User login
Hypertension in the elderly: Some practical considerations
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
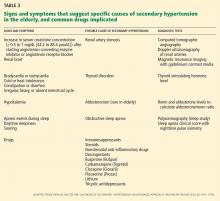
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
The management of hypertension has advanced significantly in the last few decades. But the race for more effective means to control this epidemic and its associated complications is far from won. A high percentage of patients in the United States have hypertension that is uncontrolled. Most of these belong to the most rapidly growing demographic group in the United States, ie, the elderly.
It is estimated that more than 70% of medical practice will be directed to geriatric needs in the coming years. It is therefore very important for clinicians to be comfortable with managing hypertension in the elderly.
A GROWING PROBLEM IN AN AGING POPULATION
Between 1980 and 2009, the US population age 65 and older increased from 25.6 million to 39.6 million, of which 42% are men and 58% women.1 This number is expected to reach 75 million by the year 2040. People over 85 years of age are the fastest growing subset of the US population.2 As many as 50% of people who were born recently in countries such as the United States, the United Kingdom, France, Denmark, and Japan will live to celebrate their 100th birthday.3
According to the Framingham Heart Study, by age 60 approximately 60% of the population develops hypertension, and by 70 years about 65% of men and about 75% of women have the disease. In the same study, 90% of those who were normotensive at age 55 went on to develop hypertension. The elderly also are more likely to suffer from the complications of hypertension and are more likely to have uncontrolled disease.
Compared with younger patients with similar blood pressure, elderly hypertensive patients have lower cardiac output, higher peripheral resistance, wider pulse pressure, lower intravascular volume, and lower renal blood flow.4 These age-related pathophysiologic differences must be considered when treating antihypertension in the elderly.
IS TREATING THE ELDERLY BENEFICIAL?
Most elderly hypertensive patients have multiple comorbidities, which tremendously affect the management of their hypertension. They are also more likely than younger patients to have resistant hypertension and to need multiple drugs to control their blood pressure. In the process, these frail patients are exposed to a host of drug-related adverse effects. Thus, it is relevant to question the net benefit of treatment in this age group.
Many studies have indeed shown that treating hypertension reduces the risk of stroke and other adverse cardiovascular events. A decade ago, Staessen et al,5 in a meta-analysis of more than 15,000 patients between ages 62 and 76, showed that treating isolated systolic hypertension substantially reduced morbidity and mortality rates. Moreover, a 2011 meta-analysis of randomized controlled trials in hypertensive patients age 75 and over also concluded that treatment reduced cardiovascular morbidity and mortality rates and the incidence of heart failure, even though the total mortality rate was not affected.6
Opinion on treating the very elderly (≥ 80 years of age) was divided until the results of the Hypertension in the Very Elderly trial (HYVET)7 came out in 2008. This study documented major benefits of treatment in the very elderly age group as well.
The consensus, therefore, is that it is appropriate, even imperative, to treat elderly hypertensive patients (with some cautions—see the sections that follow).
GOAL OF TREATMENT IN THE ELDERLY
Targets for blood pressure management have been based primarily on observational data in middle-aged patients. There is no such thing as an ideal blood pressure that has been derived from randomized controlled trials for any population, let alone the elderly. The generally recommended blood pressure goal of 140/90 mm Hg for elderly hypertensive patients is based on expert opinion.
Moreover, it is unclear if the same target should apply to octogenarians. According to a 2011 American College of Cardiology/American Heart Association (ACC/AHA) expert consensus report,8 an achieved systolic blood pressure of 140 to 145 mm Hg, if tolerated, can be acceptable in this age group.
An orthostatic decline in blood pressure accompanies advanced age and is an inevitable adverse effect of some antihypertensive drugs. Accordingly, systolic blood pressure lower than 130 and diastolic blood pressure lower than 70 mm Hg are best avoided in octogenarians.8 Therefore, when hypertension is complicated by coexisting conditions that require a specific blood pressure goal, it would seem reasonable to not pursue the lower target as aggressively in octogenarians as in elderly patients under age 80.
Having stated the limitations in the quality of data at hand—largely observational—it is relevant to mention the Systolic Blood Pressure Intervention trial (SPRINT).9 This ongoing randomized, multicenter trial, launched by the National Institutes of Health, is assessing whether maintaining blood pressure levels lower than current recommendations further reduces the risk of cardiovascular and kidney diseases or, in the SPRINT-MIND substudy, of age-related cognitive decline, regardless of the type of antihypertensive drug taken. Initially planning to enroll close to 10,000 participants over the age of 55 without specifying any agegroup ranges, the investigators later decided to conduct a substudy called SPRINT Senior that will enroll about 1,750 participants over the age of 75 to determine whether a lower blood pressure range will have the same beneficial effects in older adults.
Given the limitations in the quality and applicability of published data (coming from small, nonrandomized studies with no long-term follow-up), SPRINT is expected to provide the evidence needed to support standard vs aggressive hypertension control among the elderly. The trial is projected to run until late 2018.
MANAGEMENT APPROACH IN THE ELDERLY
Blood pressure should be recorded in both arms before a diagnosis is made. In a number of patients, particularly the elderly, there are significant differences in blood pressure readings between the two arms. The higher reading should be relied on and the corresponding arm used for subsequent measurements.
Lifestyle interventions
Similar to the approach in younger patients with hypertension, lifestyle interventions are the first step to managing high blood pressure in the elderly. The diet and exercise interventions in the Dietary Approaches to Stop Hypertension (DASH) trial have both been shown to lower blood pressure.10,11
Restricting sodium intake has been shown to lower blood pressure more in older adults than in younger adults. In the DASH trial,12 systolic blood pressure decreased by 8.1 mm Hg with sodium restriction in hypertensive patients age 55 to 76 years, compared with 4.8 mm Hg for adults aged 23 to 41 years. In the Trial of Nonpharmacologic Interventions in the Elderly (TONE),13 in people ages 60 to 80 who were randomized to reduce their salt intake, urinary sodium excretion was 40 mmol/day lower and blood pressure was 4.3/2.0 mm Hg lower than in a group that received usual care. Accordingly, reducing salt intake is particularly valuable for blood pressure management in the salt-sensitive elderly.14
Drug therapy
The hypertension pandemic has driven extensive pharmaceutical research, and new drugs continue to be introduced. The major classes of drugs commonly used for treating hypertension are diuretics, calcium channel blockers, and renin-angiotensin system blockers. Each class has specific benefits and adverse-effect profiles.
It is appropriate to start antihypertensive drug therapy with the lowest dose and to monitor for adverse effects, including orthostatic hypotension. The choice of drug should be guided by the patient’s comorbid conditions (Table 1) and the other drugs the patient is taking.15 If the blood pressure response is inadequate, a second drug from a different class should be added. In the same manner, a third drug from a different class should be added if the blood pressure remains outside the optimal range on two drugs.
The average elderly American is on more than six medications.16 Some of these are for high blood pressure, but others interact with antihypertensive drugs (Table 2), and some, including nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids, directly affect blood pressure. Therefore, the drug regimen of an elderly hypertensive patient should be reviewed carefully at every visit. The Screening Tool of Older Person’s Prescriptions (STOPP), a list of 65 rules relating to the most common and most potentially dangerous instances of inappropriate prescribing and overprescribing in the elderly,17 has been found to be a reliable tool in this regard, with a kappa-coefficient of 0.75. Together with the Screening Tool to Alert Doctors to Right [ie, Appropriate, Indicated] Treatment (START),17 which lists 22 evidence-based prescribing indicators for common conditions in the elderly, these criteria provide clinicians with an easy screening tool to combat polypharmacy.
Given the multitude of factors that go into deciding on a specific management strategy in the elderly, it is not possible to discuss individualized care in all patients in the scope of one paper. Below, we present several case scenarios that internists commonly encounter, and suggest ways to approach each.
CASE 1: SECONDARY HYPERTENSION
A 69-year-old obese man who has hypertension of recent onset, long-standing gastroesophageal reflux disease, and benign prostatic hypertrophy comes to your office, accompanied by his wife. He has never had hypertension before. His body mass index is 34 kg/m2. On physical examination, his blood pressure is 180/112 mm Hg.
We start with this case to emphasize the importance of considering causes of secondary hypertension in all patients with the disease (Table 3).18 Further workup should be pursued in those who appear to have “inappropriate” hypertension. This could present as refractory hypertension, abrupt-onset hypertension, hypertension that is first diagnosed before age 20 or after age 60, or loss of control over previously well-controlled blood pressure. Secondary hypertension must always be considered when the history or physical examination suggests a possible cause.
Renal artery stenosis increases in incidence with age. Its prevalence is reported to be as high as 50% in elderly patients with other signs of atherosclerosis such as widespread peripheral artery disease.19
Obstructive sleep apnea also commonly coexists with hypertension and its prevalence also increases with age. In addition, elderly patients with obstructive sleep apnea have a higher incidence of cardiovascular complications, including hypertension, than middle-aged people.20 Numerous studies have found that the severity of obstructive sleep apnea corresponds with the likelihood of systemic hypertension.21–23 Randomized trials and meta-analyses have also concluded that effective treatment with continuous positive airway pressure reduces systemic blood pressure,24–27 although by less than antihypertensive medications do.
A causal relationship between obstructive sleep apnea and hypertension has not been established with certainty. It is recommended, however, that patients with resistant hypertension be screened for obstructive sleep apnea as a possible cause of their disease.
Other causes of secondary hypertension to keep in mind when evaluating patients who have inappropriate hypertension include thyroid disorders, alcohol and tobacco use, and chronic steroid or NSAID use. Pheochromocytoma and adrenal adenoma, though possible, are less prevalent in the elderly.
Case continued
Physical examination in the above patient revealed an epigastric systolic-diastolic bruit, a sign that, although not sensitive, is highly specific for renal artery stenosis, raising the suspicion of this condition. Duplex ultrasonography of the renal arteries confirmed this suspicion. The patient underwent angiography and revascularization, resulting in a distinct fall in, but not normalization of, his blood pressure.
Detecting and treating renal artery stenosis
Though we do not intend to detail the diagnostic approaches and treatments for the various causes of secondary hypertension, we need to briefly mention those for renal artery stenosis.
According to the 2006 ACC/AHA guidelines on peripheral artery disease,28 testing for renal artery stenosis is indicated only if a subsequent corrective procedure is a viable option.
Renal arteriography remains the gold standard for diagnosing renal artery stenosis. However, noninvasive imaging has largely replaced it.
