User login
6 ‘D’s: Next steps after an insufficient antipsychotic response
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
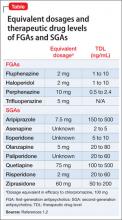
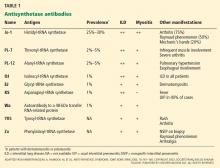
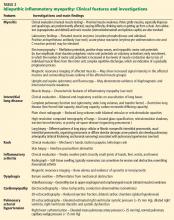
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
There are a lack of research on, and strategies for dealing with, an insufficient response to antipsychotics. Treatment often is guided by what is described in published case reports or anecdotal evidence, rather than the findings of systematic studies.
We propose that a patient be considered “difficult-to-treat” or “treatment-resistant” after experiencing limited or negative responses to 3 different antipsychotics—with ≥1 being a second-generation antipsychotic (SGA)—that the patient has taken for at least 6 to 8 weeks at the maximum recommended dosage. Furthermore, switching to clozapine is an important strategy; do not consider it solely a last resort.
These 6 ‘D’s can remind you of other problems to consider when evaluating a treatment-resistant patient.
Diagnosis. Is the diagnosis, including the presence of comorbid conditions, accurate? Are significant psychosocial stressors undermining treatment response? Treat any comorbid conditions and consider instituting adjunctive psychosocial interventions, including cognitive-behavioral therapy.
Dosage. Have the patient try the maximum recommended dosage if he (she) can tolerate it. Equivalent dosages of antipsychotics are shown in the Table,1,2 and can guide off-label use of higher dosages. Research does not support use of chlorpromazine equivalents >1,000 mg/d, and usually should not be employed. If using a higher than normal dosage, perform an ECG before the increase.3
Beneficial and adverse effects should be monitored carefully. Reduce the dosage after 3 months if the risk-benefit ratio does not justify the higher dosage.3
Duration. Try a treatment for at least 6 weeks at the maximum tolerated
dosage—even extending it to 12 weeks—before considering abandoning it because of insufficient response.
Drug interactions. Use a drug interaction tool to ensure drug-drug interactions are not reducing antipsychotic levels. A recent increase in smoking or decrease in caffeine intake can reduce the blood level of olanzapine and clozapine.4 Ultra-rapid metabolizers of cytochrome P450 isoenzymes may have a lower blood level of antipsychotic.
Consider pharmacogenetic testing in patients in whom you observe an unexpected lack of efficacy or adverse effects at customary dosages.
Double up. You might need to add another medication to the antipsychotic. Symptoms might help determine which medication to add:
- a combination of an SGA and a first-generation antipsychotic may be more effective than antipsychotic monotherapy5
- for prominent negative symptoms, consider using 2 SGAs of different potency together. Use caution when prescribing ziprasidone with another antipsychotic because this could prolong the QTc interval
- if a mood stabilizer is appropriate, consider lamotrigine because of its possible potentiating effect on SGAs6
- benzodiazepines can be used to reduce agitation or anxiety but are ineffective for psychosis.7
Drug levels. Measurement of the blood level of the drug is most useful when administering clozapine; focus on the clozapine, not on the norclozapine level that also is reported. Ensure a clozapine level of 350 to 600 ng/mL.
Therapeutic levels have been established for most antipsychotics (Table).1,2 Occasionally, knowing these levels can be helpful in evaluating patients for potential problems with absorption and metabolism of the drug, and with nonadherence.
Disclosures
Drs. Faden and Pinninti report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Mago receives grant/research support from Bristol-Myers Squibb, Forest Institute, Genomind, and Shire.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
1. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693.
2. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
3. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. http://www.rcpsych.ac.uk/files/pdfversion/CR138.pdf. Published May 2006. Accessed March 26, 2013.
4. Pinninti NR, Mago R, de Leon J. Coffee, cigarettes and meds: what are the metabolic effects? Psychiatric Times. 2005;22(6):20-23.
5. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
6. Citrome L. Treatment-resistant schizophrenia: what can we do about it? Current Psychiatry. 2011;10(6):52-58.
7. Volz A, Khorsand V, Gillies D, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1): CD006391.
Auditory musical hallucinations: When a patient complains, ‘I hear a symphony!’
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nonpsychotic auditory musical hallucinations—hearing singing voices, musical tones, song lyrics, or instrumental music—occur in >20% of outpatients who have a diagnosis of an anxiety, affective, or schizophrenic disorder, with the highest prevalence (41%) in patients with obsessive-compulsive disorder (OCD).1 OCD comorbidity with other psychiatric disorders increases the frequency of auditory musical hallucinations. Auditory musical hallucinations mainly affect older (mean age, 61.5 years) females who have tinnitus and severe, high-frequency, sensorineural hearing loss.1 Auditory musical hallucinations occur in psychiatric diseases, ictal states of complex partial seizures, abnormalities of the auditory cortex, thalamic infarcts, subarachnoid hemorrhage, tumors of the brain stem, intoxication, and progressive deafness.1,2
What patients report hearing
Some patients identify 1 musical instrument that dominates others. The musical tones are reported to have a vibrating quality, similar to the sound produced by blowing air through a paper-covered comb. Some patients hear singing voices, predominantly deep in tone, although the words usually are not clear.
Patients with auditory musical hallucinations associated with deafness may not have dementia or psychosis. Both sensorineural and conductive involvement indicates a mixed type of deafness. Pure tone audiograms show a bilateral loss of >30 decibels, affecting the higher and lower ranges.2,3 Cerebral atrophy and microangiopathic changes are common co-occurring findings on MRI.
Treatment options
Reassure your patient that the experience is not necessarily associated with a psychotic disorder. Perform a complete history, physical, and neurologic examination. Rule out unilateral symptoms, tinnitus, and hearing loss. If she (he) is experiencing unilateral symptoms, pulsatile tinnitus, unilateral hearing loss, and a constant feeling of unsteadiness, further evaluation is necessary to exclude underlying pathology. Treating concurrent insomnia, depression, or anxiety might resolve the hallucinations.4
Nonpharmacotherapeutic treatments include hearing amplification, and masking tinnitus with a hearing aid emitting low-volume music or sounds of nature (ie, rainfall).4 Two cases have reported successful carbamazepine therapy; 2 other cases demonstrated success with clomipramine.5 Frequently, symptoms spontaneously remit.
Consider electroconvulsive therapy (ECT) for patients with musical hallucinations that are refractory to medical treatment and cause distress; 3 patients with concurrent major depressive disorder showed improvement after ECT.6 Antipsychotics are not recommended as first-line treatment.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
1. Hermesh H, Konas S, Shiloh R, et al. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65(2):191-197.
2. Schakenraad SM, Teunisse RJ, Olde Rikkert MG. Musical hallucinations in psychiatric patients. Int J Geriatr Psychiatry. 2006;21(4):394-397.
3. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65.
4. Zegarra NM, Cuetter AC, Briones DF, et al. Nonpsychotic auditory musical hallucinations in elderly persons with progressive deafness. Clin Geriatr. 2007;15(11):33-37.
5. Mahendran R. The psychopathology of musical hallucinations. Singapore Med J. 2007;48(2):e68-e70.
6. Wengel SP, Burke WJ, Holemon D. Musical hallucinations. The sounds of silence? J Am Geriatr Soc. 1989;37(2):163-166.
Know your BIOTEACHERS when you assess sexual health
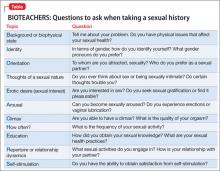
Psychiatrists often are required to obtain basic sexual information as part of a thorough clinical assessment.1 Although a detailed sexual health assessment comprises multiple elaborate domains,2 it’s important that mental health professionals remember a set of topics and related questions (Table) that can help investigate, diagnose, and treat sexual dysfunction, and contribute to a biopsychosocial formulation of your patient’s sexuality. The BIOTEACHERS mnemonic can remind you what to ask when taking a patient’s sexual history; it is not intended to be an alternative to a comprehensive sexual health evaluation.
Each letter stands for a component of the sexual assessment. The grouped letters of the acronym break down into different relevant areas that aid in remembering each category.
BIO
Background of the problem or the patient’s biophysical state
Identity
Orientation
BIO gathers basic medical information, then creates an opportunity to understand the patient’s gender identity. This step allows for nonjudgmental discussions about the patient’s sexual orientation.
TEACH
Thoughts of a sexual nature
Erotic desire or sexual interest
Arousal
Climax during sex
How often
TEACH incorporates a common chronology of sexual response and activity, starting with sexual thoughts, feelings of erotic desire, development of sexual arousal, ability and quality of a patient’s orgasm, as well as frequency of sexual activity.
ERS
Education
Repertoire or relationship dynamics
Self-stimulation
ERS comprises somewhat more complicated subjects: education (questions about a person’s sexual awareness and communication style); formal sexual knowledge; and health practices. These areas of questioning also normalize discussion of the patient’s sexual repertoire (what activities he [she] does and avoids), reviews qualities of the sexual relationship, and broaches the issue of self-stimulation.
Remember: All discussions of sexuality should be appropriate to the clinical context and considerate of the deeply personal nature of the information.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Sallie Foley, LMSW, Daniela Wittmann, LMSW, and the University of Michigan Sexual Health Certificate program for their assistance.
1. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
2. Downey JI, Friedman RC. Taking a sexual history: the adult psychiatric patient. Focus. 2009;7(4):435-440.
Psychiatrists often are required to obtain basic sexual information as part of a thorough clinical assessment.1 Although a detailed sexual health assessment comprises multiple elaborate domains,2 it’s important that mental health professionals remember a set of topics and related questions (Table) that can help investigate, diagnose, and treat sexual dysfunction, and contribute to a biopsychosocial formulation of your patient’s sexuality. The BIOTEACHERS mnemonic can remind you what to ask when taking a patient’s sexual history; it is not intended to be an alternative to a comprehensive sexual health evaluation.
Each letter stands for a component of the sexual assessment. The grouped letters of the acronym break down into different relevant areas that aid in remembering each category.
BIO
Background of the problem or the patient’s biophysical state
Identity
Orientation
BIO gathers basic medical information, then creates an opportunity to understand the patient’s gender identity. This step allows for nonjudgmental discussions about the patient’s sexual orientation.
TEACH
Thoughts of a sexual nature
Erotic desire or sexual interest
Arousal
Climax during sex
How often
TEACH incorporates a common chronology of sexual response and activity, starting with sexual thoughts, feelings of erotic desire, development of sexual arousal, ability and quality of a patient’s orgasm, as well as frequency of sexual activity.
ERS
Education
Repertoire or relationship dynamics
Self-stimulation
ERS comprises somewhat more complicated subjects: education (questions about a person’s sexual awareness and communication style); formal sexual knowledge; and health practices. These areas of questioning also normalize discussion of the patient’s sexual repertoire (what activities he [she] does and avoids), reviews qualities of the sexual relationship, and broaches the issue of self-stimulation.
Remember: All discussions of sexuality should be appropriate to the clinical context and considerate of the deeply personal nature of the information.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Sallie Foley, LMSW, Daniela Wittmann, LMSW, and the University of Michigan Sexual Health Certificate program for their assistance.
Psychiatrists often are required to obtain basic sexual information as part of a thorough clinical assessment.1 Although a detailed sexual health assessment comprises multiple elaborate domains,2 it’s important that mental health professionals remember a set of topics and related questions (Table) that can help investigate, diagnose, and treat sexual dysfunction, and contribute to a biopsychosocial formulation of your patient’s sexuality. The BIOTEACHERS mnemonic can remind you what to ask when taking a patient’s sexual history; it is not intended to be an alternative to a comprehensive sexual health evaluation.
Each letter stands for a component of the sexual assessment. The grouped letters of the acronym break down into different relevant areas that aid in remembering each category.
BIO
Background of the problem or the patient’s biophysical state
Identity
Orientation
BIO gathers basic medical information, then creates an opportunity to understand the patient’s gender identity. This step allows for nonjudgmental discussions about the patient’s sexual orientation.
TEACH
Thoughts of a sexual nature
Erotic desire or sexual interest
Arousal
Climax during sex
How often
TEACH incorporates a common chronology of sexual response and activity, starting with sexual thoughts, feelings of erotic desire, development of sexual arousal, ability and quality of a patient’s orgasm, as well as frequency of sexual activity.
ERS
Education
Repertoire or relationship dynamics
Self-stimulation
ERS comprises somewhat more complicated subjects: education (questions about a person’s sexual awareness and communication style); formal sexual knowledge; and health practices. These areas of questioning also normalize discussion of the patient’s sexual repertoire (what activities he [she] does and avoids), reviews qualities of the sexual relationship, and broaches the issue of self-stimulation.
Remember: All discussions of sexuality should be appropriate to the clinical context and considerate of the deeply personal nature of the information.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Sallie Foley, LMSW, Daniela Wittmann, LMSW, and the University of Michigan Sexual Health Certificate program for their assistance.
1. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
2. Downey JI, Friedman RC. Taking a sexual history: the adult psychiatric patient. Focus. 2009;7(4):435-440.
1. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
2. Downey JI, Friedman RC. Taking a sexual history: the adult psychiatric patient. Focus. 2009;7(4):435-440.
Use of the JAK1/JAK2 inhibitor ruxolitinib in the treatment of patients with myelofibrosis
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Myelofibrosis (MF), including primary MF and MF secondary to polycythemia vera or essential thrombocythemia, is a chronic, clinically heterogeneous hematologic malignancy characterized by inefficient hematopoiesis, bone marrow fibrosis, and shortened survival. Typical clinical manifestations include progressive splenomegaly, debilitating symptoms, and anemia. MF is associated with dysregulation of Janus kinase (JAK)-signal transducer and activator of transcription (JAK/STAT) pathway affecting hematopoiesis and inflammation. Ruxolitinib, an oral JAK1/JAK2 inhibitor, was approved for the treatment of patients with intermediate or high-risk MF based on the results of 2 phase 3 studies (Controlled MyeloFibrosis Study with Oral JAK Inhibitor Treatment [COMFORT]-I and COMFORT-II). In these trials, ruxolitinib treatment was associated with reductions in spleen size and symptom burden, and improvements in quality of life. The most common adverse events were dose-dependent cytopenias, which were managed by dose modifications, treatment interruptions, and red blood cell transfusions (for anemia). Ruxolitinib was effective regardless of MF type, risk status, or JAK2V617F mutation status, and across various other MF subpopulations. Two-year follow-up data from the COMFORT trials also demonstrate that ruxolitinib has durable efficacy and may be associated with a survival advantage relative to placebo and best available therapy. Preliminary data from ongoing studies support possible dosing strategies for patients with low platelet counts.
Click on the PDF icon at the top of this introduction to read the full article.
Electronic Communication
INTRODUCTION
Coordination of care within a practice, during transitions of care, and between primary and specialty care teams requires more than data exchange; it requires effective communication among healthcare providers.[1, 2, 3] In clinical terms, data exchange, communication, and care coordination are related, but they represent distinct concepts.[4] Data exchange refers to transfer of information between settings, independent of the individuals involved, whereas communication is the multistep process that enables information exchange between two people.[5] Care coordination, as defined by O'Malley, is integration of care in consultation with patients, their families and caregivers across all of a patient's conditions, needs, clinicians and settings.[3]
Strong collaboration among providers has been associated with improved patient outcomes.[2, 6] Yet, despite the significant role of communication in healthcare, communication may not take place at all, even at high‐stakes events like transitions of care,[7, 8] or it may be done poorly at the risk of substantial clinical morbidity and mortality.[9, 10, 11, 12, 13, 14, 15, 16]
Proof of the global effectiveness of health information technology (HIT) to improve patient care is lacking, but data from some studies demonstrate real improvements in quality and safety in specific areas,[17, 18, 19] especially with computerized physician order entry[20] and electronic prescribing.[21]
The limited information about the effect of HIT on communication focuses largely on the anticipated improvements in patient‐physician communication[22, 23, 24, 25, 26, 27]; provider‐to‐provider communication within the electronic domain is not as well understood. A recent review of interventions involving communication devices such as pagers and mobile phones found limited high‐quality evidence in the literature.[28] Clinicians have described what they consider to be key characteristics of clinical electronic communications systems such as security/reliability, cross coverage, overall convenience, and message prioritization.[29] Although the electronic health record (EHR) is expected to assist with this communication,[30] it also has the potential to impede effective communication, leading physicians to resort to more traditional workarounds.[31, 32, 33]
Measuring and improving the use of EHRs nationally were driving forces behind the creation of the Meaningful Use incentive program in the United States.[34] To receive the incentive payments, providers must meet and report on a series of measures set in three stages over the course of five years.[35] In the current state, Meaningful Use does not reward provider‐to‐provider communication within the EHR.[36, 37] The main communication objectives for stages 1 and 2 concentrate on patient‐to‐provider communication, such as patient portals and patient‐to‐provider messaging.[36, 37]
Understanding the current evidence for provider‐to‐provider communication within EHRs, its reported effectiveness, and its shortcomings may help to develop a roadmap for identifying next‐generation solutions to support coordination of care.[38, 39] This review assesses the literature regarding provider‐to‐provider electronic communication tools (as supported within or external to an EHR). It is intended as a comprehensive view of studies reporting quantitative measures of the impact of electronic communication on providers and patients.
METHODS
Definitions and Conceptual Model of Provider‐to‐Provider Communication
We conducted a systematic review of studies of provider‐to‐provider electronic communication. This review included only formal clinical communication between providers and was informed by the Coiera communications paradigm.[5] This paradigm consists of four steps: (1) task identification, when a task is identified and associated with the appropriate individual; (2) connection, when an attempt is made to contact that person; (3) communication, when task‐specific information is exchanged between the parties; and (4) disconnection, when the task reaches some stage of completion.
Literature Review
We examined written electronic communication between providers including e‐mail, text messaging, and instant messaging. We did not review provider‐to‐provider telephone or telehealth communication, as these are not generally supported within EHR systems. Communication in all clinical contexts was included among providers within an individual clinic or hospital and among providers across specialties or practice settings.[40] We excluded physician handoff communication because it has been extensively reviewed elsewhere and because handoff occurs largely through verbal exchange not recorded in the EHR.[41, 42] Communication from clinical information systems to providers, such as automated notification of unacknowledged orders, was also excluded, as it is not within the scope of provider‐to‐provider interaction.
Data Sources and Searches
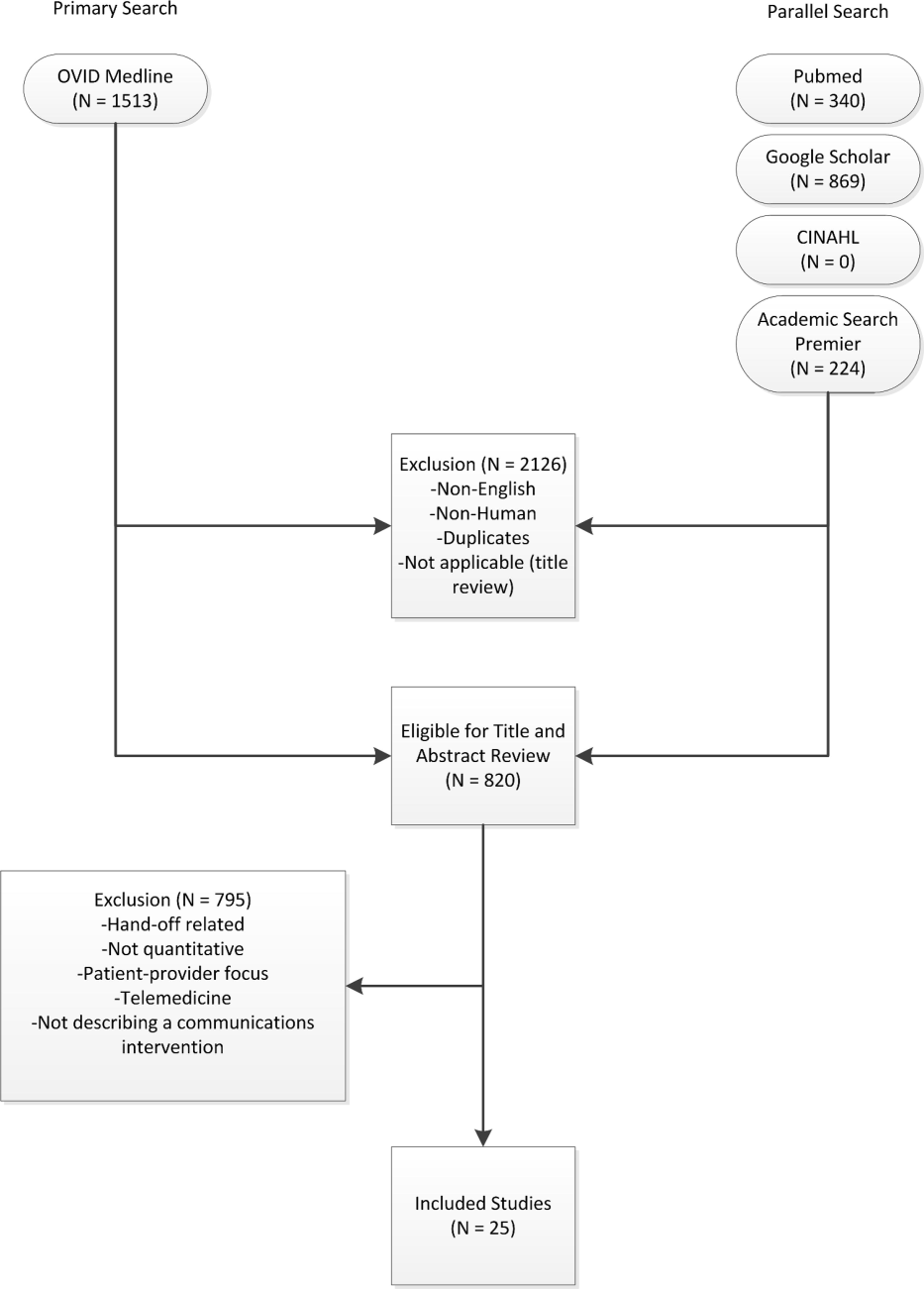
A comprehensive literature search was conducted in Ovid MEDLINE with the input of a medical librarian, and a parallel search was performed using PubMed. The Ovid MEDLINE query and parallel database search terms are documented in Table 1. Subsearches were conducted in Google Scholar, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Academic Search Premier for peer‐reviewed journals. Subsequent studies citing the initially detected articles were found through citation maps.
| Database | Strategy | Items Reviewed |
|---|---|---|
| ||
| Ovid MEDLINE | Query terms: exp medicine/ or physicians or exp outpatient clinics/ or exp hospitals/ AND *communication/ or *computer communication networks/ or *interprofessional relations/ or *continuity of patient care/ AND electronic mail or referral and consultation or text messaging/ or reminder systems. | 1513 |
| PubMed | Healthcare, provider, communication, messaging, e‐mail, texting, text messaging, instant messaging, paging, coordination, referral, EHR, EMR, electronic health record, electronic medical record, electronic, and physician. Excluding patient‐provider and patient‐physician | 340 |
| Google Scholar | Physician‐physician electronic communication excluding physician‐patient | 940 |
| CINAHL | Medical records and communication; or computerized patient records and communication | None |
| Academic Search Premier (peer‐reviewed journals) | Electronic health record and communication | 54 |
| Communication and electronic health record | 80 | |
| Physician‐physician communication | 2 | |
| Physicians and electronic health records | 88 | |
Study Selection
Paper Inclusion Criteria
Requirements included publication in English‐language peer‐reviewed journals. Included studies provided quantitative provider‐to‐provider communication data, provider satisfaction statistics, or EHR communication data. Provider‐to‐staff communication was also included if it fell within the scope of studies of communication between providers.
Paper Exclusion Criteria
Studies excluded in this review were articles that reviewed EHR systems without any focus on communication between providers and those that discussed EHR models and strategies but did not include actual testing and quantitative results. Results that included nontraditional online documents or that were found on nonpeer‐reviewed websites were also discarded. Duplicate records or publications that covered the same study were also removed. The most common reason for exclusion was the lack of quantitative evaluation.
Data Extraction and Quality Assessment
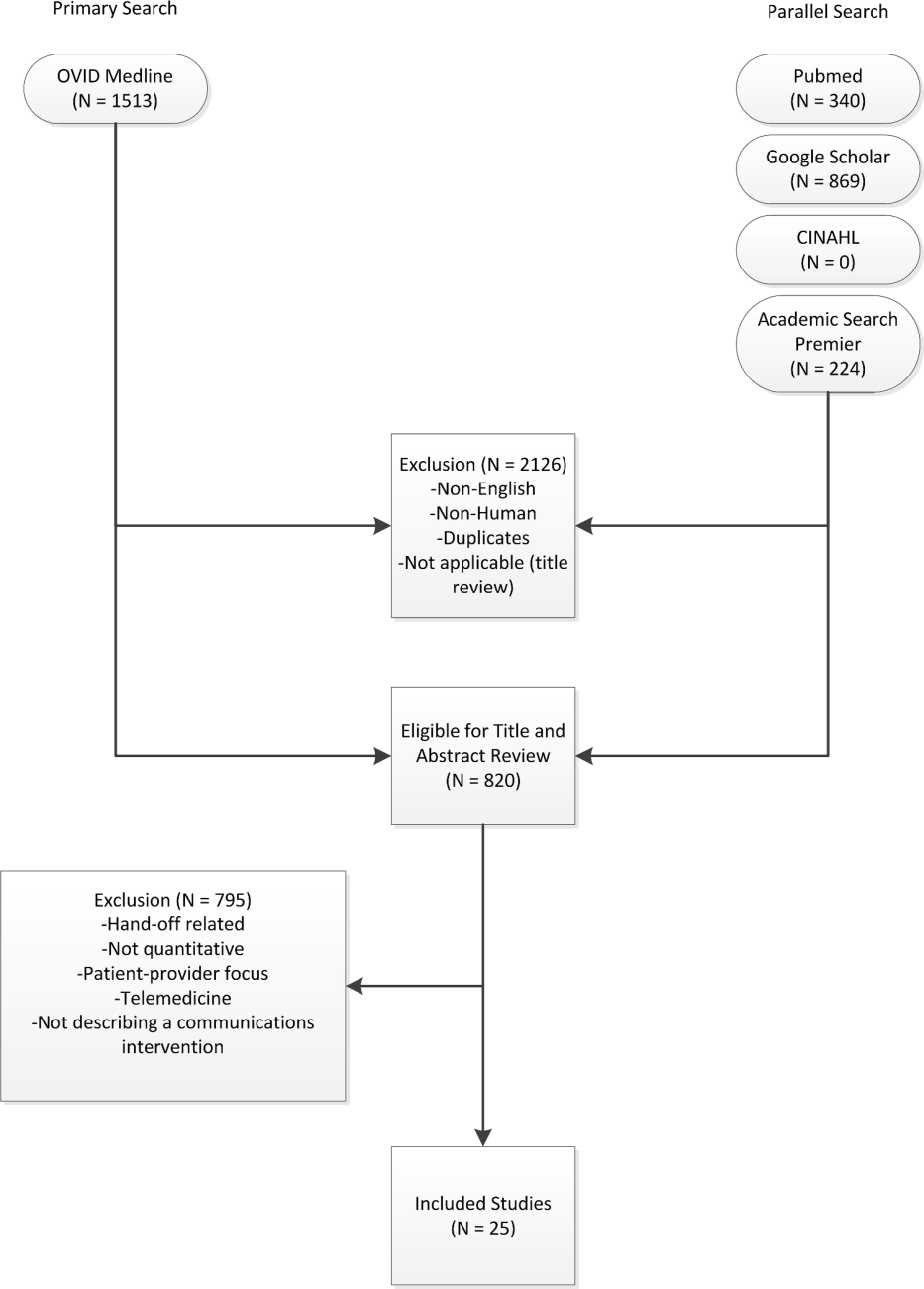
Three authors (Walsh, Siegler, Stetson) reviewed titles and abstracts of resultant studies against inclusion and exclusion criteria (Figure 1). Studies were evaluated qualitatively and findings summarized. Given the heterogeneous nature of data reported, statistical analysis was not possible.
RESULTS
The primary and parallel searches produced 2946 results that were weaned through title review and exclusion of duplicates, nonEnglish‐language, and nonhuman studies to 820 articles for title and abstract review (Figure 1). After careful review of the articles' titles, abstracts, or full content (where appropriate), twenty‐five articles met inclusion criteria and presented data about provider‐to‐provider electronic communication, either within an EHR or through a system designed to promote provider‐to‐provider communication. All of the studies that met inclusion criteria focused on physicians as providers. Five studies (20%) described trial design, three (12%) were pilot studies, and seventeen (68%) were observational studies. Thirteen of twenty‐five articles (52%) described studies conducted in the United States and twelve in Europe.
