User login
Rule out pulmonary tuberculosis: Clinical and radiographic clues for the internist
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
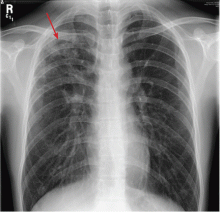
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
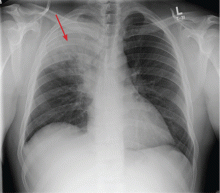
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
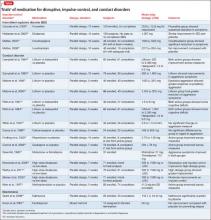
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
KEY POINTS
- Tuberculosis continues to be in the differential diagnosis for patients hospitalized in the United States.
- Clinical, demographic, and radiologic data obtained during the patient’s initial evaluation are helpful in determining the likelihood of tuberculosis.
- Sputum smears for acid-fast bacilli and either skin testing with purified protein derivative or blood testing with an interferon-gamma-release assay continue to be the mainstays of the initial evaluation for pulmonary tuberculosis.
- Nucleic acid amplification testing of sputum or bronchoscopy specimens can provide additional information and should be considered when pulmonary tuberculosis is part of the differential diagnosis.
Enterovirus D68: A clinically important respiratory enterovirus
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
KEY POINTS
- EV-D68 is a respiratory virus that has genetic and biologic features that blur the distinction between the rhinoviruses and enteroviruses.
- Recognition of EV-D68 as an important cause of viral lower respiratory tract illness in children underscores the role of specific strain typing in advancing our understanding of the epidemiology of respiratory virus infections.
- Given the inability of commonly used clinical tests for rhinovirus to distinguish EV-D68 in the absence of strain-specific sequence data, caution needs to be used in attributing severe or acute lower respiratory illness to rhinovirus and in interpreting epidemiologic associations between asthma and rhinovirus.
- Emerging data suggest that, in addition to its important role in pediatric respiratory illness, EV-D68 may cause systemic disease, especially acute neurologic disease.
Inpatient vs Outpatient Hospitalization
Status determinations (outpatient versus inpatient) for hospitalized patients have become a routine part of patient care in the United States. Under the guidance provided by the Medicare Benefits Policy Manual, hospitalized Medicare beneficiaries are assigned 1 of these 2 statuses. The status assignment does not affect the care a patient can receive, but rather how the hospital services provided are billed to Medicare. Hospital services provided under inpatient status are billed under Medicare Part A. Hospital services provided under outpatient status, which includes all patients receiving observation services (commonly referred to as under observation), are billed under Medicare Part B. Whether hospital services are billed under Part A or Part B is important to hospitals and Medicare beneficiaries, as both the hospital reimbursement and beneficiary liability can vary greatly depending on whether services are billed under Part A versus Part B. Hospitals are generally reimbursed at a higher rate for services provided as an inpatient (Part A). The Office of the Inspector General (OIG) recently found that Medicare paid nearly three times more for a short inpatient stay than an [outpatient] stay for the same condition.[1] Medicare beneficiary liability also varies based on status. First, beneficiaries hospitalized as inpatients are subject to a deductible under Part A ($1,216 in 2014) for hospital services associated with that hospitalization and any future inpatient hospitalization beyond 60 days of discharge.[2] Beneficiaries hospitalized as outpatients are subject to the Medicare Part B deductible ($147 in 2014), and then a 20% copay on each individual outpatient hospital service, with no cumulative limit.[2, 3] In addition, hospital pharmacy charges for Medicare beneficiaries hospitalized as inpatients are covered under Medicare A. However, for Medicare patients hospitalized as outpatients, many medications are not covered by Medicare Part B benefits. Finally, time spent hospitalized as an outpatient does not count toward the Medicare 3‐day medically necessary inpatient stay requirement to qualify for the skilled nursing facility care benefit following discharge.
HISTORY AND INTENT OF INPATIENT AND OUTPATIENT STATUS DETERMINATIONS
Prior to October 1, 2013, the Centers for Medicare & Medicaid Services (CMS) stated that physician judgment and an expectation of at least an overnight hospitalization should determine inpatient status of hospitalized Medicare beneficiaries. Guidance as to when inpatient services were covered was found in the Medicare Benefits Policy Manual (MBPM)[4]:
An inpatient is a person who has been admitted to a hospital for bed occupancy for purposes of receiving inpatient hospital services. Generally, a patient is considered an inpatient if formally admitted as inpatient with the expectation that he or she will remain at least overnight and occupy a bed even though it later develops that the patient can be discharged or transferred to another hospital and not actually use a hospital bed overnight. The physician or other practitioner responsible for a patient's care at the hospital is also responsible for deciding whether the patient should be admitted as an inpatient. Physicians should use a 24‐hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis. However, the decision to admit a patient is a complex medical judgment that can be made only after the physician has considered a number of factors, including the patient's medical history and current medical needs, the types of facilities available to inpatients and to outpatients, the hospital's by‐laws and admissions policies, and the relative appropriateness of treatment in each setting.
For a subset of patients who are hospitalized under outpatient status, billing for observation services is allowed. CMS defines observation as a well defined set of services, that should last less than 24 hours and in only rare and exceptional casesspan more than 48 hours.[5] Many providers recognize the utility of a few additional hours of hospital care and/or testing in a hospital setting to determine whether a patient can go home or needs additional evaluation, monitoring, and/or treatment that can only be provided in a hospital, consistent with the CMS definition of observation.[6] It is important to note that although observation and outpatient are frequently used interchangeably, only outpatient is technically a CMS status. Patients in observation or under observation are, in fact, a subset of patients who are hospitalized under an outpatient status.
Outpatient status may also be appropriate for patients who require hospitalization for routine and expected overnight monitoring following a procedure. These patients are often not eligible for billing of observation services or as an inpatient because alternative methods of billing for the recovery time following the procedure exist. When determining the appropriate status of a Medicare beneficiary for a hospitalization following a procedure, physicians need to be aware of whether a specific procedure appears on the Medicare inpatient‐only procedures list.[7] Per CMS, procedures designated as inpatient only are reimbursed only when the patient is admitted as an inpatient at the time the procedure is performed.[8] Conversely, outpatient status for an overnight hospitalization associated with a procedure not on the inpatient‐only list is generally appropriate. Therefore, patients hospitalized for a procedure that appears on this list should always be hospitalized under inpatient status, regardless of the amount of time that the patient is expected to be hospitalized following the procedure, including those cases for which the hospitalization is expected to be only overnight.[7, 8] Only a limited number of Current Procedural Technology (CPT) codes, mostly surgical, automatically qualify for inpatient status and do not have outpatient prospective payment system eligibility. Although most procedures on the inpatient‐only list are associated with a hospitalization that commonly span at least 2 midnights, such as coronary artery bypass grafting, some potentially overnight stay cases, such as cholecystectomy (CPT 47600) appear on the 2014 inpatient‐only list.[9]
As noted above, prior to October 1, 2013, the Medicare definitions governing outpatient versus inpatient status included a 24‐hour benchmark. However, the MBPM also notes that: Admissions of particular patients are not covered or non‐covered solely on the basis of the length of time the patient actually spends in the hospital.[10]
In practice, status determination was ultimately dependent on physician or other practitioner's complex medical judgment as specified by CMS. To validate this judgment, CMS recommended that reviewers use a screening tool as part of their medical review. This screening tool could include practice guidelines that are well accepted by the medical community but did not require or identify a specific criteria set.[11] Not surprisingly, there was and continues to be great variability in the application of outpatient versus inpatient status across hospitals in actual practice.[1, 12, 13] The ambiguity in the definition of a hospitalized patient's status helped spawn commercial clinical decision tools, such as InterQual (McKesson Corporation, San Francisco, CA) and MCG (formally known as Milliman Care Guidelines; MCG Health, LLC, Seattle, WA), to help define inpatients versus outpatients.[14, 15] However, these guidelines are complex, can be difficult to interpret and apply, and have been criticized for poor predictive value and attempting to replace physician judgment.[16, 17, 18] Furthermore, CMS has never formally endorsed any specific decision tool.
INPATIENT AND OUTPATIENT PAYMENTS AND THE RECOVERY AUDIT CONTRACTOR PROGRAM
In 2000, CMS started using Ambulatory Payment Classifications for hospital services, which made inpatient care more financially favorable for hospitals. In response to concerns that hospitals would be incentivized to overuse inpatient status, CMS made a number of changes to their payment system, including the creation of the Recovery Audit Program in 2003. This program was originally called the Recovery Audit Contractor (RAC) Program and continues to be most commonly referred to as the RAC program. The RAC program, tasked with finding and correcting improper claims to the Medicare program, began as a demonstration required in the Medicare Prescription Drug Improvement and Modernization Act of 2003 (MMA), and subsequently became a nationwide audit program under the Tax Relief and Health Care Act of 2006. Under this program, private contractors review hospital and billing records of Medicare patients and are paid on a contingency fee (8%12.5%) for all underpayments and overpayments that are identified and corrected.[19] Importantly, the RACs are not subject to any financial penalties for cases improperly denied.
RACs initially targeted many overnight inpatient stays for recoupment. These cases were attractive audit targets because the RACs could argue that the inpatient hospital services were delivered in the improper status based solely on the length of stay, without having to consider in their audit the complexity of decision making or medical necessity of the services provided. However, it is worth noting that with improvement in efficiency and advancements in medical technology, hospitals and physicians have been increasingly able to safely evaluate and treat medically complex and severely ill patients quickly, sometimes with just an overnight stay. As perspective, in 1965, the average length of stay for a Medicare patient was 13 days; in 2010, it was 5.4 days, with over one‐third of hospitalizations lasting <3 days.[20]
Concurrent with the increased RAC denials for services provided in an inpatient status, the use of observation services changed significantly from 2007 to 2012. The average length of stay for Medicare patients under outpatient status with observation services exceeded 24 hours in 2007, was 28.2 hours by 2009,[21] and grew to 29 hours by 2012.[22] Between July 2010 and December 2011, at the University of Wisconsin Hospital, 1 in 6 observation stays lasted longer than 48 hours, suggesting that long observation stays were no longer rare and exceptional as stated in CMS' own definition.[23] This same University of Wisconsin study also found that observation services were not well defined, with 1141 distinct diagnosis codes used for these services.[23]
Additionally, a Medicare Payment Advisory Commission (MedPAC; described on their website,
Hospitals have also expressed concern that the RAC contingency fee payment model and a lack of penalty for improper denials promotes overzealous auditing.[24, 25] RAC recoupment has increased from approximately $939 million in 2011, to $2.4 billion in 2012, to $3.8 billion in 2013.[26, 27, 28] Given the money now at stake, it is not surprising that hospitals have become very active in appealing RAC denials. Self‐reported data submitted to the American Hospital Association (AHA) for the months January 2014 to March of 2014 show that hospitals now appeal 50% of RAC denials and win 66% of these appeals.[29] The AHA data also show that 69% of self‐reporting hospitals spent over $10,000 to manage their audit and appeals process over this same 3‐month time period, with 11% spending more than $100,000.
This appeals process is not only costly to hospitals, it is also lengthy. As of January 2014, the average wait time for an appeal hearing with an administrative law judge (level 3 appeal) exceeded 16 months.[30] In fact, the appeals process has become so backlogged that hospitals' rights to assignment of level 3 (administrative law judge) appeals have been temporarily suspended.[30] In August 2014, CMS offered a $0.68 on the dollar partial payment for hospitals willing to settle all eligible outstanding appeals in an attempt to relieve the appeals backlog.[31] In addition, the AHA currently has a suit against the US Department of Health & Human Services over the RAC appeals backlog.[32]
Increased use of outpatient status may be driven by pressures from the RAC program and, potentially, by improvements in the efficiency of care. Because hospitals are paid less for care provided under outpatient status than they are for the identical care provided under inpatient status, hospitals faced both potential financial penalty for improvements in efficiency and the threat of RAC audits.
THE 2‐MIDNIGHT RULE: A FIX?
Given the challenges in defining inpatient versus outpatient hospitalization, the increasing use of outpatient status and the increasing length of stay of outpatient hospitalizations with observation services, in 2013, CMS responded with new policies to define the visit status for hospitalized patients. On August 2, 2013, CMS announced the fiscal year 2014 hospital Inpatient Prospective Payment System final rule (IPPS‐2014) to become effective October 1, 2013. This document was formally issued as part of the Federal Register on August 19, 2013.[33] Central to the CMS IPPS‐2014 was a 2‐midnight benchmark that offered a major change in how physicians were to determine the status (inpatient vs outpatient) of hospitalized patients. With this 2‐midnight benchmark, now informally known as the 2‐midnight rule, CMS finalized its proposal to generally consider patients that are expected by a practitioner (with knowledge of the case and with admitting privileges) to need hospitalization that will span 2 or more midnights as inpatient. The IPPS‐2014 also finalized the converse of this: hospitalizations expected to span <2 midnights are to be regarded as outpatient with 2 exceptions:
- If the hospitalization is associated with a procedure appearing on the previously described Medicare inpatient‐only procedures list, or
- A rare and unusual circumstance in which an inpatient admission would be reasonable regardless of length of stay. Currently, unanticipated mechanical ventilation initiated during the hospitalization visit is the only rare and unusual circumstance that qualifies as such an exception.[7]
CMS' stated goals and expectations for the 2‐midnight benchmark were:
- Reduce the growing number of prolonged hospitalizations (>48 hours) for Medicare beneficiaries under outpatient status.
- Decrease billing disputes between hospitals and Medicare auditors, especially RACs, by establishing more clearly defined, time‐based status criteria.
- Reduce the number of outpatient encounters overall. Because CMS expected the rule to convert a net increase of cases from outpatient to inpatient, resulting in higher payments to hospitals, CMS included a 0.2% payment cut in hospital reimbursement in the IPPS‐2014 as an offset.[33, 34]
Although unrelated to the goals and expectations above, the IPPS‐2014 also included a requirement that:
[T]he order [for inpatient admission] must be furnished by a qualified and licensed practitioner who has admitting privileges at the hospital as permitted by State law, and who is knowledgeable about the patient's hospital course, medical plan of care and current condition.
CMS allowed for an authentication (generally regarded as a cosignature that is timed and dated) of the inpatient admission order by an attending physician with admitting privileges, done prior to discharge, in cases where the inpatient order had been placed by a practitioner (such as a resident, fellow, or physician assistant) without admitting privileges. Attending physician authentication of the inpatient admission order must be done prior to discharge [a]s a condition of payment for hospital inpatient services under Medicare Part A.[35]
From the August 2, 2013 announcement until the effective date of October 1, 2013, hospitals had just 2 months to interpret and comply with the IPPS‐2014, a complex 546‐page document that required hospitals to make extensive changes to admission procedures, workflows, and electronic health records (EHRs). In addition, extensive physician, provider, and administrator education was needed. During these 2 months, hospitals continued to request additional information and clarification from CMS regarding many aspects of the IPPS‐2014, including basic questions that included (1) how to apply the 2‐midnight benchmark to patients who were transferred from 1 hospital to another and (2) when the clock started for hospital services in determining a patient's expected length of hospitalization.
