User login
HIV: How to provide compassionate care
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
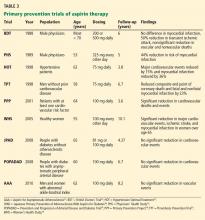
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
Post-transplant Lymphoproliferative Disorders
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
Evaluation and management of premature ventricular complexes
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
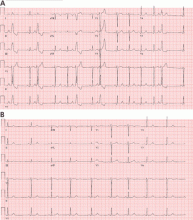
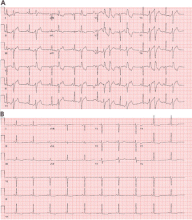
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
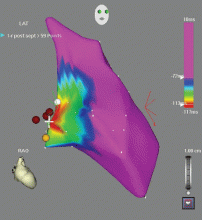
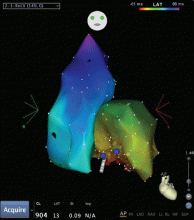
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
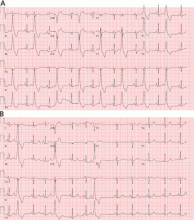
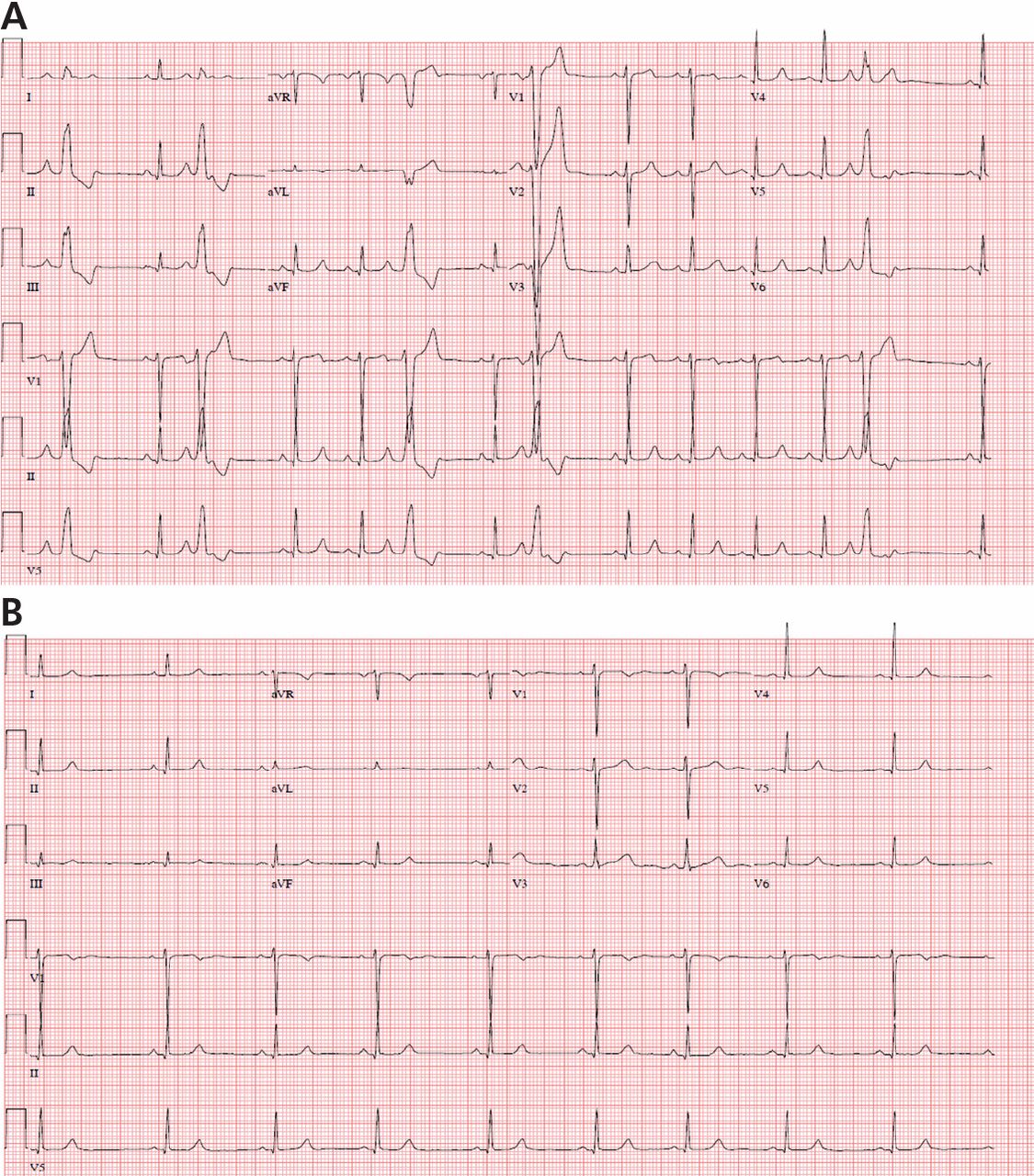
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
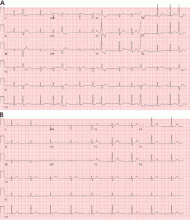
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
KEY POINTS
- Diagnostic evaluation should include an assessment for structural heart disease and quantification of the total PVC burden by ambulatory Holter monitoring.
- Patients without structural heart disease and low-to-modest PVC burdens do not always require treatment. PVCs at higher burdens (typically more than 15% to 20% of heartbeats) or strung together in runs of ventricular tachycardia pose a higher risk of tachycardia-related cardiomyopathy and heart failure, even if asymptomatic.
- When necessary, treatment for PVCs involves beta-blockers, calcium channel blockers, or other antiarrhythmic drugs and catheter ablation in selected cases.
- Catheter ablation can be curative, but it is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Medication-assisted treatment of opiate dependence is gaining favor
Experts have argued for decades about how best to manage opiate dependence, with practitioners generally subscribing to one of two strategies: either total abstinence or medication-assisted treatment (MAT).
Although MAT has proven efficacy, it has been slow to gain acceptance, and the gold standard of care since the 1930s has been abstinence-based treatment. Among elite institutional holdouts against MAT was the Hazelden Treatment Center, a leading treatment institution and publishing house that had been wedded to the abstinence model since it was founded in 1949.1 Now, Hazelden has gone on record as embracing MAT, raising the possibility that the two predominant treatment philosophies for opiate-dependent patients may no longer be at odds.
FROM ABSTINENCE TO METHADONE MAINTENANCE
The modern day abstinence-based movement in this country started in the decade before the founding of Hazelden. In 1935, the US government opened the first of two federal drug treatment centers, known as the United States Narcotic Farm, in Lexington, KY.2 The move by the government to get into the addiction treatment business largely stemmed from frustration over the growing problem of addiction at that time, coupled with a dearth of treatment options for addicts in the wake of the 1914 Harrison Narcotics Act.
The Narcotic Farm was an impressive facility—for all intents and purposes, a specialized prison—that initially housed 1,200 people. In addition to prisoners, it also accepted voluntary, nonprisoner patients. In many ways, it was ahead of its time. It offered a wide variety of services, including detoxification, group therapy, individual therapy, psychiatric and medical services, and vocational rehabilitation.2 Housed on the premises was the Addiction Research Center at Lexington, the first intramural research branch of the National Institute of Mental Health. After the “Blue Grass” mandatory commitment laws were passed in the 1940s, even the voluntary patients were ultimately committed for a 1-year sentence at Lexington. This facility, and its sister facility in Ft. Worth, TX, would have been the envy of any modern-day abstinence-based treatment center in terms of the services offered and the long lengths of stay.
The quality of the program, as evidenced by the impressive array of services and long stays, would lead one to expect that its treatment outcomes over nearly 40 years of operation were equally stellar. However, in terms of outcomes the Farm was an abysmal failure, as shown by numerous studies demonstrating relapse rates of more than 90% in the patients discharged from it.2,3
Similar frustrations at other abstinence-based treatment centers from the 1940s through the 1960s led Dr. Vincent Dole, the “father of methadone maintenance,” to conclude in 1971 that after detoxification from opiates, “human addicts almost always return to use of narcotics after they leave the hospital where they have been detoxified.”4 That realization inspired Dr. Dole and his wife and colleague Dr. Marie Nyswander to revisit the idea of medication-assisted treatment, an approach previously used by the morphine maintenance clinics of the early 1900s. This work led to the development of government-sanctioned methadone clinics across America and to the realization that long-term recovery was possible with medication, even without a lengthy hospital stay. For this revolutionary work on opiate addiction, Dr. Dole won the prestigious Lasker Award in 1988.
The major reason for the success of methadone was that, because of its pharmacokinetic profile, it could stabilize the patient through once-daily dosing without sedation or narcosis. As noted by Dr. Dole, once patients are on a stable dosing regimen, the obsessive preoccupation with drug use fades away.5
Despite its success, methadone maintenance had its share of detractors. It was fraught with controversy because it was viewed as a crutch, and those who were on it were often not considered by their abstinent peers as being in true recovery. The reasons for the negative attitudes toward MAT are unclear but may reflect antiquated beliefs that addiction may be indicative of a failure of morals or will, and that patients ought to be able to simply stop using.
Whatever the reason for the animosity surrounding MAT, it should be noted that an expert consensus panel convened by the Betty Ford Center in 2007 agreed that patients on MAT met their consensus definition of sobriety.6 The issue of what constitutes recovery remains a very complex and hotly debated topic that is beyond the scope of this paper and that has been discussed elsewhere.6,7
For more than 3 decades, methadone was the only medication available for MAT. Federal regulations limit the dispensing of methadone to licensed clinics, most of which are located in major metropolitan areas. Patients must go to the clinic every day to receive their dose of methadone—a major inconvenience, especially to those with transportation issues. Adding to the lack of appeal of methadone maintenance is that the clinics are typically located in the higher-crime areas of cities. Savvy drug dealers know the location of these clinics and often loiter on nearby street corners in an attempt to lure addicts away from recovery by flaunting their illicit drugs.
A final, very significant drawback of methadone is its safety profile. It is a full-agonist narcotic that can be fatal in overdose or in the induction phase, especially if taken with other drugs, such as benzodiazepines.
2003: BUPRENORPHINE-NALOXONE IS APPROVED
Such concerns led researchers to search for other medications to be used for MAT that could perhaps be prescribed in a typical outpatient physician practice. For many reasons, buprenorphine became the most promising candidate. In 2003, the US Food and Drug Administration approved the combination medication buprenorphine-naloxone (Sub-oxone) as only the second drug indicated for maintenance treatment of opioid dependence in the United States.
Buprenorphine differs from methadone in that it is a partial agonist at mu opiate receptors, and therefore has a “ceiling” or “plateau” effect in terms of dose-response and a much improved safety profile. Unlike methadone, buprenorphine can be prescribed in a doctor’s office and does not have to be dispensed at a government-approved clinic.
Unfortunately, buprenorphine-maintained patients seem to carry the same stigma in the recovery community as those maintained on methadone—that they are simply substituting one drug for another. Detractors usually fail to consider that, as with methadone, patients do not report getting “high” from taking buprenorphine. Patients will often state that when they first start taking it, they “feel something,” but after a few days of adjustment, they simply feel normal. They don’t feel high, they are no longer in withdrawal, their cravings are virtually eliminated, and their opiate receptors are effectively occupied and blocked, so there is no “high” in the event of a relapse.
What’s more, buprenorphine is not a medication that will help them deal with life’s stressors by “chemical coping.” Sober coping is a skill they must learn by actively participating in a solid 12-step-based recovery program and, in some cases, in psychotherapy. By removing the drug obsession, buprenorphine promotes and facilitates the important recovery goal of learning how to deal with life on life’s terms.
ADDICTION AS CHRONIC ILLNESS
Outcomes studies of addiction treatment have focused largely on rates of relapse after discharge from acute treatments such as residential rehabilitation, partial hospitalization, and intensive outpatient programs. With MAT, however, outcomes research has primarily looked at the duration of retention in treatment.
The change in focus between the two types of treatment coincides with a paradigm shift that views addiction as a chronic condition that requires ongoing care. Continued participation in prescribed care with demonstrated efficacy is considered to be the major indicator of success. Under the chronic illness model employed by MAT providers, if a patient reverted to briefly using a drug of abuse, this would be an issue to address in his ongoing treatment and would not necessarily indicate treatment failure as with the acute care model. Beyond retention rates, research has demonstrated that MAT with methadone results in reductions in rates of criminal activity, illicit drug use, acquisition of human immunodeficiency virus, and overall mortality.8–10
In outcomes studies, MAT has repeatedly shown better efficacy than abstinence-based approaches. During the first 5 years of its implementation, in 4,000 patients, methadone maintenance boasted 1-year retention rates exceeding 98%.11 Over the subsequent 3 years, with the number of patients approaching 35,000, the 1-year retention rates fell to around 60%—still far exceeding results of abstinence-based treatment and approximating the number cited in most modern studies.11
The retention rates in buprenorphine programs are similarly promising. Studies of 12 to 13 weeks duration have shown retention rates of 52% to 79%.12–15 Six-month studies have demonstrated retention rates of 43% to 100%.16–19 Another study showed that 38% of opiate-dependent patients remained in treatment with buprenorphine at 5 years.20 Surprisingly, most of the buprenorphine studies have been conducted in office-based practices, which are less structured than outpatient methadone programs.
MEDICATION-ASSISTED TREATMENT IS GAINING ACCEPTANCE
Data from decades of experience with MAT strongly support the conclusion that it is superior to abstinence-based approaches.
The importance of a patient staying in treatment cannot be overemphasized, as the consequence of failing in recovery may well be an early death. On average, heroin addicts lose about 18 years of life expectancy, and the mortality rate for injection users is roughly 2% per year.21 The mortality rate for heroin users is 6 to 20 times greater than for age-matched peers who are not drug users.22
As high as these numbers are, they are even higher for abusers of prescription narcotics. The annual death rate associated with opioid pain relievers (4.8 per 100,000) is nearly double that associated with illicit drugs (2.8 per 100,000).23
The recent and rather radical change in treatment philosophy by Hazelden came as a shock to some, a disappointment to others, and a welcome change to many who saw this as a move by one of the more respected treatment centers in the country to fall in line with the body of evidence that supports MAT for those suffering from opiate dependence. It remains a mystery why so many, if not most, addiction treatment centers in the United States cling to the abstinence-based philosophy despite the overwhelming data from decades of research and experience that show that abstinence does not work for the majority of opiate addicts.
Complete abstinence from opiate drugs of abuse and potentially addictive medications is a noble but perhaps unreachable goal for many sufferers. Hazelden’s announced acceptance of MAT gives credence to the value of recovery goals that are not entirely drug-free.
Dr. Dole was correct in stating that opiate addicts usually return to drugs if not provided with MAT. Treatment programs need to inform opiate-dependent patients that abstinence-based treatment offers only a 1 in 10 chance of success. Perhaps some patients, armed with the daunting statistics regarding abstinence, will be inspired to devote themselves wholeheartedly to their recovery in an effort to make it into that elite 10% group that achieves long-lasting recovery without the aid of medications. But for the other 90%, it is encouraging to hear that Hazelden, the model treatment center for most abstinence-based programs in this country, may now lead other abstinence-based centers to reconsider their treatment philosophies.
Historically, US doctors were not allowed by federal law to prescribe opiates for addiction treatment. With the passage of DATA 2000, buprenorphine (alone or in combination with naloxone) can be prescribed for addiction treatment only by providers who obtain a waiver from the US Drug Enforcement Administration (DEA). Any doctor can become qualified to prescribe buprenorphine or buprenorphinenaloxone after completing an 8-hour online training course (available at www.buppractice.com and at www.aaap.org/buprenorphine) and by obtaining a DATA 2000 waiver and a new prescribing number from the DEA. Doctors are initially limited to treating only 30 patients with buprenorphine-naloxone at any given time, but can apply for an extension to 100 patients after having had their waiver for 1 year.
As MAT continues to gain favor, demand will grow for more providers to obtain their waivers to prescribe buprenorphine and buprenorphine-naloxone. Historically, there have always been too few methadone clinics to meet the demand. One can hope that the growing number of waivered providers will greatly improve access to care by opiate addicts, no matter where they reside. Qualified prescribers of buprenorphine and buprenorphine-naloxone are limited by the federal restrictions on the numbers of patients they can treat. If the chronic disease of addiction is to be integrated into the continuing-care approach of modern medicine and managed alongside other chronic diseases, primary care providers who are not specialized in treating addiction will need to be become comfortable with maintaining patients on buprenorphine-naloxone.7 Presumably, such patients will have already been stabilized through participation in addiction treatment programs in their respective geographic areas. Primary care providers will need to develop relationships with local addictionologists and treatment programs so that they will be able to refer those in active addiction for induction and stabilization on MAT and will be able to refer those already stabilized on MAT back to such specialists when relapses occur.
We may finally be approaching a time when structured residential treatment and MAT are not mutually exclusive options for our patients. These treatment options must work together for optimal outcomes. Based on our experience with hundreds of patients at Cleveland Clinic’s Alcohol and Drug Recovery Center, we believe this change of treatment philosophy is long overdue. In clinical settings, patients do not fit cleanly into one treatment arm or another and often require a blended approach to effect long-lasting change. Hazelden’s shift of treatment philosophy is an indication that this research-supported viewpoint is gaining acceptance in the traditionally drug-free halls of addiction treatment programs.
- White WL. Slaying the Dragon. The History of Addiction Treatment and Recovery in America. Bloomington, IL: Chestnut Health Systems/Lighthouse Institute; 1998:124–125,201.
- Kosten TR, Gorelick DA. The Lexington narcotic farm. Am J Psychiatry 2002; 159:22.
- Hunt GH, Odoroff ME. Followup study of narcotic drug addicts after hospitalization. Public Health Rep 1962; 77:41–54.
- Dole VP. Narcotic addiction, physical dependence and relapse. N Engl J Med 1972; 286:988–992.
- Dole VP. Implications of methadone maintenance for theories of narcotic addiction. JAMA 1988; 260:3025–3029.
- Betty Ford Institute Consensus Panel. What is recovery? A working definition from the Betty Ford Institute. J Subst Abuse Treat 2007; 33:221–228.
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction 2002; 97:249–252.
- Grönbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand 1990; 82:223–227.
- Ball JC, Lange WR, Myers CP, Friedman SR. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav 1988; 29:214–226.
- Martin J, Zweben JE, Payte JT. Opioid maintenance treatment. In:Ries RK, Fiellin DA, Miller SC, Saitzeds R, editors. Principles of Addiction Medicine. 4th ed. Philadelphia, PA: Lippincottt Williams & Wilkins, 2009:671–688.
- Dole VP, Nyswander ME. Methadone maintenance treatment. A tenyear perspective. JAMA 1976; 235:2117–2119.
- Cunningham C, Giovanniello A, Sacajiu G, et al. Buprenorphine treatment in an urban community health center: what to expect. Fam Med 2008; 40:500–506.
- Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002; 28:231–241.
- Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003; 349:949–958.
- O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med 1998; 105:100–105.
- Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med 2006; 355:365–374.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med 2007; 5:146–150.
- O’Connor PG, Oliveto AH, Shi JM, et al. A pilot study of primary-carebased buprenorphine maintenance for heroin dependence. Am J Drug Alcohol Abuse 1996; 22:523–531.
- Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict 2008; 17:116–120.
- Smyth B, Hoffman V, Fan J, Hser YI. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med 2007; 44:369–374.
- Sporer KA. Acute heroin overdose. Ann Intern Med 1999; 130:584–590.
- Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60:1487–1492.
Experts have argued for decades about how best to manage opiate dependence, with practitioners generally subscribing to one of two strategies: either total abstinence or medication-assisted treatment (MAT).
Although MAT has proven efficacy, it has been slow to gain acceptance, and the gold standard of care since the 1930s has been abstinence-based treatment. Among elite institutional holdouts against MAT was the Hazelden Treatment Center, a leading treatment institution and publishing house that had been wedded to the abstinence model since it was founded in 1949.1 Now, Hazelden has gone on record as embracing MAT, raising the possibility that the two predominant treatment philosophies for opiate-dependent patients may no longer be at odds.
FROM ABSTINENCE TO METHADONE MAINTENANCE
The modern day abstinence-based movement in this country started in the decade before the founding of Hazelden. In 1935, the US government opened the first of two federal drug treatment centers, known as the United States Narcotic Farm, in Lexington, KY.2 The move by the government to get into the addiction treatment business largely stemmed from frustration over the growing problem of addiction at that time, coupled with a dearth of treatment options for addicts in the wake of the 1914 Harrison Narcotics Act.
The Narcotic Farm was an impressive facility—for all intents and purposes, a specialized prison—that initially housed 1,200 people. In addition to prisoners, it also accepted voluntary, nonprisoner patients. In many ways, it was ahead of its time. It offered a wide variety of services, including detoxification, group therapy, individual therapy, psychiatric and medical services, and vocational rehabilitation.2 Housed on the premises was the Addiction Research Center at Lexington, the first intramural research branch of the National Institute of Mental Health. After the “Blue Grass” mandatory commitment laws were passed in the 1940s, even the voluntary patients were ultimately committed for a 1-year sentence at Lexington. This facility, and its sister facility in Ft. Worth, TX, would have been the envy of any modern-day abstinence-based treatment center in terms of the services offered and the long lengths of stay.
The quality of the program, as evidenced by the impressive array of services and long stays, would lead one to expect that its treatment outcomes over nearly 40 years of operation were equally stellar. However, in terms of outcomes the Farm was an abysmal failure, as shown by numerous studies demonstrating relapse rates of more than 90% in the patients discharged from it.2,3
Similar frustrations at other abstinence-based treatment centers from the 1940s through the 1960s led Dr. Vincent Dole, the “father of methadone maintenance,” to conclude in 1971 that after detoxification from opiates, “human addicts almost always return to use of narcotics after they leave the hospital where they have been detoxified.”4 That realization inspired Dr. Dole and his wife and colleague Dr. Marie Nyswander to revisit the idea of medication-assisted treatment, an approach previously used by the morphine maintenance clinics of the early 1900s. This work led to the development of government-sanctioned methadone clinics across America and to the realization that long-term recovery was possible with medication, even without a lengthy hospital stay. For this revolutionary work on opiate addiction, Dr. Dole won the prestigious Lasker Award in 1988.
The major reason for the success of methadone was that, because of its pharmacokinetic profile, it could stabilize the patient through once-daily dosing without sedation or narcosis. As noted by Dr. Dole, once patients are on a stable dosing regimen, the obsessive preoccupation with drug use fades away.5
Despite its success, methadone maintenance had its share of detractors. It was fraught with controversy because it was viewed as a crutch, and those who were on it were often not considered by their abstinent peers as being in true recovery. The reasons for the negative attitudes toward MAT are unclear but may reflect antiquated beliefs that addiction may be indicative of a failure of morals or will, and that patients ought to be able to simply stop using.
Whatever the reason for the animosity surrounding MAT, it should be noted that an expert consensus panel convened by the Betty Ford Center in 2007 agreed that patients on MAT met their consensus definition of sobriety.6 The issue of what constitutes recovery remains a very complex and hotly debated topic that is beyond the scope of this paper and that has been discussed elsewhere.6,7
For more than 3 decades, methadone was the only medication available for MAT. Federal regulations limit the dispensing of methadone to licensed clinics, most of which are located in major metropolitan areas. Patients must go to the clinic every day to receive their dose of methadone—a major inconvenience, especially to those with transportation issues. Adding to the lack of appeal of methadone maintenance is that the clinics are typically located in the higher-crime areas of cities. Savvy drug dealers know the location of these clinics and often loiter on nearby street corners in an attempt to lure addicts away from recovery by flaunting their illicit drugs.
A final, very significant drawback of methadone is its safety profile. It is a full-agonist narcotic that can be fatal in overdose or in the induction phase, especially if taken with other drugs, such as benzodiazepines.
2003: BUPRENORPHINE-NALOXONE IS APPROVED
Such concerns led researchers to search for other medications to be used for MAT that could perhaps be prescribed in a typical outpatient physician practice. For many reasons, buprenorphine became the most promising candidate. In 2003, the US Food and Drug Administration approved the combination medication buprenorphine-naloxone (Sub-oxone) as only the second drug indicated for maintenance treatment of opioid dependence in the United States.
Buprenorphine differs from methadone in that it is a partial agonist at mu opiate receptors, and therefore has a “ceiling” or “plateau” effect in terms of dose-response and a much improved safety profile. Unlike methadone, buprenorphine can be prescribed in a doctor’s office and does not have to be dispensed at a government-approved clinic.
Unfortunately, buprenorphine-maintained patients seem to carry the same stigma in the recovery community as those maintained on methadone—that they are simply substituting one drug for another. Detractors usually fail to consider that, as with methadone, patients do not report getting “high” from taking buprenorphine. Patients will often state that when they first start taking it, they “feel something,” but after a few days of adjustment, they simply feel normal. They don’t feel high, they are no longer in withdrawal, their cravings are virtually eliminated, and their opiate receptors are effectively occupied and blocked, so there is no “high” in the event of a relapse.
What’s more, buprenorphine is not a medication that will help them deal with life’s stressors by “chemical coping.” Sober coping is a skill they must learn by actively participating in a solid 12-step-based recovery program and, in some cases, in psychotherapy. By removing the drug obsession, buprenorphine promotes and facilitates the important recovery goal of learning how to deal with life on life’s terms.