Most of the studies (56%) focused on electronic referrals between primary care and subspecialty providers. The clinical need was to communicate information on a specific patient with a specialist who shared responsibility for the overall plan of care. Only two studies evaluated curbside consultation, where providers ask for clinical recommendations without formally engaging a specialist in the plan of care for a particular patient. Table 2 summarizes included studies and has been organized with respect to clinical need under evaluation. The major themes that emerged from this review included: studies of penetration of communication tools either within the EHR system (intra‐EHR IT) or external to the EHR (extra‐EHR IT); electronic referrals; curbside consultations; and test results reporting (results notification).
| Primary Author, Year | Design | Intervention | Measurement | Results |
|---|---|---|---|---|
| ||||
| Need: Communicate care across clinical settings (inpatient‐outpatient) | ||||
| Branger, 1992[4, 6] | Observational study | Introduction of electronic messaging system in the Netherlands between hospital and PCPs. | Satisfaction survey data using Likert scale of usefulness. | Free text messaging to exchange patient data was rated very useful or useful by 20 of 27 PCP respondents. |
| Reponen, 2004[66] | Observational study | Finnish study of electronic referrals XML messages between EHRs or secure web links. | User questionnaire. No description of respondents was provided. | Internists surveyed estimated that electronic referrals accelerate the referral process by 1 week. |
| Need: Communicate care across specialties (primary care physicians‐specialists) | ||||
| Kooijman, 1998[67] | Observational study | Survey of 45 PCPs who received notes from specialists via Electronic Data Interchange. | User questionnaire with 5‐point Likert scale of satisfaction, from 1 (much better) to 5 (much worse). | Highest satisfaction scores for speed (1.51.8) and efficiency (1.51.7) for electronic messages, with lower scores for reliability (2.52.7) and clarity (2.5). |
| Harno, 2000[4][8] | Nonrandomized trial | Eight‐month prospective comparative study in Finland of outpatient clinics in hospitals with and without intranet referral systems. | Comparison of numbers of electronic referrals, clinic visits, costs. | There were 43% of electronic referrals and 79% of outpatient referrals that resulted in outpatient visits. A 3‐fold increase in productivity overall and 7‐fold reduction in visit costs per patient using e‐mail consultation. |
| Moorman, 2001[4][7] | Observational study | Supersedes Branger, 1999.[68] Analyzes intra‐EHR communications between PCPs and consultant in Netherlands re: diabetes management of patients (19941998). | Descriptive statistics of number of messages, content, whether message had been copied into EMR; survey of PCPs (12 of 15 responded). | Decline in integration by PCPs of messages in the EHR from 75% to 51% over first 3 years. Despite this, most PCPs wanted to extend messaging to other patient groups. |
| Bergus, 2006[69] | Observational study | Follow‐up of Bergus, 1998[54]; evaluated formulation of clinical referrals to specialists at the University of Iowa by retrospective review of e‐mail transcripts. | Analyzed taxonomy of clinical questions; assessed need for clinical consultation of 1618 clinical questions. | Specialists less likely to recommend clinic consultation if referral specified the clinical task (OR: 0.36, P<0.001), intervention (OR: 0.62, P=0.004), or outcome (OR: 0.49, P<0.001). This effect was independent of clinical content (P>0.05). |
| Dennison, 2006[70] | Pilot study | Construction of an electronic referral pro forma to facilitate referral of patients to colorectal surgeons. | Descriptive statistics. Comparisons of patient attendance rate, delays to booking and to actual appointment between 54 electronic referrals and 189 paper referrals. | Compared to paper referrals, electronic referrals were booked more quickly (same day vs 1 week later on average) and patients had lower nonattendance rates (8.5% vs 22.5%). Both results stated as statistically significant, but P values were not provided. |
| Shaw, 2007[49] | Observational study | Dermatology electronic referral in England. | Content of 131 electronic vs 139 paper referrals to dermatologists(NHS Choose and Book).[71] | Paper superior to electronic for clinical data such as current treatments (included in 68% of paper vs 39% of electronic referrals, P<0.001); electronic superior for demographic data. |
| Gandhi, 2008[50] | Nonrandomized trial | Electronic referral tool in the Partners Healthcare System in Massachusetts that included a structured referral‐letter generator and referral status tracker. Assigned to 1 intervention site and 1 control site. | Survey assessment. Fifty‐four of 117 PCPs responded (46%), 235 of 430 specialists responded (55%), 143 out of 210 patients responded (69%). | Intervention group showed high voluntary adoption (99%), higher information transfer rates prior to subspecialty visit (62% vs 12%), and lower rates of conflicting information being given to patients (6% vs 20%). |
| John, 2008[72] | Pilot study | Validation study of the Lower Gastrointestinal e‐RP (through the Choose and Book System in the United Kingdom) intended to improve yield of colon cancers diagnosed and to reduce delays in diagnosis. | Comparison of actual to simulated referral patterns through e‐RP for 300 patients divided into colorectal cancer, 2‐week wait suspected cancer, and routine referral groups. | e‐RP was more accurate than traditional referral at upgrading patients who had cancer to the appropriate suspected cancer referral group (85% vs 43%, P=0.002). |
| Kim, 2009[73] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Included reply functionality and ability to forward messaging to a scheduler for calendaring. | Impact of electronic referral system as measured by questionnaire to referring providers. A total of 298/368 participated (24 clinics); 53.5% attending physicians. | Electronic referrals improved overall quality of care (reported by 72%), guidance of presubspecialty visit (73%), and the ability to track referrals (89%). Small change in access for urgent issues (35% better, 49% reported no change). |
| Scott, 2009[74] | Pilot study | Pilot of urgent electronic referral system from PCPs to oncologists at South West Wales Cancer Centre. | Satisfaction statistics (10‐point Likert scale) collected from PCPs via interview. | Over 6 months, 99 referrals submitted; 81% were processed within 1 hour with high satisfaction scores. |
| Were, 2009[75] | Nonrandomized trial | Geriatrics consultants were provided system to make electronic recommendations (consultant‐recommended orders) in the native CPOE system along with consult notes in the intervention vs consult notes alone in the control. | Rates of implementation of consultant recommendations. Qualitative survey of users of the new system. | Higher total number of recommendations (247 vs 192, P<0.05) and higher implementation rates of consultant‐recommended orders in the intervention group vs control (78% vs 59%, P=0.01). High satisfaction scores on 5‐point Likert scale for the intervention system with good survey response rate (83%). |
| Dixon, 2010[52] | Observational study | Comparison of 2 extra‐EHR systems (NHS Choose and Book, Dutch ZorgDomein) for booking referrals. Patients choose doctor or hospital and the system transfers demographic and clinical information between PCP and specialist. | National data, patient and provider surveys, focus groups, observational studies. Focus was on patient choice, but evaluations included all aspects of the systems. | Resistance from PCPs during implementation; 78% of ZorgDomein PCPs felt referrals took more time; general displeasure on the part of specialists re: quality of referrals, although not quantified. |
| Patterson, 2010[51] | Observational study | E‐mail referral system to a neurologist in Northern Ireland. Referrals were template based and recorded as clinical episode in the patient administration system. Comparison of this system to conventional referrals to another neurologist. | Evaluated effectiveness, cost, safety for period 20022007. | Decreased referral wait times (4 vs 13 weeks) and 35% cost reduction per patient for the e‐mail referral vs conventional referrals. |
| No diminution in safety. Limitation: single neurologist participated. | ||||
| Singh, 2011[76] | Observational study | Chart review of electronic referrals to specialist practices in a Veterans Affairs outpatient system. | Follow‐up actions taken by subspecialists within 30 days of receiving referral. | An intra‐EHR referral system was still affected by communication breakdowns. Of 61,931 referrals, 36.4% were discontinued for inappropriate or incomplete referral requests. |
| Kim‐Hwang, 2010[77] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Follow‐up to Kim, 2009.[73] | Survey of medical and surgical subspecialty consultants. | Statistically significant differences in clarity of consult request in both medical and surgical clinics, in decreased inappropriate referrals in surgical clinics, in decreased use of follow‐up appointments by surgical specialists, and in decreased avoidable follow‐up surgical visits. |
| Warren, 2011[53] | Observational study | Electronic referrals from general medical practices to public referral network of Hutt Hospital in New Zealand (20072010). | Retrospective analysis of transactional data from messaging system and from general inpatient tracking system. Qualitative data collection via interviews. | Estimated 71% of 10,367 referrals were electronic referrals over 3 years. Statistically significant improvement in referral latency without change in staffing. Clinicians appreciate shared transparency of referrals but cite usability issues as barriers. |
| Need: Curbside consults (primary care physicians‐specialists) | ||||
| Bergus, 1998[54] | Observational study | Evaluation of the ECS for curbside consultations between family physicians and subspecialists. | Descriptive statistics of usage data; survey of users. | Median response time 16.1 hours; 92% of questions answered; almost 90% concerned specific patients. Both groups expressed satisfaction. |
| Abbott, 2002[55] | Observational study | Evaluation of Department of Defense Ask a Doc physician‐to‐physicians e‐mail consultation system over network of 21 states (19982000). | Descriptive statistics; qualitative assessment. | There were 3121 consultations. Average response time <12 hours. Minimal cost and effort to initiate and sustain. Felt to mirror clinical practice. Barriers were security and assignation of credit for consultation. |
| Need: Communication of results (primary care physicians ‐specialists) | ||||
| Singh, 2007[5][6] | Nonrandomized trial | Concurrent prospective evaluation of responses to 1017 critical imaging alert notifications in a Veterans Affairs outpatient system (2006). Radiologists generated alerts. Included receipt system. | Measured percentage of unacknowledged alerts and imaging lost to follow‐up. | There were 368 of 1017 transmitted alerts unacknowledged (36%); 45 were completely lost to follow‐up. There were 0.2% outpatient imaging results lost to follow‐up overall. |
| Singh, 2009[5][7] | Nonrandomized trial | Concurrent evaluation of responses to 1196 critical imaging alert notifications in a Veterans Affairs outpatient system (20072008). Similar coding system to Singh, 2007.[56] | Measured percentage of alerts acknowledged, timely follow‐up; compared electronic alerts alone to combination of alerts and phone calls or admission. | Percentage of alerts acknowledged did not differ by type of communication; combination of electronic alerts with phone follow‐up (OR: 0.12, P<0.001) or admission (OR: 0.22, P<0.001) decreased likelihood of delayed follow‐up. Alerts to 2 providers increased the likelihood of delayed follow‐up (OR: 1.99, P=0.03). |
| Abujudeh, 2009[5][8] | Observational study | Retrospective review of e‐mailbased alert system for abnormal imaging results at Massachusetts General Hospital 20052007. E‐mail alerting by radiologist to ordering physician of nonurgent findings. | Descriptive statistics; survey of referring physicians (12/26). | There were 56,691 out of 1,540,254 reports for important but not urgent findings; 93.3% generated e‐mail message (6.7% failure rate); 80% of alerts were viewed. Higher satisfaction for e‐mail alerts over conventional methods (eg, facsimile) for nonurgent but important findings. |
| Need: Communicate within 1 care setting (primary care physicians) | ||||
| Lanham, 2012[78] | Observational study | Comparison of practice‐level EHR use with communication patterns among physicians, nurses, medical assistants, practice managers, and nonclinical staff within individual practices in Texas. | Observation and semistructured interviews. Within‐practice communication patterns were categorized as fragmented or cohesive. Practice‐level EHR use was categorized as homogeneous or heterogeneous. | Clinical practices with cohesive within‐practice communication patterns were associated with homogeneous patterns of practice‐level EHR use. |
| Murphy, 2012[79] | Observational study | Review of note‐based messaging within the EHR in outpatient clinics of large tertiary Veterans Affairs facility. Clinic staff send additional signature request alerts linked to parent notes in the EHR to primary care physicians. | Reason for and origin of alerts. Parent note linked to alert was also reviewed for 3 value attributes: urgency; potential harm if alert was missed; subjective value to PCP of the alert. | Of the alerts reviewed, 53.7% of 525 were deemed of high value but required PCPs to review significant amounts of extraneous text (80.3% of words in parent notes) to get relevant information. Most alerts (40%) were medication, prescription, or refill related. |
Extra‐EHR IT
A review of electronic communication in 2000 examined electronic communication among primary care physicians but notably did not distinguish between communication and data exchange.[43] Of the thirty included publications in that review, seventeen publications dealt with electronically communicated information in general; the remaining studies focused on notifications of test results or transitions of care, reports from specialists, or electronic communication as replacement of traditional referral.[43] Although many studies of electronic communication described positive benefits, few included objective data, and most did not analyze provider‐to‐provider communication specifically. A survey of IT use outside of the EHR in 2006 documented that approximately 30% of clinicians used e‐mail to communicate with other clinicians, fewer than those who consulted on‐line journals (40.8%), but many more than those who communicated with patients by e‐mail at that time (3.6%).[44]
Intra‐EHR IT
A comparison of two physician surveys of EHR use in Massachusetts (the first in 2005 and the second in 2007) documented an increase in the percentage of practices with an EHR, from 23% to 35%; in those practices with EHRs, only the use of electronic prescribing increased over time. Use of secure electronic referrals or messaging including secure e‐mail remained unchanged; of note, referrals and messaging were considered a singular clinical function in that study. Between 2005 and 2007, referrals or clinical messaging were available in 62% and 63% of EHR systems, respectively, and they were used most or all of the time by 29% to 33% of the physicians who had an EHR.[45]
Electronic Referrals
Fourteen articles focused on electronic referrals. Two had a prepost or longitudinal study design,[46, 47] and five included a control group.[48, 49, 50, 51] The rest were descriptive. In most cases, electronic referral improved the transfer of information, especially when standardized message templates were created. Use of electronic referral appeared to result in reduced waiting time for appointments and enabled more efficient triage.
Barriers to integration of electronic referral in the EHR were also assessed. An intra‐EHR communication system requiring a primary care physician to integrate information e‐mailed by the consultant into the record showed the percentage of integrated notes decreasing over time.[47] Practitioners had mixed feelings about the system; although the majority (92% of respondents) felt that the system improved patient care and wanted to extend messaging to other patient groups, they also felt that electronic messaging decreased the ease of reviewing data (83%) and confused tasks and responsibilities (59%). A study of British and Dutch electronic referral systems described significant resistance on the part of practitioners to electronic referrals and concern on the part of specialists about the quality of referrals.[52] Another study demonstrated improvement in quality of demographic data but degradation in quality of clinical information when referrals were submitted electronically.[49] A recent transactional analysis of electronic referrals in New Zealand showed high uptake and reduced referral latency compared to conventional referral; clinicians cited usability concerns as the major barrier to use.[53]
Curbside Consultations via E‐mail
Two studies evaluated curbside consultations via e‐mail and documented high provider satisfaction and rapid turnaround.[54, 55] The preliminary nature of these studies raises questions of sustainability and long‐term implementation.
Results Notification
Three studies focused on test‐result reporting from radiologists. In these studies, a radiologist could designate a result as high priority and have an e‐mail notification sent to the ordering physicians.[56, 57, 58] Urgent results were relayed by telephone. Lack of acknowledgement of alerts impacted the results of every study, and in one of these studies, alerting two physicians, rather than just one, decreased the likelihood that the results would be followed up.[57] Providers did prefer e‐mail to fax notification.[58]
DISCUSSION
The principal findings of the literature review demonstrate the paucity of quantitative data surrounding provider‐to‐provider communication. The majority of studies focused on physicians as providers without emphasis on other provider types on the care team. Most of the quantitative studies investigated electronic referrals. Data collected largely represented measures of provider satisfaction and process measures. Few quantitative studies used established models or measures of team coordination or communication.
This study extends the work of others by compiling a comprehensive view of electronic provider‐to‐provider communication. A recent review of devices for clinical communication tells a part of the story,[28] and our review adds a comprehensive, device‐agnostic look at the systems physicians and other providers use every day.
Limitations of this review include the small number of eligible studies and a homogenous provider type (physicians). The latter is both an important finding and a limitation to generalizability of our results. Reviewed studies were in English only. The literature review by its nature is subject to publication bias.
Intra‐EHR communication cannot serve all purposes, and is it not a panacea for effective care coordination. One recent qualitative study warns about the pitfalls of electronic communication. Interviews with physicians from twenty‐six practices elicited some concerns about the resulting decrease in face‐to‐face communication that has resulted from the adoption of electronic communication tools.[32] This finding brings implications: (1) a false sense of security may reduce verbal communications when they are needed mostduring emergencies or when caring for complex patients who require detailed, nuanced discussion; and (2) fewer conversations within a practice can reduce both knowledge sharing and basic social interactions necessary for the maintenance of a collaboration. Last, privacy and confidentiality are top priorities. Common electronic communication tools are susceptible to security breaches,[47, 59] and innovations within this domain must conform to Health Insurance Portability and Accountability Act of 1996 and Health Information Technology for Economic and Clinical Health Act regulations.[60]
Although electronic communication is not a complete solution for clinical collaboration, it is difficult to use face‐to‐face communication and telephone communication to convey large amounts of patient information while simultaneously generating a record of the transaction. Moreover, paging functions, telephone calls, and face‐to‐face encounters can be highly interruptive, increasing cognitive load, burdening working memory, and shifting attention from the task at hand.[14] Interruptions contribute to inefficiency and to the potential for errors.[61]
Effective coordination of care for the chronically ill is one of the essential goals of the health system; it is an ongoing process that depends on constant, effective communication. Bates and Bitton have recognized this and described the crucial role that HIT will play in creating an effective medical home by enumerating seven domains of HIT especially in need of research.[62] In particular, they note that effective team care and care transitions will depend on an EHR that promotes both implicit and real‐time communication: it will be essential to develop communication tools that allow practices to record goals shared by providers and patients alike, and to track medical interventions and progress.[62]
Future research could investigate a number of open questions. Overall, an emphasis should be placed on rigorous qualitative and quantitative evaluation of electronic communication. Process measures, such as length of stay, hospital readmission rates, and measures of care coordination, should be framed ultimately with respect to patient health outcomes. Such data are beginning to be reported.[63]
It is unclear which types of communications would be best served within the EHR and which should remain external to it. Instant communication or chat has not been studied sufficiently to show a demonstrable impact on patient care. Cross‐coverage and team identification within the EHR can be further studied with respect to workflows and best practices. Studies using structured observation or time‐and‐motion analysis could provide insight into use cases and workflows that providers implement to discuss patients. Future research should incorporate established models of communication[5] and coordination.[64] Data on unintended consequences or harms of provider‐to‐provider electronic communication have been limited, and this area should be considered in subsequent work. Finally, although the scope of this review focused on communication between providers, transformative electronic communication systems should bridge communication gaps between providers and patients as well.
As adoption of EHRs in US hospitals has increased from 15.1% of US hospitals in 2010 to 26.6% in 2011 for any type of EHR and 3.6% to 8.7% for comprehensive EHRs,[65] it is worth noting that Meaningful Use, as it stands, incentivizes patient‐provider communication, but not communication between providers. Inclusion of certification criteria focused on provider‐to‐provider communication may spur additional innovation.
CONCLUSIONS
The optimal features to support electronic communication between providers remain under‐assessed, although there is preliminary evidence for the acceptability of electronic referrals. Without better understanding of electronic communication on workflow, provider satisfaction, and patient outcomes, the impact of such tools on coordination of complex medical care will be an open question, and it remains an important one to answer.
Acknowledgments
The authors would like to express their gratitude to Dr. Thomas Payne, Medical Director of IT Services at the University of Washington, for sharing his expertise, and to Marina Chilov, medical librarian at Columbia University, for her assistance with the literature search. The authors would like to thank Paul Sun, MA, for his assistance with the literature review.
Disclosures: This work was funded by 5K22LM8805 (PDS) and T15 LM007079 (CW, SC) grants. Dr. Stetson serves on the advisory board of the Allscripts Enterprise EHR.
- , . Physician‐physician communication: what's the hang‐up? J Gen Intern Med. 2009;24:437–439.
- , , , et al. Meta‐analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152(4):247–258.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2009;25:177–185.
- , , , , , . Development of an ontology to model medical errors, information needs, and the clinical communication space. Proc AMIA Symp. 2001:672–676.
- . Clinical communication: a new informatics paradigm. Proc AMIA Annu Fall Symp. 1996:17–21.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Dis Mon. 2002;48(4):218–229.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Analysing potential harm in Australian general practice: an incident‐monitoring study. J Am Med Inform Assoc. 1998;169:73–76.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , , . Communication loads on clinical staff in the emergency department. J Am Med Inform Assoc. 2002;176(9):415–418.
- , , , , , . Deficit in communicaiton and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297:831–841.
- , . Improving clinical communication: a view from psychology. J Am Med Inform Assoc. 2000;7(5):453–461.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–471.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , . Costs and benefits of health information technology. Evid Rep Technol Assess. 2006;132:1–71.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48:203–209.
- , , , et al. Decrease in hospital‐wide mortality rate after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2010;126:e1–e8.
- , , , , . Electronic prescribing improves medication safety in community‐based office practices. J Gen Intern Med. 2010;25:530–536.
- . Patient‐centered Care. Radiol Technol. 2009;81(2):133–147.
- , . The missing link: bridging the patient‐provider health information gap. Health Aff (Millwood). 2005;24(5):1290–1295.
- , , , , . Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006;13(2):121–126.
- , . Web messaging: a new tool for patient‐physician communication. J Am Med Inform Assoc. 2003;10(3):260–270.
- , , , . Expanding the guidelines for electronic communication with patients. J Am Med Inform Assoc. 2001;8(4):344–348.
- , , , . The utility of electronic mail as a medium for patient‐physician communication. Arch Fam Med. 1994;3(3):268–271.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , . Desiderata for personal electronic communication in clinical systems. J Am Med Inform Assoc. 2002;9(3):209–216.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25:174–176.
- , , . Electronic medical records and communication with patients and other clinicians: are we talking less? Issue Brief Cent Stud Health Syst Change. 2010;(131):1–4.
- , . Referral and consultation communication between primary care and specialist physicians: finding common ground. Arch Intern Med. 2011;171(1):56–65.
- , . The "meaningful use" regulation for electronic health records. N Engl J Med. 2010;363(6):501–504.
- Centers for Medicare 25(3):177–185.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25(3):174–176.
- , , , . A framework for clinical communication supporting healthcare delivery. AMIA Annu Symp Proc. 2005:375–379.
- Understanding and improving patient handoffs. Jt Comm J Qual Improv. 2010:49–96.
- , , , . Signout: a collaborative document with implications for the future of clinical information systems. AMIA Annu Symp Proc. 2007:696–700.
- , , . Effects of electronic communication in general practice. Int J Med Inform. 2000;60(1):59–70.
- , , , , . Prevalence of basic information technology use by U.S. physicians. J Gen Intern Med. 2006;21:1150–1155.
- , , , et al. Physicians' use of key functions in electronic health records from 2005 to 2007: a statewide survey. J Am Med Inform Assoc. 2009;16:465–470.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , , . Electronic messaging between primary and secondary care: a four‐year case report. J Am Med Inform Assoc. 2001;8(4):372–378.
- , , , . Patient referral by telemedicine: effectiveness and cost anslysis of an intranet system. J Telemed Telecare. 2000;6(6):320–329.
- , . Strengths and weaknesses of electronic referral: comparison of data content and clinical value of electronic and paper referrals in dermatology. Br J Gen Pract. 2007;57(536):223–224.
- , , , et al. Improving referral communication using a referral tool within an electronic medical record. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:63–74.
- , , , . Email triage is an effective, efficient, and safe way of managing new referrals to a neurologist. Qual Safety Health Care. 2010;19(5):e51.
- , , . The experience of implementing choice at point of referral: a comparison of the Netherlands and England. Health Econ Policy Law. 2010;13:1–23.
- , , , , . Introduction of electronic referral from community associated with more timely review by secondary services. Appl Clin Inform. 2011;2(4):546–564.
- , , , . Use of an e‐mail curbside consultation service by family physicians. J Fam Pract. 1998;47:357–360.
- , , , , , . Physician‐to‐physician consultation via electronic mail: the Walter Reed Army Medical Center Ask a Doc system. Mil Med. 2002;167:200–204.
- , , , , , . Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14:459–466.
- , , , et al. Timely follow‐up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch of Intern Med. 2009;169(17):1578–1586.
- , , , , . Important imaging finding e‐mail alert system: experience after 3 years of implementation. Radiology. 2009;252:747–753.
- . Instant insecurity: security issues of instant messaging. Symantec website. Available at: http://www.symantec.com/connect/articles/instant‐insecurity‐security‐issues‐instant‐messaging.
- . Secure messaging in healthcare. Tech solutions for HIPAA‐compliant messaging. J AHIMA. 2008;79(6):50–51.
- , , , , . Association of interruptions with an increased risk and severity of medication administration errors. Arch Intern Med. 2010;170(8):683–690.
- , . The future of health information technology in the patient‐centered medical home. Health Aff (Millwood). 2010;29(4):614–621.
- , , . eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450–2453.
- , , , et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol 7. Care Coordination. Rockville, MD; 2007.
- , , , , . Small, nonteaching, and rural hospitals continue to be slow in adopting electronic health record systems. Health Aff (Millwood). 2012;31(5):1092–1099.
- , , , . Extending a multimedia medical record to a regional service with electronic referral and discharge letters. J Telemed Telecare. 2004;10S:81–83.
- , . Sending specialist reports to GPs using EDI. Stud Health Tech Inform. 1998;52(pt 1):408–411.
- , , , , . Shared care for diabetes: supporting communication between primary and secondary care. Int J Med Inform. 1999;53(2-3):133–142.
- , , , . Email teleconsultations: well formulated clinical referrals reduce the need for clinic consultation. J Telemed Telecare. 2006;12(1):33–38.
- , , , . An effective electronic surgical referral system. Ann R Coll Surg Engl. 2006;88(6):554–556.
- . Choose and book. Clin Med. 2006;6(5):473–476.
- , , , . Validation of the lower gastrointestinal electronic referral protocol. Brit J Surg. 2008;95:506–514.
- , , , , . Not perfect, but better: primary care providers' experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24:614–619.
- . The Swansea electronic referrals project. J Telemed Telecare. 2009;15:156–158.
- , , , , . Using computerized provider order entry and clinical decision support to improve referring physicians' implementation of consultants' medical recommendations. J Am Med Inform Assoc. 2009;16(2):196–202.
- , , , et al. Follow‐up actions on electronic referral communication in a multispecialty outpatient setting. J Gen Intern Med. 2011;26(1):64–69.
- , , , , , . Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123–11028.
- , , . Same organization, same electronic health records (EHRs) system, different use: exploring the linkage between practice member communication patterns and EHR use patterns in an ambulatory care setting. J Am Med Inform Assoc. 2012;19(3):382–391.
- , , , et al. Electronic health record‐based messages to primary care providers: valuable information or just noise? Arch Intern Med. 2012;172(3):283–285.
INTRODUCTION
Coordination of care within a practice, during transitions of care, and between primary and specialty care teams requires more than data exchange; it requires effective communication among healthcare providers.[1, 2, 3] In clinical terms, data exchange, communication, and care coordination are related, but they represent distinct concepts.[4] Data exchange refers to transfer of information between settings, independent of the individuals involved, whereas communication is the multistep process that enables information exchange between two people.[5] Care coordination, as defined by O'Malley, is integration of care in consultation with patients, their families and caregivers across all of a patient's conditions, needs, clinicians and settings.[3]
Strong collaboration among providers has been associated with improved patient outcomes.[2, 6] Yet, despite the significant role of communication in healthcare, communication may not take place at all, even at high‐stakes events like transitions of care,[7, 8] or it may be done poorly at the risk of substantial clinical morbidity and mortality.[9, 10, 11, 12, 13, 14, 15, 16]
Proof of the global effectiveness of health information technology (HIT) to improve patient care is lacking, but data from some studies demonstrate real improvements in quality and safety in specific areas,[17, 18, 19] especially with computerized physician order entry[20] and electronic prescribing.[21]
The limited information about the effect of HIT on communication focuses largely on the anticipated improvements in patient‐physician communication[22, 23, 24, 25, 26, 27]; provider‐to‐provider communication within the electronic domain is not as well understood. A recent review of interventions involving communication devices such as pagers and mobile phones found limited high‐quality evidence in the literature.[28] Clinicians have described what they consider to be key characteristics of clinical electronic communications systems such as security/reliability, cross coverage, overall convenience, and message prioritization.[29] Although the electronic health record (EHR) is expected to assist with this communication,[30] it also has the potential to impede effective communication, leading physicians to resort to more traditional workarounds.[31, 32, 33]
Measuring and improving the use of EHRs nationally were driving forces behind the creation of the Meaningful Use incentive program in the United States.[34] To receive the incentive payments, providers must meet and report on a series of measures set in three stages over the course of five years.[35] In the current state, Meaningful Use does not reward provider‐to‐provider communication within the EHR.[36, 37] The main communication objectives for stages 1 and 2 concentrate on patient‐to‐provider communication, such as patient portals and patient‐to‐provider messaging.[36, 37]
Understanding the current evidence for provider‐to‐provider communication within EHRs, its reported effectiveness, and its shortcomings may help to develop a roadmap for identifying next‐generation solutions to support coordination of care.[38, 39] This review assesses the literature regarding provider‐to‐provider electronic communication tools (as supported within or external to an EHR). It is intended as a comprehensive view of studies reporting quantitative measures of the impact of electronic communication on providers and patients.
METHODS
Definitions and Conceptual Model of Provider‐to‐Provider Communication
We conducted a systematic review of studies of provider‐to‐provider electronic communication. This review included only formal clinical communication between providers and was informed by the Coiera communications paradigm.[5] This paradigm consists of four steps: (1) task identification, when a task is identified and associated with the appropriate individual; (2) connection, when an attempt is made to contact that person; (3) communication, when task‐specific information is exchanged between the parties; and (4) disconnection, when the task reaches some stage of completion.
Literature Review
We examined written electronic communication between providers including e‐mail, text messaging, and instant messaging. We did not review provider‐to‐provider telephone or telehealth communication, as these are not generally supported within EHR systems. Communication in all clinical contexts was included among providers within an individual clinic or hospital and among providers across specialties or practice settings.[40] We excluded physician handoff communication because it has been extensively reviewed elsewhere and because handoff occurs largely through verbal exchange not recorded in the EHR.[41, 42] Communication from clinical information systems to providers, such as automated notification of unacknowledged orders, was also excluded, as it is not within the scope of provider‐to‐provider interaction.
Data Sources and Searches
A comprehensive literature search was conducted in Ovid MEDLINE with the input of a medical librarian, and a parallel search was performed using PubMed. The Ovid MEDLINE query and parallel database search terms are documented in Table 1. Subsearches were conducted in Google Scholar, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Academic Search Premier for peer‐reviewed journals. Subsequent studies citing the initially detected articles were found through citation maps.
| Database | Strategy | Items Reviewed |
|---|---|---|
| ||
| Ovid MEDLINE | Query terms: exp medicine/ or physicians or exp outpatient clinics/ or exp hospitals/ AND *communication/ or *computer communication networks/ or *interprofessional relations/ or *continuity of patient care/ AND electronic mail or referral and consultation or text messaging/ or reminder systems. | 1513 |
| PubMed | Healthcare, provider, communication, messaging, e‐mail, texting, text messaging, instant messaging, paging, coordination, referral, EHR, EMR, electronic health record, electronic medical record, electronic, and physician. Excluding patient‐provider and patient‐physician | 340 |
| Google Scholar | Physician‐physician electronic communication excluding physician‐patient | 940 |
| CINAHL | Medical records and communication; or computerized patient records and communication | None |
| Academic Search Premier (peer‐reviewed journals) | Electronic health record and communication | 54 |
| Communication and electronic health record | 80 | |
| Physician‐physician communication | 2 | |
| Physicians and electronic health records | 88 | |
Study Selection
Paper Inclusion Criteria
Requirements included publication in English‐language peer‐reviewed journals. Included studies provided quantitative provider‐to‐provider communication data, provider satisfaction statistics, or EHR communication data. Provider‐to‐staff communication was also included if it fell within the scope of studies of communication between providers.
Paper Exclusion Criteria
Studies excluded in this review were articles that reviewed EHR systems without any focus on communication between providers and those that discussed EHR models and strategies but did not include actual testing and quantitative results. Results that included nontraditional online documents or that were found on nonpeer‐reviewed websites were also discarded. Duplicate records or publications that covered the same study were also removed. The most common reason for exclusion was the lack of quantitative evaluation.
Data Extraction and Quality Assessment
Three authors (Walsh, Siegler, Stetson) reviewed titles and abstracts of resultant studies against inclusion and exclusion criteria (Figure 1). Studies were evaluated qualitatively and findings summarized. Given the heterogeneous nature of data reported, statistical analysis was not possible.

RESULTS
The primary and parallel searches produced 2946 results that were weaned through title review and exclusion of duplicates, nonEnglish‐language, and nonhuman studies to 820 articles for title and abstract review (Figure 1). After careful review of the articles' titles, abstracts, or full content (where appropriate), twenty‐five articles met inclusion criteria and presented data about provider‐to‐provider electronic communication, either within an EHR or through a system designed to promote provider‐to‐provider communication. All of the studies that met inclusion criteria focused on physicians as providers. Five studies (20%) described trial design, three (12%) were pilot studies, and seventeen (68%) were observational studies. Thirteen of twenty‐five articles (52%) described studies conducted in the United States and twelve in Europe.