Duplex Doppler ultrasonography, compared with angiography, has a sensitivity of 84% to 98% depending on operator experience, and a specificity of 62% to 99% for detecting renal artery stenosis.29 Some of its limiting factors are the time needed to do the study, its steep learning curve and operator-dependence, and interference with the results by body fat and intestinal gas.
Computed tomographic angiography has a sensitivity of over 90% for detecting renal artery stenosis, and its specificity has been shown to be as high as 99% in certain studies.29 Use of contrast can be a limiting factor in some clinical settings.
Magnetic resonance angiography also offers a sensitivity of 90% to 100% and specificities of 76% to 94% for detecting renal artery stenosis.29 On the other hand, it is costly, and the gadolinium contrast solution used is nephrotoxic, though not as toxic as the contrast used in computed tomographic angiography.
As previously stated, these imaging studies should be used only if corrective measures will be undertaken if clinically significant renal artery stenosis is found. Even in such cases, revascularization may not be curative in all cases. Its effectiveness has been compared with that of medical management alone in a number of studies.30,31 A meta-analysis32 of six key trials involving more than 1,200 patients showed no difference in systolic and diastolic blood pressures and other clinical outcomes, including all-cause mortality, between the two treatment groups over a 29-month follow-up period.
Hence, although we advise that causes of secondary hypertension be considered in cases of inappropriate hypertension, aggressive management must be pursued on a case-by-case basis.
CASE 2: DRUG ADVERSE EFFECTS
A 75-year-old Hispanic woman with a history of treated breast cancer was recently diagnosed with hypertension. Her blood pressure is controlled on amlodipine (Norvasc) 10 mg daily, and her blood pressure today is 128/80 mm Hg. Her only complaint during this office visit is some swelling of her ankles.
Edema and dihydropyridine calcium channel blockers
Like all drugs, antihypertensive medications come with their own set of adverse effects. These are more common as people age—hence the importance of identifying and effectively managing them in the elderly population.
Calcium channel blockers, especially the dihydropyridines—ie, nifedipine (Adalat), amlodipine, felodipine (Plendil), and isradipine (DynaCirc)—are known to cause peripheral vasodilation. Peripheral edema is a common dose-related effect in people on these drugs. In one study, median leg weight increased by 80 g after amlodipine 5 mg was given for 4 weeks, and by another 68 g on a 10-mg dose.33
Ankle swelling, encountered more in women, can be very bothersome. The swelling is related to hyperfiltration of fluid into the interstitial space secondary to intracapillary hypertension. Calcium channel blockers predominantly cause arteriolar dilation by paralyzing the precapillary sphincter, thereby elevating intracapillary pressure.
Traditionally, physicians have lowered the dose of the calcium channel blocker, switched to another drug, or added a diuretic to alleviate the swelling. However, giving a diuretic for edema induced by a calcium channel blocker may not relieve the edema.34
Peripheral edema is much less encountered when a calcium channel blocker is given with an inhibitor of the renin-angiotensin system.35 A meta-analysis concluded that the incidence of peripheral edema was lowered by 38% with such a combination. The same study found angiotensin-converting enzyme (ACE) inhibitors significantly more efficacious for this effect than angiotensin receptor blockers (ARBs).35
ACE inhibitors and ARBs are known to cause venodilation, thereby lowering intra-capillary pressure. It is probable that this effect helps remove the extra fluid sequestered in the capillary bed by the arteriolar dilation from the calcium channel blocker.
Pedal edema associated with use of a calcium channel blocker occurs much more commonly in the elderly than in the young. It is clearly dose-dependent, and the incidence peaks after 6 months of therapy. In the patient described above, adding a low dose of an ACE inhibitor or an ARB (if the patient is ACE inhibitor-intolerant) should relieve the swelling.
Hyponatremia and diuretics
Electrolyte imbalances are another common problem encountered in the elderly. Even though for years attention has been directed to the potassium level, hyponatremia has been equally associated with adverse effects in the elderly, such as an increased risk of fractures as shown in the Rotterdam study.36
In 180 hypertensive inpatients, mean age 76.4, Sharabi et al37 found the incidence of hyponatremia to be three times higher in women than in men (odds ratio 3.10, 95% confidence interval 2.07 to 4.67). Patients were 10 times more likely to be affected after age 65 and 14 times more likely after age 75. Most of the patients affected (74.5%) used a thiazide-type diuretic. Even though in many of the patients diuretics were used for more than 1 year before hyponatremia developed, susceptible patients—such as the frail elderly—can develop severe hyponatremia within days of starting to use a thiazide.38
Severe hyponatremia is potentially life-threatening. Most cases are caused by thiazide rather than loop diuretics.38 Thiazides inhibit electrolyte transport at the cortical diluting sites. As they decrease the glomerular filtration rate acutely, they increase proximal water reabsorption (making the plasma hypotonic), reducing water delivery distally. Loop diuretics, on the other hand, have their main effect at the thick ascending limb, reducing the osmolality at the medullary interstitium and not affecting proximal water reabsorption. Additionally, loop diuretics have a shorter half-life than thiazides, which makes hyponatremia more likely to happen with thiazides.
In patients who develop hyponatremia secondary to diuretic use, appropriate treatment includes stopping the medication, restricting water intake, and repleting electrolyte stores.38 As with any cause of chronic hyponatremia, correction must be cautiously monitored and not hastily done.
Therefore, we advise adding a thiazide diuric with caution in the elderly, and we advise avoiding thiazides in patients with high water or alcohol intake.
CASE 3: DEMENTIA AND HYPERTENSION
A 74-year-old man with long-standing hypertension, gout, and chronic obstructive pulmonary disease was recently diagnosed with Alzheimer dementia. He takes enalapril (Vasotec) 10 mg daily for his blood pressure. His blood pressure is 130/78 mm Hg.
Dementia is one of the most important and common neurologic disorders in the elderly. With the rise in average life expectancy, its magnitude has grown to cause a substantial emotional and economic burden on society and health care.
Midlife hypertension has been demonstrated to be an important modifiable risk factor for late-life cognitive decline,39 mild cognitive impairment,40 and dementia of all causes.41 It has been suggested that hypertension might be part of the pathogenesis of dementia, and targeting high blood pressure might prevent its onset.
Moreover, a significant reduction in both Alzheimer and vascular dementia was demonstrated (risk reduction 55%) with the use of a long-acting dihydropyridine calcium channel blocker (nitrendipine) in the Syst-Eur study.42 However, data from studies such as Systolic Hypertension in the Elderly Program (SHEP) and the HYVET substudy of cognitive function assessement43 showed no difference in dementia between placebo and active therapy with chlorthalidone (Hygroton) (in SHEP) or indapamide (Lozol) (in the HYVET substudy).
Disorders of calcium homeostasis are associated with the brain’s aging process. Probably, the neuroprotective effect of nitrendipine seen in Syst-Eur was due to its ability to affect this process, independent of its blood pressure-lowering effect.
In another prospective study, people over 60 years of age who complained of subjective memory loss showed a significant and positive association between memory scores and the use of calcium channel blockers (+0.14 ± 0.09 in users vs −0.12 ± 0.06 in nonusers; P = .016) independently of age, sex, white matter hyperintensities, and carotid wall cross-sectional area, all of which were associated with worse memory scores.44
Drugs that block the renin-angiotensin system have also been proposed to delay the onset and slow the progression of dementia.45 A small randomized, controlled trial suggested that centrally active ACE inhibitors—those that cross the blood-brain barrier, such as captopril (Capoten), lisinopril (Prinivil), ramipril (Altace), and fosinopril (Monopril)—slow cognitive decline in Alzheimer dementia more than non-centrally active ACE inhibitors or calcium channel blockers.46
Sink et al47 examined data from participants in the Cognition Substudy of the Cardiovascular Health Study48 on the effect of ACE inhibitors on cognitive decline. ACE inhibitors, as a class, showed no benefit in reducing the risk of dementia compared with other antihypertensive drug classes. However, centrally active ACE inhibitors, compared with other medications, were associated with a 65% reduction in cognitive decline per year of drug exposure (P = .01). Non-centrally active ACE inhibitors worsened cognitive decline.
It appears that the brain’s renin-angiotensin system plays a role in the pathogenesis of dementia. Indeed, ACE has been shown to degrade amyloid-beta protein, and its level was increased in brain tissue of Alzheimer patients postmortem.49
The relationship between blood pressure and cognitive function appears to be curvilinear, so that low blood pressure in late life is also associated with dementia and Alzheimer dementia.50 In 5,816 patients age 65 and older, Morris et al51 calculated the percentile scores of four cognitive tests according to the level of blood pressure. Patients with systolic blood pressures of 100 mm Hg, 120 mm Hg, and 180 mm Hg scored lower on the Mini Mental State Examination than those in the 140 to 160 mm Hg range. Patients with diastolic blood pressures between 80 and 90 mm Hg appeared to have the best cognitive function. This further emphasizes that blood pressure control must be pursued in the very elderly, albeit less aggressively. The MIND substudy of the SPRINT trial9 is likely to shed more light on this relationship.
When needed for optimal blood pressure control in a hypertensive patient at risk of dementia, a calcium channel blocker of the dihydropyridine type or a centrally active ACE inhibitor, or both, is preferable.
CASE 4: LABILE HYPERTENSION
A 74-year-old man with hypertension and diabetes mellitus comes to see you in the office. On physical examination, his blood pressure is 175/110 mm Hg. His blood pressure during his last visit 3 months ago was 120/75. He brings a log with him that shows random fluctuations in his blood pressure readings. He takes hydrochlorothiazide 25 mg daily for his blood pressure.
Hypertension in some patients continuously fluctuates between low and high levels. A study in Canada found that up to 15% of all adult hypertensive patients might have labile hypertension.52 In the presence of a normal average blood pressure, visit-to-visit blood pressure variability is usually considered a trivial matter. However, some but not all studies have shown that such visit-to-visit variability in blood pressure is an independent predictor of future cardiovascular events in hypertensive patients, independent of the mean systolic blood pressure.52–54
Blood pressure fluctuates from heartbeat to heartbeat, from morning to night, from winter to summer, and from sitting to standing, and it is prone to increase with exertion, stress, and other factors. But excessive fluctuations in the elderly are most likely the result of excessive stiffness of the arterial tree and a decrease in the windkessel (cushioning) function of the aorta. As a consequence, even small-volume changes in the intravascular system can trigger large blood pressure fluctuations.