ADDICTION AS CHRONIC ILLNESS
Outcomes studies of addiction treatment have focused largely on rates of relapse after discharge from acute treatments such as residential rehabilitation, partial hospitalization, and intensive outpatient programs. With MAT, however, outcomes research has primarily looked at the duration of retention in treatment.
The change in focus between the two types of treatment coincides with a paradigm shift that views addiction as a chronic condition that requires ongoing care. Continued participation in prescribed care with demonstrated efficacy is considered to be the major indicator of success. Under the chronic illness model employed by MAT providers, if a patient reverted to briefly using a drug of abuse, this would be an issue to address in his ongoing treatment and would not necessarily indicate treatment failure as with the acute care model. Beyond retention rates, research has demonstrated that MAT with methadone results in reductions in rates of criminal activity, illicit drug use, acquisition of human immunodeficiency virus, and overall mortality.8–10
In outcomes studies, MAT has repeatedly shown better efficacy than abstinence-based approaches. During the first 5 years of its implementation, in 4,000 patients, methadone maintenance boasted 1-year retention rates exceeding 98%.11 Over the subsequent 3 years, with the number of patients approaching 35,000, the 1-year retention rates fell to around 60%—still far exceeding results of abstinence-based treatment and approximating the number cited in most modern studies.11
The retention rates in buprenorphine programs are similarly promising. Studies of 12 to 13 weeks duration have shown retention rates of 52% to 79%.12–15 Six-month studies have demonstrated retention rates of 43% to 100%.16–19 Another study showed that 38% of opiate-dependent patients remained in treatment with buprenorphine at 5 years.20 Surprisingly, most of the buprenorphine studies have been conducted in office-based practices, which are less structured than outpatient methadone programs.
MEDICATION-ASSISTED TREATMENT IS GAINING ACCEPTANCE
Data from decades of experience with MAT strongly support the conclusion that it is superior to abstinence-based approaches.
The importance of a patient staying in treatment cannot be overemphasized, as the consequence of failing in recovery may well be an early death. On average, heroin addicts lose about 18 years of life expectancy, and the mortality rate for injection users is roughly 2% per year.21 The mortality rate for heroin users is 6 to 20 times greater than for age-matched peers who are not drug users.22
As high as these numbers are, they are even higher for abusers of prescription narcotics. The annual death rate associated with opioid pain relievers (4.8 per 100,000) is nearly double that associated with illicit drugs (2.8 per 100,000).23
The recent and rather radical change in treatment philosophy by Hazelden came as a shock to some, a disappointment to others, and a welcome change to many who saw this as a move by one of the more respected treatment centers in the country to fall in line with the body of evidence that supports MAT for those suffering from opiate dependence. It remains a mystery why so many, if not most, addiction treatment centers in the United States cling to the abstinence-based philosophy despite the overwhelming data from decades of research and experience that show that abstinence does not work for the majority of opiate addicts.
Complete abstinence from opiate drugs of abuse and potentially addictive medications is a noble but perhaps unreachable goal for many sufferers. Hazelden’s announced acceptance of MAT gives credence to the value of recovery goals that are not entirely drug-free.
Dr. Dole was correct in stating that opiate addicts usually return to drugs if not provided with MAT. Treatment programs need to inform opiate-dependent patients that abstinence-based treatment offers only a 1 in 10 chance of success. Perhaps some patients, armed with the daunting statistics regarding abstinence, will be inspired to devote themselves wholeheartedly to their recovery in an effort to make it into that elite 10% group that achieves long-lasting recovery without the aid of medications. But for the other 90%, it is encouraging to hear that Hazelden, the model treatment center for most abstinence-based programs in this country, may now lead other abstinence-based centers to reconsider their treatment philosophies.
Historically, US doctors were not allowed by federal law to prescribe opiates for addiction treatment. With the passage of DATA 2000, buprenorphine (alone or in combination with naloxone) can be prescribed for addiction treatment only by providers who obtain a waiver from the US Drug Enforcement Administration (DEA). Any doctor can become qualified to prescribe buprenorphine or buprenorphinenaloxone after completing an 8-hour online training course (available at www.buppractice.com and at www.aaap.org/buprenorphine) and by obtaining a DATA 2000 waiver and a new prescribing number from the DEA. Doctors are initially limited to treating only 30 patients with buprenorphine-naloxone at any given time, but can apply for an extension to 100 patients after having had their waiver for 1 year.
As MAT continues to gain favor, demand will grow for more providers to obtain their waivers to prescribe buprenorphine and buprenorphine-naloxone. Historically, there have always been too few methadone clinics to meet the demand. One can hope that the growing number of waivered providers will greatly improve access to care by opiate addicts, no matter where they reside. Qualified prescribers of buprenorphine and buprenorphine-naloxone are limited by the federal restrictions on the numbers of patients they can treat. If the chronic disease of addiction is to be integrated into the continuing-care approach of modern medicine and managed alongside other chronic diseases, primary care providers who are not specialized in treating addiction will need to be become comfortable with maintaining patients on buprenorphine-naloxone.7 Presumably, such patients will have already been stabilized through participation in addiction treatment programs in their respective geographic areas. Primary care providers will need to develop relationships with local addictionologists and treatment programs so that they will be able to refer those in active addiction for induction and stabilization on MAT and will be able to refer those already stabilized on MAT back to such specialists when relapses occur.
We may finally be approaching a time when structured residential treatment and MAT are not mutually exclusive options for our patients. These treatment options must work together for optimal outcomes. Based on our experience with hundreds of patients at Cleveland Clinic’s Alcohol and Drug Recovery Center, we believe this change of treatment philosophy is long overdue. In clinical settings, patients do not fit cleanly into one treatment arm or another and often require a blended approach to effect long-lasting change. Hazelden’s shift of treatment philosophy is an indication that this research-supported viewpoint is gaining acceptance in the traditionally drug-free halls of addiction treatment programs.
Experts have argued for decades about how best to manage opiate dependence, with practitioners generally subscribing to one of two strategies: either total abstinence or medication-assisted treatment (MAT).
Although MAT has proven efficacy, it has been slow to gain acceptance, and the gold standard of care since the 1930s has been abstinence-based treatment. Among elite institutional holdouts against MAT was the Hazelden Treatment Center, a leading treatment institution and publishing house that had been wedded to the abstinence model since it was founded in 1949.1 Now, Hazelden has gone on record as embracing MAT, raising the possibility that the two predominant treatment philosophies for opiate-dependent patients may no longer be at odds.
FROM ABSTINENCE TO METHADONE MAINTENANCE
The modern day abstinence-based movement in this country started in the decade before the founding of Hazelden. In 1935, the US government opened the first of two federal drug treatment centers, known as the United States Narcotic Farm, in Lexington, KY.2 The move by the government to get into the addiction treatment business largely stemmed from frustration over the growing problem of addiction at that time, coupled with a dearth of treatment options for addicts in the wake of the 1914 Harrison Narcotics Act.
The Narcotic Farm was an impressive facility—for all intents and purposes, a specialized prison—that initially housed 1,200 people. In addition to prisoners, it also accepted voluntary, nonprisoner patients. In many ways, it was ahead of its time. It offered a wide variety of services, including detoxification, group therapy, individual therapy, psychiatric and medical services, and vocational rehabilitation.2 Housed on the premises was the Addiction Research Center at Lexington, the first intramural research branch of the National Institute of Mental Health. After the “Blue Grass” mandatory commitment laws were passed in the 1940s, even the voluntary patients were ultimately committed for a 1-year sentence at Lexington. This facility, and its sister facility in Ft. Worth, TX, would have been the envy of any modern-day abstinence-based treatment center in terms of the services offered and the long lengths of stay.
The quality of the program, as evidenced by the impressive array of services and long stays, would lead one to expect that its treatment outcomes over nearly 40 years of operation were equally stellar. However, in terms of outcomes the Farm was an abysmal failure, as shown by numerous studies demonstrating relapse rates of more than 90% in the patients discharged from it.2,3
Similar frustrations at other abstinence-based treatment centers from the 1940s through the 1960s led Dr. Vincent Dole, the “father of methadone maintenance,” to conclude in 1971 that after detoxification from opiates, “human addicts almost always return to use of narcotics after they leave the hospital where they have been detoxified.”4 That realization inspired Dr. Dole and his wife and colleague Dr. Marie Nyswander to revisit the idea of medication-assisted treatment, an approach previously used by the morphine maintenance clinics of the early 1900s. This work led to the development of government-sanctioned methadone clinics across America and to the realization that long-term recovery was possible with medication, even without a lengthy hospital stay. For this revolutionary work on opiate addiction, Dr. Dole won the prestigious Lasker Award in 1988.
The major reason for the success of methadone was that, because of its pharmacokinetic profile, it could stabilize the patient through once-daily dosing without sedation or narcosis. As noted by Dr. Dole, once patients are on a stable dosing regimen, the obsessive preoccupation with drug use fades away.5
Despite its success, methadone maintenance had its share of detractors. It was fraught with controversy because it was viewed as a crutch, and those who were on it were often not considered by their abstinent peers as being in true recovery. The reasons for the negative attitudes toward MAT are unclear but may reflect antiquated beliefs that addiction may be indicative of a failure of morals or will, and that patients ought to be able to simply stop using.
Whatever the reason for the animosity surrounding MAT, it should be noted that an expert consensus panel convened by the Betty Ford Center in 2007 agreed that patients on MAT met their consensus definition of sobriety.6 The issue of what constitutes recovery remains a very complex and hotly debated topic that is beyond the scope of this paper and that has been discussed elsewhere.6,7
For more than 3 decades, methadone was the only medication available for MAT. Federal regulations limit the dispensing of methadone to licensed clinics, most of which are located in major metropolitan areas. Patients must go to the clinic every day to receive their dose of methadone—a major inconvenience, especially to those with transportation issues. Adding to the lack of appeal of methadone maintenance is that the clinics are typically located in the higher-crime areas of cities. Savvy drug dealers know the location of these clinics and often loiter on nearby street corners in an attempt to lure addicts away from recovery by flaunting their illicit drugs.
A final, very significant drawback of methadone is its safety profile. It is a full-agonist narcotic that can be fatal in overdose or in the induction phase, especially if taken with other drugs, such as benzodiazepines.
2003: BUPRENORPHINE-NALOXONE IS APPROVED
Such concerns led researchers to search for other medications to be used for MAT that could perhaps be prescribed in a typical outpatient physician practice. For many reasons, buprenorphine became the most promising candidate. In 2003, the US Food and Drug Administration approved the combination medication buprenorphine-naloxone (Sub-oxone) as only the second drug indicated for maintenance treatment of opioid dependence in the United States.
Buprenorphine differs from methadone in that it is a partial agonist at mu opiate receptors, and therefore has a “ceiling” or “plateau” effect in terms of dose-response and a much improved safety profile. Unlike methadone, buprenorphine can be prescribed in a doctor’s office and does not have to be dispensed at a government-approved clinic.
Unfortunately, buprenorphine-maintained patients seem to carry the same stigma in the recovery community as those maintained on methadone—that they are simply substituting one drug for another. Detractors usually fail to consider that, as with methadone, patients do not report getting “high” from taking buprenorphine. Patients will often state that when they first start taking it, they “feel something,” but after a few days of adjustment, they simply feel normal. They don’t feel high, they are no longer in withdrawal, their cravings are virtually eliminated, and their opiate receptors are effectively occupied and blocked, so there is no “high” in the event of a relapse.
What’s more, buprenorphine is not a medication that will help them deal with life’s stressors by “chemical coping.” Sober coping is a skill they must learn by actively participating in a solid 12-step-based recovery program and, in some cases, in psychotherapy. By removing the drug obsession, buprenorphine promotes and facilitates the important recovery goal of learning how to deal with life on life’s terms.
ADDICTION AS CHRONIC ILLNESS
Outcomes studies of addiction treatment have focused largely on rates of relapse after discharge from acute treatments such as residential rehabilitation, partial hospitalization, and intensive outpatient programs. With MAT, however, outcomes research has primarily looked at the duration of retention in treatment.
The change in focus between the two types of treatment coincides with a paradigm shift that views addiction as a chronic condition that requires ongoing care. Continued participation in prescribed care with demonstrated efficacy is considered to be the major indicator of success. Under the chronic illness model employed by MAT providers, if a patient reverted to briefly using a drug of abuse, this would be an issue to address in his ongoing treatment and would not necessarily indicate treatment failure as with the acute care model. Beyond retention rates, research has demonstrated that MAT with methadone results in reductions in rates of criminal activity, illicit drug use, acquisition of human immunodeficiency virus, and overall mortality.8–10
In outcomes studies, MAT has repeatedly shown better efficacy than abstinence-based approaches. During the first 5 years of its implementation, in 4,000 patients, methadone maintenance boasted 1-year retention rates exceeding 98%.11 Over the subsequent 3 years, with the number of patients approaching 35,000, the 1-year retention rates fell to around 60%—still far exceeding results of abstinence-based treatment and approximating the number cited in most modern studies.11
The retention rates in buprenorphine programs are similarly promising. Studies of 12 to 13 weeks duration have shown retention rates of 52% to 79%.12–15 Six-month studies have demonstrated retention rates of 43% to 100%.16–19 Another study showed that 38% of opiate-dependent patients remained in treatment with buprenorphine at 5 years.20 Surprisingly, most of the buprenorphine studies have been conducted in office-based practices, which are less structured than outpatient methadone programs.
MEDICATION-ASSISTED TREATMENT IS GAINING ACCEPTANCE
Data from decades of experience with MAT strongly support the conclusion that it is superior to abstinence-based approaches.
The importance of a patient staying in treatment cannot be overemphasized, as the consequence of failing in recovery may well be an early death. On average, heroin addicts lose about 18 years of life expectancy, and the mortality rate for injection users is roughly 2% per year.21 The mortality rate for heroin users is 6 to 20 times greater than for age-matched peers who are not drug users.22
As high as these numbers are, they are even higher for abusers of prescription narcotics. The annual death rate associated with opioid pain relievers (4.8 per 100,000) is nearly double that associated with illicit drugs (2.8 per 100,000).23
The recent and rather radical change in treatment philosophy by Hazelden came as a shock to some, a disappointment to others, and a welcome change to many who saw this as a move by one of the more respected treatment centers in the country to fall in line with the body of evidence that supports MAT for those suffering from opiate dependence. It remains a mystery why so many, if not most, addiction treatment centers in the United States cling to the abstinence-based philosophy despite the overwhelming data from decades of research and experience that show that abstinence does not work for the majority of opiate addicts.
Complete abstinence from opiate drugs of abuse and potentially addictive medications is a noble but perhaps unreachable goal for many sufferers. Hazelden’s announced acceptance of MAT gives credence to the value of recovery goals that are not entirely drug-free.
Dr. Dole was correct in stating that opiate addicts usually return to drugs if not provided with MAT. Treatment programs need to inform opiate-dependent patients that abstinence-based treatment offers only a 1 in 10 chance of success. Perhaps some patients, armed with the daunting statistics regarding abstinence, will be inspired to devote themselves wholeheartedly to their recovery in an effort to make it into that elite 10% group that achieves long-lasting recovery without the aid of medications. But for the other 90%, it is encouraging to hear that Hazelden, the model treatment center for most abstinence-based programs in this country, may now lead other abstinence-based centers to reconsider their treatment philosophies.
Historically, US doctors were not allowed by federal law to prescribe opiates for addiction treatment. With the passage of DATA 2000, buprenorphine (alone or in combination with naloxone) can be prescribed for addiction treatment only by providers who obtain a waiver from the US Drug Enforcement Administration (DEA). Any doctor can become qualified to prescribe buprenorphine or buprenorphinenaloxone after completing an 8-hour online training course (available at www.buppractice.com and at www.aaap.org/buprenorphine) and by obtaining a DATA 2000 waiver and a new prescribing number from the DEA. Doctors are initially limited to treating only 30 patients with buprenorphine-naloxone at any given time, but can apply for an extension to 100 patients after having had their waiver for 1 year.
As MAT continues to gain favor, demand will grow for more providers to obtain their waivers to prescribe buprenorphine and buprenorphine-naloxone. Historically, there have always been too few methadone clinics to meet the demand. One can hope that the growing number of waivered providers will greatly improve access to care by opiate addicts, no matter where they reside. Qualified prescribers of buprenorphine and buprenorphine-naloxone are limited by the federal restrictions on the numbers of patients they can treat. If the chronic disease of addiction is to be integrated into the continuing-care approach of modern medicine and managed alongside other chronic diseases, primary care providers who are not specialized in treating addiction will need to be become comfortable with maintaining patients on buprenorphine-naloxone.7 Presumably, such patients will have already been stabilized through participation in addiction treatment programs in their respective geographic areas. Primary care providers will need to develop relationships with local addictionologists and treatment programs so that they will be able to refer those in active addiction for induction and stabilization on MAT and will be able to refer those already stabilized on MAT back to such specialists when relapses occur.
We may finally be approaching a time when structured residential treatment and MAT are not mutually exclusive options for our patients. These treatment options must work together for optimal outcomes. Based on our experience with hundreds of patients at Cleveland Clinic’s Alcohol and Drug Recovery Center, we believe this change of treatment philosophy is long overdue. In clinical settings, patients do not fit cleanly into one treatment arm or another and often require a blended approach to effect long-lasting change. Hazelden’s shift of treatment philosophy is an indication that this research-supported viewpoint is gaining acceptance in the traditionally drug-free halls of addiction treatment programs.
- White WL. Slaying the Dragon. The History of Addiction Treatment and Recovery in America. Bloomington, IL: Chestnut Health Systems/Lighthouse Institute; 1998:124–125,201.
- Kosten TR, Gorelick DA. The Lexington narcotic farm. Am J Psychiatry 2002; 159:22.
- Hunt GH, Odoroff ME. Followup study of narcotic drug addicts after hospitalization. Public Health Rep 1962; 77:41–54.
- Dole VP. Narcotic addiction, physical dependence and relapse. N Engl J Med 1972; 286:988–992.
- Dole VP. Implications of methadone maintenance for theories of narcotic addiction. JAMA 1988; 260:3025–3029.
- Betty Ford Institute Consensus Panel. What is recovery? A working definition from the Betty Ford Institute. J Subst Abuse Treat 2007; 33:221–228.
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction 2002; 97:249–252.
- Grönbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand 1990; 82:223–227.
- Ball JC, Lange WR, Myers CP, Friedman SR. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav 1988; 29:214–226.
- Martin J, Zweben JE, Payte JT. Opioid maintenance treatment. In:Ries RK, Fiellin DA, Miller SC, Saitzeds R, editors. Principles of Addiction Medicine. 4th ed. Philadelphia, PA: Lippincottt Williams & Wilkins, 2009:671–688.
- Dole VP, Nyswander ME. Methadone maintenance treatment. A tenyear perspective. JAMA 1976; 235:2117–2119.
- Cunningham C, Giovanniello A, Sacajiu G, et al. Buprenorphine treatment in an urban community health center: what to expect. Fam Med 2008; 40:500–506.
- Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002; 28:231–241.
- Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003; 349:949–958.
- O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med 1998; 105:100–105.
- Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med 2006; 355:365–374.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med 2007; 5:146–150.
- O’Connor PG, Oliveto AH, Shi JM, et al. A pilot study of primary-carebased buprenorphine maintenance for heroin dependence. Am J Drug Alcohol Abuse 1996; 22:523–531.
- Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict 2008; 17:116–120.
- Smyth B, Hoffman V, Fan J, Hser YI. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med 2007; 44:369–374.
- Sporer KA. Acute heroin overdose. Ann Intern Med 1999; 130:584–590.
- Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60:1487–1492.
- White WL. Slaying the Dragon. The History of Addiction Treatment and Recovery in America. Bloomington, IL: Chestnut Health Systems/Lighthouse Institute; 1998:124–125,201.
- Kosten TR, Gorelick DA. The Lexington narcotic farm. Am J Psychiatry 2002; 159:22.
- Hunt GH, Odoroff ME. Followup study of narcotic drug addicts after hospitalization. Public Health Rep 1962; 77:41–54.
- Dole VP. Narcotic addiction, physical dependence and relapse. N Engl J Med 1972; 286:988–992.
- Dole VP. Implications of methadone maintenance for theories of narcotic addiction. JAMA 1988; 260:3025–3029.
- Betty Ford Institute Consensus Panel. What is recovery? A working definition from the Betty Ford Institute. J Subst Abuse Treat 2007; 33:221–228.
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction 2002; 97:249–252.
- Grönbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand 1990; 82:223–227.
- Ball JC, Lange WR, Myers CP, Friedman SR. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav 1988; 29:214–226.
- Martin J, Zweben JE, Payte JT. Opioid maintenance treatment. In:Ries RK, Fiellin DA, Miller SC, Saitzeds R, editors. Principles of Addiction Medicine. 4th ed. Philadelphia, PA: Lippincottt Williams & Wilkins, 2009:671–688.
- Dole VP, Nyswander ME. Methadone maintenance treatment. A tenyear perspective. JAMA 1976; 235:2117–2119.
- Cunningham C, Giovanniello A, Sacajiu G, et al. Buprenorphine treatment in an urban community health center: what to expect. Fam Med 2008; 40:500–506.
- Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002; 28:231–241.
- Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003; 349:949–958.
- O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med 1998; 105:100–105.
- Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med 2006; 355:365–374.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med 2007; 5:146–150.
- O’Connor PG, Oliveto AH, Shi JM, et al. A pilot study of primary-carebased buprenorphine maintenance for heroin dependence. Am J Drug Alcohol Abuse 1996; 22:523–531.
- Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict 2008; 17:116–120.
- Smyth B, Hoffman V, Fan J, Hser YI. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med 2007; 44:369–374.
- Sporer KA. Acute heroin overdose. Ann Intern Med 1999; 130:584–590.
- Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60:1487–1492.
KEY POINTS
- Recidivism rates are high after detoxification without medication-assisted treatment.
- Whether staying in a maintenance program truly constitutes recovery continues to be debated, but patients on methadone or buprenorphine maintenance do not report getting “high”—they merely feel normal.
- Methadone is dispensed only in special clinics, whereas buprenorphine can be prescribed by a physician. Prescribing physicians must complete an 8-hour course online at www.buppractice.com or www.aaap.org/buprenorphine and obtain a waiver from the US Drug Enforcement Administration.
- With or without medication-assisted treatment, recovering addicts must learn the skill of sober coping by actively participating in a solid 12-step-based program and, in some cases, in psychotherapy.
Applied molecular profiling: evidence-based decision-making for anticancer therapy
Applied molecular profiling is a method for helping clinicians select the most appropriate therapy for a patient with cancer by determining the level of gene and/or protein expression within the cancer and comparing that expression pattern with the expression profiles of cancers with known outcomes. This approach facilitates the development and selection of tumor-specific therapies based on the identification of biomarkers within a tumor. Molecular characterization techniques such as immunohistochemistry, microarray analysis, and fluorescence in situ hybridization have facilitated identification and validation of a number of important solid tumor biomarkers, including HER2/neu, EGFR, EML4/ALK, and KIT, and can also be used to identify biomarkers (eg, BCR-ABL, CD20, CD30) in various hematologic malignancies. It is of note that molecular profiling can be used to identify targets in tumors for which a therapeutic agent may already be available, thus avoiding the administration of an unproven investigational agent. As the field of molecular profiling continues to evolve and next-generation techniques such as exome sequencing – sequencing 1% of the genome – and whole gene sequencing gain currency, biomarker identification and analysis will become less expensive and more efficient, and possibly allow for a pathway-oriented approach to treatment selection. Wider acceptance and use of molecular profiling should therefore help practicing physicians and oncology researchers keep pace with advances in the understanding of oncogenic expression in various malignancies and encourage the use of molecular profiling in earlier stages of cancer rather than as an option of last resort...
*Click on the link to the left for a PDF of the full article.
Applied molecular profiling is a method for helping clinicians select the most appropriate therapy for a patient with cancer by determining the level of gene and/or protein expression within the cancer and comparing that expression pattern with the expression profiles of cancers with known outcomes. This approach facilitates the development and selection of tumor-specific therapies based on the identification of biomarkers within a tumor. Molecular characterization techniques such as immunohistochemistry, microarray analysis, and fluorescence in situ hybridization have facilitated identification and validation of a number of important solid tumor biomarkers, including HER2/neu, EGFR, EML4/ALK, and KIT, and can also be used to identify biomarkers (eg, BCR-ABL, CD20, CD30) in various hematologic malignancies. It is of note that molecular profiling can be used to identify targets in tumors for which a therapeutic agent may already be available, thus avoiding the administration of an unproven investigational agent. As the field of molecular profiling continues to evolve and next-generation techniques such as exome sequencing – sequencing 1% of the genome – and whole gene sequencing gain currency, biomarker identification and analysis will become less expensive and more efficient, and possibly allow for a pathway-oriented approach to treatment selection. Wider acceptance and use of molecular profiling should therefore help practicing physicians and oncology researchers keep pace with advances in the understanding of oncogenic expression in various malignancies and encourage the use of molecular profiling in earlier stages of cancer rather than as an option of last resort...
*Click on the link to the left for a PDF of the full article.