Most of the studies (56%) focused on electronic referrals between primary care and subspecialty providers. The clinical need was to communicate information on a specific patient with a specialist who shared responsibility for the overall plan of care. Only two studies evaluated curbside consultation, where providers ask for clinical recommendations without formally engaging a specialist in the plan of care for a particular patient. Table 2 summarizes included studies and has been organized with respect to clinical need under evaluation. The major themes that emerged from this review included: studies of penetration of communication tools either within the EHR system (intra‐EHR IT) or external to the EHR (extra‐EHR IT); electronic referrals; curbside consultations; and test results reporting (results notification).
| Primary Author, Year | Design | Intervention | Measurement | Results |
|---|---|---|---|---|
| ||||
| Need: Communicate care across clinical settings (inpatient‐outpatient) | ||||
| Branger, 1992[4, 6] | Observational study | Introduction of electronic messaging system in the Netherlands between hospital and PCPs. | Satisfaction survey data using Likert scale of usefulness. | Free text messaging to exchange patient data was rated very useful or useful by 20 of 27 PCP respondents. |
| Reponen, 2004[66] | Observational study | Finnish study of electronic referrals XML messages between EHRs or secure web links. | User questionnaire. No description of respondents was provided. | Internists surveyed estimated that electronic referrals accelerate the referral process by 1 week. |
| Need: Communicate care across specialties (primary care physicians‐specialists) | ||||
| Kooijman, 1998[67] | Observational study | Survey of 45 PCPs who received notes from specialists via Electronic Data Interchange. | User questionnaire with 5‐point Likert scale of satisfaction, from 1 (much better) to 5 (much worse). | Highest satisfaction scores for speed (1.51.8) and efficiency (1.51.7) for electronic messages, with lower scores for reliability (2.52.7) and clarity (2.5). |
| Harno, 2000[4][8] | Nonrandomized trial | Eight‐month prospective comparative study in Finland of outpatient clinics in hospitals with and without intranet referral systems. | Comparison of numbers of electronic referrals, clinic visits, costs. | There were 43% of electronic referrals and 79% of outpatient referrals that resulted in outpatient visits. A 3‐fold increase in productivity overall and 7‐fold reduction in visit costs per patient using e‐mail consultation. |
| Moorman, 2001[4][7] | Observational study | Supersedes Branger, 1999.[68] Analyzes intra‐EHR communications between PCPs and consultant in Netherlands re: diabetes management of patients (19941998). | Descriptive statistics of number of messages, content, whether message had been copied into EMR; survey of PCPs (12 of 15 responded). | Decline in integration by PCPs of messages in the EHR from 75% to 51% over first 3 years. Despite this, most PCPs wanted to extend messaging to other patient groups. |
| Bergus, 2006[69] | Observational study | Follow‐up of Bergus, 1998[54]; evaluated formulation of clinical referrals to specialists at the University of Iowa by retrospective review of e‐mail transcripts. | Analyzed taxonomy of clinical questions; assessed need for clinical consultation of 1618 clinical questions. | Specialists less likely to recommend clinic consultation if referral specified the clinical task (OR: 0.36, P<0.001), intervention (OR: 0.62, P=0.004), or outcome (OR: 0.49, P<0.001). This effect was independent of clinical content (P>0.05). |
| Dennison, 2006[70] | Pilot study | Construction of an electronic referral pro forma to facilitate referral of patients to colorectal surgeons. | Descriptive statistics. Comparisons of patient attendance rate, delays to booking and to actual appointment between 54 electronic referrals and 189 paper referrals. | Compared to paper referrals, electronic referrals were booked more quickly (same day vs 1 week later on average) and patients had lower nonattendance rates (8.5% vs 22.5%). Both results stated as statistically significant, but P values were not provided. |
| Shaw, 2007[49] | Observational study | Dermatology electronic referral in England. | Content of 131 electronic vs 139 paper referrals to dermatologists(NHS Choose and Book).[71] | Paper superior to electronic for clinical data such as current treatments (included in 68% of paper vs 39% of electronic referrals, P<0.001); electronic superior for demographic data. |
| Gandhi, 2008[50] | Nonrandomized trial | Electronic referral tool in the Partners Healthcare System in Massachusetts that included a structured referral‐letter generator and referral status tracker. Assigned to 1 intervention site and 1 control site. | Survey assessment. Fifty‐four of 117 PCPs responded (46%), 235 of 430 specialists responded (55%), 143 out of 210 patients responded (69%). | Intervention group showed high voluntary adoption (99%), higher information transfer rates prior to subspecialty visit (62% vs 12%), and lower rates of conflicting information being given to patients (6% vs 20%). |
| John, 2008[72] | Pilot study | Validation study of the Lower Gastrointestinal e‐RP (through the Choose and Book System in the United Kingdom) intended to improve yield of colon cancers diagnosed and to reduce delays in diagnosis. | Comparison of actual to simulated referral patterns through e‐RP for 300 patients divided into colorectal cancer, 2‐week wait suspected cancer, and routine referral groups. | e‐RP was more accurate than traditional referral at upgrading patients who had cancer to the appropriate suspected cancer referral group (85% vs 43%, P=0.002). |
| Kim, 2009[73] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Included reply functionality and ability to forward messaging to a scheduler for calendaring. | Impact of electronic referral system as measured by questionnaire to referring providers. A total of 298/368 participated (24 clinics); 53.5% attending physicians. | Electronic referrals improved overall quality of care (reported by 72%), guidance of presubspecialty visit (73%), and the ability to track referrals (89%). Small change in access for urgent issues (35% better, 49% reported no change). |
| Scott, 2009[74] | Pilot study | Pilot of urgent electronic referral system from PCPs to oncologists at South West Wales Cancer Centre. | Satisfaction statistics (10‐point Likert scale) collected from PCPs via interview. | Over 6 months, 99 referrals submitted; 81% were processed within 1 hour with high satisfaction scores. |
| Were, 2009[75] | Nonrandomized trial | Geriatrics consultants were provided system to make electronic recommendations (consultant‐recommended orders) in the native CPOE system along with consult notes in the intervention vs consult notes alone in the control. | Rates of implementation of consultant recommendations. Qualitative survey of users of the new system. | Higher total number of recommendations (247 vs 192, P<0.05) and higher implementation rates of consultant‐recommended orders in the intervention group vs control (78% vs 59%, P=0.01). High satisfaction scores on 5‐point Likert scale for the intervention system with good survey response rate (83%). |
| Dixon, 2010[52] | Observational study | Comparison of 2 extra‐EHR systems (NHS Choose and Book, Dutch ZorgDomein) for booking referrals. Patients choose doctor or hospital and the system transfers demographic and clinical information between PCP and specialist. | National data, patient and provider surveys, focus groups, observational studies. Focus was on patient choice, but evaluations included all aspects of the systems. | Resistance from PCPs during implementation; 78% of ZorgDomein PCPs felt referrals took more time; general displeasure on the part of specialists re: quality of referrals, although not quantified. |
| Patterson, 2010[51] | Observational study | E‐mail referral system to a neurologist in Northern Ireland. Referrals were template based and recorded as clinical episode in the patient administration system. Comparison of this system to conventional referrals to another neurologist. | Evaluated effectiveness, cost, safety for period 20022007. | Decreased referral wait times (4 vs 13 weeks) and 35% cost reduction per patient for the e‐mail referral vs conventional referrals. |
| No diminution in safety. Limitation: single neurologist participated. | ||||
| Singh, 2011[76] | Observational study | Chart review of electronic referrals to specialist practices in a Veterans Affairs outpatient system. | Follow‐up actions taken by subspecialists within 30 days of receiving referral. | An intra‐EHR referral system was still affected by communication breakdowns. Of 61,931 referrals, 36.4% were discontinued for inappropriate or incomplete referral requests. |
| Kim‐Hwang, 2010[77] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Follow‐up to Kim, 2009.[73] | Survey of medical and surgical subspecialty consultants. | Statistically significant differences in clarity of consult request in both medical and surgical clinics, in decreased inappropriate referrals in surgical clinics, in decreased use of follow‐up appointments by surgical specialists, and in decreased avoidable follow‐up surgical visits. |
| Warren, 2011[53] | Observational study | Electronic referrals from general medical practices to public referral network of Hutt Hospital in New Zealand (20072010). | Retrospective analysis of transactional data from messaging system and from general inpatient tracking system. Qualitative data collection via interviews. | Estimated 71% of 10,367 referrals were electronic referrals over 3 years. Statistically significant improvement in referral latency without change in staffing. Clinicians appreciate shared transparency of referrals but cite usability issues as barriers. |
| Need: Curbside consults (primary care physicians‐specialists) | ||||
| Bergus, 1998[54] | Observational study | Evaluation of the ECS for curbside consultations between family physicians and subspecialists. | Descriptive statistics of usage data; survey of users. | Median response time 16.1 hours; 92% of questions answered; almost 90% concerned specific patients. Both groups expressed satisfaction. |
| Abbott, 2002[55] | Observational study | Evaluation of Department of Defense Ask a Doc physician‐to‐physicians e‐mail consultation system over network of 21 states (19982000). | Descriptive statistics; qualitative assessment. | There were 3121 consultations. Average response time <12 hours. Minimal cost and effort to initiate and sustain. Felt to mirror clinical practice. Barriers were security and assignation of credit for consultation. |
| Need: Communication of results (primary care physicians ‐specialists) | ||||
| Singh, 2007[5][6] | Nonrandomized trial | Concurrent prospective evaluation of responses to 1017 critical imaging alert notifications in a Veterans Affairs outpatient system (2006). Radiologists generated alerts. Included receipt system. | Measured percentage of unacknowledged alerts and imaging lost to follow‐up. | There were 368 of 1017 transmitted alerts unacknowledged (36%); 45 were completely lost to follow‐up. There were 0.2% outpatient imaging results lost to follow‐up overall. |
| Singh, 2009[5][7] | Nonrandomized trial | Concurrent evaluation of responses to 1196 critical imaging alert notifications in a Veterans Affairs outpatient system (20072008). Similar coding system to Singh, 2007.[56] | Measured percentage of alerts acknowledged, timely follow‐up; compared electronic alerts alone to combination of alerts and phone calls or admission. | Percentage of alerts acknowledged did not differ by type of communication; combination of electronic alerts with phone follow‐up (OR: 0.12, P<0.001) or admission (OR: 0.22, P<0.001) decreased likelihood of delayed follow‐up. Alerts to 2 providers increased the likelihood of delayed follow‐up (OR: 1.99, P=0.03). |
| Abujudeh, 2009[5][8] | Observational study | Retrospective review of e‐mailbased alert system for abnormal imaging results at Massachusetts General Hospital 20052007. E‐mail alerting by radiologist to ordering physician of nonurgent findings. | Descriptive statistics; survey of referring physicians (12/26). | There were 56,691 out of 1,540,254 reports for important but not urgent findings; 93.3% generated e‐mail message (6.7% failure rate); 80% of alerts were viewed. Higher satisfaction for e‐mail alerts over conventional methods (eg, facsimile) for nonurgent but important findings. |
| Need: Communicate within 1 care setting (primary care physicians) | ||||
| Lanham, 2012[78] | Observational study | Comparison of practice‐level EHR use with communication patterns among physicians, nurses, medical assistants, practice managers, and nonclinical staff within individual practices in Texas. | Observation and semistructured interviews. Within‐practice communication patterns were categorized as fragmented or cohesive. Practice‐level EHR use was categorized as homogeneous or heterogeneous. | Clinical practices with cohesive within‐practice communication patterns were associated with homogeneous patterns of practice‐level EHR use. |
| Murphy, 2012[79] | Observational study | Review of note‐based messaging within the EHR in outpatient clinics of large tertiary Veterans Affairs facility. Clinic staff send additional signature request alerts linked to parent notes in the EHR to primary care physicians. | Reason for and origin of alerts. Parent note linked to alert was also reviewed for 3 value attributes: urgency; potential harm if alert was missed; subjective value to PCP of the alert. | Of the alerts reviewed, 53.7% of 525 were deemed of high value but required PCPs to review significant amounts of extraneous text (80.3% of words in parent notes) to get relevant information. Most alerts (40%) were medication, prescription, or refill related. |
Extra‐EHR IT
A review of electronic communication in 2000 examined electronic communication among primary care physicians but notably did not distinguish between communication and data exchange.[43] Of the thirty included publications in that review, seventeen publications dealt with electronically communicated information in general; the remaining studies focused on notifications of test results or transitions of care, reports from specialists, or electronic communication as replacement of traditional referral.[43] Although many studies of electronic communication described positive benefits, few included objective data, and most did not analyze provider‐to‐provider communication specifically. A survey of IT use outside of the EHR in 2006 documented that approximately 30% of clinicians used e‐mail to communicate with other clinicians, fewer than those who consulted on‐line journals (40.8%), but many more than those who communicated with patients by e‐mail at that time (3.6%).[44]
Intra‐EHR IT
A comparison of two physician surveys of EHR use in Massachusetts (the first in 2005 and the second in 2007) documented an increase in the percentage of practices with an EHR, from 23% to 35%; in those practices with EHRs, only the use of electronic prescribing increased over time. Use of secure electronic referrals or messaging including secure e‐mail remained unchanged; of note, referrals and messaging were considered a singular clinical function in that study. Between 2005 and 2007, referrals or clinical messaging were available in 62% and 63% of EHR systems, respectively, and they were used most or all of the time by 29% to 33% of the physicians who had an EHR.[45]
Electronic Referrals
Fourteen articles focused on electronic referrals. Two had a prepost or longitudinal study design,[46, 47] and five included a control group.[48, 49, 50, 51] The rest were descriptive. In most cases, electronic referral improved the transfer of information, especially when standardized message templates were created. Use of electronic referral appeared to result in reduced waiting time for appointments and enabled more efficient triage.
Barriers to integration of electronic referral in the EHR were also assessed. An intra‐EHR communication system requiring a primary care physician to integrate information e‐mailed by the consultant into the record showed the percentage of integrated notes decreasing over time.[47] Practitioners had mixed feelings about the system; although the majority (92% of respondents) felt that the system improved patient care and wanted to extend messaging to other patient groups, they also felt that electronic messaging decreased the ease of reviewing data (83%) and confused tasks and responsibilities (59%). A study of British and Dutch electronic referral systems described significant resistance on the part of practitioners to electronic referrals and concern on the part of specialists about the quality of referrals.[52] Another study demonstrated improvement in quality of demographic data but degradation in quality of clinical information when referrals were submitted electronically.[49] A recent transactional analysis of electronic referrals in New Zealand showed high uptake and reduced referral latency compared to conventional referral; clinicians cited usability concerns as the major barrier to use.[53]
Curbside Consultations via E‐mail
Two studies evaluated curbside consultations via e‐mail and documented high provider satisfaction and rapid turnaround.[54, 55] The preliminary nature of these studies raises questions of sustainability and long‐term implementation.
Results Notification
Three studies focused on test‐result reporting from radiologists. In these studies, a radiologist could designate a result as high priority and have an e‐mail notification sent to the ordering physicians.[56, 57, 58] Urgent results were relayed by telephone. Lack of acknowledgement of alerts impacted the results of every study, and in one of these studies, alerting two physicians, rather than just one, decreased the likelihood that the results would be followed up.[57] Providers did prefer e‐mail to fax notification.[58]
DISCUSSION
The principal findings of the literature review demonstrate the paucity of quantitative data surrounding provider‐to‐provider communication. The majority of studies focused on physicians as providers without emphasis on other provider types on the care team. Most of the quantitative studies investigated electronic referrals. Data collected largely represented measures of provider satisfaction and process measures. Few quantitative studies used established models or measures of team coordination or communication.
This study extends the work of others by compiling a comprehensive view of electronic provider‐to‐provider communication. A recent review of devices for clinical communication tells a part of the story,[28] and our review adds a comprehensive, device‐agnostic look at the systems physicians and other providers use every day.
Limitations of this review include the small number of eligible studies and a homogenous provider type (physicians). The latter is both an important finding and a limitation to generalizability of our results. Reviewed studies were in English only. The literature review by its nature is subject to publication bias.
Intra‐EHR communication cannot serve all purposes, and is it not a panacea for effective care coordination. One recent qualitative study warns about the pitfalls of electronic communication. Interviews with physicians from twenty‐six practices elicited some concerns about the resulting decrease in face‐to‐face communication that has resulted from the adoption of electronic communication tools.[32] This finding brings implications: (1) a false sense of security may reduce verbal communications when they are needed mostduring emergencies or when caring for complex patients who require detailed, nuanced discussion; and (2) fewer conversations within a practice can reduce both knowledge sharing and basic social interactions necessary for the maintenance of a collaboration. Last, privacy and confidentiality are top priorities. Common electronic communication tools are susceptible to security breaches,[47, 59] and innovations within this domain must conform to Health Insurance Portability and Accountability Act of 1996 and Health Information Technology for Economic and Clinical Health Act regulations.[60]
Although electronic communication is not a complete solution for clinical collaboration, it is difficult to use face‐to‐face communication and telephone communication to convey large amounts of patient information while simultaneously generating a record of the transaction. Moreover, paging functions, telephone calls, and face‐to‐face encounters can be highly interruptive, increasing cognitive load, burdening working memory, and shifting attention from the task at hand.[14] Interruptions contribute to inefficiency and to the potential for errors.[61]
Effective coordination of care for the chronically ill is one of the essential goals of the health system; it is an ongoing process that depends on constant, effective communication. Bates and Bitton have recognized this and described the crucial role that HIT will play in creating an effective medical home by enumerating seven domains of HIT especially in need of research.[62] In particular, they note that effective team care and care transitions will depend on an EHR that promotes both implicit and real‐time communication: it will be essential to develop communication tools that allow practices to record goals shared by providers and patients alike, and to track medical interventions and progress.[62]
Future research could investigate a number of open questions. Overall, an emphasis should be placed on rigorous qualitative and quantitative evaluation of electronic communication. Process measures, such as length of stay, hospital readmission rates, and measures of care coordination, should be framed ultimately with respect to patient health outcomes. Such data are beginning to be reported.[63]
It is unclear which types of communications would be best served within the EHR and which should remain external to it. Instant communication or chat has not been studied sufficiently to show a demonstrable impact on patient care. Cross‐coverage and team identification within the EHR can be further studied with respect to workflows and best practices. Studies using structured observation or time‐and‐motion analysis could provide insight into use cases and workflows that providers implement to discuss patients. Future research should incorporate established models of communication[5] and coordination.[64] Data on unintended consequences or harms of provider‐to‐provider electronic communication have been limited, and this area should be considered in subsequent work. Finally, although the scope of this review focused on communication between providers, transformative electronic communication systems should bridge communication gaps between providers and patients as well.
As adoption of EHRs in US hospitals has increased from 15.1% of US hospitals in 2010 to 26.6% in 2011 for any type of EHR and 3.6% to 8.7% for comprehensive EHRs,[65] it is worth noting that Meaningful Use, as it stands, incentivizes patient‐provider communication, but not communication between providers. Inclusion of certification criteria focused on provider‐to‐provider communication may spur additional innovation.
CONCLUSIONS
The optimal features to support electronic communication between providers remain under‐assessed, although there is preliminary evidence for the acceptability of electronic referrals. Without better understanding of electronic communication on workflow, provider satisfaction, and patient outcomes, the impact of such tools on coordination of complex medical care will be an open question, and it remains an important one to answer.
Acknowledgments
The authors would like to express their gratitude to Dr. Thomas Payne, Medical Director of IT Services at the University of Washington, for sharing his expertise, and to Marina Chilov, medical librarian at Columbia University, for her assistance with the literature search. The authors would like to thank Paul Sun, MA, for his assistance with the literature review.
Disclosures: This work was funded by 5K22LM8805 (PDS) and T15 LM007079 (CW, SC) grants. Dr. Stetson serves on the advisory board of the Allscripts Enterprise EHR.
INTRODUCTION
Coordination of care within a practice, during transitions of care, and between primary and specialty care teams requires more than data exchange; it requires effective communication among healthcare providers.[1, 2, 3] In clinical terms, data exchange, communication, and care coordination are related, but they represent distinct concepts.[4] Data exchange refers to transfer of information between settings, independent of the individuals involved, whereas communication is the multistep process that enables information exchange between two people.[5] Care coordination, as defined by O'Malley, is integration of care in consultation with patients, their families and caregivers across all of a patient's conditions, needs, clinicians and settings.[3]
Strong collaboration among providers has been associated with improved patient outcomes.[2, 6] Yet, despite the significant role of communication in healthcare, communication may not take place at all, even at high‐stakes events like transitions of care,[7, 8] or it may be done poorly at the risk of substantial clinical morbidity and mortality.[9, 10, 11, 12, 13, 14, 15, 16]
Proof of the global effectiveness of health information technology (HIT) to improve patient care is lacking, but data from some studies demonstrate real improvements in quality and safety in specific areas,[17, 18, 19] especially with computerized physician order entry[20] and electronic prescribing.[21]
The limited information about the effect of HIT on communication focuses largely on the anticipated improvements in patient‐physician communication[22, 23, 24, 25, 26, 27]; provider‐to‐provider communication within the electronic domain is not as well understood. A recent review of interventions involving communication devices such as pagers and mobile phones found limited high‐quality evidence in the literature.[28] Clinicians have described what they consider to be key characteristics of clinical electronic communications systems such as security/reliability, cross coverage, overall convenience, and message prioritization.[29] Although the electronic health record (EHR) is expected to assist with this communication,[30] it also has the potential to impede effective communication, leading physicians to resort to more traditional workarounds.[31, 32, 33]
Measuring and improving the use of EHRs nationally were driving forces behind the creation of the Meaningful Use incentive program in the United States.[34] To receive the incentive payments, providers must meet and report on a series of measures set in three stages over the course of five years.[35] In the current state, Meaningful Use does not reward provider‐to‐provider communication within the EHR.[36, 37] The main communication objectives for stages 1 and 2 concentrate on patient‐to‐provider communication, such as patient portals and patient‐to‐provider messaging.[36, 37]
Understanding the current evidence for provider‐to‐provider communication within EHRs, its reported effectiveness, and its shortcomings may help to develop a roadmap for identifying next‐generation solutions to support coordination of care.[38, 39] This review assesses the literature regarding provider‐to‐provider electronic communication tools (as supported within or external to an EHR). It is intended as a comprehensive view of studies reporting quantitative measures of the impact of electronic communication on providers and patients.
METHODS
Definitions and Conceptual Model of Provider‐to‐Provider Communication
We conducted a systematic review of studies of provider‐to‐provider electronic communication. This review included only formal clinical communication between providers and was informed by the Coiera communications paradigm.[5] This paradigm consists of four steps: (1) task identification, when a task is identified and associated with the appropriate individual; (2) connection, when an attempt is made to contact that person; (3) communication, when task‐specific information is exchanged between the parties; and (4) disconnection, when the task reaches some stage of completion.
Literature Review
We examined written electronic communication between providers including e‐mail, text messaging, and instant messaging. We did not review provider‐to‐provider telephone or telehealth communication, as these are not generally supported within EHR systems. Communication in all clinical contexts was included among providers within an individual clinic or hospital and among providers across specialties or practice settings.[40] We excluded physician handoff communication because it has been extensively reviewed elsewhere and because handoff occurs largely through verbal exchange not recorded in the EHR.[41, 42] Communication from clinical information systems to providers, such as automated notification of unacknowledged orders, was also excluded, as it is not within the scope of provider‐to‐provider interaction.
Data Sources and Searches
A comprehensive literature search was conducted in Ovid MEDLINE with the input of a medical librarian, and a parallel search was performed using PubMed. The Ovid MEDLINE query and parallel database search terms are documented in Table 1. Subsearches were conducted in Google Scholar, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Academic Search Premier for peer‐reviewed journals. Subsequent studies citing the initially detected articles were found through citation maps.
| Database | Strategy | Items Reviewed |
|---|---|---|
| ||
| Ovid MEDLINE | Query terms: exp medicine/ or physicians or exp outpatient clinics/ or exp hospitals/ AND *communication/ or *computer communication networks/ or *interprofessional relations/ or *continuity of patient care/ AND electronic mail or referral and consultation or text messaging/ or reminder systems. | 1513 |
| PubMed | Healthcare, provider, communication, messaging, e‐mail, texting, text messaging, instant messaging, paging, coordination, referral, EHR, EMR, electronic health record, electronic medical record, electronic, and physician. Excluding patient‐provider and patient‐physician | 340 |
| Google Scholar | Physician‐physician electronic communication excluding physician‐patient | 940 |
| CINAHL | Medical records and communication; or computerized patient records and communication | None |
| Academic Search Premier (peer‐reviewed journals) | Electronic health record and communication | 54 |
| Communication and electronic health record | 80 | |
| Physician‐physician communication | 2 | |
| Physicians and electronic health records | 88 | |
Study Selection
Paper Inclusion Criteria
Requirements included publication in English‐language peer‐reviewed journals. Included studies provided quantitative provider‐to‐provider communication data, provider satisfaction statistics, or EHR communication data. Provider‐to‐staff communication was also included if it fell within the scope of studies of communication between providers.
Paper Exclusion Criteria
Studies excluded in this review were articles that reviewed EHR systems without any focus on communication between providers and those that discussed EHR models and strategies but did not include actual testing and quantitative results. Results that included nontraditional online documents or that were found on nonpeer‐reviewed websites were also discarded. Duplicate records or publications that covered the same study were also removed. The most common reason for exclusion was the lack of quantitative evaluation.
Data Extraction and Quality Assessment
Three authors (Walsh, Siegler, Stetson) reviewed titles and abstracts of resultant studies against inclusion and exclusion criteria (Figure 1). Studies were evaluated qualitatively and findings summarized. Given the heterogeneous nature of data reported, statistical analysis was not possible.

RESULTS
The primary and parallel searches produced 2946 results that were weaned through title review and exclusion of duplicates, nonEnglish‐language, and nonhuman studies to 820 articles for title and abstract review (Figure 1). After careful review of the articles' titles, abstracts, or full content (where appropriate), twenty‐five articles met inclusion criteria and presented data about provider‐to‐provider electronic communication, either within an EHR or through a system designed to promote provider‐to‐provider communication. All of the studies that met inclusion criteria focused on physicians as providers. Five studies (20%) described trial design, three (12%) were pilot studies, and seventeen (68%) were observational studies. Thirteen of twenty‐five articles (52%) described studies conducted in the United States and twelve in Europe.
Most of the studies (56%) focused on electronic referrals between primary care and subspecialty providers. The clinical need was to communicate information on a specific patient with a specialist who shared responsibility for the overall plan of care. Only two studies evaluated curbside consultation, where providers ask for clinical recommendations without formally engaging a specialist in the plan of care for a particular patient. Table 2 summarizes included studies and has been organized with respect to clinical need under evaluation. The major themes that emerged from this review included: studies of penetration of communication tools either within the EHR system (intra‐EHR IT) or external to the EHR (extra‐EHR IT); electronic referrals; curbside consultations; and test results reporting (results notification).
| Primary Author, Year | Design | Intervention | Measurement | Results |
|---|---|---|---|---|
| ||||
| Need: Communicate care across clinical settings (inpatient‐outpatient) | ||||
| Branger, 1992[4, 6] | Observational study | Introduction of electronic messaging system in the Netherlands between hospital and PCPs. | Satisfaction survey data using Likert scale of usefulness. | Free text messaging to exchange patient data was rated very useful or useful by 20 of 27 PCP respondents. |
| Reponen, 2004[66] | Observational study | Finnish study of electronic referrals XML messages between EHRs or secure web links. | User questionnaire. No description of respondents was provided. | Internists surveyed estimated that electronic referrals accelerate the referral process by 1 week. |
| Need: Communicate care across specialties (primary care physicians‐specialists) | ||||
| Kooijman, 1998[67] | Observational study | Survey of 45 PCPs who received notes from specialists via Electronic Data Interchange. | User questionnaire with 5‐point Likert scale of satisfaction, from 1 (much better) to 5 (much worse). | Highest satisfaction scores for speed (1.51.8) and efficiency (1.51.7) for electronic messages, with lower scores for reliability (2.52.7) and clarity (2.5). |
| Harno, 2000[4][8] | Nonrandomized trial | Eight‐month prospective comparative study in Finland of outpatient clinics in hospitals with and without intranet referral systems. | Comparison of numbers of electronic referrals, clinic visits, costs. | There were 43% of electronic referrals and 79% of outpatient referrals that resulted in outpatient visits. A 3‐fold increase in productivity overall and 7‐fold reduction in visit costs per patient using e‐mail consultation. |
| Moorman, 2001[4][7] | Observational study | Supersedes Branger, 1999.[68] Analyzes intra‐EHR communications between PCPs and consultant in Netherlands re: diabetes management of patients (19941998). | Descriptive statistics of number of messages, content, whether message had been copied into EMR; survey of PCPs (12 of 15 responded). | Decline in integration by PCPs of messages in the EHR from 75% to 51% over first 3 years. Despite this, most PCPs wanted to extend messaging to other patient groups. |
| Bergus, 2006[69] | Observational study | Follow‐up of Bergus, 1998[54]; evaluated formulation of clinical referrals to specialists at the University of Iowa by retrospective review of e‐mail transcripts. | Analyzed taxonomy of clinical questions; assessed need for clinical consultation of 1618 clinical questions. | Specialists less likely to recommend clinic consultation if referral specified the clinical task (OR: 0.36, P<0.001), intervention (OR: 0.62, P=0.004), or outcome (OR: 0.49, P<0.001). This effect was independent of clinical content (P>0.05). |
| Dennison, 2006[70] | Pilot study | Construction of an electronic referral pro forma to facilitate referral of patients to colorectal surgeons. | Descriptive statistics. Comparisons of patient attendance rate, delays to booking and to actual appointment between 54 electronic referrals and 189 paper referrals. | Compared to paper referrals, electronic referrals were booked more quickly (same day vs 1 week later on average) and patients had lower nonattendance rates (8.5% vs 22.5%). Both results stated as statistically significant, but P values were not provided. |
| Shaw, 2007[49] | Observational study | Dermatology electronic referral in England. | Content of 131 electronic vs 139 paper referrals to dermatologists(NHS Choose and Book).[71] | Paper superior to electronic for clinical data such as current treatments (included in 68% of paper vs 39% of electronic referrals, P<0.001); electronic superior for demographic data. |
| Gandhi, 2008[50] | Nonrandomized trial | Electronic referral tool in the Partners Healthcare System in Massachusetts that included a structured referral‐letter generator and referral status tracker. Assigned to 1 intervention site and 1 control site. | Survey assessment. Fifty‐four of 117 PCPs responded (46%), 235 of 430 specialists responded (55%), 143 out of 210 patients responded (69%). | Intervention group showed high voluntary adoption (99%), higher information transfer rates prior to subspecialty visit (62% vs 12%), and lower rates of conflicting information being given to patients (6% vs 20%). |
| John, 2008[72] | Pilot study | Validation study of the Lower Gastrointestinal e‐RP (through the Choose and Book System in the United Kingdom) intended to improve yield of colon cancers diagnosed and to reduce delays in diagnosis. | Comparison of actual to simulated referral patterns through e‐RP for 300 patients divided into colorectal cancer, 2‐week wait suspected cancer, and routine referral groups. | e‐RP was more accurate than traditional referral at upgrading patients who had cancer to the appropriate suspected cancer referral group (85% vs 43%, P=0.002). |
| Kim, 2009[73] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Included reply functionality and ability to forward messaging to a scheduler for calendaring. | Impact of electronic referral system as measured by questionnaire to referring providers. A total of 298/368 participated (24 clinics); 53.5% attending physicians. | Electronic referrals improved overall quality of care (reported by 72%), guidance of presubspecialty visit (73%), and the ability to track referrals (89%). Small change in access for urgent issues (35% better, 49% reported no change). |
| Scott, 2009[74] | Pilot study | Pilot of urgent electronic referral system from PCPs to oncologists at South West Wales Cancer Centre. | Satisfaction statistics (10‐point Likert scale) collected from PCPs via interview. | Over 6 months, 99 referrals submitted; 81% were processed within 1 hour with high satisfaction scores. |
| Were, 2009[75] | Nonrandomized trial | Geriatrics consultants were provided system to make electronic recommendations (consultant‐recommended orders) in the native CPOE system along with consult notes in the intervention vs consult notes alone in the control. | Rates of implementation of consultant recommendations. Qualitative survey of users of the new system. | Higher total number of recommendations (247 vs 192, P<0.05) and higher implementation rates of consultant‐recommended orders in the intervention group vs control (78% vs 59%, P=0.01). High satisfaction scores on 5‐point Likert scale for the intervention system with good survey response rate (83%). |
| Dixon, 2010[52] | Observational study | Comparison of 2 extra‐EHR systems (NHS Choose and Book, Dutch ZorgDomein) for booking referrals. Patients choose doctor or hospital and the system transfers demographic and clinical information between PCP and specialist. | National data, patient and provider surveys, focus groups, observational studies. Focus was on patient choice, but evaluations included all aspects of the systems. | Resistance from PCPs during implementation; 78% of ZorgDomein PCPs felt referrals took more time; general displeasure on the part of specialists re: quality of referrals, although not quantified. |
| Patterson, 2010[51] | Observational study | E‐mail referral system to a neurologist in Northern Ireland. Referrals were template based and recorded as clinical episode in the patient administration system. Comparison of this system to conventional referrals to another neurologist. | Evaluated effectiveness, cost, safety for period 20022007. | Decreased referral wait times (4 vs 13 weeks) and 35% cost reduction per patient for the e‐mail referral vs conventional referrals. |
| No diminution in safety. Limitation: single neurologist participated. | ||||
| Singh, 2011[76] | Observational study | Chart review of electronic referrals to specialist practices in a Veterans Affairs outpatient system. | Follow‐up actions taken by subspecialists within 30 days of receiving referral. | An intra‐EHR referral system was still affected by communication breakdowns. Of 61,931 referrals, 36.4% were discontinued for inappropriate or incomplete referral requests. |
| Kim‐Hwang, 2010[77] | Observational study | Electronic referrals via a portal to San Francisco General Hospital. Follow‐up to Kim, 2009.[73] | Survey of medical and surgical subspecialty consultants. | Statistically significant differences in clarity of consult request in both medical and surgical clinics, in decreased inappropriate referrals in surgical clinics, in decreased use of follow‐up appointments by surgical specialists, and in decreased avoidable follow‐up surgical visits. |
| Warren, 2011[53] | Observational study | Electronic referrals from general medical practices to public referral network of Hutt Hospital in New Zealand (20072010). | Retrospective analysis of transactional data from messaging system and from general inpatient tracking system. Qualitative data collection via interviews. | Estimated 71% of 10,367 referrals were electronic referrals over 3 years. Statistically significant improvement in referral latency without change in staffing. Clinicians appreciate shared transparency of referrals but cite usability issues as barriers. |
| Need: Curbside consults (primary care physicians‐specialists) | ||||
| Bergus, 1998[54] | Observational study | Evaluation of the ECS for curbside consultations between family physicians and subspecialists. | Descriptive statistics of usage data; survey of users. | Median response time 16.1 hours; 92% of questions answered; almost 90% concerned specific patients. Both groups expressed satisfaction. |
| Abbott, 2002[55] | Observational study | Evaluation of Department of Defense Ask a Doc physician‐to‐physicians e‐mail consultation system over network of 21 states (19982000). | Descriptive statistics; qualitative assessment. | There were 3121 consultations. Average response time <12 hours. Minimal cost and effort to initiate and sustain. Felt to mirror clinical practice. Barriers were security and assignation of credit for consultation. |
| Need: Communication of results (primary care physicians ‐specialists) | ||||
| Singh, 2007[5][6] | Nonrandomized trial | Concurrent prospective evaluation of responses to 1017 critical imaging alert notifications in a Veterans Affairs outpatient system (2006). Radiologists generated alerts. Included receipt system. | Measured percentage of unacknowledged alerts and imaging lost to follow‐up. | There were 368 of 1017 transmitted alerts unacknowledged (36%); 45 were completely lost to follow‐up. There were 0.2% outpatient imaging results lost to follow‐up overall. |
| Singh, 2009[5][7] | Nonrandomized trial | Concurrent evaluation of responses to 1196 critical imaging alert notifications in a Veterans Affairs outpatient system (20072008). Similar coding system to Singh, 2007.[56] | Measured percentage of alerts acknowledged, timely follow‐up; compared electronic alerts alone to combination of alerts and phone calls or admission. | Percentage of alerts acknowledged did not differ by type of communication; combination of electronic alerts with phone follow‐up (OR: 0.12, P<0.001) or admission (OR: 0.22, P<0.001) decreased likelihood of delayed follow‐up. Alerts to 2 providers increased the likelihood of delayed follow‐up (OR: 1.99, P=0.03). |
| Abujudeh, 2009[5][8] | Observational study | Retrospective review of e‐mailbased alert system for abnormal imaging results at Massachusetts General Hospital 20052007. E‐mail alerting by radiologist to ordering physician of nonurgent findings. | Descriptive statistics; survey of referring physicians (12/26). | There were 56,691 out of 1,540,254 reports for important but not urgent findings; 93.3% generated e‐mail message (6.7% failure rate); 80% of alerts were viewed. Higher satisfaction for e‐mail alerts over conventional methods (eg, facsimile) for nonurgent but important findings. |
| Need: Communicate within 1 care setting (primary care physicians) | ||||
| Lanham, 2012[78] | Observational study | Comparison of practice‐level EHR use with communication patterns among physicians, nurses, medical assistants, practice managers, and nonclinical staff within individual practices in Texas. | Observation and semistructured interviews. Within‐practice communication patterns were categorized as fragmented or cohesive. Practice‐level EHR use was categorized as homogeneous or heterogeneous. | Clinical practices with cohesive within‐practice communication patterns were associated with homogeneous patterns of practice‐level EHR use. |
| Murphy, 2012[79] | Observational study | Review of note‐based messaging within the EHR in outpatient clinics of large tertiary Veterans Affairs facility. Clinic staff send additional signature request alerts linked to parent notes in the EHR to primary care physicians. | Reason for and origin of alerts. Parent note linked to alert was also reviewed for 3 value attributes: urgency; potential harm if alert was missed; subjective value to PCP of the alert. | Of the alerts reviewed, 53.7% of 525 were deemed of high value but required PCPs to review significant amounts of extraneous text (80.3% of words in parent notes) to get relevant information. Most alerts (40%) were medication, prescription, or refill related. |
Extra‐EHR IT
A review of electronic communication in 2000 examined electronic communication among primary care physicians but notably did not distinguish between communication and data exchange.[43] Of the thirty included publications in that review, seventeen publications dealt with electronically communicated information in general; the remaining studies focused on notifications of test results or transitions of care, reports from specialists, or electronic communication as replacement of traditional referral.[43] Although many studies of electronic communication described positive benefits, few included objective data, and most did not analyze provider‐to‐provider communication specifically. A survey of IT use outside of the EHR in 2006 documented that approximately 30% of clinicians used e‐mail to communicate with other clinicians, fewer than those who consulted on‐line journals (40.8%), but many more than those who communicated with patients by e‐mail at that time (3.6%).[44]
Intra‐EHR IT
A comparison of two physician surveys of EHR use in Massachusetts (the first in 2005 and the second in 2007) documented an increase in the percentage of practices with an EHR, from 23% to 35%; in those practices with EHRs, only the use of electronic prescribing increased over time. Use of secure electronic referrals or messaging including secure e‐mail remained unchanged; of note, referrals and messaging were considered a singular clinical function in that study. Between 2005 and 2007, referrals or clinical messaging were available in 62% and 63% of EHR systems, respectively, and they were used most or all of the time by 29% to 33% of the physicians who had an EHR.[45]
Electronic Referrals
Fourteen articles focused on electronic referrals. Two had a prepost or longitudinal study design,[46, 47] and five included a control group.[48, 49, 50, 51] The rest were descriptive. In most cases, electronic referral improved the transfer of information, especially when standardized message templates were created. Use of electronic referral appeared to result in reduced waiting time for appointments and enabled more efficient triage.