There is some evidence that antihypertensive drug classes may differ in their effects on visit-to-visit blood pressure variability. In a 2010 study comparing the effects of different antihypertensive drugs on blood pressure variation, calcium channel blockers and non-loop diuretics were associated with less variation in systolic blood pressure, and calcium channel blockers reduced it the most.55
In the patient described above, switching to a low-dose calcium channel blocker with a thorough follow-up is a reasonable plan.
CASE 5: ORTHOSTATIC HYPOTENSION
A 73-year-old woman with long-standing hypertension complains of some dizziness, especially when getting out of bed in the morning. On physical examination, her blood pressure is 134/100 mm Hg sitting and 115/90 standing. She takes amlodipine 10 mg daily, enalapril 10 mg daily, and chlorthalidone 25 mg daily. Chlorthalidone had been added on her last visit 1 month before.
As a result of the increase in the number of elderly patients with hypertension and guidelines recommending better control in this age group, the number of elderly patients on anti-hypertensive drugs has risen significantly. At the same time, the elderly have increasingly presented with adverse effects of treatment.
Orthostatic hypotension is a drop in systolic pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more on standing, with or without symptoms. These are caused by cerebral hypoperfusion and include dizziness, lightheadedness, generalized weakness, visual blurring, and, in severe cases, syncope.
Alpha-blockers and nitrates have been most commonly implicated in causing orthostatic hypotension, due to venous pooling. Clearly, not all antihypertensive drugs are equal with regard to their venodilatory effects. Thiazide diuretics, by causing fluid volume depletion, and beta-blockers, by interfering with compensatory cardioacceleration with upright posture, are also commonly involved in causing an excessive blood pressure drop with standing.
Systolic orthostatic hypotension has been shown to be a significant and independent predictor of cardiovascular morbidity and death.56 Moreover, syncope and subsequent falls are an important cause of injury and death in the elderly.57 The clinical combination of hypertension and orthostatic hypotension is, therefore, especially challenging. A compromise between accepting a higher cardiovascular risk at either end of the spectrum with an added higher risk for fall at the lower end has to be made.
To prevent orthostatic hypotension in the elderly, it is important to avoid prescribing high-risk drugs. When starting antihypertensive therapy, a low dose should be used, and the dose should be titrated upward very slowly and cautiously. If orthostatic hypotension is suggested by the history or by the orthostatic test, which is warranted in all elderly hypertensive patients before starting or significantly altering therapy, the potential culprit drug should be withdrawn and the patient reassessed. Improved hydration, elevating the head of the bed, and taking the antihypertensive drug at night are ways to improve symptoms, but these remain largely unproven.
In this patient, the newly added chlorthalidone was stopped, and her symptoms improved.
PSEUDOHYPERTENSION
Since hypertension is defined by a numerical value, it is prudent that this value be accurate. Treating a falsely high reading or leaving a falsely low reading untreated will predispose the elderly patient to increased risk either way. One rare condition in the elderly that can give a falsely high blood pressure reading is pseudohypertension.
Pseudohypertension is a condition in which indirect blood pressure measured by the cuff method overestimates the true intra-arterial blood pressure due to marked underlying arteriosclerosis. The Osler maneuver can be used to differentiate true hypertension from pseudohypertension.58 This is performed by palpating the pulseless radial or brachial artery distal to the inflated cuff. If the artery is palpable despite being pulseless, the patient is said to be “Osler-positive” and likely has pseudohypertension.58
Pseudohypertension should be suspected if the patient has orthostatic hypotension despite normal blood pressure sitting and standing. Also, elevated blood pressure without appropriate target organ disease should raise the suspicion of pseudohypertension. Apart from the Osler maneuver, measuring the intraarterial pressure can confirm this diagnosis.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
- US Census Bureau. The 2011 statistical abstract. The national data book. http://www.census.gov/compendia/statab/2011/2011edition.html. Accessed July 24, 2012.
- He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau. Current Population Reports, P23-209. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf. Accessed July 24, 2012.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374:1196–1208.
- Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2:983–986.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Schall P, Wehling M. Treatment of arterial hypertension in the very elderly: a meta-analysis of clinical trials. Arzneimittelforschung 2011; 61:221–228.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5:259–352.
- Wake Forest University, Winston-Salem NC. SPRINT: Systolic Blood Pressure Intervention Trial. http://www.sprinttrial.org/public/dspHome.cfm. Accessed July 24, 2012.
- Sacks FM, Svetkey LP, Vollmer WM, et al; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503.
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012; 125:433–439.
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. http://www.ncbi.nlm.nih.gov/books/NBK9630/Accessed July 30, 2012.
- Mitka M. New guidance covers ways to prevent and treat hypertension in elderly patients. JAMA 2011; 305:2394,2398.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician 2010; 82:1471–1478.
- Rihal CS, Textor SC, Breen JF, et al. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc 2002; 77:309–316.
- Wang Y, Li Y. [Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome]. [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008; 22:222–225.
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160:2289–2295.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359:204–210.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive air-way pressure on blood pressure: a placebo trial. Hypertension 2000; 35:144–147.
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011; 365:2277–2286.
- White CJ, Jaff MR, Haskal ZJ, et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation 2006; 114:1892–1895.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–1312.
- Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848,W150–W151.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622.e1–630.e1.
- Pedrinelli R, Dell’Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension 2000; 35:621–625.
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens 1996; 14:1041–1045.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135.
- Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 2011; 26:1822–1828.
- Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens 2002; 16:631–635.
- Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999; 19:447–452.
- Knopman D, Boland LL, Mosley T, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007; 64:1734–1740.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Forette F, Seux ML, Staessen JA, et al Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351.
- Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008. www.thelancet.com/journals/laneur/article/PIIS1474-4422(08)70143-1/fulltext. Accessed August 23, 2012.
- Watfa G, Rossignol P, Kearney-Schwartz A, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens 2010; 28:2485–2493.
- Tzourio C, Anderson C, Chapman N, et al; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075.
- Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004; 63:1324–1325.
- Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med 2009; 169:1195–1202.
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22:1–12.
- Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem 2005; 280:37644–37650.
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007; 19:41–47.
- Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002; 21:123–130.
- Joffres MR, Hamet P, Rabkin SW, Gelskey D, Hogan K, Fodor G. Prevalence, control and awareness of high blood pressure among Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ 1992; 146:1997–2005.
- Schillaci G, Pucci G. The importance of instability and visit-to-visit variability of blood pressure. Expert Rev Cardiovasc Ther 2010; 8:1095–1097.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012; 126:569–578.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375:906–915.
- Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010; 56:56–61.
- Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther 2009; 7:557–560.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
KEY POINTS
- Therapy should be considered in all aging hypertensive patients, even the very elderly (> 80 years old).
- Most antihypertensive drugs can be used as first-line treatment in the absence of a compelling indication for a specific class, with the possible exception of alpha-blockers and beta-blockers.
- An initial goal of less than 140/90 mm Hg is reasonable in elderly patients, and an achieved systolic blood pressure of 140 to 145 mm Hg is acceptable in octogenarians.
- Start with low doses; titrate upward slowly; and monitor closely for adverse effects.
- Thiazide diuretics should be used with caution in the elderly because of the risk of hyponatremia.
Home testing for HIV: Hopefully, a step forward
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
In July 2012, the US Food and Drug Administration approved the first over-the-counter test kit for human immunodeficiency virus (HIV) infection, the OraQuick In-Home HIV Test (OraSure Technologies, Bethlehem, PA). This test is a variation of the currently available OraQuick ADVANCE Rapid HIV-1/2 Antibody Test used in clinical settings by trained personnel for rapid detection of HIV.
The home HIV test is expected to become available in the fall of 2012 from the company’s Web site and at retail drugstores. This will put the power of HIV testing into the hands of anyone able to afford the estimated $60 price and willing to purchase the item online or in stores.
GOAL: TO REDUCE THE NUMBER OF INFECTED PEOPLE WHO ARE UNAWARE
How home testing will change the demographics of HIV testing is not clear, but the intention is to reduce the number of HIV-infected people who are unaware of their infection and to get them in for care. Anthony Fauci, MD, the director of the National Institutes of Allergy and Infectious Diseases, has called the new test a “positive step forward” in bringing the HIV epidemic under control.1
Recent figures from the US Centers for Disease Control and Prevention (CDC) indicate that, of the 1.2 million HIV-infected people in the United States, up to 220,000 are unaware of their infection.2,3 Since antiretroviral therapy is now considered beneficial even in the early stages of HIV infection, those who are unaware of their infection are missing an opportunity for the most effective therapies.
They may also be unknowingly transmitting the virus, thus perpetuating the HIV epidemic. Awareness of one’s HIV infection may lead to behavioral changes that can reduce the risk of transmission. It has also become clear that antiretroviral therapy can dramatically reduce transmission rates, a concept known as “treatment as prevention.” 4 Thus, access to care and initiation of antiretroviral therapy have the potential to prevent progression to acquired immunodeficiency syndrome (AIDS) in the individual and to interrupt the spread of the virus in the community.
There are several steps between awareness of HIV infection and full engagement in HIV care that require attention from the health care community.5 Only a quarter of those with known HIV infection are in care and adherent to antiretroviral therapy, leaving much work to be done on removing barriers to effective treatment.5 The first step is still to identify those infected. The effort to increase the percentage of HIV-infected individuals who know their HIV status is one of the goals of the National HIV/AIDS Strategy and HealthyPeople2020.6
HOW THE TEST IS USED
The OraQuick In-Home Test consists of the device and reagents, instructional materials, information on interpreting the results, and contact information for the OraQuick Answer Center for information, support, and local medical referral.7 The overall time needed for testing is 20 to 40 minutes.
To perform the test, an oral fluid specimen is collected by swabbing the upper and lower buccal mucosa along the gum line. Once inserted into the developer solution the swabbed sample is carried onto a membrane strip containing HIV-1/2 antigens.
The device has two windows, one labelled “T” (for test) and the other labelled “C” (for control). If the patient has sufficient antibodies to HIV proteins, the “T” window indicates a positive result if a band is visible. The “C” (control) window displays a band to indicate if the device and reagents are working. If the control window does not show a band, then the kit has not functioned properly and the test result is not reliable.
SOME PEOPLE MAY STILL NEED HELP
For the test to succeed in informing people of their HIV status, it must be used effectively and the results must be interpretable. Of 5,662 participants in phase III investigational-device studies, 99% were able to use the kit and determine a result.7 While the test’s simplicity is similar to that of pregnancy test kits, it is possible that some people (at least 1% of those using the kit) may seek guidance from medical practitioners because they are unable to understand the test results.
