User login
6 Strategies to address risk factors for school violence
School shootings engender the deepest of public concern. They violate strongly held cross-culture beliefs about the sanctity of childhood and the obligation to protect children from harm.
Prevention and intervention approaches to school shootings have emerged (1) in the literature, from case studies, and (2) from discourse among experts.1 Approaches include:
• bolstering security at schools
• reducing the facilities’ vulnerability to intrusion
• increasing the capacity to respond at the moment of threat
• transforming the school climate
• increasing attachment and bonding.1,2
Psychiatrists often are consulted by school districts to provide expertise for the latter 2 approaches. Using the following strategies, you can help address risk factors for school violence.
Strengthen school attachment. Develop curricular and extracurricular programs for students that create, and contribute to, a sense of belonging. This, in turn, decreases alienation and reduces hostility. Unaddressed hostility can lead to depression, anger, and, subsequently, violence.
Reduce social aggression. Social aggression, such as teasing, taunting, humiliating, and bullying, is an important predictor of developmental outcomes in victims and perpetrators.3 Social aggression has been linked to peer victimization and low school attachment. Implement social skills programs, such as Making Choices, which have yielded positive effects on social aggression in elementary school students.4
Break codes of silence. This can involve encouraging schools to:
• develop an anonymous mechanism of voicing concerns
• take diligent action based on students’ concerns
• treat disclosures discreetly.
Establish resources for troubled and rejected students. Develop routine emergency modes of communication, such as a protocol for high-priority referral to mental health resources. These could reduce the likelihood of students acting out against the school.
Recommend that security be enhanced. Establishing the position of school resource officer might increase confidence and decrease feelings of vulnerability among teachers, students, and parents. This can increase the perception of school security, potentially helps school attachment, and promotes breaking down codes of silence.5
Increase communication within the school, and between the school and law enforcement agencies. Effective communication can help identify the location of an attacker and disrupt a developing event. Create an alert system to notify students, faculty, and parents with an automated text message or phone call during an emergency. Increased accessibility of the students by the school alert system might be a quicker way to reach the school community. Work with security agencies to develop a protocol for communicating and assessing threat potential. Also, develop guidelines to outline referral and assessing procedures for students whose writings may present indication for possible attack or whose class behavior may be alienating or intimidating to either faculty or other students. Behavior that can lead to school violence is outlined in the Table.
You also can educate school administrators about the following:
• School violence has been significantly associated with mental health problems, such as depression and inability to form age appropriate social connections,6 which in combination with extreme social rejection and specific personality-related issues (eg, antisocial personality disorder) can culminate in violent outbreaks.7 Work closely with school nurses and counselors to identify and treat vulnerable students.
• In most multiple-victim incidents, more than 1 person had information about the attack before it occurred that was not communicated to an authority figure. Educate school officials about being sensitive to warnings or threats about possible attack, and help develop ways get counseling for potential attackers.2
• Zero-tolerance policies are ineffective at preventing school shootings, mostly because of literal interpretation and inconsistent implementation of such policies.8 Help circumvent a more stringent zero-tolerance policy with adequate availability of mental health care for students who are identified as being at risk of perpetrating an attack.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Culley MR, Conkling M, Emshoff J, et al. Environmental and contextual influences on school violence and its prevention. J Prim Prev. 2006;27(3):217-227.
2. Wike TL, Fraser MW. School shooting: making sense of the senseless. Aggress Violent Behav. 2009;14(3):162-169.
3. Rudatsikira E, Singh P, Job J, et al. Variables associated with weapon-carrying among young adolescents in southern California. J Adolesc Health. 2007;40(5):470-473.
4. Fraser MW, Galinsky MJ, Smokowski PR, et al. Social information-processing skills training to promote social competence and prevent aggressive behavior in the third grades. J Consult Clin Psychol. 2005;73(6):1045-1055.
5. Finn P. School resource officer programs. Finding the funding, reaping the benefits. FBI Law Enforcement Bulletin. 2006;75(8):1-13.
6. Ferguson C, Coulson M, Barnett J. Psychological profiles of school shooters: positive directions and one big wrong turn. J Police Crisis Negot. 2011;11:1-17.
7. Leary MR, Kowalski RM, Smith L, et al. Teasing, rejection and violence: case studies of the school shootings. Aggressive Behavior. 2003;29(3):202-214.
8. American Psychological Association Zero Tolerance Task Force. Are zero tolerance policies effective in the schools?: an evidentiary review and recommendation. Am Psychol. 2008;63(9):852-862.
School shootings engender the deepest of public concern. They violate strongly held cross-culture beliefs about the sanctity of childhood and the obligation to protect children from harm.
Prevention and intervention approaches to school shootings have emerged (1) in the literature, from case studies, and (2) from discourse among experts.1 Approaches include:
• bolstering security at schools
• reducing the facilities’ vulnerability to intrusion
• increasing the capacity to respond at the moment of threat
• transforming the school climate
• increasing attachment and bonding.1,2
Psychiatrists often are consulted by school districts to provide expertise for the latter 2 approaches. Using the following strategies, you can help address risk factors for school violence.
Strengthen school attachment. Develop curricular and extracurricular programs for students that create, and contribute to, a sense of belonging. This, in turn, decreases alienation and reduces hostility. Unaddressed hostility can lead to depression, anger, and, subsequently, violence.
Reduce social aggression. Social aggression, such as teasing, taunting, humiliating, and bullying, is an important predictor of developmental outcomes in victims and perpetrators.3 Social aggression has been linked to peer victimization and low school attachment. Implement social skills programs, such as Making Choices, which have yielded positive effects on social aggression in elementary school students.4
Break codes of silence. This can involve encouraging schools to:
• develop an anonymous mechanism of voicing concerns
• take diligent action based on students’ concerns
• treat disclosures discreetly.
Establish resources for troubled and rejected students. Develop routine emergency modes of communication, such as a protocol for high-priority referral to mental health resources. These could reduce the likelihood of students acting out against the school.
Recommend that security be enhanced. Establishing the position of school resource officer might increase confidence and decrease feelings of vulnerability among teachers, students, and parents. This can increase the perception of school security, potentially helps school attachment, and promotes breaking down codes of silence.5
Increase communication within the school, and between the school and law enforcement agencies. Effective communication can help identify the location of an attacker and disrupt a developing event. Create an alert system to notify students, faculty, and parents with an automated text message or phone call during an emergency. Increased accessibility of the students by the school alert system might be a quicker way to reach the school community. Work with security agencies to develop a protocol for communicating and assessing threat potential. Also, develop guidelines to outline referral and assessing procedures for students whose writings may present indication for possible attack or whose class behavior may be alienating or intimidating to either faculty or other students. Behavior that can lead to school violence is outlined in the Table.
You also can educate school administrators about the following:
• School violence has been significantly associated with mental health problems, such as depression and inability to form age appropriate social connections,6 which in combination with extreme social rejection and specific personality-related issues (eg, antisocial personality disorder) can culminate in violent outbreaks.7 Work closely with school nurses and counselors to identify and treat vulnerable students.
• In most multiple-victim incidents, more than 1 person had information about the attack before it occurred that was not communicated to an authority figure. Educate school officials about being sensitive to warnings or threats about possible attack, and help develop ways get counseling for potential attackers.2
• Zero-tolerance policies are ineffective at preventing school shootings, mostly because of literal interpretation and inconsistent implementation of such policies.8 Help circumvent a more stringent zero-tolerance policy with adequate availability of mental health care for students who are identified as being at risk of perpetrating an attack.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
School shootings engender the deepest of public concern. They violate strongly held cross-culture beliefs about the sanctity of childhood and the obligation to protect children from harm.
Prevention and intervention approaches to school shootings have emerged (1) in the literature, from case studies, and (2) from discourse among experts.1 Approaches include:
• bolstering security at schools
• reducing the facilities’ vulnerability to intrusion
• increasing the capacity to respond at the moment of threat
• transforming the school climate
• increasing attachment and bonding.1,2
Psychiatrists often are consulted by school districts to provide expertise for the latter 2 approaches. Using the following strategies, you can help address risk factors for school violence.
Strengthen school attachment. Develop curricular and extracurricular programs for students that create, and contribute to, a sense of belonging. This, in turn, decreases alienation and reduces hostility. Unaddressed hostility can lead to depression, anger, and, subsequently, violence.
Reduce social aggression. Social aggression, such as teasing, taunting, humiliating, and bullying, is an important predictor of developmental outcomes in victims and perpetrators.3 Social aggression has been linked to peer victimization and low school attachment. Implement social skills programs, such as Making Choices, which have yielded positive effects on social aggression in elementary school students.4
Break codes of silence. This can involve encouraging schools to:
• develop an anonymous mechanism of voicing concerns
• take diligent action based on students’ concerns
• treat disclosures discreetly.
Establish resources for troubled and rejected students. Develop routine emergency modes of communication, such as a protocol for high-priority referral to mental health resources. These could reduce the likelihood of students acting out against the school.
Recommend that security be enhanced. Establishing the position of school resource officer might increase confidence and decrease feelings of vulnerability among teachers, students, and parents. This can increase the perception of school security, potentially helps school attachment, and promotes breaking down codes of silence.5
Increase communication within the school, and between the school and law enforcement agencies. Effective communication can help identify the location of an attacker and disrupt a developing event. Create an alert system to notify students, faculty, and parents with an automated text message or phone call during an emergency. Increased accessibility of the students by the school alert system might be a quicker way to reach the school community. Work with security agencies to develop a protocol for communicating and assessing threat potential. Also, develop guidelines to outline referral and assessing procedures for students whose writings may present indication for possible attack or whose class behavior may be alienating or intimidating to either faculty or other students. Behavior that can lead to school violence is outlined in the Table.
You also can educate school administrators about the following:
• School violence has been significantly associated with mental health problems, such as depression and inability to form age appropriate social connections,6 which in combination with extreme social rejection and specific personality-related issues (eg, antisocial personality disorder) can culminate in violent outbreaks.7 Work closely with school nurses and counselors to identify and treat vulnerable students.
• In most multiple-victim incidents, more than 1 person had information about the attack before it occurred that was not communicated to an authority figure. Educate school officials about being sensitive to warnings or threats about possible attack, and help develop ways get counseling for potential attackers.2
• Zero-tolerance policies are ineffective at preventing school shootings, mostly because of literal interpretation and inconsistent implementation of such policies.8 Help circumvent a more stringent zero-tolerance policy with adequate availability of mental health care for students who are identified as being at risk of perpetrating an attack.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Culley MR, Conkling M, Emshoff J, et al. Environmental and contextual influences on school violence and its prevention. J Prim Prev. 2006;27(3):217-227.
2. Wike TL, Fraser MW. School shooting: making sense of the senseless. Aggress Violent Behav. 2009;14(3):162-169.
3. Rudatsikira E, Singh P, Job J, et al. Variables associated with weapon-carrying among young adolescents in southern California. J Adolesc Health. 2007;40(5):470-473.
4. Fraser MW, Galinsky MJ, Smokowski PR, et al. Social information-processing skills training to promote social competence and prevent aggressive behavior in the third grades. J Consult Clin Psychol. 2005;73(6):1045-1055.
5. Finn P. School resource officer programs. Finding the funding, reaping the benefits. FBI Law Enforcement Bulletin. 2006;75(8):1-13.
6. Ferguson C, Coulson M, Barnett J. Psychological profiles of school shooters: positive directions and one big wrong turn. J Police Crisis Negot. 2011;11:1-17.
7. Leary MR, Kowalski RM, Smith L, et al. Teasing, rejection and violence: case studies of the school shootings. Aggressive Behavior. 2003;29(3):202-214.
8. American Psychological Association Zero Tolerance Task Force. Are zero tolerance policies effective in the schools?: an evidentiary review and recommendation. Am Psychol. 2008;63(9):852-862.
1. Culley MR, Conkling M, Emshoff J, et al. Environmental and contextual influences on school violence and its prevention. J Prim Prev. 2006;27(3):217-227.
2. Wike TL, Fraser MW. School shooting: making sense of the senseless. Aggress Violent Behav. 2009;14(3):162-169.
3. Rudatsikira E, Singh P, Job J, et al. Variables associated with weapon-carrying among young adolescents in southern California. J Adolesc Health. 2007;40(5):470-473.
4. Fraser MW, Galinsky MJ, Smokowski PR, et al. Social information-processing skills training to promote social competence and prevent aggressive behavior in the third grades. J Consult Clin Psychol. 2005;73(6):1045-1055.
5. Finn P. School resource officer programs. Finding the funding, reaping the benefits. FBI Law Enforcement Bulletin. 2006;75(8):1-13.
6. Ferguson C, Coulson M, Barnett J. Psychological profiles of school shooters: positive directions and one big wrong turn. J Police Crisis Negot. 2011;11:1-17.
7. Leary MR, Kowalski RM, Smith L, et al. Teasing, rejection and violence: case studies of the school shootings. Aggressive Behavior. 2003;29(3):202-214.
8. American Psychological Association Zero Tolerance Task Force. Are zero tolerance policies effective in the schools?: an evidentiary review and recommendation. Am Psychol. 2008;63(9):852-862.
‘No SAD Me’: A memory device for treating bipolar depression with an antidepressant
Depression is the first affective episode in >50% of patients with bipolar disorder, and is associated with considerable morbidity and mortality.
The mean duration of a bipolar depressive episode is considerably longer than a manic episode; >20% of bipolar depressive episodes run a chronic course.1 Evidence suggests that depressive episodes and symptoms are equal to, or more disabling than, corresponding levels of manic or hypomanic symptoms.2
Debate over appropriate therapy
Using antidepressants to treat bipolar depression remains controversial. Much of the debate surrounds concern that antidepressants have the potential to switch a patient to mania/hypomania or to destabilize mood over the longitudinal course of illness.2
Several guidelines for informing the use of antidepressants in bipolar depression have been published, including the International Society for Bipolar Disorders task force report on antidepressant use in bipolar disorders3 and the guideline of the World Federation of Societies of Biological Psychiatry.4 To summarize the most recent consensus on treating bipolar depression, we devised the mnemonic No SAD Me:
No n-antidepressant treatments should be considered as monotherapy before antidepressants are used. Consider lithium, lamotrigine, olanzapine, quetiapine, or lurasidone first for bipolar depression.3
S afe-to-use adjunctive antidepressants can be considered if the patient relapses to a depressive episode after antidepressant therapy is stopped. Consider using a selective serotonin reuptake inhibitor (SSRI) and bupropion (1) for an acute bipolar I or II depressive episode when the patient has a history of a positive response to an antidepressant and (2) as maintenance treatment with SSRIs and bupropion as adjunctive therapy.2,3
A void antidepressants as monotherapy. If using an antidepressant to treat bipolar I disorder, prescribe a mood-stabilizer concomitantly, even though the evidence for antidepressant-associated mood-switching is mixed and the ability of mood stabilizers to prevent such responses to antidepressant treatment is unproven.
D o not use tricyclic antidepressants (TCAs) or venlafaxine. Evidence does not show 1 type of antidepressant is more or less effective or dangerous than another. Nevertheless, TCAs and venlafaxine appear to carry a particularly high risk of inducing pathologically elevated states of mood and behavior.3
M onitor closely. Bipolar disorder patients who are being started on an antidepressant should be closely monitored for signs of hypomania or mania and increased psychomotor agitation. Discontinue the antidepressant if such signs are observed or emerge.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sidor MM, MacQueen GM. An update on antidepressant use in bipolar depression. Curr Psychiatry Rep. 2012;14(6):696-704.
2. Pacchiarotti I, Mazzarini L, Colom F, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120(6):429-440.
3. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013; 170(11):1249-1262.
4. Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force On Treatment Guidelines For Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11:81-109.
Depression is the first affective episode in >50% of patients with bipolar disorder, and is associated with considerable morbidity and mortality.
The mean duration of a bipolar depressive episode is considerably longer than a manic episode; >20% of bipolar depressive episodes run a chronic course.1 Evidence suggests that depressive episodes and symptoms are equal to, or more disabling than, corresponding levels of manic or hypomanic symptoms.2
Debate over appropriate therapy
Using antidepressants to treat bipolar depression remains controversial. Much of the debate surrounds concern that antidepressants have the potential to switch a patient to mania/hypomania or to destabilize mood over the longitudinal course of illness.2
Several guidelines for informing the use of antidepressants in bipolar depression have been published, including the International Society for Bipolar Disorders task force report on antidepressant use in bipolar disorders3 and the guideline of the World Federation of Societies of Biological Psychiatry.4 To summarize the most recent consensus on treating bipolar depression, we devised the mnemonic No SAD Me:
No n-antidepressant treatments should be considered as monotherapy before antidepressants are used. Consider lithium, lamotrigine, olanzapine, quetiapine, or lurasidone first for bipolar depression.3
S afe-to-use adjunctive antidepressants can be considered if the patient relapses to a depressive episode after antidepressant therapy is stopped. Consider using a selective serotonin reuptake inhibitor (SSRI) and bupropion (1) for an acute bipolar I or II depressive episode when the patient has a history of a positive response to an antidepressant and (2) as maintenance treatment with SSRIs and bupropion as adjunctive therapy.2,3
A void antidepressants as monotherapy. If using an antidepressant to treat bipolar I disorder, prescribe a mood-stabilizer concomitantly, even though the evidence for antidepressant-associated mood-switching is mixed and the ability of mood stabilizers to prevent such responses to antidepressant treatment is unproven.
D o not use tricyclic antidepressants (TCAs) or venlafaxine. Evidence does not show 1 type of antidepressant is more or less effective or dangerous than another. Nevertheless, TCAs and venlafaxine appear to carry a particularly high risk of inducing pathologically elevated states of mood and behavior.3
M onitor closely. Bipolar disorder patients who are being started on an antidepressant should be closely monitored for signs of hypomania or mania and increased psychomotor agitation. Discontinue the antidepressant if such signs are observed or emerge.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is the first affective episode in >50% of patients with bipolar disorder, and is associated with considerable morbidity and mortality.
The mean duration of a bipolar depressive episode is considerably longer than a manic episode; >20% of bipolar depressive episodes run a chronic course.1 Evidence suggests that depressive episodes and symptoms are equal to, or more disabling than, corresponding levels of manic or hypomanic symptoms.2
Debate over appropriate therapy
Using antidepressants to treat bipolar depression remains controversial. Much of the debate surrounds concern that antidepressants have the potential to switch a patient to mania/hypomania or to destabilize mood over the longitudinal course of illness.2
Several guidelines for informing the use of antidepressants in bipolar depression have been published, including the International Society for Bipolar Disorders task force report on antidepressant use in bipolar disorders3 and the guideline of the World Federation of Societies of Biological Psychiatry.4 To summarize the most recent consensus on treating bipolar depression, we devised the mnemonic No SAD Me:
No n-antidepressant treatments should be considered as monotherapy before antidepressants are used. Consider lithium, lamotrigine, olanzapine, quetiapine, or lurasidone first for bipolar depression.3
S afe-to-use adjunctive antidepressants can be considered if the patient relapses to a depressive episode after antidepressant therapy is stopped. Consider using a selective serotonin reuptake inhibitor (SSRI) and bupropion (1) for an acute bipolar I or II depressive episode when the patient has a history of a positive response to an antidepressant and (2) as maintenance treatment with SSRIs and bupropion as adjunctive therapy.2,3
A void antidepressants as monotherapy. If using an antidepressant to treat bipolar I disorder, prescribe a mood-stabilizer concomitantly, even though the evidence for antidepressant-associated mood-switching is mixed and the ability of mood stabilizers to prevent such responses to antidepressant treatment is unproven.
D o not use tricyclic antidepressants (TCAs) or venlafaxine. Evidence does not show 1 type of antidepressant is more or less effective or dangerous than another. Nevertheless, TCAs and venlafaxine appear to carry a particularly high risk of inducing pathologically elevated states of mood and behavior.3
M onitor closely. Bipolar disorder patients who are being started on an antidepressant should be closely monitored for signs of hypomania or mania and increased psychomotor agitation. Discontinue the antidepressant if such signs are observed or emerge.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sidor MM, MacQueen GM. An update on antidepressant use in bipolar depression. Curr Psychiatry Rep. 2012;14(6):696-704.
2. Pacchiarotti I, Mazzarini L, Colom F, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120(6):429-440.
3. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013; 170(11):1249-1262.
4. Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force On Treatment Guidelines For Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11:81-109.
1. Sidor MM, MacQueen GM. An update on antidepressant use in bipolar depression. Curr Psychiatry Rep. 2012;14(6):696-704.
2. Pacchiarotti I, Mazzarini L, Colom F, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120(6):429-440.
3. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013; 170(11):1249-1262.
4. Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force On Treatment Guidelines For Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11:81-109.
Can social media help mental health practitioners prevent suicides?
Suicide is the tenth leading cause of death among Americans and the third leading cause among those age 15 to 24.1 As many as 36% of suicide victims leave a suicide note.2 Researchers have analyzed such notes with the aim of identifying specific content and patterns that might aid in creating more effective strategies for preventing suicide.3-5
One study found that the presence of a suicide note is an indicator of serious intent; that is, when the initial attempt fails, those who had left a suicide note were found to be at increased risk of subsequent completed suicide.4 Researchers also found that 75% of suicide notes contained the theme “apology/shame,” suggesting that many suicide victims might have welcomed an alternative to suicide to solve their personal predicament. Tragically, however, most suicide notes are not discovered until suicide has been attempted or completed.4
That’s where social media comes in. As platforms for self-expression, social networking sites such as Facebook, Twitter, and Tumblr are sources of real-time information that could aid in suicide prevention.6 With that in mind, we:
• present 2 cases in which a patient announced her suicidal ideation on Facebook
• consider the opportunities that social media present for early intervention
• propose high-tech monitoring methods for high-risk patients.
CASE 1 Major depressive disorder (MDD) and nonadherence
Ms. S, age 24, has a 4-year history of MDD and treatment nonadherence. She had no history of suicide attempt or inpatient treatment, but she had briefly engaged in psychotherapy before discontinuing visits. Physically healthy and employed as a security officer, Ms. S recently broke up with her boyfriend who had abused her physically—and against whom she had an order of protection.
On the day in question, Ms. S posted several status updates on Facebook expressing hopelessness, which, over the course of the day, escalated to expression of frank suicidal ideation:
• “I am ugly, no man would ever want to live with me.”
• “I have made no effect on the world and I’m just a waste of space.”
• “It’s sad that I want to die but such is life. We all die one day.”
• “I’m going to kill myself. It was nice knowing you world. Goodbye everyone.”
CASE 2 Substance abuse and previous suicide attempt
Ms. B, age 21, had a remote (approximately age 16) history of a suicide attempt and was actively abusing 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy,” “Molly”]) and Cannabis. She was not receiving outpatient care. One afternoon, Ms. B walked into the emergency department (ED) and said she had just taken 17 ibuprofen pills with the intent of killing herself.
On initial evaluation, Ms. B was irritable and uncooperative, denying all psychiatric symptoms and refusing to divulge details of her recent behavior. Her mother, who had not accompanied her daughter to the ED, reported that Ms. B had engaged in excessive risk-taking—speeding, driving while intoxicated, having multiple sex partners—for the past 5 years, resulting in several arrests for minor offenses, and she had been depressed and was sleeping and eating poorly in the 2 weeks leading up to the suicide attempt.
Two days ago, her mother added, Ms. B had posted disturbing notes on Facebook: ”Life is useless,” she declared in one post; “I’d be better off dead,” in another.
Suicidal content online
Worldwide, Facebook has 1.35 billion active users each month.7 Thus far, a limited number of posts indicating suicidal intent have been reported in the lay press,8 but evidence suggests that the use of social media for this purpose is an emerging trend.9
A search of the literature yielded only 3 case reports.8,10,11 In one case, a delayed response to a suicide note resulted in a failure to prevent the suicide.8 In another, a clinician’s discovery of a patient’s explicitly suicidal Facebook post led to what the team leader described as a more meaningful therapeutic relationship.10 The clinician’s discovery might have been pivotal in preventing the patient from committing suicide.
The authors of these case reports explored the idea of using Facebook for suicide prevention, raising a number of practical and ethical issues. Among them are the potential for immediate intervention by other Facebook users and the extent to which suicidal posts on social media sites induce copycat suicides.8
Issues associated with clinicians’ use of social media to follow or monitor patients include the ethical concepts of beneficence and nonmaleficence, privacy and confidentiality, clinical judgment, and informed consent,8,10 including potential benefit and harm and the difference between actual and perceived privacy violations. Bennett et al11 recommend developing guidelines for the use of social media to enhance medical care and provide appropriate protections to both patients and providers.
Reporting suicidal content. Although the primary purpose of Facebook is to give users the opportunity to share life events and thoughts with friends and family, the company does address the question of suicidal content in its Help Center (Box 1).12 As our 2 cases illustrate, however, intervention can be significantly delayed.
CASE 1 CONTINUED Call to 911
Fortunately for Ms. S, a friend who read her Facebook posts called 911; even then, however, 16 hours passed between the initial postings and the patient’s arrival at the ED. When emergency medical services brought Ms. S to the Comprehensive Psychiatry Emergency Program, she acknowledged suicidal ideation without an active plan. Other symptoms included depressed mood, a sense of hopelessness, feelings of worthlessness lasting >2 months, low self-esteem, dissatisfaction with body image, and a recent verbal altercation with a friend.
Ms. S was admitted to the inpatient unit for further observation and stabilization.
CASE 2 CONTINUED No one answered her calls
Ms. B, who did not arrive at the ED until 2 days after her suicidal posts, corroborated the history given by her mother. She also reported that she had attempted to reach out to her friends for support, but no one had answered her phone calls. She felt hurt because of this, Ms. B said, and impulsively ingested the pills.
Ms. B said she regretted the suicide attempt. Nevertheless, in light of her recent attempt and persistent distress, she was admitted to the inpatient unit for observation and stabilization.
Can artificial intelligence help?
There is no effective means of tracking high-risk patients after their first contact with the mental health system, despite the fact that (1) those who attempt suicide are at high risk of subsequent suicide attempts3 and (2) we have the potential to prevent future attempts based on self-expressed online cues. We propose machine learning algorithms—a branch of artificial intelligence—to capture and process suicide notes on Facebook in real time.
Machine learning can be broadly defined as computational methods using experience to improve performance or make accurate predictions. In this context, “experience” refers to past information, typically in the form of electronic data collected and analyzed to design accurate and efficient predictive algorithms. Machine learning, which incorporates fundamental concepts in computer science, as well as statistics, probability, and optimization, already has been established in a variety of applications, such as detecting e-mail spam, natural language processing, and computational biology.13
Affective computing, known as emotion-oriented computing, is a branch of artificial intelligence that involves the design of systems and devices that can recognize, interpret, and process human moods and emotions (Box 2).14
Prediction models, developed by Poulin et al15 to estimate the risk of suicide (based on keywords and multiword phrases from unstructured clinical notes from a national sample of U.S. Veterans Administration medical records), resulted in an inference accuracy of ≥65%. Pestian et al16 created and annotated a collection of suicide notes—a vital resource for scientists to use for machine learning and data mining. Machine learning algorithms based on such notes and clinical data might be used to capture alarming social media posts by high-risk patients and activate crisis management, with potentially life-saving results.
But limitations remain
It is not easy to identify or analyze people’s emotions based on social media posts; emotions can be implicit, based on specific events or situations. To distinguish among different emotions purely on the basis of keywords is to deal in great subtlety. Framing algorithms to include multiple parameters—the duration of suicidal content and the number of suicidal posts, for example—would help mitigate the risk of false alarms.
Another problem is that not all Facebook profiles are public. In fact, only 28% of users share all or most of their posts with anyone other than their friends.17 This limitation could be addressed by urging patients identified as being at high risk of suicide during an initial clinical encounter with a mental health provider to “friend” a generic Web page created by the hospital or clinic to protect patients’ privacy.
As Levahot et al10 wrote in their report of the patient whose clinician discovered a patient’s explicitly suicidal Facebook post, the incident “did not hinder the therapeutic alliance.” Instead, the team leader said, the discovery deepened the therapeutic relationship and helped the patient “better understand his mental illness and need for increased support.”
Bottom Line
Machine learning algorithms offer the possibility of analyzing status updates from patients who express suicidal ideation on social media and alerting clinicians to the need for early intervention. There are steps clinicians can take now, however, to take advantage of Facebook, in particular, to monitor and potentially prevent suicide attempts by those at high risk.
Related Resource
• Ahuja AK, Biesaga K, Sudak DM, et al. Suicide on Facebook. J Psychiatr Pract. 2014;20(2):141-146.
Acknowledgement
Zafar Sharif MD, Associate Clinical Professor of Psychiatry, Columbia University College of Physicians and Surgeons, and Director of Psychiatry, Harlem Hospital Center, New York, New York, and Michael Yogman MD, Assistant Clinical Professor of Pediatrics, Harvard Medical School, Boston Children’s Hospital, Boston, Massachusetts, provided insight into the topic and useful feedback on the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) 2010. http://www.cdc.gov/injury/wisqars/index.html. Updated July 7, 2014. Accessed January 19, 2015.
2. Shioiri T, Nishimura A, Akazawa K, et al. Incidence of note-leaving remains constant despite increasing suicide rates. Psychiatr Clin Neurosci. 2005;59(2):226-228.
3. Barr W, Leitner M, Thomas J. Self-harm or attempted suicide? Do suicide notes help us decide the level of intent in those who survive? Accid Emerg Nurs. 2007;15(3):122-127.
4. Foster T. Suicide note themes and suicide prevention. Int J Psychiatry Med. 2003;33(4):323-331.
5. Bhatia MS, Verma SK, Murty OP. Suicide notes: psychological and clinical profile. Int J Psychiatry Med. 2006;36(2):163-170.
6. Jashinsky J, Burton SH, Hanson CL, et al. Tracking suicide risk factors through Twitter in the US. Crisis. 2014;35(1):51-59.
7. Facebook news room. Company info. http://newsroom. fb.com/company-info. Accessed January 7, 2015.
8. Ruder TD, Hatch GM, Ampanozi G, et al. Suicide announcement on Facebook. Crisis. 2011;32(5):280-282.
9. Luxton DD, June JD, Fairall JM. Social media and suicide: a public health perspective. Am J Public Health. 2012;102(suppl 2):S195-S200.
10. Lehavot K, Ben-Zeev D, Neville RE. Ethical considerations and social media: a case of suicidal postings on Facebook. Journal of Dual Diagnosis. 2012;8(4):341-346.
11. Bennett A, Pourmand A, Shokoohi H, et al. Impacts of social networking sites on patient care in the emergency department. Telemed J E Health. 2014;20(1):94-96.
12. How to report suicidal content/threats on Facebook. h t tps ://www. facebook.com/notes/amer ican-foundation-for-suicide-prevention/how-to-report-suicidal-contentthreats-on-facebook/10150090259398144. Published February 15, 2011. Accessed January 22, 2015.
13. Mohri M, Rostamizadeh A, Talwalker A. Foundations of machine learning (adaptive computation and machine learning series). Cambridge, MA: MIT Press; 2012:14.
14. Blázquez Gil G, Berlanga de Jesús A, Molina Lopéz JM. Combining machine learning techniques and natural language processing to infer emotions using Spanish Twitter corpus. Communications in Computer and Information Science. 2013;365:149-157.
15. Poulin C, Shiner B, Thompson P, et al. Predicting the risk of suicide by analyzing the text of clinical notes. PLoS One. 2014;9(1):e85733.
16. Pestian JP, Matykiewicz P, Linn-Gust M. What’s in a note: construction of a suicide note corpus. Biomed Inform Insights. 2012;5:1-6.
17. ConsumerReports.org. Facebook & your privacy. http:// www.consumerreports.org/cro/magazine/2012/06/ facebook-your-privacy/index.html. Published June 2012. Accessed January 22, 2015
Suicide is the tenth leading cause of death among Americans and the third leading cause among those age 15 to 24.1 As many as 36% of suicide victims leave a suicide note.2 Researchers have analyzed such notes with the aim of identifying specific content and patterns that might aid in creating more effective strategies for preventing suicide.3-5
One study found that the presence of a suicide note is an indicator of serious intent; that is, when the initial attempt fails, those who had left a suicide note were found to be at increased risk of subsequent completed suicide.4 Researchers also found that 75% of suicide notes contained the theme “apology/shame,” suggesting that many suicide victims might have welcomed an alternative to suicide to solve their personal predicament. Tragically, however, most suicide notes are not discovered until suicide has been attempted or completed.4
That’s where social media comes in. As platforms for self-expression, social networking sites such as Facebook, Twitter, and Tumblr are sources of real-time information that could aid in suicide prevention.6 With that in mind, we:
• present 2 cases in which a patient announced her suicidal ideation on Facebook
• consider the opportunities that social media present for early intervention
• propose high-tech monitoring methods for high-risk patients.
CASE 1 Major depressive disorder (MDD) and nonadherence
Ms. S, age 24, has a 4-year history of MDD and treatment nonadherence. She had no history of suicide attempt or inpatient treatment, but she had briefly engaged in psychotherapy before discontinuing visits. Physically healthy and employed as a security officer, Ms. S recently broke up with her boyfriend who had abused her physically—and against whom she had an order of protection.
On the day in question, Ms. S posted several status updates on Facebook expressing hopelessness, which, over the course of the day, escalated to expression of frank suicidal ideation:
• “I am ugly, no man would ever want to live with me.”
• “I have made no effect on the world and I’m just a waste of space.”
• “It’s sad that I want to die but such is life. We all die one day.”
• “I’m going to kill myself. It was nice knowing you world. Goodbye everyone.”
CASE 2 Substance abuse and previous suicide attempt
Ms. B, age 21, had a remote (approximately age 16) history of a suicide attempt and was actively abusing 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy,” “Molly”]) and Cannabis. She was not receiving outpatient care. One afternoon, Ms. B walked into the emergency department (ED) and said she had just taken 17 ibuprofen pills with the intent of killing herself.
On initial evaluation, Ms. B was irritable and uncooperative, denying all psychiatric symptoms and refusing to divulge details of her recent behavior. Her mother, who had not accompanied her daughter to the ED, reported that Ms. B had engaged in excessive risk-taking—speeding, driving while intoxicated, having multiple sex partners—for the past 5 years, resulting in several arrests for minor offenses, and she had been depressed and was sleeping and eating poorly in the 2 weeks leading up to the suicide attempt.
Two days ago, her mother added, Ms. B had posted disturbing notes on Facebook: ”Life is useless,” she declared in one post; “I’d be better off dead,” in another.
Suicidal content online
Worldwide, Facebook has 1.35 billion active users each month.7 Thus far, a limited number of posts indicating suicidal intent have been reported in the lay press,8 but evidence suggests that the use of social media for this purpose is an emerging trend.9
A search of the literature yielded only 3 case reports.8,10,11 In one case, a delayed response to a suicide note resulted in a failure to prevent the suicide.8 In another, a clinician’s discovery of a patient’s explicitly suicidal Facebook post led to what the team leader described as a more meaningful therapeutic relationship.10 The clinician’s discovery might have been pivotal in preventing the patient from committing suicide.
The authors of these case reports explored the idea of using Facebook for suicide prevention, raising a number of practical and ethical issues. Among them are the potential for immediate intervention by other Facebook users and the extent to which suicidal posts on social media sites induce copycat suicides.8
Issues associated with clinicians’ use of social media to follow or monitor patients include the ethical concepts of beneficence and nonmaleficence, privacy and confidentiality, clinical judgment, and informed consent,8,10 including potential benefit and harm and the difference between actual and perceived privacy violations. Bennett et al11 recommend developing guidelines for the use of social media to enhance medical care and provide appropriate protections to both patients and providers.