Barriers to integration of electronic referral in the EHR were also assessed. An intra‐EHR communication system requiring a primary care physician to integrate information e‐mailed by the consultant into the record showed the percentage of integrated notes decreasing over time.[47] Practitioners had mixed feelings about the system; although the majority (92% of respondents) felt that the system improved patient care and wanted to extend messaging to other patient groups, they also felt that electronic messaging decreased the ease of reviewing data (83%) and confused tasks and responsibilities (59%). A study of British and Dutch electronic referral systems described significant resistance on the part of practitioners to electronic referrals and concern on the part of specialists about the quality of referrals.[52] Another study demonstrated improvement in quality of demographic data but degradation in quality of clinical information when referrals were submitted electronically.[49] A recent transactional analysis of electronic referrals in New Zealand showed high uptake and reduced referral latency compared to conventional referral; clinicians cited usability concerns as the major barrier to use.[53]
Curbside Consultations via E‐mail
Two studies evaluated curbside consultations via e‐mail and documented high provider satisfaction and rapid turnaround.[54, 55] The preliminary nature of these studies raises questions of sustainability and long‐term implementation.
Results Notification
Three studies focused on test‐result reporting from radiologists. In these studies, a radiologist could designate a result as high priority and have an e‐mail notification sent to the ordering physicians.[56, 57, 58] Urgent results were relayed by telephone. Lack of acknowledgement of alerts impacted the results of every study, and in one of these studies, alerting two physicians, rather than just one, decreased the likelihood that the results would be followed up.[57] Providers did prefer e‐mail to fax notification.[58]
DISCUSSION
The principal findings of the literature review demonstrate the paucity of quantitative data surrounding provider‐to‐provider communication. The majority of studies focused on physicians as providers without emphasis on other provider types on the care team. Most of the quantitative studies investigated electronic referrals. Data collected largely represented measures of provider satisfaction and process measures. Few quantitative studies used established models or measures of team coordination or communication.
This study extends the work of others by compiling a comprehensive view of electronic provider‐to‐provider communication. A recent review of devices for clinical communication tells a part of the story,[28] and our review adds a comprehensive, device‐agnostic look at the systems physicians and other providers use every day.
Limitations of this review include the small number of eligible studies and a homogenous provider type (physicians). The latter is both an important finding and a limitation to generalizability of our results. Reviewed studies were in English only. The literature review by its nature is subject to publication bias.
Intra‐EHR communication cannot serve all purposes, and is it not a panacea for effective care coordination. One recent qualitative study warns about the pitfalls of electronic communication. Interviews with physicians from twenty‐six practices elicited some concerns about the resulting decrease in face‐to‐face communication that has resulted from the adoption of electronic communication tools.[32] This finding brings implications: (1) a false sense of security may reduce verbal communications when they are needed mostduring emergencies or when caring for complex patients who require detailed, nuanced discussion; and (2) fewer conversations within a practice can reduce both knowledge sharing and basic social interactions necessary for the maintenance of a collaboration. Last, privacy and confidentiality are top priorities. Common electronic communication tools are susceptible to security breaches,[47, 59] and innovations within this domain must conform to Health Insurance Portability and Accountability Act of 1996 and Health Information Technology for Economic and Clinical Health Act regulations.[60]
Although electronic communication is not a complete solution for clinical collaboration, it is difficult to use face‐to‐face communication and telephone communication to convey large amounts of patient information while simultaneously generating a record of the transaction. Moreover, paging functions, telephone calls, and face‐to‐face encounters can be highly interruptive, increasing cognitive load, burdening working memory, and shifting attention from the task at hand.[14] Interruptions contribute to inefficiency and to the potential for errors.[61]
Effective coordination of care for the chronically ill is one of the essential goals of the health system; it is an ongoing process that depends on constant, effective communication. Bates and Bitton have recognized this and described the crucial role that HIT will play in creating an effective medical home by enumerating seven domains of HIT especially in need of research.[62] In particular, they note that effective team care and care transitions will depend on an EHR that promotes both implicit and real‐time communication: it will be essential to develop communication tools that allow practices to record goals shared by providers and patients alike, and to track medical interventions and progress.[62]
Future research could investigate a number of open questions. Overall, an emphasis should be placed on rigorous qualitative and quantitative evaluation of electronic communication. Process measures, such as length of stay, hospital readmission rates, and measures of care coordination, should be framed ultimately with respect to patient health outcomes. Such data are beginning to be reported.[63]
It is unclear which types of communications would be best served within the EHR and which should remain external to it. Instant communication or chat has not been studied sufficiently to show a demonstrable impact on patient care. Cross‐coverage and team identification within the EHR can be further studied with respect to workflows and best practices. Studies using structured observation or time‐and‐motion analysis could provide insight into use cases and workflows that providers implement to discuss patients. Future research should incorporate established models of communication[5] and coordination.[64] Data on unintended consequences or harms of provider‐to‐provider electronic communication have been limited, and this area should be considered in subsequent work. Finally, although the scope of this review focused on communication between providers, transformative electronic communication systems should bridge communication gaps between providers and patients as well.
As adoption of EHRs in US hospitals has increased from 15.1% of US hospitals in 2010 to 26.6% in 2011 for any type of EHR and 3.6% to 8.7% for comprehensive EHRs,[65] it is worth noting that Meaningful Use, as it stands, incentivizes patient‐provider communication, but not communication between providers. Inclusion of certification criteria focused on provider‐to‐provider communication may spur additional innovation.
CONCLUSIONS
The optimal features to support electronic communication between providers remain under‐assessed, although there is preliminary evidence for the acceptability of electronic referrals. Without better understanding of electronic communication on workflow, provider satisfaction, and patient outcomes, the impact of such tools on coordination of complex medical care will be an open question, and it remains an important one to answer.
Acknowledgments
The authors would like to express their gratitude to Dr. Thomas Payne, Medical Director of IT Services at the University of Washington, for sharing his expertise, and to Marina Chilov, medical librarian at Columbia University, for her assistance with the literature search. The authors would like to thank Paul Sun, MA, for his assistance with the literature review.
Disclosures: This work was funded by 5K22LM8805 (PDS) and T15 LM007079 (CW, SC) grants. Dr. Stetson serves on the advisory board of the Allscripts Enterprise EHR.
- , . Physician‐physician communication: what's the hang‐up? J Gen Intern Med. 2009;24:437–439.
- , , , et al. Meta‐analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152(4):247–258.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2009;25:177–185.
- , , , , , . Development of an ontology to model medical errors, information needs, and the clinical communication space. Proc AMIA Symp. 2001:672–676.
- . Clinical communication: a new informatics paradigm. Proc AMIA Annu Fall Symp. 1996:17–21.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Dis Mon. 2002;48(4):218–229.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Analysing potential harm in Australian general practice: an incident‐monitoring study. J Am Med Inform Assoc. 1998;169:73–76.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , , . Communication loads on clinical staff in the emergency department. J Am Med Inform Assoc. 2002;176(9):415–418.
- , , , , , . Deficit in communicaiton and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297:831–841.
- , . Improving clinical communication: a view from psychology. J Am Med Inform Assoc. 2000;7(5):453–461.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–471.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , . Costs and benefits of health information technology. Evid Rep Technol Assess. 2006;132:1–71.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48:203–209.
- , , , et al. Decrease in hospital‐wide mortality rate after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2010;126:e1–e8.
- , , , , . Electronic prescribing improves medication safety in community‐based office practices. J Gen Intern Med. 2010;25:530–536.
- . Patient‐centered Care. Radiol Technol. 2009;81(2):133–147.
- , . The missing link: bridging the patient‐provider health information gap. Health Aff (Millwood). 2005;24(5):1290–1295.
- , , , , . Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006;13(2):121–126.
- , . Web messaging: a new tool for patient‐physician communication. J Am Med Inform Assoc. 2003;10(3):260–270.
- , , , . Expanding the guidelines for electronic communication with patients. J Am Med Inform Assoc. 2001;8(4):344–348.
- , , , . The utility of electronic mail as a medium for patient‐physician communication. Arch Fam Med. 1994;3(3):268–271.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , . Desiderata for personal electronic communication in clinical systems. J Am Med Inform Assoc. 2002;9(3):209–216.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25:174–176.
- , , . Electronic medical records and communication with patients and other clinicians: are we talking less? Issue Brief Cent Stud Health Syst Change. 2010;(131):1–4.
- , . Referral and consultation communication between primary care and specialist physicians: finding common ground. Arch Intern Med. 2011;171(1):56–65.
- , . The "meaningful use" regulation for electronic health records. N Engl J Med. 2010;363(6):501–504.
- Centers for Medicare 25(3):177–185.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25(3):174–176.
- , , , . A framework for clinical communication supporting healthcare delivery. AMIA Annu Symp Proc. 2005:375–379.
- Understanding and improving patient handoffs. Jt Comm J Qual Improv. 2010:49–96.
- , , , . Signout: a collaborative document with implications for the future of clinical information systems. AMIA Annu Symp Proc. 2007:696–700.
- , , . Effects of electronic communication in general practice. Int J Med Inform. 2000;60(1):59–70.
- , , , , . Prevalence of basic information technology use by U.S. physicians. J Gen Intern Med. 2006;21:1150–1155.
- , , , et al. Physicians' use of key functions in electronic health records from 2005 to 2007: a statewide survey. J Am Med Inform Assoc. 2009;16:465–470.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , , . Electronic messaging between primary and secondary care: a four‐year case report. J Am Med Inform Assoc. 2001;8(4):372–378.
- , , , . Patient referral by telemedicine: effectiveness and cost anslysis of an intranet system. J Telemed Telecare. 2000;6(6):320–329.
- , . Strengths and weaknesses of electronic referral: comparison of data content and clinical value of electronic and paper referrals in dermatology. Br J Gen Pract. 2007;57(536):223–224.
- , , , et al. Improving referral communication using a referral tool within an electronic medical record. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:63–74.
- , , , . Email triage is an effective, efficient, and safe way of managing new referrals to a neurologist. Qual Safety Health Care. 2010;19(5):e51.
- , , . The experience of implementing choice at point of referral: a comparison of the Netherlands and England. Health Econ Policy Law. 2010;13:1–23.
- , , , , . Introduction of electronic referral from community associated with more timely review by secondary services. Appl Clin Inform. 2011;2(4):546–564.
- , , , . Use of an e‐mail curbside consultation service by family physicians. J Fam Pract. 1998;47:357–360.
- , , , , , . Physician‐to‐physician consultation via electronic mail: the Walter Reed Army Medical Center Ask a Doc system. Mil Med. 2002;167:200–204.
- , , , , , . Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14:459–466.
- , , , et al. Timely follow‐up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch of Intern Med. 2009;169(17):1578–1586.
- , , , , . Important imaging finding e‐mail alert system: experience after 3 years of implementation. Radiology. 2009;252:747–753.
- . Instant insecurity: security issues of instant messaging. Symantec website. Available at: http://www.symantec.com/connect/articles/instant‐insecurity‐security‐issues‐instant‐messaging.
- . Secure messaging in healthcare. Tech solutions for HIPAA‐compliant messaging. J AHIMA. 2008;79(6):50–51.
- , , , , . Association of interruptions with an increased risk and severity of medication administration errors. Arch Intern Med. 2010;170(8):683–690.
- , . The future of health information technology in the patient‐centered medical home. Health Aff (Millwood). 2010;29(4):614–621.
- , , . eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450–2453.
- , , , et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol 7. Care Coordination. Rockville, MD; 2007.
- , , , , . Small, nonteaching, and rural hospitals continue to be slow in adopting electronic health record systems. Health Aff (Millwood). 2012;31(5):1092–1099.
- , , , . Extending a multimedia medical record to a regional service with electronic referral and discharge letters. J Telemed Telecare. 2004;10S:81–83.
- , . Sending specialist reports to GPs using EDI. Stud Health Tech Inform. 1998;52(pt 1):408–411.
- , , , , . Shared care for diabetes: supporting communication between primary and secondary care. Int J Med Inform. 1999;53(2-3):133–142.
- , , , . Email teleconsultations: well formulated clinical referrals reduce the need for clinic consultation. J Telemed Telecare. 2006;12(1):33–38.
- , , , . An effective electronic surgical referral system. Ann R Coll Surg Engl. 2006;88(6):554–556.
- . Choose and book. Clin Med. 2006;6(5):473–476.
- , , , . Validation of the lower gastrointestinal electronic referral protocol. Brit J Surg. 2008;95:506–514.
- , , , , . Not perfect, but better: primary care providers' experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24:614–619.
- . The Swansea electronic referrals project. J Telemed Telecare. 2009;15:156–158.
- , , , , . Using computerized provider order entry and clinical decision support to improve referring physicians' implementation of consultants' medical recommendations. J Am Med Inform Assoc. 2009;16(2):196–202.
- , , , et al. Follow‐up actions on electronic referral communication in a multispecialty outpatient setting. J Gen Intern Med. 2011;26(1):64–69.
- , , , , , . Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123–11028.
- , , . Same organization, same electronic health records (EHRs) system, different use: exploring the linkage between practice member communication patterns and EHR use patterns in an ambulatory care setting. J Am Med Inform Assoc. 2012;19(3):382–391.
- , , , et al. Electronic health record‐based messages to primary care providers: valuable information or just noise? Arch Intern Med. 2012;172(3):283–285.
- , . Physician‐physician communication: what's the hang‐up? J Gen Intern Med. 2009;24:437–439.
- , , , et al. Meta‐analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152(4):247–258.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2009;25:177–185.
- , , , , , . Development of an ontology to model medical errors, information needs, and the clinical communication space. Proc AMIA Symp. 2001:672–676.
- . Clinical communication: a new informatics paradigm. Proc AMIA Annu Fall Symp. 1996:17–21.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Dis Mon. 2002;48(4):218–229.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–1316.
- , , , . Analysing potential harm in Australian general practice: an incident‐monitoring study. J Am Med Inform Assoc. 1998;169:73–76.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277–286.
- , , , , . Communication loads on clinical staff in the emergency department. J Am Med Inform Assoc. 2002;176(9):415–418.
- , , , , , . Deficit in communicaiton and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297:831–841.
- , . Improving clinical communication: a view from psychology. J Am Med Inform Assoc. 2000;7(5):453–461.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–471.
- , , , et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752.
- , , . Costs and benefits of health information technology. Evid Rep Technol Assess. 2006;132:1–71.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48:203–209.
- , , , et al. Decrease in hospital‐wide mortality rate after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2010;126:e1–e8.
- , , , , . Electronic prescribing improves medication safety in community‐based office practices. J Gen Intern Med. 2010;25:530–536.
- . Patient‐centered Care. Radiol Technol. 2009;81(2):133–147.
- , . The missing link: bridging the patient‐provider health information gap. Health Aff (Millwood). 2005;24(5):1290–1295.
- , , , , . Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006;13(2):121–126.
- , . Web messaging: a new tool for patient‐physician communication. J Am Med Inform Assoc. 2003;10(3):260–270.
- , , , . Expanding the guidelines for electronic communication with patients. J Am Med Inform Assoc. 2001;8(4):344–348.
- , , , . The utility of electronic mail as a medium for patient‐physician communication. Arch Fam Med. 1994;3(3):268–271.
- , , , et al. Effects of clinical communication interventions in hospitals: a systematic review of information and communication technology adoptions for improved communication between clinicians. Int J Med Inform. 2012;81(11):723–732.
- , . Desiderata for personal electronic communication in clinical systems. J Am Med Inform Assoc. 2002;9(3):209–216.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25:174–176.
- , , . Electronic medical records and communication with patients and other clinicians: are we talking less? Issue Brief Cent Stud Health Syst Change. 2010;(131):1–4.
- , . Referral and consultation communication between primary care and specialist physicians: finding common ground. Arch Intern Med. 2011;171(1):56–65.
- , . The "meaningful use" regulation for electronic health records. N Engl J Med. 2010;363(6):501–504.
- Centers for Medicare 25(3):177–185.
- . Getting in step: electronic health records and their role in care coordination. J Gen Intern Med. 2010;25(3):174–176.
- , , , . A framework for clinical communication supporting healthcare delivery. AMIA Annu Symp Proc. 2005:375–379.
- Understanding and improving patient handoffs. Jt Comm J Qual Improv. 2010:49–96.
- , , , . Signout: a collaborative document with implications for the future of clinical information systems. AMIA Annu Symp Proc. 2007:696–700.
- , , . Effects of electronic communication in general practice. Int J Med Inform. 2000;60(1):59–70.
- , , , , . Prevalence of basic information technology use by U.S. physicians. J Gen Intern Med. 2006;21:1150–1155.
- , , , et al. Physicians' use of key functions in electronic health records from 2005 to 2007: a statewide survey. J Am Med Inform Assoc. 2009;16:465–470.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , , . Electronic messaging between primary and secondary care: a four‐year case report. J Am Med Inform Assoc. 2001;8(4):372–378.
- , , , . Patient referral by telemedicine: effectiveness and cost anslysis of an intranet system. J Telemed Telecare. 2000;6(6):320–329.
- , . Strengths and weaknesses of electronic referral: comparison of data content and clinical value of electronic and paper referrals in dermatology. Br J Gen Pract. 2007;57(536):223–224.
- , , , et al. Improving referral communication using a referral tool within an electronic medical record. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:63–74.
- , , , . Email triage is an effective, efficient, and safe way of managing new referrals to a neurologist. Qual Safety Health Care. 2010;19(5):e51.
- , , . The experience of implementing choice at point of referral: a comparison of the Netherlands and England. Health Econ Policy Law. 2010;13:1–23.
- , , , , . Introduction of electronic referral from community associated with more timely review by secondary services. Appl Clin Inform. 2011;2(4):546–564.
- , , , . Use of an e‐mail curbside consultation service by family physicians. J Fam Pract. 1998;47:357–360.
- , , , , , . Physician‐to‐physician consultation via electronic mail: the Walter Reed Army Medical Center Ask a Doc system. Mil Med. 2002;167:200–204.
- , , , , , . Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14:459–466.
- , , , et al. Timely follow‐up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch of Intern Med. 2009;169(17):1578–1586.
- , , , , . Important imaging finding e‐mail alert system: experience after 3 years of implementation. Radiology. 2009;252:747–753.
- . Instant insecurity: security issues of instant messaging. Symantec website. Available at: http://www.symantec.com/connect/articles/instant‐insecurity‐security‐issues‐instant‐messaging.
- . Secure messaging in healthcare. Tech solutions for HIPAA‐compliant messaging. J AHIMA. 2008;79(6):50–51.
- , , , , . Association of interruptions with an increased risk and severity of medication administration errors. Arch Intern Med. 2010;170(8):683–690.
- , . The future of health information technology in the patient‐centered medical home. Health Aff (Millwood). 2010;29(4):614–621.
- , , . eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450–2453.
- , , , et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol 7. Care Coordination. Rockville, MD; 2007.
- , , , , . Small, nonteaching, and rural hospitals continue to be slow in adopting electronic health record systems. Health Aff (Millwood). 2012;31(5):1092–1099.
- , , , . Extending a multimedia medical record to a regional service with electronic referral and discharge letters. J Telemed Telecare. 2004;10S:81–83.
- , . Sending specialist reports to GPs using EDI. Stud Health Tech Inform. 1998;52(pt 1):408–411.
- , , , , . Shared care for diabetes: supporting communication between primary and secondary care. Int J Med Inform. 1999;53(2-3):133–142.
- , , , . Email teleconsultations: well formulated clinical referrals reduce the need for clinic consultation. J Telemed Telecare. 2006;12(1):33–38.
- , , , . An effective electronic surgical referral system. Ann R Coll Surg Engl. 2006;88(6):554–556.
- . Choose and book. Clin Med. 2006;6(5):473–476.
- , , , . Validation of the lower gastrointestinal electronic referral protocol. Brit J Surg. 2008;95:506–514.
- , , , , . Not perfect, but better: primary care providers' experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24:614–619.
- . The Swansea electronic referrals project. J Telemed Telecare. 2009;15:156–158.
- , , , , . Using computerized provider order entry and clinical decision support to improve referring physicians' implementation of consultants' medical recommendations. J Am Med Inform Assoc. 2009;16(2):196–202.
- , , , et al. Follow‐up actions on electronic referral communication in a multispecialty outpatient setting. J Gen Intern Med. 2011;26(1):64–69.
- , , , , , . Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123–11028.
- , , . Same organization, same electronic health records (EHRs) system, different use: exploring the linkage between practice member communication patterns and EHR use patterns in an ambulatory care setting. J Am Med Inform Assoc. 2012;19(3):382–391.
- , , , et al. Electronic health record‐based messages to primary care providers: valuable information or just noise? Arch Intern Med. 2012;172(3):283–285.
Hepatocellular carcinoma: Options for diagnosing and managing a deadly disease
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
KEY POINTS
- Surveillance for HCC is indicated in all patients with cirrhosis, regardless of the cause of the cirrhosis.
- Liver biopsy is not needed to make the diagnosis if the findings on four-phase multidetector computed tomography or dynamic contrast-enhanced magnetic resonance imaging are typical of HCC (arterial hyperenhancement with venous-phase or delayed-phase washout).
- Many treatments are available, including surgical resection, liver transplantation, ablative therapy, perfusion-based therapy, chemotherapy, and palliative therapy.
The jugular venous pressure revisited
In this age of technological marvels, it is easy to become so reliant on them as to neglect the value of bedside physical signs. Yet these signs provide information that adds no cost, is immediately available, and can be repeated at will.
Few physical findings are as useful but as undervalued as is the estimation of the jugular venous pressure. Unfortunately, many practitioners at many levels of seniority and experience do not measure it correctly, leading to a vicious circle of unreliable information, lack of confidence, and underuse. Another reason for its underuse is that the jugular venous pressure does not correlate precisely with the right atrial pressure, as we will see below.
In this review, we will attempt to clarify physiologic principles and describe technical details. Much of this is simple but, as always, the devil is in the details.
ANATOMIC CONSIDERATIONS
Think of the systemic veins as a soft-walled and mildly distensible reservoir with fingerlike projections, analogous to a partially fluidfilled surgical glove.1 In a semi-upright position, the venous system is partially filled with blood and is collapsed above the level that this blood reaches up to.
Blood is constantly flowing in and out of this reservoir, flowing in by venous return and flowing out by the pumping action of the right side of the heart. The volume in the venous reservoir and hence the pressure are normally maintained by the variability of right ventricular stroke volume in accordance with the Frank-Starling law. Excess volume and pressure indicate failure of this homeostatic mechanism.
The internal jugular veins, being continuous with the superior vena cava, provide a visible measure of the degree to which the systemic venous reservoir is filled, a manometer that reflects the pressure in the right atrium—at least in theory.2 Thus, the vertical height above the right atrium to which they are distended and above which they are in a collapsed state should reflect the right atrial pressure.
(In fact, the jugular venous pressure may underestimate the right atrial pressure, for reasons still not understood. This will be discussed below.)
In a healthy person, the visible jugular veins are fully collapsed when the person is standing and are often distended to a variable degree when the person is supine. Selecting an appropriate intermediate position permits the top of the column (the meniscus) to become visible in the neck between the clavicle and the mandible.
DISCREPANCY BETWEEN JUGULAR VENOUS AND RIGHT ATRIAL PRESSURE
Several reports have indicated that the jugular venous pressure may underestimate the right atrial pressure. Deol et al3 confirmed this, while establishing an excellent correlation between the level of venous collapse (observed on ultrasonography) and the jugular venous pressure. The difference between the right atrial pressure and the jugular venous pressure tended to be greater at higher venous pressures.3
Most people have a valve near the termination of the internal jugular vein, with variable competence. Inhibition of reflux of blood from the superior vena cava into the internal jugular vein by this valve is the most plausible cause of this disparity.4
The failure of the jugular venous pressure to correlate with the right atrial pressure has been cited by some as a reason to doubt the value of a sign that cardiologists have long relied on. How do we reconcile this apparent paradox? Careful review of the literature that has demonstrated this lack of correlation reveals the following:
- When unequal, the jugular venous pressure always underestimates the right atrial pressure.
- The lack of correlation is less evident at lower venous pressures.
This indicates the following:
- In the presence of congestive heart failure, the right atrial pressure is at least as high and perhaps higher than the jugular venous pressure. Hence, if the jugular venous pressure is high, further treatment, especially diuresis, is needed.
- A jugular venous pressure of zero implies a euvolemic state.
Thus, the jugular venous pressure provides excellent guidance when administering diuresis in congestive heart failure. These deductions obviously require the clinical judgment that the elevated right atrial pressure and jugular venous pressure do indeed reflect elevation of pulmonary capillary wedge pressure rather than other conditions discussed later in this article.
WHICH REFERENCE POINT TO USE?
The two points that can be used as references above which the jugular venous pressure is expressed are the center of the right atrium and the sternal angle. While the former may reflect physiology, the latter is preferred, as it is always visible and has the added advantage of being close to the upper limit of normal, which is about 3 cm above this level.
The difference in height between these two reference points has often been quoted as 5 cm, but this is an underestimate in the body positions used in examination.5 Seth et al6 found a mean of 8 cm at 30° elevation, 9.7 cm at 45°, and 9.8 cm at 60°. The difference also varied between patients, being larger in association with smoking, older age, large body mass index, and large anterior-posterior diameter. These factors should be considered when trying to evaluate the significance of a particular jugular venous pressure.
The junction of the midaxillary line and the fourth left intercostal space (“the phlebostatic point”) has been recommended as a reference point by some, as it is level with the mid-right atrium. However, using the phlebostatic point as a reference position is cumbersome and results in a valid measurement only with the patient in the supine position.7
TECHNIQUE IS VITAL
Close adherence to technical details is vital in reliably and reproducibly measuring the pressure in the internal jugular veins (Figure 1).
The right side is usually observed first, as it is the side on which the examiner usually stands. Using the right side also avoids the rare occurrence of external compression of the left brachiocephalic vein.
Head and shoulders
The sternocleidomastoid muscle lies anterior to each internal jugular vein.8 When tense, it impedes good observation. Shortening, and hence relaxing, this muscle permits the meniscus to be observed. Correct positioning is achieved by:
- Placing a folded pillow behind the patient’s head
- Keeping the shoulders on the mattress
- Turning the head away and elevating the jaw, both slightly; this is often best achieved by gentle pressure of the palm of the observer's hand on the patient's forehead.
Degree of head elevation
Although the proper degree of head elevation is sometimes said to be between 30° and 60°, these numbers are approximate. The correct angle is that which brings the venous meniscus into the window of visibility in the neck between the clavicle and mandible.
Lighting
Shining a flashlight tangentially to the skin is often helpful, casting shadows that improve the visibility of vein motion. Dimming the room lighting may further enhance this effect. Directing a light perpendicular to the skin is not helpful.
Also check the external jugular vein
Checking the external jugular vein can help establish that the jugular venous pressure is normal. If the vein is initially collapsed, light finger pressure at the base of the neck will distend it. If the distention rapidly clears after release of this pressure, the jugular venous pressure is not elevated. However, if external jugular venous distention persists, this does not prove true jugular venous pressure elevation, since it may reflect external compression of the vein by the cervical fascia or delayed blood flow caused by sclerotic venous valves.9 In these instances, the internal jugular pulsation level must be sought.