For a test result to have the desired outcome of leading to HIV care, individuals must act on a positive result. When home test results are positive, the instructions indicate that “you may have HIV” and provide contact information for the OraQuick Answer Center. It is unclear how reliable the counseling, information, and referral process from OraSure will be and if people will use the service.
Individuals may access medical care at a variety of levels for further assistance if they have a positive test result. These may include primary care offices, emergency and urgent care settings, health departments, and HIV clinics.
LESS SENSITIVE THAN BLOOD TESTS
To provide additional care, clinicians must understand the performance of the home HIV test. Most importantly, the test result must be confirmed.
The In-Home test is less sensitive than currently available HIV blood tests used in the clinical setting, particularly the HIV-1/2 enzyme immunoassay (EIA) with confirmatory Western blot testing. The In-Home test is less likely to detect HIV infection during the 90-day “window period” when seroconversion is occurring, and so it should not be relied on to rule out HIV during this early period after infection.
The sensitivity and specificity of the OraQuick In-Home HIV test were determined in a phase III trial in 5,662 people (80% at risk of HIV), who were tested concurrently with the “gold standard” blood tests (EIA and Western blot). The sensitivity was 93% (giving a positive result in 106 of 114 patients who had a positive result on blood testing), and the specificity was 99.9% (giving a negative result in 5,384 of 5,385 patients who had a negative result on blood testing).7
Therefore, a positive In-Home test result is likely to be truly positive, but a negative result is not as reliably truly negative. False-negative results may occur particularly in the window period early after HIV infection, so the test should not be relied on within 90 days of high-risk behavior. In contrast, with the fourth-generation blood HIV tests, the window period is approximately 16 days.
The predictive value of the test will depend on the population using it and on the patient’s pretest probability of disease at the time of testing. In the population tested by OraQuick, the positive predictive value was 99.1% and the negative predictive value was 99.9%.7 Mathematical modeling has been done to examine the potential outcomes for use in subpopulations at lower risk and at higher risk.
As clinicians, we will have to address the potential for both false-positive and false-negative test results. False-positive results may be more likely in low-risk populations and may occur in the setting of cross-reactive antibodies from pregnancy, autoimmune diseases, or previous receipt of an experimental HIV vaccination. False-negative results may occur in the setting of acute HIV infection and in those with severely impaired immunity (eg, from agammaglobulinemia or immunosuppressive drugs) and will be more likely in higher-risk populations, such as men who have sex with men, intravenous drug users, blacks, and Hispanics ages 18 to 35 with multiple sexual partners. A positive In-Home HIV test should be followed up with a blood EIA and confirmed with Western blot in all patients.
WHO WILL USE THIS TEST?
It is unclear who will use this new test. In OraSure’s clinical trial, the percentages of people who indicated they would “definitely or probably buy” the test were:
- 20% of the general population
- 27% of those ages 18 to 35
- 49% of blacks ages 18 to 35
- 47% of homosexual men
- 43% of people who said they had more than two sexual partners per year
- 32% who said they use condoms inconsistently.
If this is true, the test may appropriately target several populations that are not currently being tested, either because they lack access to care or because they do not see themselves as being at high risk. Of those with newly diagnosed HIV infection from 2006 to 2009, 40% had had no prior testing, and the groups with the highest percentages of people in this category were black, men with injection drug use as their sole risk factor, those older than 50 years, and those with heterosexual contact as their sole risk factor.8 Because of difficulties in identifying some of these groups as “at risk,” the current CDC guidelines recommend that HIV testing be offered to all patients ages 13 to 64, regardless of their risk factors.9
The home HIV test may fill a gap in testing, extending it to those still not tested in the health care setting or to those who have not sought health care. For the home test to fill that gap, people still have to perceive themselves as at risk and then purchase the test. Through public health strategies and at clinical points of care, we must continue to inform our patients about HIV risk and work to identify new or ongoing risk factors that would prompt additional testing.
MANY QUESTIONS REMAIN
- Will those who need testing want to use this test? People will buy the test only if they perceive themselves to be at risk.
- Is this test affordable for the target populations? $60 will be unaffordable to some.
- Will the directions be followed effectively?
- Will home testing reduce opportunities to counsel patients on their HIV risk factors?
- Will there be situations in which individuals are socially pressured to take the test?
- Can users of the test expect the appropriate amount of privacy? Availability on the Internet and in drug stores is not a guarantee of privacy when purchasing the test, although the result presumably will not be known.
- Will those with positive results seek medical care?
- Will those with negative results who are still at high risk forgo more sensitive testing and continue to engage in high-risk activities?
Nevertheless, since early and continued treatment prevents disease progression and reduces HIV transmission, testing is the first step toward access to effective HIV care. The home HIV test is a step forward in providing high-quality HIV testing to the wider population.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
- McNeil DG. Rapid H.I.V. Home Test Wins Federal Approval. New York Times, July 3, 2012. http://www.nytimes.com/2012/07/04/health/oraquick-at-home-hiv-test-wins-fda-approval.html. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 US Dependent Areas—2010 HIV Surveillance Supplemental Report, Volume 17, Number 3 (Part A). http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol-17no3/index.htm. Accessed August 27, 2012.
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2010 HIV Surveillance Report, Volume 22. http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed August 27, 2012.
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800.
- Centers for Disease Control and Prevention (CDC). Healthy People 2020 Summary of Objectives. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HIV.pdf. Accessed August 27, 2012.
- Food and Drug Administration (FDA). 102nd Meeting of The Blood Product Advisory Committee (BPAC). Evaluation of the Safety and Effectiveness of the OraQuick In-Home HIV Test. May 15, 2012.
- Centers for Disease Control and Prevention (CDC). Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morb Mortal Wkly Rep 2012; 61:441–445.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17.
KEY POINTS
- The new test is highly (99.9%) specific for HIV but is not quite as reliable at ruling out infection (93% sensitivity). Therefore, it may miss some cases of HIV, especially during the 90-day window after initial infection.
- False-positive test results may occur, especially in people at low risk. A positive result must be confirmed with a laboratory-based third- or fourth-generation blood test.
- It is important to continue to assess and counsel patients on how to modify their risk of HIV infection.
- Providers are urged to offer HIV testing to all patients ages 13 to 64 at least once, regardless of their risk.
- At least once a year, patients at high risk should get one of the more sensitive laboratory blood tests.
- People who choose to test themselves at home should seek medical care for verification of the test result and for HIV counseling, and, if the result is confirmed positive, access to HIV care.
Safer use of benzodiazepines for alcohol detoxification
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.
Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.

Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.

Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
How to talk to patients about religion and spirituality
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
Electroconvulsive therapy: How modern techniques improve patient outcomes
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
Can combining triptans with SSRIs or SNRIs cause serotonin syndrome?
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
The link between schizophrenia and diabetes
Discuss this article at www.facebook.com/CurrentPsychiatry
Although diabetes and schizophrenia are common companions, it is unclear how this association should influence our practice. What do we need to know about diabetes, and what are the key intervention points for psychiatrists?
This article is informed by my experience monitoring >1,000 patients with schizophrenia in a large urban mental health facility using an electronic metabolic monitoring system and consulting on hundreds of individuals with comorbid schizophrenia and diabetes in a mental health metabolic clinic.
A significant link
The association between schizophrenia and diabetes has been recognized for more than a century.1 The prevalence of diabetes is increased 2- to 3-fold in patients with schizophrenia.2,3 This relationship is specific to type 2 diabetes mellitus (T2DM); type 1 diabetes mellitus, an autoimmune disease, is less common in patients with schizophrenia.4 Factors that contribute to comorbidity between schizophrenia and T2DM include:
- illness susceptibility: the mechanisms remain unclear but include the thrifty phenotype hypothesis,5 autonomic hyperactivity,6 and potential cellular and genetic links7,8
- lifestyle: diet, physical inactivity, and cigarette smoking9-12
- antipsychotic use13
- social health determinants, such as income, housing, and food insecurity.14
When evaluating a patient’s risk for a cardiac event, we consider having a diabetes diagnosis equivalent to having had a myocardial infarction.15 Likely, the high prevalence of T2DM among schizophrenia patients and challenges in managing diabetes and prediabetes underlies these patients’ reduced life expectancy.16 Self-care, a cornerstone of diabetes management, is challenging for patients with schizophrenia because of deficits in executive functioning, working memory, and motivation, coupled with negative symptoms and social and economic disadvantages that often accompany schizophrenia.
How diabetes impacts practice
What psychiatrists need to know. Insulin resistance—reduced biologic effectiveness of insulin—is the precursor of T2DM. Insulin is required to move glucose from the blood into cells. Weight gain, particularly abdominal adiposity, is the principal driver of insulin resistance. The body responds by producing more insulin (hyperinsulinemia) to maintain glucose homeostasis. Hyperinsulinemia underlies metabolic syndrome, an important risk marker for developing T2DM. Diabetes usually develops after many years when the pancreas fails to compensate for insulin resistance.
In most cases the development of diabetes in patients with schizophrenia follows this course. Weight gain, a consequence of lifestyle factors as well as antipsychotics and other psychotropics that promote obesity, leads to progressive insulin resistance. Consequently, metabolic syndrome is twice as prevalent among patients with schizophrenia compared with matched controls.17,18
Occasionally patients develop T2DM within a few weeks or months of starting antipsychotic treatment—usually with clozapine or olanzapine—before they gain weight, which suggests a second mechanism may be involved. Animal studies have documented rapid development of insulin resistance after a single subcutaneous injection of antipsychotics that have high metabolic liability, possibly through a direct effect on insulin signaling.19 This phenomenon has been difficult to demonstrate in humans.20
Managing diabetes is complex and ideally involves a range of health practitioners who work with patients to provide education, promote self-care behaviors, and direct complex health care. These services are outside the scope of psychiatric practice, but given the functional deficits in seriously mentally ill patients, it is important to have an overview of diabetes care (Table 3).
In addition to diagnosing diabetes, psychiatrists should be able to identify patients at risk for developing diabetes and initiate prevention strategies. Interventions are focused on lifestyle—weight reduction, increased physical activity, diet, and smoking cessation—as well as pharmacologic strategies such as metformin.
What patients need to know. Similar to schizophrenia, a diabetes diagnosis may be difficult for patients to accept. Initially, a patient may have no manifestations or symptoms. However, untreated diabetes has serious long-term health consequences—including blindness, amputations, kidney disease, and early death from heart attacks.