Reporting suicidal content. Although the primary purpose of Facebook is to give users the opportunity to share life events and thoughts with friends and family, the company does address the question of suicidal content in its Help Center (Box 1).12 As our 2 cases illustrate, however, intervention can be significantly delayed.
CASE 1 CONTINUED Call to 911
Fortunately for Ms. S, a friend who read her Facebook posts called 911; even then, however, 16 hours passed between the initial postings and the patient’s arrival at the ED. When emergency medical services brought Ms. S to the Comprehensive Psychiatry Emergency Program, she acknowledged suicidal ideation without an active plan. Other symptoms included depressed mood, a sense of hopelessness, feelings of worthlessness lasting >2 months, low self-esteem, dissatisfaction with body image, and a recent verbal altercation with a friend.
Ms. S was admitted to the inpatient unit for further observation and stabilization.
CASE 2 CONTINUED No one answered her calls
Ms. B, who did not arrive at the ED until 2 days after her suicidal posts, corroborated the history given by her mother. She also reported that she had attempted to reach out to her friends for support, but no one had answered her phone calls. She felt hurt because of this, Ms. B said, and impulsively ingested the pills.
Ms. B said she regretted the suicide attempt. Nevertheless, in light of her recent attempt and persistent distress, she was admitted to the inpatient unit for observation and stabilization.
Can artificial intelligence help?
There is no effective means of tracking high-risk patients after their first contact with the mental health system, despite the fact that (1) those who attempt suicide are at high risk of subsequent suicide attempts3 and (2) we have the potential to prevent future attempts based on self-expressed online cues. We propose machine learning algorithms—a branch of artificial intelligence—to capture and process suicide notes on Facebook in real time.
Machine learning can be broadly defined as computational methods using experience to improve performance or make accurate predictions. In this context, “experience” refers to past information, typically in the form of electronic data collected and analyzed to design accurate and efficient predictive algorithms. Machine learning, which incorporates fundamental concepts in computer science, as well as statistics, probability, and optimization, already has been established in a variety of applications, such as detecting e-mail spam, natural language processing, and computational biology.13
Affective computing, known as emotion-oriented computing, is a branch of artificial intelligence that involves the design of systems and devices that can recognize, interpret, and process human moods and emotions (Box 2).14
Prediction models, developed by Poulin et al15 to estimate the risk of suicide (based on keywords and multiword phrases from unstructured clinical notes from a national sample of U.S. Veterans Administration medical records), resulted in an inference accuracy of ≥65%. Pestian et al16 created and annotated a collection of suicide notes—a vital resource for scientists to use for machine learning and data mining. Machine learning algorithms based on such notes and clinical data might be used to capture alarming social media posts by high-risk patients and activate crisis management, with potentially life-saving results.
But limitations remain
It is not easy to identify or analyze people’s emotions based on social media posts; emotions can be implicit, based on specific events or situations. To distinguish among different emotions purely on the basis of keywords is to deal in great subtlety. Framing algorithms to include multiple parameters—the duration of suicidal content and the number of suicidal posts, for example—would help mitigate the risk of false alarms.
Another problem is that not all Facebook profiles are public. In fact, only 28% of users share all or most of their posts with anyone other than their friends.17 This limitation could be addressed by urging patients identified as being at high risk of suicide during an initial clinical encounter with a mental health provider to “friend” a generic Web page created by the hospital or clinic to protect patients’ privacy.
As Levahot et al10 wrote in their report of the patient whose clinician discovered a patient’s explicitly suicidal Facebook post, the incident “did not hinder the therapeutic alliance.” Instead, the team leader said, the discovery deepened the therapeutic relationship and helped the patient “better understand his mental illness and need for increased support.”
Bottom Line
Machine learning algorithms offer the possibility of analyzing status updates from patients who express suicidal ideation on social media and alerting clinicians to the need for early intervention. There are steps clinicians can take now, however, to take advantage of Facebook, in particular, to monitor and potentially prevent suicide attempts by those at high risk.
Related Resource
• Ahuja AK, Biesaga K, Sudak DM, et al. Suicide on Facebook. J Psychiatr Pract. 2014;20(2):141-146.
Acknowledgement
Zafar Sharif MD, Associate Clinical Professor of Psychiatry, Columbia University College of Physicians and Surgeons, and Director of Psychiatry, Harlem Hospital Center, New York, New York, and Michael Yogman MD, Assistant Clinical Professor of Pediatrics, Harvard Medical School, Boston Children’s Hospital, Boston, Massachusetts, provided insight into the topic and useful feedback on the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Suicide is the tenth leading cause of death among Americans and the third leading cause among those age 15 to 24.1 As many as 36% of suicide victims leave a suicide note.2 Researchers have analyzed such notes with the aim of identifying specific content and patterns that might aid in creating more effective strategies for preventing suicide.3-5
One study found that the presence of a suicide note is an indicator of serious intent; that is, when the initial attempt fails, those who had left a suicide note were found to be at increased risk of subsequent completed suicide.4 Researchers also found that 75% of suicide notes contained the theme “apology/shame,” suggesting that many suicide victims might have welcomed an alternative to suicide to solve their personal predicament. Tragically, however, most suicide notes are not discovered until suicide has been attempted or completed.4
That’s where social media comes in. As platforms for self-expression, social networking sites such as Facebook, Twitter, and Tumblr are sources of real-time information that could aid in suicide prevention.6 With that in mind, we:
• present 2 cases in which a patient announced her suicidal ideation on Facebook
• consider the opportunities that social media present for early intervention
• propose high-tech monitoring methods for high-risk patients.
CASE 1 Major depressive disorder (MDD) and nonadherence
Ms. S, age 24, has a 4-year history of MDD and treatment nonadherence. She had no history of suicide attempt or inpatient treatment, but she had briefly engaged in psychotherapy before discontinuing visits. Physically healthy and employed as a security officer, Ms. S recently broke up with her boyfriend who had abused her physically—and against whom she had an order of protection.
On the day in question, Ms. S posted several status updates on Facebook expressing hopelessness, which, over the course of the day, escalated to expression of frank suicidal ideation:
• “I am ugly, no man would ever want to live with me.”
• “I have made no effect on the world and I’m just a waste of space.”
• “It’s sad that I want to die but such is life. We all die one day.”
• “I’m going to kill myself. It was nice knowing you world. Goodbye everyone.”
CASE 2 Substance abuse and previous suicide attempt
Ms. B, age 21, had a remote (approximately age 16) history of a suicide attempt and was actively abusing 3,4-methylenedioxymethamphetamine (MDMA [“Ecstasy,” “Molly”]) and Cannabis. She was not receiving outpatient care. One afternoon, Ms. B walked into the emergency department (ED) and said she had just taken 17 ibuprofen pills with the intent of killing herself.
On initial evaluation, Ms. B was irritable and uncooperative, denying all psychiatric symptoms and refusing to divulge details of her recent behavior. Her mother, who had not accompanied her daughter to the ED, reported that Ms. B had engaged in excessive risk-taking—speeding, driving while intoxicated, having multiple sex partners—for the past 5 years, resulting in several arrests for minor offenses, and she had been depressed and was sleeping and eating poorly in the 2 weeks leading up to the suicide attempt.
Two days ago, her mother added, Ms. B had posted disturbing notes on Facebook: ”Life is useless,” she declared in one post; “I’d be better off dead,” in another.
Suicidal content online
Worldwide, Facebook has 1.35 billion active users each month.7 Thus far, a limited number of posts indicating suicidal intent have been reported in the lay press,8 but evidence suggests that the use of social media for this purpose is an emerging trend.9
A search of the literature yielded only 3 case reports.8,10,11 In one case, a delayed response to a suicide note resulted in a failure to prevent the suicide.8 In another, a clinician’s discovery of a patient’s explicitly suicidal Facebook post led to what the team leader described as a more meaningful therapeutic relationship.10 The clinician’s discovery might have been pivotal in preventing the patient from committing suicide.
The authors of these case reports explored the idea of using Facebook for suicide prevention, raising a number of practical and ethical issues. Among them are the potential for immediate intervention by other Facebook users and the extent to which suicidal posts on social media sites induce copycat suicides.8
Issues associated with clinicians’ use of social media to follow or monitor patients include the ethical concepts of beneficence and nonmaleficence, privacy and confidentiality, clinical judgment, and informed consent,8,10 including potential benefit and harm and the difference between actual and perceived privacy violations. Bennett et al11 recommend developing guidelines for the use of social media to enhance medical care and provide appropriate protections to both patients and providers.
Reporting suicidal content. Although the primary purpose of Facebook is to give users the opportunity to share life events and thoughts with friends and family, the company does address the question of suicidal content in its Help Center (Box 1).12 As our 2 cases illustrate, however, intervention can be significantly delayed.
CASE 1 CONTINUED Call to 911
Fortunately for Ms. S, a friend who read her Facebook posts called 911; even then, however, 16 hours passed between the initial postings and the patient’s arrival at the ED. When emergency medical services brought Ms. S to the Comprehensive Psychiatry Emergency Program, she acknowledged suicidal ideation without an active plan. Other symptoms included depressed mood, a sense of hopelessness, feelings of worthlessness lasting >2 months, low self-esteem, dissatisfaction with body image, and a recent verbal altercation with a friend.
Ms. S was admitted to the inpatient unit for further observation and stabilization.
CASE 2 CONTINUED No one answered her calls
Ms. B, who did not arrive at the ED until 2 days after her suicidal posts, corroborated the history given by her mother. She also reported that she had attempted to reach out to her friends for support, but no one had answered her phone calls. She felt hurt because of this, Ms. B said, and impulsively ingested the pills.
Ms. B said she regretted the suicide attempt. Nevertheless, in light of her recent attempt and persistent distress, she was admitted to the inpatient unit for observation and stabilization.
Can artificial intelligence help?
There is no effective means of tracking high-risk patients after their first contact with the mental health system, despite the fact that (1) those who attempt suicide are at high risk of subsequent suicide attempts3 and (2) we have the potential to prevent future attempts based on self-expressed online cues. We propose machine learning algorithms—a branch of artificial intelligence—to capture and process suicide notes on Facebook in real time.
Machine learning can be broadly defined as computational methods using experience to improve performance or make accurate predictions. In this context, “experience” refers to past information, typically in the form of electronic data collected and analyzed to design accurate and efficient predictive algorithms. Machine learning, which incorporates fundamental concepts in computer science, as well as statistics, probability, and optimization, already has been established in a variety of applications, such as detecting e-mail spam, natural language processing, and computational biology.13
Affective computing, known as emotion-oriented computing, is a branch of artificial intelligence that involves the design of systems and devices that can recognize, interpret, and process human moods and emotions (Box 2).14
Prediction models, developed by Poulin et al15 to estimate the risk of suicide (based on keywords and multiword phrases from unstructured clinical notes from a national sample of U.S. Veterans Administration medical records), resulted in an inference accuracy of ≥65%. Pestian et al16 created and annotated a collection of suicide notes—a vital resource for scientists to use for machine learning and data mining. Machine learning algorithms based on such notes and clinical data might be used to capture alarming social media posts by high-risk patients and activate crisis management, with potentially life-saving results.
But limitations remain
It is not easy to identify or analyze people’s emotions based on social media posts; emotions can be implicit, based on specific events or situations. To distinguish among different emotions purely on the basis of keywords is to deal in great subtlety. Framing algorithms to include multiple parameters—the duration of suicidal content and the number of suicidal posts, for example—would help mitigate the risk of false alarms.
Another problem is that not all Facebook profiles are public. In fact, only 28% of users share all or most of their posts with anyone other than their friends.17 This limitation could be addressed by urging patients identified as being at high risk of suicide during an initial clinical encounter with a mental health provider to “friend” a generic Web page created by the hospital or clinic to protect patients’ privacy.
As Levahot et al10 wrote in their report of the patient whose clinician discovered a patient’s explicitly suicidal Facebook post, the incident “did not hinder the therapeutic alliance.” Instead, the team leader said, the discovery deepened the therapeutic relationship and helped the patient “better understand his mental illness and need for increased support.”
Bottom Line
Machine learning algorithms offer the possibility of analyzing status updates from patients who express suicidal ideation on social media and alerting clinicians to the need for early intervention. There are steps clinicians can take now, however, to take advantage of Facebook, in particular, to monitor and potentially prevent suicide attempts by those at high risk.
Related Resource
• Ahuja AK, Biesaga K, Sudak DM, et al. Suicide on Facebook. J Psychiatr Pract. 2014;20(2):141-146.
Acknowledgement
Zafar Sharif MD, Associate Clinical Professor of Psychiatry, Columbia University College of Physicians and Surgeons, and Director of Psychiatry, Harlem Hospital Center, New York, New York, and Michael Yogman MD, Assistant Clinical Professor of Pediatrics, Harvard Medical School, Boston Children’s Hospital, Boston, Massachusetts, provided insight into the topic and useful feedback on the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) 2010. http://www.cdc.gov/injury/wisqars/index.html. Updated July 7, 2014. Accessed January 19, 2015.
2. Shioiri T, Nishimura A, Akazawa K, et al. Incidence of note-leaving remains constant despite increasing suicide rates. Psychiatr Clin Neurosci. 2005;59(2):226-228.
3. Barr W, Leitner M, Thomas J. Self-harm or attempted suicide? Do suicide notes help us decide the level of intent in those who survive? Accid Emerg Nurs. 2007;15(3):122-127.
4. Foster T. Suicide note themes and suicide prevention. Int J Psychiatry Med. 2003;33(4):323-331.
5. Bhatia MS, Verma SK, Murty OP. Suicide notes: psychological and clinical profile. Int J Psychiatry Med. 2006;36(2):163-170.
6. Jashinsky J, Burton SH, Hanson CL, et al. Tracking suicide risk factors through Twitter in the US. Crisis. 2014;35(1):51-59.
7. Facebook news room. Company info. http://newsroom. fb.com/company-info. Accessed January 7, 2015.
8. Ruder TD, Hatch GM, Ampanozi G, et al. Suicide announcement on Facebook. Crisis. 2011;32(5):280-282.
9. Luxton DD, June JD, Fairall JM. Social media and suicide: a public health perspective. Am J Public Health. 2012;102(suppl 2):S195-S200.
10. Lehavot K, Ben-Zeev D, Neville RE. Ethical considerations and social media: a case of suicidal postings on Facebook. Journal of Dual Diagnosis. 2012;8(4):341-346.
11. Bennett A, Pourmand A, Shokoohi H, et al. Impacts of social networking sites on patient care in the emergency department. Telemed J E Health. 2014;20(1):94-96.
12. How to report suicidal content/threats on Facebook. h t tps ://www. facebook.com/notes/amer ican-foundation-for-suicide-prevention/how-to-report-suicidal-contentthreats-on-facebook/10150090259398144. Published February 15, 2011. Accessed January 22, 2015.
13. Mohri M, Rostamizadeh A, Talwalker A. Foundations of machine learning (adaptive computation and machine learning series). Cambridge, MA: MIT Press; 2012:14.
14. Blázquez Gil G, Berlanga de Jesús A, Molina Lopéz JM. Combining machine learning techniques and natural language processing to infer emotions using Spanish Twitter corpus. Communications in Computer and Information Science. 2013;365:149-157.
15. Poulin C, Shiner B, Thompson P, et al. Predicting the risk of suicide by analyzing the text of clinical notes. PLoS One. 2014;9(1):e85733.
16. Pestian JP, Matykiewicz P, Linn-Gust M. What’s in a note: construction of a suicide note corpus. Biomed Inform Insights. 2012;5:1-6.
17. ConsumerReports.org. Facebook & your privacy. http:// www.consumerreports.org/cro/magazine/2012/06/ facebook-your-privacy/index.html. Published June 2012. Accessed January 22, 2015
1. Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) 2010. http://www.cdc.gov/injury/wisqars/index.html. Updated July 7, 2014. Accessed January 19, 2015.
2. Shioiri T, Nishimura A, Akazawa K, et al. Incidence of note-leaving remains constant despite increasing suicide rates. Psychiatr Clin Neurosci. 2005;59(2):226-228.
3. Barr W, Leitner M, Thomas J. Self-harm or attempted suicide? Do suicide notes help us decide the level of intent in those who survive? Accid Emerg Nurs. 2007;15(3):122-127.
4. Foster T. Suicide note themes and suicide prevention. Int J Psychiatry Med. 2003;33(4):323-331.
5. Bhatia MS, Verma SK, Murty OP. Suicide notes: psychological and clinical profile. Int J Psychiatry Med. 2006;36(2):163-170.
6. Jashinsky J, Burton SH, Hanson CL, et al. Tracking suicide risk factors through Twitter in the US. Crisis. 2014;35(1):51-59.
7. Facebook news room. Company info. http://newsroom. fb.com/company-info. Accessed January 7, 2015.
8. Ruder TD, Hatch GM, Ampanozi G, et al. Suicide announcement on Facebook. Crisis. 2011;32(5):280-282.
9. Luxton DD, June JD, Fairall JM. Social media and suicide: a public health perspective. Am J Public Health. 2012;102(suppl 2):S195-S200.
10. Lehavot K, Ben-Zeev D, Neville RE. Ethical considerations and social media: a case of suicidal postings on Facebook. Journal of Dual Diagnosis. 2012;8(4):341-346.
11. Bennett A, Pourmand A, Shokoohi H, et al. Impacts of social networking sites on patient care in the emergency department. Telemed J E Health. 2014;20(1):94-96.
12. How to report suicidal content/threats on Facebook. h t tps ://www. facebook.com/notes/amer ican-foundation-for-suicide-prevention/how-to-report-suicidal-contentthreats-on-facebook/10150090259398144. Published February 15, 2011. Accessed January 22, 2015.
13. Mohri M, Rostamizadeh A, Talwalker A. Foundations of machine learning (adaptive computation and machine learning series). Cambridge, MA: MIT Press; 2012:14.
14. Blázquez Gil G, Berlanga de Jesús A, Molina Lopéz JM. Combining machine learning techniques and natural language processing to infer emotions using Spanish Twitter corpus. Communications in Computer and Information Science. 2013;365:149-157.
15. Poulin C, Shiner B, Thompson P, et al. Predicting the risk of suicide by analyzing the text of clinical notes. PLoS One. 2014;9(1):e85733.
16. Pestian JP, Matykiewicz P, Linn-Gust M. What’s in a note: construction of a suicide note corpus. Biomed Inform Insights. 2012;5:1-6.
17. ConsumerReports.org. Facebook & your privacy. http:// www.consumerreports.org/cro/magazine/2012/06/ facebook-your-privacy/index.html. Published June 2012. Accessed January 22, 2015
Prescriber’s guide to using 3 new antidepressants: Vilazodone, levomilnacipran, vortioxetine
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
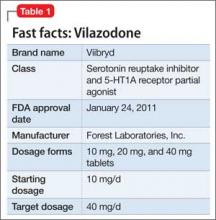
Vilazodone
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
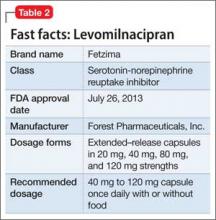
Levomilnacipran
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
Vilazodone
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
Levomilnacipran
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
Vilazodone
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
Levomilnacipran
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
Maximizing Teaching on the Wards
An important role of the hospitalist educator is to teach residents and medical students how to diagnose and manage acute medical problems. However, clinical reasoning is complex and nuanced, and there are many challenges to teaching this important process. Medical inpatients are increasingly complex, older, and more seriously ill.[1] Documentation requirements and productivity obligations compete with teaching time. Hospitalists must adjust their teaching for learners from different professions and at various levels of training. In addition, hospitalists tend to be less experienced, and must balance the need to learn their roles as clinicians with developing their own skills as educators.[2]
Despite the challenges inherent to the setting, inpatient rotations provide tremendous teaching and learning opportunities. Patients with undifferentiated complaints or known diagnoses in need of management decisions are available to stimulate discussion. Hospitalist educators have the opportunity to assess residents' progress along the developmental milestones, which residency programs are now required to report for accreditation,[3] and provide role modeling for residents who are developing their own teaching skills.
To maximize these opportunities, attendings must engage trainees to practice clinical reasoning and identify their own knowledge gaps. Various strategies for facilitating the clinical reasoning discussion exist, but two frameworksthe One‐Minute Preceptor (OMP) and SNAPPShave been well studied, albeit mainly in the outpatient setting. Both models offer ways to maximize teaching and assess clinical reasoning, but they have different methods and strengths. This article provides a narrative review of the two frameworks and discusses how they can be applied to the inpatient teaching environment. Hospitalists can utilize these models or components of each framework to facilitate teaching on inpatient teams and enhance their roles as educators.
ONE‐MINUTE PRECEPTOR
The OMP was first described in 1992 by Neher and colleagues as an alternative to the traditional model of precepting.[4] It gives preceptors a method to facilitate learners presentation of their thought process and then for the preceptor to provide targeted teaching points.[4] The OMP helps diagnose both learner and patient, whereas the traditional model focuses on diagnosing the patient.[5] In the traditional model, the attending questions the learner to diagnose the patient, which does not often make clear the learner's thinking process. Thus, there may be a mismatch between the teaching points the preceptor makes and what the learner really needs to know.[5] There are several key benefits to the OMP compared to the traditional model; broadly, these relate to improved ability to assess the learner and provide targeted teaching,[4, 5, 6, 7] improved integration of feedback,[4, 8, 9, 10] learner preference,[11] and ease with which it is learned by faculty members.[4]
The OMP model consists of five steps outlined in Table 1. Step 1, getting a commitment, can involve any aspect of the casediagnosis, treatment, or follow‐upand learners should be challenged to make intellectual commitments just beyond their level of comfort.[12] Steps 1 and 2 bring to light the learner's individual learning needs,[11] then the preceptor follows up with personalized teaching. The OMP is efficient; no increase in time was needed to precept a case in an outpatient study.[9] In a separate outpatient study, the OMP led preceptors to be more likely to teach about disease‐specific points and differential diagnosis, as compared to generic items such as history taking and presentation skills with the traditional model.[5]
| A 5‐step framework in which the preceptor does the following: |
| 1. Get a commitment |
| 2. Probe for supporting evidence |
| 3. Provide general rules |
| 4. Reinforce what was done correctly |
| 5. Correct mistakes |
Faculty feel better prepared to assess learners and provide feedback with the OMP model.[6, 9] Aagaard and colleagues provided 116 mostly ambulatory preceptors with scripted, videotaped encounters of the OMP and traditional models. The OMP improved preceptors' confidence at rating students' presentation skills, clinical reasoning, and fund of knowledge. It was rated more efficient and effective, and preceptors were able to diagnose the patient with the same or improved accuracy compared to the traditional model.[6] In a pre‐post study assessing the efficacy of a faculty development workshop, students rated ambulatory teaching encounters incorporating the OMP model as having increased quantity and quality of feedback. Furthermore, faculty reported improved ability to evaluate students and were more likely to let students reach their own conclusions and create their own postencounter learning plans.[9]
The OMP is also well‐received by trainees. Teherani and colleagues analyzed medical students' responses to videotaped teaching encounters of the OMP and traditional models. Students gave higher mean ratings for all studied items (including feedback, involving the student in decision‐making, and overall effectiveness) to the OMP model, and preferred it over the traditional model.[11]
Several studies have evaluated the OMP for use by residents as teachers,[10, 13, 14] and it is one of the most common models taught to residents.[13] One study evaluated the impact of a one‐day workshop for 276 residents that included the five‐step microskills model (also known as the OMP).[10] Residents felt more prepared to teach, set expectations, and provide feedback.[10] The OMP model, despite brief training, is effective in improving residents' teaching effectiveness and confidence.[13]
The only study we found that exclusively evaluated the OMP in the inpatient setting was a randomized trial[8] involving 57 internal medicine residents. Interns and students rated OMP‐trained residents more highly in 4 of 5 behaviors. The behavior that showed no difference from the control group was teaching general rules.[8] However, there was no difference in ratings of overall teaching effectiveness between groups.[8]
Our review of the literature on the OMP shows it is a quickly learned, easily implemented framework for teaching clinical reasoning. It has been used across specialties and settings, provides a built‐in mechanism for feedback, and allows educators to assess trainees' reasoning while extracting the clinical information needed to work efficiently.
SNAPPS
SNAPPS was first described in 2003 by Wolpaw and colleagues. It is a six‐step learner‐centered model as outlined in Table 2.[15] Unlike the OMP, SNAPPS requires both trainee and teacher to learn the framework. In doing so, the responsibility for directing the teaching encounter is shifted toward the learner.[15] Consequently, this model may be best suited to advanced or motivated learners. Like the OMP, SNAPPS was originally described for the ambulatory environment. However, it has been studied in the inpatient setting as well.
| A 6‐step framework in which the learner does the following: |
| 1. Summarize briefly the history and findings |
| 2. Narrow the differential to 2 or 3 possibilities |
| 3. Analyze the differential by comparing/contrasting the possibilities |
| 4. Probe the preceptor by asking questions |
| 5. Plan management for the patient's medical issues |
| 6. Select a case‐related issue for self‐directed learning |
With SNAPPS, the teaching encounter is learner driven. The trainee presents the case and directs the discussion of differential diagnosis. The educator does not have an active role until the fourth step, where the learner asks questions or identifies areas of uncertainty. But even at this stage, the discussion is learner driven. Step 5, planning management, is collaborative, with trainees suggesting management plans with appropriate attending guidance. Depending on learner skill level or case difficulty, the preceptor may need to play more or less of an active role. The final step, picking a case‐related issue to examine, extends the learning beyond the initial encounter, and ensures that it is individualized and relevant. This step also encourages learner progression toward the Accreditation Council for Graduate Medical Education (ACGME) competency of practice‐based learning and improvement.[3]
A handful of studies have evaluated the SNAPPS model. A randomized comparison group trial found that SNAPPS‐trained students outperformed students trained to elicit feedback and students who received the usual and customary preparation.[16] Notably, SNAPPS students expressed more than twice as many differential diagnoses, justified their reasoning more than five times as often, and expressed more questions and uncertainties. The SNAPPS students' presentations were no longer than in the usual and customary group, and were just one minute longer than in the group trained to elicit feedback.[16] A follow‐up analysis found that 100% of the SNAPPS students expressed an uncertainty (i.e. step 4) compared with 54% of the comparison group, and that most of these uncertainties related to diagnostic reasoning.[17]
A study of medicine clerkship students evaluated the impact of extending SNAPPS to the inpatient setting and including educational prescriptions.[18] The goal was to facilitate the formulation and answering of clinical questions by using the patient, intervention, comparison, outcome (PICO) format for step 6 (selecting a case‐based issue to learn about). Dubbing this SNAPPS‐Plus, the authors found that 99% of cases included a question, and 93% of those were answered. Most questions related to therapeutics, and there was a positive correlation between questions more closely corresponding to the PICO format and higher quality answers.[18]
As with the OMP, SNAPPS does not require additional time for case presentations compared to the usual method.[16] From the perspective of a busy hospitalist, this model takes some responsibility for education away from faculty and places it on the learner. This is an important process for fostering self‐directed learning. As with the OMP, SNAPPS appears easily translatable from the outpatient to inpatient setting. Its main downside is the training time required for both parties to implement it.
TRANSLATING THE MODELS TO THE INPATIENT SETTING
The OMP and SNAPPS have largely been used in the outpatient setting. However, we propose that hospitalists can adapt either model for teaching on ward rotations, as the steps of each framework are not exclusive to one clinical setting.
Although the OMP is typically used between a preceptor and single trainee, it is well suited to engaging the entire group on inpatient rounds (Table 3). For example, a student could commit to and support a diagnosis (steps 1 and 2), whereas the intern could commit to and provide evidence for a treatment or management option. Attendings can repeat steps 1 and 2 for patients' secondary problems, encouraging learners to commit to other items on the problem list.
| Attending/ Senior Resident | Learner | Practical Tips | |
|---|---|---|---|
| |||
| Active listening. | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Learners may end their presentation here and expect you to fill in with your assessment and plan. Rather than jumping in, turn it back to the learner following the OMP model. | |
| Get a commitment | What do you think is going on? | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? |
| If the student does not expand the differential, direct this question to the intern. | |||
| Probe for supporting evidence | Why do you think this? | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (e.g. absence of tachycardia) are often key findings. |
| Provide general rules | When a patient with a history of alcohol abuse has a GI bleed, you should consider whether she has underlying liver disease or a coagulopathy. If she did have liver disease, what other sources of bleeding should you consider? | Esophageal varices? | This is the step the residents tend to struggle with when teaching.[8] If your senior resident is leading the case discussion, be prepared to step in with some clinical pearls. |
| Reinforce what was done correctly | You did a nice job considering her predisposing factors, including NSAIDS and alcohol. This helped you prioritize the most likely diagnoses. | Thank you. | Tell them what they did right and the effect it had. |
| Correct mistakes | You did not address her risk for alcohol withdrawal. This increases in patients who are hospitalized for a medical illness. Next time be sure to include substance abuse in your problem list. | I'll make sure to do that. | Tell them what they did not do right and how to improve for the next time. If the student is presenting, consider asking the intern or senior resident for a management plan. |
While teaching general rules (step 3) in the group setting, hospitalists should emphasize basic principles for students (which will serve as reinforcement for residents) as well as discuss more complex rules for the edification of all team members. Hospitalists should encourage senior residents to speak up during this step and share their knowledge with the group. This is an opportunity for residents to practice their role as teachers, and for faculty to assess their clinical acumen. However, residents struggled with teaching general rules in Furney and colleagues' randomized trial.[8] Successful clinical teachers use a mix of improvisational teaching and curriculum scripts developed through years of experience.[19] Hospitalists can model this method of instruction for residents who are learning to teach. For more junior hospitalists who may still be developing their own teaching scripts, the OMP provides an opportunity to regularly integrate these scripts into rounds.
The OMP teaching encounter ends with feedback. Providing real‐time feedback to an individual in the group setting could feel awkward. Reassuringly, in Furney and colleagues' study, some of the greatest gains were in the realm of feedback, as reported by both the senior residents providing the feedback and the interns and students on the receiving end.[8] Although the OMP builds in a space for feedback, it does not teach one how to give feedback. Although it is possible that not all feedback is beneficial, trainees are eager to receive constructive input, and hospitalists should not fear providing this in front of the group. Thoughtful critique of one trainee can provide learning opportunities for others listening in.
SNAPPS is also well suited to inpatient education (Table 4). Because it emphasizes a discussion of differential diagnosis, it works well for new admissions. Because hospitalized patients usually have multiple problems, learners may repeat steps 2 and 3 for each problem, or just for the primary issue. On subsequent days, a standard presentation may work better, but if new problems arise (e.g. fever), hospitalists can ask learners to go through the SNAPPS steps for the new issue.
| Learner | Attending/ Senior Resident | Practical Tips | |
|---|---|---|---|
| |||
| 1. Summarize | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Active listening. | Rather than a complete, detailed history and physical, we emphasize tailoring the oral presentation to include only those components relevant to this admission. Then, transition to the SNAPPS presentation with a summary statement as presented here. |
| 2. Narrow the differential | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? | Hospitalized patients often have multiple problems. Learners can go through this process of SNAPPS for each problem or only the primary problem. |
| 3. Analyze the differential | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | That's a very reasonable differential. You did a nice job considering her predisposing factors. What do her vital signs tell you about how much blood she has lost? | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (eg, absence of tachycardia) are often key findings. |
| 4. Probe the preceptor | I know alcohol increases the risk of esophageal cancer, but I was not sure if that could present like this. | You are right that she has a higher risk of cancer. Because most tumors are slow‐growing, what kind of symptoms do you think a mass in the esophagus might cause? | Guide learners to the correct answer, helping them connect pre‐existing knowledge to the question at hand. This is also a good spot to provide real‐time feedback. |
| NOTE: This is a great place for learners to ask questions that might be harder to look up, or to ask about physical findings (eg, I thought I heard crackles but was not sure. Could somebody check this with me?) | Does anyone else on the team have thoughts about this question? | Alternatively, give the senior resident an opportunity to address the question. This allows the attending to assess the senior resident's clinical reasoning and gives him or her an opportunity to practice teaching. | |
| 5. Plan management | For the suspected GI bleed, I would like to start a proton pump inhibitor, call a GI consult for an EGD, and check the hematocrit every 8 hours. We can use sequential compression devices for DVT prophylaxis. We will also counsel on alcohol cessation and monitor for withdrawal. | Good start. Does anyone else on the team want to add to the management plan? We have a pharmacist rounding with us today. Is there a difference in outcomes or costs with BID dosing versus continuous infusion of a proton pump inhibitor? | If a student is presenting, offer the intern and/or senior resident an opportunity to add to the plan.Incorporate the expertise of ancillary providers rounding with the team. |
| 6. Select a case‐related issue for self‐directed learning | I would like to look up the best way to treat her alcohol withdrawal if she develops it. | Great! We do have a protocol at the hospital, but it is a good idea to review the literature behind it. | Set aside 10 minutes before rounds each day for learners to present their findings. |
| Consider having learners write educational prescriptions following the PICO format. | |||
Step 6 of SNAPPS provides trainees an opportunity to search for and present relevant information to guide patient management. To incorporate more formal teaching time each day, set aside 10 minutes before rounds for learners to present their answers to the team. Also, because SNAPPS has the learner ask about uncertainties, faculty can use their on‐the‐fly teaching time to answer questions for which trainees do not know the answer. In the era of problem‐based learning (PBL) and medical school curricula that foster self‐directed learning from day one, many students should find SNAPPS a natural extension of PBL‐style learning from the preclinical into the clinical years.
Unlike the OMP, SNAPPS does not build in a step for feedback. Therefore, preceptors should focus on step 4 as an opportunity for this. Because feedback is paired with discussion of an uncertainty, it focuses on a trainee's immediate needs and can maximize learning opportunities.[17]
Clinical educators must simultaneously diagnose and manage patients as well as assess learners' abilities.[20] Workplace‐based assessment is particularly important for residents, and hospitalists play a pivotal role in determining their progression along the developmental milestones for achieving the ACGME competencies in medical knowledge, patient care, and practice‐based learning and improvement.[3] Both the OMP and SNAPPS frameworks encourage trainees to think out loud, providing some transparency to their thought process and enabling faculty to more accurately assess their clinical reasoning.
CONCLUSION
Many hospitalists may already use a teaching approach resembling the OMP. It has a familiar, back‐and‐forth rhythm. By explicitly following its steps, however, attendings can ensure they are providing feedback and individualized teaching with each case. SNAPPS, on the other hand, relieves faculty of their familiar role of leading the thought process and imparting teaching points. Instead, the trainee directs the encounter, leaving the attending in the role of guide.[15] SNAPPS aims to help students and residents take charge of their education and develop lifelong learning skills.
Both frameworks can be transferred from the ambulatory to inpatient setting with little modification. The OMP is older and better studied. It is easy to learn, and can be utilized by attendings and residents as teachers. In contrast, SNAPPS requires both teacher and trainee to learn the framework. Typically, this means that SNAPPS needs to be implemented systematically, via a clerkship or residency program. However, if a team was motivated, they could learn and apply it for their time together on service. Though it requires more effort to put in place, SNAPPS provides a novel approach to teaching clinical reasoning. Finally, hospitalists need not implement all steps of either framework for every teaching encounter, but can use components of either model, depending on the individual learners, team composition, time available, or clinical case.
Additional studies examining both frameworks' use for inpatient teaching and assessment would be helpful. Potential questions to address include how the team structure of inpatient rotations impacts the effectiveness of either model (e.g. which trainees benefit when committing to diagnoses or getting feedback in front of a group?), whether either model improves senior residents' ability to lead rounds and teach, whether written faculty assessments of residents are more specific and accurate with either model, and the impact of not following all steps of either model. Higher level outcomes for both models would be another area for investigation, including change in clinical performance, exam performance of students and residents, or patient outcomes, such as length of stay, cost per case, or need for rapid response/emntensive care unit transfer.