Applied molecular profiling is a method for helping clinicians select the most appropriate therapy for a patient with cancer by determining the level of gene and/or protein expression within the cancer and comparing that expression pattern with the expression profiles of cancers with known outcomes. This approach facilitates the development and selection of tumor-specific therapies based on the identification of biomarkers within a tumor. Molecular characterization techniques such as immunohistochemistry, microarray analysis, and fluorescence in situ hybridization have facilitated identification and validation of a number of important solid tumor biomarkers, including HER2/neu, EGFR, EML4/ALK, and KIT, and can also be used to identify biomarkers (eg, BCR-ABL, CD20, CD30) in various hematologic malignancies. It is of note that molecular profiling can be used to identify targets in tumors for which a therapeutic agent may already be available, thus avoiding the administration of an unproven investigational agent. As the field of molecular profiling continues to evolve and next-generation techniques such as exome sequencing – sequencing 1% of the genome – and whole gene sequencing gain currency, biomarker identification and analysis will become less expensive and more efficient, and possibly allow for a pathway-oriented approach to treatment selection. Wider acceptance and use of molecular profiling should therefore help practicing physicians and oncology researchers keep pace with advances in the understanding of oncogenic expression in various malignancies and encourage the use of molecular profiling in earlier stages of cancer rather than as an option of last resort...
*Click on the link to the left for a PDF of the full article.
Biomarkers of small intestinal mucosal damage induced by chemotherapy: an emerging role for the 13C sucrose breath test
Gastrointestinal mucosal toxicity is extremely common following cytotoxic therapies. The alimentary mucosa is particularly susceptible to injury and dysfunction, leading to many debilitating complications. Despite much research, there is currently no single noninvasive biomarker to detect gut injury. Several biomarkers have been investigated in the context of gastrointestinal diseases, which may prove useful in the oncology arena. Identification of a biomarker that is easy to obtain and measure and that accurately identifies mucosal damage would allow for improved patient diagnosis of toxicities and for personalized treatment regimens. In this review, we highlight the effectiveness of urine and breath tests as potential clinically effective biomarkers, with significant focus placed on the emerging role of the carbon-13 sucrose breath test (13C SBT). The 13C SBT provides a simple, noninvasive, and integrated measure of gut function. The 13C SBT also has the potential to monitor gut function in the setting of cytotoxic therapy–induced mucositis, or in the assessment of the efficacy of antimucositis agents.
*For a PDF of the full article, click on the link to the left of this introduction.
Gastrointestinal mucosal toxicity is extremely common following cytotoxic therapies. The alimentary mucosa is particularly susceptible to injury and dysfunction, leading to many debilitating complications. Despite much research, there is currently no single noninvasive biomarker to detect gut injury. Several biomarkers have been investigated in the context of gastrointestinal diseases, which may prove useful in the oncology arena. Identification of a biomarker that is easy to obtain and measure and that accurately identifies mucosal damage would allow for improved patient diagnosis of toxicities and for personalized treatment regimens. In this review, we highlight the effectiveness of urine and breath tests as potential clinically effective biomarkers, with significant focus placed on the emerging role of the carbon-13 sucrose breath test (13C SBT). The 13C SBT provides a simple, noninvasive, and integrated measure of gut function. The 13C SBT also has the potential to monitor gut function in the setting of cytotoxic therapy–induced mucositis, or in the assessment of the efficacy of antimucositis agents.
*For a PDF of the full article, click on the link to the left of this introduction.
Gastrointestinal mucosal toxicity is extremely common following cytotoxic therapies. The alimentary mucosa is particularly susceptible to injury and dysfunction, leading to many debilitating complications. Despite much research, there is currently no single noninvasive biomarker to detect gut injury. Several biomarkers have been investigated in the context of gastrointestinal diseases, which may prove useful in the oncology arena. Identification of a biomarker that is easy to obtain and measure and that accurately identifies mucosal damage would allow for improved patient diagnosis of toxicities and for personalized treatment regimens. In this review, we highlight the effectiveness of urine and breath tests as potential clinically effective biomarkers, with significant focus placed on the emerging role of the carbon-13 sucrose breath test (13C SBT). The 13C SBT provides a simple, noninvasive, and integrated measure of gut function. The 13C SBT also has the potential to monitor gut function in the setting of cytotoxic therapy–induced mucositis, or in the assessment of the efficacy of antimucositis agents.
*For a PDF of the full article, click on the link to the left of this introduction.
Predicting life expectancy in patients with advanced incurable cancer: a review
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
ABSTRACT
Oncologists frequently face the difficult task of estimating prognosis in patients with incurable malignancies. Their prediction of prognosis informs decision-making ranging from recommendations of cancer treatments to hospice enrollment. Unfortunately, physicians’ estimates of prognosis are often inaccurate and overly optimistic. Further, physicians often fail to disclose their prognosis estimates, despite patient wishes to the contrary. Several studies have examined patient factors that might improve physicians’ prognostic accuracy, including performance status, clinical symptoms and laboratory values. Prognostic models have been developed and validated but, to date, none are able to provide accurate estimates throughout the spectrum of advanced illness. This review examines tools utilized to predict life expectancy for patients with advanced, incurable cancer.
*For a PDF of the full article, click on the link to the left of this introduction.
Resistance exercise interventions during and following cancer treatment: a systematic review
Findings from prior systematic reviews suggest that exercise results in meaningful improvements in many clinically relevant physiologic and quality of life (QoL) outcomes during and following cancer treatment. However, the majority of exercise-cancer studies have focused upon the benefits of aerobic exercise (AE) and knowledge of the efficacy of resistance exercise (RE) alone as a supportive care intervention for cancer patients and survivors remains limited. Consequently, the purpose of this review was to provide the first systematic evaluation of the effects of RE alone upon clinically relevant physiologic and QoL outcomes during and following cancer treatment. Literature searches were conducted to identify studies examining RE interventions in cancer patients and survivors. Data were extracted on physiologic (fitness, physical function, and body composition) and QoL (fatigue, psychological well-being, and cancer-specific and global QoL outcomes. Cohen’s d effect sizes were calculated for each outcome. A total of 15 studies (6 in samples undergoing active cancer treatment and 9 in samples having completed cancer treatment) involving 1,077 participants met the inclusion criteria. Findings revealed that, on average, RE resulted in large effectsize improvements in muscular strength (d 0.86), moderate effect-size improvements in physical function (d 0.66), and small effect-size improvements in body composition (d 0.28) and QoL (d 0.25) outcomes. The effect sizes observed following RE are comparable in magnitude to the effects of exercise interventions reported in prior comprehensive reviews of the exercise cancer literature which primarily focused upon AE. Additionally, the methodologic quality of the studies was generally strong. Taken collectively, results of this systematic review suggest that RE is a promising supportive care intervention that results in meaningful improvements in clinically relevant physiologic and QoL outcomes during and following cancer treatment.
Click on the PDF icon at the top of this introduction to read the full article.
Findings from prior systematic reviews suggest that exercise results in meaningful improvements in many clinically relevant physiologic and quality of life (QoL) outcomes during and following cancer treatment. However, the majority of exercise-cancer studies have focused upon the benefits of aerobic exercise (AE) and knowledge of the efficacy of resistance exercise (RE) alone as a supportive care intervention for cancer patients and survivors remains limited. Consequently, the purpose of this review was to provide the first systematic evaluation of the effects of RE alone upon clinically relevant physiologic and QoL outcomes during and following cancer treatment. Literature searches were conducted to identify studies examining RE interventions in cancer patients and survivors. Data were extracted on physiologic (fitness, physical function, and body composition) and QoL (fatigue, psychological well-being, and cancer-specific and global QoL outcomes. Cohen’s d effect sizes were calculated for each outcome. A total of 15 studies (6 in samples undergoing active cancer treatment and 9 in samples having completed cancer treatment) involving 1,077 participants met the inclusion criteria. Findings revealed that, on average, RE resulted in large effectsize improvements in muscular strength (d 0.86), moderate effect-size improvements in physical function (d 0.66), and small effect-size improvements in body composition (d 0.28) and QoL (d 0.25) outcomes. The effect sizes observed following RE are comparable in magnitude to the effects of exercise interventions reported in prior comprehensive reviews of the exercise cancer literature which primarily focused upon AE. Additionally, the methodologic quality of the studies was generally strong. Taken collectively, results of this systematic review suggest that RE is a promising supportive care intervention that results in meaningful improvements in clinically relevant physiologic and QoL outcomes during and following cancer treatment.
Click on the PDF icon at the top of this introduction to read the full article.
Findings from prior systematic reviews suggest that exercise results in meaningful improvements in many clinically relevant physiologic and quality of life (QoL) outcomes during and following cancer treatment. However, the majority of exercise-cancer studies have focused upon the benefits of aerobic exercise (AE) and knowledge of the efficacy of resistance exercise (RE) alone as a supportive care intervention for cancer patients and survivors remains limited. Consequently, the purpose of this review was to provide the first systematic evaluation of the effects of RE alone upon clinically relevant physiologic and QoL outcomes during and following cancer treatment. Literature searches were conducted to identify studies examining RE interventions in cancer patients and survivors. Data were extracted on physiologic (fitness, physical function, and body composition) and QoL (fatigue, psychological well-being, and cancer-specific and global QoL outcomes. Cohen’s d effect sizes were calculated for each outcome. A total of 15 studies (6 in samples undergoing active cancer treatment and 9 in samples having completed cancer treatment) involving 1,077 participants met the inclusion criteria. Findings revealed that, on average, RE resulted in large effectsize improvements in muscular strength (d 0.86), moderate effect-size improvements in physical function (d 0.66), and small effect-size improvements in body composition (d 0.28) and QoL (d 0.25) outcomes. The effect sizes observed following RE are comparable in magnitude to the effects of exercise interventions reported in prior comprehensive reviews of the exercise cancer literature which primarily focused upon AE. Additionally, the methodologic quality of the studies was generally strong. Taken collectively, results of this systematic review suggest that RE is a promising supportive care intervention that results in meaningful improvements in clinically relevant physiologic and QoL outcomes during and following cancer treatment.
Click on the PDF icon at the top of this introduction to read the full article.
MDS: What Do Hospitalists Need to Know?
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal hematopoietic stem cell neoplasms characterized by dysplasia, ineffective hematopoiesis resulting in peripheral blood (PB) cytopenias affecting one or more cell lines, and a variable risk of progression to acute myeloid leukemia (AML). The last 15 years have witnessed significant advances in our understanding of the complex pathogenesis, classification and prognostication, and therapeutic approaches to MDS. As more elderly patients are diagnosed with MDS, encounters with hospitalized MDS patients or patients in whom MDS should be considered in the differential diagnosis are common events for today's hospitalists. In this review, we discuss the epidemiology, diagnosis, pathogenesis, prognostication, and therapies for MDS, with an emphasis on practical aspects that would be useful for hospitalists caring for these patients.
EPIDEMIOLOGY OF MDS
Although MDS is one of the most common hematologic malignancies, MDS remains understudied epidemiologically.[1, 2] Our understanding of the epidemiology improved after the implementation of reporting requirements to cancer registries, especially the Surveillance, Epidemiology, and End Results (SEER) database in 2001.[1, 2, 3] Age‐adjusted incidence of MDS in the United States ranged between 3.3 to 4.6 per 100,000 persons per year in the period between 2001 and 2008.[1, 2, 4] The majority of MDS patients are elderly, and because MDS incidence increases with age, the number of patients diagnosed with MDS is expected to continue to rise with the aging population.[1, 2, 5] MDS is more common in men compared to women, and in Caucasians compared to African Americans.[1, 2] Different estimates put MDS prevalence in the United States somewhere between 60,000 and 170,000 persons.[2, 6]
DIAGNOSIS OF MDS
Many patients with MDS are asymptomatic at diagnosis and only come to medical attention due to abnormal blood counts done routinely or for other reasons. This contributes to MDS being underdiagnosed. When cytopenias are not severe enough to cause symptoms, it is also frequently overlooked in patients with mild anemia or other cytopenias.[7] Together, being asymptomatic and having relatively mild cytopenias are probably the most important factors that lead to under‐recognition of MDS among primary care physicians (PCPs).[7, 8, 9] There is a misconception that anemia is normal in the elderly, and when patients are not symptomatic that a workup is not needed.[6, 7] This is compounded by a lack of awareness of the importance of making a diagnosis in these patients and of currently available therapies for MDS.[7, 8, 9]
Anemia is not a normal consequence of aging and is always a pathologic state with an underlying etiology.[6, 7] Because a significant number of elderly patients with unexplained anemia could have MDS, patients with symptomatic or progressive anemia, especially if associated with other cytopenias, should be considered for further evaluation.[7, 9] Diagnosis is important given the recent availability of effective therapies for MDS that can improve anemia, decrease transfusion needs, improve life quality, and potentially increase survival. MDS is generally an indolent disease with a relative stability of blood counts in comparison to AML, so prior blood counts and the tempo of the process is an important consideration.[9, 10] The National Comprehensive Cancer Network clinical practice guidelines recommend exclusion of nutritional deficiencies (iron, vitamin B12, folate) and other causes of anemia (eg, gastrointestinal bleeding, renal insufficiency, and anemia of inflammation), assessment of reticulocyte count and serum erythropoietin level, and evaluation of a PB smear for evidence of dysplasia as important initial steps.[10, 11] Eventually the diagnosis of MDS requires a bone marrow (BM) evaluation to confirm the diagnosis and exclude other BM failure states by evaluating for BM cellularity, cell maturation, dysplasia (which should be present in at least 10% of any the myeloid lineages), percentage of blasts (20%), iron stores and sideroblasts, cytogenetics, MDS‐specific fluorescence in situ hybridization (FISH) panels, flow cytometry, and other special testing.[9, 10] Despite extensive testing, MDS can sometimes be very difficult to differentiate from other bone marrow failure states (eg, hypoplastic MDS from aplastic anemia) (Table 1).[10, 11] In the absence of significant morbidity related to MDS, the definitive diagnosis of MDS can be usually made on an outpatient basis. It is important to ensure adequate follow‐up with PCPs postdischarge and/or outpatient hematologist referral for patients with unexplained cytopenias.
|
| Idiopathic cytopenia of undetermined significance: no significant dysplasia or MDS‐associated karyotypic aberrations |
| Acute myeloid leukemia: BM blasts 20%, presence of core‐binding characteristic cytogenetic aberrations: t(8;21), t(15;17), inv(16) defines AML regardless of BM blast count; AML can be associated with hepatosplenomegaly or myeloid sarcomas |
| Chronic myeloid leukemia: presence of Philadelphia chromosome t(9;22) positive, basophilia, and splenomegaly |
| Myelofibrosis: significant BM fibrosis, splenomegaly, and leukoerythroblastic picture in PB (teardrop and nucleated RBCs, left‐shifted myeloid cells) |
| Chronic myelomonocytic leukemia: significant PB monocytosis |
| MDS/MPN overlap syndromes: dysplasia with myeloproliferative characteristics such as splenomegaly, thrombocytosis, or leukocytosis |
| Infections: for example, HIV and parvovirus B19 infections |
| Myelophthisis: infiltration of BM with other tumors (eg, melanoma) with resultant PB cytopenias |
| Nutritional disturbances: B12, folate, and copper deficiency, and zinc and arsenic excess can mimic MDS |
| Medications: drugs that interfere with DNA synthesis such as HIV medications, chemotherapeutic agents, cotrimoxazole, methotrexate, azathioprine, and G‐CSF |
| Immune disorders: for example, LGL leukemia, lupus, or rheumatoid arthritis |
| Other acquired or congenital hematological disorders: for example, paroxysmal nocturnal hemoglobinuria, congenital dyserythropoietic anemia, dyskeratosis congenita |
PATHOGENESIS AND ETIOLOGY OF MDS
Ineffective hematopoiesis due to excessive apoptosis of hematopoietic precursors is a prominent feature of MDS, which explains the apparent paradox of hypercellular BM and PB cytopenias. Although not fully understood, complex epigenetic, genetic, and immunologic mechanisms contribute to the pathogenesis of MDS and account for disease heterogeneity. Aberrant silencing of tumor‐suppressor and DNA repair genes mediated by hypermethylation of their promoters is believed to play an important part in the pathogenesis of MDS.[12] This theory is supported by the unique sensitivity of MDS to drugs that reverse DNA methylation. Genetic abnormalities not only contribute to the pathogenesis of MDS, but are also among the strongest prognostic indicators for MDS patients, and can also affect therapeutic decisions. Clonal karyotypic abnormalities are observed in 50% of patients with MDS using conventional karyotyping.[12, 13] The most common chromosomal aberrations in MDS include deletions of the long arm of chromosome 5 (del5q), monosomy Y, monosomy 7 (del7) or deletion of its long arm (del7q), trisomy 8, del20q, and complex karyotypes (3 chromosomal aberrations).[12, 13] These cytogenetic abnormalities correlate with the prognosis of MDS (eg, poor prognosis with complex karyotypes and chromosome 7 deletions vs better prognosis with isolated del5q).[12, 13]
Recently, FISH assays and genome‐wide screening techniques (eg, single nucleotide polymorphism arrays, array‐based comparative genomic hybridization, whole genome or exome sequencing) have enabled detection of an increasing number of genetic aberrations and recurrent somatic molecular abnormalities in a significant number of MDS patients (eg, abnormalities of ASXL1, IDH1/IDH2, DNMT3, EZH2, TET2, and SF3B1 genes).[12, 14] Most affected genes are involved in the epigenetic regulation of transcription (DNA methylation and demethylation, histone posttranslational modification) or mRNA splicing.[12, 13, 14]
Immunologic aberrations have also been proposed to contribute to pathogenesis of MDS. For example, in early‐stage MDS, an aberrant immune attack on myeloid progenitors resulting in increased apoptosis can contribute to BM failure.[15] This is supported by association of some forms of MDS with autoimmune diseases and observed responses in some patients to immunosuppressive therapies. The relative contribution of pathogenetic mechanisms varies between the different MDS subtypes. For example, haploinsufficiency of cell‐cycle regulatory and ribosomal protein genes located in the commonly deleted region of 5q play an important role in the pathogenesis of MDS with isolated del5q (5q syndrome).[16] Mutations in the RNA spliceosomal machinery gene SF3B have been shown to play a role in the pathogenesis of the MDS subtype refractory anemia with ringed sideroblasts (RARS), with those patients with RARS carrying this mutation having a more favorable prognosis than those with the wild‐type gene.[14] Several excellent recent reviews provide detailed discussion of the complex pathophysiology of MDS.[12, 13, 14, 17]
Approximately 10% of MDS patients have secondary MDS (MDS occurring after chemotherapy or radiation therapy administration for treatment of another malignancy).[2] Aside from advancing age, the causative factors for the other 90% of cases (primary MDS) are unknown in most patients, although environmental and occupational exposures (eg, smoking, painting, insecticides, pesticides, organic solvents), and genetic syndromes (eg, DNA repair defects such as Fanconi's anemia) are implicated in some patients.[2, 10] Recently, an epidemiologic study found an increased MDS risk with obesity.[18]
PROGNOSTICATION OF MDS
MDS is a form of cancer, and most affected patients eventually die from cytopenic complications or leukemic progression. MDS is not a single disease but rather encompasses a group of heterogeneous subtypes with significantly different natural histories and pace of progression. Therefore, accurate risk stratification of MDS is necessary not only to predict survival and risk of leukemic progression, but also to help choose the most appropriate therapeutic option for individual patients. Information about prognosis should also be utilized when making management decisions with patients for other comorbid conditions (eg, major surgery). Two morphologically based classification systems are commonly used for MDS: the French‐American‐British (FAB) system and the World Health Organization (WHO) classification (Table 2), which most recently has supplanted the FAB system as the primary pathologic classification system.[19, 20, 21] Several prognostic models have been developed around the morphologic classifications to better account for relevant clinical and cytogenetic modifiers of this disease. Although some of these models have been validated by different groups, each of these models has limitations. Although the predictions generated by these models are generally accurate for the different prognostic categories to which the patient is assigned, the extent to which the prediction applies to an individual MDS patient can vary significantly. In addition, comorbid conditions affect survival of MDS patients and are not included in the specific scoring systems. For example, congestive heart failure and chronic obstructive lung disease were associated with shortened survival in MDS patients.[18]
| MDS WHO Class | PB Findings | BM Findings |
|---|---|---|
| ||
| Refractory cytopenias with unilineage dysplasia: includes refractory anemia; refractory neutropenia; refractory thrombocytopenia | Unicytopenia or bicytopenia; PB blasts 1% | BM blasts 5%; unilineage dysplasia (10% of cells in any myeloid lineage); 15% of erythroid precursors are ringed sideroblasts |
| Refractory anemia with ring sideroblasts | Anemia; PB blasts 1% | BM blasts 5%; erythroid dysplasia only; 15% of erythroid precursors are ringed sideroblasts |
| Refractory cytopenia with multilineage dysplasia | Cytopenia(s); PB blasts 1%; no Auer rods; 1 106/L monocytes | BM blasts 5% ; dysplasia (10% of cells in at least 2 myeloid lineages); no Auer rods |
| Refractory anemia with excess blasts‐1 | Cytopenia(s); PB blasts 5%; no Auer rods; 1 106/L monocytes | BM blasts 5%9%; unilineage or multilineage dysplasia; no Auer rods |
| Refractory anemia with excess blasts‐2 | Cytopenia(s); PB blasts 5%19%; Auer rods; 1 106/L monocytes | BM blasts 10%19%; unilineage or multilineage dysplasia; Auer rods |
| Myelodysplastic syndromeunclassified | Cytopenias; PB blasts 1% | BM blasts 5%; unequivocal dysplasia in 10% of cells at least one myeloid cell lines when accompanied by a cytogenetic abnormality considered as presumptive evidence for a diagnosis of MDS |
| MDS associated with isolated del5q | Anemia; normal to elevated platelet count; PB blasts 1% | BM blasts 5%; normal to elevated megakaryocytes with hypolobated nuclei; isolated del5q karyotypic abnormality; no Auer rods |
The International Prognostic Scoring System (IPSS) is the most widely used prognostic tool for MDS (Table 3).[22] In this model, an aggregate score is calculated based on points assigned to the percentage of blasts in BM, the number of PB cell lines affected by cytopenias, and the karyotype. Based on this point score, the patient is assigned to 1 of 4 categories that portend significantly different outcomes: low, intermediate‐1 (INT‐1), intermediate‐2 (INT‐2), and high risk. The IPSS was developed from a database of mostly untreated MDS patients and does not account for other important prognostic parameters such as transfusion dependence, depth of cytopenias, and extent/severity of lineage dysplasia.[22] The WHO Prognostic Scoring System was proposed to overcome some of these shortcomings.[23, 24] Efforts to continue to improve the prognostic models further led to a large international collaboration that compiled a much larger database and resulted in the development of a revised IPSS (IPSS‐R).[25] New discoveries of novel prognostic epigenetic, genetic, and immunologic determinants will likely result in the ongoing evolution of the current prognostic systems to further improve their discriminatory power.[26]
| Calculation of Score Value Based on Prognostic Variables | |||||
|---|---|---|---|---|---|
| Score Value | |||||
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| |||||
| Prognostic variable | |||||
| Bone marrow blasts (%)a | 5 | 510 | 1120 | 2130 | |
| Karyotypeb | Good | Intermediate | Poor | ||
| Number of peripheral blood cell line affected by cytopeniasc | 0 or 1 | 2 or 3 | |||
| Median Survival and Risk of Progression to AML According to the IPSS Risk Category in Absence of Therapy | |||||
| Overall Score | Risk Category | Percentage in the IPSS Population | Median Survival (Years) | Median Time From Diagnosis at Which 25% of Patients Progress to AML (Years) | |
| 0 | Low | 33% | 5.7 | 9.4 | |
| 0.51.0 | INT‐1 | 38% | 3.5 | 3.3 | |
| 1.52.0 | INT‐2 | 22% | 1.1 | 1.1 | |
| >2.5 | High | 7% | 0.4 | 0.2 | |
MANAGEMENT OF MDS
Most patients with MDS were treated historically with supportive measures only. The approval of 3 agents for treatment of MDS including the DNA methyltransferase inhibitors (DNMTi) azacitidine and decitabine, as well as the immunomodulatory agent lenalidomide, in the last decade advanced the care of MDS patients significantly (Table 4). Nonetheless, the use of allogeneic hematopoietic stem cell transplantation (alloHSCT) remains the only known curative modality for patients with MDS and should always be considered as a possible therapeutic option.[27] Unfortunately, the majority of patients with MDS are not considered candidates for alloHSCT due to age, comorbidities, and lack of suitable donors.[27] Therefore, most patients with MDS are managed with noncurative treatment and supportive paradigms. Treatment goals generally depend on the risk stratification for the particular individual, age, functional status, comorbidities, and importantly, the patient's individual preference. For medical decision‐making purposes, MDS is traditionally divided into 2 major risk categories: low‐risk (LR) and high‐risk (HR) groups. LR‐MDS includes the IPSS risk categories of low or INT‐1, whereas HR‐MDS is usually defined by the IPSS risk categories of INT‐2 and high. Newer classification tools (eg, IPSS‐R) and better molecular markers are expected to impact such categories as well as treatment recommendations in the future.[26]
|
| Azacitidine (5‐azacytidine, Vidaza) and decitabine (5‐aza,2‐deoxycytidine, Dacogen) |
| Class |
| Hypomethylating agents, azanucleosides |
| Mechanism of action |
| Epigenetic modulation by inhibition of DNA methyltransferase enzymes and other mechanisms |
| Indication |
| First line therapy for HR‐MDS, second line therapy for LR‐MDS after failure of other therapies such as ESAs, lenalidomide, or immunosuppressive agents |
| Approved regimens for MDS |
| Azacitidine: 75 mg/m2/day IV or SC for 7 days Q 4 weeks |
| Decitabine: 15 mg/m2 IV infusion over 3 hours, Q 8 hours for 3 days, Q 6 weeks or 20 mg/m2 IV infusion over 1 hour daily for 5 days Q 4 weeks |
| Common side effects |
| Fatigue |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
| Oral ulcers and rarely mucositis |
| Injection site reactions (redness, pain) |
| Lenalidomide (Revlimid) |
| Class |
| Immunomodulatory agent |
| Mechanism of action |
| Modulation of immune responses, gene expression, angiogenesis, cytokines and cell‐cycle regulatory phosphatases, and possibly other mechanisms |
| Indication |
| First line therapy for LR‐MDS with del5q (also used commonly off label for LR‐MDS without del5q as second line of therapy after ESAs) |
| Approved regimens for MDS |
| 10 mg orally once daily |
| Common side effects |
| Skin rash, dryness, and pruritus |
| Fatigue |
| Muscle cramps |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
Despite recent advances, supportive care for all patients with MDS remains a very important aspect of management, either in combination with other therapies or as sole therapy for frail patients who cannot tolerate further interventions. Supportive therapy focuses on maintaining a high quality of life and includes careful blood count monitoring, use of growth factors, use of transfusions and antibiotics as needed, and use of iron chelation therapy in some patients. Some of the common situations in which hospitalists encounter patients with MDS are listed in Table 5.
|
| Complications of cytopenias |
| Bleeding: local management based on bleeding site, platelet transfusions, and other blood products (eg, red blood cells, fresh frozen plasma) as appropriate, antifibrinolytics |
| Infections and neutropenic fevers: Antibiotics, antifungals, use of colony granulocyte‐stimulating factors or granulocyte infusions advised only in cases of uncontrolled severe infections or sepsis |
| Severe or symptomatic anemia: red blood transfusions as appropriate based on patient's comorbidities, all disease‐modifying drugs (lenalidomide, azacitidine, decitabine) and ESAs are slow acting and can take weeks to months before improving anemia |
| Complications of therapies |
| Neutropenic fevers: as above plus holding therapy |
| Most other side effects (see Table 4) are well tolerated and are managed symptomatically without requiring hospitalization. If needed hospitalization for side effects: symptomatic management and holding the drug |
| Other medical or surgical condition in a patient with MDS |
| Therapy as per the underlying medical condition. For therapeutic decisions (eg, decision to undergo major surgery), prognostication tools such as the IPSS and newer models can be used to inform medical decision making in consultation with an experienced hematologist |
MANAGEMENT OF LR‐MDS
In addition to supportive care or enrollment in clinical trials, therapies for LR‐MDS include erythropoiesis‐stimulating agents, lenalidomide, and immunosuppressive therapy.