Patients should actively participate in treatment that involves learning about the illness, making lifestyle changes, working on self-care, and keeping regular medical appointments. Three components of lifestyle change must be addressed:
- Diet: counseling with a dietician or other health professional to reduce or stabilize body weight and make changes in diet quality, portion size, and meal frequency to improve glucose control and reduce long-term diabetes complications
- Physical activity: increasing physical activity, initially by walking daily, to benefit glucose control and weight maintenance
- Smoking: reducing or stopping cigarette smoking to improve glucose control and reduce diabetes complications.
American Diabetes Association diagnostic criteria for diabetes
| Testa | Threshold | Qualifier |
|---|---|---|
| A1C, or | ≥6.5% | Lab NGSP certified, standardized DCCT assay |
| Fasting glucose, or | ≥126 mg/dL | No caloric intake for at least 8 hours |
| 2-hour glucose, or | ≥200 mg/dL | After 75 g of anhydrous glucose |
| Random glucose | ≥200 mg/dL | Plus classic hyperglycemic symptoms or crisis |
| aResults should be confirmed by repeat testing DCCT: Diabetes Control and Complications Trial; NGSP: National Glycohemoglobin Standardization Program Source: References 21-23 | ||
Signs and symptoms of diabetes and diabetic ketoacidosis
| Diabetes |
| Frequent urination |
| Excessive thirst |
| Extreme hunger |
| Unusual weight loss |
| Increased fatigue |
| Irritability |
| Blurry vision |
| Diabetic ketoacidosisa |
| Thirst or very dry mouth |
| Constantly feeling tired |
| Dry or flushed skin |
| Nausea, vomiting, or abdominal pain |
| Difficulty breathing (short, deep breaths) |
| Fruity odor on breath |
| Difficulty paying attention or confusion |
| aVomiting is a sign of escalation Source: References 24,25 |
Components of diabetes care
| Self-care tasks | Tests/annual assessments |
|---|---|
| Self-monitoring of glucose | A1C (2 to 4 times/year) |
| Medication management | Urinary microalbumin |
| Meal planning | Fasting lipids |
| Exercise | Blood pressure |
| Smoking cessation | Dilated eye exam |
| Foot self-examination and foot care | Foot exam |
| Stress management | General health and cardiovascular exam |
Managing patients at risk for diabetes
| Prediabetes21 | Management |
|---|---|
| Impaired fasting glucose (100 to 125 mg/dL) | Weight reduction (7%) Activity (150 minutes/week) At least yearly glucose monitoring |
| Impaired glucose tolerance (2-hour plasma glucose: 140 to 199 mg/dL) | |
| Prediabetic A1C (5.7% to 6.4%) | |
| Metabolic syndrome (any 3)26 | Management |
| Waist circumferencea (men >40 inches; women >35 inches) | Weight reduction Reduce consumption of refined carbohydrates Exercise Focused interventions for individual criteria |
| Fasting triglycerides (≥150 mg/dL) | |
| Fasting high-density lipoprotein cholesterol (men | |
| Fasting glucose (≥100 mg/dL or taking medication) | |
| Blood pressure (≥130/85 mm Hg or taking medication) | |
| aWaist circumference guidelines are ethnicity specific. The International Diabetes Federation27 has published specific cutoffs for those of Asian background (men: ≥90 cm [35 inches] and women: ≥80 cm [31 inches]) | |
Metabolic monitoring
Metabolic monitoring is the key to keeping patients with schizophrenia well. Treating metabolic conditions falls outside of psychiatric practice; however, many argue that mental health clinicians should monitor basic metabolic parameters during antipsychotic treatment and advocate medical interventions when indicated because:
- most antipsychotics are associated with weight gain and metabolic side effects
- patients with schizophrenia have cognitive deficits that impact health maintenance
- mental health providers often are the primary health care contacts for patients with serious mental illness.
- identify treatable medical conditions such as diabetes, dyslipidemia, and hypertension when treatment delay or no treatment has consequences
- identify individuals with prediabetes and metabolic syndrome for targeted prevention
- determine the association between antipsychotic treatment and metabolic disturbance to evaluate the risk of treatment vs antipsychotic switching.
How to intervene
To switch or not to switch? For many psychiatrists, deciding whether or when to switch from a high or intermediate metabolic liability antipsychotic to one with low metabolic liability is difficult. Clinicians must balance potential metabolic benefits against the risk of psychotic decompensation and side effects. Ultimately, patients and their families make the decision, taking into account information provided to them. For medical-legal purposes, document the discussion of potential risks and benefits. These are difficult decisions and there are no clear guidelines. In my clinical experience, the following issues need to be considered:
- The antipsychotics that many clinicians consider to be the most effective—clozapine and olanzapine—also have the greatest metabolic liability and risk for emergent T2DM.
- Patients who are stable and in psychotic remission may risk a relapse of their illness if switched.
- The clearest indication for switching is when a patient who does not have diabetes develops the condition shortly after starting an antipsychotic. This scenario is rare, but evidence suggests that diabetes may resolve or reverse with an antipsychotic switch.33
- In patients who gain weight while taking a high- or intermediate-liability antipsychotic and are able to tolerate a switch to a low-liability antipsychotic, the effect size of weight reduction can be large and may result in a patient returning to their pretreatment weight.
- To reduce relapse risk, patients switching antipsychotics should be closely monitored at least weekly for ≥1 month. A plateau cross-taper—building the new antipsychotic up to therapeutic levels before gradually reducing the first antipsychotic—may be safer than abrupt discontinuation or standard cross-titration.
- Switching from one high or intermediate liability antipsychotic to another (eg, olanzapine to quetiapine or risperidone) often provides little if any metabolic benefit on body weight or diabetes control.
- Established diabetes (type 1 or type 2) should not be a contraindication to antipsychotic treatment, including clozapine, if clinically warranted. Monitor metabolic parameters more closely for 6 to 12 months after the switch. In most cases, patients experience limited, if any, metabolic consequences. If so, diabetes medication can be adjusted.
- Patients who have experienced significant weight gain on an atypical antipsychotic often do not gain more weight when switched to clozapine. A patient may reach a “ceiling” in terms of weight gain and medication-related metabolic effects.
Data from metabolic monitoring informs the decision to switch and metabolic consequences of switching. Conducting monitoring at baseline, when starting an antipsychotic, when switching to a high-liability agent, 3 months after the switch, and then annually provides data needed to consider switching or initiating medical and behavioral or lifestyle interventions.
Facilitate early diabetes treatment. Clinicians who are most closely involved in caring for patients with schizophrenia often are best situated to screen for diabetes. I have found that without a close working relationship with my patients’ primary care practitioners, patients may experience a long delay in receiving care. After your patient is diagnosed with diabetes, establish a relationship with diabetes treatment providers and work with your patient to ensure they engage in diabetes care.
Contribute to diabetes chronic disease management. Mental health practitioners can complement diabetes care in patients with serious mental illness by:
- navigating the health system and negotiating for service on patients’ behalf
- promoting positive relationships among diabetes and mental health treatment teams
- evaluating and treating depression that may be comorbid with diabetes
- assessing treatment capacity, self-care deficits, cognitive functioning, psychotic symptoms, negative symptoms, etc., that impact diabetes self-care and collaborating with diabetes care providers to support patients.
Start with a low-liability agent
Patients who are early in the course of psychotic illness are most susceptible to the metabolic effects of antipsychotics.13 The average weight gain observed with olanzapine was 34 lbs at 2 years in first episode psychosis patients (mean age 24 ± 4.9).34 Metabolic consequences with medium-liability second-generation antipsychotics—such as quetiapine and risperidone—are extreme, particularly in children, adolescents, and young adults (age 35,36 Although frank diabetes is uncommon in early psychosis because patients are, to a certain extent, protected by insulin compensation—increased insulin secretion maintains glucose levels within a therapeutic range—diabetes risk is increased, and hyperinsulinemia and hypertriglyceridemia are early markers of metabolic strain. Also, response to initial antipsychotic treatment—possibly independent of the agent selected—is robust in early psychosis.37
Related Resources
- American Diabetes Association. www.diabetes.org.
- International Diabetes Federation. www.idf.org.
- Framingham Heart Study. Coronary heart disease (10-year risk). www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. Risk assessment tool for estimating your 10-year risk of having a heart attack. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
Dr. Cohn is a speaker for Pfizer Canada.
1. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903-912.
3. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemol Ment Health. 2006;2:14.-
4. Juvonen H, Reunanen A, Haukka J, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. 2007;64(8):894-899.
5. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20.
6. Ryan MC, Sharifi N, Condren R, et al. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065-1070.
7. Odawara M, Isaka M, Tada K, et al. Diabetes mellitus associated with mitochondrial myopathy and schizophrenia: a possible link between diabetes mellitus and schizophrenia. Diabet Med. 1997;14(6):503.-
8. Siuta MA, Robertson SD, Kocalis H, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8(6):e1000393.-
9. Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29(2):393-397.
10. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. 2005;193(10):641-646.
11. Ussher M, Stanbury L, Cheeseman V, et al. Physical activity p and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr Serv. 2007;58(3):405-408.
12. Cho NH, Chan JC, Jang HC, et al. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf). 2009;71(5):679-685.
13. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
14. Yu VL, Raphael D. Identifying and addressing the social determinants of the incidence and successful management of type 2 diabetes mellitus in Canada. Can J Public Health. 2004;95(5):366-368.
15. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151-157.
16. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
17. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49(11):753-760.
18. Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009;119(1):4-14.
19. Chintoh AF, Mann SW, Lam L, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28(5):494-499.
20. Hahn MK, Arenovich T, Wolever T, et al. Single dose administration of olanzapine: effects on glucose metabolism, endocrine and inflammatory markers in healthy volunteers. Poster presented at: Schizophrenia International Research Society 3rd Biennial Conference; April 14-18, 2012; Florence, Italy.
21. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
22. Little RR. Glycated hemoglobin standardization—National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191-1198.
23. Keen H. The Diabetes Control and Complications Trial (DCCT). Health Trends. 1994;26(2):41-43.
24. American Diabetes Association. Symptoms. http://www.diabetes.org/diabetes-basics/symptoms. Accessed August 27 2012.
25. American Diabetes Association. Ketoacidosis (DKA). http://www.diabetes.org/living-with-diabetes/complications/ketoacidosis-dka.html. Accessed August 27 2012.
26. Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735-2752.
27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-480.
28. Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
29. Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51(8):492-501.
30. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
31. Newcomer JW, Nasrallah HA, Loebel AD. The Atypical Antipsychotic Therapy and Metabolic Issues National Survey: practice patterns and knowledge of psychiatrists. J Clin Psychopharmacol. 2004;24(5 suppl 1):S1-S6.
32. Khoury A, Sproule BA, Cohn TA. Development and implementation of the Metabolic Health Monitor at the Centre for Addiction and Mental Health. Poster presented at: BC Psychopharmacology Conference; February 15-16 2008; Vancouver, British Columbia, Canada.
33. Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy. 2002;22(7):841-852.
34. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
35. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45(7):771-791.
36. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
37. Nicol G, Newcomer J. Review: children and adolescents with schizophrenia spectrum disorders respond to antipsychotics but are susceptible to adverse events. Evid Based Ment Health. 2008;11(3):81.-
Discuss this article at www.facebook.com/CurrentPsychiatry
Although diabetes and schizophrenia are common companions, it is unclear how this association should influence our practice. What do we need to know about diabetes, and what are the key intervention points for psychiatrists?
This article is informed by my experience monitoring >1,000 patients with schizophrenia in a large urban mental health facility using an electronic metabolic monitoring system and consulting on hundreds of individuals with comorbid schizophrenia and diabetes in a mental health metabolic clinic.
A significant link
The association between schizophrenia and diabetes has been recognized for more than a century.1 The prevalence of diabetes is increased 2- to 3-fold in patients with schizophrenia.2,3 This relationship is specific to type 2 diabetes mellitus (T2DM); type 1 diabetes mellitus, an autoimmune disease, is less common in patients with schizophrenia.4 Factors that contribute to comorbidity between schizophrenia and T2DM include:
- illness susceptibility: the mechanisms remain unclear but include the thrifty phenotype hypothesis,5 autonomic hyperactivity,6 and potential cellular and genetic links7,8
- lifestyle: diet, physical inactivity, and cigarette smoking9-12
- antipsychotic use13
- social health determinants, such as income, housing, and food insecurity.14
When evaluating a patient’s risk for a cardiac event, we consider having a diabetes diagnosis equivalent to having had a myocardial infarction.15 Likely, the high prevalence of T2DM among schizophrenia patients and challenges in managing diabetes and prediabetes underlies these patients’ reduced life expectancy.16 Self-care, a cornerstone of diabetes management, is challenging for patients with schizophrenia because of deficits in executive functioning, working memory, and motivation, coupled with negative symptoms and social and economic disadvantages that often accompany schizophrenia.
How diabetes impacts practice
What psychiatrists need to know. Insulin resistance—reduced biologic effectiveness of insulin—is the precursor of T2DM. Insulin is required to move glucose from the blood into cells. Weight gain, particularly abdominal adiposity, is the principal driver of insulin resistance. The body responds by producing more insulin (hyperinsulinemia) to maintain glucose homeostasis. Hyperinsulinemia underlies metabolic syndrome, an important risk marker for developing T2DM. Diabetes usually develops after many years when the pancreas fails to compensate for insulin resistance.
In most cases the development of diabetes in patients with schizophrenia follows this course. Weight gain, a consequence of lifestyle factors as well as antipsychotics and other psychotropics that promote obesity, leads to progressive insulin resistance. Consequently, metabolic syndrome is twice as prevalent among patients with schizophrenia compared with matched controls.17,18
Occasionally patients develop T2DM within a few weeks or months of starting antipsychotic treatment—usually with clozapine or olanzapine—before they gain weight, which suggests a second mechanism may be involved. Animal studies have documented rapid development of insulin resistance after a single subcutaneous injection of antipsychotics that have high metabolic liability, possibly through a direct effect on insulin signaling.19 This phenomenon has been difficult to demonstrate in humans.20
Managing diabetes is complex and ideally involves a range of health practitioners who work with patients to provide education, promote self-care behaviors, and direct complex health care. These services are outside the scope of psychiatric practice, but given the functional deficits in seriously mentally ill patients, it is important to have an overview of diabetes care (Table 3).
In addition to diagnosing diabetes, psychiatrists should be able to identify patients at risk for developing diabetes and initiate prevention strategies. Interventions are focused on lifestyle—weight reduction, increased physical activity, diet, and smoking cessation—as well as pharmacologic strategies such as metformin.
What patients need to know. Similar to schizophrenia, a diabetes diagnosis may be difficult for patients to accept. Initially, a patient may have no manifestations or symptoms. However, untreated diabetes has serious long-term health consequences—including blindness, amputations, kidney disease, and early death from heart attacks.
Patients should actively participate in treatment that involves learning about the illness, making lifestyle changes, working on self-care, and keeping regular medical appointments. Three components of lifestyle change must be addressed:
- Diet: counseling with a dietician or other health professional to reduce or stabilize body weight and make changes in diet quality, portion size, and meal frequency to improve glucose control and reduce long-term diabetes complications
- Physical activity: increasing physical activity, initially by walking daily, to benefit glucose control and weight maintenance
- Smoking: reducing or stopping cigarette smoking to improve glucose control and reduce diabetes complications.
American Diabetes Association diagnostic criteria for diabetes
| Testa | Threshold | Qualifier |
|---|---|---|
| A1C, or | ≥6.5% | Lab NGSP certified, standardized DCCT assay |
| Fasting glucose, or | ≥126 mg/dL | No caloric intake for at least 8 hours |
| 2-hour glucose, or | ≥200 mg/dL | After 75 g of anhydrous glucose |
| Random glucose | ≥200 mg/dL | Plus classic hyperglycemic symptoms or crisis |
| aResults should be confirmed by repeat testing DCCT: Diabetes Control and Complications Trial; NGSP: National Glycohemoglobin Standardization Program Source: References 21-23 | ||
Signs and symptoms of diabetes and diabetic ketoacidosis
| Diabetes |
| Frequent urination |
| Excessive thirst |
| Extreme hunger |
| Unusual weight loss |
| Increased fatigue |
| Irritability |
| Blurry vision |
| Diabetic ketoacidosisa |
| Thirst or very dry mouth |
| Constantly feeling tired |
| Dry or flushed skin |
| Nausea, vomiting, or abdominal pain |
| Difficulty breathing (short, deep breaths) |
| Fruity odor on breath |
| Difficulty paying attention or confusion |
| aVomiting is a sign of escalation Source: References 24,25 |
Components of diabetes care
| Self-care tasks | Tests/annual assessments |
|---|---|
| Self-monitoring of glucose | A1C (2 to 4 times/year) |
| Medication management | Urinary microalbumin |
| Meal planning | Fasting lipids |
| Exercise | Blood pressure |
| Smoking cessation | Dilated eye exam |
| Foot self-examination and foot care | Foot exam |
| Stress management | General health and cardiovascular exam |
Managing patients at risk for diabetes
| Prediabetes21 | Management |
|---|---|
| Impaired fasting glucose (100 to 125 mg/dL) | Weight reduction (7%) Activity (150 minutes/week) At least yearly glucose monitoring |
| Impaired glucose tolerance (2-hour plasma glucose: 140 to 199 mg/dL) | |
| Prediabetic A1C (5.7% to 6.4%) | |
| Metabolic syndrome (any 3)26 | Management |
| Waist circumferencea (men >40 inches; women >35 inches) | Weight reduction Reduce consumption of refined carbohydrates Exercise Focused interventions for individual criteria |
| Fasting triglycerides (≥150 mg/dL) | |
| Fasting high-density lipoprotein cholesterol (men | |
| Fasting glucose (≥100 mg/dL or taking medication) | |
| Blood pressure (≥130/85 mm Hg or taking medication) | |
| aWaist circumference guidelines are ethnicity specific. The International Diabetes Federation27 has published specific cutoffs for those of Asian background (men: ≥90 cm [35 inches] and women: ≥80 cm [31 inches]) | |
Metabolic monitoring
Metabolic monitoring is the key to keeping patients with schizophrenia well. Treating metabolic conditions falls outside of psychiatric practice; however, many argue that mental health clinicians should monitor basic metabolic parameters during antipsychotic treatment and advocate medical interventions when indicated because:
- most antipsychotics are associated with weight gain and metabolic side effects
- patients with schizophrenia have cognitive deficits that impact health maintenance
- mental health providers often are the primary health care contacts for patients with serious mental illness.
- identify treatable medical conditions such as diabetes, dyslipidemia, and hypertension when treatment delay or no treatment has consequences
- identify individuals with prediabetes and metabolic syndrome for targeted prevention
- determine the association between antipsychotic treatment and metabolic disturbance to evaluate the risk of treatment vs antipsychotic switching.
How to intervene
To switch or not to switch? For many psychiatrists, deciding whether or when to switch from a high or intermediate metabolic liability antipsychotic to one with low metabolic liability is difficult. Clinicians must balance potential metabolic benefits against the risk of psychotic decompensation and side effects. Ultimately, patients and their families make the decision, taking into account information provided to them. For medical-legal purposes, document the discussion of potential risks and benefits. These are difficult decisions and there are no clear guidelines. In my clinical experience, the following issues need to be considered:
- The antipsychotics that many clinicians consider to be the most effective—clozapine and olanzapine—also have the greatest metabolic liability and risk for emergent T2DM.
- Patients who are stable and in psychotic remission may risk a relapse of their illness if switched.
- The clearest indication for switching is when a patient who does not have diabetes develops the condition shortly after starting an antipsychotic. This scenario is rare, but evidence suggests that diabetes may resolve or reverse with an antipsychotic switch.33
- In patients who gain weight while taking a high- or intermediate-liability antipsychotic and are able to tolerate a switch to a low-liability antipsychotic, the effect size of weight reduction can be large and may result in a patient returning to their pretreatment weight.
- To reduce relapse risk, patients switching antipsychotics should be closely monitored at least weekly for ≥1 month. A plateau cross-taper—building the new antipsychotic up to therapeutic levels before gradually reducing the first antipsychotic—may be safer than abrupt discontinuation or standard cross-titration.
- Switching from one high or intermediate liability antipsychotic to another (eg, olanzapine to quetiapine or risperidone) often provides little if any metabolic benefit on body weight or diabetes control.
- Established diabetes (type 1 or type 2) should not be a contraindication to antipsychotic treatment, including clozapine, if clinically warranted. Monitor metabolic parameters more closely for 6 to 12 months after the switch. In most cases, patients experience limited, if any, metabolic consequences. If so, diabetes medication can be adjusted.
- Patients who have experienced significant weight gain on an atypical antipsychotic often do not gain more weight when switched to clozapine. A patient may reach a “ceiling” in terms of weight gain and medication-related metabolic effects.