ACKNOWLEDGMENTS
Disclosure: Nothing to report.
- , , , . 2006 national hospital discharge survey. Natl Health Stat Report. 2008;(5):1–20.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Hosp Med. 2009;4(4):240–246.
- Accreditation Council for Graduate Medical Education. Program and institutional accreditation: next accreditation system: Milestones. Available at: https://www.acgme.org/acgmeweb/tabid/430/ProgramandInstitutionalAccreditation/NextAccreditationSystem/Milestones.aspx. Accessed July 28, 2014.
- , , , . A five‐step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5(4):419–424.
- , , . Teaching points identified by preceptors observing one‐minute preceptor and traditional preceptor encounters. Acad Med. 2004;79(1):50–55.
- , , . Effectiveness of the one‐minute preceptor model for diagnosing the patient and the learner: proof of concept. Acad Med. 2004;79(1):42–49.
- , , . Measuring outcomes of a one‐minute preceptor faculty development workshop. J Gen Intern Med. 2006;21(5):410–414.
- , , , , , . Teaching the one‐minute preceptor. A randomized controlled trial. J Gen Intern Med. 2001;16(9):620–624.
- , , , , , . Faculty development seminars based on the one‐minute preceptor improve feedback in the ambulatory setting. J Gen Intern Med. 2002;17(10):779–787.
- , , , , . Change in residents' perceptions of teaching: following a one day "residents as teachers" (RasT) workshop. South Med J. 2008;101(5):495–502.
- , , , , . Student perceptions of the one minute preceptor and traditional preceptor models. Med Teach. 2007;29(4):323–327.
- , . The one‐minute preceptor: shaping the teaching conversation. Fam Med. 2003;35(6):391–393.
- , , . Residents‐as‐teachers curricula: a critical review. Acad Med. 2009;84(3):374–380.
- , , . Teaching to teach in Toronto. Acad Psychiatry. 2010;34(4):277–281.
- , , . SNAPPS: a learner‐centered model for outpatient education. Acad Med. 2003;78(9):893–898.
- , , . Using SNAPPS to facilitate the expression of clinical reasoning and uncertainties: A randomized comparison group trial. Acad Med. 2009;84(4):517–524.
- , , , . Student uncertainties drive teaching during case presentations: more so with SNAPPS. Acad Med. 2012;87(9):1210–1217.
- , , , , , . SNAPPS‐plus: an educational prescription for students to facilitate formulating and answering clinical questions. Acad Med. 2014;89(8):1174–1179.
- . How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
An important role of the hospitalist educator is to teach residents and medical students how to diagnose and manage acute medical problems. However, clinical reasoning is complex and nuanced, and there are many challenges to teaching this important process. Medical inpatients are increasingly complex, older, and more seriously ill.[1] Documentation requirements and productivity obligations compete with teaching time. Hospitalists must adjust their teaching for learners from different professions and at various levels of training. In addition, hospitalists tend to be less experienced, and must balance the need to learn their roles as clinicians with developing their own skills as educators.[2]
Despite the challenges inherent to the setting, inpatient rotations provide tremendous teaching and learning opportunities. Patients with undifferentiated complaints or known diagnoses in need of management decisions are available to stimulate discussion. Hospitalist educators have the opportunity to assess residents' progress along the developmental milestones, which residency programs are now required to report for accreditation,[3] and provide role modeling for residents who are developing their own teaching skills.
To maximize these opportunities, attendings must engage trainees to practice clinical reasoning and identify their own knowledge gaps. Various strategies for facilitating the clinical reasoning discussion exist, but two frameworksthe One‐Minute Preceptor (OMP) and SNAPPShave been well studied, albeit mainly in the outpatient setting. Both models offer ways to maximize teaching and assess clinical reasoning, but they have different methods and strengths. This article provides a narrative review of the two frameworks and discusses how they can be applied to the inpatient teaching environment. Hospitalists can utilize these models or components of each framework to facilitate teaching on inpatient teams and enhance their roles as educators.
ONE‐MINUTE PRECEPTOR
The OMP was first described in 1992 by Neher and colleagues as an alternative to the traditional model of precepting.[4] It gives preceptors a method to facilitate learners presentation of their thought process and then for the preceptor to provide targeted teaching points.[4] The OMP helps diagnose both learner and patient, whereas the traditional model focuses on diagnosing the patient.[5] In the traditional model, the attending questions the learner to diagnose the patient, which does not often make clear the learner's thinking process. Thus, there may be a mismatch between the teaching points the preceptor makes and what the learner really needs to know.[5] There are several key benefits to the OMP compared to the traditional model; broadly, these relate to improved ability to assess the learner and provide targeted teaching,[4, 5, 6, 7] improved integration of feedback,[4, 8, 9, 10] learner preference,[11] and ease with which it is learned by faculty members.[4]
The OMP model consists of five steps outlined in Table 1. Step 1, getting a commitment, can involve any aspect of the casediagnosis, treatment, or follow‐upand learners should be challenged to make intellectual commitments just beyond their level of comfort.[12] Steps 1 and 2 bring to light the learner's individual learning needs,[11] then the preceptor follows up with personalized teaching. The OMP is efficient; no increase in time was needed to precept a case in an outpatient study.[9] In a separate outpatient study, the OMP led preceptors to be more likely to teach about disease‐specific points and differential diagnosis, as compared to generic items such as history taking and presentation skills with the traditional model.[5]
| A 5‐step framework in which the preceptor does the following: |
| 1. Get a commitment |
| 2. Probe for supporting evidence |
| 3. Provide general rules |
| 4. Reinforce what was done correctly |
| 5. Correct mistakes |
Faculty feel better prepared to assess learners and provide feedback with the OMP model.[6, 9] Aagaard and colleagues provided 116 mostly ambulatory preceptors with scripted, videotaped encounters of the OMP and traditional models. The OMP improved preceptors' confidence at rating students' presentation skills, clinical reasoning, and fund of knowledge. It was rated more efficient and effective, and preceptors were able to diagnose the patient with the same or improved accuracy compared to the traditional model.[6] In a pre‐post study assessing the efficacy of a faculty development workshop, students rated ambulatory teaching encounters incorporating the OMP model as having increased quantity and quality of feedback. Furthermore, faculty reported improved ability to evaluate students and were more likely to let students reach their own conclusions and create their own postencounter learning plans.[9]
The OMP is also well‐received by trainees. Teherani and colleagues analyzed medical students' responses to videotaped teaching encounters of the OMP and traditional models. Students gave higher mean ratings for all studied items (including feedback, involving the student in decision‐making, and overall effectiveness) to the OMP model, and preferred it over the traditional model.[11]
Several studies have evaluated the OMP for use by residents as teachers,[10, 13, 14] and it is one of the most common models taught to residents.[13] One study evaluated the impact of a one‐day workshop for 276 residents that included the five‐step microskills model (also known as the OMP).[10] Residents felt more prepared to teach, set expectations, and provide feedback.[10] The OMP model, despite brief training, is effective in improving residents' teaching effectiveness and confidence.[13]
The only study we found that exclusively evaluated the OMP in the inpatient setting was a randomized trial[8] involving 57 internal medicine residents. Interns and students rated OMP‐trained residents more highly in 4 of 5 behaviors. The behavior that showed no difference from the control group was teaching general rules.[8] However, there was no difference in ratings of overall teaching effectiveness between groups.[8]
Our review of the literature on the OMP shows it is a quickly learned, easily implemented framework for teaching clinical reasoning. It has been used across specialties and settings, provides a built‐in mechanism for feedback, and allows educators to assess trainees' reasoning while extracting the clinical information needed to work efficiently.
SNAPPS
SNAPPS was first described in 2003 by Wolpaw and colleagues. It is a six‐step learner‐centered model as outlined in Table 2.[15] Unlike the OMP, SNAPPS requires both trainee and teacher to learn the framework. In doing so, the responsibility for directing the teaching encounter is shifted toward the learner.[15] Consequently, this model may be best suited to advanced or motivated learners. Like the OMP, SNAPPS was originally described for the ambulatory environment. However, it has been studied in the inpatient setting as well.
| A 6‐step framework in which the learner does the following: |
| 1. Summarize briefly the history and findings |
| 2. Narrow the differential to 2 or 3 possibilities |
| 3. Analyze the differential by comparing/contrasting the possibilities |
| 4. Probe the preceptor by asking questions |
| 5. Plan management for the patient's medical issues |
| 6. Select a case‐related issue for self‐directed learning |
With SNAPPS, the teaching encounter is learner driven. The trainee presents the case and directs the discussion of differential diagnosis. The educator does not have an active role until the fourth step, where the learner asks questions or identifies areas of uncertainty. But even at this stage, the discussion is learner driven. Step 5, planning management, is collaborative, with trainees suggesting management plans with appropriate attending guidance. Depending on learner skill level or case difficulty, the preceptor may need to play more or less of an active role. The final step, picking a case‐related issue to examine, extends the learning beyond the initial encounter, and ensures that it is individualized and relevant. This step also encourages learner progression toward the Accreditation Council for Graduate Medical Education (ACGME) competency of practice‐based learning and improvement.[3]
A handful of studies have evaluated the SNAPPS model. A randomized comparison group trial found that SNAPPS‐trained students outperformed students trained to elicit feedback and students who received the usual and customary preparation.[16] Notably, SNAPPS students expressed more than twice as many differential diagnoses, justified their reasoning more than five times as often, and expressed more questions and uncertainties. The SNAPPS students' presentations were no longer than in the usual and customary group, and were just one minute longer than in the group trained to elicit feedback.[16] A follow‐up analysis found that 100% of the SNAPPS students expressed an uncertainty (i.e. step 4) compared with 54% of the comparison group, and that most of these uncertainties related to diagnostic reasoning.[17]
A study of medicine clerkship students evaluated the impact of extending SNAPPS to the inpatient setting and including educational prescriptions.[18] The goal was to facilitate the formulation and answering of clinical questions by using the patient, intervention, comparison, outcome (PICO) format for step 6 (selecting a case‐based issue to learn about). Dubbing this SNAPPS‐Plus, the authors found that 99% of cases included a question, and 93% of those were answered. Most questions related to therapeutics, and there was a positive correlation between questions more closely corresponding to the PICO format and higher quality answers.[18]
As with the OMP, SNAPPS does not require additional time for case presentations compared to the usual method.[16] From the perspective of a busy hospitalist, this model takes some responsibility for education away from faculty and places it on the learner. This is an important process for fostering self‐directed learning. As with the OMP, SNAPPS appears easily translatable from the outpatient to inpatient setting. Its main downside is the training time required for both parties to implement it.
TRANSLATING THE MODELS TO THE INPATIENT SETTING
The OMP and SNAPPS have largely been used in the outpatient setting. However, we propose that hospitalists can adapt either model for teaching on ward rotations, as the steps of each framework are not exclusive to one clinical setting.
Although the OMP is typically used between a preceptor and single trainee, it is well suited to engaging the entire group on inpatient rounds (Table 3). For example, a student could commit to and support a diagnosis (steps 1 and 2), whereas the intern could commit to and provide evidence for a treatment or management option. Attendings can repeat steps 1 and 2 for patients' secondary problems, encouraging learners to commit to other items on the problem list.
| Attending/ Senior Resident | Learner | Practical Tips | |
|---|---|---|---|
| |||
| Active listening. | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Learners may end their presentation here and expect you to fill in with your assessment and plan. Rather than jumping in, turn it back to the learner following the OMP model. | |
| Get a commitment | What do you think is going on? | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? |
| If the student does not expand the differential, direct this question to the intern. | |||
| Probe for supporting evidence | Why do you think this? | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (e.g. absence of tachycardia) are often key findings. |
| Provide general rules | When a patient with a history of alcohol abuse has a GI bleed, you should consider whether she has underlying liver disease or a coagulopathy. If she did have liver disease, what other sources of bleeding should you consider? | Esophageal varices? | This is the step the residents tend to struggle with when teaching.[8] If your senior resident is leading the case discussion, be prepared to step in with some clinical pearls. |
| Reinforce what was done correctly | You did a nice job considering her predisposing factors, including NSAIDS and alcohol. This helped you prioritize the most likely diagnoses. | Thank you. | Tell them what they did right and the effect it had. |
| Correct mistakes | You did not address her risk for alcohol withdrawal. This increases in patients who are hospitalized for a medical illness. Next time be sure to include substance abuse in your problem list. | I'll make sure to do that. | Tell them what they did not do right and how to improve for the next time. If the student is presenting, consider asking the intern or senior resident for a management plan. |
While teaching general rules (step 3) in the group setting, hospitalists should emphasize basic principles for students (which will serve as reinforcement for residents) as well as discuss more complex rules for the edification of all team members. Hospitalists should encourage senior residents to speak up during this step and share their knowledge with the group. This is an opportunity for residents to practice their role as teachers, and for faculty to assess their clinical acumen. However, residents struggled with teaching general rules in Furney and colleagues' randomized trial.[8] Successful clinical teachers use a mix of improvisational teaching and curriculum scripts developed through years of experience.[19] Hospitalists can model this method of instruction for residents who are learning to teach. For more junior hospitalists who may still be developing their own teaching scripts, the OMP provides an opportunity to regularly integrate these scripts into rounds.
The OMP teaching encounter ends with feedback. Providing real‐time feedback to an individual in the group setting could feel awkward. Reassuringly, in Furney and colleagues' study, some of the greatest gains were in the realm of feedback, as reported by both the senior residents providing the feedback and the interns and students on the receiving end.[8] Although the OMP builds in a space for feedback, it does not teach one how to give feedback. Although it is possible that not all feedback is beneficial, trainees are eager to receive constructive input, and hospitalists should not fear providing this in front of the group. Thoughtful critique of one trainee can provide learning opportunities for others listening in.
SNAPPS is also well suited to inpatient education (Table 4). Because it emphasizes a discussion of differential diagnosis, it works well for new admissions. Because hospitalized patients usually have multiple problems, learners may repeat steps 2 and 3 for each problem, or just for the primary issue. On subsequent days, a standard presentation may work better, but if new problems arise (e.g. fever), hospitalists can ask learners to go through the SNAPPS steps for the new issue.
| Learner | Attending/ Senior Resident | Practical Tips | |
|---|---|---|---|
| |||
| 1. Summarize | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Active listening. | Rather than a complete, detailed history and physical, we emphasize tailoring the oral presentation to include only those components relevant to this admission. Then, transition to the SNAPPS presentation with a summary statement as presented here. |
| 2. Narrow the differential | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? | Hospitalized patients often have multiple problems. Learners can go through this process of SNAPPS for each problem or only the primary problem. |
| 3. Analyze the differential | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | That's a very reasonable differential. You did a nice job considering her predisposing factors. What do her vital signs tell you about how much blood she has lost? | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (eg, absence of tachycardia) are often key findings. |
| 4. Probe the preceptor | I know alcohol increases the risk of esophageal cancer, but I was not sure if that could present like this. | You are right that she has a higher risk of cancer. Because most tumors are slow‐growing, what kind of symptoms do you think a mass in the esophagus might cause? | Guide learners to the correct answer, helping them connect pre‐existing knowledge to the question at hand. This is also a good spot to provide real‐time feedback. |
| NOTE: This is a great place for learners to ask questions that might be harder to look up, or to ask about physical findings (eg, I thought I heard crackles but was not sure. Could somebody check this with me?) | Does anyone else on the team have thoughts about this question? | Alternatively, give the senior resident an opportunity to address the question. This allows the attending to assess the senior resident's clinical reasoning and gives him or her an opportunity to practice teaching. | |
| 5. Plan management | For the suspected GI bleed, I would like to start a proton pump inhibitor, call a GI consult for an EGD, and check the hematocrit every 8 hours. We can use sequential compression devices for DVT prophylaxis. We will also counsel on alcohol cessation and monitor for withdrawal. | Good start. Does anyone else on the team want to add to the management plan? We have a pharmacist rounding with us today. Is there a difference in outcomes or costs with BID dosing versus continuous infusion of a proton pump inhibitor? | If a student is presenting, offer the intern and/or senior resident an opportunity to add to the plan.Incorporate the expertise of ancillary providers rounding with the team. |
| 6. Select a case‐related issue for self‐directed learning | I would like to look up the best way to treat her alcohol withdrawal if she develops it. | Great! We do have a protocol at the hospital, but it is a good idea to review the literature behind it. | Set aside 10 minutes before rounds each day for learners to present their findings. |
| Consider having learners write educational prescriptions following the PICO format. | |||
Step 6 of SNAPPS provides trainees an opportunity to search for and present relevant information to guide patient management. To incorporate more formal teaching time each day, set aside 10 minutes before rounds for learners to present their answers to the team. Also, because SNAPPS has the learner ask about uncertainties, faculty can use their on‐the‐fly teaching time to answer questions for which trainees do not know the answer. In the era of problem‐based learning (PBL) and medical school curricula that foster self‐directed learning from day one, many students should find SNAPPS a natural extension of PBL‐style learning from the preclinical into the clinical years.
Unlike the OMP, SNAPPS does not build in a step for feedback. Therefore, preceptors should focus on step 4 as an opportunity for this. Because feedback is paired with discussion of an uncertainty, it focuses on a trainee's immediate needs and can maximize learning opportunities.[17]
Clinical educators must simultaneously diagnose and manage patients as well as assess learners' abilities.[20] Workplace‐based assessment is particularly important for residents, and hospitalists play a pivotal role in determining their progression along the developmental milestones for achieving the ACGME competencies in medical knowledge, patient care, and practice‐based learning and improvement.[3] Both the OMP and SNAPPS frameworks encourage trainees to think out loud, providing some transparency to their thought process and enabling faculty to more accurately assess their clinical reasoning.
CONCLUSION
Many hospitalists may already use a teaching approach resembling the OMP. It has a familiar, back‐and‐forth rhythm. By explicitly following its steps, however, attendings can ensure they are providing feedback and individualized teaching with each case. SNAPPS, on the other hand, relieves faculty of their familiar role of leading the thought process and imparting teaching points. Instead, the trainee directs the encounter, leaving the attending in the role of guide.[15] SNAPPS aims to help students and residents take charge of their education and develop lifelong learning skills.
Both frameworks can be transferred from the ambulatory to inpatient setting with little modification. The OMP is older and better studied. It is easy to learn, and can be utilized by attendings and residents as teachers. In contrast, SNAPPS requires both teacher and trainee to learn the framework. Typically, this means that SNAPPS needs to be implemented systematically, via a clerkship or residency program. However, if a team was motivated, they could learn and apply it for their time together on service. Though it requires more effort to put in place, SNAPPS provides a novel approach to teaching clinical reasoning. Finally, hospitalists need not implement all steps of either framework for every teaching encounter, but can use components of either model, depending on the individual learners, team composition, time available, or clinical case.
Additional studies examining both frameworks' use for inpatient teaching and assessment would be helpful. Potential questions to address include how the team structure of inpatient rotations impacts the effectiveness of either model (e.g. which trainees benefit when committing to diagnoses or getting feedback in front of a group?), whether either model improves senior residents' ability to lead rounds and teach, whether written faculty assessments of residents are more specific and accurate with either model, and the impact of not following all steps of either model. Higher level outcomes for both models would be another area for investigation, including change in clinical performance, exam performance of students and residents, or patient outcomes, such as length of stay, cost per case, or need for rapid response/emntensive care unit transfer.
ACKNOWLEDGMENTS
Disclosure: Nothing to report.
An important role of the hospitalist educator is to teach residents and medical students how to diagnose and manage acute medical problems. However, clinical reasoning is complex and nuanced, and there are many challenges to teaching this important process. Medical inpatients are increasingly complex, older, and more seriously ill.[1] Documentation requirements and productivity obligations compete with teaching time. Hospitalists must adjust their teaching for learners from different professions and at various levels of training. In addition, hospitalists tend to be less experienced, and must balance the need to learn their roles as clinicians with developing their own skills as educators.[2]
Despite the challenges inherent to the setting, inpatient rotations provide tremendous teaching and learning opportunities. Patients with undifferentiated complaints or known diagnoses in need of management decisions are available to stimulate discussion. Hospitalist educators have the opportunity to assess residents' progress along the developmental milestones, which residency programs are now required to report for accreditation,[3] and provide role modeling for residents who are developing their own teaching skills.
To maximize these opportunities, attendings must engage trainees to practice clinical reasoning and identify their own knowledge gaps. Various strategies for facilitating the clinical reasoning discussion exist, but two frameworksthe One‐Minute Preceptor (OMP) and SNAPPShave been well studied, albeit mainly in the outpatient setting. Both models offer ways to maximize teaching and assess clinical reasoning, but they have different methods and strengths. This article provides a narrative review of the two frameworks and discusses how they can be applied to the inpatient teaching environment. Hospitalists can utilize these models or components of each framework to facilitate teaching on inpatient teams and enhance their roles as educators.
ONE‐MINUTE PRECEPTOR
The OMP was first described in 1992 by Neher and colleagues as an alternative to the traditional model of precepting.[4] It gives preceptors a method to facilitate learners presentation of their thought process and then for the preceptor to provide targeted teaching points.[4] The OMP helps diagnose both learner and patient, whereas the traditional model focuses on diagnosing the patient.[5] In the traditional model, the attending questions the learner to diagnose the patient, which does not often make clear the learner's thinking process. Thus, there may be a mismatch between the teaching points the preceptor makes and what the learner really needs to know.[5] There are several key benefits to the OMP compared to the traditional model; broadly, these relate to improved ability to assess the learner and provide targeted teaching,[4, 5, 6, 7] improved integration of feedback,[4, 8, 9, 10] learner preference,[11] and ease with which it is learned by faculty members.[4]
The OMP model consists of five steps outlined in Table 1. Step 1, getting a commitment, can involve any aspect of the casediagnosis, treatment, or follow‐upand learners should be challenged to make intellectual commitments just beyond their level of comfort.[12] Steps 1 and 2 bring to light the learner's individual learning needs,[11] then the preceptor follows up with personalized teaching. The OMP is efficient; no increase in time was needed to precept a case in an outpatient study.[9] In a separate outpatient study, the OMP led preceptors to be more likely to teach about disease‐specific points and differential diagnosis, as compared to generic items such as history taking and presentation skills with the traditional model.[5]
| A 5‐step framework in which the preceptor does the following: |
| 1. Get a commitment |
| 2. Probe for supporting evidence |
| 3. Provide general rules |
| 4. Reinforce what was done correctly |
| 5. Correct mistakes |
Faculty feel better prepared to assess learners and provide feedback with the OMP model.[6, 9] Aagaard and colleagues provided 116 mostly ambulatory preceptors with scripted, videotaped encounters of the OMP and traditional models. The OMP improved preceptors' confidence at rating students' presentation skills, clinical reasoning, and fund of knowledge. It was rated more efficient and effective, and preceptors were able to diagnose the patient with the same or improved accuracy compared to the traditional model.[6] In a pre‐post study assessing the efficacy of a faculty development workshop, students rated ambulatory teaching encounters incorporating the OMP model as having increased quantity and quality of feedback. Furthermore, faculty reported improved ability to evaluate students and were more likely to let students reach their own conclusions and create their own postencounter learning plans.[9]
The OMP is also well‐received by trainees. Teherani and colleagues analyzed medical students' responses to videotaped teaching encounters of the OMP and traditional models. Students gave higher mean ratings for all studied items (including feedback, involving the student in decision‐making, and overall effectiveness) to the OMP model, and preferred it over the traditional model.[11]
Several studies have evaluated the OMP for use by residents as teachers,[10, 13, 14] and it is one of the most common models taught to residents.[13] One study evaluated the impact of a one‐day workshop for 276 residents that included the five‐step microskills model (also known as the OMP).[10] Residents felt more prepared to teach, set expectations, and provide feedback.[10] The OMP model, despite brief training, is effective in improving residents' teaching effectiveness and confidence.[13]
The only study we found that exclusively evaluated the OMP in the inpatient setting was a randomized trial[8] involving 57 internal medicine residents. Interns and students rated OMP‐trained residents more highly in 4 of 5 behaviors. The behavior that showed no difference from the control group was teaching general rules.[8] However, there was no difference in ratings of overall teaching effectiveness between groups.[8]
Our review of the literature on the OMP shows it is a quickly learned, easily implemented framework for teaching clinical reasoning. It has been used across specialties and settings, provides a built‐in mechanism for feedback, and allows educators to assess trainees' reasoning while extracting the clinical information needed to work efficiently.
SNAPPS
SNAPPS was first described in 2003 by Wolpaw and colleagues. It is a six‐step learner‐centered model as outlined in Table 2.[15] Unlike the OMP, SNAPPS requires both trainee and teacher to learn the framework. In doing so, the responsibility for directing the teaching encounter is shifted toward the learner.[15] Consequently, this model may be best suited to advanced or motivated learners. Like the OMP, SNAPPS was originally described for the ambulatory environment. However, it has been studied in the inpatient setting as well.
| A 6‐step framework in which the learner does the following: |
| 1. Summarize briefly the history and findings |
| 2. Narrow the differential to 2 or 3 possibilities |
| 3. Analyze the differential by comparing/contrasting the possibilities |
| 4. Probe the preceptor by asking questions |
| 5. Plan management for the patient's medical issues |
| 6. Select a case‐related issue for self‐directed learning |
With SNAPPS, the teaching encounter is learner driven. The trainee presents the case and directs the discussion of differential diagnosis. The educator does not have an active role until the fourth step, where the learner asks questions or identifies areas of uncertainty. But even at this stage, the discussion is learner driven. Step 5, planning management, is collaborative, with trainees suggesting management plans with appropriate attending guidance. Depending on learner skill level or case difficulty, the preceptor may need to play more or less of an active role. The final step, picking a case‐related issue to examine, extends the learning beyond the initial encounter, and ensures that it is individualized and relevant. This step also encourages learner progression toward the Accreditation Council for Graduate Medical Education (ACGME) competency of practice‐based learning and improvement.[3]
A handful of studies have evaluated the SNAPPS model. A randomized comparison group trial found that SNAPPS‐trained students outperformed students trained to elicit feedback and students who received the usual and customary preparation.[16] Notably, SNAPPS students expressed more than twice as many differential diagnoses, justified their reasoning more than five times as often, and expressed more questions and uncertainties. The SNAPPS students' presentations were no longer than in the usual and customary group, and were just one minute longer than in the group trained to elicit feedback.[16] A follow‐up analysis found that 100% of the SNAPPS students expressed an uncertainty (i.e. step 4) compared with 54% of the comparison group, and that most of these uncertainties related to diagnostic reasoning.[17]
A study of medicine clerkship students evaluated the impact of extending SNAPPS to the inpatient setting and including educational prescriptions.[18] The goal was to facilitate the formulation and answering of clinical questions by using the patient, intervention, comparison, outcome (PICO) format for step 6 (selecting a case‐based issue to learn about). Dubbing this SNAPPS‐Plus, the authors found that 99% of cases included a question, and 93% of those were answered. Most questions related to therapeutics, and there was a positive correlation between questions more closely corresponding to the PICO format and higher quality answers.[18]
As with the OMP, SNAPPS does not require additional time for case presentations compared to the usual method.[16] From the perspective of a busy hospitalist, this model takes some responsibility for education away from faculty and places it on the learner. This is an important process for fostering self‐directed learning. As with the OMP, SNAPPS appears easily translatable from the outpatient to inpatient setting. Its main downside is the training time required for both parties to implement it.
TRANSLATING THE MODELS TO THE INPATIENT SETTING
The OMP and SNAPPS have largely been used in the outpatient setting. However, we propose that hospitalists can adapt either model for teaching on ward rotations, as the steps of each framework are not exclusive to one clinical setting.
Although the OMP is typically used between a preceptor and single trainee, it is well suited to engaging the entire group on inpatient rounds (Table 3). For example, a student could commit to and support a diagnosis (steps 1 and 2), whereas the intern could commit to and provide evidence for a treatment or management option. Attendings can repeat steps 1 and 2 for patients' secondary problems, encouraging learners to commit to other items on the problem list.
| Attending/ Senior Resident | Learner | Practical Tips | |
|---|---|---|---|
| |||
| Active listening. | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Learners may end their presentation here and expect you to fill in with your assessment and plan. Rather than jumping in, turn it back to the learner following the OMP model. | |
| Get a commitment | What do you think is going on? | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? |
| If the student does not expand the differential, direct this question to the intern. | |||
| Probe for supporting evidence | Why do you think this? | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (e.g. absence of tachycardia) are often key findings. |
| Provide general rules | When a patient with a history of alcohol abuse has a GI bleed, you should consider whether she has underlying liver disease or a coagulopathy. If she did have liver disease, what other sources of bleeding should you consider? | Esophageal varices? | This is the step the residents tend to struggle with when teaching.[8] If your senior resident is leading the case discussion, be prepared to step in with some clinical pearls. |
| Reinforce what was done correctly | You did a nice job considering her predisposing factors, including NSAIDS and alcohol. This helped you prioritize the most likely diagnoses. | Thank you. | Tell them what they did right and the effect it had. |
| Correct mistakes | You did not address her risk for alcohol withdrawal. This increases in patients who are hospitalized for a medical illness. Next time be sure to include substance abuse in your problem list. | I'll make sure to do that. | Tell them what they did not do right and how to improve for the next time. If the student is presenting, consider asking the intern or senior resident for a management plan. |
While teaching general rules (step 3) in the group setting, hospitalists should emphasize basic principles for students (which will serve as reinforcement for residents) as well as discuss more complex rules for the edification of all team members. Hospitalists should encourage senior residents to speak up during this step and share their knowledge with the group. This is an opportunity for residents to practice their role as teachers, and for faculty to assess their clinical acumen. However, residents struggled with teaching general rules in Furney and colleagues' randomized trial.[8] Successful clinical teachers use a mix of improvisational teaching and curriculum scripts developed through years of experience.[19] Hospitalists can model this method of instruction for residents who are learning to teach. For more junior hospitalists who may still be developing their own teaching scripts, the OMP provides an opportunity to regularly integrate these scripts into rounds.
The OMP teaching encounter ends with feedback. Providing real‐time feedback to an individual in the group setting could feel awkward. Reassuringly, in Furney and colleagues' study, some of the greatest gains were in the realm of feedback, as reported by both the senior residents providing the feedback and the interns and students on the receiving end.[8] Although the OMP builds in a space for feedback, it does not teach one how to give feedback. Although it is possible that not all feedback is beneficial, trainees are eager to receive constructive input, and hospitalists should not fear providing this in front of the group. Thoughtful critique of one trainee can provide learning opportunities for others listening in.
SNAPPS is also well suited to inpatient education (Table 4). Because it emphasizes a discussion of differential diagnosis, it works well for new admissions. Because hospitalized patients usually have multiple problems, learners may repeat steps 2 and 3 for each problem, or just for the primary issue. On subsequent days, a standard presentation may work better, but if new problems arise (e.g. fever), hospitalists can ask learners to go through the SNAPPS steps for the new issue.
| Learner | Attending/ Senior Resident | Practical Tips | |
|---|---|---|---|
| |||
| 1. Summarize | Ms. Weinstein is a 60 year old with a history of alcohol abuse and osteoarthritis, admitted with 1 day of epigastric pain and coffee ground emesis. Workup revealed normal vital signs, mild epigastric tenderness, and mild anemia, with normal pancreatic and liver enzymes. | Active listening. | Rather than a complete, detailed history and physical, we emphasize tailoring the oral presentation to include only those components relevant to this admission. Then, transition to the SNAPPS presentation with a summary statement as presented here. |
| 2. Narrow the differential | The most likely diagnoses are upper GI bleed due to peptic ulcer disease, gastritis, or Mallory‐Weiss tear. | If the diagnosis is already established or the leaner prematurely closes the differential, ask What else could this be? | Hospitalized patients often have multiple problems. Learners can go through this process of SNAPPS for each problem or only the primary problem. |
| 3. Analyze the differential | Peptic ulcer disease is most likely because of her alcohol abuse and her daily use of NSAIDs for arthritis pain. Gastritis is equally likely for the same reasons. Mallory‐Weiss tear is less likely, as she was not retching prior to the episode of bleeding. | That's a very reasonable differential. You did a nice job considering her predisposing factors. What do her vital signs tell you about how much blood she has lost? | Learners should use the key findings to argue for or against each diagnostic hypothesis. Novice learners often need reminders that vital signs and negative findings (eg, absence of tachycardia) are often key findings. |
| 4. Probe the preceptor | I know alcohol increases the risk of esophageal cancer, but I was not sure if that could present like this. | You are right that she has a higher risk of cancer. Because most tumors are slow‐growing, what kind of symptoms do you think a mass in the esophagus might cause? | Guide learners to the correct answer, helping them connect pre‐existing knowledge to the question at hand. This is also a good spot to provide real‐time feedback. |
| NOTE: This is a great place for learners to ask questions that might be harder to look up, or to ask about physical findings (eg, I thought I heard crackles but was not sure. Could somebody check this with me?) | Does anyone else on the team have thoughts about this question? | Alternatively, give the senior resident an opportunity to address the question. This allows the attending to assess the senior resident's clinical reasoning and gives him or her an opportunity to practice teaching. | |
| 5. Plan management | For the suspected GI bleed, I would like to start a proton pump inhibitor, call a GI consult for an EGD, and check the hematocrit every 8 hours. We can use sequential compression devices for DVT prophylaxis. We will also counsel on alcohol cessation and monitor for withdrawal. | Good start. Does anyone else on the team want to add to the management plan? We have a pharmacist rounding with us today. Is there a difference in outcomes or costs with BID dosing versus continuous infusion of a proton pump inhibitor? | If a student is presenting, offer the intern and/or senior resident an opportunity to add to the plan.Incorporate the expertise of ancillary providers rounding with the team. |
| 6. Select a case‐related issue for self‐directed learning | I would like to look up the best way to treat her alcohol withdrawal if she develops it. | Great! We do have a protocol at the hospital, but it is a good idea to review the literature behind it. | Set aside 10 minutes before rounds each day for learners to present their findings. |
| Consider having learners write educational prescriptions following the PICO format. | |||
Step 6 of SNAPPS provides trainees an opportunity to search for and present relevant information to guide patient management. To incorporate more formal teaching time each day, set aside 10 minutes before rounds for learners to present their answers to the team. Also, because SNAPPS has the learner ask about uncertainties, faculty can use their on‐the‐fly teaching time to answer questions for which trainees do not know the answer. In the era of problem‐based learning (PBL) and medical school curricula that foster self‐directed learning from day one, many students should find SNAPPS a natural extension of PBL‐style learning from the preclinical into the clinical years.
Unlike the OMP, SNAPPS does not build in a step for feedback. Therefore, preceptors should focus on step 4 as an opportunity for this. Because feedback is paired with discussion of an uncertainty, it focuses on a trainee's immediate needs and can maximize learning opportunities.[17]
Clinical educators must simultaneously diagnose and manage patients as well as assess learners' abilities.[20] Workplace‐based assessment is particularly important for residents, and hospitalists play a pivotal role in determining their progression along the developmental milestones for achieving the ACGME competencies in medical knowledge, patient care, and practice‐based learning and improvement.[3] Both the OMP and SNAPPS frameworks encourage trainees to think out loud, providing some transparency to their thought process and enabling faculty to more accurately assess their clinical reasoning.
