stillbirth in the presence of intrauterine growth restriction.
PHYSICIANS’ DEFENSE The mother’s smoking was mentioned at trial as a possible explanation of why fetal development was delayed. The ObGyn denied negligence.
VERDICT A $800,000 Maryland verdict was awarded to the parents.
Three BrCa patients share $72.6 M
THREE MENOPAUSAL WOMEN took Premarin (conjugated estrogens) plus Provera (medroxyprogesterone), and/or Prempro (conjugated estrogens/medroxyprogesterone acetate). Each discontinued hormone therapy after being diagnosed with hormone-positive breast cancer.
PATIENTS’ CLAIM The only source of hormonal stimulation for their cancer was the use of estrogen plus progestin.
DEFENDANTS’ DEFENSE Science is currently unable to determine precisely what causes breast cancer. Each plaintiff had risk factors.
VERDICT The three cases were consolidated to a reverse-bifurcated trial, with causation and damages assessed first. The Pennsylvania jury found the Wyeth Pharmaceutical products to be factual causes of the patients’ cancer, and awarded a total of $72.6 million in compensatory damages. The parties settled for confidential amounts before the liability phase began.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Income declined for many ObGyns from 2011 to 2012
“You may not have noticed but your workload is lighter. So is your wallet.”
Louis Weinstein, MD (March 2010)
Most ObGyns saw their income decline or remain flat from 2011 to 2012, according to a survey from Medscape. Thirty-five percent of ObGyns reported lower earnings than in the preceding year, and another 39% reported no change. Overall, the specialty earned 3% less than in the preceding year. For physicians as a whole, income also declined.
The survey was conducted in February 2012 among 24,216 US physicians across 25 specialties. It found that ObGyns earned a mean of $220,000—a slight decline from the previous year. About 26% of ObGyns reported an increase in earnings, however. For physicians as a whole, 34% reported an increase in earnings over the past year.
Top earners among the 25 specialties represented in the survey were radiologists and orthopedic surgeons (both earning a mean of $315,000), followed by cardiologists ($314,000), anesthesiologists ($309,000), and urologists ($309,000). The lowest income was reported by internists ($165,000), family physicians ($158,000), and pediatricians ($156,000).
Compensation for employed physicians comprised salary, any bonus, and profit-sharing contributions. For physicians in private practice, compensation consisted of earnings after the deduction of business expenses but before the payment of income tax. Compensation did not include income for nonclinical activities, such as speaking engagements and expert witness testimony.
Other findings
Men made more than women. Among physicians as a whole, male practitioners earned approximately 40% more than female practitioners. In the ObGyn specialty, however, the gap was narrower: Men earned approximately 12% more than women ($234,000 vs $206,000).
Some regions of the United States were more lucrative. The most profitable region of the United States for ObGyns was the Great Lakes region (Illinois, Indiana, Ohio, Michigan, Minnesota, and Wisconsin), with physicians there reporting a mean income of $245,000. Least profitable were the northeast and mid-Atlantic regions, with a mean income of $205,000 and $207,000, respectively.
ObGyns in private practice earned more. When income was broken down by practice setting, the single-specialty group was most profitable (mean income of $242,000), followed by health care organizations ($239,000), the multi-specialty group ($233,000), solo practice ($229,000), the hospital setting ($194,000), academia ($173,000), and outpatient clinic ($154,000).
Some paradigms remained on the margins. Only 1% of ObGyns reported working in a concierge practice, 3% required cash only, 3% were part of an accountable care organization, and 5% planned to join or form an accountable care organization over the coming year.
Most ObGyns would choose another specialty. Although most ObGyns (55%) reported that they would choose medicine again as a career, only 37% said they would choose the same specialty and 23% said they would choose the same practice setting.
For the full report, click here.
We want to hear from you! Tell us what you think.
“You may not have noticed but your workload is lighter. So is your wallet.”
Louis Weinstein, MD (March 2010)
Most ObGyns saw their income decline or remain flat from 2011 to 2012, according to a survey from Medscape. Thirty-five percent of ObGyns reported lower earnings than in the preceding year, and another 39% reported no change. Overall, the specialty earned 3% less than in the preceding year. For physicians as a whole, income also declined.
The survey was conducted in February 2012 among 24,216 US physicians across 25 specialties. It found that ObGyns earned a mean of $220,000—a slight decline from the previous year. About 26% of ObGyns reported an increase in earnings, however. For physicians as a whole, 34% reported an increase in earnings over the past year.
Top earners among the 25 specialties represented in the survey were radiologists and orthopedic surgeons (both earning a mean of $315,000), followed by cardiologists ($314,000), anesthesiologists ($309,000), and urologists ($309,000). The lowest income was reported by internists ($165,000), family physicians ($158,000), and pediatricians ($156,000).