Data from metabolic monitoring informs the decision to switch and metabolic consequences of switching. Conducting monitoring at baseline, when starting an antipsychotic, when switching to a high-liability agent, 3 months after the switch, and then annually provides data needed to consider switching or initiating medical and behavioral or lifestyle interventions.
Facilitate early diabetes treatment. Clinicians who are most closely involved in caring for patients with schizophrenia often are best situated to screen for diabetes. I have found that without a close working relationship with my patients’ primary care practitioners, patients may experience a long delay in receiving care. After your patient is diagnosed with diabetes, establish a relationship with diabetes treatment providers and work with your patient to ensure they engage in diabetes care.
Contribute to diabetes chronic disease management. Mental health practitioners can complement diabetes care in patients with serious mental illness by:
- navigating the health system and negotiating for service on patients’ behalf
- promoting positive relationships among diabetes and mental health treatment teams
- evaluating and treating depression that may be comorbid with diabetes
- assessing treatment capacity, self-care deficits, cognitive functioning, psychotic symptoms, negative symptoms, etc., that impact diabetes self-care and collaborating with diabetes care providers to support patients.
Start with a low-liability agent
Patients who are early in the course of psychotic illness are most susceptible to the metabolic effects of antipsychotics.13 The average weight gain observed with olanzapine was 34 lbs at 2 years in first episode psychosis patients (mean age 24 ± 4.9).34 Metabolic consequences with medium-liability second-generation antipsychotics—such as quetiapine and risperidone—are extreme, particularly in children, adolescents, and young adults (age 35,36 Although frank diabetes is uncommon in early psychosis because patients are, to a certain extent, protected by insulin compensation—increased insulin secretion maintains glucose levels within a therapeutic range—diabetes risk is increased, and hyperinsulinemia and hypertriglyceridemia are early markers of metabolic strain. Also, response to initial antipsychotic treatment—possibly independent of the agent selected—is robust in early psychosis.37
Related Resources
- American Diabetes Association. www.diabetes.org.
- International Diabetes Federation. www.idf.org.
- Framingham Heart Study. Coronary heart disease (10-year risk). www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. Risk assessment tool for estimating your 10-year risk of having a heart attack. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
Dr. Cohn is a speaker for Pfizer Canada.
Discuss this article at www.facebook.com/CurrentPsychiatry
Although diabetes and schizophrenia are common companions, it is unclear how this association should influence our practice. What do we need to know about diabetes, and what are the key intervention points for psychiatrists?
This article is informed by my experience monitoring >1,000 patients with schizophrenia in a large urban mental health facility using an electronic metabolic monitoring system and consulting on hundreds of individuals with comorbid schizophrenia and diabetes in a mental health metabolic clinic.
A significant link
The association between schizophrenia and diabetes has been recognized for more than a century.1 The prevalence of diabetes is increased 2- to 3-fold in patients with schizophrenia.2,3 This relationship is specific to type 2 diabetes mellitus (T2DM); type 1 diabetes mellitus, an autoimmune disease, is less common in patients with schizophrenia.4 Factors that contribute to comorbidity between schizophrenia and T2DM include:
- illness susceptibility: the mechanisms remain unclear but include the thrifty phenotype hypothesis,5 autonomic hyperactivity,6 and potential cellular and genetic links7,8
- lifestyle: diet, physical inactivity, and cigarette smoking9-12
- antipsychotic use13
- social health determinants, such as income, housing, and food insecurity.14
When evaluating a patient’s risk for a cardiac event, we consider having a diabetes diagnosis equivalent to having had a myocardial infarction.15 Likely, the high prevalence of T2DM among schizophrenia patients and challenges in managing diabetes and prediabetes underlies these patients’ reduced life expectancy.16 Self-care, a cornerstone of diabetes management, is challenging for patients with schizophrenia because of deficits in executive functioning, working memory, and motivation, coupled with negative symptoms and social and economic disadvantages that often accompany schizophrenia.
How diabetes impacts practice
What psychiatrists need to know. Insulin resistance—reduced biologic effectiveness of insulin—is the precursor of T2DM. Insulin is required to move glucose from the blood into cells. Weight gain, particularly abdominal adiposity, is the principal driver of insulin resistance. The body responds by producing more insulin (hyperinsulinemia) to maintain glucose homeostasis. Hyperinsulinemia underlies metabolic syndrome, an important risk marker for developing T2DM. Diabetes usually develops after many years when the pancreas fails to compensate for insulin resistance.
In most cases the development of diabetes in patients with schizophrenia follows this course. Weight gain, a consequence of lifestyle factors as well as antipsychotics and other psychotropics that promote obesity, leads to progressive insulin resistance. Consequently, metabolic syndrome is twice as prevalent among patients with schizophrenia compared with matched controls.17,18
Occasionally patients develop T2DM within a few weeks or months of starting antipsychotic treatment—usually with clozapine or olanzapine—before they gain weight, which suggests a second mechanism may be involved. Animal studies have documented rapid development of insulin resistance after a single subcutaneous injection of antipsychotics that have high metabolic liability, possibly through a direct effect on insulin signaling.19 This phenomenon has been difficult to demonstrate in humans.20
Managing diabetes is complex and ideally involves a range of health practitioners who work with patients to provide education, promote self-care behaviors, and direct complex health care. These services are outside the scope of psychiatric practice, but given the functional deficits in seriously mentally ill patients, it is important to have an overview of diabetes care (Table 3).
In addition to diagnosing diabetes, psychiatrists should be able to identify patients at risk for developing diabetes and initiate prevention strategies. Interventions are focused on lifestyle—weight reduction, increased physical activity, diet, and smoking cessation—as well as pharmacologic strategies such as metformin.
What patients need to know. Similar to schizophrenia, a diabetes diagnosis may be difficult for patients to accept. Initially, a patient may have no manifestations or symptoms. However, untreated diabetes has serious long-term health consequences—including blindness, amputations, kidney disease, and early death from heart attacks.
Patients should actively participate in treatment that involves learning about the illness, making lifestyle changes, working on self-care, and keeping regular medical appointments. Three components of lifestyle change must be addressed:
- Diet: counseling with a dietician or other health professional to reduce or stabilize body weight and make changes in diet quality, portion size, and meal frequency to improve glucose control and reduce long-term diabetes complications
- Physical activity: increasing physical activity, initially by walking daily, to benefit glucose control and weight maintenance
- Smoking: reducing or stopping cigarette smoking to improve glucose control and reduce diabetes complications.
American Diabetes Association diagnostic criteria for diabetes
| Testa | Threshold | Qualifier |
|---|---|---|
| A1C, or | ≥6.5% | Lab NGSP certified, standardized DCCT assay |
| Fasting glucose, or | ≥126 mg/dL | No caloric intake for at least 8 hours |
| 2-hour glucose, or | ≥200 mg/dL | After 75 g of anhydrous glucose |
| Random glucose | ≥200 mg/dL | Plus classic hyperglycemic symptoms or crisis |
| aResults should be confirmed by repeat testing DCCT: Diabetes Control and Complications Trial; NGSP: National Glycohemoglobin Standardization Program Source: References 21-23 | ||
Signs and symptoms of diabetes and diabetic ketoacidosis
| Diabetes |
| Frequent urination |
| Excessive thirst |
| Extreme hunger |
| Unusual weight loss |
| Increased fatigue |
| Irritability |
| Blurry vision |
| Diabetic ketoacidosisa |
| Thirst or very dry mouth |
| Constantly feeling tired |
| Dry or flushed skin |
| Nausea, vomiting, or abdominal pain |
| Difficulty breathing (short, deep breaths) |
| Fruity odor on breath |
| Difficulty paying attention or confusion |
| aVomiting is a sign of escalation Source: References 24,25 |
Components of diabetes care
| Self-care tasks | Tests/annual assessments |
|---|---|
| Self-monitoring of glucose | A1C (2 to 4 times/year) |
| Medication management | Urinary microalbumin |
| Meal planning | Fasting lipids |
| Exercise | Blood pressure |
| Smoking cessation | Dilated eye exam |
| Foot self-examination and foot care | Foot exam |
| Stress management | General health and cardiovascular exam |
Managing patients at risk for diabetes
| Prediabetes21 | Management |
|---|---|
| Impaired fasting glucose (100 to 125 mg/dL) | Weight reduction (7%) Activity (150 minutes/week) At least yearly glucose monitoring |
| Impaired glucose tolerance (2-hour plasma glucose: 140 to 199 mg/dL) | |
| Prediabetic A1C (5.7% to 6.4%) | |
| Metabolic syndrome (any 3)26 | Management |
| Waist circumferencea (men >40 inches; women >35 inches) | Weight reduction Reduce consumption of refined carbohydrates Exercise Focused interventions for individual criteria |
| Fasting triglycerides (≥150 mg/dL) | |
| Fasting high-density lipoprotein cholesterol (men | |
| Fasting glucose (≥100 mg/dL or taking medication) | |
| Blood pressure (≥130/85 mm Hg or taking medication) | |
| aWaist circumference guidelines are ethnicity specific. The International Diabetes Federation27 has published specific cutoffs for those of Asian background (men: ≥90 cm [35 inches] and women: ≥80 cm [31 inches]) | |
Metabolic monitoring
Metabolic monitoring is the key to keeping patients with schizophrenia well. Treating metabolic conditions falls outside of psychiatric practice; however, many argue that mental health clinicians should monitor basic metabolic parameters during antipsychotic treatment and advocate medical interventions when indicated because:
- most antipsychotics are associated with weight gain and metabolic side effects
- patients with schizophrenia have cognitive deficits that impact health maintenance
- mental health providers often are the primary health care contacts for patients with serious mental illness.
- identify treatable medical conditions such as diabetes, dyslipidemia, and hypertension when treatment delay or no treatment has consequences
- identify individuals with prediabetes and metabolic syndrome for targeted prevention
- determine the association between antipsychotic treatment and metabolic disturbance to evaluate the risk of treatment vs antipsychotic switching.
How to intervene
To switch or not to switch? For many psychiatrists, deciding whether or when to switch from a high or intermediate metabolic liability antipsychotic to one with low metabolic liability is difficult. Clinicians must balance potential metabolic benefits against the risk of psychotic decompensation and side effects. Ultimately, patients and their families make the decision, taking into account information provided to them. For medical-legal purposes, document the discussion of potential risks and benefits. These are difficult decisions and there are no clear guidelines. In my clinical experience, the following issues need to be considered:
- The antipsychotics that many clinicians consider to be the most effective—clozapine and olanzapine—also have the greatest metabolic liability and risk for emergent T2DM.