Jugular venous collapse with inspiration
Collapse of the inferior vena cava with forced inspiration is routinely evaluated during echocardiography as a way to estimate right atrial pressure. This finding has been extrapolated to the jugular veins, wherein the absence of venous collapse during vigorous inspiration or sniffing indicates elevated central venous pressures.10
Distinguishing venous from arterial pulsation
Features indicating venous rather than arterial pulsation were listed by Wood more than 50 years ago11 and are still relevant today. These include internal jugular pulsation that:
- Is soft, diffuse, undulant
- Is not palpable
- Has two crests and two troughs per cardiac cycle
- Has crests that do not coincide with the palpated carotid pulse (exceptions may be seen with the systolic timing of the v wave of tricuspid regurgitation)
- Has higher pressure in expiration, lower in inspiration (exceptions may be seen when Kussmaul physiology is present)
- Has pressure that rises with abdominal pressure
- Is obliterated by light pressure at the base of the neck.
In addition to the above criteria, a wave whose movement is predominantly a descent is nearly always venous.
Abdominojugular reflex
Firm, steady pressure over the abdomen will often result in a small rise in jugular venous pressure. In healthy people, this normalizes in a few seconds, even while manual pressure is maintained. Persistence of jugular venous pressure elevation beyond 10 seconds, followed by an abrupt fall upon withdrawal of manual pressure, is abnormal. This finding has implications similar to those of an elevated baseline jugular venous pressure.
SIGNIFICANCE OF JUGULAR VENOUS PRESSURE ELEVATION
Elevated jugular venous pressure is a manifestation of abnormal right heart dynamics, mostly commonly reflecting elevated pulmonary capillary wedge pressure from left heart failure.12 This usually implies fluid overload, indicating the need for diuresis.
Exceptions to this therapeutic implication include the presence of a primary right heart condition, pericardial disease, certain arrhythmias, and conditions that elevate intrathoracic pressure. These will be discussed below. One important example is the acute jugular venous pressure elevation seen in right ventricular infarction, in which the high venous pressure is compensatory and its reduction can produce hypotension and shock.13
Primary right heart conditions also include right-sided valvular disease, cor pulmonale (including pulmonary embolism and pulmonary hypertension), and the compressive effect of pericardial tamponade or constriction. A normal or near-normal jugular venous pressure significantly decreases the likelihood of significant constriction or of tamponade of a degree necessitating urgent pericardiocentesis.14
SPECIAL CIRCUMSTANCES
Presence of an intravenous line in the neck
An intravenous line in the neck will often prevent observation of the jugular venous pressure. A simple measure can often compensate for this. If the venous line can be temporarily disconnected, the central venous pressure can be measured directly. Using sterile technique, the line can be flushed with saline and aspirated to bring blood into the transparent tubing. Leaving the proximal end open to the air, and alternately raising and lowering it to confirm free flow, the level to which the blood rises can be easily observed. Observing small cardiac and respiratory variations of the meniscus confirms free communication with the central veins. Attaching the line to a transducer is another option, but this may be time-consuming, and establishing an accurate zero point is often difficult.
The previously described discrepancy between jugular venous pressure and central venous pressure has to be considered when drawing conclusions from this measurement.
Intrathoracic pressure elevators
Positive pressure ventilation will elevate intrathoracic pressure (including right atrial pressure) and hence the jugular venous pressure, making interpretation difficult.15 Large pleural effusions or pneumothorax may have a similar effect.16
Superior vena cava syndrome
Markedly elevated jugular venous pressure is here associated with absent or very diminished pulsation, as the caval obstruction has eliminated free communication with the right atrium.17 Associated facial plethora and edema, papilledema, and superficial venous distention over the chest wall will often confirm this diagnosis.
THE WAVEFORM
While the main purpose of viewing the neck veins is to establish the mean pressure, useful information can often be obtained by assessing the waveform. Abnormalities reflect arrhythmias, right heart hemodynamics, or pericardial disease.18 Changes may be subtle and difficult to detect, but some patterns can be quite readily appreciated (Figure 2). A limited selection follows.
Arrhythmias
Cannon a waves. These intermittent sharp positive deflections in the venous pulse represent right atrial contraction against a closed tricuspid valve. They are most commonly associated with premature ventricular complexes, but they occur in other conditions in which atrial and ventricular beating are dissociated, including complete heart block, atrioventricular dissociation, and electronic ventricular pacing.19–21
Repetitive cannon waves. These may be seen with atrioventricular junctional tachycardia or ventricular tachycardia with 1:1 retrograde ventriculoatrial conduction in which the tricuspid valve is closed to every atrial beat.
Fine rapid regular pulsation may be seen in atrial flutter and may be a useful clue in distinguishing this from sinus rhythm when there is 4:1 atrioventricular conduction and a normal ventricular rate.
Abnormal right heart hemodynamics
Large v waves (Lancisi sign). These surges, replacing the usual x descent in systole, are seen in tricuspid insufficiency when the right atrium and its venous attachments are not protected from the right ventricular systolic pressure.22 High right ventricular pressure will obviously enhance this systolic surge.
Large a waves. These reflect resistance to right atrial outflow and may be seen when right ventricular compliance is reduced by hypertrophy from chronic pressure overload or in tricuspid stenosis.23
Pericardial disease
Kussmaul sign is the paradoxical increase in jugular venous pressure with inspiration, observed in conditions associated with limited filling of the right ventricle. It is typically associated with constrictive pericarditis, although it occurs in only a minority of people with this condition.24 It may also be seen in restrictive cardiomyopathy, massive pulmonary embolism, right ventricular infarction, and tricuspid stenosis.25
Diaphragmatic descent during inspiration increases intra-abdominal pressure and decreases intrathoracic pressure. The resulting increased gradient between the abdomen and thorax enhances venous return from splanchnic vessels, which in the setting of a noncompliant right ventricle may result in increased right atrial (and, hence, jugular venous) pressure.26
It is important to point out that the Kussmaul sign does not occur with cardiac tamponade in the absence of associated pericardial constriction.
Exaggerated y descent is typically seen in pericardial constriction, in which the high pressure of the v wave falls rapidly at the onset of diastole, given initial minimal right ventricular resistance. Flow is abruptly stopped when the intrapericardial space is filled.
- Sherwood L. Human Physiology: From Cells to Systems. 8th ed. Belmont, CA: Brooks/Cole; 2012.
- Constant J. Using internal jugular pulsations as a manometer for right atrial pressure measurements. Cardiology 2000; 93:26–30.
- Deol GR, Collett N, Ashby A, Schmidt GA. Ultrasound accurately reflects the jugular venous examination but underestimates central venous pressure. Chest 2011; 139:95–100.
- Wu X, Studer W, Erb T, Skarvan K, Seeberger MD. Competence of the internal jugular vein valve is damaged by cannulation and catheterization of the internal jugular vein. Anesthesiology 2000; 93:319–324.
- Ramana RK, Sanagala T, Lichtenberg R. A new angle on the angle of Louis. Congest Heart Fail 2006; 12:196–199.
- Seth R, Magner P, Matzinger F, van Walraven C. How far is the sternal angle from the mid-right atrium? J Gen Intern Med 2002; 17:852–856.
- Kee LL, Simonson JS, Stotts NA, Skov P, Schiller NB. Echocardiographic determination of valid zero reference levels in supine and lateral positions. Am J Crit Care 1993; 2:72–80.
- Park SY, Kim MJ, Kim MG, et al. Changes in the relationship between the right internal jugular vein and an anatomical landmark after head rotation. Korean J Anesthesiol 2011; 61:107–111.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Conn RD, O’Keefe JH. Simplified evaluation of the jugular venous pressure: significance of inspiratory collapse of jugular veins. Mo Med 2012; 109:150–152.
- Wood PH. Diseases of the Heart and Circulation. 2nd ed. Philadelphia, PA: Lippincott; 1956.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Clark G, Strauss HD, Roberts R. Dobutamine vs furosemide in the treatment of cardiac failure due to right ventricular infarction. Chest 1980; 77:220–223.
- Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA 2007; 297:1810–1818.
- Zhou Q, Xiao W, An E, Zhou H, Yan M. Effects of four different positive airway pressures on right internal jugular vein catheterisation. Eur J Anaesthesiol 2012; 29:223–228.
- Jolobe OM. Disproportionate elevation of jugular venous pressure in pleural effusion. Br J Hosp Med (Lond) 2011; 72:582–585.
- Seo M, Shin WJ, Jun IG. Central venous catheter-related superior vena cava syndrome following renal transplantation—a case report. Korean J Anesthesiol 2012; 63:550–554.
- Applefeld MM. The jugular venous pressure and pulse contour. In:Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- El Gamal MI, Van Gelder LM. Chronic ventricular pacing with ventriculo-atrial conduction versus atrial pacing in three patients with symptomatic sinus bradycardia. Pacing Clin Electrophysiol 1981; 4:100–105.
- Berman ND, Waxman MB. Cannon waves with A-V association. Am Heart J 1976; 91:643–644.
- Luisada AA, Singhal A, Kim K. The jugular and hepatic tracings in normal subjects and in conduction defects. Acta Cardiol 1983; 38:405–424.
- Miller MJ, McKay RG, Ferguson JJ, et al. Right atrial pressure-volume relationships in tricuspid regurgitation. Circulation 1986; 73:799–808.
- Wooley CF, Fontana ME, Kilman JW, Ryan JM. Tricuspid stenosis. Atrial systolic murmur, tricuspid opening snap, and right atrial pressure pulse. Am J Med 1985; 78:375–384.
- McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Mittal SR, Garg S, Lalgarhia M. Jugular venous pressure and pulse wave form in the diagnosis of right ventricular infarction. Int J Cardiol 1996; 53:253–256.
- Bilchick KC, Wise RA. Paradoxical physical findings described by Kussmaul: pulsus paradoxus and Kussmaul’s sign. Lancet 2002; 359:1940–1942.
In this age of technological marvels, it is easy to become so reliant on them as to neglect the value of bedside physical signs. Yet these signs provide information that adds no cost, is immediately available, and can be repeated at will.
Few physical findings are as useful but as undervalued as is the estimation of the jugular venous pressure. Unfortunately, many practitioners at many levels of seniority and experience do not measure it correctly, leading to a vicious circle of unreliable information, lack of confidence, and underuse. Another reason for its underuse is that the jugular venous pressure does not correlate precisely with the right atrial pressure, as we will see below.
In this review, we will attempt to clarify physiologic principles and describe technical details. Much of this is simple but, as always, the devil is in the details.
ANATOMIC CONSIDERATIONS
Think of the systemic veins as a soft-walled and mildly distensible reservoir with fingerlike projections, analogous to a partially fluidfilled surgical glove.1 In a semi-upright position, the venous system is partially filled with blood and is collapsed above the level that this blood reaches up to.
Blood is constantly flowing in and out of this reservoir, flowing in by venous return and flowing out by the pumping action of the right side of the heart. The volume in the venous reservoir and hence the pressure are normally maintained by the variability of right ventricular stroke volume in accordance with the Frank-Starling law. Excess volume and pressure indicate failure of this homeostatic mechanism.
The internal jugular veins, being continuous with the superior vena cava, provide a visible measure of the degree to which the systemic venous reservoir is filled, a manometer that reflects the pressure in the right atrium—at least in theory.2 Thus, the vertical height above the right atrium to which they are distended and above which they are in a collapsed state should reflect the right atrial pressure.
(In fact, the jugular venous pressure may underestimate the right atrial pressure, for reasons still not understood. This will be discussed below.)
In a healthy person, the visible jugular veins are fully collapsed when the person is standing and are often distended to a variable degree when the person is supine. Selecting an appropriate intermediate position permits the top of the column (the meniscus) to become visible in the neck between the clavicle and the mandible.
DISCREPANCY BETWEEN JUGULAR VENOUS AND RIGHT ATRIAL PRESSURE
Several reports have indicated that the jugular venous pressure may underestimate the right atrial pressure. Deol et al3 confirmed this, while establishing an excellent correlation between the level of venous collapse (observed on ultrasonography) and the jugular venous pressure. The difference between the right atrial pressure and the jugular venous pressure tended to be greater at higher venous pressures.3
Most people have a valve near the termination of the internal jugular vein, with variable competence. Inhibition of reflux of blood from the superior vena cava into the internal jugular vein by this valve is the most plausible cause of this disparity.4
The failure of the jugular venous pressure to correlate with the right atrial pressure has been cited by some as a reason to doubt the value of a sign that cardiologists have long relied on. How do we reconcile this apparent paradox? Careful review of the literature that has demonstrated this lack of correlation reveals the following:
- When unequal, the jugular venous pressure always underestimates the right atrial pressure.
- The lack of correlation is less evident at lower venous pressures.
This indicates the following:
- In the presence of congestive heart failure, the right atrial pressure is at least as high and perhaps higher than the jugular venous pressure. Hence, if the jugular venous pressure is high, further treatment, especially diuresis, is needed.
- A jugular venous pressure of zero implies a euvolemic state.
Thus, the jugular venous pressure provides excellent guidance when administering diuresis in congestive heart failure. These deductions obviously require the clinical judgment that the elevated right atrial pressure and jugular venous pressure do indeed reflect elevation of pulmonary capillary wedge pressure rather than other conditions discussed later in this article.
WHICH REFERENCE POINT TO USE?
The two points that can be used as references above which the jugular venous pressure is expressed are the center of the right atrium and the sternal angle. While the former may reflect physiology, the latter is preferred, as it is always visible and has the added advantage of being close to the upper limit of normal, which is about 3 cm above this level.
The difference in height between these two reference points has often been quoted as 5 cm, but this is an underestimate in the body positions used in examination.5 Seth et al6 found a mean of 8 cm at 30° elevation, 9.7 cm at 45°, and 9.8 cm at 60°. The difference also varied between patients, being larger in association with smoking, older age, large body mass index, and large anterior-posterior diameter. These factors should be considered when trying to evaluate the significance of a particular jugular venous pressure.
The junction of the midaxillary line and the fourth left intercostal space (“the phlebostatic point”) has been recommended as a reference point by some, as it is level with the mid-right atrium. However, using the phlebostatic point as a reference position is cumbersome and results in a valid measurement only with the patient in the supine position.7
TECHNIQUE IS VITAL
Close adherence to technical details is vital in reliably and reproducibly measuring the pressure in the internal jugular veins (Figure 1).
The right side is usually observed first, as it is the side on which the examiner usually stands. Using the right side also avoids the rare occurrence of external compression of the left brachiocephalic vein.
Head and shoulders
The sternocleidomastoid muscle lies anterior to each internal jugular vein.8 When tense, it impedes good observation. Shortening, and hence relaxing, this muscle permits the meniscus to be observed. Correct positioning is achieved by:
- Placing a folded pillow behind the patient’s head
- Keeping the shoulders on the mattress
- Turning the head away and elevating the jaw, both slightly; this is often best achieved by gentle pressure of the palm of the observer's hand on the patient's forehead.
Degree of head elevation
Although the proper degree of head elevation is sometimes said to be between 30° and 60°, these numbers are approximate. The correct angle is that which brings the venous meniscus into the window of visibility in the neck between the clavicle and mandible.
Lighting
Shining a flashlight tangentially to the skin is often helpful, casting shadows that improve the visibility of vein motion. Dimming the room lighting may further enhance this effect. Directing a light perpendicular to the skin is not helpful.
Also check the external jugular vein
Checking the external jugular vein can help establish that the jugular venous pressure is normal. If the vein is initially collapsed, light finger pressure at the base of the neck will distend it. If the distention rapidly clears after release of this pressure, the jugular venous pressure is not elevated. However, if external jugular venous distention persists, this does not prove true jugular venous pressure elevation, since it may reflect external compression of the vein by the cervical fascia or delayed blood flow caused by sclerotic venous valves.9 In these instances, the internal jugular pulsation level must be sought.
Jugular venous collapse with inspiration
Collapse of the inferior vena cava with forced inspiration is routinely evaluated during echocardiography as a way to estimate right atrial pressure. This finding has been extrapolated to the jugular veins, wherein the absence of venous collapse during vigorous inspiration or sniffing indicates elevated central venous pressures.10
Distinguishing venous from arterial pulsation
Features indicating venous rather than arterial pulsation were listed by Wood more than 50 years ago11 and are still relevant today. These include internal jugular pulsation that:
- Is soft, diffuse, undulant
- Is not palpable
- Has two crests and two troughs per cardiac cycle
- Has crests that do not coincide with the palpated carotid pulse (exceptions may be seen with the systolic timing of the v wave of tricuspid regurgitation)
- Has higher pressure in expiration, lower in inspiration (exceptions may be seen when Kussmaul physiology is present)
- Has pressure that rises with abdominal pressure
- Is obliterated by light pressure at the base of the neck.
In addition to the above criteria, a wave whose movement is predominantly a descent is nearly always venous.
Abdominojugular reflex
Firm, steady pressure over the abdomen will often result in a small rise in jugular venous pressure. In healthy people, this normalizes in a few seconds, even while manual pressure is maintained. Persistence of jugular venous pressure elevation beyond 10 seconds, followed by an abrupt fall upon withdrawal of manual pressure, is abnormal. This finding has implications similar to those of an elevated baseline jugular venous pressure.
SIGNIFICANCE OF JUGULAR VENOUS PRESSURE ELEVATION
Elevated jugular venous pressure is a manifestation of abnormal right heart dynamics, mostly commonly reflecting elevated pulmonary capillary wedge pressure from left heart failure.12 This usually implies fluid overload, indicating the need for diuresis.
Exceptions to this therapeutic implication include the presence of a primary right heart condition, pericardial disease, certain arrhythmias, and conditions that elevate intrathoracic pressure. These will be discussed below. One important example is the acute jugular venous pressure elevation seen in right ventricular infarction, in which the high venous pressure is compensatory and its reduction can produce hypotension and shock.13
Primary right heart conditions also include right-sided valvular disease, cor pulmonale (including pulmonary embolism and pulmonary hypertension), and the compressive effect of pericardial tamponade or constriction. A normal or near-normal jugular venous pressure significantly decreases the likelihood of significant constriction or of tamponade of a degree necessitating urgent pericardiocentesis.14
SPECIAL CIRCUMSTANCES
Presence of an intravenous line in the neck
An intravenous line in the neck will often prevent observation of the jugular venous pressure. A simple measure can often compensate for this. If the venous line can be temporarily disconnected, the central venous pressure can be measured directly. Using sterile technique, the line can be flushed with saline and aspirated to bring blood into the transparent tubing. Leaving the proximal end open to the air, and alternately raising and lowering it to confirm free flow, the level to which the blood rises can be easily observed. Observing small cardiac and respiratory variations of the meniscus confirms free communication with the central veins. Attaching the line to a transducer is another option, but this may be time-consuming, and establishing an accurate zero point is often difficult.
The previously described discrepancy between jugular venous pressure and central venous pressure has to be considered when drawing conclusions from this measurement.
Intrathoracic pressure elevators
Positive pressure ventilation will elevate intrathoracic pressure (including right atrial pressure) and hence the jugular venous pressure, making interpretation difficult.15 Large pleural effusions or pneumothorax may have a similar effect.16
Superior vena cava syndrome
Markedly elevated jugular venous pressure is here associated with absent or very diminished pulsation, as the caval obstruction has eliminated free communication with the right atrium.17 Associated facial plethora and edema, papilledema, and superficial venous distention over the chest wall will often confirm this diagnosis.
THE WAVEFORM
While the main purpose of viewing the neck veins is to establish the mean pressure, useful information can often be obtained by assessing the waveform. Abnormalities reflect arrhythmias, right heart hemodynamics, or pericardial disease.18 Changes may be subtle and difficult to detect, but some patterns can be quite readily appreciated (Figure 2). A limited selection follows.
Arrhythmias
Cannon a waves. These intermittent sharp positive deflections in the venous pulse represent right atrial contraction against a closed tricuspid valve. They are most commonly associated with premature ventricular complexes, but they occur in other conditions in which atrial and ventricular beating are dissociated, including complete heart block, atrioventricular dissociation, and electronic ventricular pacing.19–21
Repetitive cannon waves. These may be seen with atrioventricular junctional tachycardia or ventricular tachycardia with 1:1 retrograde ventriculoatrial conduction in which the tricuspid valve is closed to every atrial beat.
Fine rapid regular pulsation may be seen in atrial flutter and may be a useful clue in distinguishing this from sinus rhythm when there is 4:1 atrioventricular conduction and a normal ventricular rate.
Abnormal right heart hemodynamics
Large v waves (Lancisi sign). These surges, replacing the usual x descent in systole, are seen in tricuspid insufficiency when the right atrium and its venous attachments are not protected from the right ventricular systolic pressure.22 High right ventricular pressure will obviously enhance this systolic surge.
Large a waves. These reflect resistance to right atrial outflow and may be seen when right ventricular compliance is reduced by hypertrophy from chronic pressure overload or in tricuspid stenosis.23
Pericardial disease
Kussmaul sign is the paradoxical increase in jugular venous pressure with inspiration, observed in conditions associated with limited filling of the right ventricle. It is typically associated with constrictive pericarditis, although it occurs in only a minority of people with this condition.24 It may also be seen in restrictive cardiomyopathy, massive pulmonary embolism, right ventricular infarction, and tricuspid stenosis.25
Diaphragmatic descent during inspiration increases intra-abdominal pressure and decreases intrathoracic pressure. The resulting increased gradient between the abdomen and thorax enhances venous return from splanchnic vessels, which in the setting of a noncompliant right ventricle may result in increased right atrial (and, hence, jugular venous) pressure.26
It is important to point out that the Kussmaul sign does not occur with cardiac tamponade in the absence of associated pericardial constriction.
Exaggerated y descent is typically seen in pericardial constriction, in which the high pressure of the v wave falls rapidly at the onset of diastole, given initial minimal right ventricular resistance. Flow is abruptly stopped when the intrapericardial space is filled.
In this age of technological marvels, it is easy to become so reliant on them as to neglect the value of bedside physical signs. Yet these signs provide information that adds no cost, is immediately available, and can be repeated at will.
Few physical findings are as useful but as undervalued as is the estimation of the jugular venous pressure. Unfortunately, many practitioners at many levels of seniority and experience do not measure it correctly, leading to a vicious circle of unreliable information, lack of confidence, and underuse. Another reason for its underuse is that the jugular venous pressure does not correlate precisely with the right atrial pressure, as we will see below.
In this review, we will attempt to clarify physiologic principles and describe technical details. Much of this is simple but, as always, the devil is in the details.
ANATOMIC CONSIDERATIONS
Think of the systemic veins as a soft-walled and mildly distensible reservoir with fingerlike projections, analogous to a partially fluidfilled surgical glove.1 In a semi-upright position, the venous system is partially filled with blood and is collapsed above the level that this blood reaches up to.
Blood is constantly flowing in and out of this reservoir, flowing in by venous return and flowing out by the pumping action of the right side of the heart. The volume in the venous reservoir and hence the pressure are normally maintained by the variability of right ventricular stroke volume in accordance with the Frank-Starling law. Excess volume and pressure indicate failure of this homeostatic mechanism.
The internal jugular veins, being continuous with the superior vena cava, provide a visible measure of the degree to which the systemic venous reservoir is filled, a manometer that reflects the pressure in the right atrium—at least in theory.2 Thus, the vertical height above the right atrium to which they are distended and above which they are in a collapsed state should reflect the right atrial pressure.
(In fact, the jugular venous pressure may underestimate the right atrial pressure, for reasons still not understood. This will be discussed below.)
In a healthy person, the visible jugular veins are fully collapsed when the person is standing and are often distended to a variable degree when the person is supine. Selecting an appropriate intermediate position permits the top of the column (the meniscus) to become visible in the neck between the clavicle and the mandible.
DISCREPANCY BETWEEN JUGULAR VENOUS AND RIGHT ATRIAL PRESSURE
Several reports have indicated that the jugular venous pressure may underestimate the right atrial pressure. Deol et al3 confirmed this, while establishing an excellent correlation between the level of venous collapse (observed on ultrasonography) and the jugular venous pressure. The difference between the right atrial pressure and the jugular venous pressure tended to be greater at higher venous pressures.3
Most people have a valve near the termination of the internal jugular vein, with variable competence. Inhibition of reflux of blood from the superior vena cava into the internal jugular vein by this valve is the most plausible cause of this disparity.4
The failure of the jugular venous pressure to correlate with the right atrial pressure has been cited by some as a reason to doubt the value of a sign that cardiologists have long relied on. How do we reconcile this apparent paradox? Careful review of the literature that has demonstrated this lack of correlation reveals the following:
- When unequal, the jugular venous pressure always underestimates the right atrial pressure.
- The lack of correlation is less evident at lower venous pressures.
This indicates the following:
- In the presence of congestive heart failure, the right atrial pressure is at least as high and perhaps higher than the jugular venous pressure. Hence, if the jugular venous pressure is high, further treatment, especially diuresis, is needed.
- A jugular venous pressure of zero implies a euvolemic state.
Thus, the jugular venous pressure provides excellent guidance when administering diuresis in congestive heart failure. These deductions obviously require the clinical judgment that the elevated right atrial pressure and jugular venous pressure do indeed reflect elevation of pulmonary capillary wedge pressure rather than other conditions discussed later in this article.
WHICH REFERENCE POINT TO USE?
The two points that can be used as references above which the jugular venous pressure is expressed are the center of the right atrium and the sternal angle. While the former may reflect physiology, the latter is preferred, as it is always visible and has the added advantage of being close to the upper limit of normal, which is about 3 cm above this level.
The difference in height between these two reference points has often been quoted as 5 cm, but this is an underestimate in the body positions used in examination.5 Seth et al6 found a mean of 8 cm at 30° elevation, 9.7 cm at 45°, and 9.8 cm at 60°. The difference also varied between patients, being larger in association with smoking, older age, large body mass index, and large anterior-posterior diameter. These factors should be considered when trying to evaluate the significance of a particular jugular venous pressure.
The junction of the midaxillary line and the fourth left intercostal space (“the phlebostatic point”) has been recommended as a reference point by some, as it is level with the mid-right atrium. However, using the phlebostatic point as a reference position is cumbersome and results in a valid measurement only with the patient in the supine position.7
TECHNIQUE IS VITAL
Close adherence to technical details is vital in reliably and reproducibly measuring the pressure in the internal jugular veins (Figure 1).
The right side is usually observed first, as it is the side on which the examiner usually stands. Using the right side also avoids the rare occurrence of external compression of the left brachiocephalic vein.
Head and shoulders
The sternocleidomastoid muscle lies anterior to each internal jugular vein.8 When tense, it impedes good observation. Shortening, and hence relaxing, this muscle permits the meniscus to be observed. Correct positioning is achieved by:
- Placing a folded pillow behind the patient’s head
- Keeping the shoulders on the mattress
- Turning the head away and elevating the jaw, both slightly; this is often best achieved by gentle pressure of the palm of the observer's hand on the patient's forehead.
Degree of head elevation
Although the proper degree of head elevation is sometimes said to be between 30° and 60°, these numbers are approximate. The correct angle is that which brings the venous meniscus into the window of visibility in the neck between the clavicle and mandible.
Lighting
Shining a flashlight tangentially to the skin is often helpful, casting shadows that improve the visibility of vein motion. Dimming the room lighting may further enhance this effect. Directing a light perpendicular to the skin is not helpful.
Also check the external jugular vein
Checking the external jugular vein can help establish that the jugular venous pressure is normal. If the vein is initially collapsed, light finger pressure at the base of the neck will distend it. If the distention rapidly clears after release of this pressure, the jugular venous pressure is not elevated. However, if external jugular venous distention persists, this does not prove true jugular venous pressure elevation, since it may reflect external compression of the vein by the cervical fascia or delayed blood flow caused by sclerotic venous valves.9 In these instances, the internal jugular pulsation level must be sought.
Jugular venous collapse with inspiration
Collapse of the inferior vena cava with forced inspiration is routinely evaluated during echocardiography as a way to estimate right atrial pressure. This finding has been extrapolated to the jugular veins, wherein the absence of venous collapse during vigorous inspiration or sniffing indicates elevated central venous pressures.10
Distinguishing venous from arterial pulsation
Features indicating venous rather than arterial pulsation were listed by Wood more than 50 years ago11 and are still relevant today. These include internal jugular pulsation that:
- Is soft, diffuse, undulant
- Is not palpable
- Has two crests and two troughs per cardiac cycle
- Has crests that do not coincide with the palpated carotid pulse (exceptions may be seen with the systolic timing of the v wave of tricuspid regurgitation)
- Has higher pressure in expiration, lower in inspiration (exceptions may be seen when Kussmaul physiology is present)
- Has pressure that rises with abdominal pressure
- Is obliterated by light pressure at the base of the neck.
In addition to the above criteria, a wave whose movement is predominantly a descent is nearly always venous.
Abdominojugular reflex
Firm, steady pressure over the abdomen will often result in a small rise in jugular venous pressure. In healthy people, this normalizes in a few seconds, even while manual pressure is maintained. Persistence of jugular venous pressure elevation beyond 10 seconds, followed by an abrupt fall upon withdrawal of manual pressure, is abnormal. This finding has implications similar to those of an elevated baseline jugular venous pressure.
SIGNIFICANCE OF JUGULAR VENOUS PRESSURE ELEVATION
Elevated jugular venous pressure is a manifestation of abnormal right heart dynamics, mostly commonly reflecting elevated pulmonary capillary wedge pressure from left heart failure.12 This usually implies fluid overload, indicating the need for diuresis.
Exceptions to this therapeutic implication include the presence of a primary right heart condition, pericardial disease, certain arrhythmias, and conditions that elevate intrathoracic pressure. These will be discussed below. One important example is the acute jugular venous pressure elevation seen in right ventricular infarction, in which the high venous pressure is compensatory and its reduction can produce hypotension and shock.13
Primary right heart conditions also include right-sided valvular disease, cor pulmonale (including pulmonary embolism and pulmonary hypertension), and the compressive effect of pericardial tamponade or constriction. A normal or near-normal jugular venous pressure significantly decreases the likelihood of significant constriction or of tamponade of a degree necessitating urgent pericardiocentesis.14
SPECIAL CIRCUMSTANCES
Presence of an intravenous line in the neck
An intravenous line in the neck will often prevent observation of the jugular venous pressure. A simple measure can often compensate for this. If the venous line can be temporarily disconnected, the central venous pressure can be measured directly. Using sterile technique, the line can be flushed with saline and aspirated to bring blood into the transparent tubing. Leaving the proximal end open to the air, and alternately raising and lowering it to confirm free flow, the level to which the blood rises can be easily observed. Observing small cardiac and respiratory variations of the meniscus confirms free communication with the central veins. Attaching the line to a transducer is another option, but this may be time-consuming, and establishing an accurate zero point is often difficult.
The previously described discrepancy between jugular venous pressure and central venous pressure has to be considered when drawing conclusions from this measurement.
Intrathoracic pressure elevators
Positive pressure ventilation will elevate intrathoracic pressure (including right atrial pressure) and hence the jugular venous pressure, making interpretation difficult.15 Large pleural effusions or pneumothorax may have a similar effect.16
Superior vena cava syndrome
Markedly elevated jugular venous pressure is here associated with absent or very diminished pulsation, as the caval obstruction has eliminated free communication with the right atrium.17 Associated facial plethora and edema, papilledema, and superficial venous distention over the chest wall will often confirm this diagnosis.
THE WAVEFORM
While the main purpose of viewing the neck veins is to establish the mean pressure, useful information can often be obtained by assessing the waveform. Abnormalities reflect arrhythmias, right heart hemodynamics, or pericardial disease.18 Changes may be subtle and difficult to detect, but some patterns can be quite readily appreciated (Figure 2). A limited selection follows.
Arrhythmias
Cannon a waves. These intermittent sharp positive deflections in the venous pulse represent right atrial contraction against a closed tricuspid valve. They are most commonly associated with premature ventricular complexes, but they occur in other conditions in which atrial and ventricular beating are dissociated, including complete heart block, atrioventricular dissociation, and electronic ventricular pacing.19–21
Repetitive cannon waves. These may be seen with atrioventricular junctional tachycardia or ventricular tachycardia with 1:1 retrograde ventriculoatrial conduction in which the tricuspid valve is closed to every atrial beat.
Fine rapid regular pulsation may be seen in atrial flutter and may be a useful clue in distinguishing this from sinus rhythm when there is 4:1 atrioventricular conduction and a normal ventricular rate.
Abnormal right heart hemodynamics
Large v waves (Lancisi sign). These surges, replacing the usual x descent in systole, are seen in tricuspid insufficiency when the right atrium and its venous attachments are not protected from the right ventricular systolic pressure.22 High right ventricular pressure will obviously enhance this systolic surge.
