User login
Teamwork makes the dream work – maximizing the relationship between physicians and advanced practice providers
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Coding and payment changes could hit GIs in 2021
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at [email protected] .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).
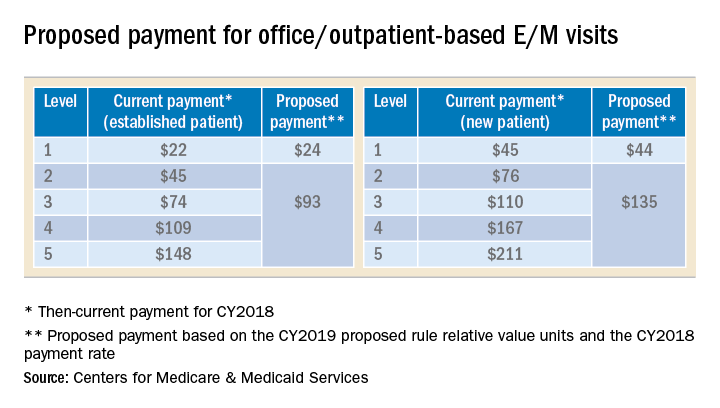
In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at [email protected] .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).

In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at [email protected] .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).

In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
Implementation of a population-based cirrhosis identification and management system
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).
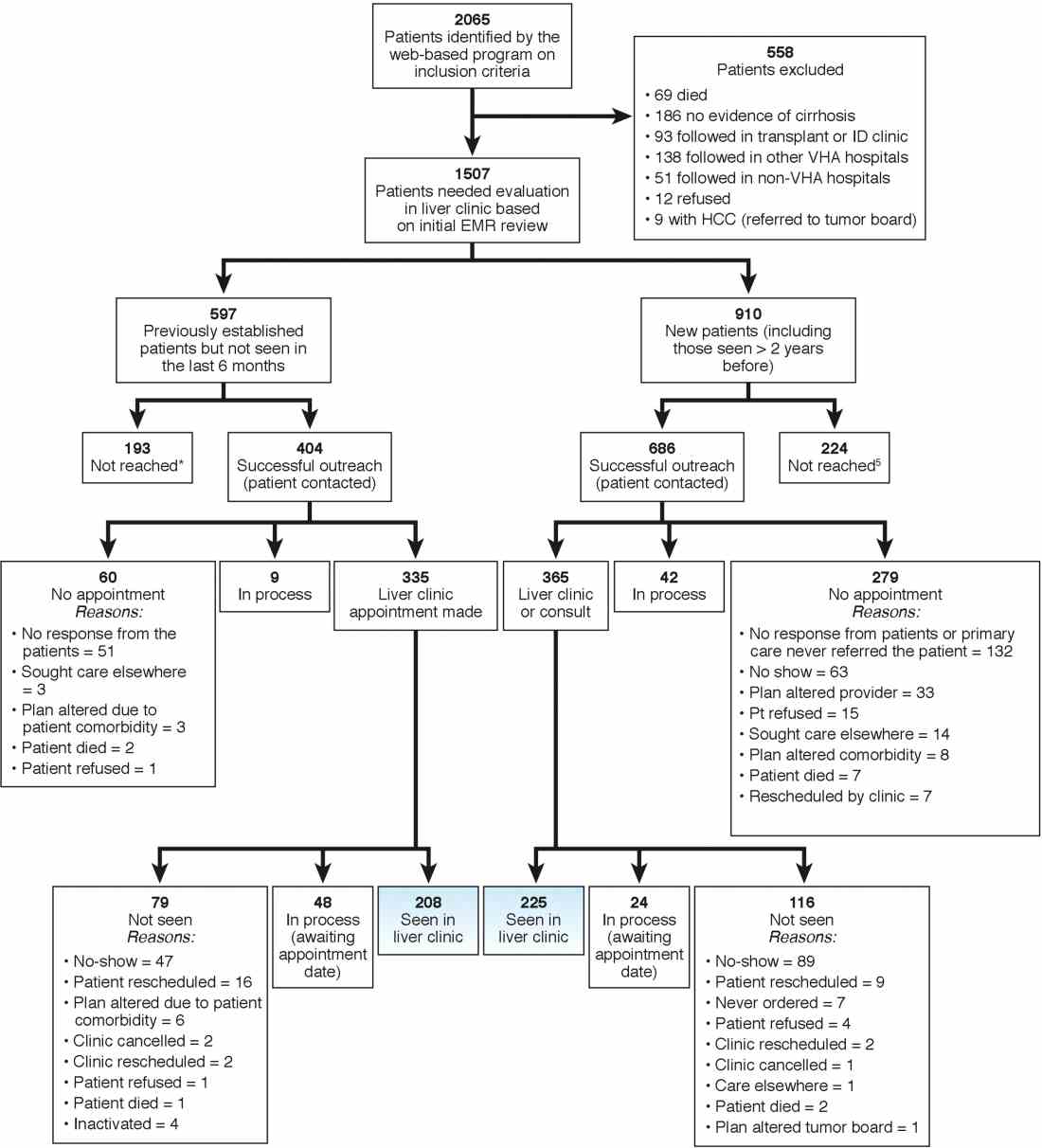
We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Training the endo-athlete – an update in ergonomics in endoscopy
As physicians, we work hard to take excellent care of our patients. Years of thoughtful practice and continuous learning allow us to deliver the best that medicine can provide. We often take poor care of ourselves, which can lead to burnout and physical injuries. As gastroenterologists, we spend substantial time performing endoscopic procedures that require repetitive motions such as flexion and extension of the wrist and fingers and torsional movements of the right hand, which may lead to overuse injuries. The volume of endoscopic procedures performed by a typical gastroenterologist has increased significantly in the past 20 years. Moreover, experts predict that by 2020 we will have too few endoscopists to meet clinical demands.1 It is imperative that we do whatever possible to ensure overuse injuries do not prematurely prevent us from providing much-needed care. One way to achieve this goal is to focus on ergonomics. The study of ergonomics, derived from the Greek words ergo (work) and nomos (law), seeks to optimize the interface between the worker, the equipment, and the work environment. This article reviews basic ergonomic principles that endoscopists can apply today and possible innovations that may improve endoscopic ergonomics in the future.