Compensation for employed physicians comprised salary, any bonus, and profit-sharing contributions. For physicians in private practice, compensation consisted of earnings after the deduction of business expenses but before the payment of income tax. Compensation did not include income for nonclinical activities, such as speaking engagements and expert witness testimony.
Other findings
Men made more than women. Among physicians as a whole, male practitioners earned approximately 40% more than female practitioners. In the ObGyn specialty, however, the gap was narrower: Men earned approximately 12% more than women ($234,000 vs $206,000).
Some regions of the United States were more lucrative. The most profitable region of the United States for ObGyns was the Great Lakes region (Illinois, Indiana, Ohio, Michigan, Minnesota, and Wisconsin), with physicians there reporting a mean income of $245,000. Least profitable were the northeast and mid-Atlantic regions, with a mean income of $205,000 and $207,000, respectively.
ObGyns in private practice earned more. When income was broken down by practice setting, the single-specialty group was most profitable (mean income of $242,000), followed by health care organizations ($239,000), the multi-specialty group ($233,000), solo practice ($229,000), the hospital setting ($194,000), academia ($173,000), and outpatient clinic ($154,000).
Some paradigms remained on the margins. Only 1% of ObGyns reported working in a concierge practice, 3% required cash only, 3% were part of an accountable care organization, and 5% planned to join or form an accountable care organization over the coming year.
Most ObGyns would choose another specialty. Although most ObGyns (55%) reported that they would choose medicine again as a career, only 37% said they would choose the same specialty and 23% said they would choose the same practice setting.
For the full report, click here.
We want to hear from you! Tell us what you think.
“You may not have noticed but your workload is lighter. So is your wallet.”
Louis Weinstein, MD (March 2010)
Most ObGyns saw their income decline or remain flat from 2011 to 2012, according to a survey from Medscape. Thirty-five percent of ObGyns reported lower earnings than in the preceding year, and another 39% reported no change. Overall, the specialty earned 3% less than in the preceding year. For physicians as a whole, income also declined.
The survey was conducted in February 2012 among 24,216 US physicians across 25 specialties. It found that ObGyns earned a mean of $220,000—a slight decline from the previous year. About 26% of ObGyns reported an increase in earnings, however. For physicians as a whole, 34% reported an increase in earnings over the past year.
Top earners among the 25 specialties represented in the survey were radiologists and orthopedic surgeons (both earning a mean of $315,000), followed by cardiologists ($314,000), anesthesiologists ($309,000), and urologists ($309,000). The lowest income was reported by internists ($165,000), family physicians ($158,000), and pediatricians ($156,000).
Compensation for employed physicians comprised salary, any bonus, and profit-sharing contributions. For physicians in private practice, compensation consisted of earnings after the deduction of business expenses but before the payment of income tax. Compensation did not include income for nonclinical activities, such as speaking engagements and expert witness testimony.
Other findings
Men made more than women. Among physicians as a whole, male practitioners earned approximately 40% more than female practitioners. In the ObGyn specialty, however, the gap was narrower: Men earned approximately 12% more than women ($234,000 vs $206,000).
Some regions of the United States were more lucrative. The most profitable region of the United States for ObGyns was the Great Lakes region (Illinois, Indiana, Ohio, Michigan, Minnesota, and Wisconsin), with physicians there reporting a mean income of $245,000. Least profitable were the northeast and mid-Atlantic regions, with a mean income of $205,000 and $207,000, respectively.
ObGyns in private practice earned more. When income was broken down by practice setting, the single-specialty group was most profitable (mean income of $242,000), followed by health care organizations ($239,000), the multi-specialty group ($233,000), solo practice ($229,000), the hospital setting ($194,000), academia ($173,000), and outpatient clinic ($154,000).
Some paradigms remained on the margins. Only 1% of ObGyns reported working in a concierge practice, 3% required cash only, 3% were part of an accountable care organization, and 5% planned to join or form an accountable care organization over the coming year.
Most ObGyns would choose another specialty. Although most ObGyns (55%) reported that they would choose medicine again as a career, only 37% said they would choose the same specialty and 23% said they would choose the same practice setting.
For the full report, click here.
We want to hear from you! Tell us what you think.
How to respond to an in-flight emergency
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Requests for a physician to assist during in-flight medical emergencies are becoming more common as travelers age and more people have access to air travel.1 Yet in this age of medical specialization, all physicians are not created equal, particularly in providing acute care. Responding to in-flight medical emergencies can be stressful, particularly for specialists who may doubt their skill base in a medical crisis.
The in-flight medical consulting service Medlink reported that only 3.5% of calls it receives are related to psychiatric emergencies.2 This means that psychiatrists who answer an in-flight distress call will almost invariably find themselves confronting a medical issue.
Discomfort with possibly having to deliver acute or invasive medical care may deter psychiatrists from responding. However, psychiatrists should be familiar with many medical problems and their basic management, particularly if their institution requires up-to-date advanced cardiac life support certification. Understanding your role and conceptualizing a general approach before you find yourself in the midst of an in-flight medical emergency can be helpful. We suggest the following principles for responding to in-flight emergencies:
- Respond to a call only if you are a licensed, currently practicing physician.