Large a waves. These reflect resistance to right atrial outflow and may be seen when right ventricular compliance is reduced by hypertrophy from chronic pressure overload or in tricuspid stenosis.23
Pericardial disease
Kussmaul sign is the paradoxical increase in jugular venous pressure with inspiration, observed in conditions associated with limited filling of the right ventricle. It is typically associated with constrictive pericarditis, although it occurs in only a minority of people with this condition.24 It may also be seen in restrictive cardiomyopathy, massive pulmonary embolism, right ventricular infarction, and tricuspid stenosis.25
Diaphragmatic descent during inspiration increases intra-abdominal pressure and decreases intrathoracic pressure. The resulting increased gradient between the abdomen and thorax enhances venous return from splanchnic vessels, which in the setting of a noncompliant right ventricle may result in increased right atrial (and, hence, jugular venous) pressure.26
It is important to point out that the Kussmaul sign does not occur with cardiac tamponade in the absence of associated pericardial constriction.
Exaggerated y descent is typically seen in pericardial constriction, in which the high pressure of the v wave falls rapidly at the onset of diastole, given initial minimal right ventricular resistance. Flow is abruptly stopped when the intrapericardial space is filled.
- Sherwood L. Human Physiology: From Cells to Systems. 8th ed. Belmont, CA: Brooks/Cole; 2012.
- Constant J. Using internal jugular pulsations as a manometer for right atrial pressure measurements. Cardiology 2000; 93:26–30.
- Deol GR, Collett N, Ashby A, Schmidt GA. Ultrasound accurately reflects the jugular venous examination but underestimates central venous pressure. Chest 2011; 139:95–100.
- Wu X, Studer W, Erb T, Skarvan K, Seeberger MD. Competence of the internal jugular vein valve is damaged by cannulation and catheterization of the internal jugular vein. Anesthesiology 2000; 93:319–324.
- Ramana RK, Sanagala T, Lichtenberg R. A new angle on the angle of Louis. Congest Heart Fail 2006; 12:196–199.
- Seth R, Magner P, Matzinger F, van Walraven C. How far is the sternal angle from the mid-right atrium? J Gen Intern Med 2002; 17:852–856.
- Kee LL, Simonson JS, Stotts NA, Skov P, Schiller NB. Echocardiographic determination of valid zero reference levels in supine and lateral positions. Am J Crit Care 1993; 2:72–80.
- Park SY, Kim MJ, Kim MG, et al. Changes in the relationship between the right internal jugular vein and an anatomical landmark after head rotation. Korean J Anesthesiol 2011; 61:107–111.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Conn RD, O’Keefe JH. Simplified evaluation of the jugular venous pressure: significance of inspiratory collapse of jugular veins. Mo Med 2012; 109:150–152.
- Wood PH. Diseases of the Heart and Circulation. 2nd ed. Philadelphia, PA: Lippincott; 1956.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Clark G, Strauss HD, Roberts R. Dobutamine vs furosemide in the treatment of cardiac failure due to right ventricular infarction. Chest 1980; 77:220–223.
- Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA 2007; 297:1810–1818.
- Zhou Q, Xiao W, An E, Zhou H, Yan M. Effects of four different positive airway pressures on right internal jugular vein catheterisation. Eur J Anaesthesiol 2012; 29:223–228.
- Jolobe OM. Disproportionate elevation of jugular venous pressure in pleural effusion. Br J Hosp Med (Lond) 2011; 72:582–585.
- Seo M, Shin WJ, Jun IG. Central venous catheter-related superior vena cava syndrome following renal transplantation—a case report. Korean J Anesthesiol 2012; 63:550–554.
- Applefeld MM. The jugular venous pressure and pulse contour. In:Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- El Gamal MI, Van Gelder LM. Chronic ventricular pacing with ventriculo-atrial conduction versus atrial pacing in three patients with symptomatic sinus bradycardia. Pacing Clin Electrophysiol 1981; 4:100–105.
- Berman ND, Waxman MB. Cannon waves with A-V association. Am Heart J 1976; 91:643–644.
- Luisada AA, Singhal A, Kim K. The jugular and hepatic tracings in normal subjects and in conduction defects. Acta Cardiol 1983; 38:405–424.
- Miller MJ, McKay RG, Ferguson JJ, et al. Right atrial pressure-volume relationships in tricuspid regurgitation. Circulation 1986; 73:799–808.
- Wooley CF, Fontana ME, Kilman JW, Ryan JM. Tricuspid stenosis. Atrial systolic murmur, tricuspid opening snap, and right atrial pressure pulse. Am J Med 1985; 78:375–384.
- McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Mittal SR, Garg S, Lalgarhia M. Jugular venous pressure and pulse wave form in the diagnosis of right ventricular infarction. Int J Cardiol 1996; 53:253–256.
- Bilchick KC, Wise RA. Paradoxical physical findings described by Kussmaul: pulsus paradoxus and Kussmaul’s sign. Lancet 2002; 359:1940–1942.
- Sherwood L. Human Physiology: From Cells to Systems. 8th ed. Belmont, CA: Brooks/Cole; 2012.
- Constant J. Using internal jugular pulsations as a manometer for right atrial pressure measurements. Cardiology 2000; 93:26–30.
- Deol GR, Collett N, Ashby A, Schmidt GA. Ultrasound accurately reflects the jugular venous examination but underestimates central venous pressure. Chest 2011; 139:95–100.
- Wu X, Studer W, Erb T, Skarvan K, Seeberger MD. Competence of the internal jugular vein valve is damaged by cannulation and catheterization of the internal jugular vein. Anesthesiology 2000; 93:319–324.
- Ramana RK, Sanagala T, Lichtenberg R. A new angle on the angle of Louis. Congest Heart Fail 2006; 12:196–199.
- Seth R, Magner P, Matzinger F, van Walraven C. How far is the sternal angle from the mid-right atrium? J Gen Intern Med 2002; 17:852–856.
- Kee LL, Simonson JS, Stotts NA, Skov P, Schiller NB. Echocardiographic determination of valid zero reference levels in supine and lateral positions. Am J Crit Care 1993; 2:72–80.
- Park SY, Kim MJ, Kim MG, et al. Changes in the relationship between the right internal jugular vein and an anatomical landmark after head rotation. Korean J Anesthesiol 2011; 61:107–111.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Conn RD, O’Keefe JH. Simplified evaluation of the jugular venous pressure: significance of inspiratory collapse of jugular veins. Mo Med 2012; 109:150–152.
- Wood PH. Diseases of the Heart and Circulation. 2nd ed. Philadelphia, PA: Lippincott; 1956.
- Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant 2012; 31:67–72.
- Clark G, Strauss HD, Roberts R. Dobutamine vs furosemide in the treatment of cardiac failure due to right ventricular infarction. Chest 1980; 77:220–223.
- Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA 2007; 297:1810–1818.
- Zhou Q, Xiao W, An E, Zhou H, Yan M. Effects of four different positive airway pressures on right internal jugular vein catheterisation. Eur J Anaesthesiol 2012; 29:223–228.
- Jolobe OM. Disproportionate elevation of jugular venous pressure in pleural effusion. Br J Hosp Med (Lond) 2011; 72:582–585.
- Seo M, Shin WJ, Jun IG. Central venous catheter-related superior vena cava syndrome following renal transplantation—a case report. Korean J Anesthesiol 2012; 63:550–554.
- Applefeld MM. The jugular venous pressure and pulse contour. In:Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- El Gamal MI, Van Gelder LM. Chronic ventricular pacing with ventriculo-atrial conduction versus atrial pacing in three patients with symptomatic sinus bradycardia. Pacing Clin Electrophysiol 1981; 4:100–105.
- Berman ND, Waxman MB. Cannon waves with A-V association. Am Heart J 1976; 91:643–644.
- Luisada AA, Singhal A, Kim K. The jugular and hepatic tracings in normal subjects and in conduction defects. Acta Cardiol 1983; 38:405–424.
- Miller MJ, McKay RG, Ferguson JJ, et al. Right atrial pressure-volume relationships in tricuspid regurgitation. Circulation 1986; 73:799–808.
- Wooley CF, Fontana ME, Kilman JW, Ryan JM. Tricuspid stenosis. Atrial systolic murmur, tricuspid opening snap, and right atrial pressure pulse. Am J Med 1985; 78:375–384.
- McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Mittal SR, Garg S, Lalgarhia M. Jugular venous pressure and pulse wave form in the diagnosis of right ventricular infarction. Int J Cardiol 1996; 53:253–256.
- Bilchick KC, Wise RA. Paradoxical physical findings described by Kussmaul: pulsus paradoxus and Kussmaul’s sign. Lancet 2002; 359:1940–1942.
KEY POINTS
- If the jugular venous pressure differs from the true right atrial pressure, the jugular venous pressure is always the lower value.
- The jugular venous pressure is useful to observe when diagnosing congestive heart failure and when considering the need for or the adequacy of diuresis.
- The jugular venous wave form is more difficult to observe than its elevation but can yield useful information in the assessment of certain arrhythmias, right-heart conditions, and pericardial disease.
Antisynthetase syndrome: Not just an inflammatory myopathy
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
KEY POINTS
- Antisynthetase syndrome can present with a wide variety of clinical manifestations, including myositis and interstitial lung disease.
- The type and severity of interstitial lung disease usually determine the long-term outcome.
- In the appropriate clinical setting, the diagnosis is usually confirmed by the detection of antibodies to various aminoacyl-transfer RNA synthetases, anti-Jo-1 antibody being the most common.
- Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine or methotrexate are often required as steroid-sparing agents and also to achieve disease control.
- In the case of severe pulmonary involvement, cyclophosphamide is recommended.
ACOG’s push for medical liability reform: What’s the latest?
It’s a conundrum. There seems to be no doubt about the need for medical liability reform—in fact, there is wide-spread support for it. And yet....
Four years after Captain Chesley “Sully” Sullenberger saved a planeload of passengers during an emergency landing—the “miracle on the Hudson”—he’s become a national champion of medical liability reform. In a recent interview with Politico, Sullenberger equated the 200,000 lives estimated to be lost each year due to medical errors to “20 jetliners crashing per week,” a situation he insists would close airports and ground flights until the problem was solved. But these 200,000 deaths cause little more than a ripple of concern, he claims.1
Among the solutions he proposes is “a whole different approach to reviewing medical errors, figuring out what’s behind them, not just blaming doctors and nurses.”1
Captain Sullenberger is discovering the difficult reality we’ve experienced for too many years: Solutions just don’t come very fast to medical liability reform, despite wide-spread support for it.
At the American Congress of Obstetricians and Gynecologists (ACOG), our campaign for medical liability reform has focused, as always, on patients, using the campaign line: “Who will delivery my baby?” ACOG supports caps on noneconomic damages and other reforms, such as those contained in the California Medical Injury Compensation Reform Act (MICRA), the gold standard for medical liability reform. We will continue to push for national MICRA reform until we’ve won that important protection for all ObGyns and their patients.
Until we reach that goal, we’re working to accomplish meaningful steps to liability reform where we can, including testing state alternatives. And our colleague organizations? Many of them, once insisting on federal adoption of MICRA or nothing at all, now actively support meaningful alternatives, too.
What do we want?
Proposals for tort reform, based on California’s MICRA statute, include:
- mandatory periodic payments of all future damages exceeding $100,000
- a $250,000 ceiling on noneconomic damage awards
- a requirement that claims must be filed within 2 years of the date by which the alleged injury reasonably should have been discovered but in no event more than 4 years from the time of the alleged injury. In the case of alleged injury to children under 4 years of age, claims must be filed by the child’s 8th birthday.
- limits on punitive damages, with 50% of punitive damage awards going to a state disciplinary fund
- limits on attorney contingency fees
- reductions in awards based on the amount paid from another source, such as health or disability insurance
- a requirement for “clear and convincing evidence” rather than the usual “preponderance of evidence” when a health-care professional who provided delivery services but not prenatal care is sued
- alternative systems for dispute resolution.
10 alternative reforms
Good ideas include:
1. Require a certificate of merit from the plaintiff
This proposal would require the plaintiff to file an affidavit with the court to demonstrate that the case has merit before the complaint can move forward. Certificates would necessitate the written opinion of a legally qualified health-care provider affirming that the defendant failed to meet the care standards that would be followed by a reasonably prudent health-care provider—and that this failure caused or directly contributed to the damages claimed.
2. Facilitate early settlement offers
Under this idea, a physician or hospital would be allowed to offer economic damages to an injured party without involving the courts. This offer would not constitute an admission of liability and would be inadmissible if a lawsuit were later filed in the case. Physicians would have an incentive to make a good-faith offer as early as possible after the injury is discovered, and patients would have an incentive to accept legitimate offers of compensation. Early-offer programs would require the injured party to meet a higher burden of proof for alleged negligence if that party chooses to reject the offer and file a lawsuit.
3. Create health-care courts
Health-care courts would allow for a bench or jury trial presided over by a specially trained judge to exclusively hear medical liability cases. Such courts have the potential to correct severe deficiencies in the current medical justice system and to reduce health-system errors and improve patient safety.
4. Allow a physician to say, “I’m sorry”
This proposal would encourage physicians to directly discuss errors and injuries with patients, to apologize and outline corrective action. Such discussions would be inadmissible if a patient later files a lawsuit.
5. Establish medical review panels
Any claim against a physician would be reviewed by a panel of experts who would provide an opinion on whether the physician failed to act within the relevant standards of care.
6. Require a claim to be screened and mediated
A plaintiff ’s claim would have to be evaluated by a screening panel before it could proceed to litigation. The panel would identify claims hat merit compensation and encourage early resolution of those claims. It also would encourage withdrawal or dismissal of non-meritorious claims.
7. Protect physicians who follow evidence-based guidelines
Health-care providers who follow guidelines based on solid evidence, and those who have legitimate justifications for departing from guidelines, would be protected from liability claims.
8. Allow the voluntary resolution of disputes
This proposal would motivate states to encourage the creation of other innovative systems to compensate individuals who are injured in the course of receiving health-care services.
9. Require expert witnesses to meet certain standards
This alternative would limit expert-witness standing to individuals who:
- are licensed and trained in the same specialty as the defendant
- have particular expertise in the disease process or procedure performed in the case
- have been in active medical practice in the same specialty as the defendant within 5 years of the claim or who have been taught at an accredited medical school on the care and type of treatment at issue.
10. Create catastrophic injury systems
These systems would establish a fund for individuals who have experienced bad outcomes. Birth injury funds are an example of this model.
Who’s on our side?
Congressional policy wonks give liability reform a thumbs up
In early 2010, the Medicare Payment Advisory Commission (MedPAC), a nonpartisan advisory counsel to the US Congress, identified three important ways that our current malpractice system harms the Medicare program and Medicare beneficiaries, the aged, and disabled:
- Medicare payments to providers include some liability costs (folded into hospital diagnosis-related group [DRG] payments; factored into physician fee schedule calculation)
- Defensive medicine drives up costs for Medicare
- Malpractice impairs the quality and safety of care to beneficiaries. That is, the current system does not improve patient safety.
MedPAC staff recommended that the commissioners urge Congress to pass government-subsidized malpractice reinsurance for providers who meet certain safety criteria or create a federal administrative adjudication process. The commissioners expressed an interest in alternatives to address the costs of medical malpractice, including ways to encourage states and providers to address medical malpractice in a manner most appropriate for them. However, when MedPAC returned to this topic at its next meeting later the same year, the commissioners mentioned medical liability only to dismiss it as an incidental issue in opening remarks.
The Congressional Budget Office (CBO) estimates that medical malpractice costs our health-care system $35 billion in direct costs, with billions more as a result of defensive medicine.
CBO has scored these medical liability reform proposals as providing significant savings to our federal budget:
- a $250,000 cap on subjective, noneconomic damages (with no limit on economic damages)
- collateral source rule allowing evidence of outside payments to be submitted in court
- a ban on subrogation by certain collateral sources
- caps on attorney contingency fees
- periodic payments of future damages
- a reasonable statute of limitations.
In addition, in 2011, CBO scored comprehensive medical liability reform as saving the federal government $62.4 billion over 10 years. As longtime Illinois Senator Everett Dirksen was known to say, “A billion here, a billion there, and pretty soon you’re talking real money.”
Many Republican congressional leaders “walk the walk”
Republicans have long claimed medical liability reform as their issue. And they walk the walk.
Representative Phil Gingrey, MD, of Georgia, an ACOG Fellow, has led the medical liability reform fight on Capitol Hill for a number of years. His bill, the Protecting Access to Healthcare Act (HR 5), which would have brought MICRA to the national level, was repeatedly passed by the Republican majority of the House of Representatives, only to be ignored by the Democrats controlling the Senate.
Again this year, Dr. Gingrey introduced legislation to protect physicians from unexpected liability. His Standard of Care Protection Act (HR 1473) would ensure that provisions of the Affordable Care Act (ACA) cannot be used to create new causes of action against medical professionals. HR 1473 would ensure that Medicare, Medicaid, and other federal programs that establish government standards and guidelines for health-care providers cannot be used to create new causes of action.
Federal health-care programs are changing to ensure that payment reflects quality of care. As a result, new payment rules, guidelines, and standards are being written into federal laws and regulations. HR 1473 would make clear that these cannot be used to define the applicable standard of care or duty of care in a medical liability lawsuit.
ACOG supports Dr. Gingrey’s bill, as well as a second, companion approach that would ensure that ObGyns who follow guidelines and standards of care developed by their medical society are protected from liability, with sensible exceptions for egregious harm and negligence.
Representative Charlie Dent, Republican of Pennsylvania, also has introduced ACOG-supported medical liability legislation. The Health Care Safety Net Enhancement Act (HR 36) would provide federal liability protection for physicians providing care under the Emergency Medical Treatment and Active Labor Act (EMTALA). HR 36 is commonly referred to as Good Samaritan legislation, intended to protect doctors who rush to the aid of a sick individual. The likelihood of any of these bills getting enacted into law is slim. Even some conservative Republicans oppose federal liability reform as an intrusion into states’ rights.
Some Democrats have said good things
In his proposed budget for fiscal year 2012, President Barack Obama asked Congress for funding to address medical liability issues.
He proposed “to restrain health-care costs” through “a more aggressive effort to reform our medical malpractice system to reduce defensive medicine, promote patient safety, and improve patient outcomes.” He encouraged Republicans to work constructively with him on medical malpractice as part of an overall effort to restrain health-care costs.2
The President asked Congress for “$250 million in grants to states to reform the way they resolve medical malpractice disputes,” including health courts, safe harbors, early disclosure and offer, and other legal reforms such as joint and several liability and collateral source rules.2
Congress never funded the President’s request.
President Obama repeated his request in his fiscal year 2013 budget proposal. Congress didn’t fund it then, either.
Earlier, in March 2009, in remarks to the Business Roundtable, President Obama noted that “the cost issue is the thing that we actually think is the big driver in this whole debate...things like comparative effectiveness, health IT, prevention, figuring out how our reimbursement structures are designed under Medicare and Medicaid. Medical liability issues—I think all those things have to be on the table.”3
In an interview the same month, Senator Ron Wyden, Democrat of Oregon, said, “I think [medical liability reform is] an essential piece for there to be enduring reform, reform that will stick and will get a significant bipar-tisan vote in the United States Senate.”4
Senator Wyden’s Healthy Americans Act (S 391) included incentives to get states to enact malpractice reforms as a key to overhauling the health-care system.
Also in March 2009, Representative Rob Andrews, Democrat of New Jersey, Chairman of the House Education and Labor, Health Subcommittee, pointed to the need for medical liability reform.
“It’s hard for me to imagine a [health-care reform] result that gets to the president’s desk that doesn’t deal with the medical mal-practice issue in some way.”4
And Senator Max Baucus, Democrat of Montana, Chairman of the Senate Finance Committee, proposed providing states grant money to develop alternative litigation models, such as encouraging disclosure and compensation in the case of error, and establishing health courts whose judges have health-care expertise.
As early as May 2006, President Obama (then a Senator from Illinois) and Senator Hillary Rodham Clinton, Democrat of New York, urged a focus on patient safety.
“Instead of focusing on the few areas of intense disagreement,” they wrote in the New England Journal of Medicine, “such as the possibility of mandating caps on the financial damages awarded to patients, we believe that the discussion should center on a more fundamental issue: the need to improve patient safety....”
“To improve both patient safety and the medical liability climate, the tort system must achieve four goals: reduce the rates of preventable patient injuries, promote open communication between physicians and patients, ensure patients access to fair compensation for legitimate medical injuries, and reduce liability insurance premiums for health-care providers. Addressing just one of these issues is not sufficient.”5
And then there are the trial lawyers
Readers of OBG Management know all too well that the role of trial lawyers in medical liability reform has been to block meaningful reforms from passing and to repeal reforms currently in place. The Association of Trial Lawyers of America, now known as the American Association for Justice, tries to portray itself as defending vulnerable patients against a few bad apples. Its Web site (www.justice.org) points to recent National Practitioner Data Bank (NPDB) figures indicating that “just 6% of doctors are responsible for 58% of all negligence incidents. The civil justice system seeks to weed out those few doctors whose actions have such devastating impact on patients.”
The Web site includes these bullet points:
- 6% of doctors have been responsible for 58% of all malpractice payments since 1991
- 2% of doctors having three or more mal-practice payments were responsible for 33% of all payments
- 1% of doctors having four or more malprac-tice payments were responsible for 20% of all payments
- 82% of doctors have never had a medical malpractice payment.
Tell that to ObGyns, who, in 2012, paid an average of 12.4% of their gross income for liability insurance premiums in 2012, and nearly 60% of whom changed their practices based on the risk or fear of professional liability claims or litigation. And this despite the fact that 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn.
Action at the state level
We need a federal solution, but since that isn’t within reach, we’re looking to the states for action. And there’s a lot of action in some states, including Connecticut, Florida, Georgia, Hawaii, Illinois, Iowa, Missouri, Oregon, Rhode Island, Tennessee, and Utah.
Advocates in these states are trying a number of different approaches, hoping that some type of meaningful reform will be signed into law. Here’s a sampling of what’s under way.
Connecticut
HB 6687, amend certificates of merit in medical liability actions. Status: April 1, 2013: Joint Committee on Judiciary hearing. The bill would eliminate the need for a detailed basis for the formation of an opinion and replace it with a lower threshold stating the appearance of one or more specific breaches of the prevailing professional standard of care.
In addition, HB 6687 would allow any expert who may testify in court to satisfy the certificate of merit requirement, but at trial the “expert,” in order to testify, needs to have the court determine him or her to be qualified to testify based on discovery and evidentiary issues that are decided at trial. This expert then could sign a certificate of merit but have the court determine that he or she is indeed not an expert for that case. HB 6687 delays the challenging of qualifications of an expert only after the completion of discovery, adding substantial time and cost to defending meritless suits. Finally, the bill allows for a second bite of the apple for cases that did not meet this watered down standard for certificate of merit and would eliminate the automatic dismissal of cases filed with inadequate certificates that did not meet the rules of the court.
SB 1154, amend Connecticut’s failure of suit statute to allow a plaintiff whose lawsuit was dismissed due to a failure to file a certificate of good faith as required by statute, to commence a new action.
HB 5229, limit noneconomic damages in medical liability cases to $250,000 for each health-care provider and institution per event, and $750,000 overall for each event.
HB 5270, establish peer-review panels in medical liability actions. The panels would consist of physicians, medical professionals, and individuals outside the medical profession who would review claims of alleged negligence and determine whether there is probable cause that the medical liability claims have been made in good faith prior to the action being referred to mandatory mediation.
SB 97, extend the statute of limitations in medical liability cases, allowing for an action to be brought no more than 10 years from the date of the act or omission that serves as the basis for the claim.
Florida
The Birth-Related Neurological Injury Compensation Association (NICA). NICA is a statutory organization that manages the compensation plan used to pay for the care of infants born with certain neurological injuries. This plan is available to eligible families statewide without litigation. By eliminating costly legal proceedings, and through professional management of its disbursements, NICA ensures that birth-injured infants receive the care they need while reducing the financial burden on medical providers and families. Defensive work continues on the NICA Board and trial bar.
HB 7015, expert witness. Status: March 28, 2013, the House Justice Appropriations Subcommittee reported favorably. This bill would adopt the Daubert standard for expert witness testimony. It provides that a witness qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion as to the facts at issue in a case.
Georgia
HB 499, Provider Shield Act. Georgia is the first state to introduce legislation based on the American Medical Association’s model bill, “The Provider Shield Act,” which clarifies language in the Affordable Care Act by providing that a physician’s failure to comply with, or a breach of, any federal statute, regulation, program, guideline, or other provision shall not: (1) be admissible; (2) be used to determine the standard of care; or (3) be the legal basis for a presumption of negligence.
Status: Enacted May 6, 2013. The law prohibits the use of payer guidelines and quality criteria outlined in federal law as a legal basis for negligence or standard of care in determining medical liability. Physicians are concerned that without such protections, the medical profession could be exposed to charges of negligence that aren’t based on clinical standards or the patient’s unique medical needs. Implementation of any guideline by any public or private payor, or the establishment of any payment standard or reimbursement criteria under any federal laws or regulations related to health care, shall not be construed, without competent expert testimony establishing the appropriate standard of care, to establish a legal basis for negligence or the standard of care or duty of care owed by a health-care provider to a patient in any civil action for medical malpractice or product liability.
This first-of-its-kind legislation reinforces the concept that medical decisions should be based on a patient’s unique medical needs. HB 499 makes it clear that federal standards or guidelines designed to enhance access to high-quality health care cannot be used to invent new legal actions against physicians.
Hawaii
SB 1308, health-care provider benevolent gesture legislation.
Illinois
On March 22, 2013, several pieces of tort-reform legislation were re-referred to the House Committee on Rules, effectively killing the bills for the session. The House Speaker would have to choose to “release” any of the bills in order for them to move again; this is highly unlikely.
HB 138 would have deleted existing-venue language providing that an action may commence in any county if all defendants are nonresidents of the state, and replaced it with language providing that, if no defendants that are joined in good faith and with probable cause for the purpose of obtaining a judgment against them are residents of the state, an action may be commenced only in the county in which the transaction or some part thereof occurred out of which the cause of action arose.
HB 2220 and HB 2222 provided that, with respect to certain types of actions, for any defendant whose fault is less than 25% of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendant who could have been sued by the plaintiff (instead of any third-party defendant except the plaintiff’s employer), shall be severally liable for all other damages. In addition, these bills provided that, for any defendant whose fault is 25% or greater of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendants who could have been sued by the plaintiff (instead of any third-party defendants except the plaintiff’s employer), shall be jointly and severally liable for all other damages.
HB 2221 created requirements regarding qualifications, testimony, disclosure and compensation of expert testimony and standards for reviewing courts to follow in ruling on the admissibility of expert testimony.
Iowa
SSB 1054 and HSB 36, expert’s certificate of merit affidavit and noneconomic cap. These bills provide that in any medical liability action, the plaintiff is required, within 180 days of the defendant’s answer, to serve the defendant with an expert’s certificate of merit affidavit for each expert scheduled to testify. They also would limit noneconomic damage awards in medical liability cases to $1 million.
Missouri
HJR 6 proposes a constitutional amendment allowing the legislature to cap noneconomic damages in medical liability cases.
SJR 1 grants the legislature the power to limit, by statute, jury awards for noneconomic damages.
SB 64 changes the evidentiary standard in medical liability cases to “clear and convincing” for noneconomic damages.
Oregon
SB 483, early discussion and resolution. Status: Passed by the legislature. This bill establishes an early discussion and resolution (EDC) process within the Oregon Patient Safety Commission. This voluntary process is intended to facilitate open communication about all outcomes of care, including serious events, between the provider, health-care facility, and the patient. When an adverse health-care incident occurs, the patient, health-care provider or health-care facility where the incident occurred may file a notice of adverse health-care incident with the Commission. This notice triggers discussion of the health-care incident and, if appropriate, an offer of compensation. If discussion does not result in the resolution of the claim, the bill gives the parties the option of participating in Commission-facilitated mediation. The entire process is voluntary.
SJR 30, proposed amendment to constitution, $1 million limit on noneconomic awards in medical liability cases. Slated for next general election.
Rhode Island
HB 5380, apology bill. Status: Heard in House Judiciary Committee on March 27, 2013; no action was taken. This bill provides that statements by a health-care provider to a patient or to the patient’s family regarding the outcome of such patient’s medical care and treatment, such as an apology or expression of sympathy, shall be inadmissible as evidence or an admission of liability in any claim or action against the provider.
Tennessee
Joint and several liability. Status: On March 26, 2013, the House Civil Justice Subcommittee reported favorably. This bill would codify current state law by providing that if multiple defendants are found liable in a civil action governed by comparative fault, a defendant shall only be severally liable for the percentage of damages for which fault is attributed to such defendant by the trier of fact, and no defendant shall be held jointly liable for any damages.
SB 274, medical liability expert witness reform.
Utah
HB 135, rules, arbitration. Status: March 21, 2013, sent to Governor Gary Herbert for his approval. HB 135 provides that a party in a medical liability action or arbitration may not attempt to allocate fault to any health-care provider unless a certificate of compliance has been issued. HB 135 also requires that evidence from a medical review panel remain unreportable to a health-care facility or health insurance plan.
Summing up
Medical liability reform—the obvious need for it, the good reasons to do it, and the fact that it remains beyond reach—is a constant source of frustration among many ObGyns. Maybe Captain Sully can save the day.
How medical liability affects the ObGyn specialty
ACOG’s 2012 Survey on Professional Liability, our 11th survey since 1983, assessed the effects of professional liability litigation and insurance issues on the practice of obstetrics and gynecology.6 The survey, conducted under the direction of ACOG’s Vice President for Fellowship and Deputy Executive Vice President Albert Strunk, MD, JD, included segments on demographics, patient care, liability claims experience, and practice changes associated with the cost of liability insurance and the fear of litigation. The survey went to 32,238 Fellows and Junior Fellows. Of these, 9,006 completed the questionnaire. Here are major findings.
Provider profiles
A total of 72.5% of respondents provided both obstetric and gynecologic care, slightly lower than the percentage identified in the 2009 survey, which was 74.3%. Fewer than 7% of respondents provided obstetric care only; 19.8% provided gynecologic care only. Of those restricting their services to gynecology, 88.9% had previously offered obstetric care. The average age at which these physicians stopped practicing obstetrics was 49 years.
Cost of liability insurance
ObGyns spent an average of 12.4% of their gross income on liability insurance premiums in 2012, down from 18% in 2009.
How liability issues affected practice
Since the previous survey in 2009, 57.9% of respondents made one or more changes to their practice to mitigate the risk or fear of professional liability claims or litigation.
Obstetric practice. Among respondents who made changes to their obstetric practice, 27.4% decreased the number of high-risk patients they see, 23.8% increased the number of cesarean deliveries they perform, 18.9% stopped offering and performing vaginal birth after cesarean (VBAC), 11.5% reduced the total number of deliveries, and 6.2% stopped practicing obstetrics altogether.
Gynecologic practice. Respondents who changed their gynecologic practice cut back on surgical procedures (18.9%), stopped performing major gynecologic surgery (6.7%), and stopped performing all surgery (1.8%).
Other changes. Medical liability issues contributed to the decisions of 12.3% of respondents to choose salaried employment with a hospital, government, or other institution.
Claims experience
Obstetric claims were likely to involve a neurologically impaired infant (28.8%) as the primary allegation, followed by stillbirth or neonatal death (14.4%).
Other variables involved in obstetric claims included electronic fetal monitoring (20.9%), shoulder dystocia and/or brachial plexus injury (15.5%), and actions of ObGyn residents (11.4%).
Gynecologic claims. Survey respondents reported a total of 1,496 gynecologic claims. Major injury to the patient was the primary allegation of 29.1% of these claims. A delay in diagnosis or failure to diagnose was the second most common primary allegation (22.1%), followed by minor injury to the patient (20.7%).Of the claims involving a delayed or missed diagnosis, 41.8% involved cancer. Of these, breast cancer was the most frequent type of cancer (39.1%), followed by uterine cancer (20.3%), ovarian cancer (14.5%), and cervical cancer (10.9%).
Many gynecologic claims (44.4%) involved surgical complications arising from hysterectomy (28.7%) and laparoscopic procedures (14.6%).