Erythropoiesis‐Stimulating Agents
Anemia in MDS is a multifactorial process that includes ineffective erythropoiesis and suboptimal serum erythropoietin responses.[10, 28, 29] There are no randomized studies to suggest that erythropoiesis‐stimulating agents (ESA) therapy prolongs survival in MDS patients. Nonetheless, ESAs improve anemia significantly in some patients and are widely used.[30, 31] Approximately 20% to 30% of unselected MDS patients and about 40% of LR‐MDS patients achieve clinically meaningful erythroid responses with ESA therapy with a median response duration of 2 years.[30, 31] It is important to correct coexisting nutritional deficiencies (eg, iron or folate deficiency) to optimize responses to ESA.[10] Granulocyte colony‐stimulating factor can be synergistic with ESAs especially in patients with RARS.[10] Patients with LR‐MDS who have low endogenous serum erythropoietin levels (200500 mU/mL) and lower red blood cell (RBC) transfusion requirements (2 U per month) are more likely to respond to ESA therapy.[32, 33] Compared to certain solid tumors, ESA therapy in MDS has not been associated with an increased risk of thromboembolic events.[34]
Lenalidomide
5q syndrome is a subtype of MDS characterized by refractory macrocytic anemia, normal or elevated platelet counts, low BM blast percentage, small hypolobated dysplastic megakaryocytes, an isolated interstitial deletion in 5q, and an indolent natural history.[17, 35] Lenalidomide, an oral derivative of thalidomide, induces high response rates in LR‐MDS patients with 5q deletions, including hematologic improvements, RBC transfusion independence (TI) (56%67%, median duration >104 weeks), cytogenetic responses (50%76%), and complete remissions.[35, 36] These findings resulted in approval of lenalidomide (Revlimid; Celgene Corp., Summit, NJ) for patients with IPSS low or INT‐1 MDS with transfusion‐dependent anemia and 5q deletions with or without additional cytogenetic abnormalities. In addition, lenalidomide has some activity against LR‐MDS without 5q deletions (TI, 26%, median duration 41 weeks) and some patients with HR‐MDS and 5q deletions (TI, 25.5%, median duration 26 weeks.[37, 38] Therefore, lenalidomide is a reasonable consideration in some patients with LR‐MDS without 5q deletions with primary or secondary resistance to ESA therapy.[10]
Immunosuppressive Therapy
Some patients with LR‐MDS respond to immunosuppressive therapy with antithymocyte globulin with or without cyclosporine. Characteristics that correlate with higher response rates: LR‐MDS, younger age (60 years), hypoplastic MDS, normal karyotype, human leukocyte antigen‐DR15 histocompatibility type, and presence of a paroxysmal nocturnal hemoglobinuria clone.[10, 39]
MANAGEMENT OF HR‐MDS
The goal of management for HR‐MDS is to modify the natural history of the disease and to prolong survival. In addition to a supportive care‐only approach or clinical trial referral, 3 standard therapeutic approaches are used for patients with HR‐MDS: alloHSCT, intensive chemotherapy, and DNMTi therapy. The use of intensive AML‐like chemotherapy for HR‐MDS is associated with high toxicity and very limited long‐term success. Despite recent innovations in the field of transplantation, only a minority of MDS patients undergo alloHSCT, as most patients with HR‐MDS are elderly and/or medically infirm. Even for the minority of patients who do undergo alloHSCT, relapse after alloHSCT remains a major challenge.
DNA Methyltransferase Inhibitor Therapy
5‐azacitidine (AZA), (Vidaza; Celgene Corp.) and decitabine (DAC) (Dacogen; Eisai, Inc.) are potent inhibitors of DNA methyltransferases, which are enzymes responsible for cytosine methylation.[38, 40] These so‐called differentiation agents appear to restore normal hematopoiesis for many MDS patients, and the approved regimens of DNMTi in MDS result in overall response rates in about 40% to 60% of patients. Unfortunately, complete remissions (CR) are rare (10%20%) and the duration of responses are also somewhat limited (median CR duration, 10 to 14 months).[41, 42, 43, 44] In randomized clinical trials, both AZA and DAC resulted in significant improvements in blood counts, reduction in transfusion needs, reduced infection rates, decreased risk of progression to AML, and improvements in patient‐reported quality‐of‐life measures.[41, 42, 43, 44] AZA, but not DAC, prolonged survival in HR‐MDS patients in a large randomized trial (median overall survival for the AZA group was 24.5 months compared to 15 months for a group of patients treated with 1 of 3 conventional care regimens).[41, 42, 43, 44] AZA and DAC have not been compared head to head in trials, but most experts recommend AZA for first‐line use in HR‐MDS based on its effect on survival.[10]
AZA and DAC have also been studied as treatments for patients with AML. These agents differ from traditional intensive chemotherapy, as both agents are commonly administered on an outpatient basis, and hematologic responses are generally expected after 4 to 6 cycles of treatment as compared to a single course of intensive cytarabine‐based induction chemotherapy used to treat AML.[45] Additionally, the impact on survival may not require the achievement of a CR based on the finding that MDS patients saw improved survival even in patients whose best responses were hematologic improvements.[46] However, therapy with DNMTi is not curative, and patients are maintained on treatment as long as they are responding and not experiencing major side effects. Still, all patients will eventually lose response to DNMTi.
CONCLUSIONS
MDS is a form of cancer that largely affects elderly patients and leads to a BM failure state and increased risk of leukemic transformation. MDS is underdiagnosed and is frequently overlooked in the differential diagnosis of anemia in the elderly. DNMTi, lenalidomide, and ESA therapy offer effective therapeutic options for many MDS patients, including some considered too old or frail for intensive medical interventions. The use of prognostic models help physicians and patients better understand the common course of patients with MDS and facilitate tailoring of risk‐adapted therapy. It is expected that our improved understanding of the genetic, epigenetic, and immunologic mechanisms that operate in MDS will help develop better classification tools and rationally design effective new therapies.
Acknowledgments
The authors thank Dr. Balazs Zsenits (Medical Director of the Rochester General Hospitalist Group, Rochester General Hospital, Rochester, NY) for his critical review of the article.
Disclosures: Dr. Steven Gore owned stock in Celgene until November 2011, received research support from Celgene and Novartis, and consulted for Celgene. Drs. B. Douglas Smith, Amer Zeidan, and Bishoy Faltas have no relevant disclosures.
- . Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125:S2–S5.
- . Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9:57–63.
- . Myelodysplastic syndromes: increasing disease awareness. Introduction. Am J Med. 2012;125:S1.
- , , , et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52.
- , , , . Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542.
- , , , , . Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.
- . Myelodysplastic syndromes: increasing disease awareness. Discussion. Am J Med. 2012;125:S33–S34.
- . Why are myelodysplastic syndromes unrecognized and underdiagnosed? A primary care perspective. Am J Med. 2012;125:S15–S17.
- , . Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125:S6–S13.
- , , , et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56.
- . Dysplasia has A differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr Hematol Malig Rep. 2012;7:310–320.
- , . Interpreting new molecular genetics in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2012;2012:56–64.
- , , , , . Updates in cytogenetics and molecular markers in MDS. Curr Hematol Malig Rep. 2011;6:126–135.
- , , , . Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia. 2012;26:2447–2454.
- , , , et al. Reduced natural killer (NK) function associated with high‐risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824.
- , , , et al. Identification of RPS14 as a 5q− syndrome gene by RNA interference screen. Nature. 2008;451:335–339.
- , , . Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29:504–515.
- , , , et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169:1492–1499.
- , , , et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199.
- , , , et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting–Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849.
- , , , et al. WHO classification of MDS. In: World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
- , , , et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088.
- , , , et al. Time‐dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510.
- , , , et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification‐based Prognostic Scoring System (WPSS). Haematologica. 2011;96:1433–1440.
- , , , et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465.
- , , , . Prognostication in myelodysplastic syndromes: beyond the International Prognostic Scoring System (IPSS). Am J Med. 2013;126:e25.
- , . Myelodysplastic syndromes: who and when in the course of disease to transplant. Hematology Am Soc Hematol Educ Program. 2012;2012:49–55.
- , , , , , . “Low‐risk” myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro‐ versus anti‐apoptotic bcl‐2‐related proteins. Br J Haematol. 1998;103:1075–1082.
- , . Ineffective haemopoiesis and apoptosis in myelodysplastic syndromes. Br J Haematol. 1998;101:220–230.
- . Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38:635–647.
- , , , et al. Patient and physician characteristics associated with erythropoiesis‐stimulating agent use in patients with myelodysplastic syndromes. Haematologica. 2012;97:128–132.
- , , , et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G‐CSF: the GFM experience. Blood. 2008;111:574–582.
- , , , et al. Erythroid response to treatment with G‐CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99:344–351.
- , , , et al. Erythropoiesis‐stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97:15–20.
- , , , et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465.
- , , , et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion‐dependent patients with low‐/‐ntermediate‐1‐risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776.
- , , , et al. Phase 2 study of lenalidomide in transfusion‐dependent, low‐risk, and intermediate‐1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93.
- , , , et al. Efficacy and safety of lenalidomide in intermediate‐2 or high‐risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952.
- , , , , . Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511.
- , . DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30.
- , , , et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10:223–232.
- , , , et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440.
- , , , et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803.
- , , , et al. Low‐dose decitabine versus best supportive care in elderly patients with intermediate‐ or high‐risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996.
- , , , et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903.
- , , , et al. Prognostic factors for response and overall survival in 282 patients with higher‐risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411.
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal hematopoietic stem cell neoplasms characterized by dysplasia, ineffective hematopoiesis resulting in peripheral blood (PB) cytopenias affecting one or more cell lines, and a variable risk of progression to acute myeloid leukemia (AML). The last 15 years have witnessed significant advances in our understanding of the complex pathogenesis, classification and prognostication, and therapeutic approaches to MDS. As more elderly patients are diagnosed with MDS, encounters with hospitalized MDS patients or patients in whom MDS should be considered in the differential diagnosis are common events for today's hospitalists. In this review, we discuss the epidemiology, diagnosis, pathogenesis, prognostication, and therapies for MDS, with an emphasis on practical aspects that would be useful for hospitalists caring for these patients.
EPIDEMIOLOGY OF MDS
Although MDS is one of the most common hematologic malignancies, MDS remains understudied epidemiologically.[1, 2] Our understanding of the epidemiology improved after the implementation of reporting requirements to cancer registries, especially the Surveillance, Epidemiology, and End Results (SEER) database in 2001.[1, 2, 3] Age‐adjusted incidence of MDS in the United States ranged between 3.3 to 4.6 per 100,000 persons per year in the period between 2001 and 2008.[1, 2, 4] The majority of MDS patients are elderly, and because MDS incidence increases with age, the number of patients diagnosed with MDS is expected to continue to rise with the aging population.[1, 2, 5] MDS is more common in men compared to women, and in Caucasians compared to African Americans.[1, 2] Different estimates put MDS prevalence in the United States somewhere between 60,000 and 170,000 persons.[2, 6]
DIAGNOSIS OF MDS
Many patients with MDS are asymptomatic at diagnosis and only come to medical attention due to abnormal blood counts done routinely or for other reasons. This contributes to MDS being underdiagnosed. When cytopenias are not severe enough to cause symptoms, it is also frequently overlooked in patients with mild anemia or other cytopenias.[7] Together, being asymptomatic and having relatively mild cytopenias are probably the most important factors that lead to under‐recognition of MDS among primary care physicians (PCPs).[7, 8, 9] There is a misconception that anemia is normal in the elderly, and when patients are not symptomatic that a workup is not needed.[6, 7] This is compounded by a lack of awareness of the importance of making a diagnosis in these patients and of currently available therapies for MDS.[7, 8, 9]
Anemia is not a normal consequence of aging and is always a pathologic state with an underlying etiology.[6, 7] Because a significant number of elderly patients with unexplained anemia could have MDS, patients with symptomatic or progressive anemia, especially if associated with other cytopenias, should be considered for further evaluation.[7, 9] Diagnosis is important given the recent availability of effective therapies for MDS that can improve anemia, decrease transfusion needs, improve life quality, and potentially increase survival. MDS is generally an indolent disease with a relative stability of blood counts in comparison to AML, so prior blood counts and the tempo of the process is an important consideration.[9, 10] The National Comprehensive Cancer Network clinical practice guidelines recommend exclusion of nutritional deficiencies (iron, vitamin B12, folate) and other causes of anemia (eg, gastrointestinal bleeding, renal insufficiency, and anemia of inflammation), assessment of reticulocyte count and serum erythropoietin level, and evaluation of a PB smear for evidence of dysplasia as important initial steps.[10, 11] Eventually the diagnosis of MDS requires a bone marrow (BM) evaluation to confirm the diagnosis and exclude other BM failure states by evaluating for BM cellularity, cell maturation, dysplasia (which should be present in at least 10% of any the myeloid lineages), percentage of blasts (20%), iron stores and sideroblasts, cytogenetics, MDS‐specific fluorescence in situ hybridization (FISH) panels, flow cytometry, and other special testing.[9, 10] Despite extensive testing, MDS can sometimes be very difficult to differentiate from other bone marrow failure states (eg, hypoplastic MDS from aplastic anemia) (Table 1).[10, 11] In the absence of significant morbidity related to MDS, the definitive diagnosis of MDS can be usually made on an outpatient basis. It is important to ensure adequate follow‐up with PCPs postdischarge and/or outpatient hematologist referral for patients with unexplained cytopenias.
|
| Idiopathic cytopenia of undetermined significance: no significant dysplasia or MDS‐associated karyotypic aberrations |
| Acute myeloid leukemia: BM blasts 20%, presence of core‐binding characteristic cytogenetic aberrations: t(8;21), t(15;17), inv(16) defines AML regardless of BM blast count; AML can be associated with hepatosplenomegaly or myeloid sarcomas |
| Chronic myeloid leukemia: presence of Philadelphia chromosome t(9;22) positive, basophilia, and splenomegaly |
| Myelofibrosis: significant BM fibrosis, splenomegaly, and leukoerythroblastic picture in PB (teardrop and nucleated RBCs, left‐shifted myeloid cells) |
| Chronic myelomonocytic leukemia: significant PB monocytosis |
| MDS/MPN overlap syndromes: dysplasia with myeloproliferative characteristics such as splenomegaly, thrombocytosis, or leukocytosis |
| Infections: for example, HIV and parvovirus B19 infections |
| Myelophthisis: infiltration of BM with other tumors (eg, melanoma) with resultant PB cytopenias |
| Nutritional disturbances: B12, folate, and copper deficiency, and zinc and arsenic excess can mimic MDS |
| Medications: drugs that interfere with DNA synthesis such as HIV medications, chemotherapeutic agents, cotrimoxazole, methotrexate, azathioprine, and G‐CSF |
| Immune disorders: for example, LGL leukemia, lupus, or rheumatoid arthritis |
| Other acquired or congenital hematological disorders: for example, paroxysmal nocturnal hemoglobinuria, congenital dyserythropoietic anemia, dyskeratosis congenita |
PATHOGENESIS AND ETIOLOGY OF MDS
Ineffective hematopoiesis due to excessive apoptosis of hematopoietic precursors is a prominent feature of MDS, which explains the apparent paradox of hypercellular BM and PB cytopenias. Although not fully understood, complex epigenetic, genetic, and immunologic mechanisms contribute to the pathogenesis of MDS and account for disease heterogeneity. Aberrant silencing of tumor‐suppressor and DNA repair genes mediated by hypermethylation of their promoters is believed to play an important part in the pathogenesis of MDS.[12] This theory is supported by the unique sensitivity of MDS to drugs that reverse DNA methylation. Genetic abnormalities not only contribute to the pathogenesis of MDS, but are also among the strongest prognostic indicators for MDS patients, and can also affect therapeutic decisions. Clonal karyotypic abnormalities are observed in 50% of patients with MDS using conventional karyotyping.[12, 13] The most common chromosomal aberrations in MDS include deletions of the long arm of chromosome 5 (del5q), monosomy Y, monosomy 7 (del7) or deletion of its long arm (del7q), trisomy 8, del20q, and complex karyotypes (3 chromosomal aberrations).[12, 13] These cytogenetic abnormalities correlate with the prognosis of MDS (eg, poor prognosis with complex karyotypes and chromosome 7 deletions vs better prognosis with isolated del5q).[12, 13]
Recently, FISH assays and genome‐wide screening techniques (eg, single nucleotide polymorphism arrays, array‐based comparative genomic hybridization, whole genome or exome sequencing) have enabled detection of an increasing number of genetic aberrations and recurrent somatic molecular abnormalities in a significant number of MDS patients (eg, abnormalities of ASXL1, IDH1/IDH2, DNMT3, EZH2, TET2, and SF3B1 genes).[12, 14] Most affected genes are involved in the epigenetic regulation of transcription (DNA methylation and demethylation, histone posttranslational modification) or mRNA splicing.[12, 13, 14]
Immunologic aberrations have also been proposed to contribute to pathogenesis of MDS. For example, in early‐stage MDS, an aberrant immune attack on myeloid progenitors resulting in increased apoptosis can contribute to BM failure.[15] This is supported by association of some forms of MDS with autoimmune diseases and observed responses in some patients to immunosuppressive therapies. The relative contribution of pathogenetic mechanisms varies between the different MDS subtypes. For example, haploinsufficiency of cell‐cycle regulatory and ribosomal protein genes located in the commonly deleted region of 5q play an important role in the pathogenesis of MDS with isolated del5q (5q syndrome).[16] Mutations in the RNA spliceosomal machinery gene SF3B have been shown to play a role in the pathogenesis of the MDS subtype refractory anemia with ringed sideroblasts (RARS), with those patients with RARS carrying this mutation having a more favorable prognosis than those with the wild‐type gene.[14] Several excellent recent reviews provide detailed discussion of the complex pathophysiology of MDS.[12, 13, 14, 17]
Approximately 10% of MDS patients have secondary MDS (MDS occurring after chemotherapy or radiation therapy administration for treatment of another malignancy).[2] Aside from advancing age, the causative factors for the other 90% of cases (primary MDS) are unknown in most patients, although environmental and occupational exposures (eg, smoking, painting, insecticides, pesticides, organic solvents), and genetic syndromes (eg, DNA repair defects such as Fanconi's anemia) are implicated in some patients.[2, 10] Recently, an epidemiologic study found an increased MDS risk with obesity.[18]
PROGNOSTICATION OF MDS
MDS is a form of cancer, and most affected patients eventually die from cytopenic complications or leukemic progression. MDS is not a single disease but rather encompasses a group of heterogeneous subtypes with significantly different natural histories and pace of progression. Therefore, accurate risk stratification of MDS is necessary not only to predict survival and risk of leukemic progression, but also to help choose the most appropriate therapeutic option for individual patients. Information about prognosis should also be utilized when making management decisions with patients for other comorbid conditions (eg, major surgery). Two morphologically based classification systems are commonly used for MDS: the French‐American‐British (FAB) system and the World Health Organization (WHO) classification (Table 2), which most recently has supplanted the FAB system as the primary pathologic classification system.[19, 20, 21] Several prognostic models have been developed around the morphologic classifications to better account for relevant clinical and cytogenetic modifiers of this disease. Although some of these models have been validated by different groups, each of these models has limitations. Although the predictions generated by these models are generally accurate for the different prognostic categories to which the patient is assigned, the extent to which the prediction applies to an individual MDS patient can vary significantly. In addition, comorbid conditions affect survival of MDS patients and are not included in the specific scoring systems. For example, congestive heart failure and chronic obstructive lung disease were associated with shortened survival in MDS patients.[18]
| MDS WHO Class | PB Findings | BM Findings |
|---|---|---|
| ||
| Refractory cytopenias with unilineage dysplasia: includes refractory anemia; refractory neutropenia; refractory thrombocytopenia | Unicytopenia or bicytopenia; PB blasts 1% | BM blasts 5%; unilineage dysplasia (10% of cells in any myeloid lineage); 15% of erythroid precursors are ringed sideroblasts |
| Refractory anemia with ring sideroblasts | Anemia; PB blasts 1% | BM blasts 5%; erythroid dysplasia only; 15% of erythroid precursors are ringed sideroblasts |
| Refractory cytopenia with multilineage dysplasia | Cytopenia(s); PB blasts 1%; no Auer rods; 1 106/L monocytes | BM blasts 5% ; dysplasia (10% of cells in at least 2 myeloid lineages); no Auer rods |
| Refractory anemia with excess blasts‐1 | Cytopenia(s); PB blasts 5%; no Auer rods; 1 106/L monocytes | BM blasts 5%9%; unilineage or multilineage dysplasia; no Auer rods |
| Refractory anemia with excess blasts‐2 | Cytopenia(s); PB blasts 5%19%; Auer rods; 1 106/L monocytes | BM blasts 10%19%; unilineage or multilineage dysplasia; Auer rods |
| Myelodysplastic syndromeunclassified | Cytopenias; PB blasts 1% | BM blasts 5%; unequivocal dysplasia in 10% of cells at least one myeloid cell lines when accompanied by a cytogenetic abnormality considered as presumptive evidence for a diagnosis of MDS |
| MDS associated with isolated del5q | Anemia; normal to elevated platelet count; PB blasts 1% | BM blasts 5%; normal to elevated megakaryocytes with hypolobated nuclei; isolated del5q karyotypic abnormality; no Auer rods |
The International Prognostic Scoring System (IPSS) is the most widely used prognostic tool for MDS (Table 3).[22] In this model, an aggregate score is calculated based on points assigned to the percentage of blasts in BM, the number of PB cell lines affected by cytopenias, and the karyotype. Based on this point score, the patient is assigned to 1 of 4 categories that portend significantly different outcomes: low, intermediate‐1 (INT‐1), intermediate‐2 (INT‐2), and high risk. The IPSS was developed from a database of mostly untreated MDS patients and does not account for other important prognostic parameters such as transfusion dependence, depth of cytopenias, and extent/severity of lineage dysplasia.[22] The WHO Prognostic Scoring System was proposed to overcome some of these shortcomings.[23, 24] Efforts to continue to improve the prognostic models further led to a large international collaboration that compiled a much larger database and resulted in the development of a revised IPSS (IPSS‐R).[25] New discoveries of novel prognostic epigenetic, genetic, and immunologic determinants will likely result in the ongoing evolution of the current prognostic systems to further improve their discriminatory power.[26]
| Calculation of Score Value Based on Prognostic Variables | |||||
|---|---|---|---|---|---|
| Score Value | |||||
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| |||||
| Prognostic variable | |||||
| Bone marrow blasts (%)a | 5 | 510 | 1120 | 2130 | |
| Karyotypeb | Good | Intermediate | Poor | ||
| Number of peripheral blood cell line affected by cytopeniasc | 0 or 1 | 2 or 3 | |||
| Median Survival and Risk of Progression to AML According to the IPSS Risk Category in Absence of Therapy | |||||
| Overall Score | Risk Category | Percentage in the IPSS Population | Median Survival (Years) | Median Time From Diagnosis at Which 25% of Patients Progress to AML (Years) | |
| 0 | Low | 33% | 5.7 | 9.4 | |
| 0.51.0 | INT‐1 | 38% | 3.5 | 3.3 | |
| 1.52.0 | INT‐2 | 22% | 1.1 | 1.1 | |
| >2.5 | High | 7% | 0.4 | 0.2 | |
MANAGEMENT OF MDS
Most patients with MDS were treated historically with supportive measures only. The approval of 3 agents for treatment of MDS including the DNA methyltransferase inhibitors (DNMTi) azacitidine and decitabine, as well as the immunomodulatory agent lenalidomide, in the last decade advanced the care of MDS patients significantly (Table 4). Nonetheless, the use of allogeneic hematopoietic stem cell transplantation (alloHSCT) remains the only known curative modality for patients with MDS and should always be considered as a possible therapeutic option.[27] Unfortunately, the majority of patients with MDS are not considered candidates for alloHSCT due to age, comorbidities, and lack of suitable donors.[27] Therefore, most patients with MDS are managed with noncurative treatment and supportive paradigms. Treatment goals generally depend on the risk stratification for the particular individual, age, functional status, comorbidities, and importantly, the patient's individual preference. For medical decision‐making purposes, MDS is traditionally divided into 2 major risk categories: low‐risk (LR) and high‐risk (HR) groups. LR‐MDS includes the IPSS risk categories of low or INT‐1, whereas HR‐MDS is usually defined by the IPSS risk categories of INT‐2 and high. Newer classification tools (eg, IPSS‐R) and better molecular markers are expected to impact such categories as well as treatment recommendations in the future.[26]
|
| Azacitidine (5‐azacytidine, Vidaza) and decitabine (5‐aza,2‐deoxycytidine, Dacogen) |
| Class |
| Hypomethylating agents, azanucleosides |
| Mechanism of action |
| Epigenetic modulation by inhibition of DNA methyltransferase enzymes and other mechanisms |
| Indication |
| First line therapy for HR‐MDS, second line therapy for LR‐MDS after failure of other therapies such as ESAs, lenalidomide, or immunosuppressive agents |
| Approved regimens for MDS |
| Azacitidine: 75 mg/m2/day IV or SC for 7 days Q 4 weeks |
| Decitabine: 15 mg/m2 IV infusion over 3 hours, Q 8 hours for 3 days, Q 6 weeks or 20 mg/m2 IV infusion over 1 hour daily for 5 days Q 4 weeks |
| Common side effects |
| Fatigue |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
| Oral ulcers and rarely mucositis |
| Injection site reactions (redness, pain) |
| Lenalidomide (Revlimid) |
| Class |
| Immunomodulatory agent |
| Mechanism of action |
| Modulation of immune responses, gene expression, angiogenesis, cytokines and cell‐cycle regulatory phosphatases, and possibly other mechanisms |
| Indication |
| First line therapy for LR‐MDS with del5q (also used commonly off label for LR‐MDS without del5q as second line of therapy after ESAs) |
| Approved regimens for MDS |
| 10 mg orally once daily |
| Common side effects |
| Skin rash, dryness, and pruritus |
| Fatigue |
| Muscle cramps |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
Despite recent advances, supportive care for all patients with MDS remains a very important aspect of management, either in combination with other therapies or as sole therapy for frail patients who cannot tolerate further interventions. Supportive therapy focuses on maintaining a high quality of life and includes careful blood count monitoring, use of growth factors, use of transfusions and antibiotics as needed, and use of iron chelation therapy in some patients. Some of the common situations in which hospitalists encounter patients with MDS are listed in Table 5.