CONCLUSION
Many hospitalists may already use a teaching approach resembling the OMP. It has a familiar, back‐and‐forth rhythm. By explicitly following its steps, however, attendings can ensure they are providing feedback and individualized teaching with each case. SNAPPS, on the other hand, relieves faculty of their familiar role of leading the thought process and imparting teaching points. Instead, the trainee directs the encounter, leaving the attending in the role of guide.[15] SNAPPS aims to help students and residents take charge of their education and develop lifelong learning skills.
Both frameworks can be transferred from the ambulatory to inpatient setting with little modification. The OMP is older and better studied. It is easy to learn, and can be utilized by attendings and residents as teachers. In contrast, SNAPPS requires both teacher and trainee to learn the framework. Typically, this means that SNAPPS needs to be implemented systematically, via a clerkship or residency program. However, if a team was motivated, they could learn and apply it for their time together on service. Though it requires more effort to put in place, SNAPPS provides a novel approach to teaching clinical reasoning. Finally, hospitalists need not implement all steps of either framework for every teaching encounter, but can use components of either model, depending on the individual learners, team composition, time available, or clinical case.
Additional studies examining both frameworks' use for inpatient teaching and assessment would be helpful. Potential questions to address include how the team structure of inpatient rotations impacts the effectiveness of either model (e.g. which trainees benefit when committing to diagnoses or getting feedback in front of a group?), whether either model improves senior residents' ability to lead rounds and teach, whether written faculty assessments of residents are more specific and accurate with either model, and the impact of not following all steps of either model. Higher level outcomes for both models would be another area for investigation, including change in clinical performance, exam performance of students and residents, or patient outcomes, such as length of stay, cost per case, or need for rapid response/emntensive care unit transfer.
ACKNOWLEDGMENTS
Disclosure: Nothing to report.
- , , , . 2006 national hospital discharge survey. Natl Health Stat Report. 2008;(5):1–20.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Hosp Med. 2009;4(4):240–246.
- Accreditation Council for Graduate Medical Education. Program and institutional accreditation: next accreditation system: Milestones. Available at: https://www.acgme.org/acgmeweb/tabid/430/ProgramandInstitutionalAccreditation/NextAccreditationSystem/Milestones.aspx. Accessed July 28, 2014.
- , , , . A five‐step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5(4):419–424.
- , , . Teaching points identified by preceptors observing one‐minute preceptor and traditional preceptor encounters. Acad Med. 2004;79(1):50–55.
- , , . Effectiveness of the one‐minute preceptor model for diagnosing the patient and the learner: proof of concept. Acad Med. 2004;79(1):42–49.
- , , . Measuring outcomes of a one‐minute preceptor faculty development workshop. J Gen Intern Med. 2006;21(5):410–414.
- , , , , , . Teaching the one‐minute preceptor. A randomized controlled trial. J Gen Intern Med. 2001;16(9):620–624.
- , , , , , . Faculty development seminars based on the one‐minute preceptor improve feedback in the ambulatory setting. J Gen Intern Med. 2002;17(10):779–787.
- , , , , . Change in residents' perceptions of teaching: following a one day "residents as teachers" (RasT) workshop. South Med J. 2008;101(5):495–502.
- , , , , . Student perceptions of the one minute preceptor and traditional preceptor models. Med Teach. 2007;29(4):323–327.
- , . The one‐minute preceptor: shaping the teaching conversation. Fam Med. 2003;35(6):391–393.
- , , . Residents‐as‐teachers curricula: a critical review. Acad Med. 2009;84(3):374–380.
- , , . Teaching to teach in Toronto. Acad Psychiatry. 2010;34(4):277–281.
- , , . SNAPPS: a learner‐centered model for outpatient education. Acad Med. 2003;78(9):893–898.
- , , . Using SNAPPS to facilitate the expression of clinical reasoning and uncertainties: A randomized comparison group trial. Acad Med. 2009;84(4):517–524.
- , , , . Student uncertainties drive teaching during case presentations: more so with SNAPPS. Acad Med. 2012;87(9):1210–1217.
- , , , , , . SNAPPS‐plus: an educational prescription for students to facilitate formulating and answering clinical questions. Acad Med. 2014;89(8):1174–1179.
- . How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
- , , , . 2006 national hospital discharge survey. Natl Health Stat Report. 2008;(5):1–20.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Hosp Med. 2009;4(4):240–246.
- Accreditation Council for Graduate Medical Education. Program and institutional accreditation: next accreditation system: Milestones. Available at: https://www.acgme.org/acgmeweb/tabid/430/ProgramandInstitutionalAccreditation/NextAccreditationSystem/Milestones.aspx. Accessed July 28, 2014.
- , , , . A five‐step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5(4):419–424.
- , , . Teaching points identified by preceptors observing one‐minute preceptor and traditional preceptor encounters. Acad Med. 2004;79(1):50–55.
- , , . Effectiveness of the one‐minute preceptor model for diagnosing the patient and the learner: proof of concept. Acad Med. 2004;79(1):42–49.
- , , . Measuring outcomes of a one‐minute preceptor faculty development workshop. J Gen Intern Med. 2006;21(5):410–414.
- , , , , , . Teaching the one‐minute preceptor. A randomized controlled trial. J Gen Intern Med. 2001;16(9):620–624.
- , , , , , . Faculty development seminars based on the one‐minute preceptor improve feedback in the ambulatory setting. J Gen Intern Med. 2002;17(10):779–787.
- , , , , . Change in residents' perceptions of teaching: following a one day "residents as teachers" (RasT) workshop. South Med J. 2008;101(5):495–502.
- , , , , . Student perceptions of the one minute preceptor and traditional preceptor models. Med Teach. 2007;29(4):323–327.
- , . The one‐minute preceptor: shaping the teaching conversation. Fam Med. 2003;35(6):391–393.
- , , . Residents‐as‐teachers curricula: a critical review. Acad Med. 2009;84(3):374–380.
- , , . Teaching to teach in Toronto. Acad Psychiatry. 2010;34(4):277–281.
- , , . SNAPPS: a learner‐centered model for outpatient education. Acad Med. 2003;78(9):893–898.
- , , . Using SNAPPS to facilitate the expression of clinical reasoning and uncertainties: A randomized comparison group trial. Acad Med. 2009;84(4):517–524.
- , , , . Student uncertainties drive teaching during case presentations: more so with SNAPPS. Acad Med. 2012;87(9):1210–1217.
- , , , , , . SNAPPS‐plus: an educational prescription for students to facilitate formulating and answering clinical questions. Acad Med. 2014;89(8):1174–1179.
- . How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638.
- . Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217–2225.
Acute Kidney Injury for Hospitalists
Acute kidney injury (AKI) is a clinical syndrome broadly defined as an abrupt decline in renal function occurring over a period of hours to days resulting in the retention of nitrogenous and metabolic waste products. Although the initial clinical manifestation of AKI may be oliguria, urine volume can remain normal or even increase. Patients may be asymptomatic, especially early in the course of AKI. The diagnosis is often made in hospitalized patients when biochemical screening reveals a recent increase in serum creatinine and/or blood urea nitrogen concentrations, or when there is a dramatic decrease in urine output.
Older studies looking at the incidence of AKI in hospitalized patients are difficult to interpret due to variable definitions of AKI. Those based on administrative databases were limited by lack of clinical context and/or variation in coding for AKI.[1]
There is no universally accepted operational definition of AKI, and more than 30 different criteria have been employed in various clinical studies. Difficulty in defining AKI lies in the lag time in the rise and fall of the serum creatinine concentration with injury and recovery, the variability of oliguria, and in the heterogeneity of patterns of renal injury. Two classification systems that attempt to capture the spectrum of AKI are the RIFLE (Risk, Injury, Failure, Loss, End Stage) criteria and the AKIN (Acute Kidney Injury Network) criteria.[2, 3] The AKIN criteria parallel the risk, injury, and failure stages of the RIFLE criteria and are the most applicable to characterizing AKI in the hospital (Table 1). AKI is commonly classified by daily urine output as anuric (<50 mL/day), oliguric (<500 mL/day), or nonoliguric.
| Stage | Creatinine Criteria | Urine Output Criteria |
|---|---|---|
| 1 | Increase in serum creatinine of 0.3 mg/dL (26.4 mol/L) or increase of 150%200% (1.5‐fold to 2‐fold) above baseline | <0.5 mL/kg/hr for >6 hours |
| 2 | Increase in serum creatinine of >200%300% (>2‐fold to 3‐fold) above baseline | <0.5 mL/kg/hr for >12 hours |
| 3 | Increase in serum creatinine of >300% (3‐fold) above baseline or serum creatinine 5.0 mg/dL (354 mol/L) with an acute rise of 0.5 mg/dL (44 mol/L) | <0.3 mL/kg/hr 24 hours or anuria 12 hours |
With a move toward standardized definitions, recent studies have shown a rising incidence of AKI in hospitalized patients.[4, 5, 6] According to these series, AKI develops in up to 7% of hospitalized patients and in about 30% of those admitted to intensive care units. In one study of consecutive hospital admissions, patients classified by the RIFLE criteria had a sharp rise in the rate of in‐hospital mortality whether they had no change or improvement in creatinine (4.4%), or fell into a risk (15.1%), injury (29.2%), or failure (41.1%) class.[7] The in‐hospital mortality of critically ill patients with AKI is higher than 50%. AKI increases length of stay and hospital costs, and affects the clinical course after discharge.[8, 9] Small increases in serum creatinine during an intensive care unit stay predict increased 10‐year mortality above a critical illness alone.[10]
Risk factors for AKI include advanced age, male gender, African American ethnicity, and diabetes mellitus.[11] The most important risk factor, however, is preexisting chronic kidney disease (CKD).[12] AKI and CKD are tightly linked, each increasing the risk of the other.[13, 14, 15] Preexisting renal insufficiency is a key predictor of postoperative AKI and poor surgical outcomes.[16, 17]
AKI AND CLINICAL CONTEXT
The causes of AKI can be broadly divided into 3 categories: prerenal azotemia (a disorder characterized by renal hypoperfusion in which renal parenchymal tissue integrity is preserved), intrinsic kidney injury with parenchymal tissue injury, and postrenal AKI (dysfunction due to acute obstruction of the urinary tract). Table 2 lists several clinical scenarios sorted into these 3 categories.[18] The general epidemiology of AKI varies based on whether it was acquired in the community or in a hospital setting. Prerenal azotemia accounts for the bulk of community‐acquired AKI, followed in lesser frequency by postrenal and intrinsic etiologies. Prerenal azotemia continues to be the major cause of hospital‐acquired AKI, but intrinsic kidney injury becomes more common.[5, 19]
| Prerenal | Intrinsic | Postrenal |
|---|---|---|
| ||
| Hemorrhage | Acute tubular necrosis | Bilateral upper tract obstruction |
| Surgical | Ischemic | Nephrolithiasis |
| Gastrointestinal | Postoperative | Papillary necrosis |
| Retroperitoneal | Prolonged hypotension | Retroperitoneal fibrosis |
| Gastrointestinal losses | Sepsis | Retroperitoneal lymphadenopathy |
| Diarrhea | Nephrotoxins | Obstruction of solitary functioning kidney |
| Vomiting | Myoglobin | Lower tract obstruction |
| Nasogastric suction | Hemoglobin | Prostatic hypertrophy |
| Enteral fistula | Radiocontrast agents | Urethral stricture |
| Renal losses | Aminoglycosides | Bladder mass or stone |
| Diuretics | Intratubular obstruction | Obstructed urinary catheter |
| Glucosuria | Tumor lysis/uric acid | Urinary retention |
| Skin losses | Oxalosis/ethylene glycol ingestion | Neurogenic bladder |
| Excessive sweating | Phosphate nephropathy | Constipation |
| Burns | Light chain nephropathy | Medications |
| Erythroderma | Acyclovir | Anticholinergics |
| Third‐spacing | Indinavir | Antihistamines |
| Hypoalbuminemia | Methotrexate | Alpha1‐agonists |
| Pancreatitis | Acute glomerulonephritis | ‐Blockers |
| Capillary leak | Acute interstitial nephritis | Opiates |
| Reduced effective arterial volume | Proton pump inhibitors | Tricyclic antidepressants |
| Congestive heart failure | Penicillins | |
| Cirrhosis | Fluoroquinolones | |
| Renal vasoconstriction | Atheroembolic disease | |
| Hypercalcemia | Acute vascular syndrome | |
| NSAIDs | Aortic dissection | |
| ACEI/ARB | Bilateral renal artery thromboembolism | |
| Calcineurin inhibitors | Bilateral renal vein thrombosis | |
| Vasopressors | Thrombotic microangiopathy | |
| Iodinated contrast | ||
MEDICAL HISTORY
The initial goal of history taking is to establish whether the patient has AKI rather than the acute discovery of a more chronic process. A recent serum creatinine measurement can be valuable in this regard. In some cases the clinician must make a presumptive diagnosis of AKI while simultaneously reviewing past medical history and family history to assess for underlying CKD. A diagnosis of AKI is more readily established when it occurs during a hospitalization through review of urine output and serial laboratory values.
Symptoms of poor oral intake as well as salt and fluid losses from diarrhea or vomiting suggest a prerenal etiology. Subjective symptoms of lightheadedness, visual clouding, and near‐syncope with standing also suggest volume depletion. Patients should be asked about recent nonsteroidal anti‐inflammatory drug (NSAID) use, as these agents can exacerbate renal hypoperfusion through loss of prostaglandin‐mediated afferent arteriole dilatation. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, especially when combined with diuretics, can generate a hypoperfusion state. Heart failure and liver disease regularly result in an expanded extracellular fluid (ECF) compartment yet a reduced effective arterial volume and predispose to renal hypoperfusion.
A history of decreased urine output or anuria suggests postrenal AKI, but its absence does not rule out urinary tract obstruction. Voiding symptoms such as urinary frequency, hesitancy, or incontinence also raise the possibility of obstructive uropathy. Flank pain and hematuria often accompany obstruction from nephrolithiasis.
Symptoms of fever, skin rash, arthralgias, sinusitis, and/or hemoptysis raise the possibility of glomerulonephritis from infection, collagen vascular disease, or vasculitis. Risk factors for viral hepatitis and human immunodeficiency virus are important to clarify as are systemic symptoms of autoimmunity (eg, dry eyes, dry mouth, eye pain/emnflammation, or visual changes). The recent start of any new medication, including NSAIDs, antibiotics, or proton‐pump inhibitors, raises the possibility of a drug‐induced interstitial nephritis.[20] Statins are direct myotoxins, and the risk of rhabdomyolysis with renal injury increases with dose. Patients may not associate intravenous (IV) contrast or phosphate‐containing bowel preparations (eg, Fleet Phospho Soda), with the development of AKI, thus the clinician must carefully review for recent exposures that could result in intrinsic renal injury.[21]
PHYSICAL EXAMINATION
Estimation of the ECF volume and effective arterial volume are central to assessing the likelihood of renal hypoperfusion. Overt hypotension is the strongest indicator of hypoperfusion, and a careful review of initial blood pressure prior to worsening of renal function can provide significant information. Normal blood pressure does not exclude renal hypoperfusion, as acute tubular necrosis (ATN) may develop in chronically hypertensive patients whose blood pressures are acutely reduced.[22] Less‐severe volume depletion is suggested by an orthostatic pulse increase of more than 30 beats/minute, measured 1 minute after standing. Orthostatic hypotension, defined as a drop in systolic pressure of more than 20 mm Hg after standing, is less helpful, as it occurs in 10% of normovolemic subjects.[23] Dry axillae and mucous membranes with a furrowed tongue are useful signs of volume depletion. Poor skin turgor and slow capillary refill have not been shown to be reliable signs of hypovolemia in adults. The neck veins are usually flat when volume contraction exists, though engorged neck veins in the setting of elevated right‐sided pressures from heart failure or pulmonary hypertension may obscure this sign. Similarly, pulmonary rales, ascites, and peripheral edema may confound the exam in patients with underlying heart failure and/or cirrhosis.
Flank tenderness or a bladder palpable or percussable above the pelvic brim suggests possible urinary tract obstruction. Prostate exam should be performed on all men with AKI and a bimanual pelvic exam considered in women with changes in usual voiding pattern or with suspected gynecologic disease. Postvoid residual can be assessed at the bedside with either straight catheterization or bladder scan where available.
Signs of systemic disease associated with intrinsic AKI include fever, skin and joint findings of connective tissue disease, a new or changing heart murmur, purpura, and petechiae. Cholesterol emboli, disrupted by interarterial catheterization (eg, cardiac catheterization, angiography), cardiac or aortic surgeries, or, rarely, by systemic anticoagulation can shower throughout the vasculature, causing organ dysfunction and local inflammation. Kidney injury due to atheroemboli often has a stuttering course and may be separated in time from the vascular procedure by days to weeks. Physical exam findings of atheroembolic disease include livedo reticularis, blue toes, purpura, painful skin nodules, and gangrene. Retinal examination may reveal atheroembolic emboli (Hollenhorst plaques).[24, 25]
LABORATORY TESTING
Initial testing in AKI aims to assess the severity of injury as well as the likely mechanism of the injury. Estimation of glomerular filtration rate (GFR) gives an approximate measure of the number of functioning nephrons and hence an overall measure of renal function. Mathematical estimates of GFR, however, assume a steady state, and AKI, by definition, is not a steady state. This makes GFR estimates based on plasma creatinine unreliable. A rising serum creatinine concentration indicates that the renal injury is persistent or worsening, whereas a stable or falling creatinine concentration suggests recovery. Interventions that expand the ECF (eg, volume resuscitation with normal saline) will dilute the plasma creatinine concentration and must be considered when interpreting a falling creatinine concentration. A daily rise in the serum creatinine concentration of more than 1 mg/dL nearly always implies a GFR of <10 mL/min. Any change in serum creatinine must be interpreted with the nonlinear relationship of GFR and serum creatinine in mind (Figure 1).[26]

The fractional excretion of sodium (FENa) has been used to differentiate prerenal azotemia from intrinsic renal injury in patients with oligoanuria. Specifically, an FENa of <1% implies a prerenal cause for the oliguric AKI, whereas if it is >1%, then intrinsic renal injury is more likely. Unfortunately, there are significant limitations to this laboratory measure.[27] The FENa may be low (<1%) in any intrinsic process that causes tissue ischemia, such as vasculitis, acute glomerulonephritis, atheroembolic disease, or from intense vasoconstriction such as after IV contrast administration. Patients with severe heart failure or portal hypertension often have avid sodium retention, and can have a FENa <1% even in the setting of ATN. Alternatively, the FENa may be elevated (>1%) in prerenal patients on diuretics, with osmotic diuresis, or in the setting of aldosterone deficiency.
Examination of the urinalysis and urine sediment provides valuable information about the etiology of the AKI. Prerenal and postrenal AKI typically present with a bland urine, without evidence of blood, protein, or leukocyte esterase on urinalysis and few cells or hyaline casts in the sediment. The urinalysis typically has a high specific gravity in prerenal AKI, reflecting intact tubules producing a concentrated urine. An active urinary sediment suggests intrinsic renal injury that is either the mechanism of the current AKI or indicative of underlying CKD. ATN, the most common cause of intrinsic renal injury, often produces a dirty urinalysis with many epithelial cells and muddy brown granular and epithelial cell casts. The urine is generally isosthenuric (ie, specific gravity of 1.010) due to loss of tubular function. A urinalysis positive for heme pigment but without red cells on microscopic analysis suggests the presence of either myoglobin from rhabdomyolysis or hemoglobin from hemolysis. Acute glomerulonephritis disrupts the usual glomerular barrier to large proteins and red cells and results in proteinuria and hematuria. Red cells that weather the journey from the glomerulus through the nephron often become dysmorphic with Mickey Mouse ear blebs in their membrane or are bound together by Tamm‐Horsfall protein into red cell casts. Acute interstitial nephritis results in pyuria, proteinuria, and white cell casts. Urinary eosinophils are neither sensitive nor specific for interstitial nephritis and have little utility in its diagnosis.[28, 29]
Given the limitations of serum creatinine as a marker of renal injury, a number of new urinary biomarkers have been recognized over the past decade.[30, 31, 32] These molecules are normal constituents of renal tubular cells that are upregulated and released into the urine in response to renal injury. Early measurement of these biomarkers might allow for detection of AKI within hours of the insult. The 2 biomarkers with the most promise include kidney injury molecule‐1 (KIM‐1) and neutrophil gelatinase‐associated lipocalin (NGAL). KIM‐1 is expressed by proximal tubular cells, and its production is sharply upregulated in response to ischemic injury. NGAL is a protein expressed primarily in immune cells, but also by renal tubular cells. Urinary NGAL levels rapidly rise in response to renal ischemia, and return to baseline following resolution of the injury. Although these urinary biomarkers are promising, they have a relatively low (70%75%) sensitivity and specificity, and have not yet been adopted into routine clinical practice.[33]
IMAGING
Renal ultrasound is useful both in the assessment of AKI as well as in the investigation for underlying CKD. Patients with long‐standing kidney disease frequently have small, echogenic kidneys consistent with fibrosis and nephron loss, or markedly distorted renal architecture in cystic diseases. Hydronephrosis and/or hydroureter suggest an acute or chronic urinary tract obstruction. However, this may not be present in the setting of early obstruction or ureteric encasement. Doppler ultrasonography of the renal vasculature can assess patency when vascular obstruction is suspected. The use of computerized tomography, magnetic resonance imaging, or angiography may be helpful in selected clinical circumstances, but their use is often limited due to the potential risk of contrast nephrotoxicity. Nuclear renal scans use less radiation than computerized tomography and are a preferred imaging modality for pediatric patients. When volume status is uncertain, echocardiography to assess both inferior vena cava volume and change in volume with respiration may be helpful.
MANAGEMENT
The general principles for management of AKI are to limit further injury and prevent systemic complications. Management of the patient with AKI greatly depends on which category of AKI is suspected, namely prerenal, intrinsic renal injury, or a postrenal (obstructive) cause. If a prerenal etiology due to true ECF volume depletion is suspected, volume resuscitation to replace baseline and ongoing losses is imperative. Careful attention to intake and output as well as serial volume assessment should dictate the strategy for resuscitation. Hyperchloremic acidosis is an expected consequence of normal saline resuscitation but is irrelevant to clinical outcomes.[34] NSAIDs, antihypertensives, especially those that affect the angiotensin/aldosterone system, and diuretics should be discontinued. Ongoing hypotension despite volume resuscitation suggests the possibility of blood loss, infection, or autonomic nervous system dysfunction. If this occurs, the patient may need to be transferred to an intensive care unit for pressor support to keep the mean arterial pressure >70 mm Hg. When prerenal AKI from reduced effective circulating volume is suspected, as in decompensated heart failure or cirrhosis, management must be tailored to the underlying pathophysiology.
If judicious volume resuscitation produces no improvement in renal function or if oliguria develops, repeat urinalysis and urine microscopy should be considered to assess for intrinsic renal injury. Aggressive volume resuscitation in the face of oliguria will not speed recovery from the intrinsic injury and may cause signs or symptoms of volume overload. This could also potentially necessitate renal replacement therapy earlier than anticipated.
In patients where an obstructive etiology for the AKI is identified, the obstruction must be relieved as soon and as safely as possible. In this regard, a timely urologic consultation may be helpful in assuring that urethral and/or ureteral conduits are placed rapidly. Interventional radiology can also assist in those patients who need percutaneous nephrostomies for the relief of the obstruction. In many patients with obstructive nephropathy, a timely intervention will avoid the need for renal replacement therapy.
The suspected mechanism of injury influences the management of intrinsic AKI. The management of ATN is primarily supportive, paying close attention to optimizing volume status, correcting electrolyte abnormalities, avoiding further nephrotoxic agents, and adjusting medication doses to the low GFR present. Over the last several decades, multiple studies have explored treatment strategies for established ATN using various drugs and biologic agents. All have been uniformly disappointing.
When the trajectory of AKI is uncertain and the creatinine continues to rise, all medication dosing should be adjusted for GFR <10 mL/min. Antibiotics routinely will require dose reduction, but all current medications should be reviewed for risk of accumulation in renal failure. Because the half‐life of oral hypoglycemic medications is unpredictable in AKI, these medications should be discontinued and replaced with insulin. Vigilance for hypoglycemia is necessary, as renal clearance of insulin is also reduced. Narcotics such as morphine and oxycodone, which are renally cleared, can produce unwanted sedation and respiratory depression if not discontinued. Fentanyl, methadone, and hydromorphone are safer choices for controlling pain in a patient with AKI.[35] Gabapentin is regularly used to treat symptoms of neuropathic pain, but can produce encephalopathy and myoclonus if not dose reduced in renal failure.[36] Clinicians should weigh the risk of overdose with underdose for each medication, namely antibiotics in critically ill patients.
TIMING OF NEPHROLOGY CONSULTATION
The optimal timing for nephrology consultation in hospital‐acquired AKI is uncertain, though several studies have suggested better outcomes, including shorter length of stay and reduced mortality, with early consultation.[37, 38, 39] A renal consult is indicated when intrinsic ATN does not reverse in a timely fashion. Renal replacement therapy should be instituted to limit the systemic complications of prolonged AKI and to allow time for the renal injury to improve or resolve over time. If acute glomerulonephritis or interstitial nephritis is suspected, an urgent consultation may be required for consideration of biopsy, immunosuppression, and guidance for further management. Early consultation may help limit drug toxicities and volume overload in the setting of decreased renal clearance. Guidance on vascular access (eg, peripherally inserted central catheter placement) may prevent future complications with hemodialysis access if the patient ultimately develops end‐stage renal disease (ESRD).[40]
PREVENTION OF AKI
Most studies of AKI prevention have focused on clinical scenarios where the likelihood of ATN was substantial such as in vascular or open heart surgery, or with the use of intravenous contrast agents.[41, 42] This topic remains controversial, though generally supported strategies include judicious volume expansion, avoidance of hypotension, and, when using contrast, limiting the volume of contrast and using iso‐osmolar formulations. As recent studies have shown uncertain benefit, the role for pretreatment with n‐acetylcysteine remains uncertain. Many clinicians, however, continue to use it as a preventive strategy as there are few side effects with this medication.
TAKE HOME POINTS
- AKI is common in hospitalized patients, with pre‐renal azotemia being the dominant etiology in both community‐acquired and hospital‐acquired AKI.
- CKD is an important risk factor for AKI. AKI increases the long‐term risk of developing CKD and ESRD.
- The diagnosis of AKI hinges on detailed medical history, careful physical exam, and key laboratory parameters including the urinalysis and urinary sediment.
- The management of AKI is tailored to the likely mechanism of injury. Reconsideration of the likely etiology is imperative if AKI fails to respond to initial attempts to reverse or limit injury.
- Early renal consultation for AKI is indicated when the etiology remains uncertain, AKI persists despite initial management, or acute glomerulonephritis or interstitial nephritis are suspected.
- , , . Acute kidney injury. Lancet. 2012;380(9843):756–766.
- , , , , . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212.
- , , , et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis. 2007;50(1):1–4.
- , , , et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142.
- , , . Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936.
- , , , . Acute renal failure: factors influencing nephrology referral and outcome. QJM. 1997;90(12):781–785.
- , , , , . An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917.
- , , , , . Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370.
- , , , , . The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta‐analysis. Am J Kidney Dis. 2007;50(5):712–720.
- , , , , , . Small acute increases in serum creatinine are associated with decreased long‐term survival in the critically ill. Am J Respir Crit Care Med. 2014;189(9):1075–1081.
- , , , et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228.
- , , , , , . The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–107.
- , , . Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012;81(5):442–448.
- , , , et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125(6):585–593.
- , , , et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185.
- , , , et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515.
- , , , et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta‐analysis. Anesthesiology. 2013;118(4):809–824.
- , . Brenner 2008.
- , , , , . Hospital‐acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248.
- , , , . A nationwide nested case‐control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837–844.
- , , , . Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16(11):3389–3396.
- . Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797–805.
- , , . The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022–1029.
- , . Atheroembolic renal disease. Lancet. 2010;375(9726):1650–1660.
- , , , et al. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116(3):298–304.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470.
- , . Traditional urinary biomarkers in the assessment of hospital‐acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167–174.
- , . Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841–1843.
- , , . Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–1862.
- . Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219.
- , . Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62(1):165–178.
- , , , et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–2165.
- , , , et al. Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside? Ann Intensive Care. 2012;2(1):24.
- , . The case for 0.9% NaCl: is the undefendable, defensible? Kidney Int. 2014;86(6):1087–1095.
- , , , , . A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525–552.
- , , . Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med. 2010;123(4):367–373.
- , , , et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919.
- , , , et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113(6):456–461.
- , , , et al. Early nephrologist involvement in hospital‐acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011;57(2):228–234.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601–608.
- , , , , , . Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. Am J Surg. 2013;206(1):86–95.
- , , , et al. Strategies to reduce the risk of contrast‐induced nephropathy. Am J Cardiol. 2006;98(6A):59K–77K.
Acute kidney injury (AKI) is a clinical syndrome broadly defined as an abrupt decline in renal function occurring over a period of hours to days resulting in the retention of nitrogenous and metabolic waste products. Although the initial clinical manifestation of AKI may be oliguria, urine volume can remain normal or even increase. Patients may be asymptomatic, especially early in the course of AKI. The diagnosis is often made in hospitalized patients when biochemical screening reveals a recent increase in serum creatinine and/or blood urea nitrogen concentrations, or when there is a dramatic decrease in urine output.
Older studies looking at the incidence of AKI in hospitalized patients are difficult to interpret due to variable definitions of AKI. Those based on administrative databases were limited by lack of clinical context and/or variation in coding for AKI.[1]
There is no universally accepted operational definition of AKI, and more than 30 different criteria have been employed in various clinical studies. Difficulty in defining AKI lies in the lag time in the rise and fall of the serum creatinine concentration with injury and recovery, the variability of oliguria, and in the heterogeneity of patterns of renal injury. Two classification systems that attempt to capture the spectrum of AKI are the RIFLE (Risk, Injury, Failure, Loss, End Stage) criteria and the AKIN (Acute Kidney Injury Network) criteria.[2, 3] The AKIN criteria parallel the risk, injury, and failure stages of the RIFLE criteria and are the most applicable to characterizing AKI in the hospital (Table 1). AKI is commonly classified by daily urine output as anuric (<50 mL/day), oliguric (<500 mL/day), or nonoliguric.
| Stage | Creatinine Criteria | Urine Output Criteria |
|---|---|---|
| 1 | Increase in serum creatinine of 0.3 mg/dL (26.4 mol/L) or increase of 150%200% (1.5‐fold to 2‐fold) above baseline | <0.5 mL/kg/hr for >6 hours |
| 2 | Increase in serum creatinine of >200%300% (>2‐fold to 3‐fold) above baseline | <0.5 mL/kg/hr for >12 hours |
| 3 | Increase in serum creatinine of >300% (3‐fold) above baseline or serum creatinine 5.0 mg/dL (354 mol/L) with an acute rise of 0.5 mg/dL (44 mol/L) | <0.3 mL/kg/hr 24 hours or anuria 12 hours |
With a move toward standardized definitions, recent studies have shown a rising incidence of AKI in hospitalized patients.[4, 5, 6] According to these series, AKI develops in up to 7% of hospitalized patients and in about 30% of those admitted to intensive care units. In one study of consecutive hospital admissions, patients classified by the RIFLE criteria had a sharp rise in the rate of in‐hospital mortality whether they had no change or improvement in creatinine (4.4%), or fell into a risk (15.1%), injury (29.2%), or failure (41.1%) class.[7] The in‐hospital mortality of critically ill patients with AKI is higher than 50%. AKI increases length of stay and hospital costs, and affects the clinical course after discharge.[8, 9] Small increases in serum creatinine during an intensive care unit stay predict increased 10‐year mortality above a critical illness alone.[10]
Risk factors for AKI include advanced age, male gender, African American ethnicity, and diabetes mellitus.[11] The most important risk factor, however, is preexisting chronic kidney disease (CKD).[12] AKI and CKD are tightly linked, each increasing the risk of the other.[13, 14, 15] Preexisting renal insufficiency is a key predictor of postoperative AKI and poor surgical outcomes.[16, 17]
AKI AND CLINICAL CONTEXT
The causes of AKI can be broadly divided into 3 categories: prerenal azotemia (a disorder characterized by renal hypoperfusion in which renal parenchymal tissue integrity is preserved), intrinsic kidney injury with parenchymal tissue injury, and postrenal AKI (dysfunction due to acute obstruction of the urinary tract). Table 2 lists several clinical scenarios sorted into these 3 categories.[18] The general epidemiology of AKI varies based on whether it was acquired in the community or in a hospital setting. Prerenal azotemia accounts for the bulk of community‐acquired AKI, followed in lesser frequency by postrenal and intrinsic etiologies. Prerenal azotemia continues to be the major cause of hospital‐acquired AKI, but intrinsic kidney injury becomes more common.[5, 19]
| Prerenal | Intrinsic | Postrenal |
|---|---|---|
| ||
| Hemorrhage | Acute tubular necrosis | Bilateral upper tract obstruction |
| Surgical | Ischemic | Nephrolithiasis |
| Gastrointestinal | Postoperative | Papillary necrosis |
| Retroperitoneal | Prolonged hypotension | Retroperitoneal fibrosis |
| Gastrointestinal losses | Sepsis | Retroperitoneal lymphadenopathy |
| Diarrhea | Nephrotoxins | Obstruction of solitary functioning kidney |
| Vomiting | Myoglobin | Lower tract obstruction |
| Nasogastric suction | Hemoglobin | Prostatic hypertrophy |
| Enteral fistula | Radiocontrast agents | Urethral stricture |
| Renal losses | Aminoglycosides | Bladder mass or stone |
| Diuretics | Intratubular obstruction | Obstructed urinary catheter |
| Glucosuria | Tumor lysis/uric acid | Urinary retention |
| Skin losses | Oxalosis/ethylene glycol ingestion | Neurogenic bladder |
| Excessive sweating | Phosphate nephropathy | Constipation |
| Burns | Light chain nephropathy | Medications |
| Erythroderma | Acyclovir | Anticholinergics |
| Third‐spacing | Indinavir | Antihistamines |
| Hypoalbuminemia | Methotrexate | Alpha1‐agonists |
| Pancreatitis | Acute glomerulonephritis | ‐Blockers |
| Capillary leak | Acute interstitial nephritis | Opiates |
| Reduced effective arterial volume | Proton pump inhibitors | Tricyclic antidepressants |
| Congestive heart failure | Penicillins | |
| Cirrhosis | Fluoroquinolones | |
| Renal vasoconstriction | Atheroembolic disease | |
| Hypercalcemia | Acute vascular syndrome | |
| NSAIDs | Aortic dissection | |
| ACEI/ARB | Bilateral renal artery thromboembolism | |
| Calcineurin inhibitors | Bilateral renal vein thrombosis | |
| Vasopressors | Thrombotic microangiopathy | |
| Iodinated contrast | ||
MEDICAL HISTORY
The initial goal of history taking is to establish whether the patient has AKI rather than the acute discovery of a more chronic process. A recent serum creatinine measurement can be valuable in this regard. In some cases the clinician must make a presumptive diagnosis of AKI while simultaneously reviewing past medical history and family history to assess for underlying CKD. A diagnosis of AKI is more readily established when it occurs during a hospitalization through review of urine output and serial laboratory values.
Symptoms of poor oral intake as well as salt and fluid losses from diarrhea or vomiting suggest a prerenal etiology. Subjective symptoms of lightheadedness, visual clouding, and near‐syncope with standing also suggest volume depletion. Patients should be asked about recent nonsteroidal anti‐inflammatory drug (NSAID) use, as these agents can exacerbate renal hypoperfusion through loss of prostaglandin‐mediated afferent arteriole dilatation. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, especially when combined with diuretics, can generate a hypoperfusion state. Heart failure and liver disease regularly result in an expanded extracellular fluid (ECF) compartment yet a reduced effective arterial volume and predispose to renal hypoperfusion.