Claims outcomes. A total of 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn. Of these, 29.0% were dropped by the plaintiff, 11.2% were dismissed by the court, and 3.7% were settled without payment on behalf of the ObGyn.The average for all paid claims was $510,473.
The average payment for claims involving a neurologically impaired infant was $982,051. Other average payments for obstetric claims include $364,794 for “other infant injury–major” and $271,149 for stillbirth or neonatal death.
Average payments for gynecologic claims include $407,500 for a failure to diagnose breast cancer and $315,633 for “patient injury–major.”
Most challenging locales. It will come as no surprise to many readers that average medical liability payouts are especially high in six states:
New York - $677,866,050
Pennsylvania - $319,710,250
Illinois - $242,108,800
New Jersey - $221,170,750
Florida - $218,123,050
California - $215,519,200.
Fifty-eight percent of payouts nationwide were for female patients.7
Tell us what you think, at [email protected]. Please include your name and city and state.
1.Cheney K. ‘Miracle’ pilot on mission against medical errors. Politico.com. August 1, 2013. http://www.politico.com/story/2013/08/sully-sullenberger-mission-medical-erros-95009.html. Accessed August 6, 2013.
2.Office of Management and Budget. Fiscal Year 2012 Budget of the US Government. http://www.whitehouse.gov/files/documents/budget_2012.pdf. Accessed August 8, 2013.
3.Obama’s remarks to the Business Roundtable, March 12, 2009. Wall Street Journal: Washington Wire. http://blogs.wsj.com/washwire/2009/03/12/obamas-remarks-to-the-business-roundtable/. Accessed August 8, 2013.
4.Werner E. Health debate could spur malpractice changes. Salt Lake Tribune. March 17, 2009. http://www.sltrib.com/ci_11933162. Accessed August 8, 2013.
5.Clinton HR, Obama B. Perspective: Making patient safety the centerpiece of medical liability reform. N Engl J Med. 2006;354(21):2205–2208.
6.American Congress of Obstetricians and Gynecologists. Survey on Professional Liability. 2012 Survey Results. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed August 7, 2013.
7. Latner AW. Six states account for 50% of malpractice payouts. Clinical Advisor. April 17, 2012. http://www.clinicaladvisor.com/six-states-account-for-50-of-malpractice-payouts/article/236931. Accessed August 7, 2013.
It’s a conundrum. There seems to be no doubt about the need for medical liability reform—in fact, there is wide-spread support for it. And yet....
Four years after Captain Chesley “Sully” Sullenberger saved a planeload of passengers during an emergency landing—the “miracle on the Hudson”—he’s become a national champion of medical liability reform. In a recent interview with Politico, Sullenberger equated the 200,000 lives estimated to be lost each year due to medical errors to “20 jetliners crashing per week,” a situation he insists would close airports and ground flights until the problem was solved. But these 200,000 deaths cause little more than a ripple of concern, he claims.1
Among the solutions he proposes is “a whole different approach to reviewing medical errors, figuring out what’s behind them, not just blaming doctors and nurses.”1
Captain Sullenberger is discovering the difficult reality we’ve experienced for too many years: Solutions just don’t come very fast to medical liability reform, despite wide-spread support for it.
At the American Congress of Obstetricians and Gynecologists (ACOG), our campaign for medical liability reform has focused, as always, on patients, using the campaign line: “Who will delivery my baby?” ACOG supports caps on noneconomic damages and other reforms, such as those contained in the California Medical Injury Compensation Reform Act (MICRA), the gold standard for medical liability reform. We will continue to push for national MICRA reform until we’ve won that important protection for all ObGyns and their patients.
Until we reach that goal, we’re working to accomplish meaningful steps to liability reform where we can, including testing state alternatives. And our colleague organizations? Many of them, once insisting on federal adoption of MICRA or nothing at all, now actively support meaningful alternatives, too.
What do we want?
Proposals for tort reform, based on California’s MICRA statute, include:
- mandatory periodic payments of all future damages exceeding $100,000
- a $250,000 ceiling on noneconomic damage awards
- a requirement that claims must be filed within 2 years of the date by which the alleged injury reasonably should have been discovered but in no event more than 4 years from the time of the alleged injury. In the case of alleged injury to children under 4 years of age, claims must be filed by the child’s 8th birthday.
- limits on punitive damages, with 50% of punitive damage awards going to a state disciplinary fund
- limits on attorney contingency fees
- reductions in awards based on the amount paid from another source, such as health or disability insurance
- a requirement for “clear and convincing evidence” rather than the usual “preponderance of evidence” when a health-care professional who provided delivery services but not prenatal care is sued
- alternative systems for dispute resolution.
10 alternative reforms
Good ideas include:
1. Require a certificate of merit from the plaintiff
This proposal would require the plaintiff to file an affidavit with the court to demonstrate that the case has merit before the complaint can move forward. Certificates would necessitate the written opinion of a legally qualified health-care provider affirming that the defendant failed to meet the care standards that would be followed by a reasonably prudent health-care provider—and that this failure caused or directly contributed to the damages claimed.
2. Facilitate early settlement offers
Under this idea, a physician or hospital would be allowed to offer economic damages to an injured party without involving the courts. This offer would not constitute an admission of liability and would be inadmissible if a lawsuit were later filed in the case. Physicians would have an incentive to make a good-faith offer as early as possible after the injury is discovered, and patients would have an incentive to accept legitimate offers of compensation. Early-offer programs would require the injured party to meet a higher burden of proof for alleged negligence if that party chooses to reject the offer and file a lawsuit.
3. Create health-care courts
Health-care courts would allow for a bench or jury trial presided over by a specially trained judge to exclusively hear medical liability cases. Such courts have the potential to correct severe deficiencies in the current medical justice system and to reduce health-system errors and improve patient safety.
4. Allow a physician to say, “I’m sorry”
This proposal would encourage physicians to directly discuss errors and injuries with patients, to apologize and outline corrective action. Such discussions would be inadmissible if a patient later files a lawsuit.
5. Establish medical review panels
Any claim against a physician would be reviewed by a panel of experts who would provide an opinion on whether the physician failed to act within the relevant standards of care.
6. Require a claim to be screened and mediated
A plaintiff ’s claim would have to be evaluated by a screening panel before it could proceed to litigation. The panel would identify claims hat merit compensation and encourage early resolution of those claims. It also would encourage withdrawal or dismissal of non-meritorious claims.
7. Protect physicians who follow evidence-based guidelines
Health-care providers who follow guidelines based on solid evidence, and those who have legitimate justifications for departing from guidelines, would be protected from liability claims.
8. Allow the voluntary resolution of disputes
This proposal would motivate states to encourage the creation of other innovative systems to compensate individuals who are injured in the course of receiving health-care services.
9. Require expert witnesses to meet certain standards
This alternative would limit expert-witness standing to individuals who:
- are licensed and trained in the same specialty as the defendant
- have particular expertise in the disease process or procedure performed in the case
- have been in active medical practice in the same specialty as the defendant within 5 years of the claim or who have been taught at an accredited medical school on the care and type of treatment at issue.
10. Create catastrophic injury systems
These systems would establish a fund for individuals who have experienced bad outcomes. Birth injury funds are an example of this model.
Who’s on our side?
Congressional policy wonks give liability reform a thumbs up
In early 2010, the Medicare Payment Advisory Commission (MedPAC), a nonpartisan advisory counsel to the US Congress, identified three important ways that our current malpractice system harms the Medicare program and Medicare beneficiaries, the aged, and disabled:
- Medicare payments to providers include some liability costs (folded into hospital diagnosis-related group [DRG] payments; factored into physician fee schedule calculation)
- Defensive medicine drives up costs for Medicare
- Malpractice impairs the quality and safety of care to beneficiaries. That is, the current system does not improve patient safety.
MedPAC staff recommended that the commissioners urge Congress to pass government-subsidized malpractice reinsurance for providers who meet certain safety criteria or create a federal administrative adjudication process. The commissioners expressed an interest in alternatives to address the costs of medical malpractice, including ways to encourage states and providers to address medical malpractice in a manner most appropriate for them. However, when MedPAC returned to this topic at its next meeting later the same year, the commissioners mentioned medical liability only to dismiss it as an incidental issue in opening remarks.
The Congressional Budget Office (CBO) estimates that medical malpractice costs our health-care system $35 billion in direct costs, with billions more as a result of defensive medicine.
CBO has scored these medical liability reform proposals as providing significant savings to our federal budget:
- a $250,000 cap on subjective, noneconomic damages (with no limit on economic damages)
- collateral source rule allowing evidence of outside payments to be submitted in court
- a ban on subrogation by certain collateral sources
- caps on attorney contingency fees
- periodic payments of future damages
- a reasonable statute of limitations.
In addition, in 2011, CBO scored comprehensive medical liability reform as saving the federal government $62.4 billion over 10 years. As longtime Illinois Senator Everett Dirksen was known to say, “A billion here, a billion there, and pretty soon you’re talking real money.”
Many Republican congressional leaders “walk the walk”
Republicans have long claimed medical liability reform as their issue. And they walk the walk.
Representative Phil Gingrey, MD, of Georgia, an ACOG Fellow, has led the medical liability reform fight on Capitol Hill for a number of years. His bill, the Protecting Access to Healthcare Act (HR 5), which would have brought MICRA to the national level, was repeatedly passed by the Republican majority of the House of Representatives, only to be ignored by the Democrats controlling the Senate.
Again this year, Dr. Gingrey introduced legislation to protect physicians from unexpected liability. His Standard of Care Protection Act (HR 1473) would ensure that provisions of the Affordable Care Act (ACA) cannot be used to create new causes of action against medical professionals. HR 1473 would ensure that Medicare, Medicaid, and other federal programs that establish government standards and guidelines for health-care providers cannot be used to create new causes of action.
Federal health-care programs are changing to ensure that payment reflects quality of care. As a result, new payment rules, guidelines, and standards are being written into federal laws and regulations. HR 1473 would make clear that these cannot be used to define the applicable standard of care or duty of care in a medical liability lawsuit.
ACOG supports Dr. Gingrey’s bill, as well as a second, companion approach that would ensure that ObGyns who follow guidelines and standards of care developed by their medical society are protected from liability, with sensible exceptions for egregious harm and negligence.
Representative Charlie Dent, Republican of Pennsylvania, also has introduced ACOG-supported medical liability legislation. The Health Care Safety Net Enhancement Act (HR 36) would provide federal liability protection for physicians providing care under the Emergency Medical Treatment and Active Labor Act (EMTALA). HR 36 is commonly referred to as Good Samaritan legislation, intended to protect doctors who rush to the aid of a sick individual. The likelihood of any of these bills getting enacted into law is slim. Even some conservative Republicans oppose federal liability reform as an intrusion into states’ rights.
Some Democrats have said good things
In his proposed budget for fiscal year 2012, President Barack Obama asked Congress for funding to address medical liability issues.
He proposed “to restrain health-care costs” through “a more aggressive effort to reform our medical malpractice system to reduce defensive medicine, promote patient safety, and improve patient outcomes.” He encouraged Republicans to work constructively with him on medical malpractice as part of an overall effort to restrain health-care costs.2
The President asked Congress for “$250 million in grants to states to reform the way they resolve medical malpractice disputes,” including health courts, safe harbors, early disclosure and offer, and other legal reforms such as joint and several liability and collateral source rules.2
Congress never funded the President’s request.
President Obama repeated his request in his fiscal year 2013 budget proposal. Congress didn’t fund it then, either.
Earlier, in March 2009, in remarks to the Business Roundtable, President Obama noted that “the cost issue is the thing that we actually think is the big driver in this whole debate...things like comparative effectiveness, health IT, prevention, figuring out how our reimbursement structures are designed under Medicare and Medicaid. Medical liability issues—I think all those things have to be on the table.”3
In an interview the same month, Senator Ron Wyden, Democrat of Oregon, said, “I think [medical liability reform is] an essential piece for there to be enduring reform, reform that will stick and will get a significant bipar-tisan vote in the United States Senate.”4
Senator Wyden’s Healthy Americans Act (S 391) included incentives to get states to enact malpractice reforms as a key to overhauling the health-care system.
Also in March 2009, Representative Rob Andrews, Democrat of New Jersey, Chairman of the House Education and Labor, Health Subcommittee, pointed to the need for medical liability reform.
“It’s hard for me to imagine a [health-care reform] result that gets to the president’s desk that doesn’t deal with the medical mal-practice issue in some way.”4
And Senator Max Baucus, Democrat of Montana, Chairman of the Senate Finance Committee, proposed providing states grant money to develop alternative litigation models, such as encouraging disclosure and compensation in the case of error, and establishing health courts whose judges have health-care expertise.
As early as May 2006, President Obama (then a Senator from Illinois) and Senator Hillary Rodham Clinton, Democrat of New York, urged a focus on patient safety.
“Instead of focusing on the few areas of intense disagreement,” they wrote in the New England Journal of Medicine, “such as the possibility of mandating caps on the financial damages awarded to patients, we believe that the discussion should center on a more fundamental issue: the need to improve patient safety....”
“To improve both patient safety and the medical liability climate, the tort system must achieve four goals: reduce the rates of preventable patient injuries, promote open communication between physicians and patients, ensure patients access to fair compensation for legitimate medical injuries, and reduce liability insurance premiums for health-care providers. Addressing just one of these issues is not sufficient.”5
And then there are the trial lawyers
Readers of OBG Management know all too well that the role of trial lawyers in medical liability reform has been to block meaningful reforms from passing and to repeal reforms currently in place. The Association of Trial Lawyers of America, now known as the American Association for Justice, tries to portray itself as defending vulnerable patients against a few bad apples. Its Web site (www.justice.org) points to recent National Practitioner Data Bank (NPDB) figures indicating that “just 6% of doctors are responsible for 58% of all negligence incidents. The civil justice system seeks to weed out those few doctors whose actions have such devastating impact on patients.”
The Web site includes these bullet points:
- 6% of doctors have been responsible for 58% of all malpractice payments since 1991
- 2% of doctors having three or more mal-practice payments were responsible for 33% of all payments
- 1% of doctors having four or more malprac-tice payments were responsible for 20% of all payments
- 82% of doctors have never had a medical malpractice payment.
Tell that to ObGyns, who, in 2012, paid an average of 12.4% of their gross income for liability insurance premiums in 2012, and nearly 60% of whom changed their practices based on the risk or fear of professional liability claims or litigation. And this despite the fact that 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn.
Action at the state level
We need a federal solution, but since that isn’t within reach, we’re looking to the states for action. And there’s a lot of action in some states, including Connecticut, Florida, Georgia, Hawaii, Illinois, Iowa, Missouri, Oregon, Rhode Island, Tennessee, and Utah.
Advocates in these states are trying a number of different approaches, hoping that some type of meaningful reform will be signed into law. Here’s a sampling of what’s under way.
Connecticut
HB 6687, amend certificates of merit in medical liability actions. Status: April 1, 2013: Joint Committee on Judiciary hearing. The bill would eliminate the need for a detailed basis for the formation of an opinion and replace it with a lower threshold stating the appearance of one or more specific breaches of the prevailing professional standard of care.
In addition, HB 6687 would allow any expert who may testify in court to satisfy the certificate of merit requirement, but at trial the “expert,” in order to testify, needs to have the court determine him or her to be qualified to testify based on discovery and evidentiary issues that are decided at trial. This expert then could sign a certificate of merit but have the court determine that he or she is indeed not an expert for that case. HB 6687 delays the challenging of qualifications of an expert only after the completion of discovery, adding substantial time and cost to defending meritless suits. Finally, the bill allows for a second bite of the apple for cases that did not meet this watered down standard for certificate of merit and would eliminate the automatic dismissal of cases filed with inadequate certificates that did not meet the rules of the court.
SB 1154, amend Connecticut’s failure of suit statute to allow a plaintiff whose lawsuit was dismissed due to a failure to file a certificate of good faith as required by statute, to commence a new action.
HB 5229, limit noneconomic damages in medical liability cases to $250,000 for each health-care provider and institution per event, and $750,000 overall for each event.
HB 5270, establish peer-review panels in medical liability actions. The panels would consist of physicians, medical professionals, and individuals outside the medical profession who would review claims of alleged negligence and determine whether there is probable cause that the medical liability claims have been made in good faith prior to the action being referred to mandatory mediation.
SB 97, extend the statute of limitations in medical liability cases, allowing for an action to be brought no more than 10 years from the date of the act or omission that serves as the basis for the claim.
Florida
The Birth-Related Neurological Injury Compensation Association (NICA). NICA is a statutory organization that manages the compensation plan used to pay for the care of infants born with certain neurological injuries. This plan is available to eligible families statewide without litigation. By eliminating costly legal proceedings, and through professional management of its disbursements, NICA ensures that birth-injured infants receive the care they need while reducing the financial burden on medical providers and families. Defensive work continues on the NICA Board and trial bar.
HB 7015, expert witness. Status: March 28, 2013, the House Justice Appropriations Subcommittee reported favorably. This bill would adopt the Daubert standard for expert witness testimony. It provides that a witness qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion as to the facts at issue in a case.
Georgia
HB 499, Provider Shield Act. Georgia is the first state to introduce legislation based on the American Medical Association’s model bill, “The Provider Shield Act,” which clarifies language in the Affordable Care Act by providing that a physician’s failure to comply with, or a breach of, any federal statute, regulation, program, guideline, or other provision shall not: (1) be admissible; (2) be used to determine the standard of care; or (3) be the legal basis for a presumption of negligence.
Status: Enacted May 6, 2013. The law prohibits the use of payer guidelines and quality criteria outlined in federal law as a legal basis for negligence or standard of care in determining medical liability. Physicians are concerned that without such protections, the medical profession could be exposed to charges of negligence that aren’t based on clinical standards or the patient’s unique medical needs. Implementation of any guideline by any public or private payor, or the establishment of any payment standard or reimbursement criteria under any federal laws or regulations related to health care, shall not be construed, without competent expert testimony establishing the appropriate standard of care, to establish a legal basis for negligence or the standard of care or duty of care owed by a health-care provider to a patient in any civil action for medical malpractice or product liability.
This first-of-its-kind legislation reinforces the concept that medical decisions should be based on a patient’s unique medical needs. HB 499 makes it clear that federal standards or guidelines designed to enhance access to high-quality health care cannot be used to invent new legal actions against physicians.
Hawaii
SB 1308, health-care provider benevolent gesture legislation.
Illinois
On March 22, 2013, several pieces of tort-reform legislation were re-referred to the House Committee on Rules, effectively killing the bills for the session. The House Speaker would have to choose to “release” any of the bills in order for them to move again; this is highly unlikely.
HB 138 would have deleted existing-venue language providing that an action may commence in any county if all defendants are nonresidents of the state, and replaced it with language providing that, if no defendants that are joined in good faith and with probable cause for the purpose of obtaining a judgment against them are residents of the state, an action may be commenced only in the county in which the transaction or some part thereof occurred out of which the cause of action arose.
HB 2220 and HB 2222 provided that, with respect to certain types of actions, for any defendant whose fault is less than 25% of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendant who could have been sued by the plaintiff (instead of any third-party defendant except the plaintiff’s employer), shall be severally liable for all other damages. In addition, these bills provided that, for any defendant whose fault is 25% or greater of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendants who could have been sued by the plaintiff (instead of any third-party defendants except the plaintiff’s employer), shall be jointly and severally liable for all other damages.
HB 2221 created requirements regarding qualifications, testimony, disclosure and compensation of expert testimony and standards for reviewing courts to follow in ruling on the admissibility of expert testimony.
Iowa
SSB 1054 and HSB 36, expert’s certificate of merit affidavit and noneconomic cap. These bills provide that in any medical liability action, the plaintiff is required, within 180 days of the defendant’s answer, to serve the defendant with an expert’s certificate of merit affidavit for each expert scheduled to testify. They also would limit noneconomic damage awards in medical liability cases to $1 million.
Missouri
HJR 6 proposes a constitutional amendment allowing the legislature to cap noneconomic damages in medical liability cases.
SJR 1 grants the legislature the power to limit, by statute, jury awards for noneconomic damages.
SB 64 changes the evidentiary standard in medical liability cases to “clear and convincing” for noneconomic damages.
Oregon
SB 483, early discussion and resolution. Status: Passed by the legislature. This bill establishes an early discussion and resolution (EDC) process within the Oregon Patient Safety Commission. This voluntary process is intended to facilitate open communication about all outcomes of care, including serious events, between the provider, health-care facility, and the patient. When an adverse health-care incident occurs, the patient, health-care provider or health-care facility where the incident occurred may file a notice of adverse health-care incident with the Commission. This notice triggers discussion of the health-care incident and, if appropriate, an offer of compensation. If discussion does not result in the resolution of the claim, the bill gives the parties the option of participating in Commission-facilitated mediation. The entire process is voluntary.
SJR 30, proposed amendment to constitution, $1 million limit on noneconomic awards in medical liability cases. Slated for next general election.
Rhode Island
HB 5380, apology bill. Status: Heard in House Judiciary Committee on March 27, 2013; no action was taken. This bill provides that statements by a health-care provider to a patient or to the patient’s family regarding the outcome of such patient’s medical care and treatment, such as an apology or expression of sympathy, shall be inadmissible as evidence or an admission of liability in any claim or action against the provider.
Tennessee
Joint and several liability. Status: On March 26, 2013, the House Civil Justice Subcommittee reported favorably. This bill would codify current state law by providing that if multiple defendants are found liable in a civil action governed by comparative fault, a defendant shall only be severally liable for the percentage of damages for which fault is attributed to such defendant by the trier of fact, and no defendant shall be held jointly liable for any damages.
SB 274, medical liability expert witness reform.
Utah
HB 135, rules, arbitration. Status: March 21, 2013, sent to Governor Gary Herbert for his approval. HB 135 provides that a party in a medical liability action or arbitration may not attempt to allocate fault to any health-care provider unless a certificate of compliance has been issued. HB 135 also requires that evidence from a medical review panel remain unreportable to a health-care facility or health insurance plan.
Summing up
Medical liability reform—the obvious need for it, the good reasons to do it, and the fact that it remains beyond reach—is a constant source of frustration among many ObGyns. Maybe Captain Sully can save the day.
How medical liability affects the ObGyn specialty
ACOG’s 2012 Survey on Professional Liability, our 11th survey since 1983, assessed the effects of professional liability litigation and insurance issues on the practice of obstetrics and gynecology.6 The survey, conducted under the direction of ACOG’s Vice President for Fellowship and Deputy Executive Vice President Albert Strunk, MD, JD, included segments on demographics, patient care, liability claims experience, and practice changes associated with the cost of liability insurance and the fear of litigation. The survey went to 32,238 Fellows and Junior Fellows. Of these, 9,006 completed the questionnaire. Here are major findings.
Provider profiles
A total of 72.5% of respondents provided both obstetric and gynecologic care, slightly lower than the percentage identified in the 2009 survey, which was 74.3%. Fewer than 7% of respondents provided obstetric care only; 19.8% provided gynecologic care only. Of those restricting their services to gynecology, 88.9% had previously offered obstetric care. The average age at which these physicians stopped practicing obstetrics was 49 years.
Cost of liability insurance
ObGyns spent an average of 12.4% of their gross income on liability insurance premiums in 2012, down from 18% in 2009.
How liability issues affected practice
Since the previous survey in 2009, 57.9% of respondents made one or more changes to their practice to mitigate the risk or fear of professional liability claims or litigation.
Obstetric practice. Among respondents who made changes to their obstetric practice, 27.4% decreased the number of high-risk patients they see, 23.8% increased the number of cesarean deliveries they perform, 18.9% stopped offering and performing vaginal birth after cesarean (VBAC), 11.5% reduced the total number of deliveries, and 6.2% stopped practicing obstetrics altogether.
Gynecologic practice. Respondents who changed their gynecologic practice cut back on surgical procedures (18.9%), stopped performing major gynecologic surgery (6.7%), and stopped performing all surgery (1.8%).
Other changes. Medical liability issues contributed to the decisions of 12.3% of respondents to choose salaried employment with a hospital, government, or other institution.
Claims experience
Obstetric claims were likely to involve a neurologically impaired infant (28.8%) as the primary allegation, followed by stillbirth or neonatal death (14.4%).
Other variables involved in obstetric claims included electronic fetal monitoring (20.9%), shoulder dystocia and/or brachial plexus injury (15.5%), and actions of ObGyn residents (11.4%).
Gynecologic claims. Survey respondents reported a total of 1,496 gynecologic claims. Major injury to the patient was the primary allegation of 29.1% of these claims. A delay in diagnosis or failure to diagnose was the second most common primary allegation (22.1%), followed by minor injury to the patient (20.7%).Of the claims involving a delayed or missed diagnosis, 41.8% involved cancer. Of these, breast cancer was the most frequent type of cancer (39.1%), followed by uterine cancer (20.3%), ovarian cancer (14.5%), and cervical cancer (10.9%).
Many gynecologic claims (44.4%) involved surgical complications arising from hysterectomy (28.7%) and laparoscopic procedures (14.6%).
Claims outcomes. A total of 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn. Of these, 29.0% were dropped by the plaintiff, 11.2% were dismissed by the court, and 3.7% were settled without payment on behalf of the ObGyn.The average for all paid claims was $510,473.
The average payment for claims involving a neurologically impaired infant was $982,051. Other average payments for obstetric claims include $364,794 for “other infant injury–major” and $271,149 for stillbirth or neonatal death.
Average payments for gynecologic claims include $407,500 for a failure to diagnose breast cancer and $315,633 for “patient injury–major.”
Most challenging locales. It will come as no surprise to many readers that average medical liability payouts are especially high in six states:
New York - $677,866,050
Pennsylvania - $319,710,250
Illinois - $242,108,800
New Jersey - $221,170,750
Florida - $218,123,050
California - $215,519,200.
Fifty-eight percent of payouts nationwide were for female patients.7
Tell us what you think, at [email protected]. Please include your name and city and state.
It’s a conundrum. There seems to be no doubt about the need for medical liability reform—in fact, there is wide-spread support for it. And yet....
Four years after Captain Chesley “Sully” Sullenberger saved a planeload of passengers during an emergency landing—the “miracle on the Hudson”—he’s become a national champion of medical liability reform. In a recent interview with Politico, Sullenberger equated the 200,000 lives estimated to be lost each year due to medical errors to “20 jetliners crashing per week,” a situation he insists would close airports and ground flights until the problem was solved. But these 200,000 deaths cause little more than a ripple of concern, he claims.1
Among the solutions he proposes is “a whole different approach to reviewing medical errors, figuring out what’s behind them, not just blaming doctors and nurses.”1
Captain Sullenberger is discovering the difficult reality we’ve experienced for too many years: Solutions just don’t come very fast to medical liability reform, despite wide-spread support for it.
At the American Congress of Obstetricians and Gynecologists (ACOG), our campaign for medical liability reform has focused, as always, on patients, using the campaign line: “Who will delivery my baby?” ACOG supports caps on noneconomic damages and other reforms, such as those contained in the California Medical Injury Compensation Reform Act (MICRA), the gold standard for medical liability reform. We will continue to push for national MICRA reform until we’ve won that important protection for all ObGyns and their patients.
Until we reach that goal, we’re working to accomplish meaningful steps to liability reform where we can, including testing state alternatives. And our colleague organizations? Many of them, once insisting on federal adoption of MICRA or nothing at all, now actively support meaningful alternatives, too.
What do we want?
Proposals for tort reform, based on California’s MICRA statute, include:
- mandatory periodic payments of all future damages exceeding $100,000
- a $250,000 ceiling on noneconomic damage awards
- a requirement that claims must be filed within 2 years of the date by which the alleged injury reasonably should have been discovered but in no event more than 4 years from the time of the alleged injury. In the case of alleged injury to children under 4 years of age, claims must be filed by the child’s 8th birthday.
- limits on punitive damages, with 50% of punitive damage awards going to a state disciplinary fund
- limits on attorney contingency fees
- reductions in awards based on the amount paid from another source, such as health or disability insurance
- a requirement for “clear and convincing evidence” rather than the usual “preponderance of evidence” when a health-care professional who provided delivery services but not prenatal care is sued
- alternative systems for dispute resolution.
10 alternative reforms
Good ideas include:
1. Require a certificate of merit from the plaintiff
This proposal would require the plaintiff to file an affidavit with the court to demonstrate that the case has merit before the complaint can move forward. Certificates would necessitate the written opinion of a legally qualified health-care provider affirming that the defendant failed to meet the care standards that would be followed by a reasonably prudent health-care provider—and that this failure caused or directly contributed to the damages claimed.
2. Facilitate early settlement offers
Under this idea, a physician or hospital would be allowed to offer economic damages to an injured party without involving the courts. This offer would not constitute an admission of liability and would be inadmissible if a lawsuit were later filed in the case. Physicians would have an incentive to make a good-faith offer as early as possible after the injury is discovered, and patients would have an incentive to accept legitimate offers of compensation. Early-offer programs would require the injured party to meet a higher burden of proof for alleged negligence if that party chooses to reject the offer and file a lawsuit.
3. Create health-care courts
Health-care courts would allow for a bench or jury trial presided over by a specially trained judge to exclusively hear medical liability cases. Such courts have the potential to correct severe deficiencies in the current medical justice system and to reduce health-system errors and improve patient safety.
4. Allow a physician to say, “I’m sorry”
This proposal would encourage physicians to directly discuss errors and injuries with patients, to apologize and outline corrective action. Such discussions would be inadmissible if a patient later files a lawsuit.
5. Establish medical review panels
Any claim against a physician would be reviewed by a panel of experts who would provide an opinion on whether the physician failed to act within the relevant standards of care.
6. Require a claim to be screened and mediated
A plaintiff ’s claim would have to be evaluated by a screening panel before it could proceed to litigation. The panel would identify claims hat merit compensation and encourage early resolution of those claims. It also would encourage withdrawal or dismissal of non-meritorious claims.
7. Protect physicians who follow evidence-based guidelines
Health-care providers who follow guidelines based on solid evidence, and those who have legitimate justifications for departing from guidelines, would be protected from liability claims.
8. Allow the voluntary resolution of disputes
This proposal would motivate states to encourage the creation of other innovative systems to compensate individuals who are injured in the course of receiving health-care services.
9. Require expert witnesses to meet certain standards
This alternative would limit expert-witness standing to individuals who:
- are licensed and trained in the same specialty as the defendant
- have particular expertise in the disease process or procedure performed in the case
- have been in active medical practice in the same specialty as the defendant within 5 years of the claim or who have been taught at an accredited medical school on the care and type of treatment at issue.
10. Create catastrophic injury systems
These systems would establish a fund for individuals who have experienced bad outcomes. Birth injury funds are an example of this model.
Who’s on our side?
Congressional policy wonks give liability reform a thumbs up
In early 2010, the Medicare Payment Advisory Commission (MedPAC), a nonpartisan advisory counsel to the US Congress, identified three important ways that our current malpractice system harms the Medicare program and Medicare beneficiaries, the aged, and disabled:
- Medicare payments to providers include some liability costs (folded into hospital diagnosis-related group [DRG] payments; factored into physician fee schedule calculation)
- Defensive medicine drives up costs for Medicare
- Malpractice impairs the quality and safety of care to beneficiaries. That is, the current system does not improve patient safety.
MedPAC staff recommended that the commissioners urge Congress to pass government-subsidized malpractice reinsurance for providers who meet certain safety criteria or create a federal administrative adjudication process. The commissioners expressed an interest in alternatives to address the costs of medical malpractice, including ways to encourage states and providers to address medical malpractice in a manner most appropriate for them. However, when MedPAC returned to this topic at its next meeting later the same year, the commissioners mentioned medical liability only to dismiss it as an incidental issue in opening remarks.
The Congressional Budget Office (CBO) estimates that medical malpractice costs our health-care system $35 billion in direct costs, with billions more as a result of defensive medicine.
CBO has scored these medical liability reform proposals as providing significant savings to our federal budget:
- a $250,000 cap on subjective, noneconomic damages (with no limit on economic damages)
- collateral source rule allowing evidence of outside payments to be submitted in court
- a ban on subrogation by certain collateral sources
- caps on attorney contingency fees
- periodic payments of future damages
- a reasonable statute of limitations.
In addition, in 2011, CBO scored comprehensive medical liability reform as saving the federal government $62.4 billion over 10 years. As longtime Illinois Senator Everett Dirksen was known to say, “A billion here, a billion there, and pretty soon you’re talking real money.”