Despite concerns voiced by Congress and medical organizations, the new policy went into effect as scheduled.[36, 37] However, just days prior to October 1, 2013, CMS issued a 3‐month limited suspension of auditing and enforcement of the 2‐midnight rule by the RACs that was subsequently extended by CMS 2 more times, first through March 31, 2014 and then again through September 30, 2014. Other audits to be performed by RACs and all other government audits, including those performed by Medicare Administrative Contractors (MACs) were allowed to continue.[38] In particular, the MACs were instructed to conduct patient status reviews using a probe and educate strategy, which, via educational outreach efforts, would instruct hospitals how to adapt to the new rule. On April 1, 2014, the Protecting Access to Medicare Act of 2014 was signed into law, which, under section 111 of this law, permitted CMS to continue medical review activities under the MAC probe and educate process through March of 2015, and prohibited CMS from allowing RACs to conduct inpatient hospital status reviews on claims with these same dates of admission, October 1, 2013 through March 31, 2015.
The MACs were created by the MMA of 2003, which mandated that the Secretary of Health & Human Services replace Part A Fiscal Intermediaries and Part B carriers with Medicare Administrative Contractors (MACs).[39] As established by CMS, MACs are multi‐state, regional contractors responsible for administering both Medicare Part A and Medicare Part B claims and serve as the primary operational contact between the Medicare Fee‐For‐Service program, and approximately 1.5 million health care providers enrolled in the program.[39]
THE IPPS‐2014 AND CMS' STATED GOALS AND EXPECTATIONS
In the analysis that accompanied the IPPS‐2014, Medicare expected the use of outpatient services to decrease overall, as the new rules would effectively eliminate almost all outpatient hospitalizations >48 hours. Although no official data are yet available from CMS, our early experience under the 2‐midnight rule has suggested that long observation stays have declined in frequency, a favorable outcome of the new policy. However, as designed, the new 2‐midnight IPPS rule most predominately affects 1‐day stays, or more accurately, 1‐midnight stays. This is because many hospitalizations that previously met inpatient criteria (as defined by commercially available products such as MCG or InterQual), but spanned <2 midnights would have been classified as inpatient prior to October 1, 2013. However, since October 1, 2013, these same hospitalizations are now classified as outpatient. An example of such a case is a patient who presents to an emergency department with symptoms of a transient ischemic attack and has a high ABCD (age 60 years, blood pressure 140/90 mm Hg at initial evaluation, clinical features, duration of symptoms, diabetes score).[40] Prior to the 2‐midnight rule, this patient, based on the severity of the signs and symptoms upon presentation, could have been appropriately hospitalized as an inpatient.
Now, under the current IPPS and the ability of many hospitals to efficiently evaluate and treat such patient in <2 midnights, the patient should be categorized as an outpatient, at least initially, despite the severity and high risk of his/her presentation. In fiscal year 2013, The Johns Hopkins Hospital had 1791, 1‐day inpatient stays for Medicare beneficiaries, representing 15.2% of all Medicare admissions. Similarly, in the 12 months just prior to the 2‐midnight rule (October 1, 2012 to September 30, 2013), 10.4% (1280) of all Medicare encounters at the University of Wisconsin were 1‐day inpatient stays under previous criteria. Because of implementation of the 2‐midnight rule in October 2013, Medicare outpatient hospitalization for 1‐day stays at The Johns Hopkins Hospital increased by 49%, from an average of 117 patients/month to 174 patients/month. Nationally, it is possible that a reduction in long observation stays could be offset by an increase in 1‐day‐stay outpatient hospitalization encounters.
A second key expectation and goal of IPPS‐2014 was, by shifting to a more concrete, time‐based definition of inpatient, to decrease the disagreement between hospitals and auditors regarding patient status (inpatient vs outpatient). As noted earlier, many disputes with auditors for hospitalizations prior to October 2013 did not involve the need or type of hospital services provided, but rather the status under which the care was provided. However, the new time‐based criterion hinges not on actual length of hospitalization, but the expected length of hospitalization as determined by a practitioner with admitting privileges and knowledge of the patient. Accurately and consistently predicting the length of hospitalization has proven to be challenging, even for the most experienced practitioners. Since October 2013, for patients hospitalized at The Johns Hopkins Hospital through its emergency department, the admitting physicians' expectation of whether a patient would require 1 versus 2 or more midnights of necessary hospitalization was correct only half of the time. Given past experience, the RACs may challenge the medical judgment that lead practitioners to expect a hospitalization of 2 or more midnights without having to challenge whether the care provided was medically necessary.
Further, the IPPS‐2014 has not been accompanied by any significant changes to the payment scheme for auditors. RACs continue to be paid a percentage of any monies they determine to have been improperly paid by CMS, but with no penalty for cases that are overturned on appeal. Historically, the vast majority of RAC recovery fees have been due to determination of overpayments by CMS.[41, 42] Despite the 2‐midnight rule, RACs will continue to have a financial incentive to allege overpayment. In the initial probe and educate audits by MACs under the new 2014‐IPPS, despite inpatient admission orders being authenticated and certified by an attending physician, claims are being denied because the documentation does not support an expectation for a 2‐midnight hospitalization. Namely, auditors are continuing to challenge not the medical necessity of the services that hospitals provide, but rather the status in which those services were provided. Thus far, the IPPS‐2014 does not appear to fully remedy the auditing conflict that existed prior to October 2013.
As noted above, the IPPS‐2014 also requires, as of October 1, 2013, as a condition of payment for hospital services under Part A, that the inpatient admission order must be either entered by a practitioner with admitting privileges or authenticated prior to discharge by an attending physician involved in the care of the patient in cases in which the inpatient admission order was entered by a practitioner without admitting privileges (eg, resident, physician assistant, or fellow).[43] The requirement of an attending physician's cosignature has involved major changes to physician workflow and the electronic heath record (EHR) framework at The Johns Hopkins and the University of Wisconsin Hospitals, and does not keep up with modern healthcare systems in which patients are admitted 24 hours a day by a variety of providers (eg, residents, nurse practitioners) who otherwise may write stand‐alone orders. These changes have proven to be time‐consuming, costly, and have not, to our knowledge, improved patient care or utilization of resources.
The new visit status rules have also led to confusion among clinicians. A recent large survey of hospitalists conducted by the Society of Hospital Medicine demonstrated that more than half of respondents disagreed that the 2‐midnight rule improved hospitalist workflow compared to prior observation policy.[44] In addition, only 40% of hospitalists reported confidence in how to apply the rule.[44] Thus, the intent to clarify visit status policy with the IPPS‐2014 has not translated to clear and useful rules for frontline clinicians.
FUTURE DIRECTIONS
After over a year under the 2‐midnight rule, although long observation stays may be reduced, it seems unlikely these new regulations will achieve 2 of CMS' stated goals: (1) decreasing the use of outpatient status for hospitalizations and (2) resolving status disputes between auditors and hospitals. In addition, attempts at compliance with the new rules and regulations have diverted large amounts of physician time and hospital resources away from patient care. There is a clear need to reform both the hospitalization status policy and the RAC programs that enforce these rules.
One path Congress and CMS could consider is to reform the current Medicare reimbursement paradigm for hospital services to eliminate the need to distinguish inpatient from outpatient status. For example, H.R. 1179Improving Access to Medicare Coverage Act of 2013,[45] of the 113th Congress, if reintroduced, would decouple the link between the qualification for skilled nursing facility benefits from visit status by allowing time spent hospitalized as an outpatient to count toward the 3‐day benchmark. The overarching goals of any visit status policy reform should be to: (1) simplify or eliminate the 2‐track status process for hospitalized patients, (2) stop or minimize the threat of audits based on status, and (3) maintain budget neutrality. Two additional options for consideration would be to: (1) create a low‐acuity modifier for use with patients anticipated to have short stays and low resource use and (2) preselect specific Diagnosis Related Groups based on historical data and create designations for those diagnoses of lesser intensity. Accountable care organizations contracts, a new model for healthcare payment, could potentially be structured to eliminate or simplify payment based on visit status for hospitalized patients. With bundled payments on the horizon and the possible phase‐out of fee‐for‐service reimbursement, the issue may become less paramount in the coming years. No solution will be perfect and must balance costs, ease of administration, and beneficiary protection.
There are reasons to be optimistic that change may soon be realized. CMS is currently considering significant hospitalization status policy reform. In the proposed IPPS‐2015, CMS asked for input on payment for short‐stay hospitalizations and, in the final IPPS‐2015 released August 4, 2014, CMS indicated its willingness to continue to work with stakeholders in revising these policies.[46] Additionally, CMS has responded to hospitals on 3 separate occasions by delaying RAC audits pertaining to the 2‐midnight rule. Further, the current MAC probe and educate audits focus on education with respect to 2‐midnight rule implementation rather than threatening hospitals with major financial penalties.[47] Congress has also been responsive in this area. In addition to the 3 delays announced by CMS, Congress passed legislation that mandated an additional delay to RAC audits that pertain to the 2‐midnight rule. Moreover, the Subcommittee on Health of the House Ways and Means Committee held hearings that included the 2‐midnight rule and RAC reform in May 2014, and the Senate Special Committee on Aging held hearings on the impact of visit status on Medicare beneficiaries in July 2014.[48, 49] Additionally, the House Ways and Means Health Subcommittee recently issued a draft bill to address Medicare hospital issues.[50] The OIG has also been responsive to hospital concerns regarding the current RAC program with a recent report recommending that CMS develop additional performance evaluation metrics to improve RAC performance and ensure that RACs are evaluated on all contract requirements.[51] Additionally, MedPAC has been considering several short‐stay payment reform options, modifying the need for a 3‐day inpatient hospitalization to qualify for postdischarge skilled nursing facility benefits and adjusting RAC contingency fees based on overturn rates.[52, 53] These actions by CMS, Congress, and the OIG, as well as the options under consideration by MedPAC, demonstrate a degree of regulatory and legislative responsiveness to hospital and provider concerns in the area of visit status determination.
The Medicare program is vital to tens of millions of disabled and elderly Americans. Fraud and abuse of the Medicare program should not be tolerated. Yet, the current system of assigning, monitoring, and auditing outpatient versus inpatient hospital care is in need of reform. It will be up to CMS and Congress to continue to work with hospitals and physicians to find an improved way to appropriately and fairly compensate hospitals for hospital services in a way that that does not depend on a poorly defined and contentious status of a patient. Such reform must include the RAC program. It is our hope that both CMS and Congress will prioritize status determination and payment reform so that Medicare beneficiaries, physicians, and hospitals all have a sustainable, fair, and transparent process.
- Testimony of Jodi D Nudelman, Regional Inspector General for the Office of Evaluation and Inspections, Office of the Inspector General, US Department of Health and Human Services, Hearing: Current Hospital Issues in the Medicare Program, House Committee on Ways and Means, Subcommittee on Health, May 20, 2014. Available at: https://oig.hhs.gov/newsroom/testimony‐and‐speeches/index.asp. Accessed November 24, 2014.
- Centers for Medicare 173:1999–2000.
- US Department of Health 49:893–909.
- US Department of Health 28:95–111.
- . The price of admission: increasing use of decision‐support technology draws criticism for changing roles in hospital‐admissions process. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20121117/MAGAZINE/311179951. Published November 17, 2012. Accessed November 9, 2014.
- , , , et al. The accuracy of interqual criteria in determining the need for observation versus hospitalization in emergency department patients with chronic heart failure. Crit Pathw Cardiol. 2013;12:192–196.
- US Department of Health 31:1251–1259.
- MedPAC March 2104 Report to the Congress, Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed on December 22, 2014.
- , , , et al, Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173:1991–1998.
- . The recovery audit contractor program and observation status for hospitalized Medicare beneficiaries. JAMA Internal Medicine blog. Available at: http://internalmedicineblog.jamainternalmed.com/2014/02/04/the‐recovery‐audit‐contractor‐program‐and‐observation‐status‐for‐hospitalized‐medicare‐beneficiaries. Published February 4, 2014. Accessed June 15, 2014.
- . Broken RAC system continues to hurt patients, providers. The Hospital Leader blog. Available at: http://blogs.hospitalmedicine.org/Blog/broken‐rac‐system‐continues‐to‐hurt‐patients‐providers. Published April 22, 2014. Accessed June 15, 2014.
- US Department of Health 78(160). Available at: http://www.gpo.gov/fdsys/pkg/FR‐2013‐08‐19/pdf/2013‐18956.pdf. Accessed August 4, 2014.
- US Department of Health use of observation and inpatient stays for Medicare beneficiaries, OEI‐02‐12‐00040. Available at: http://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Accessed June 15, 2014.
- US Department of Health
Status determinations (outpatient versus inpatient) for hospitalized patients have become a routine part of patient care in the United States. Under the guidance provided by the Medicare Benefits Policy Manual, hospitalized Medicare beneficiaries are assigned 1 of these 2 statuses. The status assignment does not affect the care a patient can receive, but rather how the hospital services provided are billed to Medicare. Hospital services provided under inpatient status are billed under Medicare Part A. Hospital services provided under outpatient status, which includes all patients receiving observation services (commonly referred to as under observation), are billed under Medicare Part B. Whether hospital services are billed under Part A or Part B is important to hospitals and Medicare beneficiaries, as both the hospital reimbursement and beneficiary liability can vary greatly depending on whether services are billed under Part A versus Part B. Hospitals are generally reimbursed at a higher rate for services provided as an inpatient (Part A). The Office of the Inspector General (OIG) recently found that Medicare paid nearly three times more for a short inpatient stay than an [outpatient] stay for the same condition.[1] Medicare beneficiary liability also varies based on status. First, beneficiaries hospitalized as inpatients are subject to a deductible under Part A ($1,216 in 2014) for hospital services associated with that hospitalization and any future inpatient hospitalization beyond 60 days of discharge.[2] Beneficiaries hospitalized as outpatients are subject to the Medicare Part B deductible ($147 in 2014), and then a 20% copay on each individual outpatient hospital service, with no cumulative limit.[2, 3] In addition, hospital pharmacy charges for Medicare beneficiaries hospitalized as inpatients are covered under Medicare A. However, for Medicare patients hospitalized as outpatients, many medications are not covered by Medicare Part B benefits. Finally, time spent hospitalized as an outpatient does not count toward the Medicare 3‐day medically necessary inpatient stay requirement to qualify for the skilled nursing facility care benefit following discharge.
HISTORY AND INTENT OF INPATIENT AND OUTPATIENT STATUS DETERMINATIONS
Prior to October 1, 2013, the Centers for Medicare & Medicaid Services (CMS) stated that physician judgment and an expectation of at least an overnight hospitalization should determine inpatient status of hospitalized Medicare beneficiaries. Guidance as to when inpatient services were covered was found in the Medicare Benefits Policy Manual (MBPM)[4]:
An inpatient is a person who has been admitted to a hospital for bed occupancy for purposes of receiving inpatient hospital services. Generally, a patient is considered an inpatient if formally admitted as inpatient with the expectation that he or she will remain at least overnight and occupy a bed even though it later develops that the patient can be discharged or transferred to another hospital and not actually use a hospital bed overnight. The physician or other practitioner responsible for a patient's care at the hospital is also responsible for deciding whether the patient should be admitted as an inpatient. Physicians should use a 24‐hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis. However, the decision to admit a patient is a complex medical judgment that can be made only after the physician has considered a number of factors, including the patient's medical history and current medical needs, the types of facilities available to inpatients and to outpatients, the hospital's by‐laws and admissions policies, and the relative appropriateness of treatment in each setting.
For a subset of patients who are hospitalized under outpatient status, billing for observation services is allowed. CMS defines observation as a well defined set of services, that should last less than 24 hours and in only rare and exceptional casesspan more than 48 hours.[5] Many providers recognize the utility of a few additional hours of hospital care and/or testing in a hospital setting to determine whether a patient can go home or needs additional evaluation, monitoring, and/or treatment that can only be provided in a hospital, consistent with the CMS definition of observation.[6] It is important to note that although observation and outpatient are frequently used interchangeably, only outpatient is technically a CMS status. Patients in observation or under observation are, in fact, a subset of patients who are hospitalized under an outpatient status.