A history of decreased urine output or anuria suggests postrenal AKI, but its absence does not rule out urinary tract obstruction. Voiding symptoms such as urinary frequency, hesitancy, or incontinence also raise the possibility of obstructive uropathy. Flank pain and hematuria often accompany obstruction from nephrolithiasis.
Symptoms of fever, skin rash, arthralgias, sinusitis, and/or hemoptysis raise the possibility of glomerulonephritis from infection, collagen vascular disease, or vasculitis. Risk factors for viral hepatitis and human immunodeficiency virus are important to clarify as are systemic symptoms of autoimmunity (eg, dry eyes, dry mouth, eye pain/emnflammation, or visual changes). The recent start of any new medication, including NSAIDs, antibiotics, or proton‐pump inhibitors, raises the possibility of a drug‐induced interstitial nephritis.[20] Statins are direct myotoxins, and the risk of rhabdomyolysis with renal injury increases with dose. Patients may not associate intravenous (IV) contrast or phosphate‐containing bowel preparations (eg, Fleet Phospho Soda), with the development of AKI, thus the clinician must carefully review for recent exposures that could result in intrinsic renal injury.[21]
PHYSICAL EXAMINATION
Estimation of the ECF volume and effective arterial volume are central to assessing the likelihood of renal hypoperfusion. Overt hypotension is the strongest indicator of hypoperfusion, and a careful review of initial blood pressure prior to worsening of renal function can provide significant information. Normal blood pressure does not exclude renal hypoperfusion, as acute tubular necrosis (ATN) may develop in chronically hypertensive patients whose blood pressures are acutely reduced.[22] Less‐severe volume depletion is suggested by an orthostatic pulse increase of more than 30 beats/minute, measured 1 minute after standing. Orthostatic hypotension, defined as a drop in systolic pressure of more than 20 mm Hg after standing, is less helpful, as it occurs in 10% of normovolemic subjects.[23] Dry axillae and mucous membranes with a furrowed tongue are useful signs of volume depletion. Poor skin turgor and slow capillary refill have not been shown to be reliable signs of hypovolemia in adults. The neck veins are usually flat when volume contraction exists, though engorged neck veins in the setting of elevated right‐sided pressures from heart failure or pulmonary hypertension may obscure this sign. Similarly, pulmonary rales, ascites, and peripheral edema may confound the exam in patients with underlying heart failure and/or cirrhosis.
Flank tenderness or a bladder palpable or percussable above the pelvic brim suggests possible urinary tract obstruction. Prostate exam should be performed on all men with AKI and a bimanual pelvic exam considered in women with changes in usual voiding pattern or with suspected gynecologic disease. Postvoid residual can be assessed at the bedside with either straight catheterization or bladder scan where available.
Signs of systemic disease associated with intrinsic AKI include fever, skin and joint findings of connective tissue disease, a new or changing heart murmur, purpura, and petechiae. Cholesterol emboli, disrupted by interarterial catheterization (eg, cardiac catheterization, angiography), cardiac or aortic surgeries, or, rarely, by systemic anticoagulation can shower throughout the vasculature, causing organ dysfunction and local inflammation. Kidney injury due to atheroemboli often has a stuttering course and may be separated in time from the vascular procedure by days to weeks. Physical exam findings of atheroembolic disease include livedo reticularis, blue toes, purpura, painful skin nodules, and gangrene. Retinal examination may reveal atheroembolic emboli (Hollenhorst plaques).[24, 25]
LABORATORY TESTING
Initial testing in AKI aims to assess the severity of injury as well as the likely mechanism of the injury. Estimation of glomerular filtration rate (GFR) gives an approximate measure of the number of functioning nephrons and hence an overall measure of renal function. Mathematical estimates of GFR, however, assume a steady state, and AKI, by definition, is not a steady state. This makes GFR estimates based on plasma creatinine unreliable. A rising serum creatinine concentration indicates that the renal injury is persistent or worsening, whereas a stable or falling creatinine concentration suggests recovery. Interventions that expand the ECF (eg, volume resuscitation with normal saline) will dilute the plasma creatinine concentration and must be considered when interpreting a falling creatinine concentration. A daily rise in the serum creatinine concentration of more than 1 mg/dL nearly always implies a GFR of <10 mL/min. Any change in serum creatinine must be interpreted with the nonlinear relationship of GFR and serum creatinine in mind (Figure 1).[26]

The fractional excretion of sodium (FENa) has been used to differentiate prerenal azotemia from intrinsic renal injury in patients with oligoanuria. Specifically, an FENa of <1% implies a prerenal cause for the oliguric AKI, whereas if it is >1%, then intrinsic renal injury is more likely. Unfortunately, there are significant limitations to this laboratory measure.[27] The FENa may be low (<1%) in any intrinsic process that causes tissue ischemia, such as vasculitis, acute glomerulonephritis, atheroembolic disease, or from intense vasoconstriction such as after IV contrast administration. Patients with severe heart failure or portal hypertension often have avid sodium retention, and can have a FENa <1% even in the setting of ATN. Alternatively, the FENa may be elevated (>1%) in prerenal patients on diuretics, with osmotic diuresis, or in the setting of aldosterone deficiency.
Examination of the urinalysis and urine sediment provides valuable information about the etiology of the AKI. Prerenal and postrenal AKI typically present with a bland urine, without evidence of blood, protein, or leukocyte esterase on urinalysis and few cells or hyaline casts in the sediment. The urinalysis typically has a high specific gravity in prerenal AKI, reflecting intact tubules producing a concentrated urine. An active urinary sediment suggests intrinsic renal injury that is either the mechanism of the current AKI or indicative of underlying CKD. ATN, the most common cause of intrinsic renal injury, often produces a dirty urinalysis with many epithelial cells and muddy brown granular and epithelial cell casts. The urine is generally isosthenuric (ie, specific gravity of 1.010) due to loss of tubular function. A urinalysis positive for heme pigment but without red cells on microscopic analysis suggests the presence of either myoglobin from rhabdomyolysis or hemoglobin from hemolysis. Acute glomerulonephritis disrupts the usual glomerular barrier to large proteins and red cells and results in proteinuria and hematuria. Red cells that weather the journey from the glomerulus through the nephron often become dysmorphic with Mickey Mouse ear blebs in their membrane or are bound together by Tamm‐Horsfall protein into red cell casts. Acute interstitial nephritis results in pyuria, proteinuria, and white cell casts. Urinary eosinophils are neither sensitive nor specific for interstitial nephritis and have little utility in its diagnosis.[28, 29]
Given the limitations of serum creatinine as a marker of renal injury, a number of new urinary biomarkers have been recognized over the past decade.[30, 31, 32] These molecules are normal constituents of renal tubular cells that are upregulated and released into the urine in response to renal injury. Early measurement of these biomarkers might allow for detection of AKI within hours of the insult. The 2 biomarkers with the most promise include kidney injury molecule‐1 (KIM‐1) and neutrophil gelatinase‐associated lipocalin (NGAL). KIM‐1 is expressed by proximal tubular cells, and its production is sharply upregulated in response to ischemic injury. NGAL is a protein expressed primarily in immune cells, but also by renal tubular cells. Urinary NGAL levels rapidly rise in response to renal ischemia, and return to baseline following resolution of the injury. Although these urinary biomarkers are promising, they have a relatively low (70%75%) sensitivity and specificity, and have not yet been adopted into routine clinical practice.[33]
IMAGING
Renal ultrasound is useful both in the assessment of AKI as well as in the investigation for underlying CKD. Patients with long‐standing kidney disease frequently have small, echogenic kidneys consistent with fibrosis and nephron loss, or markedly distorted renal architecture in cystic diseases. Hydronephrosis and/or hydroureter suggest an acute or chronic urinary tract obstruction. However, this may not be present in the setting of early obstruction or ureteric encasement. Doppler ultrasonography of the renal vasculature can assess patency when vascular obstruction is suspected. The use of computerized tomography, magnetic resonance imaging, or angiography may be helpful in selected clinical circumstances, but their use is often limited due to the potential risk of contrast nephrotoxicity. Nuclear renal scans use less radiation than computerized tomography and are a preferred imaging modality for pediatric patients. When volume status is uncertain, echocardiography to assess both inferior vena cava volume and change in volume with respiration may be helpful.
MANAGEMENT
The general principles for management of AKI are to limit further injury and prevent systemic complications. Management of the patient with AKI greatly depends on which category of AKI is suspected, namely prerenal, intrinsic renal injury, or a postrenal (obstructive) cause. If a prerenal etiology due to true ECF volume depletion is suspected, volume resuscitation to replace baseline and ongoing losses is imperative. Careful attention to intake and output as well as serial volume assessment should dictate the strategy for resuscitation. Hyperchloremic acidosis is an expected consequence of normal saline resuscitation but is irrelevant to clinical outcomes.[34] NSAIDs, antihypertensives, especially those that affect the angiotensin/aldosterone system, and diuretics should be discontinued. Ongoing hypotension despite volume resuscitation suggests the possibility of blood loss, infection, or autonomic nervous system dysfunction. If this occurs, the patient may need to be transferred to an intensive care unit for pressor support to keep the mean arterial pressure >70 mm Hg. When prerenal AKI from reduced effective circulating volume is suspected, as in decompensated heart failure or cirrhosis, management must be tailored to the underlying pathophysiology.
If judicious volume resuscitation produces no improvement in renal function or if oliguria develops, repeat urinalysis and urine microscopy should be considered to assess for intrinsic renal injury. Aggressive volume resuscitation in the face of oliguria will not speed recovery from the intrinsic injury and may cause signs or symptoms of volume overload. This could also potentially necessitate renal replacement therapy earlier than anticipated.
In patients where an obstructive etiology for the AKI is identified, the obstruction must be relieved as soon and as safely as possible. In this regard, a timely urologic consultation may be helpful in assuring that urethral and/or ureteral conduits are placed rapidly. Interventional radiology can also assist in those patients who need percutaneous nephrostomies for the relief of the obstruction. In many patients with obstructive nephropathy, a timely intervention will avoid the need for renal replacement therapy.
The suspected mechanism of injury influences the management of intrinsic AKI. The management of ATN is primarily supportive, paying close attention to optimizing volume status, correcting electrolyte abnormalities, avoiding further nephrotoxic agents, and adjusting medication doses to the low GFR present. Over the last several decades, multiple studies have explored treatment strategies for established ATN using various drugs and biologic agents. All have been uniformly disappointing.
When the trajectory of AKI is uncertain and the creatinine continues to rise, all medication dosing should be adjusted for GFR <10 mL/min. Antibiotics routinely will require dose reduction, but all current medications should be reviewed for risk of accumulation in renal failure. Because the half‐life of oral hypoglycemic medications is unpredictable in AKI, these medications should be discontinued and replaced with insulin. Vigilance for hypoglycemia is necessary, as renal clearance of insulin is also reduced. Narcotics such as morphine and oxycodone, which are renally cleared, can produce unwanted sedation and respiratory depression if not discontinued. Fentanyl, methadone, and hydromorphone are safer choices for controlling pain in a patient with AKI.[35] Gabapentin is regularly used to treat symptoms of neuropathic pain, but can produce encephalopathy and myoclonus if not dose reduced in renal failure.[36] Clinicians should weigh the risk of overdose with underdose for each medication, namely antibiotics in critically ill patients.
TIMING OF NEPHROLOGY CONSULTATION
The optimal timing for nephrology consultation in hospital‐acquired AKI is uncertain, though several studies have suggested better outcomes, including shorter length of stay and reduced mortality, with early consultation.[37, 38, 39] A renal consult is indicated when intrinsic ATN does not reverse in a timely fashion. Renal replacement therapy should be instituted to limit the systemic complications of prolonged AKI and to allow time for the renal injury to improve or resolve over time. If acute glomerulonephritis or interstitial nephritis is suspected, an urgent consultation may be required for consideration of biopsy, immunosuppression, and guidance for further management. Early consultation may help limit drug toxicities and volume overload in the setting of decreased renal clearance. Guidance on vascular access (eg, peripherally inserted central catheter placement) may prevent future complications with hemodialysis access if the patient ultimately develops end‐stage renal disease (ESRD).[40]
PREVENTION OF AKI
Most studies of AKI prevention have focused on clinical scenarios where the likelihood of ATN was substantial such as in vascular or open heart surgery, or with the use of intravenous contrast agents.[41, 42] This topic remains controversial, though generally supported strategies include judicious volume expansion, avoidance of hypotension, and, when using contrast, limiting the volume of contrast and using iso‐osmolar formulations. As recent studies have shown uncertain benefit, the role for pretreatment with n‐acetylcysteine remains uncertain. Many clinicians, however, continue to use it as a preventive strategy as there are few side effects with this medication.
TAKE HOME POINTS
- AKI is common in hospitalized patients, with pre‐renal azotemia being the dominant etiology in both community‐acquired and hospital‐acquired AKI.
- CKD is an important risk factor for AKI. AKI increases the long‐term risk of developing CKD and ESRD.
- The diagnosis of AKI hinges on detailed medical history, careful physical exam, and key laboratory parameters including the urinalysis and urinary sediment.
- The management of AKI is tailored to the likely mechanism of injury. Reconsideration of the likely etiology is imperative if AKI fails to respond to initial attempts to reverse or limit injury.
- Early renal consultation for AKI is indicated when the etiology remains uncertain, AKI persists despite initial management, or acute glomerulonephritis or interstitial nephritis are suspected.
Acute kidney injury (AKI) is a clinical syndrome broadly defined as an abrupt decline in renal function occurring over a period of hours to days resulting in the retention of nitrogenous and metabolic waste products. Although the initial clinical manifestation of AKI may be oliguria, urine volume can remain normal or even increase. Patients may be asymptomatic, especially early in the course of AKI. The diagnosis is often made in hospitalized patients when biochemical screening reveals a recent increase in serum creatinine and/or blood urea nitrogen concentrations, or when there is a dramatic decrease in urine output.
Older studies looking at the incidence of AKI in hospitalized patients are difficult to interpret due to variable definitions of AKI. Those based on administrative databases were limited by lack of clinical context and/or variation in coding for AKI.[1]
There is no universally accepted operational definition of AKI, and more than 30 different criteria have been employed in various clinical studies. Difficulty in defining AKI lies in the lag time in the rise and fall of the serum creatinine concentration with injury and recovery, the variability of oliguria, and in the heterogeneity of patterns of renal injury. Two classification systems that attempt to capture the spectrum of AKI are the RIFLE (Risk, Injury, Failure, Loss, End Stage) criteria and the AKIN (Acute Kidney Injury Network) criteria.[2, 3] The AKIN criteria parallel the risk, injury, and failure stages of the RIFLE criteria and are the most applicable to characterizing AKI in the hospital (Table 1). AKI is commonly classified by daily urine output as anuric (<50 mL/day), oliguric (<500 mL/day), or nonoliguric.
| Stage | Creatinine Criteria | Urine Output Criteria |
|---|---|---|
| 1 | Increase in serum creatinine of 0.3 mg/dL (26.4 mol/L) or increase of 150%200% (1.5‐fold to 2‐fold) above baseline | <0.5 mL/kg/hr for >6 hours |
| 2 | Increase in serum creatinine of >200%300% (>2‐fold to 3‐fold) above baseline | <0.5 mL/kg/hr for >12 hours |
| 3 | Increase in serum creatinine of >300% (3‐fold) above baseline or serum creatinine 5.0 mg/dL (354 mol/L) with an acute rise of 0.5 mg/dL (44 mol/L) | <0.3 mL/kg/hr 24 hours or anuria 12 hours |
With a move toward standardized definitions, recent studies have shown a rising incidence of AKI in hospitalized patients.[4, 5, 6] According to these series, AKI develops in up to 7% of hospitalized patients and in about 30% of those admitted to intensive care units. In one study of consecutive hospital admissions, patients classified by the RIFLE criteria had a sharp rise in the rate of in‐hospital mortality whether they had no change or improvement in creatinine (4.4%), or fell into a risk (15.1%), injury (29.2%), or failure (41.1%) class.[7] The in‐hospital mortality of critically ill patients with AKI is higher than 50%. AKI increases length of stay and hospital costs, and affects the clinical course after discharge.[8, 9] Small increases in serum creatinine during an intensive care unit stay predict increased 10‐year mortality above a critical illness alone.[10]
Risk factors for AKI include advanced age, male gender, African American ethnicity, and diabetes mellitus.[11] The most important risk factor, however, is preexisting chronic kidney disease (CKD).[12] AKI and CKD are tightly linked, each increasing the risk of the other.[13, 14, 15] Preexisting renal insufficiency is a key predictor of postoperative AKI and poor surgical outcomes.[16, 17]
AKI AND CLINICAL CONTEXT
The causes of AKI can be broadly divided into 3 categories: prerenal azotemia (a disorder characterized by renal hypoperfusion in which renal parenchymal tissue integrity is preserved), intrinsic kidney injury with parenchymal tissue injury, and postrenal AKI (dysfunction due to acute obstruction of the urinary tract). Table 2 lists several clinical scenarios sorted into these 3 categories.[18] The general epidemiology of AKI varies based on whether it was acquired in the community or in a hospital setting. Prerenal azotemia accounts for the bulk of community‐acquired AKI, followed in lesser frequency by postrenal and intrinsic etiologies. Prerenal azotemia continues to be the major cause of hospital‐acquired AKI, but intrinsic kidney injury becomes more common.[5, 19]
| Prerenal | Intrinsic | Postrenal |
|---|---|---|
| ||
| Hemorrhage | Acute tubular necrosis | Bilateral upper tract obstruction |
| Surgical | Ischemic | Nephrolithiasis |
| Gastrointestinal | Postoperative | Papillary necrosis |
| Retroperitoneal | Prolonged hypotension | Retroperitoneal fibrosis |
| Gastrointestinal losses | Sepsis | Retroperitoneal lymphadenopathy |
| Diarrhea | Nephrotoxins | Obstruction of solitary functioning kidney |
| Vomiting | Myoglobin | Lower tract obstruction |
| Nasogastric suction | Hemoglobin | Prostatic hypertrophy |
| Enteral fistula | Radiocontrast agents | Urethral stricture |
| Renal losses | Aminoglycosides | Bladder mass or stone |
| Diuretics | Intratubular obstruction | Obstructed urinary catheter |
| Glucosuria | Tumor lysis/uric acid | Urinary retention |
| Skin losses | Oxalosis/ethylene glycol ingestion | Neurogenic bladder |
| Excessive sweating | Phosphate nephropathy | Constipation |
| Burns | Light chain nephropathy | Medications |
| Erythroderma | Acyclovir | Anticholinergics |
| Third‐spacing | Indinavir | Antihistamines |
| Hypoalbuminemia | Methotrexate | Alpha1‐agonists |
| Pancreatitis | Acute glomerulonephritis | ‐Blockers |
| Capillary leak | Acute interstitial nephritis | Opiates |
| Reduced effective arterial volume | Proton pump inhibitors | Tricyclic antidepressants |
| Congestive heart failure | Penicillins | |
| Cirrhosis | Fluoroquinolones | |
| Renal vasoconstriction | Atheroembolic disease | |
| Hypercalcemia | Acute vascular syndrome | |
| NSAIDs | Aortic dissection | |
| ACEI/ARB | Bilateral renal artery thromboembolism | |
| Calcineurin inhibitors | Bilateral renal vein thrombosis | |
| Vasopressors | Thrombotic microangiopathy | |
| Iodinated contrast | ||
MEDICAL HISTORY
The initial goal of history taking is to establish whether the patient has AKI rather than the acute discovery of a more chronic process. A recent serum creatinine measurement can be valuable in this regard. In some cases the clinician must make a presumptive diagnosis of AKI while simultaneously reviewing past medical history and family history to assess for underlying CKD. A diagnosis of AKI is more readily established when it occurs during a hospitalization through review of urine output and serial laboratory values.
Symptoms of poor oral intake as well as salt and fluid losses from diarrhea or vomiting suggest a prerenal etiology. Subjective symptoms of lightheadedness, visual clouding, and near‐syncope with standing also suggest volume depletion. Patients should be asked about recent nonsteroidal anti‐inflammatory drug (NSAID) use, as these agents can exacerbate renal hypoperfusion through loss of prostaglandin‐mediated afferent arteriole dilatation. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, especially when combined with diuretics, can generate a hypoperfusion state. Heart failure and liver disease regularly result in an expanded extracellular fluid (ECF) compartment yet a reduced effective arterial volume and predispose to renal hypoperfusion.
A history of decreased urine output or anuria suggests postrenal AKI, but its absence does not rule out urinary tract obstruction. Voiding symptoms such as urinary frequency, hesitancy, or incontinence also raise the possibility of obstructive uropathy. Flank pain and hematuria often accompany obstruction from nephrolithiasis.
Symptoms of fever, skin rash, arthralgias, sinusitis, and/or hemoptysis raise the possibility of glomerulonephritis from infection, collagen vascular disease, or vasculitis. Risk factors for viral hepatitis and human immunodeficiency virus are important to clarify as are systemic symptoms of autoimmunity (eg, dry eyes, dry mouth, eye pain/emnflammation, or visual changes). The recent start of any new medication, including NSAIDs, antibiotics, or proton‐pump inhibitors, raises the possibility of a drug‐induced interstitial nephritis.[20] Statins are direct myotoxins, and the risk of rhabdomyolysis with renal injury increases with dose. Patients may not associate intravenous (IV) contrast or phosphate‐containing bowel preparations (eg, Fleet Phospho Soda), with the development of AKI, thus the clinician must carefully review for recent exposures that could result in intrinsic renal injury.[21]
PHYSICAL EXAMINATION
Estimation of the ECF volume and effective arterial volume are central to assessing the likelihood of renal hypoperfusion. Overt hypotension is the strongest indicator of hypoperfusion, and a careful review of initial blood pressure prior to worsening of renal function can provide significant information. Normal blood pressure does not exclude renal hypoperfusion, as acute tubular necrosis (ATN) may develop in chronically hypertensive patients whose blood pressures are acutely reduced.[22] Less‐severe volume depletion is suggested by an orthostatic pulse increase of more than 30 beats/minute, measured 1 minute after standing. Orthostatic hypotension, defined as a drop in systolic pressure of more than 20 mm Hg after standing, is less helpful, as it occurs in 10% of normovolemic subjects.[23] Dry axillae and mucous membranes with a furrowed tongue are useful signs of volume depletion. Poor skin turgor and slow capillary refill have not been shown to be reliable signs of hypovolemia in adults. The neck veins are usually flat when volume contraction exists, though engorged neck veins in the setting of elevated right‐sided pressures from heart failure or pulmonary hypertension may obscure this sign. Similarly, pulmonary rales, ascites, and peripheral edema may confound the exam in patients with underlying heart failure and/or cirrhosis.
Flank tenderness or a bladder palpable or percussable above the pelvic brim suggests possible urinary tract obstruction. Prostate exam should be performed on all men with AKI and a bimanual pelvic exam considered in women with changes in usual voiding pattern or with suspected gynecologic disease. Postvoid residual can be assessed at the bedside with either straight catheterization or bladder scan where available.
Signs of systemic disease associated with intrinsic AKI include fever, skin and joint findings of connective tissue disease, a new or changing heart murmur, purpura, and petechiae. Cholesterol emboli, disrupted by interarterial catheterization (eg, cardiac catheterization, angiography), cardiac or aortic surgeries, or, rarely, by systemic anticoagulation can shower throughout the vasculature, causing organ dysfunction and local inflammation. Kidney injury due to atheroemboli often has a stuttering course and may be separated in time from the vascular procedure by days to weeks. Physical exam findings of atheroembolic disease include livedo reticularis, blue toes, purpura, painful skin nodules, and gangrene. Retinal examination may reveal atheroembolic emboli (Hollenhorst plaques).[24, 25]
LABORATORY TESTING
Initial testing in AKI aims to assess the severity of injury as well as the likely mechanism of the injury. Estimation of glomerular filtration rate (GFR) gives an approximate measure of the number of functioning nephrons and hence an overall measure of renal function. Mathematical estimates of GFR, however, assume a steady state, and AKI, by definition, is not a steady state. This makes GFR estimates based on plasma creatinine unreliable. A rising serum creatinine concentration indicates that the renal injury is persistent or worsening, whereas a stable or falling creatinine concentration suggests recovery. Interventions that expand the ECF (eg, volume resuscitation with normal saline) will dilute the plasma creatinine concentration and must be considered when interpreting a falling creatinine concentration. A daily rise in the serum creatinine concentration of more than 1 mg/dL nearly always implies a GFR of <10 mL/min. Any change in serum creatinine must be interpreted with the nonlinear relationship of GFR and serum creatinine in mind (Figure 1).[26]

The fractional excretion of sodium (FENa) has been used to differentiate prerenal azotemia from intrinsic renal injury in patients with oligoanuria. Specifically, an FENa of <1% implies a prerenal cause for the oliguric AKI, whereas if it is >1%, then intrinsic renal injury is more likely. Unfortunately, there are significant limitations to this laboratory measure.[27] The FENa may be low (<1%) in any intrinsic process that causes tissue ischemia, such as vasculitis, acute glomerulonephritis, atheroembolic disease, or from intense vasoconstriction such as after IV contrast administration. Patients with severe heart failure or portal hypertension often have avid sodium retention, and can have a FENa <1% even in the setting of ATN. Alternatively, the FENa may be elevated (>1%) in prerenal patients on diuretics, with osmotic diuresis, or in the setting of aldosterone deficiency.
Examination of the urinalysis and urine sediment provides valuable information about the etiology of the AKI. Prerenal and postrenal AKI typically present with a bland urine, without evidence of blood, protein, or leukocyte esterase on urinalysis and few cells or hyaline casts in the sediment. The urinalysis typically has a high specific gravity in prerenal AKI, reflecting intact tubules producing a concentrated urine. An active urinary sediment suggests intrinsic renal injury that is either the mechanism of the current AKI or indicative of underlying CKD. ATN, the most common cause of intrinsic renal injury, often produces a dirty urinalysis with many epithelial cells and muddy brown granular and epithelial cell casts. The urine is generally isosthenuric (ie, specific gravity of 1.010) due to loss of tubular function. A urinalysis positive for heme pigment but without red cells on microscopic analysis suggests the presence of either myoglobin from rhabdomyolysis or hemoglobin from hemolysis. Acute glomerulonephritis disrupts the usual glomerular barrier to large proteins and red cells and results in proteinuria and hematuria. Red cells that weather the journey from the glomerulus through the nephron often become dysmorphic with Mickey Mouse ear blebs in their membrane or are bound together by Tamm‐Horsfall protein into red cell casts. Acute interstitial nephritis results in pyuria, proteinuria, and white cell casts. Urinary eosinophils are neither sensitive nor specific for interstitial nephritis and have little utility in its diagnosis.[28, 29]
Given the limitations of serum creatinine as a marker of renal injury, a number of new urinary biomarkers have been recognized over the past decade.[30, 31, 32] These molecules are normal constituents of renal tubular cells that are upregulated and released into the urine in response to renal injury. Early measurement of these biomarkers might allow for detection of AKI within hours of the insult. The 2 biomarkers with the most promise include kidney injury molecule‐1 (KIM‐1) and neutrophil gelatinase‐associated lipocalin (NGAL). KIM‐1 is expressed by proximal tubular cells, and its production is sharply upregulated in response to ischemic injury. NGAL is a protein expressed primarily in immune cells, but also by renal tubular cells. Urinary NGAL levels rapidly rise in response to renal ischemia, and return to baseline following resolution of the injury. Although these urinary biomarkers are promising, they have a relatively low (70%75%) sensitivity and specificity, and have not yet been adopted into routine clinical practice.[33]
IMAGING
Renal ultrasound is useful both in the assessment of AKI as well as in the investigation for underlying CKD. Patients with long‐standing kidney disease frequently have small, echogenic kidneys consistent with fibrosis and nephron loss, or markedly distorted renal architecture in cystic diseases. Hydronephrosis and/or hydroureter suggest an acute or chronic urinary tract obstruction. However, this may not be present in the setting of early obstruction or ureteric encasement. Doppler ultrasonography of the renal vasculature can assess patency when vascular obstruction is suspected. The use of computerized tomography, magnetic resonance imaging, or angiography may be helpful in selected clinical circumstances, but their use is often limited due to the potential risk of contrast nephrotoxicity. Nuclear renal scans use less radiation than computerized tomography and are a preferred imaging modality for pediatric patients. When volume status is uncertain, echocardiography to assess both inferior vena cava volume and change in volume with respiration may be helpful.
MANAGEMENT
The general principles for management of AKI are to limit further injury and prevent systemic complications. Management of the patient with AKI greatly depends on which category of AKI is suspected, namely prerenal, intrinsic renal injury, or a postrenal (obstructive) cause. If a prerenal etiology due to true ECF volume depletion is suspected, volume resuscitation to replace baseline and ongoing losses is imperative. Careful attention to intake and output as well as serial volume assessment should dictate the strategy for resuscitation. Hyperchloremic acidosis is an expected consequence of normal saline resuscitation but is irrelevant to clinical outcomes.[34] NSAIDs, antihypertensives, especially those that affect the angiotensin/aldosterone system, and diuretics should be discontinued. Ongoing hypotension despite volume resuscitation suggests the possibility of blood loss, infection, or autonomic nervous system dysfunction. If this occurs, the patient may need to be transferred to an intensive care unit for pressor support to keep the mean arterial pressure >70 mm Hg. When prerenal AKI from reduced effective circulating volume is suspected, as in decompensated heart failure or cirrhosis, management must be tailored to the underlying pathophysiology.
If judicious volume resuscitation produces no improvement in renal function or if oliguria develops, repeat urinalysis and urine microscopy should be considered to assess for intrinsic renal injury. Aggressive volume resuscitation in the face of oliguria will not speed recovery from the intrinsic injury and may cause signs or symptoms of volume overload. This could also potentially necessitate renal replacement therapy earlier than anticipated.
In patients where an obstructive etiology for the AKI is identified, the obstruction must be relieved as soon and as safely as possible. In this regard, a timely urologic consultation may be helpful in assuring that urethral and/or ureteral conduits are placed rapidly. Interventional radiology can also assist in those patients who need percutaneous nephrostomies for the relief of the obstruction. In many patients with obstructive nephropathy, a timely intervention will avoid the need for renal replacement therapy.
The suspected mechanism of injury influences the management of intrinsic AKI. The management of ATN is primarily supportive, paying close attention to optimizing volume status, correcting electrolyte abnormalities, avoiding further nephrotoxic agents, and adjusting medication doses to the low GFR present. Over the last several decades, multiple studies have explored treatment strategies for established ATN using various drugs and biologic agents. All have been uniformly disappointing.
When the trajectory of AKI is uncertain and the creatinine continues to rise, all medication dosing should be adjusted for GFR <10 mL/min. Antibiotics routinely will require dose reduction, but all current medications should be reviewed for risk of accumulation in renal failure. Because the half‐life of oral hypoglycemic medications is unpredictable in AKI, these medications should be discontinued and replaced with insulin. Vigilance for hypoglycemia is necessary, as renal clearance of insulin is also reduced. Narcotics such as morphine and oxycodone, which are renally cleared, can produce unwanted sedation and respiratory depression if not discontinued. Fentanyl, methadone, and hydromorphone are safer choices for controlling pain in a patient with AKI.[35] Gabapentin is regularly used to treat symptoms of neuropathic pain, but can produce encephalopathy and myoclonus if not dose reduced in renal failure.[36] Clinicians should weigh the risk of overdose with underdose for each medication, namely antibiotics in critically ill patients.
TIMING OF NEPHROLOGY CONSULTATION
The optimal timing for nephrology consultation in hospital‐acquired AKI is uncertain, though several studies have suggested better outcomes, including shorter length of stay and reduced mortality, with early consultation.[37, 38, 39] A renal consult is indicated when intrinsic ATN does not reverse in a timely fashion. Renal replacement therapy should be instituted to limit the systemic complications of prolonged AKI and to allow time for the renal injury to improve or resolve over time. If acute glomerulonephritis or interstitial nephritis is suspected, an urgent consultation may be required for consideration of biopsy, immunosuppression, and guidance for further management. Early consultation may help limit drug toxicities and volume overload in the setting of decreased renal clearance. Guidance on vascular access (eg, peripherally inserted central catheter placement) may prevent future complications with hemodialysis access if the patient ultimately develops end‐stage renal disease (ESRD).[40]
PREVENTION OF AKI
Most studies of AKI prevention have focused on clinical scenarios where the likelihood of ATN was substantial such as in vascular or open heart surgery, or with the use of intravenous contrast agents.[41, 42] This topic remains controversial, though generally supported strategies include judicious volume expansion, avoidance of hypotension, and, when using contrast, limiting the volume of contrast and using iso‐osmolar formulations. As recent studies have shown uncertain benefit, the role for pretreatment with n‐acetylcysteine remains uncertain. Many clinicians, however, continue to use it as a preventive strategy as there are few side effects with this medication.
TAKE HOME POINTS
- AKI is common in hospitalized patients, with pre‐renal azotemia being the dominant etiology in both community‐acquired and hospital‐acquired AKI.
- CKD is an important risk factor for AKI. AKI increases the long‐term risk of developing CKD and ESRD.
- The diagnosis of AKI hinges on detailed medical history, careful physical exam, and key laboratory parameters including the urinalysis and urinary sediment.
- The management of AKI is tailored to the likely mechanism of injury. Reconsideration of the likely etiology is imperative if AKI fails to respond to initial attempts to reverse or limit injury.
- Early renal consultation for AKI is indicated when the etiology remains uncertain, AKI persists despite initial management, or acute glomerulonephritis or interstitial nephritis are suspected.
- , , . Acute kidney injury. Lancet. 2012;380(9843):756–766.
- , , , , . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212.
- , , , et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis. 2007;50(1):1–4.
- , , , et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142.
- , , . Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936.
- , , , . Acute renal failure: factors influencing nephrology referral and outcome. QJM. 1997;90(12):781–785.
- , , , , . An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917.
- , , , , . Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370.
- , , , , . The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta‐analysis. Am J Kidney Dis. 2007;50(5):712–720.
- , , , , , . Small acute increases in serum creatinine are associated with decreased long‐term survival in the critically ill. Am J Respir Crit Care Med. 2014;189(9):1075–1081.
- , , , et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228.
- , , , , , . The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–107.
- , , . Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012;81(5):442–448.
- , , , et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125(6):585–593.
- , , , et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185.
- , , , et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515.
- , , , et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta‐analysis. Anesthesiology. 2013;118(4):809–824.
- , . Brenner 2008.
- , , , , . Hospital‐acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248.
- , , , . A nationwide nested case‐control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837–844.
- , , , . Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16(11):3389–3396.
- . Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797–805.
- , , . The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022–1029.
- , . Atheroembolic renal disease. Lancet. 2010;375(9726):1650–1660.
- , , , et al. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116(3):298–304.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470.
- , . Traditional urinary biomarkers in the assessment of hospital‐acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167–174.
- , . Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841–1843.
- , , . Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–1862.
- . Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219.
- , . Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62(1):165–178.
- , , , et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–2165.
- , , , et al. Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside? Ann Intensive Care. 2012;2(1):24.
- , . The case for 0.9% NaCl: is the undefendable, defensible? Kidney Int. 2014;86(6):1087–1095.
- , , , , . A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525–552.
- , , . Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med. 2010;123(4):367–373.
- , , , et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919.
- , , , et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113(6):456–461.
- , , , et al. Early nephrologist involvement in hospital‐acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011;57(2):228–234.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601–608.
- , , , , , . Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. Am J Surg. 2013;206(1):86–95.