|
| Complications of cytopenias |
| Bleeding: local management based on bleeding site, platelet transfusions, and other blood products (eg, red blood cells, fresh frozen plasma) as appropriate, antifibrinolytics |
| Infections and neutropenic fevers: Antibiotics, antifungals, use of colony granulocyte‐stimulating factors or granulocyte infusions advised only in cases of uncontrolled severe infections or sepsis |
| Severe or symptomatic anemia: red blood transfusions as appropriate based on patient's comorbidities, all disease‐modifying drugs (lenalidomide, azacitidine, decitabine) and ESAs are slow acting and can take weeks to months before improving anemia |
| Complications of therapies |
| Neutropenic fevers: as above plus holding therapy |
| Most other side effects (see Table 4) are well tolerated and are managed symptomatically without requiring hospitalization. If needed hospitalization for side effects: symptomatic management and holding the drug |
| Other medical or surgical condition in a patient with MDS |
| Therapy as per the underlying medical condition. For therapeutic decisions (eg, decision to undergo major surgery), prognostication tools such as the IPSS and newer models can be used to inform medical decision making in consultation with an experienced hematologist |
MANAGEMENT OF LR‐MDS
In addition to supportive care or enrollment in clinical trials, therapies for LR‐MDS include erythropoiesis‐stimulating agents, lenalidomide, and immunosuppressive therapy.
Erythropoiesis‐Stimulating Agents
Anemia in MDS is a multifactorial process that includes ineffective erythropoiesis and suboptimal serum erythropoietin responses.[10, 28, 29] There are no randomized studies to suggest that erythropoiesis‐stimulating agents (ESA) therapy prolongs survival in MDS patients. Nonetheless, ESAs improve anemia significantly in some patients and are widely used.[30, 31] Approximately 20% to 30% of unselected MDS patients and about 40% of LR‐MDS patients achieve clinically meaningful erythroid responses with ESA therapy with a median response duration of 2 years.[30, 31] It is important to correct coexisting nutritional deficiencies (eg, iron or folate deficiency) to optimize responses to ESA.[10] Granulocyte colony‐stimulating factor can be synergistic with ESAs especially in patients with RARS.[10] Patients with LR‐MDS who have low endogenous serum erythropoietin levels (200500 mU/mL) and lower red blood cell (RBC) transfusion requirements (2 U per month) are more likely to respond to ESA therapy.[32, 33] Compared to certain solid tumors, ESA therapy in MDS has not been associated with an increased risk of thromboembolic events.[34]
Lenalidomide
5q syndrome is a subtype of MDS characterized by refractory macrocytic anemia, normal or elevated platelet counts, low BM blast percentage, small hypolobated dysplastic megakaryocytes, an isolated interstitial deletion in 5q, and an indolent natural history.[17, 35] Lenalidomide, an oral derivative of thalidomide, induces high response rates in LR‐MDS patients with 5q deletions, including hematologic improvements, RBC transfusion independence (TI) (56%67%, median duration >104 weeks), cytogenetic responses (50%76%), and complete remissions.[35, 36] These findings resulted in approval of lenalidomide (Revlimid; Celgene Corp., Summit, NJ) for patients with IPSS low or INT‐1 MDS with transfusion‐dependent anemia and 5q deletions with or without additional cytogenetic abnormalities. In addition, lenalidomide has some activity against LR‐MDS without 5q deletions (TI, 26%, median duration 41 weeks) and some patients with HR‐MDS and 5q deletions (TI, 25.5%, median duration 26 weeks.[37, 38] Therefore, lenalidomide is a reasonable consideration in some patients with LR‐MDS without 5q deletions with primary or secondary resistance to ESA therapy.[10]
Immunosuppressive Therapy
Some patients with LR‐MDS respond to immunosuppressive therapy with antithymocyte globulin with or without cyclosporine. Characteristics that correlate with higher response rates: LR‐MDS, younger age (60 years), hypoplastic MDS, normal karyotype, human leukocyte antigen‐DR15 histocompatibility type, and presence of a paroxysmal nocturnal hemoglobinuria clone.[10, 39]
MANAGEMENT OF HR‐MDS
The goal of management for HR‐MDS is to modify the natural history of the disease and to prolong survival. In addition to a supportive care‐only approach or clinical trial referral, 3 standard therapeutic approaches are used for patients with HR‐MDS: alloHSCT, intensive chemotherapy, and DNMTi therapy. The use of intensive AML‐like chemotherapy for HR‐MDS is associated with high toxicity and very limited long‐term success. Despite recent innovations in the field of transplantation, only a minority of MDS patients undergo alloHSCT, as most patients with HR‐MDS are elderly and/or medically infirm. Even for the minority of patients who do undergo alloHSCT, relapse after alloHSCT remains a major challenge.
DNA Methyltransferase Inhibitor Therapy
5‐azacitidine (AZA), (Vidaza; Celgene Corp.) and decitabine (DAC) (Dacogen; Eisai, Inc.) are potent inhibitors of DNA methyltransferases, which are enzymes responsible for cytosine methylation.[38, 40] These so‐called differentiation agents appear to restore normal hematopoiesis for many MDS patients, and the approved regimens of DNMTi in MDS result in overall response rates in about 40% to 60% of patients. Unfortunately, complete remissions (CR) are rare (10%20%) and the duration of responses are also somewhat limited (median CR duration, 10 to 14 months).[41, 42, 43, 44] In randomized clinical trials, both AZA and DAC resulted in significant improvements in blood counts, reduction in transfusion needs, reduced infection rates, decreased risk of progression to AML, and improvements in patient‐reported quality‐of‐life measures.[41, 42, 43, 44] AZA, but not DAC, prolonged survival in HR‐MDS patients in a large randomized trial (median overall survival for the AZA group was 24.5 months compared to 15 months for a group of patients treated with 1 of 3 conventional care regimens).[41, 42, 43, 44] AZA and DAC have not been compared head to head in trials, but most experts recommend AZA for first‐line use in HR‐MDS based on its effect on survival.[10]
AZA and DAC have also been studied as treatments for patients with AML. These agents differ from traditional intensive chemotherapy, as both agents are commonly administered on an outpatient basis, and hematologic responses are generally expected after 4 to 6 cycles of treatment as compared to a single course of intensive cytarabine‐based induction chemotherapy used to treat AML.[45] Additionally, the impact on survival may not require the achievement of a CR based on the finding that MDS patients saw improved survival even in patients whose best responses were hematologic improvements.[46] However, therapy with DNMTi is not curative, and patients are maintained on treatment as long as they are responding and not experiencing major side effects. Still, all patients will eventually lose response to DNMTi.
CONCLUSIONS
MDS is a form of cancer that largely affects elderly patients and leads to a BM failure state and increased risk of leukemic transformation. MDS is underdiagnosed and is frequently overlooked in the differential diagnosis of anemia in the elderly. DNMTi, lenalidomide, and ESA therapy offer effective therapeutic options for many MDS patients, including some considered too old or frail for intensive medical interventions. The use of prognostic models help physicians and patients better understand the common course of patients with MDS and facilitate tailoring of risk‐adapted therapy. It is expected that our improved understanding of the genetic, epigenetic, and immunologic mechanisms that operate in MDS will help develop better classification tools and rationally design effective new therapies.
Acknowledgments
The authors thank Dr. Balazs Zsenits (Medical Director of the Rochester General Hospitalist Group, Rochester General Hospital, Rochester, NY) for his critical review of the article.
Disclosures: Dr. Steven Gore owned stock in Celgene until November 2011, received research support from Celgene and Novartis, and consulted for Celgene. Drs. B. Douglas Smith, Amer Zeidan, and Bishoy Faltas have no relevant disclosures.
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal hematopoietic stem cell neoplasms characterized by dysplasia, ineffective hematopoiesis resulting in peripheral blood (PB) cytopenias affecting one or more cell lines, and a variable risk of progression to acute myeloid leukemia (AML). The last 15 years have witnessed significant advances in our understanding of the complex pathogenesis, classification and prognostication, and therapeutic approaches to MDS. As more elderly patients are diagnosed with MDS, encounters with hospitalized MDS patients or patients in whom MDS should be considered in the differential diagnosis are common events for today's hospitalists. In this review, we discuss the epidemiology, diagnosis, pathogenesis, prognostication, and therapies for MDS, with an emphasis on practical aspects that would be useful for hospitalists caring for these patients.
EPIDEMIOLOGY OF MDS
Although MDS is one of the most common hematologic malignancies, MDS remains understudied epidemiologically.[1, 2] Our understanding of the epidemiology improved after the implementation of reporting requirements to cancer registries, especially the Surveillance, Epidemiology, and End Results (SEER) database in 2001.[1, 2, 3] Age‐adjusted incidence of MDS in the United States ranged between 3.3 to 4.6 per 100,000 persons per year in the period between 2001 and 2008.[1, 2, 4] The majority of MDS patients are elderly, and because MDS incidence increases with age, the number of patients diagnosed with MDS is expected to continue to rise with the aging population.[1, 2, 5] MDS is more common in men compared to women, and in Caucasians compared to African Americans.[1, 2] Different estimates put MDS prevalence in the United States somewhere between 60,000 and 170,000 persons.[2, 6]
DIAGNOSIS OF MDS
Many patients with MDS are asymptomatic at diagnosis and only come to medical attention due to abnormal blood counts done routinely or for other reasons. This contributes to MDS being underdiagnosed. When cytopenias are not severe enough to cause symptoms, it is also frequently overlooked in patients with mild anemia or other cytopenias.[7] Together, being asymptomatic and having relatively mild cytopenias are probably the most important factors that lead to under‐recognition of MDS among primary care physicians (PCPs).[7, 8, 9] There is a misconception that anemia is normal in the elderly, and when patients are not symptomatic that a workup is not needed.[6, 7] This is compounded by a lack of awareness of the importance of making a diagnosis in these patients and of currently available therapies for MDS.[7, 8, 9]
Anemia is not a normal consequence of aging and is always a pathologic state with an underlying etiology.[6, 7] Because a significant number of elderly patients with unexplained anemia could have MDS, patients with symptomatic or progressive anemia, especially if associated with other cytopenias, should be considered for further evaluation.[7, 9] Diagnosis is important given the recent availability of effective therapies for MDS that can improve anemia, decrease transfusion needs, improve life quality, and potentially increase survival. MDS is generally an indolent disease with a relative stability of blood counts in comparison to AML, so prior blood counts and the tempo of the process is an important consideration.[9, 10] The National Comprehensive Cancer Network clinical practice guidelines recommend exclusion of nutritional deficiencies (iron, vitamin B12, folate) and other causes of anemia (eg, gastrointestinal bleeding, renal insufficiency, and anemia of inflammation), assessment of reticulocyte count and serum erythropoietin level, and evaluation of a PB smear for evidence of dysplasia as important initial steps.[10, 11] Eventually the diagnosis of MDS requires a bone marrow (BM) evaluation to confirm the diagnosis and exclude other BM failure states by evaluating for BM cellularity, cell maturation, dysplasia (which should be present in at least 10% of any the myeloid lineages), percentage of blasts (20%), iron stores and sideroblasts, cytogenetics, MDS‐specific fluorescence in situ hybridization (FISH) panels, flow cytometry, and other special testing.[9, 10] Despite extensive testing, MDS can sometimes be very difficult to differentiate from other bone marrow failure states (eg, hypoplastic MDS from aplastic anemia) (Table 1).[10, 11] In the absence of significant morbidity related to MDS, the definitive diagnosis of MDS can be usually made on an outpatient basis. It is important to ensure adequate follow‐up with PCPs postdischarge and/or outpatient hematologist referral for patients with unexplained cytopenias.
|
| Idiopathic cytopenia of undetermined significance: no significant dysplasia or MDS‐associated karyotypic aberrations |
| Acute myeloid leukemia: BM blasts 20%, presence of core‐binding characteristic cytogenetic aberrations: t(8;21), t(15;17), inv(16) defines AML regardless of BM blast count; AML can be associated with hepatosplenomegaly or myeloid sarcomas |
| Chronic myeloid leukemia: presence of Philadelphia chromosome t(9;22) positive, basophilia, and splenomegaly |
| Myelofibrosis: significant BM fibrosis, splenomegaly, and leukoerythroblastic picture in PB (teardrop and nucleated RBCs, left‐shifted myeloid cells) |
| Chronic myelomonocytic leukemia: significant PB monocytosis |
| MDS/MPN overlap syndromes: dysplasia with myeloproliferative characteristics such as splenomegaly, thrombocytosis, or leukocytosis |
| Infections: for example, HIV and parvovirus B19 infections |
| Myelophthisis: infiltration of BM with other tumors (eg, melanoma) with resultant PB cytopenias |
| Nutritional disturbances: B12, folate, and copper deficiency, and zinc and arsenic excess can mimic MDS |
| Medications: drugs that interfere with DNA synthesis such as HIV medications, chemotherapeutic agents, cotrimoxazole, methotrexate, azathioprine, and G‐CSF |
| Immune disorders: for example, LGL leukemia, lupus, or rheumatoid arthritis |
| Other acquired or congenital hematological disorders: for example, paroxysmal nocturnal hemoglobinuria, congenital dyserythropoietic anemia, dyskeratosis congenita |
PATHOGENESIS AND ETIOLOGY OF MDS
Ineffective hematopoiesis due to excessive apoptosis of hematopoietic precursors is a prominent feature of MDS, which explains the apparent paradox of hypercellular BM and PB cytopenias. Although not fully understood, complex epigenetic, genetic, and immunologic mechanisms contribute to the pathogenesis of MDS and account for disease heterogeneity. Aberrant silencing of tumor‐suppressor and DNA repair genes mediated by hypermethylation of their promoters is believed to play an important part in the pathogenesis of MDS.[12] This theory is supported by the unique sensitivity of MDS to drugs that reverse DNA methylation. Genetic abnormalities not only contribute to the pathogenesis of MDS, but are also among the strongest prognostic indicators for MDS patients, and can also affect therapeutic decisions. Clonal karyotypic abnormalities are observed in 50% of patients with MDS using conventional karyotyping.[12, 13] The most common chromosomal aberrations in MDS include deletions of the long arm of chromosome 5 (del5q), monosomy Y, monosomy 7 (del7) or deletion of its long arm (del7q), trisomy 8, del20q, and complex karyotypes (3 chromosomal aberrations).[12, 13] These cytogenetic abnormalities correlate with the prognosis of MDS (eg, poor prognosis with complex karyotypes and chromosome 7 deletions vs better prognosis with isolated del5q).[12, 13]
Recently, FISH assays and genome‐wide screening techniques (eg, single nucleotide polymorphism arrays, array‐based comparative genomic hybridization, whole genome or exome sequencing) have enabled detection of an increasing number of genetic aberrations and recurrent somatic molecular abnormalities in a significant number of MDS patients (eg, abnormalities of ASXL1, IDH1/IDH2, DNMT3, EZH2, TET2, and SF3B1 genes).[12, 14] Most affected genes are involved in the epigenetic regulation of transcription (DNA methylation and demethylation, histone posttranslational modification) or mRNA splicing.[12, 13, 14]
Immunologic aberrations have also been proposed to contribute to pathogenesis of MDS. For example, in early‐stage MDS, an aberrant immune attack on myeloid progenitors resulting in increased apoptosis can contribute to BM failure.[15] This is supported by association of some forms of MDS with autoimmune diseases and observed responses in some patients to immunosuppressive therapies. The relative contribution of pathogenetic mechanisms varies between the different MDS subtypes. For example, haploinsufficiency of cell‐cycle regulatory and ribosomal protein genes located in the commonly deleted region of 5q play an important role in the pathogenesis of MDS with isolated del5q (5q syndrome).[16] Mutations in the RNA spliceosomal machinery gene SF3B have been shown to play a role in the pathogenesis of the MDS subtype refractory anemia with ringed sideroblasts (RARS), with those patients with RARS carrying this mutation having a more favorable prognosis than those with the wild‐type gene.[14] Several excellent recent reviews provide detailed discussion of the complex pathophysiology of MDS.[12, 13, 14, 17]
Approximately 10% of MDS patients have secondary MDS (MDS occurring after chemotherapy or radiation therapy administration for treatment of another malignancy).[2] Aside from advancing age, the causative factors for the other 90% of cases (primary MDS) are unknown in most patients, although environmental and occupational exposures (eg, smoking, painting, insecticides, pesticides, organic solvents), and genetic syndromes (eg, DNA repair defects such as Fanconi's anemia) are implicated in some patients.[2, 10] Recently, an epidemiologic study found an increased MDS risk with obesity.[18]
PROGNOSTICATION OF MDS
MDS is a form of cancer, and most affected patients eventually die from cytopenic complications or leukemic progression. MDS is not a single disease but rather encompasses a group of heterogeneous subtypes with significantly different natural histories and pace of progression. Therefore, accurate risk stratification of MDS is necessary not only to predict survival and risk of leukemic progression, but also to help choose the most appropriate therapeutic option for individual patients. Information about prognosis should also be utilized when making management decisions with patients for other comorbid conditions (eg, major surgery). Two morphologically based classification systems are commonly used for MDS: the French‐American‐British (FAB) system and the World Health Organization (WHO) classification (Table 2), which most recently has supplanted the FAB system as the primary pathologic classification system.[19, 20, 21] Several prognostic models have been developed around the morphologic classifications to better account for relevant clinical and cytogenetic modifiers of this disease. Although some of these models have been validated by different groups, each of these models has limitations. Although the predictions generated by these models are generally accurate for the different prognostic categories to which the patient is assigned, the extent to which the prediction applies to an individual MDS patient can vary significantly. In addition, comorbid conditions affect survival of MDS patients and are not included in the specific scoring systems. For example, congestive heart failure and chronic obstructive lung disease were associated with shortened survival in MDS patients.[18]
| MDS WHO Class | PB Findings | BM Findings |
|---|---|---|
| ||
| Refractory cytopenias with unilineage dysplasia: includes refractory anemia; refractory neutropenia; refractory thrombocytopenia | Unicytopenia or bicytopenia; PB blasts 1% | BM blasts 5%; unilineage dysplasia (10% of cells in any myeloid lineage); 15% of erythroid precursors are ringed sideroblasts |
| Refractory anemia with ring sideroblasts | Anemia; PB blasts 1% | BM blasts 5%; erythroid dysplasia only; 15% of erythroid precursors are ringed sideroblasts |
| Refractory cytopenia with multilineage dysplasia | Cytopenia(s); PB blasts 1%; no Auer rods; 1 106/L monocytes | BM blasts 5% ; dysplasia (10% of cells in at least 2 myeloid lineages); no Auer rods |
| Refractory anemia with excess blasts‐1 | Cytopenia(s); PB blasts 5%; no Auer rods; 1 106/L monocytes | BM blasts 5%9%; unilineage or multilineage dysplasia; no Auer rods |
| Refractory anemia with excess blasts‐2 | Cytopenia(s); PB blasts 5%19%; Auer rods; 1 106/L monocytes | BM blasts 10%19%; unilineage or multilineage dysplasia; Auer rods |
| Myelodysplastic syndromeunclassified | Cytopenias; PB blasts 1% | BM blasts 5%; unequivocal dysplasia in 10% of cells at least one myeloid cell lines when accompanied by a cytogenetic abnormality considered as presumptive evidence for a diagnosis of MDS |
| MDS associated with isolated del5q | Anemia; normal to elevated platelet count; PB blasts 1% | BM blasts 5%; normal to elevated megakaryocytes with hypolobated nuclei; isolated del5q karyotypic abnormality; no Auer rods |
The International Prognostic Scoring System (IPSS) is the most widely used prognostic tool for MDS (Table 3).[22] In this model, an aggregate score is calculated based on points assigned to the percentage of blasts in BM, the number of PB cell lines affected by cytopenias, and the karyotype. Based on this point score, the patient is assigned to 1 of 4 categories that portend significantly different outcomes: low, intermediate‐1 (INT‐1), intermediate‐2 (INT‐2), and high risk. The IPSS was developed from a database of mostly untreated MDS patients and does not account for other important prognostic parameters such as transfusion dependence, depth of cytopenias, and extent/severity of lineage dysplasia.[22] The WHO Prognostic Scoring System was proposed to overcome some of these shortcomings.[23, 24] Efforts to continue to improve the prognostic models further led to a large international collaboration that compiled a much larger database and resulted in the development of a revised IPSS (IPSS‐R).[25] New discoveries of novel prognostic epigenetic, genetic, and immunologic determinants will likely result in the ongoing evolution of the current prognostic systems to further improve their discriminatory power.[26]
| Calculation of Score Value Based on Prognostic Variables | |||||
|---|---|---|---|---|---|
| Score Value | |||||
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| |||||
| Prognostic variable | |||||
| Bone marrow blasts (%)a | 5 | 510 | 1120 | 2130 | |
| Karyotypeb | Good | Intermediate | Poor | ||
| Number of peripheral blood cell line affected by cytopeniasc | 0 or 1 | 2 or 3 | |||
| Median Survival and Risk of Progression to AML According to the IPSS Risk Category in Absence of Therapy | |||||
| Overall Score | Risk Category | Percentage in the IPSS Population | Median Survival (Years) | Median Time From Diagnosis at Which 25% of Patients Progress to AML (Years) | |
| 0 | Low | 33% | 5.7 | 9.4 | |
| 0.51.0 | INT‐1 | 38% | 3.5 | 3.3 | |
| 1.52.0 | INT‐2 | 22% | 1.1 | 1.1 | |
| >2.5 | High | 7% | 0.4 | 0.2 | |
MANAGEMENT OF MDS
Most patients with MDS were treated historically with supportive measures only. The approval of 3 agents for treatment of MDS including the DNA methyltransferase inhibitors (DNMTi) azacitidine and decitabine, as well as the immunomodulatory agent lenalidomide, in the last decade advanced the care of MDS patients significantly (Table 4). Nonetheless, the use of allogeneic hematopoietic stem cell transplantation (alloHSCT) remains the only known curative modality for patients with MDS and should always be considered as a possible therapeutic option.[27] Unfortunately, the majority of patients with MDS are not considered candidates for alloHSCT due to age, comorbidities, and lack of suitable donors.[27] Therefore, most patients with MDS are managed with noncurative treatment and supportive paradigms. Treatment goals generally depend on the risk stratification for the particular individual, age, functional status, comorbidities, and importantly, the patient's individual preference. For medical decision‐making purposes, MDS is traditionally divided into 2 major risk categories: low‐risk (LR) and high‐risk (HR) groups. LR‐MDS includes the IPSS risk categories of low or INT‐1, whereas HR‐MDS is usually defined by the IPSS risk categories of INT‐2 and high. Newer classification tools (eg, IPSS‐R) and better molecular markers are expected to impact such categories as well as treatment recommendations in the future.[26]
|
| Azacitidine (5‐azacytidine, Vidaza) and decitabine (5‐aza,2‐deoxycytidine, Dacogen) |
| Class |
| Hypomethylating agents, azanucleosides |
| Mechanism of action |
| Epigenetic modulation by inhibition of DNA methyltransferase enzymes and other mechanisms |
| Indication |
| First line therapy for HR‐MDS, second line therapy for LR‐MDS after failure of other therapies such as ESAs, lenalidomide, or immunosuppressive agents |
| Approved regimens for MDS |
| Azacitidine: 75 mg/m2/day IV or SC for 7 days Q 4 weeks |
| Decitabine: 15 mg/m2 IV infusion over 3 hours, Q 8 hours for 3 days, Q 6 weeks or 20 mg/m2 IV infusion over 1 hour daily for 5 days Q 4 weeks |
| Common side effects |
| Fatigue |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
| Oral ulcers and rarely mucositis |
| Injection site reactions (redness, pain) |
| Lenalidomide (Revlimid) |
| Class |
| Immunomodulatory agent |
| Mechanism of action |
| Modulation of immune responses, gene expression, angiogenesis, cytokines and cell‐cycle regulatory phosphatases, and possibly other mechanisms |
| Indication |
| First line therapy for LR‐MDS with del5q (also used commonly off label for LR‐MDS without del5q as second line of therapy after ESAs) |
| Approved regimens for MDS |
| 10 mg orally once daily |
| Common side effects |
| Skin rash, dryness, and pruritus |
| Fatigue |
| Muscle cramps |
| Development of or worsening cytopenias (neutropenia, thrombocytopenia, and anemia) and their complications (eg, infections, bleeding) |
| Gastrointestinal disturbances (nausea, vomiting, or diarrhea) |
Despite recent advances, supportive care for all patients with MDS remains a very important aspect of management, either in combination with other therapies or as sole therapy for frail patients who cannot tolerate further interventions. Supportive therapy focuses on maintaining a high quality of life and includes careful blood count monitoring, use of growth factors, use of transfusions and antibiotics as needed, and use of iron chelation therapy in some patients. Some of the common situations in which hospitalists encounter patients with MDS are listed in Table 5.