Outpatient status may also be appropriate for patients who require hospitalization for routine and expected overnight monitoring following a procedure. These patients are often not eligible for billing of observation services or as an inpatient because alternative methods of billing for the recovery time following the procedure exist. When determining the appropriate status of a Medicare beneficiary for a hospitalization following a procedure, physicians need to be aware of whether a specific procedure appears on the Medicare inpatient‐only procedures list.[7] Per CMS, procedures designated as inpatient only are reimbursed only when the patient is admitted as an inpatient at the time the procedure is performed.[8] Conversely, outpatient status for an overnight hospitalization associated with a procedure not on the inpatient‐only list is generally appropriate. Therefore, patients hospitalized for a procedure that appears on this list should always be hospitalized under inpatient status, regardless of the amount of time that the patient is expected to be hospitalized following the procedure, including those cases for which the hospitalization is expected to be only overnight.[7, 8] Only a limited number of Current Procedural Technology (CPT) codes, mostly surgical, automatically qualify for inpatient status and do not have outpatient prospective payment system eligibility. Although most procedures on the inpatient‐only list are associated with a hospitalization that commonly span at least 2 midnights, such as coronary artery bypass grafting, some potentially overnight stay cases, such as cholecystectomy (CPT 47600) appear on the 2014 inpatient‐only list.[9]
As noted above, prior to October 1, 2013, the Medicare definitions governing outpatient versus inpatient status included a 24‐hour benchmark. However, the MBPM also notes that: Admissions of particular patients are not covered or non‐covered solely on the basis of the length of time the patient actually spends in the hospital.[10]
In practice, status determination was ultimately dependent on physician or other practitioner's complex medical judgment as specified by CMS. To validate this judgment, CMS recommended that reviewers use a screening tool as part of their medical review. This screening tool could include practice guidelines that are well accepted by the medical community but did not require or identify a specific criteria set.[11] Not surprisingly, there was and continues to be great variability in the application of outpatient versus inpatient status across hospitals in actual practice.[1, 12, 13] The ambiguity in the definition of a hospitalized patient's status helped spawn commercial clinical decision tools, such as InterQual (McKesson Corporation, San Francisco, CA) and MCG (formally known as Milliman Care Guidelines; MCG Health, LLC, Seattle, WA), to help define inpatients versus outpatients.[14, 15] However, these guidelines are complex, can be difficult to interpret and apply, and have been criticized for poor predictive value and attempting to replace physician judgment.[16, 17, 18] Furthermore, CMS has never formally endorsed any specific decision tool.
INPATIENT AND OUTPATIENT PAYMENTS AND THE RECOVERY AUDIT CONTRACTOR PROGRAM
In 2000, CMS started using Ambulatory Payment Classifications for hospital services, which made inpatient care more financially favorable for hospitals. In response to concerns that hospitals would be incentivized to overuse inpatient status, CMS made a number of changes to their payment system, including the creation of the Recovery Audit Program in 2003. This program was originally called the Recovery Audit Contractor (RAC) Program and continues to be most commonly referred to as the RAC program. The RAC program, tasked with finding and correcting improper claims to the Medicare program, began as a demonstration required in the Medicare Prescription Drug Improvement and Modernization Act of 2003 (MMA), and subsequently became a nationwide audit program under the Tax Relief and Health Care Act of 2006. Under this program, private contractors review hospital and billing records of Medicare patients and are paid on a contingency fee (8%12.5%) for all underpayments and overpayments that are identified and corrected.[19] Importantly, the RACs are not subject to any financial penalties for cases improperly denied.
RACs initially targeted many overnight inpatient stays for recoupment. These cases were attractive audit targets because the RACs could argue that the inpatient hospital services were delivered in the improper status based solely on the length of stay, without having to consider in their audit the complexity of decision making or medical necessity of the services provided. However, it is worth noting that with improvement in efficiency and advancements in medical technology, hospitals and physicians have been increasingly able to safely evaluate and treat medically complex and severely ill patients quickly, sometimes with just an overnight stay. As perspective, in 1965, the average length of stay for a Medicare patient was 13 days; in 2010, it was 5.4 days, with over one‐third of hospitalizations lasting <3 days.[20]
Concurrent with the increased RAC denials for services provided in an inpatient status, the use of observation services changed significantly from 2007 to 2012. The average length of stay for Medicare patients under outpatient status with observation services exceeded 24 hours in 2007, was 28.2 hours by 2009,[21] and grew to 29 hours by 2012.[22] Between July 2010 and December 2011, at the University of Wisconsin Hospital, 1 in 6 observation stays lasted longer than 48 hours, suggesting that long observation stays were no longer rare and exceptional as stated in CMS' own definition.[23] This same University of Wisconsin study also found that observation services were not well defined, with 1141 distinct diagnosis codes used for these services.[23]
Additionally, a Medicare Payment Advisory Commission (MedPAC; described on their website,
Hospitals have also expressed concern that the RAC contingency fee payment model and a lack of penalty for improper denials promotes overzealous auditing.[24, 25] RAC recoupment has increased from approximately $939 million in 2011, to $2.4 billion in 2012, to $3.8 billion in 2013.[26, 27, 28] Given the money now at stake, it is not surprising that hospitals have become very active in appealing RAC denials. Self‐reported data submitted to the American Hospital Association (AHA) for the months January 2014 to March of 2014 show that hospitals now appeal 50% of RAC denials and win 66% of these appeals.[29] The AHA data also show that 69% of self‐reporting hospitals spent over $10,000 to manage their audit and appeals process over this same 3‐month time period, with 11% spending more than $100,000.
This appeals process is not only costly to hospitals, it is also lengthy. As of January 2014, the average wait time for an appeal hearing with an administrative law judge (level 3 appeal) exceeded 16 months.[30] In fact, the appeals process has become so backlogged that hospitals' rights to assignment of level 3 (administrative law judge) appeals have been temporarily suspended.[30] In August 2014, CMS offered a $0.68 on the dollar partial payment for hospitals willing to settle all eligible outstanding appeals in an attempt to relieve the appeals backlog.[31] In addition, the AHA currently has a suit against the US Department of Health & Human Services over the RAC appeals backlog.[32]
Increased use of outpatient status may be driven by pressures from the RAC program and, potentially, by improvements in the efficiency of care. Because hospitals are paid less for care provided under outpatient status than they are for the identical care provided under inpatient status, hospitals faced both potential financial penalty for improvements in efficiency and the threat of RAC audits.
THE 2‐MIDNIGHT RULE: A FIX?
Given the challenges in defining inpatient versus outpatient hospitalization, the increasing use of outpatient status and the increasing length of stay of outpatient hospitalizations with observation services, in 2013, CMS responded with new policies to define the visit status for hospitalized patients. On August 2, 2013, CMS announced the fiscal year 2014 hospital Inpatient Prospective Payment System final rule (IPPS‐2014) to become effective October 1, 2013. This document was formally issued as part of the Federal Register on August 19, 2013.[33] Central to the CMS IPPS‐2014 was a 2‐midnight benchmark that offered a major change in how physicians were to determine the status (inpatient vs outpatient) of hospitalized patients. With this 2‐midnight benchmark, now informally known as the 2‐midnight rule, CMS finalized its proposal to generally consider patients that are expected by a practitioner (with knowledge of the case and with admitting privileges) to need hospitalization that will span 2 or more midnights as inpatient. The IPPS‐2014 also finalized the converse of this: hospitalizations expected to span <2 midnights are to be regarded as outpatient with 2 exceptions:
- If the hospitalization is associated with a procedure appearing on the previously described Medicare inpatient‐only procedures list, or
- A rare and unusual circumstance in which an inpatient admission would be reasonable regardless of length of stay. Currently, unanticipated mechanical ventilation initiated during the hospitalization visit is the only rare and unusual circumstance that qualifies as such an exception.[7]
CMS' stated goals and expectations for the 2‐midnight benchmark were:
- Reduce the growing number of prolonged hospitalizations (>48 hours) for Medicare beneficiaries under outpatient status.
- Decrease billing disputes between hospitals and Medicare auditors, especially RACs, by establishing more clearly defined, time‐based status criteria.
- Reduce the number of outpatient encounters overall. Because CMS expected the rule to convert a net increase of cases from outpatient to inpatient, resulting in higher payments to hospitals, CMS included a 0.2% payment cut in hospital reimbursement in the IPPS‐2014 as an offset.[33, 34]
Although unrelated to the goals and expectations above, the IPPS‐2014 also included a requirement that:
[T]he order [for inpatient admission] must be furnished by a qualified and licensed practitioner who has admitting privileges at the hospital as permitted by State law, and who is knowledgeable about the patient's hospital course, medical plan of care and current condition.
CMS allowed for an authentication (generally regarded as a cosignature that is timed and dated) of the inpatient admission order by an attending physician with admitting privileges, done prior to discharge, in cases where the inpatient order had been placed by a practitioner (such as a resident, fellow, or physician assistant) without admitting privileges. Attending physician authentication of the inpatient admission order must be done prior to discharge [a]s a condition of payment for hospital inpatient services under Medicare Part A.[35]
From the August 2, 2013 announcement until the effective date of October 1, 2013, hospitals had just 2 months to interpret and comply with the IPPS‐2014, a complex 546‐page document that required hospitals to make extensive changes to admission procedures, workflows, and electronic health records (EHRs). In addition, extensive physician, provider, and administrator education was needed. During these 2 months, hospitals continued to request additional information and clarification from CMS regarding many aspects of the IPPS‐2014, including basic questions that included (1) how to apply the 2‐midnight benchmark to patients who were transferred from 1 hospital to another and (2) when the clock started for hospital services in determining a patient's expected length of hospitalization.
Despite concerns voiced by Congress and medical organizations, the new policy went into effect as scheduled.[36, 37] However, just days prior to October 1, 2013, CMS issued a 3‐month limited suspension of auditing and enforcement of the 2‐midnight rule by the RACs that was subsequently extended by CMS 2 more times, first through March 31, 2014 and then again through September 30, 2014. Other audits to be performed by RACs and all other government audits, including those performed by Medicare Administrative Contractors (MACs) were allowed to continue.[38] In particular, the MACs were instructed to conduct patient status reviews using a probe and educate strategy, which, via educational outreach efforts, would instruct hospitals how to adapt to the new rule. On April 1, 2014, the Protecting Access to Medicare Act of 2014 was signed into law, which, under section 111 of this law, permitted CMS to continue medical review activities under the MAC probe and educate process through March of 2015, and prohibited CMS from allowing RACs to conduct inpatient hospital status reviews on claims with these same dates of admission, October 1, 2013 through March 31, 2015.
The MACs were created by the MMA of 2003, which mandated that the Secretary of Health & Human Services replace Part A Fiscal Intermediaries and Part B carriers with Medicare Administrative Contractors (MACs).[39] As established by CMS, MACs are multi‐state, regional contractors responsible for administering both Medicare Part A and Medicare Part B claims and serve as the primary operational contact between the Medicare Fee‐For‐Service program, and approximately 1.5 million health care providers enrolled in the program.[39]
THE IPPS‐2014 AND CMS' STATED GOALS AND EXPECTATIONS
In the analysis that accompanied the IPPS‐2014, Medicare expected the use of outpatient services to decrease overall, as the new rules would effectively eliminate almost all outpatient hospitalizations >48 hours. Although no official data are yet available from CMS, our early experience under the 2‐midnight rule has suggested that long observation stays have declined in frequency, a favorable outcome of the new policy. However, as designed, the new 2‐midnight IPPS rule most predominately affects 1‐day stays, or more accurately, 1‐midnight stays. This is because many hospitalizations that previously met inpatient criteria (as defined by commercially available products such as MCG or InterQual), but spanned <2 midnights would have been classified as inpatient prior to October 1, 2013. However, since October 1, 2013, these same hospitalizations are now classified as outpatient. An example of such a case is a patient who presents to an emergency department with symptoms of a transient ischemic attack and has a high ABCD (age 60 years, blood pressure 140/90 mm Hg at initial evaluation, clinical features, duration of symptoms, diabetes score).[40] Prior to the 2‐midnight rule, this patient, based on the severity of the signs and symptoms upon presentation, could have been appropriately hospitalized as an inpatient.
Now, under the current IPPS and the ability of many hospitals to efficiently evaluate and treat such patient in <2 midnights, the patient should be categorized as an outpatient, at least initially, despite the severity and high risk of his/her presentation. In fiscal year 2013, The Johns Hopkins Hospital had 1791, 1‐day inpatient stays for Medicare beneficiaries, representing 15.2% of all Medicare admissions. Similarly, in the 12 months just prior to the 2‐midnight rule (October 1, 2012 to September 30, 2013), 10.4% (1280) of all Medicare encounters at the University of Wisconsin were 1‐day inpatient stays under previous criteria. Because of implementation of the 2‐midnight rule in October 2013, Medicare outpatient hospitalization for 1‐day stays at The Johns Hopkins Hospital increased by 49%, from an average of 117 patients/month to 174 patients/month. Nationally, it is possible that a reduction in long observation stays could be offset by an increase in 1‐day‐stay outpatient hospitalization encounters.
A second key expectation and goal of IPPS‐2014 was, by shifting to a more concrete, time‐based definition of inpatient, to decrease the disagreement between hospitals and auditors regarding patient status (inpatient vs outpatient). As noted earlier, many disputes with auditors for hospitalizations prior to October 2013 did not involve the need or type of hospital services provided, but rather the status under which the care was provided. However, the new time‐based criterion hinges not on actual length of hospitalization, but the expected length of hospitalization as determined by a practitioner with admitting privileges and knowledge of the patient. Accurately and consistently predicting the length of hospitalization has proven to be challenging, even for the most experienced practitioners. Since October 2013, for patients hospitalized at The Johns Hopkins Hospital through its emergency department, the admitting physicians' expectation of whether a patient would require 1 versus 2 or more midnights of necessary hospitalization was correct only half of the time. Given past experience, the RACs may challenge the medical judgment that lead practitioners to expect a hospitalization of 2 or more midnights without having to challenge whether the care provided was medically necessary.
Further, the IPPS‐2014 has not been accompanied by any significant changes to the payment scheme for auditors. RACs continue to be paid a percentage of any monies they determine to have been improperly paid by CMS, but with no penalty for cases that are overturned on appeal. Historically, the vast majority of RAC recovery fees have been due to determination of overpayments by CMS.[41, 42] Despite the 2‐midnight rule, RACs will continue to have a financial incentive to allege overpayment. In the initial probe and educate audits by MACs under the new 2014‐IPPS, despite inpatient admission orders being authenticated and certified by an attending physician, claims are being denied because the documentation does not support an expectation for a 2‐midnight hospitalization. Namely, auditors are continuing to challenge not the medical necessity of the services that hospitals provide, but rather the status in which those services were provided. Thus far, the IPPS‐2014 does not appear to fully remedy the auditing conflict that existed prior to October 2013.
As noted above, the IPPS‐2014 also requires, as of October 1, 2013, as a condition of payment for hospital services under Part A, that the inpatient admission order must be either entered by a practitioner with admitting privileges or authenticated prior to discharge by an attending physician involved in the care of the patient in cases in which the inpatient admission order was entered by a practitioner without admitting privileges (eg, resident, physician assistant, or fellow).[43] The requirement of an attending physician's cosignature has involved major changes to physician workflow and the electronic heath record (EHR) framework at The Johns Hopkins and the University of Wisconsin Hospitals, and does not keep up with modern healthcare systems in which patients are admitted 24 hours a day by a variety of providers (eg, residents, nurse practitioners) who otherwise may write stand‐alone orders. These changes have proven to be time‐consuming, costly, and have not, to our knowledge, improved patient care or utilization of resources.