- , , , et al. Strategies to reduce the risk of contrast‐induced nephropathy. Am J Cardiol. 2006;98(6A):59K–77K.
- , , . Acute kidney injury. Lancet. 2012;380(9843):756–766.
- , , , , . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212.
- , , , et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis. 2007;50(1):1–4.
- , , , et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142.
- , , . Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936.
- , , , . Acute renal failure: factors influencing nephrology referral and outcome. QJM. 1997;90(12):781–785.
- , , , , . An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917.
- , , , , . Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370.
- , , , , . The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta‐analysis. Am J Kidney Dis. 2007;50(5):712–720.
- , , , , , . Small acute increases in serum creatinine are associated with decreased long‐term survival in the critically ill. Am J Respir Crit Care Med. 2014;189(9):1075–1081.
- , , , et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228.
- , , , , , . The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–107.
- , , . Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012;81(5):442–448.
- , , , et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125(6):585–593.
- , , , et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185.
- , , , et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515.
- , , , et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta‐analysis. Anesthesiology. 2013;118(4):809–824.
- , . Brenner 2008.
- , , , , . Hospital‐acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248.
- , , , . A nationwide nested case‐control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837–844.
- , , , . Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16(11):3389–3396.
- . Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797–805.
- , , . The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022–1029.
- , . Atheroembolic renal disease. Lancet. 2010;375(9726):1650–1660.
- , , , et al. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116(3):298–304.
- , , , , , . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470.
- , . Traditional urinary biomarkers in the assessment of hospital‐acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167–174.
- , . Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841–1843.
- , , . Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–1862.
- . Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219.
- , . Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62(1):165–178.
- , , , et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–2165.
- , , , et al. Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside? Ann Intensive Care. 2012;2(1):24.
- , . The case for 0.9% NaCl: is the undefendable, defensible? Kidney Int. 2014;86(6):1087–1095.
- , , , , . A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525–552.
- , , . Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med. 2010;123(4):367–373.
- , , , et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919.
- , , , et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113(6):456–461.
- , , , et al. Early nephrologist involvement in hospital‐acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011;57(2):228–234.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601–608.
- , , , , , . Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. Am J Surg. 2013;206(1):86–95.
- , , , et al. Strategies to reduce the risk of contrast‐induced nephropathy. Am J Cardiol. 2006;98(6A):59K–77K.
Bedside Swallow Examination Review
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.
Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.
Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.
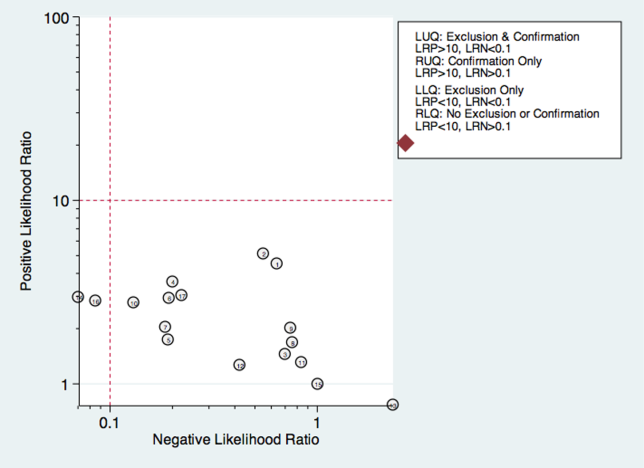
Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]
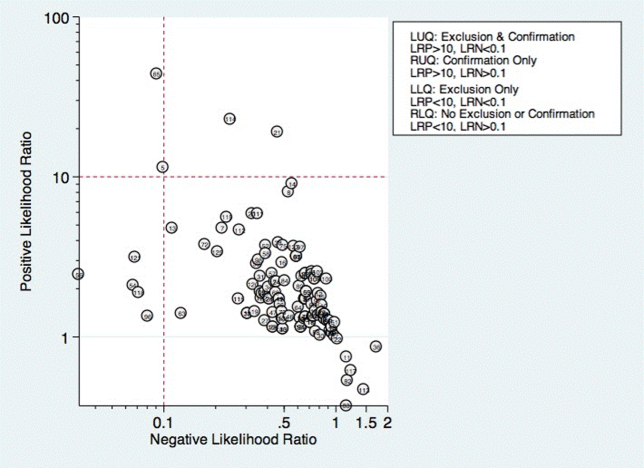
McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Management of Plasma Cell Disorders
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
The plasma cell disorders are a spectrum of conditions that include asymptomatic precursor conditions—monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM)—as well as symptomatic multiple myeloma (MM) and solitary plasmacytoma. Other plasma cell disorders include immunoglobulin light chain amyloidosis and POEMS syndrome, which are characterized by a unique set of end-organ manifestations. There are other related plasma cell and B-cell proliferations, such as light chain deposition disease and cryoglobulinemia, that are beyond the scope of this review but are relevant to the hematologist/oncologist and have been reviewed in detail elsewhere.
To read the full article in PDF:
Selecting antithrombotic therapy for patients with atrial fibrillation
Antithrombotic therapy reduces the risk of systemic embolism in patients with atrial fibrillation, but one approach does not suit all patients. The decision whether to start this therapy—and which agent to use—must take into account the patient’s risk of thromboembolism as well as bleeding.
Antithrombotic therapy encompasses antiplatelet drugs such as aspirin and clopidogrel and anticoagulants such as warfarin and the target-specific oral anticoagulants (TSOACs). Oral anticoagulation is more effective than antiplatelet therapy and is preferred in all but those at lowest risk, in whom either antiplatelet therapy or no therapy is deemed adequate.
Patients with valvular atrial fibrillation, specifically those who have rheumatic mitral stenosis or a prosthetic heart valve, are at significantly higher risk of systemic embolization. Their overall risk-benefit profile is nearly always in favor of anticoagulation. But the same is not necessarily true for patients with nonvalvular atrial fibrillation.
The following discussion sets forth our rationale for clinical decision-making, based on recommendations in the 2014 guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society.1 The second half of this review outlines the oral anticoagulants currently available.
ONE IN FOUR PEOPLE
Atrial fibrillation is common, with an incidence that increases with age. It affects more than 10% of people over age 80 and is often associated with cardiovascular disease.2 Based on Framingham Heart Study data, a person’s lifetime risk of developing it is about 25%.3
FIVEFOLD RISK OF STROKE
The most serious complication of atrial fibrillation is arterial thromboembolism, of which ischemic stroke is the most common and most feared manifestation. The risk of stroke is five times higher than normal in patients with atrial fibrillation.3 More than 15% of strokes may be attributable to atrial fibrillation, and the proportion increases with age.4
The risk of thromboembolism appears to be similar in patients with clinically manifest atrial fibrillation irrespective of the type (paroxysmal, persistent, or permanent). The Stroke Prevention in Atrial Fibrillation (SPAF) study5 and the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W)6 showed that patients who had paroxysmal atrial fibrillation and at least one risk factor for thromboembolism had stroke rates comparable to those of their counterparts who had persistent and permanent atrial fibrillation.
Subclinical atrial fibrillation may be an important cause of stroke. Clinically silent episodes can be detected by implantable electronic devices, which record episodes of atrial tachyarrhythmia (atrial high-rate events). Subclinical episodes have been detected in 10% to 28% of monitored patients who did not have a history of atrial fibrillation.7,8 Patients who have atrial high-rate events detected by implantable devices have a higher risk of future clinically manifest atrial fibrillation, thromboembolic events, or both.7–9 Yet characteristics of atrial high-rate episodes that predict risk are not well defined and warrant further investigation.
CLINICAL RISK FACTORS FOR STROKE
To date, thousands of patients with nonvalvular atrial fibrillation have participated in randomized clinical trials of stroke prevention. The placebo groups from these trials provide a sizable database for retrospectively identifying clinical characteristics associated with thromboembolism. The Atrial Fibrillation Investigators10 pooled data from five large trials and found that risk factors consistently associated with stroke in multivariate analysis included diabetes mellitus, hypertension, prior systemic embolism, and advanced age.
Though the risk of stroke increases with age with no lower limit, most studies identify age 65 as a threshold, with further escalating risk after age 75. Moreover, women were observed to be at higher risk in some but not all studies. These risk factors have become components of commonly used risk-stratification schemes.
Hypertrophic cardiomyopathy. Maron et al11 reported that atrial fibrillation in patients with hypertrophic cardiomyopathy was independently associated with thromboembolism. In 900 patients with hypertrophic cardiomyopathy, the prevalence of systemic embolism was 6%. Patients with hypertrophic cardiomyopathy and a thromboembolic complication were seven times more likely to have atrial fibrillation than matched counterparts free of thromboembolism. A notable subset of patients experienced a stroke or embolic event before age 50, and the authors advised that the risk of thromboembolism should be considered in patients of any age with hypertrophic cardiomyopathy and atrial fibrillation.
Olivotto et al12 similarly found patients with hypertrophic cardiomyopathy and atrial fibrillation to be at significantly greater risk of stroke (odds ratio [OR] 17.7, 95% confidence interval [CI] 4.1–75.9, P < .001).
Chronic kidney disease is also associated with a higher risk of thromboembolism in patients with atrial fibrillation. A glomerular filtration rate of 60 mL/min or less is independently and inversely predictive of risk.13,14
While patients with end-stage renal disease have been largely excluded from stroke prevention trials, Vázquez et al15 prospectively followed 190 dialysis patients for 12 months. In multivariate analysis, compared with matched controls without documented atrial fibrillation, patients receiving renal replacement therapy and having any form of atrial fibrillation were eight times more likely to have systemic embolization.
IMAGING-BASED RISK FACTORS
In addition to clinical factors, several imaging-based factors have been associated with stroke risk in patients with atrial fibrillation.
Complex aortic atheroma or markers of blood stasis within the left atrium, such as reduced left atrial appendage emptying flow (< 20 cm/second), dense spontaneous echo contrast, or left atrial appendage thrombus, seen on transesophageal echocardiography, were independently associated with increased systemic embolic risk in the third SPAF substudy.16 Moreover, multivariate analysis of SPAF data found both left ventricular dysfunction of any severity and increased left atrial size (diameter corrected for body surface area by M-mode > 2.5 cm/m2) to be independent predictors of thromboembolism.17
Although enlargement of the left atrium has not been incorporated into traditional risk stratification schemes, data from Osranek et al18 further implicate it as a marker of risk. The cohort was small (N = 46), but consisted of patients with lone atrial fibrillation followed for nearly 30 years. Patients with normal left atrial size enjoyed a benign course, while those with left atrial enlargement (> 32 mL/m2) at diagnosis or later during follow-up had significantly worse event-free survival (hazard ratio [HR] 4.46, 95% CI 1.56–12.74, P < .01). All embolic strokes occurred in the group with left atrial enlargement.
RISK STRATIFICATION SCHEMES
Several models for predicting systemic embolism risk in patients with nonvalvular atrial fibrillation have been proposed and validated.
The CHADS2 score has been the most widely applied, being simple to use.19,20 It assigns 1 point each for Congestive heart failure, Hypertension, Age 75 or older, and Diabetes, and 2 points for prior Stroke or systemic thromboembolism.
In patients with chronic nonvalvular atrial fibrillation, Gage et al19 reported that the stroke rate was lowest in those with a score of 0, with an annual adjusted stroke rate of 1.9% per year, and highest in those with the maximal possible score (ie, 6), with a rate of 18.2%. The rate increased by a factor of 1.5 with each point in the CHADS2 score.
CHA2DS2-VASc. Endorsed for use in both the American and European guidelines,1,21 CHA2DS2-VASc is an extension of CHADS2. Points are assigned as follows:
- Congestive heart failure or left ventricular dysfunction (moderate to severe left ventricular dysfunction or recent heart failure exacerbation requiring hospitalization irrespective of ejection fraction): 1 point
- Hypertension: 1 point
- Age ≥ 75: 2 points; age 65–74: 1 point
- Diabetes mellitus: 1 point
- Stroke, transient ischemic attack, or thromboembolism: 2 points
- Vascular disease (prior myocardial infarction, peripheral arterial disease, or aortic plaque): 1 point
- Sex, female: 1 point
- Maximum score: 9 points.
Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk. Lip et al22 found that, in untreated patients with nonvalvular atrial fibrillation, rates of stroke ranged from 0 with a score of 0 to 15.2% per year with a score of 9 points.
In a large cohort with over 11,000 patient-years of follow-up, 98% of the thromboembolic events occurred in people with a CHA2DS2-VASc score of 2 or more. Moreover, more than 99% of patients with a score of less than 2 were free of stroke and thromboembolism.23
Compared with the CHADS2 score, CHA2DS2-VASc has superior negative predictive power. Of 1,084 patients from the European Heart Survey for Atrial Fibrillation, the newer scheme classified significantly fewer patients as being at either low risk (score of 0; 9% vs 20%) or intermediate risk (score of 1; 15% vs 35%).23 Though the overall rate of stroke was low, those categorized as being at low or intermediate risk by CHA2DS2-VASc had significantly fewer thromboembolic events than their counterparts according to CHADS2 (0.6% vs 3.3%).
Olesen et al24 similarly showed that in patients with a CHADS2 score of 0, reclassification by CHA2DS2-VASc yielded a range of annual stroke rates from 0.84% with a score of 0 up to 3.2% with a score of 3.
RISK-BASED ANTITHROMBOTIC THERAPY IN NONVALVULAR ATRIAL FIBRILLATION
The 2014 atrial fibrillation guidelines1 state that the decision to give antithrombotic therapy for atrial fibrillation should be individualized, based on the absolute and relative risks of stroke and bleeding, and ought to take into consideration the patient’s preferences. For patients with nonvalvular atrial fibrillation, selection of antithrombotic therapy should take into account the risk of thromboembolism determined by the CHA2DS2-VASc score and be irrespective of the pattern of atrial fibrillation (paroxysmal, persistent, or permanent). Antithrombotic therapy is similarly recommended for patients with atrial flutter, according to the same risk profile used for atrial fibrillation.
Studies have consistently shown24–27 that the risk of ischemic stroke without anticoagulation exceeds the risk of intracranial bleeding with anticoagulation in nearly all patients except those at lowest risk of thromboembolism. The CHA2DS2-VASc score better identified those at truly low risk, in whom treatment may offer more risk than benefit.24–27
The HAS-BLED score28 assigns points as follows:
- Hypertension (systolic blood pressure > 160 mm Hg): 1 point
- Abnormal renal function (dialysis, renal transplantation, or serum creatinine > 2.6 mg/mL) or liver function (cirrhosis, bilirubin more than two times the upper limit, or aminotransferase levels more than three times the upper limit): 1 or 2 points
- Stroke: 1 point
- Bleeding (prior major bleeding event or predisposition to bleeding): 1 point
- Labile international normalized ratio (INR) (supratherapeutic or time in therapeutic range < 60%): 1 point
- Elderly (age > 65): 1 point
- Drugs (antiplatelet, nonsteroidal anti-inflammatory) or alcohol (more than eight drinks per week): 1 or 2 points
- Maximum total: 9 points.
HAS-BLED is a practical and validated approach for estimating bleeding risk and is mentioned in the guidelines, but it is not recommended for use in guiding decisions about antithrombotic therapy. Specifically, it should not be used to exclude patients, but rather to identify those at high risk (score ≥ 3) who may require closer observation and more attentive monitoring of the INR.
ANTITHROMBOTIC THERAPY
Antithrombotic agents available for use in the United States include antiplatelet drugs (eg, aspirin and clopidogrel) and anticoagulants (unfractionated heparin and low-molecular-weight heparin, vitamin K antagonists such as warfarin, and direct thrombin and factor Xa inhibitors). Anticoagulation has been shown in randomized controlled trials to be superior to both placebo and antiplatelet agents used either alone or in combination.29
Aspirin has been downgraded
Aspirin has been compared with placebo in seven randomized controlled trials. Only the original SPAF study, in which aspirin 325 mg/day was used, found that it was beneficial. This result alone accounted for the 19% reduction in relative risk (95% CI 1%–35%, P < .05) in a meta-analysis performed by Hart et al.29 Even when combined with clopidogrel 75 mg/day, aspirin 75 to 100 mg/day is still inferior to warfarin.5 While dual antiplatelet therapy resulted in a 28% relative reduction in thromboembolism (95% CI 17%–38%, P < .01) compared with aspirin alone, major bleeding significantly increased by 57% (95% CI 29%–92%, P < .01).
Although aspirin may be beneficial, differences among patients may influence its efficacy. It may be more effective in preventing noncardioembolic stroke, particularly in diabetic and hypertensive patients.30,31 To date, aspirin has not been shown to be beneficial in low-risk populations.
The 2014 guidelines downgraded the recommendation for aspirin therapy. For patients at low risk and for some at intermediate risk, it is permissible to forgo therapy altogether, including aspirin.1
ORAL ANTICOAGULANTS
The rest of this paper reviews the oral anticoagulants that are approved for reducing the risk of thromboembolism in atrial fibrillation, focusing on each agent’s mechanism of action, pharmacokinetics, clinical efficacy, and safety.
WARFARIN, A VITAMIN K ANTAGONIST
Warfarin inhibits synthesis of vitamin K-dependent clotting factors (ie, factors II, VII, IX, and X) and proteins C and S by inhibiting the C1 subunit of vitamin K epoxide reductase, thereby interfering with production of vitamin K1 epoxide and consequent regeneration of vitamin K.
Pharmacokinetics. Warfarin is nearly completely absorbed after oral administration. Its anticoagulant effect can be seen within 24 hours of administration, but its peak effect is typically apparent only after 72 hours. Elimination occurs predominantly through metabolism by cytochrome P450 enzymes, principally CYP2C9. Its effective half-life ranges from 20 to 60 hours, with a mean of 40 hours.32
Warfarin’s effect, dosage, and bleeding risk are influenced by multiple factors, including vitamin K-containing foods such as green leafy vegetables, medications that either inhibit or induce hepatic cytochrome P450 enzymes, and polymorphisms in the VKORC1 and CYP2C9 genes.32
Reversal. Warfarin’s anticoagulant effect is reversed with vitamin K, but this reversal may not become apparent for 6 to 24 hours. In contrast, fresh-frozen plasma and prothrombin protein concentrate, which contain clotting factors, reverse warfarin immediately. Currently, a three-factor prothrombin protein concentrate (factors II, IX, and X) and a four-factor concentrate (factors II, VII, IX, and X plus proteins C and S) are available in the United States. Although prothrombin protein concentrate works rapidly and has a lower volume of administration, available data do not indicate it is clinically superior to fresh-frozen plasma.33,34 The ongoing randomized PROTECT trial (NCT00618098), comparing fresh-frozen plasma and four-factor prothrombin protein concentrate for reversal of vitamin K antagonist therapy, may provide further insight.
Efficacy and safety. Randomized controlled trials in patients with nonvalvular atrial fibrillation have shown that warfarin (in doses adjusted to maintain an INR greater than 2) is highly efficacious in preventing systemic embolism, with a relative risk reduction of 61% (95% CI 47%–71%, P < .05) compared with placebo.29,35 An INR of 2 to 3 is recommended for patients with nonvalvular atrial fibrillation, and those with atrial fibrillation and either a bioprosthetic valve or rheumatic heart disease. In contrast, an INR of 2.5 to 3.5 is recommended for patients with atrial fibrillation and mechanical valves in the aortic or mitral positions.1,36
Stroke prevention with warfarin is most effective when the achieved mean time in the therapeutic range is at least 70%. The risk of intracranial hemorrhage increases significantly at INRs higher than 3. An INR of 2 to 3 offers maximum protection with minimal risk of bleeding.37,38 Systematic follow-up of patients through anticoagulation clinics produces better compliance and control and is encouraged.
TARGET-SPECIFIC ORAL ANTICOAGULANTS
Although effective, warfarin requires frequent monitoring and dosage adjustment, has a delayed onset and protracted offset, and interacts with commonly consumed vitamin K–containing foods and frequently used drugs. These drawbacks prompted evaluation of existing antiplatelet agents, in combination or in conjunction with lower adjusted or fixed-dose warfarin. These regimens proved inferior,39–42 spurring interest in developing alternative oral anticoagulants.
TSOACs act by directly inhibiting thrombin (factor IIa) or by reducing thrombin production indirectly by inhibiting factor Xa. Three TSOACs are approved. Each was compared with adjusted-dose warfarin in randomized controlled trials.
Dabigatran
Dabigatran etexilate was the first TSOAC approved in the United States.
Pharmacokinetics. Dabigatran etexilate has a bioavailability of 3% to 7% after oral administration. Its absorption is enhanced in an acidic gastric environment and is limited by P-glycoprotein-facilitated transport out of enterocytes. Dabigatran etexilate is hydrolyzed to its active metabolite dabigatran, which directly inhibits thrombin. Maximal plasma drug concentration and peak anticoagulant effect are achieved within 0.5 to 2 hours after administration.
Dabigatran is predominantly excreted by the kidneys, and has a half-life of 12 to 17 hours in patients with normal renal function. The half-life extends to 27 hours in those with moderately severe renal impairment (creatinine clearance 15–30 mL/min). The recommended dose of 150 mg twice daily should be reduced to 75 mg twice daily in patients with a creatinine clearance of 15 to 30 mL/min. This drug is contraindicated in patients with a creatinine clearance less than 15 mL/min.43,44
Efficacy. The Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial45 randomly assigned 18,113 patients with nonvalvular atrial fibrillation at risk of thromboembolism (mean CHADS2 score 2.1) to receive either dabigatran (either 150 mg twice daily or 110 mg twice daily) or warfarin (adjusted to an INR of 2.0 to 3.0). Of note, the lower approved dose of dabigatran (75 mg twice daily) was not tested in RE-LY.
At 2 years, higher-dose dabigatran was significantly more effective than both warfarin (RR 0.65, 95% CI 0.52–0.81, P < .05) and lower-dose dabigatran (RR 0.73, 95% CI 0.58–0.91, P < .05) in reducing the rate of systemic embolic events.
The risk of combined major bleeding events was no different with higher-dose dabigatran than with warfarin (RR 0.93, 95% CI 0.81–1.07, P < .05), but the rate of hemorrhagic stroke was significantly less with dabigatran than with warfarin (RR 0.26, 95% CI 0.14–0.49, P < .05). Higher rates of major gastrointestinal bleeding and dyspepsia occurred with dabigatran.
Concern about the safety of dabigatran was raised when post hoc evaluation of RE-LY found a higher incidence of myocardial infarction with dabigatran than with warfarin (RR 1.38, 95% CI 1–1.91, P = .048).46 Corroborating data were reported by Uchino and Hernandez,47 comparing dabigatran with either warfarin or low-molecular-weight heparin. However, without directly comparing dabigatran and placebo, it is unclear whether the small increase in myocardial infarction reflects a direct effect of dabigatran or absence of a protective effect of warfarin or low-molecular-weight heparin.
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor that blocks the amplified burst of thrombin production and in turn inhibits platelet aggregation and thrombus formation.
Pharmacokinetics. Rivaroxaban’s oral bioavailability is 80% to 100% after a single 15- or 20-mg dose taken with food. Its maximal anticoagulant effect is achieved within 2 hours. Two-thirds of the active drug is metabolized by either CYP450-dependent (CYP3A4, 2J2) or CYP-independent mechanisms; the inactive drug is then excreted in the urine and feces. The remaining, active drug is removed by the kidneys using the P-glycoprotein transporter.
The half-life of rivaroxaban is 5 to 9 hours. The recommended dosage of 20 mg daily should be reduced to 15 mg daily if the creatinine clearance rate is 30 to 50 mL/min, or to 10 mg if the creatinine clearance rate is 15 to 30 mL/min. Rivaroxaban is contraindicated in patients whose creatinine clearance rate is less than 15 mL/min.48–52
Efficacy and safety. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF),53 14,264 at-risk patients with nonvalvular atrial fibrillation (mean CHADS2 score 3.5) were randomly assigned to receive either rivaroxaban 20 mg daily (or 15 mg daily if their creatinine clearance was 30–49 mL/min; the lowest dose of rivaroxaban, 10 mg, was not studied in this trial) or warfarin (target INR 2.0–3.0). Outcomes with rivaroxaban compared with warfarin:
- Systemic embolism:
HR 0.79, 95% CI 0.66–0.96, P < .01, noninferiority - Total bleeding: no difference
- Intracranial bleeding:
HR 0.67, 95% CI 0.47–0.93, P = .02 - Fatal bleeding:
HR 0.50, 95% CI 0.31–0.79, P = .003 - Major gastrointestinal bleeding:
3.2% vs 2.2%, P < .001.
Apixaban
Apixaban is also a direct factor Xa inhibitor.
Pharmacokinetics. Apixaban’s oral bioavailability is 50%, with maximal blood concentration achieved at 3 to 4 hours. One-quarter of the drug is metabolized via CYP3A4. The remaining active drug is excreted by the kidneys and biliary/intestinal system via the P-glycoprotein transporter. Apixaban’s half-life is 9 to 14 hours.
The recommended dosage is 5 mg twice daily, but it should be reduced to 2.5 mg twice daily if at least two of the following characteristics are present: age 80 or older, weight 60 kg or less, and serum creatinine 1.5 mg/dL or more.54,55
Efficacy and safety. The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial56 enrolled 18,201 patients with nonvalvular atrial fibrillation (mean CHADS2 score 2.1) randomly assigned to receive either apixaban (5 mg twice daily with dosage reduction to 2.5 mg twice daily as noted above) or warfarin (target INR 2.0–3.0).
Compared with warfarin, apixaban was associated with lower risk of:
- Systemic embolism
(HR 0.79, 95% CI 0.66–0.95, P = .01) - Major bleeding
(HR 0.69, 95% CI 0.60–0.80, P < .001) - Intracranial hemorrhage
(HR 0.42, 95% CI 0.30–0.58, P < .001) - All-cause mortality
(HR 0.89 95% CI 0.80–0.99, P = .047).
Drug interactions with the novel oral anticoagulants
TSOACs were developed with the intent to avoid many of the shortcomings of warfarin. Each has a broader therapeutic window and a rapid onset of action, enabling fixed dosing without need for serial monitoring. Compared with warfarin, they have significantly fewer dietary and drug interactions.
Nonetheless, drug interactions do exist and are important to recognize (Tables 1–3). These primarily result from inhibition or induction of cytochrome P450 enzyme activity or P-glycoprotein transporter action, involved in metabolism and elimination of active drug.
Reversibility of the target-specific oral anticoagulants
While the anticoagulant effects of warfarin can be reversed by vitamin K, fresh-frozen plasma, and prothrombin complex concentrate, TSOACs have no currently approved antidotes. Management of bleeding due to these agents was recently reviewed in this journal by Fawole et al.57
Several nonspecific hemostatic agents have been suggested, including recombinant factor VIIa or prothrombin complex concentrates. The anticoagulant effect of rivaroxaban has been shown to be reversed by prothrombin complex concentrate in vitro; clinical effect has not been demonstrated.58 PRT06445 (andexanet alfa), a recombinant protein antidote specific for factor Xa inhibitors, has entered clinical studies, with a phase 2 trial reporting high reversing capability for apixaban.59
Unlike rivaroxaban and apixaban, which are highly bound to plasma protein, dabigatran can be effectively removed with hemodialysis. Liesenfeld et al60 showed that longer dialysis duration was the most relevant variable for reducing dabigatran plasma levels. Current clinical experience is limited, and standard recommendations and formal guidance are lacking.
Switching oral anticoagulants
Suggested approaches for switching between anticoagulants are listed in Table 4.61
CHOOSING ANTITHROMBOTIC THERAPY
In valvular atrial fibrillation: warfarin
Anticoagulation with warfarin is advised for valvular atrial fibrillation. Patients with bioprosthetic heart valves or rheumatic valvular disease were not evaluated in randomized controlled trials of TSOACs. Dabigatran in particular is contraindicated in patients with mechanical heart valves, as the Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement (RE-ALIGN)62 found higher rates of stroke, valve-related thrombosis, and myocardial infarction in patients receiving dabigatran.
In nonvalvular atrial fibrillation
According to the 2014 guidelines,1 oral anticoagulation is preferred in all patients with nonvalvular atrial fibrillation but those at lowest risk (CHA2DS2-VASc = 0).
Experience with TSOACs is lacking in patients with end-stage kidney disease (creatinine clearance < 15 mL/min), and warfarin is advised in this group.
TSOACs are recommended in patients with nonvalvular atrial fibrillation in whom therapeutic INR levels cannot be maintained with warfarin. For most patients with nonvalvular atrial fibrillation, TSOACs are an option equivalent to warfarin. Anticoagulant choice is largely driven by dosing convenience, out-of-pocket cost for treatment with a TSOAC, and ready availability of antidotes for warfarin in case of bleeding (Tables 5 and 6).
In patients with nonvalvular atrial fibrillation, TSOACs are as effective as warfarin in preventing systemic thromboembolism, and some of them have been shown to be superior in terms of lower rates of ischemic stroke (dabigatran), systemic embolism (apixaban), and mortality (apixaban; trend for dabigatran). All TSOACs demonstrate modestly favorable bleeding risk profiles compared with warfarin, with lower risk of intracranial hemorrhage. Potential differences in efficacy and safety among TSOACs are unknown since there have been no randomized direct comparisons between them. A summary of landmark trial results and assessment of the advantages and disadvantages of each are listed in Table 7.
Two groups of patients with nonvalvular atrial fibrillation warrant special consideration:
Patients with hypertrophic cardiomyopathy. There are no randomized controlled trials of anticoagulation therapy in patients with hypertrophic cardiomyopathy; however, because of their high risk of thromboembolism, anticoagulation is indicated irrespective of the CHA2DS2-VASc score. TSOACs are an option as an alternative to warfarin.
Patients with coronary artery disease and an indication for antiplatelet therapy. In this group the decision for concurrent anticoagulation is guided by the CHA2DS2-VASc score. For patients who have intracoronary stents, dual antiplatelet therapy is the standard of care for reducing risk of cardiovascular events after stent implantation.63 When triple therapy (ie, two antiplatelet drugs and an anticoagulant) is indicated, such as after intracoronary stent placement, the guidelines suggest trying to minimize the duration of triple therapy. For instance, a bare-metal stent may be preferred. Alternatively, after coronary revascularization, it may be reasonable to use clopidogrel 75 mg daily with an oral anticoagulant and to omit aspirin.
Interrupting and bridging anticoagulation
Patients with atrial fibrillation often require suspension of anticoagulation, most commonly before an elective invasive procedure. The duration of interruption, timing of resumption, and need for bridging anticoagulation are guided by clinical judgment, which considers risk of thromboembolism and severity of procedure-related bleeding risk.
In general, if therapy needs to be interrupted, it should be restarted as soon as possible. Short-term interruption does not seem to be associated with clinically significant risk of thromboembolic events, whereas postoperative heparin bridging therapy increases the risk of hematoma with implantation of a cardiac electronic device.64,65
To date, evidence is lacking to advise upon periprocedure bridging anticoagulation. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) study (NCT00786474)— enrolling chronically anticoagulated patients undergoing an invasive procedure to randomly receive placebo or bridging low-molecular-weight heparin—may provide guidance.
Currently, it is common practice in low-risk patients undergoing an invasive procedure with significant bleeding risk to interrupt anticoagulation for up to 1 week without bridging. Warfarin is typically held 3 to 5 days, while TSOACs are held for 24 hours if renal function is preserved or up to 2 to 3 days if renal function is severely impaired (creatinine clearance 15–30 mL/min). If complete hemostasis is necessary, it could be confirmed by a normalized INR (for warfarin), activated partial thromboplastin time (dabigatran), or prothrombin time (apixaban or rivaroxaban).
For patients at high risk (valvular atrial fibrillation or CHA2DS2-VASc ≥ 2), bridging with unfractionated heparin or low-molecular-weight heparin during periods of subtherapeutic anticoagulation is common. Alternatively, it is becoming increasingly common to perform cardiac electronic device implantation, catheter ablation, and coronary angiography and intervention without interrupting anticoagulation.66–72
Recently, concern has been raised over a possible increase in thromboembolism upon discontinuation of rivaroxaban and apixaban. ROCKET-AF reported a spike in thrombotic events in the rivaroxaban-treated group at the end of the trial (HR 1.50, 95% CI 1.05–2.15, P = .026). This raised concern for a possible “rebound” effect upon drug cessation. Yet a post hoc analysis of ROCKET-AF demonstrated that events clustered in the rivaroxaban-treated cohort who completed the study and were transitioning to open-label warfarin, and this alone accounted for the rise in stroke occurrence. In contrast, there was no increase in the cohort of patients treated with rivaroxaban who either temporarily interrupted or permanently discontinued the drug.73 The authors concluded that increased stroke was the consequence of transiently interrupted anticoagulation, rather than a rebound prothrombotic effect. Similar results were reported in ARISTOTLE.
Another possibility is that, during the transition to warfarin therapy, transient hypercoagulability could be a function of warfarin. Azoulay et al74 observed in a large cohort that warfarin was associated with a 71% increased risk of stroke in the first 30 days after initiation, compared with decreased risk thereafter. Nevertheless, there is now a black- box warning recommendation for all three TSOACs that if discontinuation is required for a reason other than pathological bleeding, bridging with another anticoagulant should at least be considered.
The perioperative management of the TSOACs was recently reviewed in this journal by Anderson et al.75
WEIGHING THE RISKS OF STROKE AND BLEEDING
Stroke is the most feared complication in patients with atrial fibrillation. Risk reduction is an important goal in management, yet decisions for individuals must take into account both stroke and bleeding risks related to antithrombotic therapy.
The 2014 guidelines1 differ from past versions. First, they endorse the use of CHA2DS2-VASc for categorizing stroke risk in patients with nonvalvular atrial fibrillation. This in turn guides antithrombotic therapy. This scheme effectively identifies patients at very low risk of stroke (men with a score of 0, women with a score of 0 or 1), in whom it is reasonable to omit antithrombotic therapy. For all patients with valvular heart disease or hypertrophic cardiomyopathy, unless bleeding risk is prohibitive, anticoagulation is recommended irrespective of the CHA2DS2-VASc score. Second, they incorporate the TSOACs, which offer convenience and improved safety in select patients.
While the guidelines mention the potential relevance of subclinical atrial tachyarrhythmias as they pertain to stroke risk, there is no specific recommendation as to their management. We do take into consideration the finding of atrial high-rate events (≥ 180 bpm, ≥ 6 minutes in duration) diagnostically confirmed by cardiac implantable electronic devices or telemetric monitoring, particularly in patients with a clinical profile of high stroke risk. In addition, atriopathy with increased left atrial size and renal insufficiency, as discussed in this review, appear to correlate with greater risk of thromboembolism, yet neither is a component of the stroke risk scheme endorsed by the guidelines.
Other risk factors, some unknown to us, undoubtedly exist. Again, our empiric judgment is to at least consider these nontraditional risk factors while guided primarily by the CHA2DS2-VASc score when assessing stroke risk in patients with atrial fibrillation.
The goal in managing patients with atrial fibrillation is to balance thromboembolic risk reduction with the risk of bleeding associated with antithrombotic therapy.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000; 35:183–187.
- Hohnloser SH, Pajitnev D, Pogue J, et al; ACTIVE W Investigators. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007; 50:2156–2161.
- Healey JS, Connolly SJ, Gold MR, et al; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366:120–129.
- Glotzer TV, Hellkamp AS, Zimmerman J, et al; MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003; 107:1614–1619.
- Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009; 2:474–480.
- Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:301–307.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001; 104:2517–2524.
- Go AS, Fang MC, Udaltsova N, et al; ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119:1363–1369.
- Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol 2011; 6:2599–2604.
- Vázquez E, Sánchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 2000; 140:886–890.