|
| Complications of cytopenias |
| Bleeding: local management based on bleeding site, platelet transfusions, and other blood products (eg, red blood cells, fresh frozen plasma) as appropriate, antifibrinolytics |
| Infections and neutropenic fevers: Antibiotics, antifungals, use of colony granulocyte‐stimulating factors or granulocyte infusions advised only in cases of uncontrolled severe infections or sepsis |
| Severe or symptomatic anemia: red blood transfusions as appropriate based on patient's comorbidities, all disease‐modifying drugs (lenalidomide, azacitidine, decitabine) and ESAs are slow acting and can take weeks to months before improving anemia |
| Complications of therapies |
| Neutropenic fevers: as above plus holding therapy |
| Most other side effects (see Table 4) are well tolerated and are managed symptomatically without requiring hospitalization. If needed hospitalization for side effects: symptomatic management and holding the drug |
| Other medical or surgical condition in a patient with MDS |
| Therapy as per the underlying medical condition. For therapeutic decisions (eg, decision to undergo major surgery), prognostication tools such as the IPSS and newer models can be used to inform medical decision making in consultation with an experienced hematologist |
MANAGEMENT OF LR‐MDS
In addition to supportive care or enrollment in clinical trials, therapies for LR‐MDS include erythropoiesis‐stimulating agents, lenalidomide, and immunosuppressive therapy.
Erythropoiesis‐Stimulating Agents
Anemia in MDS is a multifactorial process that includes ineffective erythropoiesis and suboptimal serum erythropoietin responses.[10, 28, 29] There are no randomized studies to suggest that erythropoiesis‐stimulating agents (ESA) therapy prolongs survival in MDS patients. Nonetheless, ESAs improve anemia significantly in some patients and are widely used.[30, 31] Approximately 20% to 30% of unselected MDS patients and about 40% of LR‐MDS patients achieve clinically meaningful erythroid responses with ESA therapy with a median response duration of 2 years.[30, 31] It is important to correct coexisting nutritional deficiencies (eg, iron or folate deficiency) to optimize responses to ESA.[10] Granulocyte colony‐stimulating factor can be synergistic with ESAs especially in patients with RARS.[10] Patients with LR‐MDS who have low endogenous serum erythropoietin levels (200500 mU/mL) and lower red blood cell (RBC) transfusion requirements (2 U per month) are more likely to respond to ESA therapy.[32, 33] Compared to certain solid tumors, ESA therapy in MDS has not been associated with an increased risk of thromboembolic events.[34]
Lenalidomide
5q syndrome is a subtype of MDS characterized by refractory macrocytic anemia, normal or elevated platelet counts, low BM blast percentage, small hypolobated dysplastic megakaryocytes, an isolated interstitial deletion in 5q, and an indolent natural history.[17, 35] Lenalidomide, an oral derivative of thalidomide, induces high response rates in LR‐MDS patients with 5q deletions, including hematologic improvements, RBC transfusion independence (TI) (56%67%, median duration >104 weeks), cytogenetic responses (50%76%), and complete remissions.[35, 36] These findings resulted in approval of lenalidomide (Revlimid; Celgene Corp., Summit, NJ) for patients with IPSS low or INT‐1 MDS with transfusion‐dependent anemia and 5q deletions with or without additional cytogenetic abnormalities. In addition, lenalidomide has some activity against LR‐MDS without 5q deletions (TI, 26%, median duration 41 weeks) and some patients with HR‐MDS and 5q deletions (TI, 25.5%, median duration 26 weeks.[37, 38] Therefore, lenalidomide is a reasonable consideration in some patients with LR‐MDS without 5q deletions with primary or secondary resistance to ESA therapy.[10]
Immunosuppressive Therapy
Some patients with LR‐MDS respond to immunosuppressive therapy with antithymocyte globulin with or without cyclosporine. Characteristics that correlate with higher response rates: LR‐MDS, younger age (60 years), hypoplastic MDS, normal karyotype, human leukocyte antigen‐DR15 histocompatibility type, and presence of a paroxysmal nocturnal hemoglobinuria clone.[10, 39]
MANAGEMENT OF HR‐MDS
The goal of management for HR‐MDS is to modify the natural history of the disease and to prolong survival. In addition to a supportive care‐only approach or clinical trial referral, 3 standard therapeutic approaches are used for patients with HR‐MDS: alloHSCT, intensive chemotherapy, and DNMTi therapy. The use of intensive AML‐like chemotherapy for HR‐MDS is associated with high toxicity and very limited long‐term success. Despite recent innovations in the field of transplantation, only a minority of MDS patients undergo alloHSCT, as most patients with HR‐MDS are elderly and/or medically infirm. Even for the minority of patients who do undergo alloHSCT, relapse after alloHSCT remains a major challenge.
DNA Methyltransferase Inhibitor Therapy
5‐azacitidine (AZA), (Vidaza; Celgene Corp.) and decitabine (DAC) (Dacogen; Eisai, Inc.) are potent inhibitors of DNA methyltransferases, which are enzymes responsible for cytosine methylation.[38, 40] These so‐called differentiation agents appear to restore normal hematopoiesis for many MDS patients, and the approved regimens of DNMTi in MDS result in overall response rates in about 40% to 60% of patients. Unfortunately, complete remissions (CR) are rare (10%20%) and the duration of responses are also somewhat limited (median CR duration, 10 to 14 months).[41, 42, 43, 44] In randomized clinical trials, both AZA and DAC resulted in significant improvements in blood counts, reduction in transfusion needs, reduced infection rates, decreased risk of progression to AML, and improvements in patient‐reported quality‐of‐life measures.[41, 42, 43, 44] AZA, but not DAC, prolonged survival in HR‐MDS patients in a large randomized trial (median overall survival for the AZA group was 24.5 months compared to 15 months for a group of patients treated with 1 of 3 conventional care regimens).[41, 42, 43, 44] AZA and DAC have not been compared head to head in trials, but most experts recommend AZA for first‐line use in HR‐MDS based on its effect on survival.[10]
AZA and DAC have also been studied as treatments for patients with AML. These agents differ from traditional intensive chemotherapy, as both agents are commonly administered on an outpatient basis, and hematologic responses are generally expected after 4 to 6 cycles of treatment as compared to a single course of intensive cytarabine‐based induction chemotherapy used to treat AML.[45] Additionally, the impact on survival may not require the achievement of a CR based on the finding that MDS patients saw improved survival even in patients whose best responses were hematologic improvements.[46] However, therapy with DNMTi is not curative, and patients are maintained on treatment as long as they are responding and not experiencing major side effects. Still, all patients will eventually lose response to DNMTi.
CONCLUSIONS
MDS is a form of cancer that largely affects elderly patients and leads to a BM failure state and increased risk of leukemic transformation. MDS is underdiagnosed and is frequently overlooked in the differential diagnosis of anemia in the elderly. DNMTi, lenalidomide, and ESA therapy offer effective therapeutic options for many MDS patients, including some considered too old or frail for intensive medical interventions. The use of prognostic models help physicians and patients better understand the common course of patients with MDS and facilitate tailoring of risk‐adapted therapy. It is expected that our improved understanding of the genetic, epigenetic, and immunologic mechanisms that operate in MDS will help develop better classification tools and rationally design effective new therapies.
Acknowledgments
The authors thank Dr. Balazs Zsenits (Medical Director of the Rochester General Hospitalist Group, Rochester General Hospital, Rochester, NY) for his critical review of the article.
Disclosures: Dr. Steven Gore owned stock in Celgene until November 2011, received research support from Celgene and Novartis, and consulted for Celgene. Drs. B. Douglas Smith, Amer Zeidan, and Bishoy Faltas have no relevant disclosures.
- . Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125:S2–S5.
- . Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9:57–63.
- . Myelodysplastic syndromes: increasing disease awareness. Introduction. Am J Med. 2012;125:S1.
- , , , et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52.
- , , , . Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542.
- , , , , . Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.
- . Myelodysplastic syndromes: increasing disease awareness. Discussion. Am J Med. 2012;125:S33–S34.
- . Why are myelodysplastic syndromes unrecognized and underdiagnosed? A primary care perspective. Am J Med. 2012;125:S15–S17.
- , . Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125:S6–S13.
- , , , et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56.
- . Dysplasia has A differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr Hematol Malig Rep. 2012;7:310–320.
- , . Interpreting new molecular genetics in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2012;2012:56–64.
- , , , , . Updates in cytogenetics and molecular markers in MDS. Curr Hematol Malig Rep. 2011;6:126–135.
- , , , . Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia. 2012;26:2447–2454.
- , , , et al. Reduced natural killer (NK) function associated with high‐risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824.
- , , , et al. Identification of RPS14 as a 5q− syndrome gene by RNA interference screen. Nature. 2008;451:335–339.
- , , . Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29:504–515.
- , , , et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169:1492–1499.
- , , , et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199.
- , , , et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting–Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849.
- , , , et al. WHO classification of MDS. In: World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
- , , , et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088.
- , , , et al. Time‐dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510.
- , , , et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification‐based Prognostic Scoring System (WPSS). Haematologica. 2011;96:1433–1440.
- , , , et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465.
- , , , . Prognostication in myelodysplastic syndromes: beyond the International Prognostic Scoring System (IPSS). Am J Med. 2013;126:e25.
- , . Myelodysplastic syndromes: who and when in the course of disease to transplant. Hematology Am Soc Hematol Educ Program. 2012;2012:49–55.
- , , , , , . “Low‐risk” myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro‐ versus anti‐apoptotic bcl‐2‐related proteins. Br J Haematol. 1998;103:1075–1082.
- , . Ineffective haemopoiesis and apoptosis in myelodysplastic syndromes. Br J Haematol. 1998;101:220–230.
- . Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38:635–647.
- , , , et al. Patient and physician characteristics associated with erythropoiesis‐stimulating agent use in patients with myelodysplastic syndromes. Haematologica. 2012;97:128–132.
- , , , et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G‐CSF: the GFM experience. Blood. 2008;111:574–582.
- , , , et al. Erythroid response to treatment with G‐CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99:344–351.
- , , , et al. Erythropoiesis‐stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97:15–20.
- , , , et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465.
- , , , et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion‐dependent patients with low‐/‐ntermediate‐1‐risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776.
- , , , et al. Phase 2 study of lenalidomide in transfusion‐dependent, low‐risk, and intermediate‐1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93.
- , , , et al. Efficacy and safety of lenalidomide in intermediate‐2 or high‐risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952.
- , , , , . Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511.
- , . DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30.
- , , , et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10:223–232.
- , , , et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440.
- , , , et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803.
- , , , et al. Low‐dose decitabine versus best supportive care in elderly patients with intermediate‐ or high‐risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996.
- , , , et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903.
- , , , et al. Prognostic factors for response and overall survival in 282 patients with higher‐risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411.
- . Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125:S2–S5.
- . Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9:57–63.
- . Myelodysplastic syndromes: increasing disease awareness. Introduction. Am J Med. 2012;125:S1.
- , , , et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52.
- , , , . Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542.
- , , , , . Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.
- . Myelodysplastic syndromes: increasing disease awareness. Discussion. Am J Med. 2012;125:S33–S34.
- . Why are myelodysplastic syndromes unrecognized and underdiagnosed? A primary care perspective. Am J Med. 2012;125:S15–S17.
- , . Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125:S6–S13.
- , , , et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56.
- . Dysplasia has A differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr Hematol Malig Rep. 2012;7:310–320.
- , . Interpreting new molecular genetics in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2012;2012:56–64.
- , , , , . Updates in cytogenetics and molecular markers in MDS. Curr Hematol Malig Rep. 2011;6:126–135.
- , , , . Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia. 2012;26:2447–2454.
- , , , et al. Reduced natural killer (NK) function associated with high‐risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824.
- , , , et al. Identification of RPS14 as a 5q− syndrome gene by RNA interference screen. Nature. 2008;451:335–339.
- , , . Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29:504–515.
- , , , et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169:1492–1499.
- , , , et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199.
- , , , et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting–Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849.
- , , , et al. WHO classification of MDS. In: World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
- , , , et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088.
- , , , et al. Time‐dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510.
- , , , et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification‐based Prognostic Scoring System (WPSS). Haematologica. 2011;96:1433–1440.
- , , , et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465.
- , , , . Prognostication in myelodysplastic syndromes: beyond the International Prognostic Scoring System (IPSS). Am J Med. 2013;126:e25.
- , . Myelodysplastic syndromes: who and when in the course of disease to transplant. Hematology Am Soc Hematol Educ Program. 2012;2012:49–55.
- , , , , , . “Low‐risk” myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro‐ versus anti‐apoptotic bcl‐2‐related proteins. Br J Haematol. 1998;103:1075–1082.
- , . Ineffective haemopoiesis and apoptosis in myelodysplastic syndromes. Br J Haematol. 1998;101:220–230.
- . Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38:635–647.
- , , , et al. Patient and physician characteristics associated with erythropoiesis‐stimulating agent use in patients with myelodysplastic syndromes. Haematologica. 2012;97:128–132.
- , , , et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G‐CSF: the GFM experience. Blood. 2008;111:574–582.
- , , , et al. Erythroid response to treatment with G‐CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99:344–351.
- , , , et al. Erythropoiesis‐stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97:15–20.
- , , , et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465.
- , , , et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion‐dependent patients with low‐/‐ntermediate‐1‐risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776.
- , , , et al. Phase 2 study of lenalidomide in transfusion‐dependent, low‐risk, and intermediate‐1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93.
- , , , et al. Efficacy and safety of lenalidomide in intermediate‐2 or high‐risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952.
- , , , , . Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511.
- , . DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30.
- , , , et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10:223–232.
- , , , et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440.
- , , , et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803.
- , , , et al. Low‐dose decitabine versus best supportive care in elderly patients with intermediate‐ or high‐risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996.
- , , , et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903.
- , , , et al. Prognostic factors for response and overall survival in 282 patients with higher‐risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411.
Aspirin: Its risks, benefits, and optimal use in preventing cardiovascular events
A 57-year-old woman with no history of cardiovascular disease comes to the clinic for her annual evaluation. She does not have diabetes mellitus, but she does have hypertension and chronic osteoarthritis, currently treated with acetaminophen. Additionally, she admits to active tobacco use. Her systolic blood pressure is 130 mm Hg on therapy with hydrochlorothiazide. Her electrocardiogram demonstrates left ventricular hypertrophy. Her low-density lipoprotein (LDL) cholesterol level is 140 mg/dL, and her high-density lipoprotein (HDL) cholesterol level is 50 mg/dL. Should this patient be started on aspirin therapy?
Acetylsalicylic acid (aspirin) is an analgesic, antipyretic, and anti-inflammatory agent, but its more prominent use today is as an antithrombotic agent to treat or prevent cardiovascular events. Its antithrombotic properties are due to its effects on the enzyme cyclooxygenase. However, cyclooxygenase is also involved in regulation of the gastric mucosa, and so aspirin increases the risk of gastrointestinal bleeding.
Approximately 50 million people take aspirin on a daily basis to treat or prevent cardiovascular disease.1 Of these, at least half are taking more than 100 mg per day,2 reflecting the general belief that, for aspirin dosage, “more is better”—which is not true.
Additionally, recommendations about the use of aspirin were based on studies that included relatively few members of several important subgroups, such as people with diabetes without known cardiovascular disease, women, and the elderly, and thus may not reflect appropriate indications and dosages for these groups.
Here, we examine the literature, outline an individualized approach to aspirin therapy, and highlight areas for future study.
HISTORY OF ASPIRIN USE IN CARDIOVASCULAR DISEASE
- 1700s—Willow bark is used as an analgesic.
- 1897—Synthetic aspirin is developed as an antipyretic and anti-inflammatory agent.
- 1974—First landmark trial of aspirin for secondary prevention of myocardial infarction.3
- 1982—Nobel Prize awarded for discovery of aspirin mechanism.
- 1985—US Food and Drug Administration approves aspirin for the treatment and secondary prevention of acute myocardial infarction.
- 1998—The Second International Study of Infarct Survival (ISIS-2) finds that giving aspirin to patients with myocardial infarction within 24 hours of presentation leads to a significant reduction in vascular deaths.4
Ongoing uncertainties
Aspirin now carries a class I indication for all patients with suspected myocardial infarction. Since there are an estimated 600,000 new coronary events and 325,000 recurrent ischemic events per year in the United States,5 the need for aspirin will continue to remain great. It is also approved to prevent and treat stroke and in patients with unstable angina.
However, questions continue to emerge about aspirin’s dosing and appropriate use in specific populations. The initial prevention trials used a wide range of doses and, as mentioned, included few women, few people with diabetes, and few elderly people. The uncertainties are especially pertinent for patients without known vascular disease, in whom the absolute risk reduction is much less, making the assessment of bleeding risk particularly important. Furthermore, the absolute risk-to-benefit assessment may be different in certain populations.
Guidelines on the use of aspirin to prevent cardiovascular disease (Table 1)6–10 have evolved to take into account these possible disparities, and studies are taking place to further investigate aspirin use in these groups.
ASPIRIN AND GASTROINTESTINAL BLEEDING
Aspirin’s association with bleeding, particularly gastrointestinal bleeding, was recognized early as a use-limiting side effect. With or without aspirin, gastrointestinal bleeding is a common cause of morbidity and death, with an incidence of approximately 100 per 100,000 bleeding episodes in adults per year for upper gastrointestinal bleeding and 20 to 30 per 100,000 per year for lower gastrointestinal bleeding.11,12
The standard dosage (ie, 325 mg/day) is associated with a significantly higher risk of gastrointestinal bleeding (including fatal bleeds) than is 75 mg.13 However, even with lower doses, the risk of gastrointestinal bleeding is estimated to be twice as high as with no aspirin.14
And here is the irony: studies have shown that higher doses of aspirin offer no advantage in preventing thrombotic events compared with lower doses.15 For example, the Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Organization to Assess Strategies for Ischemic Stroke Syndromes study reported a higher rate of gastrointestinal bleeding with standard-dose aspirin therapy than with low-dose aspirin, with no additional cardiovascular benefit with the higher dose.16
Furthermore, several other risk factors increase the risk of gastrointestinal bleeding with aspirin use (Table 2). These risk factors are common in the general population but were not necessarily represented in participants in clinical trials. Thus, estimates of risk based on trial data most likely underestimate actual risk in the general population, and therefore, the individual patient’s risk of gastrointestinal bleeding, based on these and other factors, needs to be taken into consideration.
ASPIRIN IN PATIENTS WITH CORONARY ARTERY DISEASE
Randomized clinical trials have validated the benefits of aspirin in secondary prevention of cardiovascular events in patients who have had a myocardial infarction. Patients with coronary disease who withdraw from aspirin therapy or otherwise do not adhere to it have a risk of cardiovascular events three times higher than those who stay with it.17
Despite the strong data, however, several issues and questions remain about the use of aspirin for secondary prevention.
Bleeding risk must be considered, since gastrointestinal bleeding is associated with a higher risk of death and myocardial infarction in patients with cardiovascular disease.18 Many patients with coronary disease are on more than one antiplatelet or anticoagulant therapy for concomitant conditions such as atrial fibrillation or because they underwent a percutaneous intervention, which further increases the risk of bleeding.
This bleeding risk is reflected in changes in the most recent recommendations for aspirin dosing after percutaneous coronary intervention. Earlier guidelines advocated use of either 162 or 325 mg after the procedure. However, the most recent update (in 2011) now supports 81 mg for maintenance dosing after intervention.7
Patients with coronary disease but without prior myocardial infarction or intervention. Current guidelines recommend 75 to 162 mg of aspirin in all patients with coronary artery disease.6 However, this group is diverse and includes patients who have undergone percutaneous coronary intervention, patients with chronic stable angina, and patients with asymptomatic coronary artery disease found on imaging studies. The magnitude of benefit is not clear for those who have no symptoms or who have stable angina.
Most of the evidence supporting aspirin use in chronic angina came from a single trial in Sweden, in which 2,000 patients with chronic stable angina were given either 75 mg daily or placebo. Those who received aspirin had a 34% lower rate of myocardial infarction and sudden death.19
A substudy of the Physicians’ Health Study, with fewer patients, also noted a significant reduction in the rate of first myocardial infarction. The dose of aspirin in this study was 325 mg every other day.20
In the Women’s Health Initiative Observational Study, 70% of women with stable cardiovascular disease taking aspirin were taking 325 mg daily.21 This study demonstrated a significant reduction in the cardiovascular mortality rate, which supports current guidelines, and found no difference in outcomes with doses of 81 mg compared with 325 mg.21 This again corroborates that low-dose aspirin is preferential to standard-dose aspirin in women with cardiovascular disease.
These findings have not been validated in larger prospective trials. Thus, current guidelines for aspirin use may reflect extrapolation of aspirin benefit from higher-risk patients to lower-risk patients.
Nevertheless, although the debate continues, it has generally been accepted that in patients who are at high risk of vascular disease or who have had a myocardial infarction, the benefits of aspirin—a 20% relative reduction in vascular events22—clearly outweigh the risks.
ASPIRIN FOR PRIMARY PREVENTION
Assessing risk vs benefit is more complex when considering populations without known cardiovascular disease.
Only a few studies have specifically evaluated the use of aspirin for primary prevention (Table 3).23–31 The initial trials were in male physicians in the United Kingdom and the United States in the late 1980s and had somewhat conflicting results. A British study did not find a significant reduction in myocardial infarction,23 but the US Physician’s Health Study study did: the relative risk was 0.56 (95% confidence interval 0.45–0.70, P < .00001).24 The US study had more than four times the number of participants, used different dosing (325 mg every other day compared with 500 or 300 mg daily in the British study), and had a higher rate of compliance.
Several studies over the next decade demonstrated variable but significant reductions in cardiovascular events as well.25–27
A meta-analysis of primary prevention trials of aspirin was published in 2009.22 Although the relative risk reduction was similar in primary and secondary prevention, the absolute risk reduction in primary prevention was not nearly as great as in secondary prevention.
These findings are somewhat difficult to interpret, as the component trials included a wide spectrum of patients, ranging from healthy people with no symptoms and no known risk factors to those with limited risk factors. The trials were also performed over several decades during which primary prevention strategies were evolving. Additionally, most of the participants were middle-aged, nondiabetic men, so the results may not necessarily apply to people with diabetes, to women, or to the elderly. Thus, the pooled data in favor of aspirin for primary prevention may not be as broadly applicable to the general population as was once thought.
Aspirin for primary prevention in women
Guidelines for aspirin use in primary prevention were initially thought to be equally applicable to both sexes. However, concerns about the relatively low number of women participating in the studies and the possible mechanistic differences in aspirin efficacy in men vs women prompted further study.