The new visit status rules have also led to confusion among clinicians. A recent large survey of hospitalists conducted by the Society of Hospital Medicine demonstrated that more than half of respondents disagreed that the 2‐midnight rule improved hospitalist workflow compared to prior observation policy.[44] In addition, only 40% of hospitalists reported confidence in how to apply the rule.[44] Thus, the intent to clarify visit status policy with the IPPS‐2014 has not translated to clear and useful rules for frontline clinicians.
FUTURE DIRECTIONS
After over a year under the 2‐midnight rule, although long observation stays may be reduced, it seems unlikely these new regulations will achieve 2 of CMS' stated goals: (1) decreasing the use of outpatient status for hospitalizations and (2) resolving status disputes between auditors and hospitals. In addition, attempts at compliance with the new rules and regulations have diverted large amounts of physician time and hospital resources away from patient care. There is a clear need to reform both the hospitalization status policy and the RAC programs that enforce these rules.
One path Congress and CMS could consider is to reform the current Medicare reimbursement paradigm for hospital services to eliminate the need to distinguish inpatient from outpatient status. For example, H.R. 1179Improving Access to Medicare Coverage Act of 2013,[45] of the 113th Congress, if reintroduced, would decouple the link between the qualification for skilled nursing facility benefits from visit status by allowing time spent hospitalized as an outpatient to count toward the 3‐day benchmark. The overarching goals of any visit status policy reform should be to: (1) simplify or eliminate the 2‐track status process for hospitalized patients, (2) stop or minimize the threat of audits based on status, and (3) maintain budget neutrality. Two additional options for consideration would be to: (1) create a low‐acuity modifier for use with patients anticipated to have short stays and low resource use and (2) preselect specific Diagnosis Related Groups based on historical data and create designations for those diagnoses of lesser intensity. Accountable care organizations contracts, a new model for healthcare payment, could potentially be structured to eliminate or simplify payment based on visit status for hospitalized patients. With bundled payments on the horizon and the possible phase‐out of fee‐for‐service reimbursement, the issue may become less paramount in the coming years. No solution will be perfect and must balance costs, ease of administration, and beneficiary protection.
There are reasons to be optimistic that change may soon be realized. CMS is currently considering significant hospitalization status policy reform. In the proposed IPPS‐2015, CMS asked for input on payment for short‐stay hospitalizations and, in the final IPPS‐2015 released August 4, 2014, CMS indicated its willingness to continue to work with stakeholders in revising these policies.[46] Additionally, CMS has responded to hospitals on 3 separate occasions by delaying RAC audits pertaining to the 2‐midnight rule. Further, the current MAC probe and educate audits focus on education with respect to 2‐midnight rule implementation rather than threatening hospitals with major financial penalties.[47] Congress has also been responsive in this area. In addition to the 3 delays announced by CMS, Congress passed legislation that mandated an additional delay to RAC audits that pertain to the 2‐midnight rule. Moreover, the Subcommittee on Health of the House Ways and Means Committee held hearings that included the 2‐midnight rule and RAC reform in May 2014, and the Senate Special Committee on Aging held hearings on the impact of visit status on Medicare beneficiaries in July 2014.[48, 49] Additionally, the House Ways and Means Health Subcommittee recently issued a draft bill to address Medicare hospital issues.[50] The OIG has also been responsive to hospital concerns regarding the current RAC program with a recent report recommending that CMS develop additional performance evaluation metrics to improve RAC performance and ensure that RACs are evaluated on all contract requirements.[51] Additionally, MedPAC has been considering several short‐stay payment reform options, modifying the need for a 3‐day inpatient hospitalization to qualify for postdischarge skilled nursing facility benefits and adjusting RAC contingency fees based on overturn rates.[52, 53] These actions by CMS, Congress, and the OIG, as well as the options under consideration by MedPAC, demonstrate a degree of regulatory and legislative responsiveness to hospital and provider concerns in the area of visit status determination.
The Medicare program is vital to tens of millions of disabled and elderly Americans. Fraud and abuse of the Medicare program should not be tolerated. Yet, the current system of assigning, monitoring, and auditing outpatient versus inpatient hospital care is in need of reform. It will be up to CMS and Congress to continue to work with hospitals and physicians to find an improved way to appropriately and fairly compensate hospitals for hospital services in a way that that does not depend on a poorly defined and contentious status of a patient. Such reform must include the RAC program. It is our hope that both CMS and Congress will prioritize status determination and payment reform so that Medicare beneficiaries, physicians, and hospitals all have a sustainable, fair, and transparent process.
Status determinations (outpatient versus inpatient) for hospitalized patients have become a routine part of patient care in the United States. Under the guidance provided by the Medicare Benefits Policy Manual, hospitalized Medicare beneficiaries are assigned 1 of these 2 statuses. The status assignment does not affect the care a patient can receive, but rather how the hospital services provided are billed to Medicare. Hospital services provided under inpatient status are billed under Medicare Part A. Hospital services provided under outpatient status, which includes all patients receiving observation services (commonly referred to as under observation), are billed under Medicare Part B. Whether hospital services are billed under Part A or Part B is important to hospitals and Medicare beneficiaries, as both the hospital reimbursement and beneficiary liability can vary greatly depending on whether services are billed under Part A versus Part B. Hospitals are generally reimbursed at a higher rate for services provided as an inpatient (Part A). The Office of the Inspector General (OIG) recently found that Medicare paid nearly three times more for a short inpatient stay than an [outpatient] stay for the same condition.[1] Medicare beneficiary liability also varies based on status. First, beneficiaries hospitalized as inpatients are subject to a deductible under Part A ($1,216 in 2014) for hospital services associated with that hospitalization and any future inpatient hospitalization beyond 60 days of discharge.[2] Beneficiaries hospitalized as outpatients are subject to the Medicare Part B deductible ($147 in 2014), and then a 20% copay on each individual outpatient hospital service, with no cumulative limit.[2, 3] In addition, hospital pharmacy charges for Medicare beneficiaries hospitalized as inpatients are covered under Medicare A. However, for Medicare patients hospitalized as outpatients, many medications are not covered by Medicare Part B benefits. Finally, time spent hospitalized as an outpatient does not count toward the Medicare 3‐day medically necessary inpatient stay requirement to qualify for the skilled nursing facility care benefit following discharge.
HISTORY AND INTENT OF INPATIENT AND OUTPATIENT STATUS DETERMINATIONS
Prior to October 1, 2013, the Centers for Medicare & Medicaid Services (CMS) stated that physician judgment and an expectation of at least an overnight hospitalization should determine inpatient status of hospitalized Medicare beneficiaries. Guidance as to when inpatient services were covered was found in the Medicare Benefits Policy Manual (MBPM)[4]:
An inpatient is a person who has been admitted to a hospital for bed occupancy for purposes of receiving inpatient hospital services. Generally, a patient is considered an inpatient if formally admitted as inpatient with the expectation that he or she will remain at least overnight and occupy a bed even though it later develops that the patient can be discharged or transferred to another hospital and not actually use a hospital bed overnight. The physician or other practitioner responsible for a patient's care at the hospital is also responsible for deciding whether the patient should be admitted as an inpatient. Physicians should use a 24‐hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis. However, the decision to admit a patient is a complex medical judgment that can be made only after the physician has considered a number of factors, including the patient's medical history and current medical needs, the types of facilities available to inpatients and to outpatients, the hospital's by‐laws and admissions policies, and the relative appropriateness of treatment in each setting.
For a subset of patients who are hospitalized under outpatient status, billing for observation services is allowed. CMS defines observation as a well defined set of services, that should last less than 24 hours and in only rare and exceptional casesspan more than 48 hours.[5] Many providers recognize the utility of a few additional hours of hospital care and/or testing in a hospital setting to determine whether a patient can go home or needs additional evaluation, monitoring, and/or treatment that can only be provided in a hospital, consistent with the CMS definition of observation.[6] It is important to note that although observation and outpatient are frequently used interchangeably, only outpatient is technically a CMS status. Patients in observation or under observation are, in fact, a subset of patients who are hospitalized under an outpatient status.
Outpatient status may also be appropriate for patients who require hospitalization for routine and expected overnight monitoring following a procedure. These patients are often not eligible for billing of observation services or as an inpatient because alternative methods of billing for the recovery time following the procedure exist. When determining the appropriate status of a Medicare beneficiary for a hospitalization following a procedure, physicians need to be aware of whether a specific procedure appears on the Medicare inpatient‐only procedures list.[7] Per CMS, procedures designated as inpatient only are reimbursed only when the patient is admitted as an inpatient at the time the procedure is performed.[8] Conversely, outpatient status for an overnight hospitalization associated with a procedure not on the inpatient‐only list is generally appropriate. Therefore, patients hospitalized for a procedure that appears on this list should always be hospitalized under inpatient status, regardless of the amount of time that the patient is expected to be hospitalized following the procedure, including those cases for which the hospitalization is expected to be only overnight.[7, 8] Only a limited number of Current Procedural Technology (CPT) codes, mostly surgical, automatically qualify for inpatient status and do not have outpatient prospective payment system eligibility. Although most procedures on the inpatient‐only list are associated with a hospitalization that commonly span at least 2 midnights, such as coronary artery bypass grafting, some potentially overnight stay cases, such as cholecystectomy (CPT 47600) appear on the 2014 inpatient‐only list.[9]
As noted above, prior to October 1, 2013, the Medicare definitions governing outpatient versus inpatient status included a 24‐hour benchmark. However, the MBPM also notes that: Admissions of particular patients are not covered or non‐covered solely on the basis of the length of time the patient actually spends in the hospital.[10]
In practice, status determination was ultimately dependent on physician or other practitioner's complex medical judgment as specified by CMS. To validate this judgment, CMS recommended that reviewers use a screening tool as part of their medical review. This screening tool could include practice guidelines that are well accepted by the medical community but did not require or identify a specific criteria set.[11] Not surprisingly, there was and continues to be great variability in the application of outpatient versus inpatient status across hospitals in actual practice.[1, 12, 13] The ambiguity in the definition of a hospitalized patient's status helped spawn commercial clinical decision tools, such as InterQual (McKesson Corporation, San Francisco, CA) and MCG (formally known as Milliman Care Guidelines; MCG Health, LLC, Seattle, WA), to help define inpatients versus outpatients.[14, 15] However, these guidelines are complex, can be difficult to interpret and apply, and have been criticized for poor predictive value and attempting to replace physician judgment.[16, 17, 18] Furthermore, CMS has never formally endorsed any specific decision tool.
INPATIENT AND OUTPATIENT PAYMENTS AND THE RECOVERY AUDIT CONTRACTOR PROGRAM
In 2000, CMS started using Ambulatory Payment Classifications for hospital services, which made inpatient care more financially favorable for hospitals. In response to concerns that hospitals would be incentivized to overuse inpatient status, CMS made a number of changes to their payment system, including the creation of the Recovery Audit Program in 2003. This program was originally called the Recovery Audit Contractor (RAC) Program and continues to be most commonly referred to as the RAC program. The RAC program, tasked with finding and correcting improper claims to the Medicare program, began as a demonstration required in the Medicare Prescription Drug Improvement and Modernization Act of 2003 (MMA), and subsequently became a nationwide audit program under the Tax Relief and Health Care Act of 2006. Under this program, private contractors review hospital and billing records of Medicare patients and are paid on a contingency fee (8%12.5%) for all underpayments and overpayments that are identified and corrected.[19] Importantly, the RACs are not subject to any financial penalties for cases improperly denied.
RACs initially targeted many overnight inpatient stays for recoupment. These cases were attractive audit targets because the RACs could argue that the inpatient hospital services were delivered in the improper status based solely on the length of stay, without having to consider in their audit the complexity of decision making or medical necessity of the services provided. However, it is worth noting that with improvement in efficiency and advancements in medical technology, hospitals and physicians have been increasingly able to safely evaluate and treat medically complex and severely ill patients quickly, sometimes with just an overnight stay. As perspective, in 1965, the average length of stay for a Medicare patient was 13 days; in 2010, it was 5.4 days, with over one‐third of hospitalizations lasting <3 days.[20]
Concurrent with the increased RAC denials for services provided in an inpatient status, the use of observation services changed significantly from 2007 to 2012. The average length of stay for Medicare patients under outpatient status with observation services exceeded 24 hours in 2007, was 28.2 hours by 2009,[21] and grew to 29 hours by 2012.[22] Between July 2010 and December 2011, at the University of Wisconsin Hospital, 1 in 6 observation stays lasted longer than 48 hours, suggesting that long observation stays were no longer rare and exceptional as stated in CMS' own definition.[23] This same University of Wisconsin study also found that observation services were not well defined, with 1141 distinct diagnosis codes used for these services.[23]
Additionally, a Medicare Payment Advisory Commission (MedPAC; described on their website,
Hospitals have also expressed concern that the RAC contingency fee payment model and a lack of penalty for improper denials promotes overzealous auditing.[24, 25] RAC recoupment has increased from approximately $939 million in 2011, to $2.4 billion in 2012, to $3.8 billion in 2013.[26, 27, 28] Given the money now at stake, it is not surprising that hospitals have become very active in appealing RAC denials. Self‐reported data submitted to the American Hospital Association (AHA) for the months January 2014 to March of 2014 show that hospitals now appeal 50% of RAC denials and win 66% of these appeals.[29] The AHA data also show that 69% of self‐reporting hospitals spent over $10,000 to manage their audit and appeals process over this same 3‐month time period, with 11% spending more than $100,000.
This appeals process is not only costly to hospitals, it is also lengthy. As of January 2014, the average wait time for an appeal hearing with an administrative law judge (level 3 appeal) exceeded 16 months.[30] In fact, the appeals process has become so backlogged that hospitals' rights to assignment of level 3 (administrative law judge) appeals have been temporarily suspended.[30] In August 2014, CMS offered a $0.68 on the dollar partial payment for hospitals willing to settle all eligible outstanding appeals in an attempt to relieve the appeals backlog.[31] In addition, the AHA currently has a suit against the US Department of Health & Human Services over the RAC appeals backlog.[32]
Increased use of outpatient status may be driven by pressures from the RAC program and, potentially, by improvements in the efficiency of care. Because hospitals are paid less for care provided under outpatient status than they are for the identical care provided under inpatient status, hospitals faced both potential financial penalty for improvements in efficiency and the threat of RAC audits.
THE 2‐MIDNIGHT RULE: A FIX?
Given the challenges in defining inpatient versus outpatient hospitalization, the increasing use of outpatient status and the increasing length of stay of outpatient hospitalizations with observation services, in 2013, CMS responded with new policies to define the visit status for hospitalized patients. On August 2, 2013, CMS announced the fiscal year 2014 hospital Inpatient Prospective Payment System final rule (IPPS‐2014) to become effective October 1, 2013. This document was formally issued as part of the Federal Register on August 19, 2013.[33] Central to the CMS IPPS‐2014 was a 2‐midnight benchmark that offered a major change in how physicians were to determine the status (inpatient vs outpatient) of hospitalized patients. With this 2‐midnight benchmark, now informally known as the 2‐midnight rule, CMS finalized its proposal to generally consider patients that are expected by a practitioner (with knowledge of the case and with admitting privileges) to need hospitalization that will span 2 or more midnights as inpatient. The IPPS‐2014 also finalized the converse of this: hospitalizations expected to span <2 midnights are to be regarded as outpatient with 2 exceptions:
- If the hospitalization is associated with a procedure appearing on the previously described Medicare inpatient‐only procedures list, or
- A rare and unusual circumstance in which an inpatient admission would be reasonable regardless of length of stay. Currently, unanticipated mechanical ventilation initiated during the hospitalization visit is the only rare and unusual circumstance that qualifies as such an exception.[7]
CMS' stated goals and expectations for the 2‐midnight benchmark were:
- Reduce the growing number of prolonged hospitalizations (>48 hours) for Medicare beneficiaries under outpatient status.