- The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998; 128:639–647.
- The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med 1992; 116:6–12.
- Osranek M, Bursi F, Bailey KR, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J 2005; 26:2556–2561.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med 2003; 163:936–943.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010; 41:2731–2738.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost 2012; 107:1172–1179.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011; 106:739–749.
- Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125:2298–2307.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med 1997; 157:1237–1240.
- Miller VT, Rothrock JF, Pearce LA, Feinberg WM, Hart RG, Anderson DC. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology 1993; 43:32–36.
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005; 165:1095–1106.
- Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery 1999; 45:1113–1119.
- Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006; 37:1465–1470.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost 2011; 106:968–977.
- Odén A, Fahlén M, Hart RG. Optimal INR for prevention of stroke and death in atrial fibrillation: a critical appraisal. Thromb Res 2006; 117:493–499.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996; 348:633–638.
- Gulløv AL, Koefoed BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med 1998; 158:1513–1521.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012; 125:669–676.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005; 78:412–421.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008; 24:2757–2765.
- Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009; 37:1056–1064.
- Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov 2011; 10:61–75.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 2011; 31:478–492.
- Carreiro J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs 2008; 17:1937–1945.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Liesenfeld KH, Staab A, Härtter S, Formella S, Clemens A, Lehr T. Pharmacometric characterization of dabigatran hemodialysis. Clin Pharmacokinet 2013; 52:453–462.
- MPR. Monthly Prescribing Reference. Anticoagulant dosing conversions. August 18, 2014. www.empr.com/anticoagulant-dosing-conversions/article/194271/. Accessed December 11, 2014.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Tischenko A, Gula LJ, Yee R, Klein GJ, Skanes AC, Krahn AD. Implantation of cardiac rhythm devices without interruption of oral anticoagulation compared with perioperative bridging with low-molecular weight heparin. Am Heart J 2009; 158:252–256.
- Robinson M, Healey JS, Eikelboom J, et al. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol 2009; 32:378–382.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Ahmed I, Gertner E, Nelson WB, et al. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm 2010; 7:745–749.
- Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm 2011; 8:536–540.
- Jamula E, Lloyd NS, Schwalm JD, Airaksinen KE, Douketis JD. Safety of uninterrupted anticoagulation in patients requiring elective coronary angiography with or without percutaneous coronary intervention: a systematic review and metaanalysis. Chest 2010; 138:840–847.
- Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost 2008; 6:1615–1621.
- Korantzopoulos P, Letsas KP, Liu T, Fragakis N, Efremidis M, Goudevenos JA. Anticoagulation and antiplatelet therapy in implantation of electrophysiological devices. Europace 2011; 13:1669–1680.
- Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010; 121:2550–2556.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Azoulay L, Dell’aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2013; Dec 18 [Epub ahead of print].
- Anderson M, Hassell KL, Trujillo TC, Wolfe B. When patients on target-specific oral anticoagulants need surgery. Cleve Clin J Med 2014; 81:629–639.
Antithrombotic therapy reduces the risk of systemic embolism in patients with atrial fibrillation, but one approach does not suit all patients. The decision whether to start this therapy—and which agent to use—must take into account the patient’s risk of thromboembolism as well as bleeding.
Antithrombotic therapy encompasses antiplatelet drugs such as aspirin and clopidogrel and anticoagulants such as warfarin and the target-specific oral anticoagulants (TSOACs). Oral anticoagulation is more effective than antiplatelet therapy and is preferred in all but those at lowest risk, in whom either antiplatelet therapy or no therapy is deemed adequate.
Patients with valvular atrial fibrillation, specifically those who have rheumatic mitral stenosis or a prosthetic heart valve, are at significantly higher risk of systemic embolization. Their overall risk-benefit profile is nearly always in favor of anticoagulation. But the same is not necessarily true for patients with nonvalvular atrial fibrillation.
The following discussion sets forth our rationale for clinical decision-making, based on recommendations in the 2014 guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society.1 The second half of this review outlines the oral anticoagulants currently available.
ONE IN FOUR PEOPLE
Atrial fibrillation is common, with an incidence that increases with age. It affects more than 10% of people over age 80 and is often associated with cardiovascular disease.2 Based on Framingham Heart Study data, a person’s lifetime risk of developing it is about 25%.3
FIVEFOLD RISK OF STROKE
The most serious complication of atrial fibrillation is arterial thromboembolism, of which ischemic stroke is the most common and most feared manifestation. The risk of stroke is five times higher than normal in patients with atrial fibrillation.3 More than 15% of strokes may be attributable to atrial fibrillation, and the proportion increases with age.4
The risk of thromboembolism appears to be similar in patients with clinically manifest atrial fibrillation irrespective of the type (paroxysmal, persistent, or permanent). The Stroke Prevention in Atrial Fibrillation (SPAF) study5 and the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W)6 showed that patients who had paroxysmal atrial fibrillation and at least one risk factor for thromboembolism had stroke rates comparable to those of their counterparts who had persistent and permanent atrial fibrillation.
Subclinical atrial fibrillation may be an important cause of stroke. Clinically silent episodes can be detected by implantable electronic devices, which record episodes of atrial tachyarrhythmia (atrial high-rate events). Subclinical episodes have been detected in 10% to 28% of monitored patients who did not have a history of atrial fibrillation.7,8 Patients who have atrial high-rate events detected by implantable devices have a higher risk of future clinically manifest atrial fibrillation, thromboembolic events, or both.7–9 Yet characteristics of atrial high-rate episodes that predict risk are not well defined and warrant further investigation.
CLINICAL RISK FACTORS FOR STROKE
To date, thousands of patients with nonvalvular atrial fibrillation have participated in randomized clinical trials of stroke prevention. The placebo groups from these trials provide a sizable database for retrospectively identifying clinical characteristics associated with thromboembolism. The Atrial Fibrillation Investigators10 pooled data from five large trials and found that risk factors consistently associated with stroke in multivariate analysis included diabetes mellitus, hypertension, prior systemic embolism, and advanced age.
Though the risk of stroke increases with age with no lower limit, most studies identify age 65 as a threshold, with further escalating risk after age 75. Moreover, women were observed to be at higher risk in some but not all studies. These risk factors have become components of commonly used risk-stratification schemes.
Hypertrophic cardiomyopathy. Maron et al11 reported that atrial fibrillation in patients with hypertrophic cardiomyopathy was independently associated with thromboembolism. In 900 patients with hypertrophic cardiomyopathy, the prevalence of systemic embolism was 6%. Patients with hypertrophic cardiomyopathy and a thromboembolic complication were seven times more likely to have atrial fibrillation than matched counterparts free of thromboembolism. A notable subset of patients experienced a stroke or embolic event before age 50, and the authors advised that the risk of thromboembolism should be considered in patients of any age with hypertrophic cardiomyopathy and atrial fibrillation.
Olivotto et al12 similarly found patients with hypertrophic cardiomyopathy and atrial fibrillation to be at significantly greater risk of stroke (odds ratio [OR] 17.7, 95% confidence interval [CI] 4.1–75.9, P < .001).
Chronic kidney disease is also associated with a higher risk of thromboembolism in patients with atrial fibrillation. A glomerular filtration rate of 60 mL/min or less is independently and inversely predictive of risk.13,14
While patients with end-stage renal disease have been largely excluded from stroke prevention trials, Vázquez et al15 prospectively followed 190 dialysis patients for 12 months. In multivariate analysis, compared with matched controls without documented atrial fibrillation, patients receiving renal replacement therapy and having any form of atrial fibrillation were eight times more likely to have systemic embolization.
IMAGING-BASED RISK FACTORS
In addition to clinical factors, several imaging-based factors have been associated with stroke risk in patients with atrial fibrillation.
Complex aortic atheroma or markers of blood stasis within the left atrium, such as reduced left atrial appendage emptying flow (< 20 cm/second), dense spontaneous echo contrast, or left atrial appendage thrombus, seen on transesophageal echocardiography, were independently associated with increased systemic embolic risk in the third SPAF substudy.16 Moreover, multivariate analysis of SPAF data found both left ventricular dysfunction of any severity and increased left atrial size (diameter corrected for body surface area by M-mode > 2.5 cm/m2) to be independent predictors of thromboembolism.17
Although enlargement of the left atrium has not been incorporated into traditional risk stratification schemes, data from Osranek et al18 further implicate it as a marker of risk. The cohort was small (N = 46), but consisted of patients with lone atrial fibrillation followed for nearly 30 years. Patients with normal left atrial size enjoyed a benign course, while those with left atrial enlargement (> 32 mL/m2) at diagnosis or later during follow-up had significantly worse event-free survival (hazard ratio [HR] 4.46, 95% CI 1.56–12.74, P < .01). All embolic strokes occurred in the group with left atrial enlargement.
RISK STRATIFICATION SCHEMES
Several models for predicting systemic embolism risk in patients with nonvalvular atrial fibrillation have been proposed and validated.
The CHADS2 score has been the most widely applied, being simple to use.19,20 It assigns 1 point each for Congestive heart failure, Hypertension, Age 75 or older, and Diabetes, and 2 points for prior Stroke or systemic thromboembolism.
In patients with chronic nonvalvular atrial fibrillation, Gage et al19 reported that the stroke rate was lowest in those with a score of 0, with an annual adjusted stroke rate of 1.9% per year, and highest in those with the maximal possible score (ie, 6), with a rate of 18.2%. The rate increased by a factor of 1.5 with each point in the CHADS2 score.
CHA2DS2-VASc. Endorsed for use in both the American and European guidelines,1,21 CHA2DS2-VASc is an extension of CHADS2. Points are assigned as follows:
- Congestive heart failure or left ventricular dysfunction (moderate to severe left ventricular dysfunction or recent heart failure exacerbation requiring hospitalization irrespective of ejection fraction): 1 point
- Hypertension: 1 point
- Age ≥ 75: 2 points; age 65–74: 1 point
- Diabetes mellitus: 1 point
- Stroke, transient ischemic attack, or thromboembolism: 2 points
- Vascular disease (prior myocardial infarction, peripheral arterial disease, or aortic plaque): 1 point
- Sex, female: 1 point
- Maximum score: 9 points.
Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk. Lip et al22 found that, in untreated patients with nonvalvular atrial fibrillation, rates of stroke ranged from 0 with a score of 0 to 15.2% per year with a score of 9 points.
In a large cohort with over 11,000 patient-years of follow-up, 98% of the thromboembolic events occurred in people with a CHA2DS2-VASc score of 2 or more. Moreover, more than 99% of patients with a score of less than 2 were free of stroke and thromboembolism.23
Compared with the CHADS2 score, CHA2DS2-VASc has superior negative predictive power. Of 1,084 patients from the European Heart Survey for Atrial Fibrillation, the newer scheme classified significantly fewer patients as being at either low risk (score of 0; 9% vs 20%) or intermediate risk (score of 1; 15% vs 35%).23 Though the overall rate of stroke was low, those categorized as being at low or intermediate risk by CHA2DS2-VASc had significantly fewer thromboembolic events than their counterparts according to CHADS2 (0.6% vs 3.3%).
Olesen et al24 similarly showed that in patients with a CHADS2 score of 0, reclassification by CHA2DS2-VASc yielded a range of annual stroke rates from 0.84% with a score of 0 up to 3.2% with a score of 3.
RISK-BASED ANTITHROMBOTIC THERAPY IN NONVALVULAR ATRIAL FIBRILLATION
The 2014 atrial fibrillation guidelines1 state that the decision to give antithrombotic therapy for atrial fibrillation should be individualized, based on the absolute and relative risks of stroke and bleeding, and ought to take into consideration the patient’s preferences. For patients with nonvalvular atrial fibrillation, selection of antithrombotic therapy should take into account the risk of thromboembolism determined by the CHA2DS2-VASc score and be irrespective of the pattern of atrial fibrillation (paroxysmal, persistent, or permanent). Antithrombotic therapy is similarly recommended for patients with atrial flutter, according to the same risk profile used for atrial fibrillation.
Studies have consistently shown24–27 that the risk of ischemic stroke without anticoagulation exceeds the risk of intracranial bleeding with anticoagulation in nearly all patients except those at lowest risk of thromboembolism. The CHA2DS2-VASc score better identified those at truly low risk, in whom treatment may offer more risk than benefit.24–27
The HAS-BLED score28 assigns points as follows:
- Hypertension (systolic blood pressure > 160 mm Hg): 1 point
- Abnormal renal function (dialysis, renal transplantation, or serum creatinine > 2.6 mg/mL) or liver function (cirrhosis, bilirubin more than two times the upper limit, or aminotransferase levels more than three times the upper limit): 1 or 2 points
- Stroke: 1 point
- Bleeding (prior major bleeding event or predisposition to bleeding): 1 point
- Labile international normalized ratio (INR) (supratherapeutic or time in therapeutic range < 60%): 1 point
- Elderly (age > 65): 1 point
- Drugs (antiplatelet, nonsteroidal anti-inflammatory) or alcohol (more than eight drinks per week): 1 or 2 points
- Maximum total: 9 points.
HAS-BLED is a practical and validated approach for estimating bleeding risk and is mentioned in the guidelines, but it is not recommended for use in guiding decisions about antithrombotic therapy. Specifically, it should not be used to exclude patients, but rather to identify those at high risk (score ≥ 3) who may require closer observation and more attentive monitoring of the INR.
ANTITHROMBOTIC THERAPY
Antithrombotic agents available for use in the United States include antiplatelet drugs (eg, aspirin and clopidogrel) and anticoagulants (unfractionated heparin and low-molecular-weight heparin, vitamin K antagonists such as warfarin, and direct thrombin and factor Xa inhibitors). Anticoagulation has been shown in randomized controlled trials to be superior to both placebo and antiplatelet agents used either alone or in combination.29
Aspirin has been downgraded
Aspirin has been compared with placebo in seven randomized controlled trials. Only the original SPAF study, in which aspirin 325 mg/day was used, found that it was beneficial. This result alone accounted for the 19% reduction in relative risk (95% CI 1%–35%, P < .05) in a meta-analysis performed by Hart et al.29 Even when combined with clopidogrel 75 mg/day, aspirin 75 to 100 mg/day is still inferior to warfarin.5 While dual antiplatelet therapy resulted in a 28% relative reduction in thromboembolism (95% CI 17%–38%, P < .01) compared with aspirin alone, major bleeding significantly increased by 57% (95% CI 29%–92%, P < .01).
Although aspirin may be beneficial, differences among patients may influence its efficacy. It may be more effective in preventing noncardioembolic stroke, particularly in diabetic and hypertensive patients.30,31 To date, aspirin has not been shown to be beneficial in low-risk populations.
The 2014 guidelines downgraded the recommendation for aspirin therapy. For patients at low risk and for some at intermediate risk, it is permissible to forgo therapy altogether, including aspirin.1
ORAL ANTICOAGULANTS
The rest of this paper reviews the oral anticoagulants that are approved for reducing the risk of thromboembolism in atrial fibrillation, focusing on each agent’s mechanism of action, pharmacokinetics, clinical efficacy, and safety.
WARFARIN, A VITAMIN K ANTAGONIST
Warfarin inhibits synthesis of vitamin K-dependent clotting factors (ie, factors II, VII, IX, and X) and proteins C and S by inhibiting the C1 subunit of vitamin K epoxide reductase, thereby interfering with production of vitamin K1 epoxide and consequent regeneration of vitamin K.
Pharmacokinetics. Warfarin is nearly completely absorbed after oral administration. Its anticoagulant effect can be seen within 24 hours of administration, but its peak effect is typically apparent only after 72 hours. Elimination occurs predominantly through metabolism by cytochrome P450 enzymes, principally CYP2C9. Its effective half-life ranges from 20 to 60 hours, with a mean of 40 hours.32
Warfarin’s effect, dosage, and bleeding risk are influenced by multiple factors, including vitamin K-containing foods such as green leafy vegetables, medications that either inhibit or induce hepatic cytochrome P450 enzymes, and polymorphisms in the VKORC1 and CYP2C9 genes.32
Reversal. Warfarin’s anticoagulant effect is reversed with vitamin K, but this reversal may not become apparent for 6 to 24 hours. In contrast, fresh-frozen plasma and prothrombin protein concentrate, which contain clotting factors, reverse warfarin immediately. Currently, a three-factor prothrombin protein concentrate (factors II, IX, and X) and a four-factor concentrate (factors II, VII, IX, and X plus proteins C and S) are available in the United States. Although prothrombin protein concentrate works rapidly and has a lower volume of administration, available data do not indicate it is clinically superior to fresh-frozen plasma.33,34 The ongoing randomized PROTECT trial (NCT00618098), comparing fresh-frozen plasma and four-factor prothrombin protein concentrate for reversal of vitamin K antagonist therapy, may provide further insight.
Efficacy and safety. Randomized controlled trials in patients with nonvalvular atrial fibrillation have shown that warfarin (in doses adjusted to maintain an INR greater than 2) is highly efficacious in preventing systemic embolism, with a relative risk reduction of 61% (95% CI 47%–71%, P < .05) compared with placebo.29,35 An INR of 2 to 3 is recommended for patients with nonvalvular atrial fibrillation, and those with atrial fibrillation and either a bioprosthetic valve or rheumatic heart disease. In contrast, an INR of 2.5 to 3.5 is recommended for patients with atrial fibrillation and mechanical valves in the aortic or mitral positions.1,36
Stroke prevention with warfarin is most effective when the achieved mean time in the therapeutic range is at least 70%. The risk of intracranial hemorrhage increases significantly at INRs higher than 3. An INR of 2 to 3 offers maximum protection with minimal risk of bleeding.37,38 Systematic follow-up of patients through anticoagulation clinics produces better compliance and control and is encouraged.
TARGET-SPECIFIC ORAL ANTICOAGULANTS
Although effective, warfarin requires frequent monitoring and dosage adjustment, has a delayed onset and protracted offset, and interacts with commonly consumed vitamin K–containing foods and frequently used drugs. These drawbacks prompted evaluation of existing antiplatelet agents, in combination or in conjunction with lower adjusted or fixed-dose warfarin. These regimens proved inferior,39–42 spurring interest in developing alternative oral anticoagulants.
TSOACs act by directly inhibiting thrombin (factor IIa) or by reducing thrombin production indirectly by inhibiting factor Xa. Three TSOACs are approved. Each was compared with adjusted-dose warfarin in randomized controlled trials.
Dabigatran
Dabigatran etexilate was the first TSOAC approved in the United States.
Pharmacokinetics. Dabigatran etexilate has a bioavailability of 3% to 7% after oral administration. Its absorption is enhanced in an acidic gastric environment and is limited by P-glycoprotein-facilitated transport out of enterocytes. Dabigatran etexilate is hydrolyzed to its active metabolite dabigatran, which directly inhibits thrombin. Maximal plasma drug concentration and peak anticoagulant effect are achieved within 0.5 to 2 hours after administration.
Dabigatran is predominantly excreted by the kidneys, and has a half-life of 12 to 17 hours in patients with normal renal function. The half-life extends to 27 hours in those with moderately severe renal impairment (creatinine clearance 15–30 mL/min). The recommended dose of 150 mg twice daily should be reduced to 75 mg twice daily in patients with a creatinine clearance of 15 to 30 mL/min. This drug is contraindicated in patients with a creatinine clearance less than 15 mL/min.43,44
Efficacy. The Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial45 randomly assigned 18,113 patients with nonvalvular atrial fibrillation at risk of thromboembolism (mean CHADS2 score 2.1) to receive either dabigatran (either 150 mg twice daily or 110 mg twice daily) or warfarin (adjusted to an INR of 2.0 to 3.0). Of note, the lower approved dose of dabigatran (75 mg twice daily) was not tested in RE-LY.
At 2 years, higher-dose dabigatran was significantly more effective than both warfarin (RR 0.65, 95% CI 0.52–0.81, P < .05) and lower-dose dabigatran (RR 0.73, 95% CI 0.58–0.91, P < .05) in reducing the rate of systemic embolic events.
The risk of combined major bleeding events was no different with higher-dose dabigatran than with warfarin (RR 0.93, 95% CI 0.81–1.07, P < .05), but the rate of hemorrhagic stroke was significantly less with dabigatran than with warfarin (RR 0.26, 95% CI 0.14–0.49, P < .05). Higher rates of major gastrointestinal bleeding and dyspepsia occurred with dabigatran.
Concern about the safety of dabigatran was raised when post hoc evaluation of RE-LY found a higher incidence of myocardial infarction with dabigatran than with warfarin (RR 1.38, 95% CI 1–1.91, P = .048).46 Corroborating data were reported by Uchino and Hernandez,47 comparing dabigatran with either warfarin or low-molecular-weight heparin. However, without directly comparing dabigatran and placebo, it is unclear whether the small increase in myocardial infarction reflects a direct effect of dabigatran or absence of a protective effect of warfarin or low-molecular-weight heparin.
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor that blocks the amplified burst of thrombin production and in turn inhibits platelet aggregation and thrombus formation.
Pharmacokinetics. Rivaroxaban’s oral bioavailability is 80% to 100% after a single 15- or 20-mg dose taken with food. Its maximal anticoagulant effect is achieved within 2 hours. Two-thirds of the active drug is metabolized by either CYP450-dependent (CYP3A4, 2J2) or CYP-independent mechanisms; the inactive drug is then excreted in the urine and feces. The remaining, active drug is removed by the kidneys using the P-glycoprotein transporter.
The half-life of rivaroxaban is 5 to 9 hours. The recommended dosage of 20 mg daily should be reduced to 15 mg daily if the creatinine clearance rate is 30 to 50 mL/min, or to 10 mg if the creatinine clearance rate is 15 to 30 mL/min. Rivaroxaban is contraindicated in patients whose creatinine clearance rate is less than 15 mL/min.48–52
Efficacy and safety. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF),53 14,264 at-risk patients with nonvalvular atrial fibrillation (mean CHADS2 score 3.5) were randomly assigned to receive either rivaroxaban 20 mg daily (or 15 mg daily if their creatinine clearance was 30–49 mL/min; the lowest dose of rivaroxaban, 10 mg, was not studied in this trial) or warfarin (target INR 2.0–3.0). Outcomes with rivaroxaban compared with warfarin:
- Systemic embolism:
HR 0.79, 95% CI 0.66–0.96, P < .01, noninferiority - Total bleeding: no difference
- Intracranial bleeding:
HR 0.67, 95% CI 0.47–0.93, P = .02 - Fatal bleeding:
HR 0.50, 95% CI 0.31–0.79, P = .003 - Major gastrointestinal bleeding:
3.2% vs 2.2%, P < .001.
Apixaban
Apixaban is also a direct factor Xa inhibitor.
Pharmacokinetics. Apixaban’s oral bioavailability is 50%, with maximal blood concentration achieved at 3 to 4 hours. One-quarter of the drug is metabolized via CYP3A4. The remaining active drug is excreted by the kidneys and biliary/intestinal system via the P-glycoprotein transporter. Apixaban’s half-life is 9 to 14 hours.
The recommended dosage is 5 mg twice daily, but it should be reduced to 2.5 mg twice daily if at least two of the following characteristics are present: age 80 or older, weight 60 kg or less, and serum creatinine 1.5 mg/dL or more.54,55
Efficacy and safety. The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial56 enrolled 18,201 patients with nonvalvular atrial fibrillation (mean CHADS2 score 2.1) randomly assigned to receive either apixaban (5 mg twice daily with dosage reduction to 2.5 mg twice daily as noted above) or warfarin (target INR 2.0–3.0).
Compared with warfarin, apixaban was associated with lower risk of:
- Systemic embolism
(HR 0.79, 95% CI 0.66–0.95, P = .01) - Major bleeding
(HR 0.69, 95% CI 0.60–0.80, P < .001) - Intracranial hemorrhage
(HR 0.42, 95% CI 0.30–0.58, P < .001) - All-cause mortality
(HR 0.89 95% CI 0.80–0.99, P = .047).
Drug interactions with the novel oral anticoagulants
TSOACs were developed with the intent to avoid many of the shortcomings of warfarin. Each has a broader therapeutic window and a rapid onset of action, enabling fixed dosing without need for serial monitoring. Compared with warfarin, they have significantly fewer dietary and drug interactions.
Nonetheless, drug interactions do exist and are important to recognize (Tables 1–3). These primarily result from inhibition or induction of cytochrome P450 enzyme activity or P-glycoprotein transporter action, involved in metabolism and elimination of active drug.
Reversibility of the target-specific oral anticoagulants
While the anticoagulant effects of warfarin can be reversed by vitamin K, fresh-frozen plasma, and prothrombin complex concentrate, TSOACs have no currently approved antidotes. Management of bleeding due to these agents was recently reviewed in this journal by Fawole et al.57
Several nonspecific hemostatic agents have been suggested, including recombinant factor VIIa or prothrombin complex concentrates. The anticoagulant effect of rivaroxaban has been shown to be reversed by prothrombin complex concentrate in vitro; clinical effect has not been demonstrated.58 PRT06445 (andexanet alfa), a recombinant protein antidote specific for factor Xa inhibitors, has entered clinical studies, with a phase 2 trial reporting high reversing capability for apixaban.59
Unlike rivaroxaban and apixaban, which are highly bound to plasma protein, dabigatran can be effectively removed with hemodialysis. Liesenfeld et al60 showed that longer dialysis duration was the most relevant variable for reducing dabigatran plasma levels. Current clinical experience is limited, and standard recommendations and formal guidance are lacking.
Switching oral anticoagulants
Suggested approaches for switching between anticoagulants are listed in Table 4.61
CHOOSING ANTITHROMBOTIC THERAPY
In valvular atrial fibrillation: warfarin
Anticoagulation with warfarin is advised for valvular atrial fibrillation. Patients with bioprosthetic heart valves or rheumatic valvular disease were not evaluated in randomized controlled trials of TSOACs. Dabigatran in particular is contraindicated in patients with mechanical heart valves, as the Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement (RE-ALIGN)62 found higher rates of stroke, valve-related thrombosis, and myocardial infarction in patients receiving dabigatran.
In nonvalvular atrial fibrillation
According to the 2014 guidelines,1 oral anticoagulation is preferred in all patients with nonvalvular atrial fibrillation but those at lowest risk (CHA2DS2-VASc = 0).
Experience with TSOACs is lacking in patients with end-stage kidney disease (creatinine clearance < 15 mL/min), and warfarin is advised in this group.
TSOACs are recommended in patients with nonvalvular atrial fibrillation in whom therapeutic INR levels cannot be maintained with warfarin. For most patients with nonvalvular atrial fibrillation, TSOACs are an option equivalent to warfarin. Anticoagulant choice is largely driven by dosing convenience, out-of-pocket cost for treatment with a TSOAC, and ready availability of antidotes for warfarin in case of bleeding (Tables 5 and 6).
In patients with nonvalvular atrial fibrillation, TSOACs are as effective as warfarin in preventing systemic thromboembolism, and some of them have been shown to be superior in terms of lower rates of ischemic stroke (dabigatran), systemic embolism (apixaban), and mortality (apixaban; trend for dabigatran). All TSOACs demonstrate modestly favorable bleeding risk profiles compared with warfarin, with lower risk of intracranial hemorrhage. Potential differences in efficacy and safety among TSOACs are unknown since there have been no randomized direct comparisons between them. A summary of landmark trial results and assessment of the advantages and disadvantages of each are listed in Table 7.
Two groups of patients with nonvalvular atrial fibrillation warrant special consideration:
Patients with hypertrophic cardiomyopathy. There are no randomized controlled trials of anticoagulation therapy in patients with hypertrophic cardiomyopathy; however, because of their high risk of thromboembolism, anticoagulation is indicated irrespective of the CHA2DS2-VASc score. TSOACs are an option as an alternative to warfarin.
Patients with coronary artery disease and an indication for antiplatelet therapy. In this group the decision for concurrent anticoagulation is guided by the CHA2DS2-VASc score. For patients who have intracoronary stents, dual antiplatelet therapy is the standard of care for reducing risk of cardiovascular events after stent implantation.63 When triple therapy (ie, two antiplatelet drugs and an anticoagulant) is indicated, such as after intracoronary stent placement, the guidelines suggest trying to minimize the duration of triple therapy. For instance, a bare-metal stent may be preferred. Alternatively, after coronary revascularization, it may be reasonable to use clopidogrel 75 mg daily with an oral anticoagulant and to omit aspirin.
Interrupting and bridging anticoagulation
Patients with atrial fibrillation often require suspension of anticoagulation, most commonly before an elective invasive procedure. The duration of interruption, timing of resumption, and need for bridging anticoagulation are guided by clinical judgment, which considers risk of thromboembolism and severity of procedure-related bleeding risk.
In general, if therapy needs to be interrupted, it should be restarted as soon as possible. Short-term interruption does not seem to be associated with clinically significant risk of thromboembolic events, whereas postoperative heparin bridging therapy increases the risk of hematoma with implantation of a cardiac electronic device.64,65
To date, evidence is lacking to advise upon periprocedure bridging anticoagulation. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) study (NCT00786474)— enrolling chronically anticoagulated patients undergoing an invasive procedure to randomly receive placebo or bridging low-molecular-weight heparin—may provide guidance.
Currently, it is common practice in low-risk patients undergoing an invasive procedure with significant bleeding risk to interrupt anticoagulation for up to 1 week without bridging. Warfarin is typically held 3 to 5 days, while TSOACs are held for 24 hours if renal function is preserved or up to 2 to 3 days if renal function is severely impaired (creatinine clearance 15–30 mL/min). If complete hemostasis is necessary, it could be confirmed by a normalized INR (for warfarin), activated partial thromboplastin time (dabigatran), or prothrombin time (apixaban or rivaroxaban).
For patients at high risk (valvular atrial fibrillation or CHA2DS2-VASc ≥ 2), bridging with unfractionated heparin or low-molecular-weight heparin during periods of subtherapeutic anticoagulation is common. Alternatively, it is becoming increasingly common to perform cardiac electronic device implantation, catheter ablation, and coronary angiography and intervention without interrupting anticoagulation.66–72
Recently, concern has been raised over a possible increase in thromboembolism upon discontinuation of rivaroxaban and apixaban. ROCKET-AF reported a spike in thrombotic events in the rivaroxaban-treated group at the end of the trial (HR 1.50, 95% CI 1.05–2.15, P = .026). This raised concern for a possible “rebound” effect upon drug cessation. Yet a post hoc analysis of ROCKET-AF demonstrated that events clustered in the rivaroxaban-treated cohort who completed the study and were transitioning to open-label warfarin, and this alone accounted for the rise in stroke occurrence. In contrast, there was no increase in the cohort of patients treated with rivaroxaban who either temporarily interrupted or permanently discontinued the drug.73 The authors concluded that increased stroke was the consequence of transiently interrupted anticoagulation, rather than a rebound prothrombotic effect. Similar results were reported in ARISTOTLE.
Another possibility is that, during the transition to warfarin therapy, transient hypercoagulability could be a function of warfarin. Azoulay et al74 observed in a large cohort that warfarin was associated with a 71% increased risk of stroke in the first 30 days after initiation, compared with decreased risk thereafter. Nevertheless, there is now a black- box warning recommendation for all three TSOACs that if discontinuation is required for a reason other than pathological bleeding, bridging with another anticoagulant should at least be considered.
The perioperative management of the TSOACs was recently reviewed in this journal by Anderson et al.75
WEIGHING THE RISKS OF STROKE AND BLEEDING
Stroke is the most feared complication in patients with atrial fibrillation. Risk reduction is an important goal in management, yet decisions for individuals must take into account both stroke and bleeding risks related to antithrombotic therapy.
The 2014 guidelines1 differ from past versions. First, they endorse the use of CHA2DS2-VASc for categorizing stroke risk in patients with nonvalvular atrial fibrillation. This in turn guides antithrombotic therapy. This scheme effectively identifies patients at very low risk of stroke (men with a score of 0, women with a score of 0 or 1), in whom it is reasonable to omit antithrombotic therapy. For all patients with valvular heart disease or hypertrophic cardiomyopathy, unless bleeding risk is prohibitive, anticoagulation is recommended irrespective of the CHA2DS2-VASc score. Second, they incorporate the TSOACs, which offer convenience and improved safety in select patients.
While the guidelines mention the potential relevance of subclinical atrial tachyarrhythmias as they pertain to stroke risk, there is no specific recommendation as to their management. We do take into consideration the finding of atrial high-rate events (≥ 180 bpm, ≥ 6 minutes in duration) diagnostically confirmed by cardiac implantable electronic devices or telemetric monitoring, particularly in patients with a clinical profile of high stroke risk. In addition, atriopathy with increased left atrial size and renal insufficiency, as discussed in this review, appear to correlate with greater risk of thromboembolism, yet neither is a component of the stroke risk scheme endorsed by the guidelines.
Other risk factors, some unknown to us, undoubtedly exist. Again, our empiric judgment is to at least consider these nontraditional risk factors while guided primarily by the CHA2DS2-VASc score when assessing stroke risk in patients with atrial fibrillation.
The goal in managing patients with atrial fibrillation is to balance thromboembolic risk reduction with the risk of bleeding associated with antithrombotic therapy.
Antithrombotic therapy reduces the risk of systemic embolism in patients with atrial fibrillation, but one approach does not suit all patients. The decision whether to start this therapy—and which agent to use—must take into account the patient’s risk of thromboembolism as well as bleeding.
Antithrombotic therapy encompasses antiplatelet drugs such as aspirin and clopidogrel and anticoagulants such as warfarin and the target-specific oral anticoagulants (TSOACs). Oral anticoagulation is more effective than antiplatelet therapy and is preferred in all but those at lowest risk, in whom either antiplatelet therapy or no therapy is deemed adequate.
Patients with valvular atrial fibrillation, specifically those who have rheumatic mitral stenosis or a prosthetic heart valve, are at significantly higher risk of systemic embolization. Their overall risk-benefit profile is nearly always in favor of anticoagulation. But the same is not necessarily true for patients with nonvalvular atrial fibrillation.
The following discussion sets forth our rationale for clinical decision-making, based on recommendations in the 2014 guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society.1 The second half of this review outlines the oral anticoagulants currently available.
ONE IN FOUR PEOPLE
Atrial fibrillation is common, with an incidence that increases with age. It affects more than 10% of people over age 80 and is often associated with cardiovascular disease.2 Based on Framingham Heart Study data, a person’s lifetime risk of developing it is about 25%.3
FIVEFOLD RISK OF STROKE
The most serious complication of atrial fibrillation is arterial thromboembolism, of which ischemic stroke is the most common and most feared manifestation. The risk of stroke is five times higher than normal in patients with atrial fibrillation.3 More than 15% of strokes may be attributable to atrial fibrillation, and the proportion increases with age.4
The risk of thromboembolism appears to be similar in patients with clinically manifest atrial fibrillation irrespective of the type (paroxysmal, persistent, or permanent). The Stroke Prevention in Atrial Fibrillation (SPAF) study5 and the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W)6 showed that patients who had paroxysmal atrial fibrillation and at least one risk factor for thromboembolism had stroke rates comparable to those of their counterparts who had persistent and permanent atrial fibrillation.
Subclinical atrial fibrillation may be an important cause of stroke. Clinically silent episodes can be detected by implantable electronic devices, which record episodes of atrial tachyarrhythmia (atrial high-rate events). Subclinical episodes have been detected in 10% to 28% of monitored patients who did not have a history of atrial fibrillation.7,8 Patients who have atrial high-rate events detected by implantable devices have a higher risk of future clinically manifest atrial fibrillation, thromboembolic events, or both.7–9 Yet characteristics of atrial high-rate episodes that predict risk are not well defined and warrant further investigation.