A meta-analysis of randomized controlled trials found that aspirin was associated with a 12% relative reduction in the incidence of cardiovascular events in women and 14% in men. On the other hand, for stroke, the relative risk reduction was 17% in women, while men had no benefit.32
Most of the women in this meta-analysis were participants in the Women’s Health Study, and they were at low baseline risk.28 Although only about 10% of patients in this study were over age 65, this older group accounted for most of the benefit: these older women had a 26% risk reduction in major adverse cardiovascular events and 30% reduction in stroke.
Thus, for women, aspirin seems to become effective for primary prevention at an older age than in men, and the guidelines have been changed accordingly (Figure 1).
More women should be taking aspirin than actually are. For example, Rivera et al33 found that only 41% of eligible women were receiving aspirin for primary prevention and 48% of eligible women were receiving it for secondary prevention.
People with diabetes
People with diabetes without overt cardiovascular disease are at higher risk of cardiovascular events than age- and sex-matched controls.34 On the other hand, people with diabetes may be more prone to aspirin resistance and may not derive as much cardiovascular benefit from aspirin.
Early primary prevention studies included few people with diabetes. Subsequent meta-analyses of trials that used a wide range of aspirin doses found a relative risk reduction of 9%, which was not statistically significant.9,35,36
But there is some evidence that people with diabetes, with37 and without22 coronary disease, may be at higher inherent risk of bleeding than people without diabetes. Although aspirin may not necessarily increase the risk of bleeding in diabetic patients, recent data suggest no benefit in terms of a reduction in vascular events.38
The balance of risk vs benefit for aspirin in this special population is not clear, although some argue that these patients should be treated somewhere on the spectrum of risk between primary and secondary prevention.
The US Preventive Services Task Force did not differentiate between people with or without diabetes in its 2009 guidelines for aspirin for primary prevention.8 However, the debate is reflected in a change in 2010 American College of Cardiology/American Diabetes Association guidelines regarding aspirin use in people with diabetes without known cardiovascular disease.39 As opposed to earlier recommendations from these organizations in favor of aspirin for all people with diabetes regardless of 10-year risk, current recommendations advise low-dose aspirin (81–162 mg) for diabetic patients without known vascular disease who have a greater than 10% risk of a cardiovascular event and are not at increased risk of bleeding.
These changes were based on the findings of two trials: the Prevention and Progression of Arterial Disease and Diabetes Trial (POPADAD) and the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) study. These did not show a statistically significant benefit in prevention of cardiovascular events with aspirin.29,30
After the new guidelines came out, a meta-analysis further bolstered its recommendations. 40 In seven randomized clinical trials in 11,000 patients, the relative risk reduction was 9% with aspirin, which did not reach statistical significance.
Statins may dilute the benefit of aspirin
The use of statins has been increasing, and this trend may have played a role in the marginal benefit of aspirin therapy in these recent studies. In the Japanese trial, approximately 25% of the patients were known to be using a statin; the percentage of statin use was not reported specifically in POPADAD, but both of these studies were published in 2008, when the proportion of diabetic patients taking a statin would be expected to be higher than in earlier primary prevention trials, which were performed primarily in the 1990s. Thus, the beneficial effects of statins may have somewhat diluted the risk reduction attributable to aspirin.
Trials under way in patients with diabetes
The evolving and somewhat conflicting guidelines highlight the need for further study in patients with diabetes. To address this area, two trials are in progress: the Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D) and A Study of Cardiovascular Events in Diabetes (ASCEND).41,42
ACCEPT-D is testing low-dose aspirin (100 mg daily) in diabetic patients who are also on simvastatin. This study also includes prespecified subgroups stratified by sex, age, and baseline lipid levels.
The ASCEND trial will use the same aspirin dose as ACCEPT-D, with a target enrollment of 10,000 patients with diabetes without known vascular disease.
More frequent dosing for people with diabetes?
Although not supported by current guidelines, recent work has suggested that people with diabetes may need more-frequent dosing of aspirin.43 This topic warrants further investigation.
Aspirin as primary prevention in elderly patients
The incidence of cardiovascular events increases with age37—but so does the incidence of gastrointestinal bleeding.44 Upper gastrointestinal bleeding is especially worrisome in the elderly, in whom the estimated case-fatality rate is high.12 Assessment of risk and benefit is particularly important in patients over age 65 without known coronary disease.
Uncertainty about aspirin use in this population is reflected in the most recent US Preventive Services Task Force guidelines, which do not advocate either for or against regular aspirin use for primary prevention in those over the age of 80.
Data on this topic from clinical trials are limited. The Antithrombotic Trialists’ Collaboration (2009) found that although age is associated with a risk of major coronary events similar to that of other traditional risk factors such as diabetes, hypertension, and tobacco use, older age is also associated with the highest risk of major extracranial bleeding.22
Because of the lack of data in this population, several studies are currently under way. The Aspirin in Reducing Events in the Elderly (ASPREE) trial is studying 100 mg daily in nondiabetic patients without known cardiovascular disease who are age 70 and older.45 An additional trial will study patients age 60 to 85 with concurrent diagnoses of hypertension, hyperlipidemia, or diabetes and will test the same aspirin dose as in ASPREE.46 These trials should provide further insight into the safety and efficacy of aspirin for primary prevention in the elderly.
FUTURE DIRECTIONS
Aspirin remains a cornerstone of therapy in patients with cardiovascular disease and in secondary prevention of adverse cardiovascular events, but its role in primary prevention remains under scrutiny. Recommendations have evolved to reflect emerging data in special populations, and an algorithm based on Framingham risk assessment in men for myocardial infarction and ischemic stroke assessment in women for assessing appropriateness of aspirin therapy based on currently available guidelines is presented in Figure 1.6,8,47–49 Targeted studies have advanced our understanding of aspirin use in women, and future studies in people with diabetes and in the elderly should provide further insight into the role of aspirin for primary prevention in these specific groups as well.
Additionally, the range of doses used in clinical studies has propagated the general misperception that higher doses of aspirin are more efficacious. Future studies should continue to use lower doses of aspirin to minimize bleeding risk with an added focus on re-examining its net benefit in the modern era of increasing statin use, which may reduce the absolute risk reduction attributable to aspirin.
One particular area of debate is whether enteric coating can result in functional aspirin resistance. Grosser et al50 found that sustained aspirin resistance was rare, and “pseudoresistance” was related to the use of a single enteric-coated aspirin instead of immediate-release aspirin in people who had not been taking aspirin up to then. This complements an earlier study, which found that enteric-coated aspirin had an appropriate effect when given for 7 days.51 Therefore, for patients who have not been taking aspirin, the first dose should always be immediate-release, not enteric coated.
SHOULD OUR PATIENT RECEIVE ASPIRIN?
The patient we described at the beginning of this article has several risk factors—hypertension, dyslipidemia, left ventricular hypertrophy, and smoking—but no known cardiovascular disease as yet. Her risk of an adverse cardiovascular event appears moderate. However, her 10-year risk of stroke by the Framingham risk calculation is 10%, which would qualify her for aspirin for primary prevention. Of particular note is that the significance of left ventricular hypertrophy as a risk factor for stroke in women is higher than in men and in our case accounts for half of this patient’s risk.
We should explain to the patient that the anticipated benefits of aspirin for stroke prevention outweigh bleeding risks, and thus aspirin therapy would be recommended. However, with her elevated LDL-cholesterol, she may benefit from a statin, which could lessen the relative risk reduction from additional aspirin use.
- Chan FK, Graham DY. Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol Ther 2004; 19:1051–1061.
- Peters RJ, Mehta SR, Fox KA, et al; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003; 108:1682–1687.
- Elwood PC, Cochrane AL, Burr ML, et al. A randomized controlled trial of acetyl salicylic acid in the secondary prevention of mortality from myocardial infarction. Br Med J 1974; 1:436–440.
- Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998; 316:1337–1343.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.
- Levine GN, Bates ER, Blankenship JC, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation 2010; 121:2694–2701.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am 2005; 34:643–664.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995; 311:222–226.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987; 69:180–186.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J 2006; 27:2667–2674.
- Berger PB, Bhatt DL, Fuster V, et al; CHARISMA Investigators. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010; 121:2575–2583.
- Juul-Möller S, Edvardsson N, Jahnmatz B, Rosén A, Sørensen S, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet 1992; 340:1421–1425.
- Ridker PM, Manson JE, Gaziano JM, Buring JE, Hennekens CH. Low-dose aspirin therapy for chronic stable angina. A randomized, placebo-controlled clinical trial. Ann Intern Med 1991; 114:835–839.
- Berger JS, Brown DL, Burke GL, et al. Aspirin use, dose, and clinical outcomes in postmenopausal women with stable cardiovascular disease: the Women’s Health Initiative Observational Study. Circ Cardiovasc Qual Outcomes 2009; 2:78–87.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- de Gaetano GCollaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Fowkes FG, Price JF, Stewart MC, et al; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010; 303:841–848.
- Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006; 295:306–313.
- Rivera CM, Song J, Copeland L, Buirge C, Ory M, McNeal CJ. Underuse of aspirin for primary and secondary prevention of cardiovascular disease events in women. J Womens Health (Larchmt) 2012; 21:379–387.
- Wilson R, Gazzala J, House J. Aspirin in primary and secondary prevention in elderly adults revisited. South Med J 2012; 105:82–86.
- De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009; 339:b4531.
- Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010; 87:211–218.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J 2009; 30:2226–2232.
- De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012; 307:2286–2294.
- Pignone M, Alberts MJ, Colwell JA, et al; American Diabetes Association; American Heart Association; American College of Cardiology Foundation. Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol 2010; 55:2878–2886.
- Butalia S, Leung AA, Ghali WA, Rabi DM. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2011; 10:25.
- De Berardis G, Sacco M, Evangelista V, et al; ACCEPT-D Study Group. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007; 8:21.
- British Heart Foundation. ASCEND: A Study of Cardiovascular Events in Diabetes. http://www.ctsu.ox.ac.uk/ascend. Accessed April 1, 2013.
- Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012; 10:1220–1230.
- Hernández-Díaz S, Garcia Rodriguez LA. Cardioprotective aspirin users and their excess risk for upper gastrointestinal complications. BMC Med 2006; 4:22.
- Nelson MR, Reid CM, Ames DA, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust 2008; 189:105–109.
- Teramoto T, Shimada K, Uchiyama S, et al. Rationale, design, and baseline data of the Japanese Primary Prevention Project (JPPP)—a randomized, open-label, controlled trial of aspirin versus no aspirin in patients with multiple risk factors for vascular events. Am Heart J 2010; 159:361–369.e4.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:671–719.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2011; 57:e16–e94.
- Wright RS, Anderson JL, Adams CD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 57:e215–e367.
- Grosser T, Fries S, Lawson JA, Kapoor SC, Grant GR, Fitzgerald GA. Drug resistance and pseudoresistance: An unintended consequence of enteric coating aspirin. Circulation 2012; Epub ahead of print.
- Karha J, Rajagopal V, Kottke-Marchant K, Bhatt DL. Lack of effect of enteric coating on aspirin-induced inhibition of platelet aggregation in healthy volunteers. Am Heart J 2006; 151:976.e7–e11.
A 57-year-old woman with no history of cardiovascular disease comes to the clinic for her annual evaluation. She does not have diabetes mellitus, but she does have hypertension and chronic osteoarthritis, currently treated with acetaminophen. Additionally, she admits to active tobacco use. Her systolic blood pressure is 130 mm Hg on therapy with hydrochlorothiazide. Her electrocardiogram demonstrates left ventricular hypertrophy. Her low-density lipoprotein (LDL) cholesterol level is 140 mg/dL, and her high-density lipoprotein (HDL) cholesterol level is 50 mg/dL. Should this patient be started on aspirin therapy?
Acetylsalicylic acid (aspirin) is an analgesic, antipyretic, and anti-inflammatory agent, but its more prominent use today is as an antithrombotic agent to treat or prevent cardiovascular events. Its antithrombotic properties are due to its effects on the enzyme cyclooxygenase. However, cyclooxygenase is also involved in regulation of the gastric mucosa, and so aspirin increases the risk of gastrointestinal bleeding.
Approximately 50 million people take aspirin on a daily basis to treat or prevent cardiovascular disease.1 Of these, at least half are taking more than 100 mg per day,2 reflecting the general belief that, for aspirin dosage, “more is better”—which is not true.
Additionally, recommendations about the use of aspirin were based on studies that included relatively few members of several important subgroups, such as people with diabetes without known cardiovascular disease, women, and the elderly, and thus may not reflect appropriate indications and dosages for these groups.
Here, we examine the literature, outline an individualized approach to aspirin therapy, and highlight areas for future study.
HISTORY OF ASPIRIN USE IN CARDIOVASCULAR DISEASE
- 1700s—Willow bark is used as an analgesic.
- 1897—Synthetic aspirin is developed as an antipyretic and anti-inflammatory agent.
- 1974—First landmark trial of aspirin for secondary prevention of myocardial infarction.3
- 1982—Nobel Prize awarded for discovery of aspirin mechanism.
- 1985—US Food and Drug Administration approves aspirin for the treatment and secondary prevention of acute myocardial infarction.
- 1998—The Second International Study of Infarct Survival (ISIS-2) finds that giving aspirin to patients with myocardial infarction within 24 hours of presentation leads to a significant reduction in vascular deaths.4
Ongoing uncertainties
Aspirin now carries a class I indication for all patients with suspected myocardial infarction. Since there are an estimated 600,000 new coronary events and 325,000 recurrent ischemic events per year in the United States,5 the need for aspirin will continue to remain great. It is also approved to prevent and treat stroke and in patients with unstable angina.
However, questions continue to emerge about aspirin’s dosing and appropriate use in specific populations. The initial prevention trials used a wide range of doses and, as mentioned, included few women, few people with diabetes, and few elderly people. The uncertainties are especially pertinent for patients without known vascular disease, in whom the absolute risk reduction is much less, making the assessment of bleeding risk particularly important. Furthermore, the absolute risk-to-benefit assessment may be different in certain populations.
Guidelines on the use of aspirin to prevent cardiovascular disease (Table 1)6–10 have evolved to take into account these possible disparities, and studies are taking place to further investigate aspirin use in these groups.
ASPIRIN AND GASTROINTESTINAL BLEEDING
Aspirin’s association with bleeding, particularly gastrointestinal bleeding, was recognized early as a use-limiting side effect. With or without aspirin, gastrointestinal bleeding is a common cause of morbidity and death, with an incidence of approximately 100 per 100,000 bleeding episodes in adults per year for upper gastrointestinal bleeding and 20 to 30 per 100,000 per year for lower gastrointestinal bleeding.11,12
The standard dosage (ie, 325 mg/day) is associated with a significantly higher risk of gastrointestinal bleeding (including fatal bleeds) than is 75 mg.13 However, even with lower doses, the risk of gastrointestinal bleeding is estimated to be twice as high as with no aspirin.14
And here is the irony: studies have shown that higher doses of aspirin offer no advantage in preventing thrombotic events compared with lower doses.15 For example, the Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Organization to Assess Strategies for Ischemic Stroke Syndromes study reported a higher rate of gastrointestinal bleeding with standard-dose aspirin therapy than with low-dose aspirin, with no additional cardiovascular benefit with the higher dose.16
Furthermore, several other risk factors increase the risk of gastrointestinal bleeding with aspirin use (Table 2). These risk factors are common in the general population but were not necessarily represented in participants in clinical trials. Thus, estimates of risk based on trial data most likely underestimate actual risk in the general population, and therefore, the individual patient’s risk of gastrointestinal bleeding, based on these and other factors, needs to be taken into consideration.
ASPIRIN IN PATIENTS WITH CORONARY ARTERY DISEASE
Randomized clinical trials have validated the benefits of aspirin in secondary prevention of cardiovascular events in patients who have had a myocardial infarction. Patients with coronary disease who withdraw from aspirin therapy or otherwise do not adhere to it have a risk of cardiovascular events three times higher than those who stay with it.17
Despite the strong data, however, several issues and questions remain about the use of aspirin for secondary prevention.
Bleeding risk must be considered, since gastrointestinal bleeding is associated with a higher risk of death and myocardial infarction in patients with cardiovascular disease.18 Many patients with coronary disease are on more than one antiplatelet or anticoagulant therapy for concomitant conditions such as atrial fibrillation or because they underwent a percutaneous intervention, which further increases the risk of bleeding.
This bleeding risk is reflected in changes in the most recent recommendations for aspirin dosing after percutaneous coronary intervention. Earlier guidelines advocated use of either 162 or 325 mg after the procedure. However, the most recent update (in 2011) now supports 81 mg for maintenance dosing after intervention.7
Patients with coronary disease but without prior myocardial infarction or intervention. Current guidelines recommend 75 to 162 mg of aspirin in all patients with coronary artery disease.6 However, this group is diverse and includes patients who have undergone percutaneous coronary intervention, patients with chronic stable angina, and patients with asymptomatic coronary artery disease found on imaging studies. The magnitude of benefit is not clear for those who have no symptoms or who have stable angina.
Most of the evidence supporting aspirin use in chronic angina came from a single trial in Sweden, in which 2,000 patients with chronic stable angina were given either 75 mg daily or placebo. Those who received aspirin had a 34% lower rate of myocardial infarction and sudden death.19
A substudy of the Physicians’ Health Study, with fewer patients, also noted a significant reduction in the rate of first myocardial infarction. The dose of aspirin in this study was 325 mg every other day.20
In the Women’s Health Initiative Observational Study, 70% of women with stable cardiovascular disease taking aspirin were taking 325 mg daily.21 This study demonstrated a significant reduction in the cardiovascular mortality rate, which supports current guidelines, and found no difference in outcomes with doses of 81 mg compared with 325 mg.21 This again corroborates that low-dose aspirin is preferential to standard-dose aspirin in women with cardiovascular disease.
These findings have not been validated in larger prospective trials. Thus, current guidelines for aspirin use may reflect extrapolation of aspirin benefit from higher-risk patients to lower-risk patients.
Nevertheless, although the debate continues, it has generally been accepted that in patients who are at high risk of vascular disease or who have had a myocardial infarction, the benefits of aspirin—a 20% relative reduction in vascular events22—clearly outweigh the risks.
ASPIRIN FOR PRIMARY PREVENTION
Assessing risk vs benefit is more complex when considering populations without known cardiovascular disease.
Only a few studies have specifically evaluated the use of aspirin for primary prevention (Table 3).23–31 The initial trials were in male physicians in the United Kingdom and the United States in the late 1980s and had somewhat conflicting results. A British study did not find a significant reduction in myocardial infarction,23 but the US Physician’s Health Study study did: the relative risk was 0.56 (95% confidence interval 0.45–0.70, P < .00001).24 The US study had more than four times the number of participants, used different dosing (325 mg every other day compared with 500 or 300 mg daily in the British study), and had a higher rate of compliance.
Several studies over the next decade demonstrated variable but significant reductions in cardiovascular events as well.25–27
A meta-analysis of primary prevention trials of aspirin was published in 2009.22 Although the relative risk reduction was similar in primary and secondary prevention, the absolute risk reduction in primary prevention was not nearly as great as in secondary prevention.
These findings are somewhat difficult to interpret, as the component trials included a wide spectrum of patients, ranging from healthy people with no symptoms and no known risk factors to those with limited risk factors. The trials were also performed over several decades during which primary prevention strategies were evolving. Additionally, most of the participants were middle-aged, nondiabetic men, so the results may not necessarily apply to people with diabetes, to women, or to the elderly. Thus, the pooled data in favor of aspirin for primary prevention may not be as broadly applicable to the general population as was once thought.
Aspirin for primary prevention in women
Guidelines for aspirin use in primary prevention were initially thought to be equally applicable to both sexes. However, concerns about the relatively low number of women participating in the studies and the possible mechanistic differences in aspirin efficacy in men vs women prompted further study.
A meta-analysis of randomized controlled trials found that aspirin was associated with a 12% relative reduction in the incidence of cardiovascular events in women and 14% in men. On the other hand, for stroke, the relative risk reduction was 17% in women, while men had no benefit.32
Most of the women in this meta-analysis were participants in the Women’s Health Study, and they were at low baseline risk.28 Although only about 10% of patients in this study were over age 65, this older group accounted for most of the benefit: these older women had a 26% risk reduction in major adverse cardiovascular events and 30% reduction in stroke.
Thus, for women, aspirin seems to become effective for primary prevention at an older age than in men, and the guidelines have been changed accordingly (Figure 1).
More women should be taking aspirin than actually are. For example, Rivera et al33 found that only 41% of eligible women were receiving aspirin for primary prevention and 48% of eligible women were receiving it for secondary prevention.
People with diabetes
People with diabetes without overt cardiovascular disease are at higher risk of cardiovascular events than age- and sex-matched controls.34 On the other hand, people with diabetes may be more prone to aspirin resistance and may not derive as much cardiovascular benefit from aspirin.
Early primary prevention studies included few people with diabetes. Subsequent meta-analyses of trials that used a wide range of aspirin doses found a relative risk reduction of 9%, which was not statistically significant.9,35,36
But there is some evidence that people with diabetes, with37 and without22 coronary disease, may be at higher inherent risk of bleeding than people without diabetes. Although aspirin may not necessarily increase the risk of bleeding in diabetic patients, recent data suggest no benefit in terms of a reduction in vascular events.38
The balance of risk vs benefit for aspirin in this special population is not clear, although some argue that these patients should be treated somewhere on the spectrum of risk between primary and secondary prevention.
The US Preventive Services Task Force did not differentiate between people with or without diabetes in its 2009 guidelines for aspirin for primary prevention.8 However, the debate is reflected in a change in 2010 American College of Cardiology/American Diabetes Association guidelines regarding aspirin use in people with diabetes without known cardiovascular disease.39 As opposed to earlier recommendations from these organizations in favor of aspirin for all people with diabetes regardless of 10-year risk, current recommendations advise low-dose aspirin (81–162 mg) for diabetic patients without known vascular disease who have a greater than 10% risk of a cardiovascular event and are not at increased risk of bleeding.
These changes were based on the findings of two trials: the Prevention and Progression of Arterial Disease and Diabetes Trial (POPADAD) and the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) study. These did not show a statistically significant benefit in prevention of cardiovascular events with aspirin.29,30
After the new guidelines came out, a meta-analysis further bolstered its recommendations. 40 In seven randomized clinical trials in 11,000 patients, the relative risk reduction was 9% with aspirin, which did not reach statistical significance.
Statins may dilute the benefit of aspirin
The use of statins has been increasing, and this trend may have played a role in the marginal benefit of aspirin therapy in these recent studies. In the Japanese trial, approximately 25% of the patients were known to be using a statin; the percentage of statin use was not reported specifically in POPADAD, but both of these studies were published in 2008, when the proportion of diabetic patients taking a statin would be expected to be higher than in earlier primary prevention trials, which were performed primarily in the 1990s. Thus, the beneficial effects of statins may have somewhat diluted the risk reduction attributable to aspirin.
Trials under way in patients with diabetes
The evolving and somewhat conflicting guidelines highlight the need for further study in patients with diabetes. To address this area, two trials are in progress: the Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D) and A Study of Cardiovascular Events in Diabetes (ASCEND).41,42
ACCEPT-D is testing low-dose aspirin (100 mg daily) in diabetic patients who are also on simvastatin. This study also includes prespecified subgroups stratified by sex, age, and baseline lipid levels.
The ASCEND trial will use the same aspirin dose as ACCEPT-D, with a target enrollment of 10,000 patients with diabetes without known vascular disease.
More frequent dosing for people with diabetes?
Although not supported by current guidelines, recent work has suggested that people with diabetes may need more-frequent dosing of aspirin.43 This topic warrants further investigation.
Aspirin as primary prevention in elderly patients
The incidence of cardiovascular events increases with age37—but so does the incidence of gastrointestinal bleeding.44 Upper gastrointestinal bleeding is especially worrisome in the elderly, in whom the estimated case-fatality rate is high.12 Assessment of risk and benefit is particularly important in patients over age 65 without known coronary disease.
Uncertainty about aspirin use in this population is reflected in the most recent US Preventive Services Task Force guidelines, which do not advocate either for or against regular aspirin use for primary prevention in those over the age of 80.
Data on this topic from clinical trials are limited. The Antithrombotic Trialists’ Collaboration (2009) found that although age is associated with a risk of major coronary events similar to that of other traditional risk factors such as diabetes, hypertension, and tobacco use, older age is also associated with the highest risk of major extracranial bleeding.22
Because of the lack of data in this population, several studies are currently under way. The Aspirin in Reducing Events in the Elderly (ASPREE) trial is studying 100 mg daily in nondiabetic patients without known cardiovascular disease who are age 70 and older.45 An additional trial will study patients age 60 to 85 with concurrent diagnoses of hypertension, hyperlipidemia, or diabetes and will test the same aspirin dose as in ASPREE.46 These trials should provide further insight into the safety and efficacy of aspirin for primary prevention in the elderly.
FUTURE DIRECTIONS
Aspirin remains a cornerstone of therapy in patients with cardiovascular disease and in secondary prevention of adverse cardiovascular events, but its role in primary prevention remains under scrutiny. Recommendations have evolved to reflect emerging data in special populations, and an algorithm based on Framingham risk assessment in men for myocardial infarction and ischemic stroke assessment in women for assessing appropriateness of aspirin therapy based on currently available guidelines is presented in Figure 1.6,8,47–49 Targeted studies have advanced our understanding of aspirin use in women, and future studies in people with diabetes and in the elderly should provide further insight into the role of aspirin for primary prevention in these specific groups as well.
Additionally, the range of doses used in clinical studies has propagated the general misperception that higher doses of aspirin are more efficacious. Future studies should continue to use lower doses of aspirin to minimize bleeding risk with an added focus on re-examining its net benefit in the modern era of increasing statin use, which may reduce the absolute risk reduction attributable to aspirin.