- Decrease billing disputes between hospitals and Medicare auditors, especially RACs, by establishing more clearly defined, time‐based status criteria.
- Reduce the number of outpatient encounters overall. Because CMS expected the rule to convert a net increase of cases from outpatient to inpatient, resulting in higher payments to hospitals, CMS included a 0.2% payment cut in hospital reimbursement in the IPPS‐2014 as an offset.[33, 34]
Although unrelated to the goals and expectations above, the IPPS‐2014 also included a requirement that:
[T]he order [for inpatient admission] must be furnished by a qualified and licensed practitioner who has admitting privileges at the hospital as permitted by State law, and who is knowledgeable about the patient's hospital course, medical plan of care and current condition.
CMS allowed for an authentication (generally regarded as a cosignature that is timed and dated) of the inpatient admission order by an attending physician with admitting privileges, done prior to discharge, in cases where the inpatient order had been placed by a practitioner (such as a resident, fellow, or physician assistant) without admitting privileges. Attending physician authentication of the inpatient admission order must be done prior to discharge [a]s a condition of payment for hospital inpatient services under Medicare Part A.[35]
From the August 2, 2013 announcement until the effective date of October 1, 2013, hospitals had just 2 months to interpret and comply with the IPPS‐2014, a complex 546‐page document that required hospitals to make extensive changes to admission procedures, workflows, and electronic health records (EHRs). In addition, extensive physician, provider, and administrator education was needed. During these 2 months, hospitals continued to request additional information and clarification from CMS regarding many aspects of the IPPS‐2014, including basic questions that included (1) how to apply the 2‐midnight benchmark to patients who were transferred from 1 hospital to another and (2) when the clock started for hospital services in determining a patient's expected length of hospitalization.
Despite concerns voiced by Congress and medical organizations, the new policy went into effect as scheduled.[36, 37] However, just days prior to October 1, 2013, CMS issued a 3‐month limited suspension of auditing and enforcement of the 2‐midnight rule by the RACs that was subsequently extended by CMS 2 more times, first through March 31, 2014 and then again through September 30, 2014. Other audits to be performed by RACs and all other government audits, including those performed by Medicare Administrative Contractors (MACs) were allowed to continue.[38] In particular, the MACs were instructed to conduct patient status reviews using a probe and educate strategy, which, via educational outreach efforts, would instruct hospitals how to adapt to the new rule. On April 1, 2014, the Protecting Access to Medicare Act of 2014 was signed into law, which, under section 111 of this law, permitted CMS to continue medical review activities under the MAC probe and educate process through March of 2015, and prohibited CMS from allowing RACs to conduct inpatient hospital status reviews on claims with these same dates of admission, October 1, 2013 through March 31, 2015.
The MACs were created by the MMA of 2003, which mandated that the Secretary of Health & Human Services replace Part A Fiscal Intermediaries and Part B carriers with Medicare Administrative Contractors (MACs).[39] As established by CMS, MACs are multi‐state, regional contractors responsible for administering both Medicare Part A and Medicare Part B claims and serve as the primary operational contact between the Medicare Fee‐For‐Service program, and approximately 1.5 million health care providers enrolled in the program.[39]
THE IPPS‐2014 AND CMS' STATED GOALS AND EXPECTATIONS
In the analysis that accompanied the IPPS‐2014, Medicare expected the use of outpatient services to decrease overall, as the new rules would effectively eliminate almost all outpatient hospitalizations >48 hours. Although no official data are yet available from CMS, our early experience under the 2‐midnight rule has suggested that long observation stays have declined in frequency, a favorable outcome of the new policy. However, as designed, the new 2‐midnight IPPS rule most predominately affects 1‐day stays, or more accurately, 1‐midnight stays. This is because many hospitalizations that previously met inpatient criteria (as defined by commercially available products such as MCG or InterQual), but spanned <2 midnights would have been classified as inpatient prior to October 1, 2013. However, since October 1, 2013, these same hospitalizations are now classified as outpatient. An example of such a case is a patient who presents to an emergency department with symptoms of a transient ischemic attack and has a high ABCD (age 60 years, blood pressure 140/90 mm Hg at initial evaluation, clinical features, duration of symptoms, diabetes score).[40] Prior to the 2‐midnight rule, this patient, based on the severity of the signs and symptoms upon presentation, could have been appropriately hospitalized as an inpatient.
Now, under the current IPPS and the ability of many hospitals to efficiently evaluate and treat such patient in <2 midnights, the patient should be categorized as an outpatient, at least initially, despite the severity and high risk of his/her presentation. In fiscal year 2013, The Johns Hopkins Hospital had 1791, 1‐day inpatient stays for Medicare beneficiaries, representing 15.2% of all Medicare admissions. Similarly, in the 12 months just prior to the 2‐midnight rule (October 1, 2012 to September 30, 2013), 10.4% (1280) of all Medicare encounters at the University of Wisconsin were 1‐day inpatient stays under previous criteria. Because of implementation of the 2‐midnight rule in October 2013, Medicare outpatient hospitalization for 1‐day stays at The Johns Hopkins Hospital increased by 49%, from an average of 117 patients/month to 174 patients/month. Nationally, it is possible that a reduction in long observation stays could be offset by an increase in 1‐day‐stay outpatient hospitalization encounters.
A second key expectation and goal of IPPS‐2014 was, by shifting to a more concrete, time‐based definition of inpatient, to decrease the disagreement between hospitals and auditors regarding patient status (inpatient vs outpatient). As noted earlier, many disputes with auditors for hospitalizations prior to October 2013 did not involve the need or type of hospital services provided, but rather the status under which the care was provided. However, the new time‐based criterion hinges not on actual length of hospitalization, but the expected length of hospitalization as determined by a practitioner with admitting privileges and knowledge of the patient. Accurately and consistently predicting the length of hospitalization has proven to be challenging, even for the most experienced practitioners. Since October 2013, for patients hospitalized at The Johns Hopkins Hospital through its emergency department, the admitting physicians' expectation of whether a patient would require 1 versus 2 or more midnights of necessary hospitalization was correct only half of the time. Given past experience, the RACs may challenge the medical judgment that lead practitioners to expect a hospitalization of 2 or more midnights without having to challenge whether the care provided was medically necessary.
Further, the IPPS‐2014 has not been accompanied by any significant changes to the payment scheme for auditors. RACs continue to be paid a percentage of any monies they determine to have been improperly paid by CMS, but with no penalty for cases that are overturned on appeal. Historically, the vast majority of RAC recovery fees have been due to determination of overpayments by CMS.[41, 42] Despite the 2‐midnight rule, RACs will continue to have a financial incentive to allege overpayment. In the initial probe and educate audits by MACs under the new 2014‐IPPS, despite inpatient admission orders being authenticated and certified by an attending physician, claims are being denied because the documentation does not support an expectation for a 2‐midnight hospitalization. Namely, auditors are continuing to challenge not the medical necessity of the services that hospitals provide, but rather the status in which those services were provided. Thus far, the IPPS‐2014 does not appear to fully remedy the auditing conflict that existed prior to October 2013.
As noted above, the IPPS‐2014 also requires, as of October 1, 2013, as a condition of payment for hospital services under Part A, that the inpatient admission order must be either entered by a practitioner with admitting privileges or authenticated prior to discharge by an attending physician involved in the care of the patient in cases in which the inpatient admission order was entered by a practitioner without admitting privileges (eg, resident, physician assistant, or fellow).[43] The requirement of an attending physician's cosignature has involved major changes to physician workflow and the electronic heath record (EHR) framework at The Johns Hopkins and the University of Wisconsin Hospitals, and does not keep up with modern healthcare systems in which patients are admitted 24 hours a day by a variety of providers (eg, residents, nurse practitioners) who otherwise may write stand‐alone orders. These changes have proven to be time‐consuming, costly, and have not, to our knowledge, improved patient care or utilization of resources.
The new visit status rules have also led to confusion among clinicians. A recent large survey of hospitalists conducted by the Society of Hospital Medicine demonstrated that more than half of respondents disagreed that the 2‐midnight rule improved hospitalist workflow compared to prior observation policy.[44] In addition, only 40% of hospitalists reported confidence in how to apply the rule.[44] Thus, the intent to clarify visit status policy with the IPPS‐2014 has not translated to clear and useful rules for frontline clinicians.
FUTURE DIRECTIONS
After over a year under the 2‐midnight rule, although long observation stays may be reduced, it seems unlikely these new regulations will achieve 2 of CMS' stated goals: (1) decreasing the use of outpatient status for hospitalizations and (2) resolving status disputes between auditors and hospitals. In addition, attempts at compliance with the new rules and regulations have diverted large amounts of physician time and hospital resources away from patient care. There is a clear need to reform both the hospitalization status policy and the RAC programs that enforce these rules.
One path Congress and CMS could consider is to reform the current Medicare reimbursement paradigm for hospital services to eliminate the need to distinguish inpatient from outpatient status. For example, H.R. 1179Improving Access to Medicare Coverage Act of 2013,[45] of the 113th Congress, if reintroduced, would decouple the link between the qualification for skilled nursing facility benefits from visit status by allowing time spent hospitalized as an outpatient to count toward the 3‐day benchmark. The overarching goals of any visit status policy reform should be to: (1) simplify or eliminate the 2‐track status process for hospitalized patients, (2) stop or minimize the threat of audits based on status, and (3) maintain budget neutrality. Two additional options for consideration would be to: (1) create a low‐acuity modifier for use with patients anticipated to have short stays and low resource use and (2) preselect specific Diagnosis Related Groups based on historical data and create designations for those diagnoses of lesser intensity. Accountable care organizations contracts, a new model for healthcare payment, could potentially be structured to eliminate or simplify payment based on visit status for hospitalized patients. With bundled payments on the horizon and the possible phase‐out of fee‐for‐service reimbursement, the issue may become less paramount in the coming years. No solution will be perfect and must balance costs, ease of administration, and beneficiary protection.
There are reasons to be optimistic that change may soon be realized. CMS is currently considering significant hospitalization status policy reform. In the proposed IPPS‐2015, CMS asked for input on payment for short‐stay hospitalizations and, in the final IPPS‐2015 released August 4, 2014, CMS indicated its willingness to continue to work with stakeholders in revising these policies.[46] Additionally, CMS has responded to hospitals on 3 separate occasions by delaying RAC audits pertaining to the 2‐midnight rule. Further, the current MAC probe and educate audits focus on education with respect to 2‐midnight rule implementation rather than threatening hospitals with major financial penalties.[47] Congress has also been responsive in this area. In addition to the 3 delays announced by CMS, Congress passed legislation that mandated an additional delay to RAC audits that pertain to the 2‐midnight rule. Moreover, the Subcommittee on Health of the House Ways and Means Committee held hearings that included the 2‐midnight rule and RAC reform in May 2014, and the Senate Special Committee on Aging held hearings on the impact of visit status on Medicare beneficiaries in July 2014.[48, 49] Additionally, the House Ways and Means Health Subcommittee recently issued a draft bill to address Medicare hospital issues.[50] The OIG has also been responsive to hospital concerns regarding the current RAC program with a recent report recommending that CMS develop additional performance evaluation metrics to improve RAC performance and ensure that RACs are evaluated on all contract requirements.[51] Additionally, MedPAC has been considering several short‐stay payment reform options, modifying the need for a 3‐day inpatient hospitalization to qualify for postdischarge skilled nursing facility benefits and adjusting RAC contingency fees based on overturn rates.[52, 53] These actions by CMS, Congress, and the OIG, as well as the options under consideration by MedPAC, demonstrate a degree of regulatory and legislative responsiveness to hospital and provider concerns in the area of visit status determination.
The Medicare program is vital to tens of millions of disabled and elderly Americans. Fraud and abuse of the Medicare program should not be tolerated. Yet, the current system of assigning, monitoring, and auditing outpatient versus inpatient hospital care is in need of reform. It will be up to CMS and Congress to continue to work with hospitals and physicians to find an improved way to appropriately and fairly compensate hospitals for hospital services in a way that that does not depend on a poorly defined and contentious status of a patient. Such reform must include the RAC program. It is our hope that both CMS and Congress will prioritize status determination and payment reform so that Medicare beneficiaries, physicians, and hospitals all have a sustainable, fair, and transparent process.
- Testimony of Jodi D Nudelman, Regional Inspector General for the Office of Evaluation and Inspections, Office of the Inspector General, US Department of Health and Human Services, Hearing: Current Hospital Issues in the Medicare Program, House Committee on Ways and Means, Subcommittee on Health, May 20, 2014. Available at: https://oig.hhs.gov/newsroom/testimony‐and‐speeches/index.asp. Accessed November 24, 2014.
- Centers for Medicare 173:1999–2000.
- US Department of Health 49:893–909.
- US Department of Health 28:95–111.
- . The price of admission: increasing use of decision‐support technology draws criticism for changing roles in hospital‐admissions process. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20121117/MAGAZINE/311179951. Published November 17, 2012. Accessed November 9, 2014.
- , , , et al. The accuracy of interqual criteria in determining the need for observation versus hospitalization in emergency department patients with chronic heart failure. Crit Pathw Cardiol. 2013;12:192–196.
- US Department of Health 31:1251–1259.
- MedPAC March 2104 Report to the Congress, Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed on December 22, 2014.
- , , , et al, Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173:1991–1998.
- . The recovery audit contractor program and observation status for hospitalized Medicare beneficiaries. JAMA Internal Medicine blog. Available at: http://internalmedicineblog.jamainternalmed.com/2014/02/04/the‐recovery‐audit‐contractor‐program‐and‐observation‐status‐for‐hospitalized‐medicare‐beneficiaries. Published February 4, 2014. Accessed June 15, 2014.
- . Broken RAC system continues to hurt patients, providers. The Hospital Leader blog. Available at: http://blogs.hospitalmedicine.org/Blog/broken‐rac‐system‐continues‐to‐hurt‐patients‐providers. Published April 22, 2014. Accessed June 15, 2014.
- US Department of Health 78(160). Available at: http://www.gpo.gov/fdsys/pkg/FR‐2013‐08‐19/pdf/2013‐18956.pdf. Accessed August 4, 2014.
- US Department of Health use of observation and inpatient stays for Medicare beneficiaries, OEI‐02‐12‐00040. Available at: http://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Accessed June 15, 2014.
- US Department of Health
- Testimony of Jodi D Nudelman, Regional Inspector General for the Office of Evaluation and Inspections, Office of the Inspector General, US Department of Health and Human Services, Hearing: Current Hospital Issues in the Medicare Program, House Committee on Ways and Means, Subcommittee on Health, May 20, 2014. Available at: https://oig.hhs.gov/newsroom/testimony‐and‐speeches/index.asp. Accessed November 24, 2014.
- Centers for Medicare 173:1999–2000.
- US Department of Health 49:893–909.
- US Department of Health 28:95–111.
- . The price of admission: increasing use of decision‐support technology draws criticism for changing roles in hospital‐admissions process. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20121117/MAGAZINE/311179951. Published November 17, 2012. Accessed November 9, 2014.