CLINICAL RISK FACTORS FOR STROKE
To date, thousands of patients with nonvalvular atrial fibrillation have participated in randomized clinical trials of stroke prevention. The placebo groups from these trials provide a sizable database for retrospectively identifying clinical characteristics associated with thromboembolism. The Atrial Fibrillation Investigators10 pooled data from five large trials and found that risk factors consistently associated with stroke in multivariate analysis included diabetes mellitus, hypertension, prior systemic embolism, and advanced age.
Though the risk of stroke increases with age with no lower limit, most studies identify age 65 as a threshold, with further escalating risk after age 75. Moreover, women were observed to be at higher risk in some but not all studies. These risk factors have become components of commonly used risk-stratification schemes.
Hypertrophic cardiomyopathy. Maron et al11 reported that atrial fibrillation in patients with hypertrophic cardiomyopathy was independently associated with thromboembolism. In 900 patients with hypertrophic cardiomyopathy, the prevalence of systemic embolism was 6%. Patients with hypertrophic cardiomyopathy and a thromboembolic complication were seven times more likely to have atrial fibrillation than matched counterparts free of thromboembolism. A notable subset of patients experienced a stroke or embolic event before age 50, and the authors advised that the risk of thromboembolism should be considered in patients of any age with hypertrophic cardiomyopathy and atrial fibrillation.
Olivotto et al12 similarly found patients with hypertrophic cardiomyopathy and atrial fibrillation to be at significantly greater risk of stroke (odds ratio [OR] 17.7, 95% confidence interval [CI] 4.1–75.9, P < .001).
Chronic kidney disease is also associated with a higher risk of thromboembolism in patients with atrial fibrillation. A glomerular filtration rate of 60 mL/min or less is independently and inversely predictive of risk.13,14
While patients with end-stage renal disease have been largely excluded from stroke prevention trials, Vázquez et al15 prospectively followed 190 dialysis patients for 12 months. In multivariate analysis, compared with matched controls without documented atrial fibrillation, patients receiving renal replacement therapy and having any form of atrial fibrillation were eight times more likely to have systemic embolization.
IMAGING-BASED RISK FACTORS
In addition to clinical factors, several imaging-based factors have been associated with stroke risk in patients with atrial fibrillation.
Complex aortic atheroma or markers of blood stasis within the left atrium, such as reduced left atrial appendage emptying flow (< 20 cm/second), dense spontaneous echo contrast, or left atrial appendage thrombus, seen on transesophageal echocardiography, were independently associated with increased systemic embolic risk in the third SPAF substudy.16 Moreover, multivariate analysis of SPAF data found both left ventricular dysfunction of any severity and increased left atrial size (diameter corrected for body surface area by M-mode > 2.5 cm/m2) to be independent predictors of thromboembolism.17
Although enlargement of the left atrium has not been incorporated into traditional risk stratification schemes, data from Osranek et al18 further implicate it as a marker of risk. The cohort was small (N = 46), but consisted of patients with lone atrial fibrillation followed for nearly 30 years. Patients with normal left atrial size enjoyed a benign course, while those with left atrial enlargement (> 32 mL/m2) at diagnosis or later during follow-up had significantly worse event-free survival (hazard ratio [HR] 4.46, 95% CI 1.56–12.74, P < .01). All embolic strokes occurred in the group with left atrial enlargement.
RISK STRATIFICATION SCHEMES
Several models for predicting systemic embolism risk in patients with nonvalvular atrial fibrillation have been proposed and validated.
The CHADS2 score has been the most widely applied, being simple to use.19,20 It assigns 1 point each for Congestive heart failure, Hypertension, Age 75 or older, and Diabetes, and 2 points for prior Stroke or systemic thromboembolism.
In patients with chronic nonvalvular atrial fibrillation, Gage et al19 reported that the stroke rate was lowest in those with a score of 0, with an annual adjusted stroke rate of 1.9% per year, and highest in those with the maximal possible score (ie, 6), with a rate of 18.2%. The rate increased by a factor of 1.5 with each point in the CHADS2 score.
CHA2DS2-VASc. Endorsed for use in both the American and European guidelines,1,21 CHA2DS2-VASc is an extension of CHADS2. Points are assigned as follows:
- Congestive heart failure or left ventricular dysfunction (moderate to severe left ventricular dysfunction or recent heart failure exacerbation requiring hospitalization irrespective of ejection fraction): 1 point
- Hypertension: 1 point
- Age ≥ 75: 2 points; age 65–74: 1 point
- Diabetes mellitus: 1 point
- Stroke, transient ischemic attack, or thromboembolism: 2 points
- Vascular disease (prior myocardial infarction, peripheral arterial disease, or aortic plaque): 1 point
- Sex, female: 1 point
- Maximum score: 9 points.
Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk. Lip et al22 found that, in untreated patients with nonvalvular atrial fibrillation, rates of stroke ranged from 0 with a score of 0 to 15.2% per year with a score of 9 points.
In a large cohort with over 11,000 patient-years of follow-up, 98% of the thromboembolic events occurred in people with a CHA2DS2-VASc score of 2 or more. Moreover, more than 99% of patients with a score of less than 2 were free of stroke and thromboembolism.23
Compared with the CHADS2 score, CHA2DS2-VASc has superior negative predictive power. Of 1,084 patients from the European Heart Survey for Atrial Fibrillation, the newer scheme classified significantly fewer patients as being at either low risk (score of 0; 9% vs 20%) or intermediate risk (score of 1; 15% vs 35%).23 Though the overall rate of stroke was low, those categorized as being at low or intermediate risk by CHA2DS2-VASc had significantly fewer thromboembolic events than their counterparts according to CHADS2 (0.6% vs 3.3%).
Olesen et al24 similarly showed that in patients with a CHADS2 score of 0, reclassification by CHA2DS2-VASc yielded a range of annual stroke rates from 0.84% with a score of 0 up to 3.2% with a score of 3.
RISK-BASED ANTITHROMBOTIC THERAPY IN NONVALVULAR ATRIAL FIBRILLATION
The 2014 atrial fibrillation guidelines1 state that the decision to give antithrombotic therapy for atrial fibrillation should be individualized, based on the absolute and relative risks of stroke and bleeding, and ought to take into consideration the patient’s preferences. For patients with nonvalvular atrial fibrillation, selection of antithrombotic therapy should take into account the risk of thromboembolism determined by the CHA2DS2-VASc score and be irrespective of the pattern of atrial fibrillation (paroxysmal, persistent, or permanent). Antithrombotic therapy is similarly recommended for patients with atrial flutter, according to the same risk profile used for atrial fibrillation.
Studies have consistently shown24–27 that the risk of ischemic stroke without anticoagulation exceeds the risk of intracranial bleeding with anticoagulation in nearly all patients except those at lowest risk of thromboembolism. The CHA2DS2-VASc score better identified those at truly low risk, in whom treatment may offer more risk than benefit.24–27
The HAS-BLED score28 assigns points as follows:
- Hypertension (systolic blood pressure > 160 mm Hg): 1 point
- Abnormal renal function (dialysis, renal transplantation, or serum creatinine > 2.6 mg/mL) or liver function (cirrhosis, bilirubin more than two times the upper limit, or aminotransferase levels more than three times the upper limit): 1 or 2 points
- Stroke: 1 point
- Bleeding (prior major bleeding event or predisposition to bleeding): 1 point
- Labile international normalized ratio (INR) (supratherapeutic or time in therapeutic range < 60%): 1 point
- Elderly (age > 65): 1 point
- Drugs (antiplatelet, nonsteroidal anti-inflammatory) or alcohol (more than eight drinks per week): 1 or 2 points
- Maximum total: 9 points.
HAS-BLED is a practical and validated approach for estimating bleeding risk and is mentioned in the guidelines, but it is not recommended for use in guiding decisions about antithrombotic therapy. Specifically, it should not be used to exclude patients, but rather to identify those at high risk (score ≥ 3) who may require closer observation and more attentive monitoring of the INR.
ANTITHROMBOTIC THERAPY
Antithrombotic agents available for use in the United States include antiplatelet drugs (eg, aspirin and clopidogrel) and anticoagulants (unfractionated heparin and low-molecular-weight heparin, vitamin K antagonists such as warfarin, and direct thrombin and factor Xa inhibitors). Anticoagulation has been shown in randomized controlled trials to be superior to both placebo and antiplatelet agents used either alone or in combination.29
Aspirin has been downgraded
Aspirin has been compared with placebo in seven randomized controlled trials. Only the original SPAF study, in which aspirin 325 mg/day was used, found that it was beneficial. This result alone accounted for the 19% reduction in relative risk (95% CI 1%–35%, P < .05) in a meta-analysis performed by Hart et al.29 Even when combined with clopidogrel 75 mg/day, aspirin 75 to 100 mg/day is still inferior to warfarin.5 While dual antiplatelet therapy resulted in a 28% relative reduction in thromboembolism (95% CI 17%–38%, P < .01) compared with aspirin alone, major bleeding significantly increased by 57% (95% CI 29%–92%, P < .01).
Although aspirin may be beneficial, differences among patients may influence its efficacy. It may be more effective in preventing noncardioembolic stroke, particularly in diabetic and hypertensive patients.30,31 To date, aspirin has not been shown to be beneficial in low-risk populations.
The 2014 guidelines downgraded the recommendation for aspirin therapy. For patients at low risk and for some at intermediate risk, it is permissible to forgo therapy altogether, including aspirin.1
ORAL ANTICOAGULANTS
The rest of this paper reviews the oral anticoagulants that are approved for reducing the risk of thromboembolism in atrial fibrillation, focusing on each agent’s mechanism of action, pharmacokinetics, clinical efficacy, and safety.
WARFARIN, A VITAMIN K ANTAGONIST
Warfarin inhibits synthesis of vitamin K-dependent clotting factors (ie, factors II, VII, IX, and X) and proteins C and S by inhibiting the C1 subunit of vitamin K epoxide reductase, thereby interfering with production of vitamin K1 epoxide and consequent regeneration of vitamin K.
Pharmacokinetics. Warfarin is nearly completely absorbed after oral administration. Its anticoagulant effect can be seen within 24 hours of administration, but its peak effect is typically apparent only after 72 hours. Elimination occurs predominantly through metabolism by cytochrome P450 enzymes, principally CYP2C9. Its effective half-life ranges from 20 to 60 hours, with a mean of 40 hours.32
Warfarin’s effect, dosage, and bleeding risk are influenced by multiple factors, including vitamin K-containing foods such as green leafy vegetables, medications that either inhibit or induce hepatic cytochrome P450 enzymes, and polymorphisms in the VKORC1 and CYP2C9 genes.32
Reversal. Warfarin’s anticoagulant effect is reversed with vitamin K, but this reversal may not become apparent for 6 to 24 hours. In contrast, fresh-frozen plasma and prothrombin protein concentrate, which contain clotting factors, reverse warfarin immediately. Currently, a three-factor prothrombin protein concentrate (factors II, IX, and X) and a four-factor concentrate (factors II, VII, IX, and X plus proteins C and S) are available in the United States. Although prothrombin protein concentrate works rapidly and has a lower volume of administration, available data do not indicate it is clinically superior to fresh-frozen plasma.33,34 The ongoing randomized PROTECT trial (NCT00618098), comparing fresh-frozen plasma and four-factor prothrombin protein concentrate for reversal of vitamin K antagonist therapy, may provide further insight.
Efficacy and safety. Randomized controlled trials in patients with nonvalvular atrial fibrillation have shown that warfarin (in doses adjusted to maintain an INR greater than 2) is highly efficacious in preventing systemic embolism, with a relative risk reduction of 61% (95% CI 47%–71%, P < .05) compared with placebo.29,35 An INR of 2 to 3 is recommended for patients with nonvalvular atrial fibrillation, and those with atrial fibrillation and either a bioprosthetic valve or rheumatic heart disease. In contrast, an INR of 2.5 to 3.5 is recommended for patients with atrial fibrillation and mechanical valves in the aortic or mitral positions.1,36
Stroke prevention with warfarin is most effective when the achieved mean time in the therapeutic range is at least 70%. The risk of intracranial hemorrhage increases significantly at INRs higher than 3. An INR of 2 to 3 offers maximum protection with minimal risk of bleeding.37,38 Systematic follow-up of patients through anticoagulation clinics produces better compliance and control and is encouraged.
TARGET-SPECIFIC ORAL ANTICOAGULANTS
Although effective, warfarin requires frequent monitoring and dosage adjustment, has a delayed onset and protracted offset, and interacts with commonly consumed vitamin K–containing foods and frequently used drugs. These drawbacks prompted evaluation of existing antiplatelet agents, in combination or in conjunction with lower adjusted or fixed-dose warfarin. These regimens proved inferior,39–42 spurring interest in developing alternative oral anticoagulants.
TSOACs act by directly inhibiting thrombin (factor IIa) or by reducing thrombin production indirectly by inhibiting factor Xa. Three TSOACs are approved. Each was compared with adjusted-dose warfarin in randomized controlled trials.
Dabigatran
Dabigatran etexilate was the first TSOAC approved in the United States.
Pharmacokinetics. Dabigatran etexilate has a bioavailability of 3% to 7% after oral administration. Its absorption is enhanced in an acidic gastric environment and is limited by P-glycoprotein-facilitated transport out of enterocytes. Dabigatran etexilate is hydrolyzed to its active metabolite dabigatran, which directly inhibits thrombin. Maximal plasma drug concentration and peak anticoagulant effect are achieved within 0.5 to 2 hours after administration.
Dabigatran is predominantly excreted by the kidneys, and has a half-life of 12 to 17 hours in patients with normal renal function. The half-life extends to 27 hours in those with moderately severe renal impairment (creatinine clearance 15–30 mL/min). The recommended dose of 150 mg twice daily should be reduced to 75 mg twice daily in patients with a creatinine clearance of 15 to 30 mL/min. This drug is contraindicated in patients with a creatinine clearance less than 15 mL/min.43,44
Efficacy. The Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial45 randomly assigned 18,113 patients with nonvalvular atrial fibrillation at risk of thromboembolism (mean CHADS2 score 2.1) to receive either dabigatran (either 150 mg twice daily or 110 mg twice daily) or warfarin (adjusted to an INR of 2.0 to 3.0). Of note, the lower approved dose of dabigatran (75 mg twice daily) was not tested in RE-LY.
At 2 years, higher-dose dabigatran was significantly more effective than both warfarin (RR 0.65, 95% CI 0.52–0.81, P < .05) and lower-dose dabigatran (RR 0.73, 95% CI 0.58–0.91, P < .05) in reducing the rate of systemic embolic events.
The risk of combined major bleeding events was no different with higher-dose dabigatran than with warfarin (RR 0.93, 95% CI 0.81–1.07, P < .05), but the rate of hemorrhagic stroke was significantly less with dabigatran than with warfarin (RR 0.26, 95% CI 0.14–0.49, P < .05). Higher rates of major gastrointestinal bleeding and dyspepsia occurred with dabigatran.
Concern about the safety of dabigatran was raised when post hoc evaluation of RE-LY found a higher incidence of myocardial infarction with dabigatran than with warfarin (RR 1.38, 95% CI 1–1.91, P = .048).46 Corroborating data were reported by Uchino and Hernandez,47 comparing dabigatran with either warfarin or low-molecular-weight heparin. However, without directly comparing dabigatran and placebo, it is unclear whether the small increase in myocardial infarction reflects a direct effect of dabigatran or absence of a protective effect of warfarin or low-molecular-weight heparin.
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor that blocks the amplified burst of thrombin production and in turn inhibits platelet aggregation and thrombus formation.
Pharmacokinetics. Rivaroxaban’s oral bioavailability is 80% to 100% after a single 15- or 20-mg dose taken with food. Its maximal anticoagulant effect is achieved within 2 hours. Two-thirds of the active drug is metabolized by either CYP450-dependent (CYP3A4, 2J2) or CYP-independent mechanisms; the inactive drug is then excreted in the urine and feces. The remaining, active drug is removed by the kidneys using the P-glycoprotein transporter.
The half-life of rivaroxaban is 5 to 9 hours. The recommended dosage of 20 mg daily should be reduced to 15 mg daily if the creatinine clearance rate is 30 to 50 mL/min, or to 10 mg if the creatinine clearance rate is 15 to 30 mL/min. Rivaroxaban is contraindicated in patients whose creatinine clearance rate is less than 15 mL/min.48–52
Efficacy and safety. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF),53 14,264 at-risk patients with nonvalvular atrial fibrillation (mean CHADS2 score 3.5) were randomly assigned to receive either rivaroxaban 20 mg daily (or 15 mg daily if their creatinine clearance was 30–49 mL/min; the lowest dose of rivaroxaban, 10 mg, was not studied in this trial) or warfarin (target INR 2.0–3.0). Outcomes with rivaroxaban compared with warfarin:
- Systemic embolism:
HR 0.79, 95% CI 0.66–0.96, P < .01, noninferiority - Total bleeding: no difference
- Intracranial bleeding:
HR 0.67, 95% CI 0.47–0.93, P = .02 - Fatal bleeding:
HR 0.50, 95% CI 0.31–0.79, P = .003 - Major gastrointestinal bleeding:
3.2% vs 2.2%, P < .001.
Apixaban
Apixaban is also a direct factor Xa inhibitor.
Pharmacokinetics. Apixaban’s oral bioavailability is 50%, with maximal blood concentration achieved at 3 to 4 hours. One-quarter of the drug is metabolized via CYP3A4. The remaining active drug is excreted by the kidneys and biliary/intestinal system via the P-glycoprotein transporter. Apixaban’s half-life is 9 to 14 hours.
The recommended dosage is 5 mg twice daily, but it should be reduced to 2.5 mg twice daily if at least two of the following characteristics are present: age 80 or older, weight 60 kg or less, and serum creatinine 1.5 mg/dL or more.54,55
Efficacy and safety. The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial56 enrolled 18,201 patients with nonvalvular atrial fibrillation (mean CHADS2 score 2.1) randomly assigned to receive either apixaban (5 mg twice daily with dosage reduction to 2.5 mg twice daily as noted above) or warfarin (target INR 2.0–3.0).
Compared with warfarin, apixaban was associated with lower risk of:
- Systemic embolism
(HR 0.79, 95% CI 0.66–0.95, P = .01) - Major bleeding
(HR 0.69, 95% CI 0.60–0.80, P < .001) - Intracranial hemorrhage
(HR 0.42, 95% CI 0.30–0.58, P < .001) - All-cause mortality
(HR 0.89 95% CI 0.80–0.99, P = .047).
Drug interactions with the novel oral anticoagulants
TSOACs were developed with the intent to avoid many of the shortcomings of warfarin. Each has a broader therapeutic window and a rapid onset of action, enabling fixed dosing without need for serial monitoring. Compared with warfarin, they have significantly fewer dietary and drug interactions.
Nonetheless, drug interactions do exist and are important to recognize (Tables 1–3). These primarily result from inhibition or induction of cytochrome P450 enzyme activity or P-glycoprotein transporter action, involved in metabolism and elimination of active drug.
Reversibility of the target-specific oral anticoagulants
While the anticoagulant effects of warfarin can be reversed by vitamin K, fresh-frozen plasma, and prothrombin complex concentrate, TSOACs have no currently approved antidotes. Management of bleeding due to these agents was recently reviewed in this journal by Fawole et al.57
Several nonspecific hemostatic agents have been suggested, including recombinant factor VIIa or prothrombin complex concentrates. The anticoagulant effect of rivaroxaban has been shown to be reversed by prothrombin complex concentrate in vitro; clinical effect has not been demonstrated.58 PRT06445 (andexanet alfa), a recombinant protein antidote specific for factor Xa inhibitors, has entered clinical studies, with a phase 2 trial reporting high reversing capability for apixaban.59
Unlike rivaroxaban and apixaban, which are highly bound to plasma protein, dabigatran can be effectively removed with hemodialysis. Liesenfeld et al60 showed that longer dialysis duration was the most relevant variable for reducing dabigatran plasma levels. Current clinical experience is limited, and standard recommendations and formal guidance are lacking.
Switching oral anticoagulants
Suggested approaches for switching between anticoagulants are listed in Table 4.61
CHOOSING ANTITHROMBOTIC THERAPY
In valvular atrial fibrillation: warfarin
Anticoagulation with warfarin is advised for valvular atrial fibrillation. Patients with bioprosthetic heart valves or rheumatic valvular disease were not evaluated in randomized controlled trials of TSOACs. Dabigatran in particular is contraindicated in patients with mechanical heart valves, as the Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement (RE-ALIGN)62 found higher rates of stroke, valve-related thrombosis, and myocardial infarction in patients receiving dabigatran.
In nonvalvular atrial fibrillation
According to the 2014 guidelines,1 oral anticoagulation is preferred in all patients with nonvalvular atrial fibrillation but those at lowest risk (CHA2DS2-VASc = 0).
Experience with TSOACs is lacking in patients with end-stage kidney disease (creatinine clearance < 15 mL/min), and warfarin is advised in this group.
TSOACs are recommended in patients with nonvalvular atrial fibrillation in whom therapeutic INR levels cannot be maintained with warfarin. For most patients with nonvalvular atrial fibrillation, TSOACs are an option equivalent to warfarin. Anticoagulant choice is largely driven by dosing convenience, out-of-pocket cost for treatment with a TSOAC, and ready availability of antidotes for warfarin in case of bleeding (Tables 5 and 6).
In patients with nonvalvular atrial fibrillation, TSOACs are as effective as warfarin in preventing systemic thromboembolism, and some of them have been shown to be superior in terms of lower rates of ischemic stroke (dabigatran), systemic embolism (apixaban), and mortality (apixaban; trend for dabigatran). All TSOACs demonstrate modestly favorable bleeding risk profiles compared with warfarin, with lower risk of intracranial hemorrhage. Potential differences in efficacy and safety among TSOACs are unknown since there have been no randomized direct comparisons between them. A summary of landmark trial results and assessment of the advantages and disadvantages of each are listed in Table 7.
Two groups of patients with nonvalvular atrial fibrillation warrant special consideration:
Patients with hypertrophic cardiomyopathy. There are no randomized controlled trials of anticoagulation therapy in patients with hypertrophic cardiomyopathy; however, because of their high risk of thromboembolism, anticoagulation is indicated irrespective of the CHA2DS2-VASc score. TSOACs are an option as an alternative to warfarin.
Patients with coronary artery disease and an indication for antiplatelet therapy. In this group the decision for concurrent anticoagulation is guided by the CHA2DS2-VASc score. For patients who have intracoronary stents, dual antiplatelet therapy is the standard of care for reducing risk of cardiovascular events after stent implantation.63 When triple therapy (ie, two antiplatelet drugs and an anticoagulant) is indicated, such as after intracoronary stent placement, the guidelines suggest trying to minimize the duration of triple therapy. For instance, a bare-metal stent may be preferred. Alternatively, after coronary revascularization, it may be reasonable to use clopidogrel 75 mg daily with an oral anticoagulant and to omit aspirin.
Interrupting and bridging anticoagulation
Patients with atrial fibrillation often require suspension of anticoagulation, most commonly before an elective invasive procedure. The duration of interruption, timing of resumption, and need for bridging anticoagulation are guided by clinical judgment, which considers risk of thromboembolism and severity of procedure-related bleeding risk.
In general, if therapy needs to be interrupted, it should be restarted as soon as possible. Short-term interruption does not seem to be associated with clinically significant risk of thromboembolic events, whereas postoperative heparin bridging therapy increases the risk of hematoma with implantation of a cardiac electronic device.64,65
To date, evidence is lacking to advise upon periprocedure bridging anticoagulation. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) study (NCT00786474)— enrolling chronically anticoagulated patients undergoing an invasive procedure to randomly receive placebo or bridging low-molecular-weight heparin—may provide guidance.
Currently, it is common practice in low-risk patients undergoing an invasive procedure with significant bleeding risk to interrupt anticoagulation for up to 1 week without bridging. Warfarin is typically held 3 to 5 days, while TSOACs are held for 24 hours if renal function is preserved or up to 2 to 3 days if renal function is severely impaired (creatinine clearance 15–30 mL/min). If complete hemostasis is necessary, it could be confirmed by a normalized INR (for warfarin), activated partial thromboplastin time (dabigatran), or prothrombin time (apixaban or rivaroxaban).
For patients at high risk (valvular atrial fibrillation or CHA2DS2-VASc ≥ 2), bridging with unfractionated heparin or low-molecular-weight heparin during periods of subtherapeutic anticoagulation is common. Alternatively, it is becoming increasingly common to perform cardiac electronic device implantation, catheter ablation, and coronary angiography and intervention without interrupting anticoagulation.66–72
Recently, concern has been raised over a possible increase in thromboembolism upon discontinuation of rivaroxaban and apixaban. ROCKET-AF reported a spike in thrombotic events in the rivaroxaban-treated group at the end of the trial (HR 1.50, 95% CI 1.05–2.15, P = .026). This raised concern for a possible “rebound” effect upon drug cessation. Yet a post hoc analysis of ROCKET-AF demonstrated that events clustered in the rivaroxaban-treated cohort who completed the study and were transitioning to open-label warfarin, and this alone accounted for the rise in stroke occurrence. In contrast, there was no increase in the cohort of patients treated with rivaroxaban who either temporarily interrupted or permanently discontinued the drug.73 The authors concluded that increased stroke was the consequence of transiently interrupted anticoagulation, rather than a rebound prothrombotic effect. Similar results were reported in ARISTOTLE.
Another possibility is that, during the transition to warfarin therapy, transient hypercoagulability could be a function of warfarin. Azoulay et al74 observed in a large cohort that warfarin was associated with a 71% increased risk of stroke in the first 30 days after initiation, compared with decreased risk thereafter. Nevertheless, there is now a black- box warning recommendation for all three TSOACs that if discontinuation is required for a reason other than pathological bleeding, bridging with another anticoagulant should at least be considered.
The perioperative management of the TSOACs was recently reviewed in this journal by Anderson et al.75
WEIGHING THE RISKS OF STROKE AND BLEEDING
Stroke is the most feared complication in patients with atrial fibrillation. Risk reduction is an important goal in management, yet decisions for individuals must take into account both stroke and bleeding risks related to antithrombotic therapy.
The 2014 guidelines1 differ from past versions. First, they endorse the use of CHA2DS2-VASc for categorizing stroke risk in patients with nonvalvular atrial fibrillation. This in turn guides antithrombotic therapy. This scheme effectively identifies patients at very low risk of stroke (men with a score of 0, women with a score of 0 or 1), in whom it is reasonable to omit antithrombotic therapy. For all patients with valvular heart disease or hypertrophic cardiomyopathy, unless bleeding risk is prohibitive, anticoagulation is recommended irrespective of the CHA2DS2-VASc score. Second, they incorporate the TSOACs, which offer convenience and improved safety in select patients.
While the guidelines mention the potential relevance of subclinical atrial tachyarrhythmias as they pertain to stroke risk, there is no specific recommendation as to their management. We do take into consideration the finding of atrial high-rate events (≥ 180 bpm, ≥ 6 minutes in duration) diagnostically confirmed by cardiac implantable electronic devices or telemetric monitoring, particularly in patients with a clinical profile of high stroke risk. In addition, atriopathy with increased left atrial size and renal insufficiency, as discussed in this review, appear to correlate with greater risk of thromboembolism, yet neither is a component of the stroke risk scheme endorsed by the guidelines.
Other risk factors, some unknown to us, undoubtedly exist. Again, our empiric judgment is to at least consider these nontraditional risk factors while guided primarily by the CHA2DS2-VASc score when assessing stroke risk in patients with atrial fibrillation.
The goal in managing patients with atrial fibrillation is to balance thromboembolic risk reduction with the risk of bleeding associated with antithrombotic therapy.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000; 35:183–187.
- Hohnloser SH, Pajitnev D, Pogue J, et al; ACTIVE W Investigators. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007; 50:2156–2161.
- Healey JS, Connolly SJ, Gold MR, et al; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366:120–129.
- Glotzer TV, Hellkamp AS, Zimmerman J, et al; MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003; 107:1614–1619.
- Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009; 2:474–480.
- Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:301–307.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001; 104:2517–2524.
- Go AS, Fang MC, Udaltsova N, et al; ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119:1363–1369.
- Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol 2011; 6:2599–2604.
- Vázquez E, Sánchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 2000; 140:886–890.
- The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998; 128:639–647.
- The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med 1992; 116:6–12.
- Osranek M, Bursi F, Bailey KR, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J 2005; 26:2556–2561.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med 2003; 163:936–943.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010; 41:2731–2738.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost 2012; 107:1172–1179.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011; 106:739–749.
- Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125:2298–2307.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med 1997; 157:1237–1240.
- Miller VT, Rothrock JF, Pearce LA, Feinberg WM, Hart RG, Anderson DC. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology 1993; 43:32–36.
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005; 165:1095–1106.
- Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery 1999; 45:1113–1119.
- Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006; 37:1465–1470.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost 2011; 106:968–977.
- Odén A, Fahlén M, Hart RG. Optimal INR for prevention of stroke and death in atrial fibrillation: a critical appraisal. Thromb Res 2006; 117:493–499.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996; 348:633–638.
- Gulløv AL, Koefoed BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med 1998; 158:1513–1521.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012; 125:669–676.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005; 78:412–421.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008; 24:2757–2765.
- Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009; 37:1056–1064.
- Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov 2011; 10:61–75.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 2011; 31:478–492.
- Carreiro J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs 2008; 17:1937–1945.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Liesenfeld KH, Staab A, Härtter S, Formella S, Clemens A, Lehr T. Pharmacometric characterization of dabigatran hemodialysis. Clin Pharmacokinet 2013; 52:453–462.
- MPR. Monthly Prescribing Reference. Anticoagulant dosing conversions. August 18, 2014. www.empr.com/anticoagulant-dosing-conversions/article/194271/. Accessed December 11, 2014.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Tischenko A, Gula LJ, Yee R, Klein GJ, Skanes AC, Krahn AD. Implantation of cardiac rhythm devices without interruption of oral anticoagulation compared with perioperative bridging with low-molecular weight heparin. Am Heart J 2009; 158:252–256.
- Robinson M, Healey JS, Eikelboom J, et al. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol 2009; 32:378–382.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Ahmed I, Gertner E, Nelson WB, et al. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm 2010; 7:745–749.
- Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm 2011; 8:536–540.
- Jamula E, Lloyd NS, Schwalm JD, Airaksinen KE, Douketis JD. Safety of uninterrupted anticoagulation in patients requiring elective coronary angiography with or without percutaneous coronary intervention: a systematic review and metaanalysis. Chest 2010; 138:840–847.
- Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost 2008; 6:1615–1621.
- Korantzopoulos P, Letsas KP, Liu T, Fragakis N, Efremidis M, Goudevenos JA. Anticoagulation and antiplatelet therapy in implantation of electrophysiological devices. Europace 2011; 13:1669–1680.
- Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010; 121:2550–2556.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Azoulay L, Dell’aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2013; Dec 18 [Epub ahead of print].
- Anderson M, Hassell KL, Trujillo TC, Wolfe B. When patients on target-specific oral anticoagulants need surgery. Cleve Clin J Med 2014; 81:629–639.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–1022.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000; 35:183–187.
- Hohnloser SH, Pajitnev D, Pogue J, et al; ACTIVE W Investigators. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007; 50:2156–2161.
- Healey JS, Connolly SJ, Gold MR, et al; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366:120–129.
- Glotzer TV, Hellkamp AS, Zimmerman J, et al; MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003; 107:1614–1619.
- Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009; 2:474–480.
- Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:301–307.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001; 104:2517–2524.
- Go AS, Fang MC, Udaltsova N, et al; ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119:1363–1369.
- Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol 2011; 6:2599–2604.
- Vázquez E, Sánchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 2000; 140:886–890.
- The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998; 128:639–647.
- The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med 1992; 116:6–12.
- Osranek M, Bursi F, Bailey KR, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J 2005; 26:2556–2561.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med 2003; 163:936–943.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010; 41:2731–2738.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost 2012; 107:1172–1179.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011; 106:739–749.
- Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125:2298–2307.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med 1997; 157:1237–1240.
- Miller VT, Rothrock JF, Pearce LA, Feinberg WM, Hart RG, Anderson DC. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology 1993; 43:32–36.
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005; 165:1095–1106.
- Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery 1999; 45:1113–1119.
- Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006; 37:1465–1470.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost 2011; 106:968–977.
- Odén A, Fahlén M, Hart RG. Optimal INR for prevention of stroke and death in atrial fibrillation: a critical appraisal. Thromb Res 2006; 117:493–499.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996; 348:633–638.
- Gulløv AL, Koefoed BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med 1998; 158:1513–1521.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012; 125:669–676.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005; 78:412–421.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008; 24:2757–2765.
- Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009; 37:1056–1064.
- Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov 2011; 10:61–75.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 2011; 31:478–492.
- Carreiro J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs 2008; 17:1937–1945.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Liesenfeld KH, Staab A, Härtter S, Formella S, Clemens A, Lehr T. Pharmacometric characterization of dabigatran hemodialysis. Clin Pharmacokinet 2013; 52:453–462.
- MPR. Monthly Prescribing Reference. Anticoagulant dosing conversions. August 18, 2014. www.empr.com/anticoagulant-dosing-conversions/article/194271/. Accessed December 11, 2014.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Tischenko A, Gula LJ, Yee R, Klein GJ, Skanes AC, Krahn AD. Implantation of cardiac rhythm devices without interruption of oral anticoagulation compared with perioperative bridging with low-molecular weight heparin. Am Heart J 2009; 158:252–256.
- Robinson M, Healey JS, Eikelboom J, et al. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol 2009; 32:378–382.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Ahmed I, Gertner E, Nelson WB, et al. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm 2010; 7:745–749.
- Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm 2011; 8:536–540.
- Jamula E, Lloyd NS, Schwalm JD, Airaksinen KE, Douketis JD. Safety of uninterrupted anticoagulation in patients requiring elective coronary angiography with or without percutaneous coronary intervention: a systematic review and metaanalysis. Chest 2010; 138:840–847.
- Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost 2008; 6:1615–1621.
- Korantzopoulos P, Letsas KP, Liu T, Fragakis N, Efremidis M, Goudevenos JA. Anticoagulation and antiplatelet therapy in implantation of electrophysiological devices. Europace 2011; 13:1669–1680.
- Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010; 121:2550–2556.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Azoulay L, Dell’aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2013; Dec 18 [Epub ahead of print].
- Anderson M, Hassell KL, Trujillo TC, Wolfe B. When patients on target-specific oral anticoagulants need surgery. Cleve Clin J Med 2014; 81:629–639.
KEY POINTS
- Valvular atrial fibrillation poses a high risk of systemic embolization, particularly stroke, and nearly all patients who have valvular atrial fibrillation need anticoagulation therapy with warfarin.
- Nonvalvular atrial fibrillation poses a somewhat lower risk. The new guidelines propose a new risk-classification scheme, called CHA2DS2-VASc; patients at very low risk of stroke may be able to forgo anticoagulation.
- The new guidelines downplay the role of aspirin, although it is still an option in some situations.
- Several novel oral anticoagulants have been approved in the past few years for thromboprophylaxis in patients with nonvalvular atrial fibrillation.
Quitting smoking: Still a challenge, but newer tools show promise
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
KEY POINTS
- Nicotine dependence is a life-threatening, biochemically based disease, driven by changes in midbrain receptors and reward mechanisms.
- The state of the art in smoking cessation involves encouragement, persistence, and evidence-based pharmacotherapy.
- Physicians should be assertive in addressing nicotine dependence, approaching patients with encouragement to quit, consistent monitoring and support, telephone “quit lines,” and counseling, as well as persistence and optimism. The combination of proactive, engaged, brief counseling and pharmacotherapy will yield the best results.