One particular area of debate is whether enteric coating can result in functional aspirin resistance. Grosser et al50 found that sustained aspirin resistance was rare, and “pseudoresistance” was related to the use of a single enteric-coated aspirin instead of immediate-release aspirin in people who had not been taking aspirin up to then. This complements an earlier study, which found that enteric-coated aspirin had an appropriate effect when given for 7 days.51 Therefore, for patients who have not been taking aspirin, the first dose should always be immediate-release, not enteric coated.
SHOULD OUR PATIENT RECEIVE ASPIRIN?
The patient we described at the beginning of this article has several risk factors—hypertension, dyslipidemia, left ventricular hypertrophy, and smoking—but no known cardiovascular disease as yet. Her risk of an adverse cardiovascular event appears moderate. However, her 10-year risk of stroke by the Framingham risk calculation is 10%, which would qualify her for aspirin for primary prevention. Of particular note is that the significance of left ventricular hypertrophy as a risk factor for stroke in women is higher than in men and in our case accounts for half of this patient’s risk.
We should explain to the patient that the anticipated benefits of aspirin for stroke prevention outweigh bleeding risks, and thus aspirin therapy would be recommended. However, with her elevated LDL-cholesterol, she may benefit from a statin, which could lessen the relative risk reduction from additional aspirin use.
A 57-year-old woman with no history of cardiovascular disease comes to the clinic for her annual evaluation. She does not have diabetes mellitus, but she does have hypertension and chronic osteoarthritis, currently treated with acetaminophen. Additionally, she admits to active tobacco use. Her systolic blood pressure is 130 mm Hg on therapy with hydrochlorothiazide. Her electrocardiogram demonstrates left ventricular hypertrophy. Her low-density lipoprotein (LDL) cholesterol level is 140 mg/dL, and her high-density lipoprotein (HDL) cholesterol level is 50 mg/dL. Should this patient be started on aspirin therapy?
Acetylsalicylic acid (aspirin) is an analgesic, antipyretic, and anti-inflammatory agent, but its more prominent use today is as an antithrombotic agent to treat or prevent cardiovascular events. Its antithrombotic properties are due to its effects on the enzyme cyclooxygenase. However, cyclooxygenase is also involved in regulation of the gastric mucosa, and so aspirin increases the risk of gastrointestinal bleeding.
Approximately 50 million people take aspirin on a daily basis to treat or prevent cardiovascular disease.1 Of these, at least half are taking more than 100 mg per day,2 reflecting the general belief that, for aspirin dosage, “more is better”—which is not true.
Additionally, recommendations about the use of aspirin were based on studies that included relatively few members of several important subgroups, such as people with diabetes without known cardiovascular disease, women, and the elderly, and thus may not reflect appropriate indications and dosages for these groups.
Here, we examine the literature, outline an individualized approach to aspirin therapy, and highlight areas for future study.
HISTORY OF ASPIRIN USE IN CARDIOVASCULAR DISEASE
- 1700s—Willow bark is used as an analgesic.
- 1897—Synthetic aspirin is developed as an antipyretic and anti-inflammatory agent.
- 1974—First landmark trial of aspirin for secondary prevention of myocardial infarction.3
- 1982—Nobel Prize awarded for discovery of aspirin mechanism.
- 1985—US Food and Drug Administration approves aspirin for the treatment and secondary prevention of acute myocardial infarction.
- 1998—The Second International Study of Infarct Survival (ISIS-2) finds that giving aspirin to patients with myocardial infarction within 24 hours of presentation leads to a significant reduction in vascular deaths.4
Ongoing uncertainties
Aspirin now carries a class I indication for all patients with suspected myocardial infarction. Since there are an estimated 600,000 new coronary events and 325,000 recurrent ischemic events per year in the United States,5 the need for aspirin will continue to remain great. It is also approved to prevent and treat stroke and in patients with unstable angina.
However, questions continue to emerge about aspirin’s dosing and appropriate use in specific populations. The initial prevention trials used a wide range of doses and, as mentioned, included few women, few people with diabetes, and few elderly people. The uncertainties are especially pertinent for patients without known vascular disease, in whom the absolute risk reduction is much less, making the assessment of bleeding risk particularly important. Furthermore, the absolute risk-to-benefit assessment may be different in certain populations.
Guidelines on the use of aspirin to prevent cardiovascular disease (Table 1)6–10 have evolved to take into account these possible disparities, and studies are taking place to further investigate aspirin use in these groups.
ASPIRIN AND GASTROINTESTINAL BLEEDING
Aspirin’s association with bleeding, particularly gastrointestinal bleeding, was recognized early as a use-limiting side effect. With or without aspirin, gastrointestinal bleeding is a common cause of morbidity and death, with an incidence of approximately 100 per 100,000 bleeding episodes in adults per year for upper gastrointestinal bleeding and 20 to 30 per 100,000 per year for lower gastrointestinal bleeding.11,12
The standard dosage (ie, 325 mg/day) is associated with a significantly higher risk of gastrointestinal bleeding (including fatal bleeds) than is 75 mg.13 However, even with lower doses, the risk of gastrointestinal bleeding is estimated to be twice as high as with no aspirin.14
And here is the irony: studies have shown that higher doses of aspirin offer no advantage in preventing thrombotic events compared with lower doses.15 For example, the Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Organization to Assess Strategies for Ischemic Stroke Syndromes study reported a higher rate of gastrointestinal bleeding with standard-dose aspirin therapy than with low-dose aspirin, with no additional cardiovascular benefit with the higher dose.16
Furthermore, several other risk factors increase the risk of gastrointestinal bleeding with aspirin use (Table 2). These risk factors are common in the general population but were not necessarily represented in participants in clinical trials. Thus, estimates of risk based on trial data most likely underestimate actual risk in the general population, and therefore, the individual patient’s risk of gastrointestinal bleeding, based on these and other factors, needs to be taken into consideration.
ASPIRIN IN PATIENTS WITH CORONARY ARTERY DISEASE
Randomized clinical trials have validated the benefits of aspirin in secondary prevention of cardiovascular events in patients who have had a myocardial infarction. Patients with coronary disease who withdraw from aspirin therapy or otherwise do not adhere to it have a risk of cardiovascular events three times higher than those who stay with it.17
Despite the strong data, however, several issues and questions remain about the use of aspirin for secondary prevention.
Bleeding risk must be considered, since gastrointestinal bleeding is associated with a higher risk of death and myocardial infarction in patients with cardiovascular disease.18 Many patients with coronary disease are on more than one antiplatelet or anticoagulant therapy for concomitant conditions such as atrial fibrillation or because they underwent a percutaneous intervention, which further increases the risk of bleeding.
This bleeding risk is reflected in changes in the most recent recommendations for aspirin dosing after percutaneous coronary intervention. Earlier guidelines advocated use of either 162 or 325 mg after the procedure. However, the most recent update (in 2011) now supports 81 mg for maintenance dosing after intervention.7
Patients with coronary disease but without prior myocardial infarction or intervention. Current guidelines recommend 75 to 162 mg of aspirin in all patients with coronary artery disease.6 However, this group is diverse and includes patients who have undergone percutaneous coronary intervention, patients with chronic stable angina, and patients with asymptomatic coronary artery disease found on imaging studies. The magnitude of benefit is not clear for those who have no symptoms or who have stable angina.
Most of the evidence supporting aspirin use in chronic angina came from a single trial in Sweden, in which 2,000 patients with chronic stable angina were given either 75 mg daily or placebo. Those who received aspirin had a 34% lower rate of myocardial infarction and sudden death.19
A substudy of the Physicians’ Health Study, with fewer patients, also noted a significant reduction in the rate of first myocardial infarction. The dose of aspirin in this study was 325 mg every other day.20
In the Women’s Health Initiative Observational Study, 70% of women with stable cardiovascular disease taking aspirin were taking 325 mg daily.21 This study demonstrated a significant reduction in the cardiovascular mortality rate, which supports current guidelines, and found no difference in outcomes with doses of 81 mg compared with 325 mg.21 This again corroborates that low-dose aspirin is preferential to standard-dose aspirin in women with cardiovascular disease.
These findings have not been validated in larger prospective trials. Thus, current guidelines for aspirin use may reflect extrapolation of aspirin benefit from higher-risk patients to lower-risk patients.
Nevertheless, although the debate continues, it has generally been accepted that in patients who are at high risk of vascular disease or who have had a myocardial infarction, the benefits of aspirin—a 20% relative reduction in vascular events22—clearly outweigh the risks.
ASPIRIN FOR PRIMARY PREVENTION
Assessing risk vs benefit is more complex when considering populations without known cardiovascular disease.
Only a few studies have specifically evaluated the use of aspirin for primary prevention (Table 3).23–31 The initial trials were in male physicians in the United Kingdom and the United States in the late 1980s and had somewhat conflicting results. A British study did not find a significant reduction in myocardial infarction,23 but the US Physician’s Health Study study did: the relative risk was 0.56 (95% confidence interval 0.45–0.70, P < .00001).24 The US study had more than four times the number of participants, used different dosing (325 mg every other day compared with 500 or 300 mg daily in the British study), and had a higher rate of compliance.
Several studies over the next decade demonstrated variable but significant reductions in cardiovascular events as well.25–27
A meta-analysis of primary prevention trials of aspirin was published in 2009.22 Although the relative risk reduction was similar in primary and secondary prevention, the absolute risk reduction in primary prevention was not nearly as great as in secondary prevention.
These findings are somewhat difficult to interpret, as the component trials included a wide spectrum of patients, ranging from healthy people with no symptoms and no known risk factors to those with limited risk factors. The trials were also performed over several decades during which primary prevention strategies were evolving. Additionally, most of the participants were middle-aged, nondiabetic men, so the results may not necessarily apply to people with diabetes, to women, or to the elderly. Thus, the pooled data in favor of aspirin for primary prevention may not be as broadly applicable to the general population as was once thought.
Aspirin for primary prevention in women
Guidelines for aspirin use in primary prevention were initially thought to be equally applicable to both sexes. However, concerns about the relatively low number of women participating in the studies and the possible mechanistic differences in aspirin efficacy in men vs women prompted further study.
A meta-analysis of randomized controlled trials found that aspirin was associated with a 12% relative reduction in the incidence of cardiovascular events in women and 14% in men. On the other hand, for stroke, the relative risk reduction was 17% in women, while men had no benefit.32
Most of the women in this meta-analysis were participants in the Women’s Health Study, and they were at low baseline risk.28 Although only about 10% of patients in this study were over age 65, this older group accounted for most of the benefit: these older women had a 26% risk reduction in major adverse cardiovascular events and 30% reduction in stroke.
Thus, for women, aspirin seems to become effective for primary prevention at an older age than in men, and the guidelines have been changed accordingly (Figure 1).
More women should be taking aspirin than actually are. For example, Rivera et al33 found that only 41% of eligible women were receiving aspirin for primary prevention and 48% of eligible women were receiving it for secondary prevention.
People with diabetes
People with diabetes without overt cardiovascular disease are at higher risk of cardiovascular events than age- and sex-matched controls.34 On the other hand, people with diabetes may be more prone to aspirin resistance and may not derive as much cardiovascular benefit from aspirin.
Early primary prevention studies included few people with diabetes. Subsequent meta-analyses of trials that used a wide range of aspirin doses found a relative risk reduction of 9%, which was not statistically significant.9,35,36
But there is some evidence that people with diabetes, with37 and without22 coronary disease, may be at higher inherent risk of bleeding than people without diabetes. Although aspirin may not necessarily increase the risk of bleeding in diabetic patients, recent data suggest no benefit in terms of a reduction in vascular events.38
The balance of risk vs benefit for aspirin in this special population is not clear, although some argue that these patients should be treated somewhere on the spectrum of risk between primary and secondary prevention.
The US Preventive Services Task Force did not differentiate between people with or without diabetes in its 2009 guidelines for aspirin for primary prevention.8 However, the debate is reflected in a change in 2010 American College of Cardiology/American Diabetes Association guidelines regarding aspirin use in people with diabetes without known cardiovascular disease.39 As opposed to earlier recommendations from these organizations in favor of aspirin for all people with diabetes regardless of 10-year risk, current recommendations advise low-dose aspirin (81–162 mg) for diabetic patients without known vascular disease who have a greater than 10% risk of a cardiovascular event and are not at increased risk of bleeding.
These changes were based on the findings of two trials: the Prevention and Progression of Arterial Disease and Diabetes Trial (POPADAD) and the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) study. These did not show a statistically significant benefit in prevention of cardiovascular events with aspirin.29,30
After the new guidelines came out, a meta-analysis further bolstered its recommendations. 40 In seven randomized clinical trials in 11,000 patients, the relative risk reduction was 9% with aspirin, which did not reach statistical significance.
Statins may dilute the benefit of aspirin
The use of statins has been increasing, and this trend may have played a role in the marginal benefit of aspirin therapy in these recent studies. In the Japanese trial, approximately 25% of the patients were known to be using a statin; the percentage of statin use was not reported specifically in POPADAD, but both of these studies were published in 2008, when the proportion of diabetic patients taking a statin would be expected to be higher than in earlier primary prevention trials, which were performed primarily in the 1990s. Thus, the beneficial effects of statins may have somewhat diluted the risk reduction attributable to aspirin.
Trials under way in patients with diabetes
The evolving and somewhat conflicting guidelines highlight the need for further study in patients with diabetes. To address this area, two trials are in progress: the Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D) and A Study of Cardiovascular Events in Diabetes (ASCEND).41,42
ACCEPT-D is testing low-dose aspirin (100 mg daily) in diabetic patients who are also on simvastatin. This study also includes prespecified subgroups stratified by sex, age, and baseline lipid levels.
The ASCEND trial will use the same aspirin dose as ACCEPT-D, with a target enrollment of 10,000 patients with diabetes without known vascular disease.
More frequent dosing for people with diabetes?
Although not supported by current guidelines, recent work has suggested that people with diabetes may need more-frequent dosing of aspirin.43 This topic warrants further investigation.
Aspirin as primary prevention in elderly patients
The incidence of cardiovascular events increases with age37—but so does the incidence of gastrointestinal bleeding.44 Upper gastrointestinal bleeding is especially worrisome in the elderly, in whom the estimated case-fatality rate is high.12 Assessment of risk and benefit is particularly important in patients over age 65 without known coronary disease.
Uncertainty about aspirin use in this population is reflected in the most recent US Preventive Services Task Force guidelines, which do not advocate either for or against regular aspirin use for primary prevention in those over the age of 80.
Data on this topic from clinical trials are limited. The Antithrombotic Trialists’ Collaboration (2009) found that although age is associated with a risk of major coronary events similar to that of other traditional risk factors such as diabetes, hypertension, and tobacco use, older age is also associated with the highest risk of major extracranial bleeding.22
Because of the lack of data in this population, several studies are currently under way. The Aspirin in Reducing Events in the Elderly (ASPREE) trial is studying 100 mg daily in nondiabetic patients without known cardiovascular disease who are age 70 and older.45 An additional trial will study patients age 60 to 85 with concurrent diagnoses of hypertension, hyperlipidemia, or diabetes and will test the same aspirin dose as in ASPREE.46 These trials should provide further insight into the safety and efficacy of aspirin for primary prevention in the elderly.
FUTURE DIRECTIONS
Aspirin remains a cornerstone of therapy in patients with cardiovascular disease and in secondary prevention of adverse cardiovascular events, but its role in primary prevention remains under scrutiny. Recommendations have evolved to reflect emerging data in special populations, and an algorithm based on Framingham risk assessment in men for myocardial infarction and ischemic stroke assessment in women for assessing appropriateness of aspirin therapy based on currently available guidelines is presented in Figure 1.6,8,47–49 Targeted studies have advanced our understanding of aspirin use in women, and future studies in people with diabetes and in the elderly should provide further insight into the role of aspirin for primary prevention in these specific groups as well.
Additionally, the range of doses used in clinical studies has propagated the general misperception that higher doses of aspirin are more efficacious. Future studies should continue to use lower doses of aspirin to minimize bleeding risk with an added focus on re-examining its net benefit in the modern era of increasing statin use, which may reduce the absolute risk reduction attributable to aspirin.
One particular area of debate is whether enteric coating can result in functional aspirin resistance. Grosser et al50 found that sustained aspirin resistance was rare, and “pseudoresistance” was related to the use of a single enteric-coated aspirin instead of immediate-release aspirin in people who had not been taking aspirin up to then. This complements an earlier study, which found that enteric-coated aspirin had an appropriate effect when given for 7 days.51 Therefore, for patients who have not been taking aspirin, the first dose should always be immediate-release, not enteric coated.
SHOULD OUR PATIENT RECEIVE ASPIRIN?
The patient we described at the beginning of this article has several risk factors—hypertension, dyslipidemia, left ventricular hypertrophy, and smoking—but no known cardiovascular disease as yet. Her risk of an adverse cardiovascular event appears moderate. However, her 10-year risk of stroke by the Framingham risk calculation is 10%, which would qualify her for aspirin for primary prevention. Of particular note is that the significance of left ventricular hypertrophy as a risk factor for stroke in women is higher than in men and in our case accounts for half of this patient’s risk.
We should explain to the patient that the anticipated benefits of aspirin for stroke prevention outweigh bleeding risks, and thus aspirin therapy would be recommended. However, with her elevated LDL-cholesterol, she may benefit from a statin, which could lessen the relative risk reduction from additional aspirin use.
- Chan FK, Graham DY. Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol Ther 2004; 19:1051–1061.
- Peters RJ, Mehta SR, Fox KA, et al; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003; 108:1682–1687.
- Elwood PC, Cochrane AL, Burr ML, et al. A randomized controlled trial of acetyl salicylic acid in the secondary prevention of mortality from myocardial infarction. Br Med J 1974; 1:436–440.
- Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998; 316:1337–1343.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.
- Levine GN, Bates ER, Blankenship JC, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation 2010; 121:2694–2701.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am 2005; 34:643–664.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995; 311:222–226.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987; 69:180–186.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J 2006; 27:2667–2674.
- Berger PB, Bhatt DL, Fuster V, et al; CHARISMA Investigators. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010; 121:2575–2583.
- Juul-Möller S, Edvardsson N, Jahnmatz B, Rosén A, Sørensen S, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet 1992; 340:1421–1425.
- Ridker PM, Manson JE, Gaziano JM, Buring JE, Hennekens CH. Low-dose aspirin therapy for chronic stable angina. A randomized, placebo-controlled clinical trial. Ann Intern Med 1991; 114:835–839.
- Berger JS, Brown DL, Burke GL, et al. Aspirin use, dose, and clinical outcomes in postmenopausal women with stable cardiovascular disease: the Women’s Health Initiative Observational Study. Circ Cardiovasc Qual Outcomes 2009; 2:78–87.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- de Gaetano GCollaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Fowkes FG, Price JF, Stewart MC, et al; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010; 303:841–848.
- Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006; 295:306–313.
- Rivera CM, Song J, Copeland L, Buirge C, Ory M, McNeal CJ. Underuse of aspirin for primary and secondary prevention of cardiovascular disease events in women. J Womens Health (Larchmt) 2012; 21:379–387.
- Wilson R, Gazzala J, House J. Aspirin in primary and secondary prevention in elderly adults revisited. South Med J 2012; 105:82–86.
- De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009; 339:b4531.
- Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010; 87:211–218.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J 2009; 30:2226–2232.
- De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012; 307:2286–2294.
- Pignone M, Alberts MJ, Colwell JA, et al; American Diabetes Association; American Heart Association; American College of Cardiology Foundation. Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol 2010; 55:2878–2886.
- Butalia S, Leung AA, Ghali WA, Rabi DM. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2011; 10:25.
- De Berardis G, Sacco M, Evangelista V, et al; ACCEPT-D Study Group. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007; 8:21.
- British Heart Foundation. ASCEND: A Study of Cardiovascular Events in Diabetes. http://www.ctsu.ox.ac.uk/ascend. Accessed April 1, 2013.
- Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012; 10:1220–1230.
- Hernández-Díaz S, Garcia Rodriguez LA. Cardioprotective aspirin users and their excess risk for upper gastrointestinal complications. BMC Med 2006; 4:22.
- Nelson MR, Reid CM, Ames DA, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust 2008; 189:105–109.
- Teramoto T, Shimada K, Uchiyama S, et al. Rationale, design, and baseline data of the Japanese Primary Prevention Project (JPPP)—a randomized, open-label, controlled trial of aspirin versus no aspirin in patients with multiple risk factors for vascular events. Am Heart J 2010; 159:361–369.e4.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:671–719.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2011; 57:e16–e94.
- Wright RS, Anderson JL, Adams CD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 57:e215–e367.
- Grosser T, Fries S, Lawson JA, Kapoor SC, Grant GR, Fitzgerald GA. Drug resistance and pseudoresistance: An unintended consequence of enteric coating aspirin. Circulation 2012; Epub ahead of print.
- Karha J, Rajagopal V, Kottke-Marchant K, Bhatt DL. Lack of effect of enteric coating on aspirin-induced inhibition of platelet aggregation in healthy volunteers. Am Heart J 2006; 151:976.e7–e11.
- Chan FK, Graham DY. Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol Ther 2004; 19:1051–1061.
- Peters RJ, Mehta SR, Fox KA, et al; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003; 108:1682–1687.
- Elwood PC, Cochrane AL, Burr ML, et al. A randomized controlled trial of acetyl salicylic acid in the secondary prevention of mortality from myocardial infarction. Br Med J 1974; 1:436–440.
- Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998; 316:1337–1343.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011; 58:2432–2446.
- Levine GN, Bates ER, Blankenship JC, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation 2010; 121:2694–2701.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am 2005; 34:643–664.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995; 311:222–226.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310:827–830.
- Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987; 69:180–186.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J 2006; 27:2667–2674.
- Berger PB, Bhatt DL, Fuster V, et al; CHARISMA Investigators. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010; 121:2575–2583.
- Juul-Möller S, Edvardsson N, Jahnmatz B, Rosén A, Sørensen S, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet 1992; 340:1421–1425.
- Ridker PM, Manson JE, Gaziano JM, Buring JE, Hennekens CH. Low-dose aspirin therapy for chronic stable angina. A randomized, placebo-controlled clinical trial. Ann Intern Med 1991; 114:835–839.
- Berger JS, Brown DL, Burke GL, et al. Aspirin use, dose, and clinical outcomes in postmenopausal women with stable cardiovascular disease: the Women’s Health Initiative Observational Study. Circ Cardiovasc Qual Outcomes 2009; 2:78–87.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- de Gaetano GCollaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Fowkes FG, Price JF, Stewart MC, et al; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010; 303:841–848.
- Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006; 295:306–313.
- Rivera CM, Song J, Copeland L, Buirge C, Ory M, McNeal CJ. Underuse of aspirin for primary and secondary prevention of cardiovascular disease events in women. J Womens Health (Larchmt) 2012; 21:379–387.
- Wilson R, Gazzala J, House J. Aspirin in primary and secondary prevention in elderly adults revisited. South Med J 2012; 105:82–86.
- De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009; 339:b4531.
- Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010; 87:211–218.
- Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J 2009; 30:2226–2232.
- De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012; 307:2286–2294.
- Pignone M, Alberts MJ, Colwell JA, et al; American Diabetes Association; American Heart Association; American College of Cardiology Foundation. Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol 2010; 55:2878–2886.
- Butalia S, Leung AA, Ghali WA, Rabi DM. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2011; 10:25.
- De Berardis G, Sacco M, Evangelista V, et al; ACCEPT-D Study Group. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007; 8:21.
- British Heart Foundation. ASCEND: A Study of Cardiovascular Events in Diabetes. http://www.ctsu.ox.ac.uk/ascend. Accessed April 1, 2013.
- Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012; 10:1220–1230.
- Hernández-Díaz S, Garcia Rodriguez LA. Cardioprotective aspirin users and their excess risk for upper gastrointestinal complications. BMC Med 2006; 4:22.
- Nelson MR, Reid CM, Ames DA, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust 2008; 189:105–109.
- Teramoto T, Shimada K, Uchiyama S, et al. Rationale, design, and baseline data of the Japanese Primary Prevention Project (JPPP)—a randomized, open-label, controlled trial of aspirin versus no aspirin in patients with multiple risk factors for vascular events. Am Heart J 2010; 159:361–369.e4.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:671–719.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2011; 57:e16–e94.
- Wright RS, Anderson JL, Adams CD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 57:e215–e367.
- Grosser T, Fries S, Lawson JA, Kapoor SC, Grant GR, Fitzgerald GA. Drug resistance and pseudoresistance: An unintended consequence of enteric coating aspirin. Circulation 2012; Epub ahead of print.
- Karha J, Rajagopal V, Kottke-Marchant K, Bhatt DL. Lack of effect of enteric coating on aspirin-induced inhibition of platelet aggregation in healthy volunteers. Am Heart J 2006; 151:976.e7–e11.
KEY POINTS
- Aspirin is as beneficial in low doses (eg, 81 mg daily) as it is in standard doses (325 mg) and poses less risk of gastrointestinal bleeding, although the bleeding risk is still twice as high as without aspirin.
- Since the absolute reduction in heart attacks and strokes is less in primary prevention than in secondary prevention, the risk of bleeding may for some groups outweigh the benefit, and the decision to use aspirin must be more individualized.
- Whether to prescribe aspirin for primary prevention depends on the combination of the individual patient’s sex, age, and 10-year risk of myocardial infarction (in men) or of stroke (in women).