- , , , et al. The accuracy of interqual criteria in determining the need for observation versus hospitalization in emergency department patients with chronic heart failure. Crit Pathw Cardiol. 2013;12:192–196.
- US Department of Health 31:1251–1259.
- MedPAC March 2104 Report to the Congress, Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed on December 22, 2014.
- , , , et al, Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173:1991–1998.
- . The recovery audit contractor program and observation status for hospitalized Medicare beneficiaries. JAMA Internal Medicine blog. Available at: http://internalmedicineblog.jamainternalmed.com/2014/02/04/the‐recovery‐audit‐contractor‐program‐and‐observation‐status‐for‐hospitalized‐medicare‐beneficiaries. Published February 4, 2014. Accessed June 15, 2014.
- . Broken RAC system continues to hurt patients, providers. The Hospital Leader blog. Available at: http://blogs.hospitalmedicine.org/Blog/broken‐rac‐system‐continues‐to‐hurt‐patients‐providers. Published April 22, 2014. Accessed June 15, 2014.
- US Department of Health 78(160). Available at: http://www.gpo.gov/fdsys/pkg/FR‐2013‐08‐19/pdf/2013‐18956.pdf. Accessed August 4, 2014.
- US Department of Health use of observation and inpatient stays for Medicare beneficiaries, OEI‐02‐12‐00040. Available at: http://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Accessed June 15, 2014.
- US Department of Health
Are ObGyns getting “bumped” out of deserved Medicaid reimbursement?
With enactment of the Affordable Care Act (ACA) came a number of significant changes to federal and state Medicaid programs to increase access to care for low-income individuals. One landmark change, which became a state option after a ruling by the US Supreme Court, is the expansion of eligibility to all adults who have an income at or below 138% of the federal poverty line, which was $16,105 annually for an individual or $32,913 for a family of four in 2014.
Pregnancy is no longer a criterion for eligibility for low-income women in states with expanded Medicaid programs—a real game changer for millions of women in need of care.
As of this writing, 27 states, including the District of Columbia, have expanded their Medicaid program. Data from the Centers for Medicare and Medicaid Services (CMS) show that total enrollment in the Children’s Health Insurance Program (CHIP) and Medicaid increased by more than 4.8 million people (from 58.9 million to 63.7 million) between July 2013 and March 2014, in the 47 states reporting data for both periods. Nearly all of this growth occurred in Medicaid expansion states.1,2 More recent data show that 7.9 million more people were enrolled in Medicaid in July 2014 than in the previous year.3,4
Is Medicaid a losing proposition for ObGyns?
In many states, it costs ObGyns more than Medicaid pays to provide primary care to Medicaid patients. Nationally, providers receive 41% less in Medicaid reimbursement than they get with Medicare for primary care services.5 In 2012, the worst offender was Rhode Island’s Medicaid program, which paid physicians only 33% of the Medicare reimbursement rates for primary care.5
The rate of Medicaid reimbursement affects a physician’s willingness to accept new Medicaid patients. Only 50% of physicians are willing to accept new Medicaid patients, compared with 70% who are willing to accept new Medicare or privately insured patients. Twenty-three percent of female Medicaid beneficiaries report a problem finding a new doctor, compared with 7% of Medicare beneficiaries and 13% of privately insured women. The main reason: low Medicaid payment rates.6
In 2007, 38% of all ObGyns accepted Medicaid gynecology patients, and 44% accepted Medicaid obstetric patients, with Medicaid accounting for 18% of revenue for the average ObGyn practice. In its 2013 survey of members, ACOG found that while 63.2% accept all Medicare patients, only 44.4% accept all Medicaid gynecology patients, and 48.7% accept all Medicaid obstetric patients, up from 2007. The percentage of ObGyns who don’t see Medicaid gynecology or obstetric patients was 22.7% and 16.3%, respectively. Only 8.2% of ObGyns see no Medicare patients.7
According to a 2014 survey, 34% of physicians report an increase in Medicaid patients; 41% of those report an increase of 11% or more.3
The American Medical Association – The AMA considers the ObGyn specialty one of four specialties that provide primary care.
Tricare – The health-care program for uniformed service members (active, Guard/Reserve, retired) and their families around the world designates ObGyns as among primary care case managers.
Community Health Teams – A grant program to support primary care practices and patient-centered medical homes includes ObGyns as primary care providers.
Medicaid – Thirty-four states and the District of Columbia define ObGyns as primary care providers.
Medicaid Health Homes – Authorized under federal law to coordinate care for Medicaid enrollees with chronic conditions, health home providers coordinate all primary care, acute, behavioral health, and long-term services and supports to treat the whole person. ObGyns are eligible home health providers.
Health Resources Services Administration – This agency delineates health professional shortage areas, providing bonuses for physicians serving in these areas. It includes ObGyns as one of four primary care specialties.
National Health Service Corps – This organization offers loan repayments and scholarships to primary care providers working in underserved communities and recognizes ObGyns as primary care physicians.
Teaching Health Center Graduate Medical Education – This program supports community-based primary care residency programs to increase the number of primary care residents and dentists trained in geographically isolated or economically or medically vulnerable
communities.
Congress addresses the discrepancy
The ACA Medicaid primary care “bump,” as it’s called, was designed to help ensure access to primary care for the huge new group of individuals covered by Medicaid. It raised Medicaid payment rates for primary care services to Medicare fee levels in 2013 and 2014, an overall average increase of 73% in Medicaid payment rates for Evaluation and Management (E/M) codes 99201–99499, and for vaccine administration codes 90461 and 90471–90474 (FIGURES 1–3).8
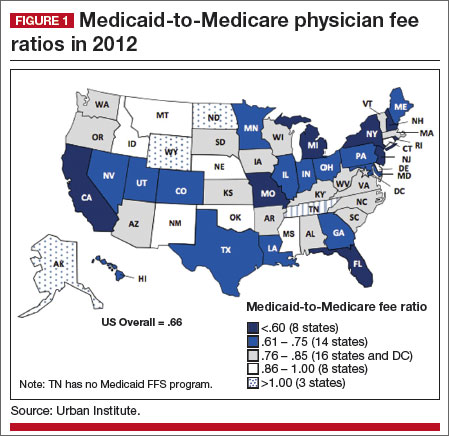 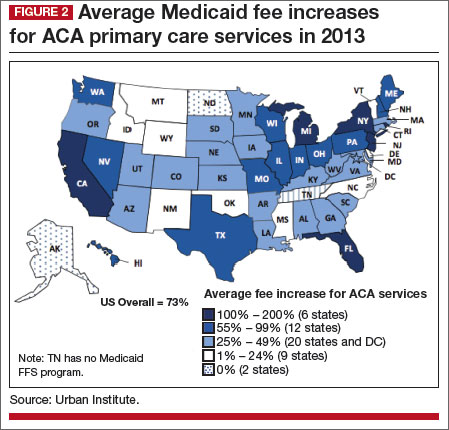  |
The catch? The bump only applies to internists, family medicine physicians, and pediatricians. The House-passed bill included ObGyns—women’s primary care providers—but the Senate bill did not, and the Senate bill was the version ultimately enacted into law.
To qualify for these additional payments, physicians must self-attest to the state Medicaid agency that she or he meets one of the following criteria:
- board certification in family medicine, general internal medicine, or pediatric medicine or a subspecialty recognized by the American Board of Medical Specialties, the American Board of Physician Specialties, or the American Osteopathic Association
- 60% of Medicaid billing involves the specified E/M and vaccine administration codes.
For newly eligible physicians, the previous month’s billing is used to determine eligibility.
What about women’s health?
In speaking with ACOG Fellows, here’s what ACOG President John Jennings, MD, had to say about the omission of ObGyns from the primary care rate bump:
This federal program, which expires at the end of this year, was designed to increase access to needed primary care services for low-income individuals. But as the program stands right now, it leaves out women’s health.
That’s just not right. And we have before us a chance to fix it.
ObGyns deliver primary and preventive care services to women; an ObGyn often is the only doctor a woman sees on a regular basis. Thirty-five state Medicaid programs classify ObGyns as primary care providers.9 Twelve percent of women aged 18 to 64 years rely on Medicaid for their health coverage, and more than 68% of adult Medicaid beneficiaries are women.10
Seeing an opportunity to expand the primary care reimbursement bump to include ObGyns, ACOG recently briefed Congress on the long and deep tradition of primary care in obstetrics and gynecology—a tradition that begins in residency. We provided Congress with ObGyn resident training requirements, as outlined by the Council on Resident Education in Obstetrics and Gynecology (CREOG).11
How ObGyns provide primary care
According to CREOG, “ObGyns provide primary health-care services to their patients both within and outside the traditional purview of reproductive medicine. As primary care physicians, ObGyns establish relationships with their patients that transcend the disease spectrum and extend to routine assessments, preventive care, early intervention, and management of medical disorders.”11
Among the services they provide are age-appropriate screening for substance use, sexual and reproductive health, sexually transmitted infection, psychosocial risks, breast disorders, cancer, and cardiovascular disease. ObGyns routinely counsel patients about diet, exercise, contraception, dental health, osteoporosis, and sexual health. And they provide front-line immunizations against such diseases as influenza, human papillomavirus, rubella, measles, meningitis, hepatitis A and B, and pneumonia.11
Certification in obstetrics and gynecology requires written and oral examinations in office practice and women’s health as a primary care content area. In fact, fully one-third of the board certification test taken by 97% of the ObGyns in this country tests their knowledge and training in primary care.11
How women view their ObGyn
A 2014 survey of women found that ObGyns play a critical role in providing primary care in the United States. Almost six in 10 women (58%) report that they see an ObGyn on a regular basis, and one-third of women (35%) view their ObGyn as their main source of care.12
Low-income women and women of color report that their ObGyn plays an even greater role in their health care. Latinas are far more likely (47%) to report that their ObGyn is their main source of care, compared with 35% of women overall. Sixty-four percent of African-American women say they see an ObGyn regularly, compared with 58% of women overall.12
Other survey findings:
- ObGyn providers are the first providers women choose as adults. They move from a pediatrician to an ObGyn.
- ObGyn providers are the “usual” providers for young women—the ones women see most frequently. They see internal medicine physicians and other providers infrequently during this period of their life.
- The ObGyn-patient relationship is intimate, comfortable, trusting, and confidential, and ObGyns often are considered “a friend.” Patients discuss issues with their ObGyns that are not raised with other providers.
- The care women receive from their -ObGyn provider is broad, ranging from an annual exam and breast exam to prenatal care, immunizations, a review of medications, blood pressure checks, and more.
- Women say their ObGyn does a better job than their family practice and internal medicine providers in providing a range of care and services.12
ObGyns may see relief in 2015
Congress has before it legislation to extend the primary care bump program into the future and expand it to include ObGyns and other women’s health clinicians. A proposal to that effect was introduced in the Senate by Patty Murray (D-WA) and Sherrod Brown (D-OH) and in the House by Reps. Frank Pallone (D-NJ) and Henry Waxman (D-CA).
At press time, ACOG was doing everything in its power to get this legislation passed in the lame duck session of Congress. We scored a big win at the November assembly of the American Medical Association (AMA) House of Delegates, when, with the support of the American Academy of Pediatricians (AAP) and the American College of Physicians, the House of Delegates overwhelmingly approved AMA policy to support extension of the program, including broadening it to include ObGyns.
ACOG also has enlisted the support of national women’s advocacy groups, including Planned Parenthood, the National Partnership for Families and Children, and the National Women’s Law Center. ACOG President Jennings led 35 ACOG leaders from across the country to Washington, DC, in November 2014, to urge members of Congress to pass this important legislation. They were joined by Thomas McInerney, MD, immediate past president of the AAP, and a number of leaders from Planned Parenthood.
Our message to Congress is clear: Not without women!
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Kaiser Family Foundation. Implementing the ACA: Medicaid Spending & Enrollment Growth for FY 2014 and FY 2015. http://kff.org/medicaid/issue-brief/implementing-the-aca-medicaid-spending-enrollment-growth-for-fy-2014-and-fy-2015/. Published October 14, 2014. Accessed December 15, 2014.
2. Center for Medicare and Medicaid Services. Medicaid and CHIP: March 2014 Monthly Applications, Eligibility Determinations, and Enrollment Report. http://www.medicaid.gov/AffordableCareAct/Medicaid-Moving-Forward-2014/Downloads/March-2014-Enrollment-Report.pdf. May 1, 2014. Accessed December 17, 2014.
3. Kane L, Peckham C. Insurer Ratings Report 2014. Medscape. http://www.medscape.com/features/slideshow /public/insurerratingsreport2014. Published October 21, 2014. Accessed December 15, 2014.
4. Kenney GM, Zuckerman S, Dubay L, et al. Opting in to the Medicaid expansion under the ACA: who are the uninsured adults who could gain health insurance coverage? Timely Analysis of Immediate Health Policy Issues. Urban Institute. http://www.urban.org/uploadedpdf/412630-opting-in-medicaid.pdf. Published August 2012. Accessed December 15, 2014.
5. Kaiser Family Foundation. Medicaid-to-Medicare Fee Index. http://kff.org/medicaid/state-indicator/medicaid-to-medicare-fee-index/. Updated November 11, 2014. Accessed December 15, 2014.
6. Kaiser Family Foundation. Women and Health Care: A National Profile. Key Findings from the Kaiser Women’s Health Survey. http://kaiserfamilyfoundation.files.wordpress.com/2013/01/women-and-health-care-a-national-profile-key-findings-from-the-kaiser-women-s-health-survey.pdf. Published July 2005. Accessed December 15, 2014.
7. ACOG 2013 survey of members. http://www.acog.org/~/media/Departments/Practice-Management-and-Managed-Care/2013SocioeconomicSurvey.pdf. Accessed December 17, 2014.
8. Zuckerman S, Goin D, Kaiser Family Foundation. How much will Medicaid physician fees for primary care rise in 2013? Evidence from a 2012 survey of Medicaid physician fees. http://kff.org/medicaid/issue-brief/how-much-will-medicaid-physician-fees-for/. Published December 13, 2012. Accessed December 15, 2014.
9. Based on an ACOG review of state Medicaid regulations, statutes, and provider manuals.
10. National Women’s Law Center. Battles over Medicaid Funding and Eligibility: What’s at Stake for Women. http://nwlc.org/sites/default/files/pdfs/national.pdf. Published June 2011. Accessed December 15, 2014.
11. Carey JC, Blanchard MH, Adams KE, et al; Education Committee of the Council on Resident Education in Obstetrics and Gynecology (CREOG). CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology, 10th ed. American Congress of Obstetricians and Gynecologists. http://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/CREOG-Educational-Objectives. Published 2013. Accessed December 15, 2014.
12. Montefiore Investigators to Present Data at American Congress of Obstetricians and Gynecologists Annual Meeting [news release]. April 25, 2014. http://www.montefiore.org/body.cfm?id=1738&action=detail&ref=1142. Accessed December 17, 2014.
With enactment of the Affordable Care Act (ACA) came a number of significant changes to federal and state Medicaid programs to increase access to care for low-income individuals. One landmark change, which became a state option after a ruling by the US Supreme Court, is the expansion of eligibility to all adults who have an income at or below 138% of the federal poverty line, which was $16,105 annually for an individual or $32,913 for a family of four in 2014.
Pregnancy is no longer a criterion for eligibility for low-income women in states with expanded Medicaid programs—a real game changer for millions of women in need of care.