- Patients who are stable and in psychotic remission may risk a relapse of their illness if switched.
- The clearest indication for switching is when a patient who does not have diabetes develops the condition shortly after starting an antipsychotic. This scenario is rare, but evidence suggests that diabetes may resolve or reverse with an antipsychotic switch.33
- In patients who gain weight while taking a high- or intermediate-liability antipsychotic and are able to tolerate a switch to a low-liability antipsychotic, the effect size of weight reduction can be large and may result in a patient returning to their pretreatment weight.
- To reduce relapse risk, patients switching antipsychotics should be closely monitored at least weekly for ≥1 month. A plateau cross-taper—building the new antipsychotic up to therapeutic levels before gradually reducing the first antipsychotic—may be safer than abrupt discontinuation or standard cross-titration.
- Switching from one high or intermediate liability antipsychotic to another (eg, olanzapine to quetiapine or risperidone) often provides little if any metabolic benefit on body weight or diabetes control.
- Established diabetes (type 1 or type 2) should not be a contraindication to antipsychotic treatment, including clozapine, if clinically warranted. Monitor metabolic parameters more closely for 6 to 12 months after the switch. In most cases, patients experience limited, if any, metabolic consequences. If so, diabetes medication can be adjusted.
- Patients who have experienced significant weight gain on an atypical antipsychotic often do not gain more weight when switched to clozapine. A patient may reach a “ceiling” in terms of weight gain and medication-related metabolic effects.
Data from metabolic monitoring informs the decision to switch and metabolic consequences of switching. Conducting monitoring at baseline, when starting an antipsychotic, when switching to a high-liability agent, 3 months after the switch, and then annually provides data needed to consider switching or initiating medical and behavioral or lifestyle interventions.
Facilitate early diabetes treatment. Clinicians who are most closely involved in caring for patients with schizophrenia often are best situated to screen for diabetes. I have found that without a close working relationship with my patients’ primary care practitioners, patients may experience a long delay in receiving care. After your patient is diagnosed with diabetes, establish a relationship with diabetes treatment providers and work with your patient to ensure they engage in diabetes care.
Contribute to diabetes chronic disease management. Mental health practitioners can complement diabetes care in patients with serious mental illness by:
- navigating the health system and negotiating for service on patients’ behalf
- promoting positive relationships among diabetes and mental health treatment teams
- evaluating and treating depression that may be comorbid with diabetes
- assessing treatment capacity, self-care deficits, cognitive functioning, psychotic symptoms, negative symptoms, etc., that impact diabetes self-care and collaborating with diabetes care providers to support patients.
Start with a low-liability agent
Patients who are early in the course of psychotic illness are most susceptible to the metabolic effects of antipsychotics.13 The average weight gain observed with olanzapine was 34 lbs at 2 years in first episode psychosis patients (mean age 24 ± 4.9).34 Metabolic consequences with medium-liability second-generation antipsychotics—such as quetiapine and risperidone—are extreme, particularly in children, adolescents, and young adults (age 35,36 Although frank diabetes is uncommon in early psychosis because patients are, to a certain extent, protected by insulin compensation—increased insulin secretion maintains glucose levels within a therapeutic range—diabetes risk is increased, and hyperinsulinemia and hypertriglyceridemia are early markers of metabolic strain. Also, response to initial antipsychotic treatment—possibly independent of the agent selected—is robust in early psychosis.37
Related Resources
- American Diabetes Association. www.diabetes.org.
- International Diabetes Federation. www.idf.org.
- Framingham Heart Study. Coronary heart disease (10-year risk). www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. Risk assessment tool for estimating your 10-year risk of having a heart attack. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Clozapine • Clozaril
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
Dr. Cohn is a speaker for Pfizer Canada.
1. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903-912.
3. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemol Ment Health. 2006;2:14.-
4. Juvonen H, Reunanen A, Haukka J, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. 2007;64(8):894-899.
5. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20.
6. Ryan MC, Sharifi N, Condren R, et al. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065-1070.
7. Odawara M, Isaka M, Tada K, et al. Diabetes mellitus associated with mitochondrial myopathy and schizophrenia: a possible link between diabetes mellitus and schizophrenia. Diabet Med. 1997;14(6):503.-
8. Siuta MA, Robertson SD, Kocalis H, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8(6):e1000393.-
9. Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29(2):393-397.
10. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. 2005;193(10):641-646.
11. Ussher M, Stanbury L, Cheeseman V, et al. Physical activity p and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr Serv. 2007;58(3):405-408.
12. Cho NH, Chan JC, Jang HC, et al. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf). 2009;71(5):679-685.
13. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
14. Yu VL, Raphael D. Identifying and addressing the social determinants of the incidence and successful management of type 2 diabetes mellitus in Canada. Can J Public Health. 2004;95(5):366-368.
15. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151-157.
16. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
17. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49(11):753-760.
18. Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009;119(1):4-14.
19. Chintoh AF, Mann SW, Lam L, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28(5):494-499.
20. Hahn MK, Arenovich T, Wolever T, et al. Single dose administration of olanzapine: effects on glucose metabolism, endocrine and inflammatory markers in healthy volunteers. Poster presented at: Schizophrenia International Research Society 3rd Biennial Conference; April 14-18, 2012; Florence, Italy.
21. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
22. Little RR. Glycated hemoglobin standardization—National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191-1198.
23. Keen H. The Diabetes Control and Complications Trial (DCCT). Health Trends. 1994;26(2):41-43.
24. American Diabetes Association. Symptoms. http://www.diabetes.org/diabetes-basics/symptoms. Accessed August 27 2012.
25. American Diabetes Association. Ketoacidosis (DKA). http://www.diabetes.org/living-with-diabetes/complications/ketoacidosis-dka.html. Accessed August 27 2012.
26. Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735-2752.
27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-480.
28. Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
29. Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51(8):492-501.
30. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
31. Newcomer JW, Nasrallah HA, Loebel AD. The Atypical Antipsychotic Therapy and Metabolic Issues National Survey: practice patterns and knowledge of psychiatrists. J Clin Psychopharmacol. 2004;24(5 suppl 1):S1-S6.
32. Khoury A, Sproule BA, Cohn TA. Development and implementation of the Metabolic Health Monitor at the Centre for Addiction and Mental Health. Poster presented at: BC Psychopharmacology Conference; February 15-16 2008; Vancouver, British Columbia, Canada.
33. Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy. 2002;22(7):841-852.
34. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
35. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45(7):771-791.
36. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
37. Nicol G, Newcomer J. Review: children and adolescents with schizophrenia spectrum disorders respond to antipsychotics but are susceptible to adverse events. Evid Based Ment Health. 2008;11(3):81.-
1. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903-912.
3. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemol Ment Health. 2006;2:14.-
4. Juvonen H, Reunanen A, Haukka J, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. 2007;64(8):894-899.
5. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20.
6. Ryan MC, Sharifi N, Condren R, et al. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065-1070.
7. Odawara M, Isaka M, Tada K, et al. Diabetes mellitus associated with mitochondrial myopathy and schizophrenia: a possible link between diabetes mellitus and schizophrenia. Diabet Med. 1997;14(6):503.-
8. Siuta MA, Robertson SD, Kocalis H, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8(6):e1000393.-
9. Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29(2):393-397.
10. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. 2005;193(10):641-646.
11. Ussher M, Stanbury L, Cheeseman V, et al. Physical activity p and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr Serv. 2007;58(3):405-408.
12. Cho NH, Chan JC, Jang HC, et al. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf). 2009;71(5):679-685.
13. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
14. Yu VL, Raphael D. Identifying and addressing the social determinants of the incidence and successful management of type 2 diabetes mellitus in Canada. Can J Public Health. 2004;95(5):366-368.
15. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151-157.
16. Colton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.-
17. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49(11):753-760.
18. Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009;119(1):4-14.
19. Chintoh AF, Mann SW, Lam L, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28(5):494-499.
20. Hahn MK, Arenovich T, Wolever T, et al. Single dose administration of olanzapine: effects on glucose metabolism, endocrine and inflammatory markers in healthy volunteers. Poster presented at: Schizophrenia International Research Society 3rd Biennial Conference; April 14-18, 2012; Florence, Italy.
21. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
22. Little RR. Glycated hemoglobin standardization—National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191-1198.
23. Keen H. The Diabetes Control and Complications Trial (DCCT). Health Trends. 1994;26(2):41-43.
24. American Diabetes Association. Symptoms. http://www.diabetes.org/diabetes-basics/symptoms. Accessed August 27 2012.
25. American Diabetes Association. Ketoacidosis (DKA). http://www.diabetes.org/living-with-diabetes/complications/ketoacidosis-dka.html. Accessed August 27 2012.
26. Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735-2752.
27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-480.
28. Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68.
29. Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51(8):492-501.
30. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
31. Newcomer JW, Nasrallah HA, Loebel AD. The Atypical Antipsychotic Therapy and Metabolic Issues National Survey: practice patterns and knowledge of psychiatrists. J Clin Psychopharmacol. 2004;24(5 suppl 1):S1-S6.
32. Khoury A, Sproule BA, Cohn TA. Development and implementation of the Metabolic Health Monitor at the Centre for Addiction and Mental Health. Poster presented at: BC Psychopharmacology Conference; February 15-16 2008; Vancouver, British Columbia, Canada.
33. Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy. 2002;22(7):841-852.
34. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
35. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45(7):771-791.
36. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
37. Nicol G, Newcomer J. Review: children and adolescents with schizophrenia spectrum disorders respond to antipsychotics but are susceptible to adverse events. Evid Based Ment Health. 2008;11(3):81.-
Management of dermatological toxicities in patients receiving EGFR inhibitors
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Hypertension in cancer patients
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Recent developments in the treatment of high-grade gliomas
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Patients with glioblastoma and other high-grade gliomas have poor outcomes and are challenging to treat. The relative rarity of these tumors has made large-scale, practice-changing trials difficult to accomplish and has led to the formation of large multinational organizations that focus on neuro-oncology. This has resulted in the rapid completion of several large trials that in some cases have set new standards of care that can offer increased progression-free and overall survivals for some patients. The incorporation of correlative tissue studies in these trials has led to the identification of prognostic and predictive genetic markers that demonstrate the heterogeneity of these tumors and will assist in developing individualized treatment strategies as research continues to uncover new therapeutic targets. This review of recently completed and in-progress phase 3 trials in high-grade gliomas highlights the developments and future directions in the treatment of these tumors...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.