- Defer to other physicians present who may have more experience delivering acute medical care.3
- If you have been drinking alcohol, do not respond unless there are no other health care providers on board. If you do respond, know your limitations and make them known to the air crew and patient.
- Identify yourself to the patient. Give him or her your name and tell the patient that you are a psychiatrist. If the chief complaint is not something you regularly deal with, tell the patient and crew.
- Perform the best history and physical exam you can, given the setting. Obtain vital signs. Document your findings for your records and for medical personnel who may later assume patient care.
- Do not attempt procedures you are unfamiliar with or not qualified to perform (eg, starting an IV, intubations). Administer only treatments you are comfortable with.
- If you are concerned the patient may face significant morbidity or death, advise the crew to divert the flight to the closest hospital.
- Realize that it is not your role to take leadership of the situation, unless you are the only physician present. Do not be afraid to ask for help from other physicians or health care providers—including nurses or emergency medical technicians—who may not have heard or acknowledged the call or a ground-based medical consulting service. Also, once another physician has taken over, you still can contribute by stabilizing the ill patient’s emotions and behavior.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article of with manufacturers of competing products.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
1. Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med. 2002;346(14):1067-1073.
2. Matsumoto K, Goebert D. In-flight psychiatric emergencies. Aviat Space Environ Med. 2001;72(10):919-923.
3. Macleod S. “If there is a doctor aboard this flight…”: issues and advice for the passenger-psychiatrist. Australas Psychiatry. 2008;16(4):233-237.
Implementing a smoking ban: Tips for success
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
The prevalence of tobacco use among psychiatric patients is up to 4 times greater than that of the general population.1 Increasing numbers of psychiatric facilities have implemented policies that ban smoking to eliminate secondhand smoke, achieve a cleaner environment, encourage healthier lifestyles for patients and staff, and reduce patient smoke breaks, which allows more time for treatment.2 The potential benefits of tobacco-free psychiatric institutions has led some clinicians to call for the total exclusion of tobacco from psychiatric and addiction settings.3
New Hampshire Hospital is a 152-bed acute inpatient psychiatric facility that has approximately 2,400 patient admissions per year. Most patients have psychotic or mood disorders, often with a co-occurring substance use or personality disorder. We report our experience in planning and implementing a campus-wide “total” smoking ban—a ban on all tobacco products in the hospital building and on hospital grounds.
Implementation and results
Our hospital’s interdisciplinary Tobacco-Free Campus Task Force developed specific recommendations and a timeline for implementing the total smoking ban. Hospital staff voiced concerns that banning smoking would lead to increased episodes of aggressive behavior. We reviewed data on the use of seclusion and restraints, patient assaults, and smoking contraband before and after initiating the total smoking ban. We found no evidence of an increase in the use of seclusion or restraints or in patient assaults with staff injury after implementing the smoking ban. However, we did see an initial increase in smoking contraband. These rates peaked and then tapered to pre-smoking ban rates within 2 years.
Why we succeeded
Several factors contributed to the successful implementation of our total smoking ban:
- Hospital administration supported having a smoke-free campus, and executive leadership allowed staff to develop strategies, programs, treatment options, and groups to maximize the possibility of success.
- Extensive communication with outside agencies, advocacy groups, and care providers allowed for discussion of potential difficulties, such as concerns regarding individuals not having access to tobacco during their hospital stay and how this could affect their treatment.
- The hospital’s Tobacco-Free Campus Task Force helped develop strategies that allowed for an effective transition to a smoke-free campus, such as increasing the number of smoking cessation groups for patients and staff and eliminating the sale of tobacco products at the hospital’s visitor shop.
- Extensive preparation, clear timelines, and achievable goals created a positive climate for a “culture of change.”
Recommendations
If you are considering a total smoking ban at your facility, we recommend the following steps:
- set a clear target date
- allow adequate time for hospital staff and administration to develop strategies for implementation
- make sure hospital administration is supportive
- involve all hospital disciplines—psychiatry, nursing, rehabilitation, psychology, social work, etc.
- address staff concerns regarding patient and staff safety
- ensure adequate nicotine replacement therapy options for patients and staff
- anticipate an initial increase in smoking-related contraband
- understand there may be differing opinions regarding the smoking ban, but remain committed to the change.
Dr. Folks is a consultant to Independent Medical Experts Consulting Services and Medical Care Management Corporation.
Drs. de Nesnera and Rauter report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.
1. Morisano D, Bacher I, Audrain-McGovern J, et al. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):356-367.
2. Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Prev Med. 2008;46(6):572-578.
3. Moss TG, Weinberger AH, Vessicchio JC, et al. A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict. 2010;19(4):293-311.