As of this writing, 27 states, including the District of Columbia, have expanded their Medicaid program. Data from the Centers for Medicare and Medicaid Services (CMS) show that total enrollment in the Children’s Health Insurance Program (CHIP) and Medicaid increased by more than 4.8 million people (from 58.9 million to 63.7 million) between July 2013 and March 2014, in the 47 states reporting data for both periods. Nearly all of this growth occurred in Medicaid expansion states.1,2 More recent data show that 7.9 million more people were enrolled in Medicaid in July 2014 than in the previous year.3,4
Is Medicaid a losing proposition for ObGyns?
In many states, it costs ObGyns more than Medicaid pays to provide primary care to Medicaid patients. Nationally, providers receive 41% less in Medicaid reimbursement than they get with Medicare for primary care services.5 In 2012, the worst offender was Rhode Island’s Medicaid program, which paid physicians only 33% of the Medicare reimbursement rates for primary care.5
The rate of Medicaid reimbursement affects a physician’s willingness to accept new Medicaid patients. Only 50% of physicians are willing to accept new Medicaid patients, compared with 70% who are willing to accept new Medicare or privately insured patients. Twenty-three percent of female Medicaid beneficiaries report a problem finding a new doctor, compared with 7% of Medicare beneficiaries and 13% of privately insured women. The main reason: low Medicaid payment rates.6
In 2007, 38% of all ObGyns accepted Medicaid gynecology patients, and 44% accepted Medicaid obstetric patients, with Medicaid accounting for 18% of revenue for the average ObGyn practice. In its 2013 survey of members, ACOG found that while 63.2% accept all Medicare patients, only 44.4% accept all Medicaid gynecology patients, and 48.7% accept all Medicaid obstetric patients, up from 2007. The percentage of ObGyns who don’t see Medicaid gynecology or obstetric patients was 22.7% and 16.3%, respectively. Only 8.2% of ObGyns see no Medicare patients.7
According to a 2014 survey, 34% of physicians report an increase in Medicaid patients; 41% of those report an increase of 11% or more.3
The American Medical Association – The AMA considers the ObGyn specialty one of four specialties that provide primary care.
Tricare – The health-care program for uniformed service members (active, Guard/Reserve, retired) and their families around the world designates ObGyns as among primary care case managers.
Community Health Teams – A grant program to support primary care practices and patient-centered medical homes includes ObGyns as primary care providers.
Medicaid – Thirty-four states and the District of Columbia define ObGyns as primary care providers.
Medicaid Health Homes – Authorized under federal law to coordinate care for Medicaid enrollees with chronic conditions, health home providers coordinate all primary care, acute, behavioral health, and long-term services and supports to treat the whole person. ObGyns are eligible home health providers.
Health Resources Services Administration – This agency delineates health professional shortage areas, providing bonuses for physicians serving in these areas. It includes ObGyns as one of four primary care specialties.
National Health Service Corps – This organization offers loan repayments and scholarships to primary care providers working in underserved communities and recognizes ObGyns as primary care physicians.
Teaching Health Center Graduate Medical Education – This program supports community-based primary care residency programs to increase the number of primary care residents and dentists trained in geographically isolated or economically or medically vulnerable
communities.
Congress addresses the discrepancy
The ACA Medicaid primary care “bump,” as it’s called, was designed to help ensure access to primary care for the huge new group of individuals covered by Medicaid. It raised Medicaid payment rates for primary care services to Medicare fee levels in 2013 and 2014, an overall average increase of 73% in Medicaid payment rates for Evaluation and Management (E/M) codes 99201–99499, and for vaccine administration codes 90461 and 90471–90474 (FIGURES 1–3).8
   |
The catch? The bump only applies to internists, family medicine physicians, and pediatricians. The House-passed bill included ObGyns—women’s primary care providers—but the Senate bill did not, and the Senate bill was the version ultimately enacted into law.
To qualify for these additional payments, physicians must self-attest to the state Medicaid agency that she or he meets one of the following criteria:
- board certification in family medicine, general internal medicine, or pediatric medicine or a subspecialty recognized by the American Board of Medical Specialties, the American Board of Physician Specialties, or the American Osteopathic Association
- 60% of Medicaid billing involves the specified E/M and vaccine administration codes.
For newly eligible physicians, the previous month’s billing is used to determine eligibility.
What about women’s health?
In speaking with ACOG Fellows, here’s what ACOG President John Jennings, MD, had to say about the omission of ObGyns from the primary care rate bump:
This federal program, which expires at the end of this year, was designed to increase access to needed primary care services for low-income individuals. But as the program stands right now, it leaves out women’s health.
That’s just not right. And we have before us a chance to fix it.
ObGyns deliver primary and preventive care services to women; an ObGyn often is the only doctor a woman sees on a regular basis. Thirty-five state Medicaid programs classify ObGyns as primary care providers.9 Twelve percent of women aged 18 to 64 years rely on Medicaid for their health coverage, and more than 68% of adult Medicaid beneficiaries are women.10
Seeing an opportunity to expand the primary care reimbursement bump to include ObGyns, ACOG recently briefed Congress on the long and deep tradition of primary care in obstetrics and gynecology—a tradition that begins in residency. We provided Congress with ObGyn resident training requirements, as outlined by the Council on Resident Education in Obstetrics and Gynecology (CREOG).11
How ObGyns provide primary care
According to CREOG, “ObGyns provide primary health-care services to their patients both within and outside the traditional purview of reproductive medicine. As primary care physicians, ObGyns establish relationships with their patients that transcend the disease spectrum and extend to routine assessments, preventive care, early intervention, and management of medical disorders.”11
Among the services they provide are age-appropriate screening for substance use, sexual and reproductive health, sexually transmitted infection, psychosocial risks, breast disorders, cancer, and cardiovascular disease. ObGyns routinely counsel patients about diet, exercise, contraception, dental health, osteoporosis, and sexual health. And they provide front-line immunizations against such diseases as influenza, human papillomavirus, rubella, measles, meningitis, hepatitis A and B, and pneumonia.11
Certification in obstetrics and gynecology requires written and oral examinations in office practice and women’s health as a primary care content area. In fact, fully one-third of the board certification test taken by 97% of the ObGyns in this country tests their knowledge and training in primary care.11
How women view their ObGyn
A 2014 survey of women found that ObGyns play a critical role in providing primary care in the United States. Almost six in 10 women (58%) report that they see an ObGyn on a regular basis, and one-third of women (35%) view their ObGyn as their main source of care.12
Low-income women and women of color report that their ObGyn plays an even greater role in their health care. Latinas are far more likely (47%) to report that their ObGyn is their main source of care, compared with 35% of women overall. Sixty-four percent of African-American women say they see an ObGyn regularly, compared with 58% of women overall.12
Other survey findings:
- ObGyn providers are the first providers women choose as adults. They move from a pediatrician to an ObGyn.
- ObGyn providers are the “usual” providers for young women—the ones women see most frequently. They see internal medicine physicians and other providers infrequently during this period of their life.
- The ObGyn-patient relationship is intimate, comfortable, trusting, and confidential, and ObGyns often are considered “a friend.” Patients discuss issues with their ObGyns that are not raised with other providers.
- The care women receive from their -ObGyn provider is broad, ranging from an annual exam and breast exam to prenatal care, immunizations, a review of medications, blood pressure checks, and more.
- Women say their ObGyn does a better job than their family practice and internal medicine providers in providing a range of care and services.12
ObGyns may see relief in 2015
Congress has before it legislation to extend the primary care bump program into the future and expand it to include ObGyns and other women’s health clinicians. A proposal to that effect was introduced in the Senate by Patty Murray (D-WA) and Sherrod Brown (D-OH) and in the House by Reps. Frank Pallone (D-NJ) and Henry Waxman (D-CA).
At press time, ACOG was doing everything in its power to get this legislation passed in the lame duck session of Congress. We scored a big win at the November assembly of the American Medical Association (AMA) House of Delegates, when, with the support of the American Academy of Pediatricians (AAP) and the American College of Physicians, the House of Delegates overwhelmingly approved AMA policy to support extension of the program, including broadening it to include ObGyns.
ACOG also has enlisted the support of national women’s advocacy groups, including Planned Parenthood, the National Partnership for Families and Children, and the National Women’s Law Center. ACOG President Jennings led 35 ACOG leaders from across the country to Washington, DC, in November 2014, to urge members of Congress to pass this important legislation. They were joined by Thomas McInerney, MD, immediate past president of the AAP, and a number of leaders from Planned Parenthood.
Our message to Congress is clear: Not without women!
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With enactment of the Affordable Care Act (ACA) came a number of significant changes to federal and state Medicaid programs to increase access to care for low-income individuals. One landmark change, which became a state option after a ruling by the US Supreme Court, is the expansion of eligibility to all adults who have an income at or below 138% of the federal poverty line, which was $16,105 annually for an individual or $32,913 for a family of four in 2014.
Pregnancy is no longer a criterion for eligibility for low-income women in states with expanded Medicaid programs—a real game changer for millions of women in need of care.
As of this writing, 27 states, including the District of Columbia, have expanded their Medicaid program. Data from the Centers for Medicare and Medicaid Services (CMS) show that total enrollment in the Children’s Health Insurance Program (CHIP) and Medicaid increased by more than 4.8 million people (from 58.9 million to 63.7 million) between July 2013 and March 2014, in the 47 states reporting data for both periods. Nearly all of this growth occurred in Medicaid expansion states.1,2 More recent data show that 7.9 million more people were enrolled in Medicaid in July 2014 than in the previous year.3,4
Is Medicaid a losing proposition for ObGyns?
In many states, it costs ObGyns more than Medicaid pays to provide primary care to Medicaid patients. Nationally, providers receive 41% less in Medicaid reimbursement than they get with Medicare for primary care services.5 In 2012, the worst offender was Rhode Island’s Medicaid program, which paid physicians only 33% of the Medicare reimbursement rates for primary care.5
The rate of Medicaid reimbursement affects a physician’s willingness to accept new Medicaid patients. Only 50% of physicians are willing to accept new Medicaid patients, compared with 70% who are willing to accept new Medicare or privately insured patients. Twenty-three percent of female Medicaid beneficiaries report a problem finding a new doctor, compared with 7% of Medicare beneficiaries and 13% of privately insured women. The main reason: low Medicaid payment rates.6
In 2007, 38% of all ObGyns accepted Medicaid gynecology patients, and 44% accepted Medicaid obstetric patients, with Medicaid accounting for 18% of revenue for the average ObGyn practice. In its 2013 survey of members, ACOG found that while 63.2% accept all Medicare patients, only 44.4% accept all Medicaid gynecology patients, and 48.7% accept all Medicaid obstetric patients, up from 2007. The percentage of ObGyns who don’t see Medicaid gynecology or obstetric patients was 22.7% and 16.3%, respectively. Only 8.2% of ObGyns see no Medicare patients.7
According to a 2014 survey, 34% of physicians report an increase in Medicaid patients; 41% of those report an increase of 11% or more.3
The American Medical Association – The AMA considers the ObGyn specialty one of four specialties that provide primary care.
Tricare – The health-care program for uniformed service members (active, Guard/Reserve, retired) and their families around the world designates ObGyns as among primary care case managers.
Community Health Teams – A grant program to support primary care practices and patient-centered medical homes includes ObGyns as primary care providers.
Medicaid – Thirty-four states and the District of Columbia define ObGyns as primary care providers.
Medicaid Health Homes – Authorized under federal law to coordinate care for Medicaid enrollees with chronic conditions, health home providers coordinate all primary care, acute, behavioral health, and long-term services and supports to treat the whole person. ObGyns are eligible home health providers.
Health Resources Services Administration – This agency delineates health professional shortage areas, providing bonuses for physicians serving in these areas. It includes ObGyns as one of four primary care specialties.
National Health Service Corps – This organization offers loan repayments and scholarships to primary care providers working in underserved communities and recognizes ObGyns as primary care physicians.
Teaching Health Center Graduate Medical Education – This program supports community-based primary care residency programs to increase the number of primary care residents and dentists trained in geographically isolated or economically or medically vulnerable
communities.
Congress addresses the discrepancy
The ACA Medicaid primary care “bump,” as it’s called, was designed to help ensure access to primary care for the huge new group of individuals covered by Medicaid. It raised Medicaid payment rates for primary care services to Medicare fee levels in 2013 and 2014, an overall average increase of 73% in Medicaid payment rates for Evaluation and Management (E/M) codes 99201–99499, and for vaccine administration codes 90461 and 90471–90474 (FIGURES 1–3).8
   |
The catch? The bump only applies to internists, family medicine physicians, and pediatricians. The House-passed bill included ObGyns—women’s primary care providers—but the Senate bill did not, and the Senate bill was the version ultimately enacted into law.
To qualify for these additional payments, physicians must self-attest to the state Medicaid agency that she or he meets one of the following criteria:
- board certification in family medicine, general internal medicine, or pediatric medicine or a subspecialty recognized by the American Board of Medical Specialties, the American Board of Physician Specialties, or the American Osteopathic Association
- 60% of Medicaid billing involves the specified E/M and vaccine administration codes.
For newly eligible physicians, the previous month’s billing is used to determine eligibility.
What about women’s health?
In speaking with ACOG Fellows, here’s what ACOG President John Jennings, MD, had to say about the omission of ObGyns from the primary care rate bump:
This federal program, which expires at the end of this year, was designed to increase access to needed primary care services for low-income individuals. But as the program stands right now, it leaves out women’s health.
That’s just not right. And we have before us a chance to fix it.
ObGyns deliver primary and preventive care services to women; an ObGyn often is the only doctor a woman sees on a regular basis. Thirty-five state Medicaid programs classify ObGyns as primary care providers.9 Twelve percent of women aged 18 to 64 years rely on Medicaid for their health coverage, and more than 68% of adult Medicaid beneficiaries are women.10
Seeing an opportunity to expand the primary care reimbursement bump to include ObGyns, ACOG recently briefed Congress on the long and deep tradition of primary care in obstetrics and gynecology—a tradition that begins in residency. We provided Congress with ObGyn resident training requirements, as outlined by the Council on Resident Education in Obstetrics and Gynecology (CREOG).11
How ObGyns provide primary care
According to CREOG, “ObGyns provide primary health-care services to their patients both within and outside the traditional purview of reproductive medicine. As primary care physicians, ObGyns establish relationships with their patients that transcend the disease spectrum and extend to routine assessments, preventive care, early intervention, and management of medical disorders.”11
Among the services they provide are age-appropriate screening for substance use, sexual and reproductive health, sexually transmitted infection, psychosocial risks, breast disorders, cancer, and cardiovascular disease. ObGyns routinely counsel patients about diet, exercise, contraception, dental health, osteoporosis, and sexual health. And they provide front-line immunizations against such diseases as influenza, human papillomavirus, rubella, measles, meningitis, hepatitis A and B, and pneumonia.11
Certification in obstetrics and gynecology requires written and oral examinations in office practice and women’s health as a primary care content area. In fact, fully one-third of the board certification test taken by 97% of the ObGyns in this country tests their knowledge and training in primary care.11
How women view their ObGyn
A 2014 survey of women found that ObGyns play a critical role in providing primary care in the United States. Almost six in 10 women (58%) report that they see an ObGyn on a regular basis, and one-third of women (35%) view their ObGyn as their main source of care.12
Low-income women and women of color report that their ObGyn plays an even greater role in their health care. Latinas are far more likely (47%) to report that their ObGyn is their main source of care, compared with 35% of women overall. Sixty-four percent of African-American women say they see an ObGyn regularly, compared with 58% of women overall.12
Other survey findings:
- ObGyn providers are the first providers women choose as adults. They move from a pediatrician to an ObGyn.