Many Republican congressional leaders “walk the walk”
Republicans have long claimed medical liability reform as their issue. And they walk the walk.
Representative Phil Gingrey, MD, of Georgia, an ACOG Fellow, has led the medical liability reform fight on Capitol Hill for a number of years. His bill, the Protecting Access to Healthcare Act (HR 5), which would have brought MICRA to the national level, was repeatedly passed by the Republican majority of the House of Representatives, only to be ignored by the Democrats controlling the Senate.
Again this year, Dr. Gingrey introduced legislation to protect physicians from unexpected liability. His Standard of Care Protection Act (HR 1473) would ensure that provisions of the Affordable Care Act (ACA) cannot be used to create new causes of action against medical professionals. HR 1473 would ensure that Medicare, Medicaid, and other federal programs that establish government standards and guidelines for health-care providers cannot be used to create new causes of action.
Federal health-care programs are changing to ensure that payment reflects quality of care. As a result, new payment rules, guidelines, and standards are being written into federal laws and regulations. HR 1473 would make clear that these cannot be used to define the applicable standard of care or duty of care in a medical liability lawsuit.
ACOG supports Dr. Gingrey’s bill, as well as a second, companion approach that would ensure that ObGyns who follow guidelines and standards of care developed by their medical society are protected from liability, with sensible exceptions for egregious harm and negligence.
Representative Charlie Dent, Republican of Pennsylvania, also has introduced ACOG-supported medical liability legislation. The Health Care Safety Net Enhancement Act (HR 36) would provide federal liability protection for physicians providing care under the Emergency Medical Treatment and Active Labor Act (EMTALA). HR 36 is commonly referred to as Good Samaritan legislation, intended to protect doctors who rush to the aid of a sick individual. The likelihood of any of these bills getting enacted into law is slim. Even some conservative Republicans oppose federal liability reform as an intrusion into states’ rights.
Some Democrats have said good things
In his proposed budget for fiscal year 2012, President Barack Obama asked Congress for funding to address medical liability issues.
He proposed “to restrain health-care costs” through “a more aggressive effort to reform our medical malpractice system to reduce defensive medicine, promote patient safety, and improve patient outcomes.” He encouraged Republicans to work constructively with him on medical malpractice as part of an overall effort to restrain health-care costs.2
The President asked Congress for “$250 million in grants to states to reform the way they resolve medical malpractice disputes,” including health courts, safe harbors, early disclosure and offer, and other legal reforms such as joint and several liability and collateral source rules.2
Congress never funded the President’s request.
President Obama repeated his request in his fiscal year 2013 budget proposal. Congress didn’t fund it then, either.
Earlier, in March 2009, in remarks to the Business Roundtable, President Obama noted that “the cost issue is the thing that we actually think is the big driver in this whole debate...things like comparative effectiveness, health IT, prevention, figuring out how our reimbursement structures are designed under Medicare and Medicaid. Medical liability issues—I think all those things have to be on the table.”3
In an interview the same month, Senator Ron Wyden, Democrat of Oregon, said, “I think [medical liability reform is] an essential piece for there to be enduring reform, reform that will stick and will get a significant bipar-tisan vote in the United States Senate.”4
Senator Wyden’s Healthy Americans Act (S 391) included incentives to get states to enact malpractice reforms as a key to overhauling the health-care system.
Also in March 2009, Representative Rob Andrews, Democrat of New Jersey, Chairman of the House Education and Labor, Health Subcommittee, pointed to the need for medical liability reform.
“It’s hard for me to imagine a [health-care reform] result that gets to the president’s desk that doesn’t deal with the medical mal-practice issue in some way.”4
And Senator Max Baucus, Democrat of Montana, Chairman of the Senate Finance Committee, proposed providing states grant money to develop alternative litigation models, such as encouraging disclosure and compensation in the case of error, and establishing health courts whose judges have health-care expertise.
As early as May 2006, President Obama (then a Senator from Illinois) and Senator Hillary Rodham Clinton, Democrat of New York, urged a focus on patient safety.
“Instead of focusing on the few areas of intense disagreement,” they wrote in the New England Journal of Medicine, “such as the possibility of mandating caps on the financial damages awarded to patients, we believe that the discussion should center on a more fundamental issue: the need to improve patient safety....”
“To improve both patient safety and the medical liability climate, the tort system must achieve four goals: reduce the rates of preventable patient injuries, promote open communication between physicians and patients, ensure patients access to fair compensation for legitimate medical injuries, and reduce liability insurance premiums for health-care providers. Addressing just one of these issues is not sufficient.”5
And then there are the trial lawyers
Readers of OBG Management know all too well that the role of trial lawyers in medical liability reform has been to block meaningful reforms from passing and to repeal reforms currently in place. The Association of Trial Lawyers of America, now known as the American Association for Justice, tries to portray itself as defending vulnerable patients against a few bad apples. Its Web site (www.justice.org) points to recent National Practitioner Data Bank (NPDB) figures indicating that “just 6% of doctors are responsible for 58% of all negligence incidents. The civil justice system seeks to weed out those few doctors whose actions have such devastating impact on patients.”
The Web site includes these bullet points:
- 6% of doctors have been responsible for 58% of all malpractice payments since 1991
- 2% of doctors having three or more mal-practice payments were responsible for 33% of all payments
- 1% of doctors having four or more malprac-tice payments were responsible for 20% of all payments
- 82% of doctors have never had a medical malpractice payment.
Tell that to ObGyns, who, in 2012, paid an average of 12.4% of their gross income for liability insurance premiums in 2012, and nearly 60% of whom changed their practices based on the risk or fear of professional liability claims or litigation. And this despite the fact that 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn.
Action at the state level
We need a federal solution, but since that isn’t within reach, we’re looking to the states for action. And there’s a lot of action in some states, including Connecticut, Florida, Georgia, Hawaii, Illinois, Iowa, Missouri, Oregon, Rhode Island, Tennessee, and Utah.
Advocates in these states are trying a number of different approaches, hoping that some type of meaningful reform will be signed into law. Here’s a sampling of what’s under way.
Connecticut
HB 6687, amend certificates of merit in medical liability actions. Status: April 1, 2013: Joint Committee on Judiciary hearing. The bill would eliminate the need for a detailed basis for the formation of an opinion and replace it with a lower threshold stating the appearance of one or more specific breaches of the prevailing professional standard of care.
In addition, HB 6687 would allow any expert who may testify in court to satisfy the certificate of merit requirement, but at trial the “expert,” in order to testify, needs to have the court determine him or her to be qualified to testify based on discovery and evidentiary issues that are decided at trial. This expert then could sign a certificate of merit but have the court determine that he or she is indeed not an expert for that case. HB 6687 delays the challenging of qualifications of an expert only after the completion of discovery, adding substantial time and cost to defending meritless suits. Finally, the bill allows for a second bite of the apple for cases that did not meet this watered down standard for certificate of merit and would eliminate the automatic dismissal of cases filed with inadequate certificates that did not meet the rules of the court.
SB 1154, amend Connecticut’s failure of suit statute to allow a plaintiff whose lawsuit was dismissed due to a failure to file a certificate of good faith as required by statute, to commence a new action.
HB 5229, limit noneconomic damages in medical liability cases to $250,000 for each health-care provider and institution per event, and $750,000 overall for each event.
HB 5270, establish peer-review panels in medical liability actions. The panels would consist of physicians, medical professionals, and individuals outside the medical profession who would review claims of alleged negligence and determine whether there is probable cause that the medical liability claims have been made in good faith prior to the action being referred to mandatory mediation.
SB 97, extend the statute of limitations in medical liability cases, allowing for an action to be brought no more than 10 years from the date of the act or omission that serves as the basis for the claim.
Florida
The Birth-Related Neurological Injury Compensation Association (NICA). NICA is a statutory organization that manages the compensation plan used to pay for the care of infants born with certain neurological injuries. This plan is available to eligible families statewide without litigation. By eliminating costly legal proceedings, and through professional management of its disbursements, NICA ensures that birth-injured infants receive the care they need while reducing the financial burden on medical providers and families. Defensive work continues on the NICA Board and trial bar.
HB 7015, expert witness. Status: March 28, 2013, the House Justice Appropriations Subcommittee reported favorably. This bill would adopt the Daubert standard for expert witness testimony. It provides that a witness qualified as an expert by knowledge, skill, experience, training, or education may testify in the form of an opinion as to the facts at issue in a case.
Georgia
HB 499, Provider Shield Act. Georgia is the first state to introduce legislation based on the American Medical Association’s model bill, “The Provider Shield Act,” which clarifies language in the Affordable Care Act by providing that a physician’s failure to comply with, or a breach of, any federal statute, regulation, program, guideline, or other provision shall not: (1) be admissible; (2) be used to determine the standard of care; or (3) be the legal basis for a presumption of negligence.
Status: Enacted May 6, 2013. The law prohibits the use of payer guidelines and quality criteria outlined in federal law as a legal basis for negligence or standard of care in determining medical liability. Physicians are concerned that without such protections, the medical profession could be exposed to charges of negligence that aren’t based on clinical standards or the patient’s unique medical needs. Implementation of any guideline by any public or private payor, or the establishment of any payment standard or reimbursement criteria under any federal laws or regulations related to health care, shall not be construed, without competent expert testimony establishing the appropriate standard of care, to establish a legal basis for negligence or the standard of care or duty of care owed by a health-care provider to a patient in any civil action for medical malpractice or product liability.
This first-of-its-kind legislation reinforces the concept that medical decisions should be based on a patient’s unique medical needs. HB 499 makes it clear that federal standards or guidelines designed to enhance access to high-quality health care cannot be used to invent new legal actions against physicians.
Hawaii
SB 1308, health-care provider benevolent gesture legislation.
Illinois
On March 22, 2013, several pieces of tort-reform legislation were re-referred to the House Committee on Rules, effectively killing the bills for the session. The House Speaker would have to choose to “release” any of the bills in order for them to move again; this is highly unlikely.
HB 138 would have deleted existing-venue language providing that an action may commence in any county if all defendants are nonresidents of the state, and replaced it with language providing that, if no defendants that are joined in good faith and with probable cause for the purpose of obtaining a judgment against them are residents of the state, an action may be commenced only in the county in which the transaction or some part thereof occurred out of which the cause of action arose.
HB 2220 and HB 2222 provided that, with respect to certain types of actions, for any defendant whose fault is less than 25% of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendant who could have been sued by the plaintiff (instead of any third-party defendant except the plaintiff’s employer), shall be severally liable for all other damages. In addition, these bills provided that, for any defendant whose fault is 25% or greater of the total fault attributable to the plaintiff, the defendants sued by the plaintiff, and any third-party defendants who could have been sued by the plaintiff (instead of any third-party defendants except the plaintiff’s employer), shall be jointly and severally liable for all other damages.
HB 2221 created requirements regarding qualifications, testimony, disclosure and compensation of expert testimony and standards for reviewing courts to follow in ruling on the admissibility of expert testimony.
Iowa
SSB 1054 and HSB 36, expert’s certificate of merit affidavit and noneconomic cap. These bills provide that in any medical liability action, the plaintiff is required, within 180 days of the defendant’s answer, to serve the defendant with an expert’s certificate of merit affidavit for each expert scheduled to testify. They also would limit noneconomic damage awards in medical liability cases to $1 million.
Missouri
HJR 6 proposes a constitutional amendment allowing the legislature to cap noneconomic damages in medical liability cases.
SJR 1 grants the legislature the power to limit, by statute, jury awards for noneconomic damages.
SB 64 changes the evidentiary standard in medical liability cases to “clear and convincing” for noneconomic damages.
Oregon
SB 483, early discussion and resolution. Status: Passed by the legislature. This bill establishes an early discussion and resolution (EDC) process within the Oregon Patient Safety Commission. This voluntary process is intended to facilitate open communication about all outcomes of care, including serious events, between the provider, health-care facility, and the patient. When an adverse health-care incident occurs, the patient, health-care provider or health-care facility where the incident occurred may file a notice of adverse health-care incident with the Commission. This notice triggers discussion of the health-care incident and, if appropriate, an offer of compensation. If discussion does not result in the resolution of the claim, the bill gives the parties the option of participating in Commission-facilitated mediation. The entire process is voluntary.
SJR 30, proposed amendment to constitution, $1 million limit on noneconomic awards in medical liability cases. Slated for next general election.
Rhode Island
HB 5380, apology bill. Status: Heard in House Judiciary Committee on March 27, 2013; no action was taken. This bill provides that statements by a health-care provider to a patient or to the patient’s family regarding the outcome of such patient’s medical care and treatment, such as an apology or expression of sympathy, shall be inadmissible as evidence or an admission of liability in any claim or action against the provider.
Tennessee
Joint and several liability. Status: On March 26, 2013, the House Civil Justice Subcommittee reported favorably. This bill would codify current state law by providing that if multiple defendants are found liable in a civil action governed by comparative fault, a defendant shall only be severally liable for the percentage of damages for which fault is attributed to such defendant by the trier of fact, and no defendant shall be held jointly liable for any damages.
SB 274, medical liability expert witness reform.
Utah
HB 135, rules, arbitration. Status: March 21, 2013, sent to Governor Gary Herbert for his approval. HB 135 provides that a party in a medical liability action or arbitration may not attempt to allocate fault to any health-care provider unless a certificate of compliance has been issued. HB 135 also requires that evidence from a medical review panel remain unreportable to a health-care facility or health insurance plan.
Summing up
Medical liability reform—the obvious need for it, the good reasons to do it, and the fact that it remains beyond reach—is a constant source of frustration among many ObGyns. Maybe Captain Sully can save the day.
How medical liability affects the ObGyn specialty
ACOG’s 2012 Survey on Professional Liability, our 11th survey since 1983, assessed the effects of professional liability litigation and insurance issues on the practice of obstetrics and gynecology.6 The survey, conducted under the direction of ACOG’s Vice President for Fellowship and Deputy Executive Vice President Albert Strunk, MD, JD, included segments on demographics, patient care, liability claims experience, and practice changes associated with the cost of liability insurance and the fear of litigation. The survey went to 32,238 Fellows and Junior Fellows. Of these, 9,006 completed the questionnaire. Here are major findings.
Provider profiles
A total of 72.5% of respondents provided both obstetric and gynecologic care, slightly lower than the percentage identified in the 2009 survey, which was 74.3%. Fewer than 7% of respondents provided obstetric care only; 19.8% provided gynecologic care only. Of those restricting their services to gynecology, 88.9% had previously offered obstetric care. The average age at which these physicians stopped practicing obstetrics was 49 years.
Cost of liability insurance
ObGyns spent an average of 12.4% of their gross income on liability insurance premiums in 2012, down from 18% in 2009.
How liability issues affected practice
Since the previous survey in 2009, 57.9% of respondents made one or more changes to their practice to mitigate the risk or fear of professional liability claims or litigation.
Obstetric practice. Among respondents who made changes to their obstetric practice, 27.4% decreased the number of high-risk patients they see, 23.8% increased the number of cesarean deliveries they perform, 18.9% stopped offering and performing vaginal birth after cesarean (VBAC), 11.5% reduced the total number of deliveries, and 6.2% stopped practicing obstetrics altogether.
Gynecologic practice. Respondents who changed their gynecologic practice cut back on surgical procedures (18.9%), stopped performing major gynecologic surgery (6.7%), and stopped performing all surgery (1.8%).
Other changes. Medical liability issues contributed to the decisions of 12.3% of respondents to choose salaried employment with a hospital, government, or other institution.
Claims experience
Obstetric claims were likely to involve a neurologically impaired infant (28.8%) as the primary allegation, followed by stillbirth or neonatal death (14.4%).
Other variables involved in obstetric claims included electronic fetal monitoring (20.9%), shoulder dystocia and/or brachial plexus injury (15.5%), and actions of ObGyn residents (11.4%).
Gynecologic claims. Survey respondents reported a total of 1,496 gynecologic claims. Major injury to the patient was the primary allegation of 29.1% of these claims. A delay in diagnosis or failure to diagnose was the second most common primary allegation (22.1%), followed by minor injury to the patient (20.7%).Of the claims involving a delayed or missed diagnosis, 41.8% involved cancer. Of these, breast cancer was the most frequent type of cancer (39.1%), followed by uterine cancer (20.3%), ovarian cancer (14.5%), and cervical cancer (10.9%).
Many gynecologic claims (44.4%) involved surgical complications arising from hysterectomy (28.7%) and laparoscopic procedures (14.6%).
Claims outcomes. A total of 43.9% of claims were dropped or settled without any payment on behalf of the ObGyn. Of these, 29.0% were dropped by the plaintiff, 11.2% were dismissed by the court, and 3.7% were settled without payment on behalf of the ObGyn.The average for all paid claims was $510,473.
The average payment for claims involving a neurologically impaired infant was $982,051. Other average payments for obstetric claims include $364,794 for “other infant injury–major” and $271,149 for stillbirth or neonatal death.
Average payments for gynecologic claims include $407,500 for a failure to diagnose breast cancer and $315,633 for “patient injury–major.”
Most challenging locales. It will come as no surprise to many readers that average medical liability payouts are especially high in six states:
New York - $677,866,050
Pennsylvania - $319,710,250
Illinois - $242,108,800
New Jersey - $221,170,750
Florida - $218,123,050
California - $215,519,200.
Fifty-eight percent of payouts nationwide were for female patients.7
Tell us what you think, at [email protected]. Please include your name and city and state.
1.Cheney K. ‘Miracle’ pilot on mission against medical errors. Politico.com. August 1, 2013. http://www.politico.com/story/2013/08/sully-sullenberger-mission-medical-erros-95009.html. Accessed August 6, 2013.
2.Office of Management and Budget. Fiscal Year 2012 Budget of the US Government. http://www.whitehouse.gov/files/documents/budget_2012.pdf. Accessed August 8, 2013.
3.Obama’s remarks to the Business Roundtable, March 12, 2009. Wall Street Journal: Washington Wire. http://blogs.wsj.com/washwire/2009/03/12/obamas-remarks-to-the-business-roundtable/. Accessed August 8, 2013.
4.Werner E. Health debate could spur malpractice changes. Salt Lake Tribune. March 17, 2009. http://www.sltrib.com/ci_11933162. Accessed August 8, 2013.
5.Clinton HR, Obama B. Perspective: Making patient safety the centerpiece of medical liability reform. N Engl J Med. 2006;354(21):2205–2208.
6.American Congress of Obstetricians and Gynecologists. Survey on Professional Liability. 2012 Survey Results. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed August 7, 2013.
7. Latner AW. Six states account for 50% of malpractice payouts. Clinical Advisor. April 17, 2012. http://www.clinicaladvisor.com/six-states-account-for-50-of-malpractice-payouts/article/236931. Accessed August 7, 2013.
1.Cheney K. ‘Miracle’ pilot on mission against medical errors. Politico.com. August 1, 2013. http://www.politico.com/story/2013/08/sully-sullenberger-mission-medical-erros-95009.html. Accessed August 6, 2013.
2.Office of Management and Budget. Fiscal Year 2012 Budget of the US Government. http://www.whitehouse.gov/files/documents/budget_2012.pdf. Accessed August 8, 2013.
3.Obama’s remarks to the Business Roundtable, March 12, 2009. Wall Street Journal: Washington Wire. http://blogs.wsj.com/washwire/2009/03/12/obamas-remarks-to-the-business-roundtable/. Accessed August 8, 2013.
4.Werner E. Health debate could spur malpractice changes. Salt Lake Tribune. March 17, 2009. http://www.sltrib.com/ci_11933162. Accessed August 8, 2013.
5.Clinton HR, Obama B. Perspective: Making patient safety the centerpiece of medical liability reform. N Engl J Med. 2006;354(21):2205–2208.
6.American Congress of Obstetricians and Gynecologists. Survey on Professional Liability. 2012 Survey Results. http://www.acog.org/About_ACOG/ACOG_Departments/Professional_Liability/2012_Survey_Results. Accessed August 7, 2013.
7. Latner AW. Six states account for 50% of malpractice payouts. Clinical Advisor. April 17, 2012. http://www.clinicaladvisor.com/six-states-account-for-50-of-malpractice-payouts/article/236931. Accessed August 7, 2013.
- ACOG proposals for reform
- Action at the state level
- How medical liability affects the ObGyn specialty
Let’s eliminate these imprecisions in chart notes of psychiatric evaluations
In private practice, government, and (especially) academically affiliated settings, chart notations that are neither erroneous nor accurate but just imprecise are seen regularly. Academic supervisors may overlook these ambiguous notations by medical students and residents because of their regularity; others may be actively taught by supervisors who use ambiguous notations themselves.
In my experience, the most frequently seen imprecisions are in diagnoses of personality disorders: for example, the terms “clusters” and “deferred,” and the symptomatic overlap between antisocial personality disorder (APD) and substance abuse. Least helpful are qualifying phrases added to substance abuse diagnoses, along with an abundance of abbreviations. The latter occurs despite efforts by the U.S. Department of Veteran Affairs and other agencies to standardize acceptable lists of abbreviations. Many imprecisions could qualify for highlighting; here are 5 of the most unhelpful:
Clusters. Personality disorders are grouped into 3 “clusters,” according to similar characteristics (eg, Cluster A includes paranoid, schizoid, and schizotypal personality disorders and focuses on patients’ oddities and eccentricities). The need for identifying “clusters” could be debated, but a “cluster” is not a diagnosis. A psychiatric evaluation that notes “Cluster B traits” in lieu of a specific personality disorder is not informative, especially to a nonpsychiatric clinician. Which Cluster B traits apply? Is the patient unstable? Self-absorbed? Needy? Dramatic? Criminal? Assaultive?
In complicated or ambiguous cases, the diagnosis of a personality disorder not otherwise specified is appropriate, indicating that traits need to be clarified.
Deferred. This notation frequently is seen under axis II, and often is carried through the medical record for months or years. Psychiatrists are reluctant to diagnose a personality disorder because of the pejorative nature a diagnosis conveys. Nevertheless, by the second or third visit—after 2 or 3 hours of interview contact—it should be evident whether a personality disorder exists. If none does, “no diagnosis” should be documented. This notation can be adjusted if such evidence comes to light.
APD (or APD traits). This diagnosis often is made mistakenly when the root problem is in fact a substance abuse disorder. A multi-decade study of alcoholism and antisocial personality attributes in university students illustrated this phenomenon.1
To be a successful substance abuser—that is, to satisfy the overwhelming urge to drink or use drugs—it’s essential to lie, cheat, and steal. Substance abusers might become belligerent when intoxicated. They might be arrested in bar fights, drive while intoxicated, and buy illegal substances. The result is incarceration, a common consequence of substance abuse and of APD. The latter diagnosis should be made only if the patient has exhibited a pattern of criminal behavior—often starting in adolescence—irrespective of substance abuse, such as breaking and entering, robbery, or assault with a deadly weapon.
Substance abusers often feel guilt and self-loathing for their “weakness,” and cannot gain control over their addiction; the APD patient, on the other hand, feels entitled to plunder and often justifies his (her) actions by attributing fault to the victim.
Keep in mind that many APD patients also are substance abusers; both diagnoses should be listed in the chart when that is the determination. Recognize that substance abuse and APD are distinct entities that should not be confused by the common denominator of having spent time in jail.
Early, late, full remission. These qualifiers often are appended to substance abuse disorders, but they do not convey useful information. How early is “early”? How late is “late”? Perhaps the most misleading term is “full” or “partial” remission,
because there is no clear definition of either.
If one is referring to length of time sober or a reduction in volume consumed, noting the date of the last use is more helpful—eg, “alcohol abuse in remission since summer of 2012.” If “partial” remission means the patient has reduced his intake, then that is not remission. The reduction can be specified—eg, “alcohol abuse, reduced to 1 or 2 beers per weekend.”
Abbreviations. Psychiatric evaluations should contain only standard, well-known medical shorthand (such as MSE for mental status exam). The military may be the biggest offender, devising acronyms and abbreviations for everything.
Two examples of abbreviations that I see in military psychiatric progress notes are AEB (“as evidenced by”) and LLGD (“linear, logical, and goal-directed”). Psychiatrists have a leg up on deciphering abbreviations in psychiatric notes; other providers might be compelled to resort to consultation. That wastes more time than typing out the words and results in frustration and lost productivity.
Reference
1. Vaillant GE. The natural history of alcoholism. Cambridge, MA: Harvard University Press; 1983.
In private practice, government, and (especially) academically affiliated settings, chart notations that are neither erroneous nor accurate but just imprecise are seen regularly. Academic supervisors may overlook these ambiguous notations by medical students and residents because of their regularity; others may be actively taught by supervisors who use ambiguous notations themselves.
In my experience, the most frequently seen imprecisions are in diagnoses of personality disorders: for example, the terms “clusters” and “deferred,” and the symptomatic overlap between antisocial personality disorder (APD) and substance abuse. Least helpful are qualifying phrases added to substance abuse diagnoses, along with an abundance of abbreviations. The latter occurs despite efforts by the U.S. Department of Veteran Affairs and other agencies to standardize acceptable lists of abbreviations. Many imprecisions could qualify for highlighting; here are 5 of the most unhelpful:
Clusters. Personality disorders are grouped into 3 “clusters,” according to similar characteristics (eg, Cluster A includes paranoid, schizoid, and schizotypal personality disorders and focuses on patients’ oddities and eccentricities). The need for identifying “clusters” could be debated, but a “cluster” is not a diagnosis. A psychiatric evaluation that notes “Cluster B traits” in lieu of a specific personality disorder is not informative, especially to a nonpsychiatric clinician. Which Cluster B traits apply? Is the patient unstable? Self-absorbed? Needy? Dramatic? Criminal? Assaultive?
In complicated or ambiguous cases, the diagnosis of a personality disorder not otherwise specified is appropriate, indicating that traits need to be clarified.
Deferred. This notation frequently is seen under axis II, and often is carried through the medical record for months or years. Psychiatrists are reluctant to diagnose a personality disorder because of the pejorative nature a diagnosis conveys. Nevertheless, by the second or third visit—after 2 or 3 hours of interview contact—it should be evident whether a personality disorder exists. If none does, “no diagnosis” should be documented. This notation can be adjusted if such evidence comes to light.
APD (or APD traits). This diagnosis often is made mistakenly when the root problem is in fact a substance abuse disorder. A multi-decade study of alcoholism and antisocial personality attributes in university students illustrated this phenomenon.1
To be a successful substance abuser—that is, to satisfy the overwhelming urge to drink or use drugs—it’s essential to lie, cheat, and steal. Substance abusers might become belligerent when intoxicated. They might be arrested in bar fights, drive while intoxicated, and buy illegal substances. The result is incarceration, a common consequence of substance abuse and of APD. The latter diagnosis should be made only if the patient has exhibited a pattern of criminal behavior—often starting in adolescence—irrespective of substance abuse, such as breaking and entering, robbery, or assault with a deadly weapon.
Substance abusers often feel guilt and self-loathing for their “weakness,” and cannot gain control over their addiction; the APD patient, on the other hand, feels entitled to plunder and often justifies his (her) actions by attributing fault to the victim.
Keep in mind that many APD patients also are substance abusers; both diagnoses should be listed in the chart when that is the determination. Recognize that substance abuse and APD are distinct entities that should not be confused by the common denominator of having spent time in jail.
Early, late, full remission. These qualifiers often are appended to substance abuse disorders, but they do not convey useful information. How early is “early”? How late is “late”? Perhaps the most misleading term is “full” or “partial” remission,
because there is no clear definition of either.
If one is referring to length of time sober or a reduction in volume consumed, noting the date of the last use is more helpful—eg, “alcohol abuse in remission since summer of 2012.” If “partial” remission means the patient has reduced his intake, then that is not remission. The reduction can be specified—eg, “alcohol abuse, reduced to 1 or 2 beers per weekend.”
Abbreviations. Psychiatric evaluations should contain only standard, well-known medical shorthand (such as MSE for mental status exam). The military may be the biggest offender, devising acronyms and abbreviations for everything.
Two examples of abbreviations that I see in military psychiatric progress notes are AEB (“as evidenced by”) and LLGD (“linear, logical, and goal-directed”). Psychiatrists have a leg up on deciphering abbreviations in psychiatric notes; other providers might be compelled to resort to consultation. That wastes more time than typing out the words and results in frustration and lost productivity.
In private practice, government, and (especially) academically affiliated settings, chart notations that are neither erroneous nor accurate but just imprecise are seen regularly. Academic supervisors may overlook these ambiguous notations by medical students and residents because of their regularity; others may be actively taught by supervisors who use ambiguous notations themselves.
In my experience, the most frequently seen imprecisions are in diagnoses of personality disorders: for example, the terms “clusters” and “deferred,” and the symptomatic overlap between antisocial personality disorder (APD) and substance abuse. Least helpful are qualifying phrases added to substance abuse diagnoses, along with an abundance of abbreviations. The latter occurs despite efforts by the U.S. Department of Veteran Affairs and other agencies to standardize acceptable lists of abbreviations. Many imprecisions could qualify for highlighting; here are 5 of the most unhelpful:
Clusters. Personality disorders are grouped into 3 “clusters,” according to similar characteristics (eg, Cluster A includes paranoid, schizoid, and schizotypal personality disorders and focuses on patients’ oddities and eccentricities). The need for identifying “clusters” could be debated, but a “cluster” is not a diagnosis. A psychiatric evaluation that notes “Cluster B traits” in lieu of a specific personality disorder is not informative, especially to a nonpsychiatric clinician. Which Cluster B traits apply? Is the patient unstable? Self-absorbed? Needy? Dramatic? Criminal? Assaultive?
In complicated or ambiguous cases, the diagnosis of a personality disorder not otherwise specified is appropriate, indicating that traits need to be clarified.
Deferred. This notation frequently is seen under axis II, and often is carried through the medical record for months or years. Psychiatrists are reluctant to diagnose a personality disorder because of the pejorative nature a diagnosis conveys. Nevertheless, by the second or third visit—after 2 or 3 hours of interview contact—it should be evident whether a personality disorder exists. If none does, “no diagnosis” should be documented. This notation can be adjusted if such evidence comes to light.
APD (or APD traits). This diagnosis often is made mistakenly when the root problem is in fact a substance abuse disorder. A multi-decade study of alcoholism and antisocial personality attributes in university students illustrated this phenomenon.1
To be a successful substance abuser—that is, to satisfy the overwhelming urge to drink or use drugs—it’s essential to lie, cheat, and steal. Substance abusers might become belligerent when intoxicated. They might be arrested in bar fights, drive while intoxicated, and buy illegal substances. The result is incarceration, a common consequence of substance abuse and of APD. The latter diagnosis should be made only if the patient has exhibited a pattern of criminal behavior—often starting in adolescence—irrespective of substance abuse, such as breaking and entering, robbery, or assault with a deadly weapon.
Substance abusers often feel guilt and self-loathing for their “weakness,” and cannot gain control over their addiction; the APD patient, on the other hand, feels entitled to plunder and often justifies his (her) actions by attributing fault to the victim.
Keep in mind that many APD patients also are substance abusers; both diagnoses should be listed in the chart when that is the determination. Recognize that substance abuse and APD are distinct entities that should not be confused by the common denominator of having spent time in jail.
Early, late, full remission. These qualifiers often are appended to substance abuse disorders, but they do not convey useful information. How early is “early”? How late is “late”? Perhaps the most misleading term is “full” or “partial” remission,
because there is no clear definition of either.
If one is referring to length of time sober or a reduction in volume consumed, noting the date of the last use is more helpful—eg, “alcohol abuse in remission since summer of 2012.” If “partial” remission means the patient has reduced his intake, then that is not remission. The reduction can be specified—eg, “alcohol abuse, reduced to 1 or 2 beers per weekend.”
Abbreviations. Psychiatric evaluations should contain only standard, well-known medical shorthand (such as MSE for mental status exam). The military may be the biggest offender, devising acronyms and abbreviations for everything.
Two examples of abbreviations that I see in military psychiatric progress notes are AEB (“as evidenced by”) and LLGD (“linear, logical, and goal-directed”). Psychiatrists have a leg up on deciphering abbreviations in psychiatric notes; other providers might be compelled to resort to consultation. That wastes more time than typing out the words and results in frustration and lost productivity.
Reference
1. Vaillant GE. The natural history of alcoholism. Cambridge, MA: Harvard University Press; 1983.
Reference
1. Vaillant GE. The natural history of alcoholism. Cambridge, MA: Harvard University Press; 1983.