- ObGyn providers are the “usual” providers for young women—the ones women see most frequently. They see internal medicine physicians and other providers infrequently during this period of their life.
- The ObGyn-patient relationship is intimate, comfortable, trusting, and confidential, and ObGyns often are considered “a friend.” Patients discuss issues with their ObGyns that are not raised with other providers.
- The care women receive from their -ObGyn provider is broad, ranging from an annual exam and breast exam to prenatal care, immunizations, a review of medications, blood pressure checks, and more.
- Women say their ObGyn does a better job than their family practice and internal medicine providers in providing a range of care and services.12
ObGyns may see relief in 2015
Congress has before it legislation to extend the primary care bump program into the future and expand it to include ObGyns and other women’s health clinicians. A proposal to that effect was introduced in the Senate by Patty Murray (D-WA) and Sherrod Brown (D-OH) and in the House by Reps. Frank Pallone (D-NJ) and Henry Waxman (D-CA).
At press time, ACOG was doing everything in its power to get this legislation passed in the lame duck session of Congress. We scored a big win at the November assembly of the American Medical Association (AMA) House of Delegates, when, with the support of the American Academy of Pediatricians (AAP) and the American College of Physicians, the House of Delegates overwhelmingly approved AMA policy to support extension of the program, including broadening it to include ObGyns.
ACOG also has enlisted the support of national women’s advocacy groups, including Planned Parenthood, the National Partnership for Families and Children, and the National Women’s Law Center. ACOG President Jennings led 35 ACOG leaders from across the country to Washington, DC, in November 2014, to urge members of Congress to pass this important legislation. They were joined by Thomas McInerney, MD, immediate past president of the AAP, and a number of leaders from Planned Parenthood.
Our message to Congress is clear: Not without women!
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Kaiser Family Foundation. Implementing the ACA: Medicaid Spending & Enrollment Growth for FY 2014 and FY 2015. http://kff.org/medicaid/issue-brief/implementing-the-aca-medicaid-spending-enrollment-growth-for-fy-2014-and-fy-2015/. Published October 14, 2014. Accessed December 15, 2014.
2. Center for Medicare and Medicaid Services. Medicaid and CHIP: March 2014 Monthly Applications, Eligibility Determinations, and Enrollment Report. http://www.medicaid.gov/AffordableCareAct/Medicaid-Moving-Forward-2014/Downloads/March-2014-Enrollment-Report.pdf. May 1, 2014. Accessed December 17, 2014.
3. Kane L, Peckham C. Insurer Ratings Report 2014. Medscape. http://www.medscape.com/features/slideshow /public/insurerratingsreport2014. Published October 21, 2014. Accessed December 15, 2014.
4. Kenney GM, Zuckerman S, Dubay L, et al. Opting in to the Medicaid expansion under the ACA: who are the uninsured adults who could gain health insurance coverage? Timely Analysis of Immediate Health Policy Issues. Urban Institute. http://www.urban.org/uploadedpdf/412630-opting-in-medicaid.pdf. Published August 2012. Accessed December 15, 2014.
5. Kaiser Family Foundation. Medicaid-to-Medicare Fee Index. http://kff.org/medicaid/state-indicator/medicaid-to-medicare-fee-index/. Updated November 11, 2014. Accessed December 15, 2014.
6. Kaiser Family Foundation. Women and Health Care: A National Profile. Key Findings from the Kaiser Women’s Health Survey. http://kaiserfamilyfoundation.files.wordpress.com/2013/01/women-and-health-care-a-national-profile-key-findings-from-the-kaiser-women-s-health-survey.pdf. Published July 2005. Accessed December 15, 2014.
7. ACOG 2013 survey of members. http://www.acog.org/~/media/Departments/Practice-Management-and-Managed-Care/2013SocioeconomicSurvey.pdf. Accessed December 17, 2014.
8. Zuckerman S, Goin D, Kaiser Family Foundation. How much will Medicaid physician fees for primary care rise in 2013? Evidence from a 2012 survey of Medicaid physician fees. http://kff.org/medicaid/issue-brief/how-much-will-medicaid-physician-fees-for/. Published December 13, 2012. Accessed December 15, 2014.
9. Based on an ACOG review of state Medicaid regulations, statutes, and provider manuals.
10. National Women’s Law Center. Battles over Medicaid Funding and Eligibility: What’s at Stake for Women. http://nwlc.org/sites/default/files/pdfs/national.pdf. Published June 2011. Accessed December 15, 2014.
11. Carey JC, Blanchard MH, Adams KE, et al; Education Committee of the Council on Resident Education in Obstetrics and Gynecology (CREOG). CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology, 10th ed. American Congress of Obstetricians and Gynecologists. http://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/CREOG-Educational-Objectives. Published 2013. Accessed December 15, 2014.
12. Montefiore Investigators to Present Data at American Congress of Obstetricians and Gynecologists Annual Meeting [news release]. April 25, 2014. http://www.montefiore.org/body.cfm?id=1738&action=detail&ref=1142. Accessed December 17, 2014.
1. Kaiser Family Foundation. Implementing the ACA: Medicaid Spending & Enrollment Growth for FY 2014 and FY 2015. http://kff.org/medicaid/issue-brief/implementing-the-aca-medicaid-spending-enrollment-growth-for-fy-2014-and-fy-2015/. Published October 14, 2014. Accessed December 15, 2014.
2. Center for Medicare and Medicaid Services. Medicaid and CHIP: March 2014 Monthly Applications, Eligibility Determinations, and Enrollment Report. http://www.medicaid.gov/AffordableCareAct/Medicaid-Moving-Forward-2014/Downloads/March-2014-Enrollment-Report.pdf. May 1, 2014. Accessed December 17, 2014.
3. Kane L, Peckham C. Insurer Ratings Report 2014. Medscape. http://www.medscape.com/features/slideshow /public/insurerratingsreport2014. Published October 21, 2014. Accessed December 15, 2014.
4. Kenney GM, Zuckerman S, Dubay L, et al. Opting in to the Medicaid expansion under the ACA: who are the uninsured adults who could gain health insurance coverage? Timely Analysis of Immediate Health Policy Issues. Urban Institute. http://www.urban.org/uploadedpdf/412630-opting-in-medicaid.pdf. Published August 2012. Accessed December 15, 2014.
5. Kaiser Family Foundation. Medicaid-to-Medicare Fee Index. http://kff.org/medicaid/state-indicator/medicaid-to-medicare-fee-index/. Updated November 11, 2014. Accessed December 15, 2014.
6. Kaiser Family Foundation. Women and Health Care: A National Profile. Key Findings from the Kaiser Women’s Health Survey. http://kaiserfamilyfoundation.files.wordpress.com/2013/01/women-and-health-care-a-national-profile-key-findings-from-the-kaiser-women-s-health-survey.pdf. Published July 2005. Accessed December 15, 2014.
7. ACOG 2013 survey of members. http://www.acog.org/~/media/Departments/Practice-Management-and-Managed-Care/2013SocioeconomicSurvey.pdf. Accessed December 17, 2014.
8. Zuckerman S, Goin D, Kaiser Family Foundation. How much will Medicaid physician fees for primary care rise in 2013? Evidence from a 2012 survey of Medicaid physician fees. http://kff.org/medicaid/issue-brief/how-much-will-medicaid-physician-fees-for/. Published December 13, 2012. Accessed December 15, 2014.
9. Based on an ACOG review of state Medicaid regulations, statutes, and provider manuals.
10. National Women’s Law Center. Battles over Medicaid Funding and Eligibility: What’s at Stake for Women. http://nwlc.org/sites/default/files/pdfs/national.pdf. Published June 2011. Accessed December 15, 2014.
11. Carey JC, Blanchard MH, Adams KE, et al; Education Committee of the Council on Resident Education in Obstetrics and Gynecology (CREOG). CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology, 10th ed. American Congress of Obstetricians and Gynecologists. http://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/CREOG-Educational-Objectives. Published 2013. Accessed December 15, 2014.
12. Montefiore Investigators to Present Data at American Congress of Obstetricians and Gynecologists Annual Meeting [news release]. April 25, 2014. http://www.montefiore.org/body.cfm?id=1738&action=detail&ref=1142. Accessed December 17, 2014.
Consider a mandibular positioning device to alleviate sleep-disordered breathing
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
Have you RULED O2uT medical illness in the presumptive psychiatric patient?
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
Second of 2 parts: The mysteries of psychiatry maintenance of certification, further unraveled
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
Choosing a treatment for disruptive, impulse-control, and conduct disorders
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
Assessing tremor to rule out psychogenic origin: It’s tricky
Tremors are a rhythmic and oscillatory movement of a body part with a relatively constant frequency.1 Several subtypes of tremors are classified on the basis of whether they occur during static or kinetic body positioning. Assessing tremors to rule out psychogenic origin is one of the trickiest tasks for a psychiatrist (Table 12). Non-organic movement disorders are not rare, and all common organic movement disorders can be mimicked by non-organic presentations.
Diagnostic approach
Start by categorizing the tremor based on its activation condition (at rest, kinetic or intentional, postural or isometric), topographic distribution, and frequency. Observe the patient sitting in a chair with his hands on his lap for resting tremor. Postural or kinetic tremors can be assessed by stretching the arms and performing a finger-to-nose test. A resting tremor can indicate parkinsonism; intention tremor may indicate a cerebellar lesion. A psychogenic tremor can occur at rest or during postural or active movement, and often will occur in all 3 situations (Table 2).1-3
Some of the maneuvers listed in Table 3 are helpful to distinguish a psychogenic from an organic cause. The key is to look for variability in direction, amplitude, and frequency. Psychogenic tremor often increases when the limb is examined and reduces upon distraction, and also might be exacerbated with movement of other limbs. Patients with psychogenic tremor often have other “non-organic” neurologic signs, such as give-way weakness, deliberate slowness carrying out requested voluntary movement, and sensory signs that contradict neuroanatomical principles.
Investigation
Proceed as follows:
1. Perform laboratory testing: thyroid function panel and serum copper and ceruloplasmin levels.2
2. Perform surface electromyography to differentiate Parkinson’s disease and benign tremor disorders.2
3. Obtain a MRI to assess atypical tremor; findings might reveal Wilson’s disease (basal ganglia and brainstem involvement) or fragile X-associated tremor/ataxia syndrome (pontocerebellar hypoplasia or cerebral white matter involvement).3
4. Consider dopaminergic functional imaging scanning. When positive, the scan can reveal symptoms of parkinsonism; negative findings can help consolidate a diagnosis of psychogenic tremor.3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology. 2000;54(11 suppl 4):S7.
2. Alty JE, Kempster PA. A practical guide to the differential diagnosis of tremor. Postgrad Med J. 2011;87(1031):623-629.
3. Crawford P, Zimmerman EE. Differentiation and diagnosis of tremor. Am Fam Physician. 2011;83(6):697-702.
Tremors are a rhythmic and oscillatory movement of a body part with a relatively constant frequency.1 Several subtypes of tremors are classified on the basis of whether they occur during static or kinetic body positioning. Assessing tremors to rule out psychogenic origin is one of the trickiest tasks for a psychiatrist (Table 12). Non-organic movement disorders are not rare, and all common organic movement disorders can be mimicked by non-organic presentations.
Diagnostic approach
Start by categorizing the tremor based on its activation condition (at rest, kinetic or intentional, postural or isometric), topographic distribution, and frequency. Observe the patient sitting in a chair with his hands on his lap for resting tremor. Postural or kinetic tremors can be assessed by stretching the arms and performing a finger-to-nose test. A resting tremor can indicate parkinsonism; intention tremor may indicate a cerebellar lesion. A psychogenic tremor can occur at rest or during postural or active movement, and often will occur in all 3 situations (Table 2).1-3
Some of the maneuvers listed in Table 3 are helpful to distinguish a psychogenic from an organic cause. The key is to look for variability in direction, amplitude, and frequency. Psychogenic tremor often increases when the limb is examined and reduces upon distraction, and also might be exacerbated with movement of other limbs. Patients with psychogenic tremor often have other “non-organic” neurologic signs, such as give-way weakness, deliberate slowness carrying out requested voluntary movement, and sensory signs that contradict neuroanatomical principles.
Investigation
Proceed as follows:
1. Perform laboratory testing: thyroid function panel and serum copper and ceruloplasmin levels.2
2. Perform surface electromyography to differentiate Parkinson’s disease and benign tremor disorders.2
3. Obtain a MRI to assess atypical tremor; findings might reveal Wilson’s disease (basal ganglia and brainstem involvement) or fragile X-associated tremor/ataxia syndrome (pontocerebellar hypoplasia or cerebral white matter involvement).3
4. Consider dopaminergic functional imaging scanning. When positive, the scan can reveal symptoms of parkinsonism; negative findings can help consolidate a diagnosis of psychogenic tremor.3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Tremors are a rhythmic and oscillatory movement of a body part with a relatively constant frequency.1 Several subtypes of tremors are classified on the basis of whether they occur during static or kinetic body positioning. Assessing tremors to rule out psychogenic origin is one of the trickiest tasks for a psychiatrist (Table 12). Non-organic movement disorders are not rare, and all common organic movement disorders can be mimicked by non-organic presentations.
Diagnostic approach
Start by categorizing the tremor based on its activation condition (at rest, kinetic or intentional, postural or isometric), topographic distribution, and frequency. Observe the patient sitting in a chair with his hands on his lap for resting tremor. Postural or kinetic tremors can be assessed by stretching the arms and performing a finger-to-nose test. A resting tremor can indicate parkinsonism; intention tremor may indicate a cerebellar lesion. A psychogenic tremor can occur at rest or during postural or active movement, and often will occur in all 3 situations (Table 2).1-3
Some of the maneuvers listed in Table 3 are helpful to distinguish a psychogenic from an organic cause. The key is to look for variability in direction, amplitude, and frequency. Psychogenic tremor often increases when the limb is examined and reduces upon distraction, and also might be exacerbated with movement of other limbs. Patients with psychogenic tremor often have other “non-organic” neurologic signs, such as give-way weakness, deliberate slowness carrying out requested voluntary movement, and sensory signs that contradict neuroanatomical principles.
Investigation
Proceed as follows:
1. Perform laboratory testing: thyroid function panel and serum copper and ceruloplasmin levels.2
2. Perform surface electromyography to differentiate Parkinson’s disease and benign tremor disorders.2
3. Obtain a MRI to assess atypical tremor; findings might reveal Wilson’s disease (basal ganglia and brainstem involvement) or fragile X-associated tremor/ataxia syndrome (pontocerebellar hypoplasia or cerebral white matter involvement).3
4. Consider dopaminergic functional imaging scanning. When positive, the scan can reveal symptoms of parkinsonism; negative findings can help consolidate a diagnosis of psychogenic tremor.3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology. 2000;54(11 suppl 4):S7.
2. Alty JE, Kempster PA. A practical guide to the differential diagnosis of tremor. Postgrad Med J. 2011;87(1031):623-629.
3. Crawford P, Zimmerman EE. Differentiation and diagnosis of tremor. Am Fam Physician. 2011;83(6):697-702.
1. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology. 2000;54(11 suppl 4):S7.
2. Alty JE, Kempster PA. A practical guide to the differential diagnosis of tremor. Postgrad Med J. 2011;87(1031):623-629.
3. Crawford P, Zimmerman EE. Differentiation and diagnosis of tremor. Am Fam Physician. 2011;83(6):697-702.
Suvorexant for sleep-onset insomnia or sleep-maintenance insomnia, or both
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.