Breadth of the problem
Examinations of injuries related to endoscopy are limited to survey-based and small controlled studies with a 39%-89% overall prevalence of pain or musculoskeletal injuries reported.2 In a survey of 684 American Society for Gastrointestinal Endoscopy members examining injury prevalence and risk factors,3 53% experienced an injury believed to be definitely or probably related to endoscopy. Risk factors included higher procedure volume (more than 20 cases/wk), greater number of hours spent performing endoscopy (more than 16 h/wk), and total number of years spent performing endoscopy2,4. Community practitioners reported injuries at higher rates than those in an academic center. Other suggested but unproven risk factors include age5, sex, hand size, room design, and level of training in ergonomics and endoscopy2. Injuries can be severe and may lead to work load reduction, missed days of work3-5, reduction of activities outside of work, and long-term disability2.
Most surveys reflect symptoms localized to the back, neck, shoulder, elbow, hands/fingers, and thumbs likely from overuse causing strain and soft-tissue microtrauma6. Without time to heal, these injuries may lead to connective tissue weakening and permanent damage. Repetitive hand movements in endoscopy include left thumb abduction, flexion, and extension while manipulating dials and right wrist flexion, extension, and deviation from torqueing the insertion tube. The use of torque is a necessary part of successful colonoscopy; during scope reduction and maneuvering through the sigmoid colon, torque forces and forces applied against the wall of the colon are highest. When of sufficient magnitude and duration, these forces are associated with an increased risk of thumb and wrist injuries. These movements may result in “endoscopist’s thumb“ (i.e., de Quervain’s tenosynovitis) and carpal tunnel syndrome2. Prolonged standing and lead aprons are implicated in back and neck injuries;2,7-9 two-piece aprons,7,10 and antifatigue mats7 are recommended to decrease pressure on the lumbar and cervical disks as well as delay muscle fatigue.
Position of equipment
Endoscopist and patient positioning can be optimized. In the absence of direct data about ergonomics in endoscopy, we rely on surgical laparoscopy data.11,12These studies show that monitors placed directly in front of surgeons at eye-level (rather than off to the side or at the head of the bed) reduced neck and shoulder muscle activity. Monitors should be placed with a height 20 cm lower than the height of the surgeon (endoscopist), suggesting that optimized monitor height should be at eye-level or lower to prevent neck strain. Estimates based on computer simulation and laparoscopy practitioners show that the optimal distance between the endoscopist/surgeon and a 14” monitor is between 52 and 182 cm, which allows for the least amount of image degradation. Many modern monitors are larger (19”-26”), which allows for placement farther from the endoscopist without losing image quality. Bed height affects both spine and arm position; surgical data again suggest that optimal bed height is between elbow height and 10 cm below elbow height.
Immediate practice points
Since poor monitor placement was identified as a major risk factor for musculoskeletal injuries, the first steps in our endoscopy unit were to improve our sightlines. Our adjustable monitors previously were locked into a specific height, and those same monitors now easily are adjusted to heights appropriate to the endoscopist. Our practice has endoscopists from 61” to 77” tall, meaning we needed monitors that could adjust over a 16” height. When designing new endoscopy suites, monitors that adjust from 93 to 162 cm would accommodate the 5th percentile of female height to the 95th percentile of male height. We use adjustable-height beds; a bed that adjusts between 85 and 120 cm would accommodate the 5th percentile of female height to the 95th percentile of male height.
We also moved our monitors to be closer to the opposite side of the bed to accommodate the 3’ to 6’ appropriate to our 16” screens. Our endoscopy suites have cushioned washable mats placed where endoscopists stand that allow for slight instability of the legs, leading to subtle movements of the legs and increased blood flow to reduce foot and leg injuries. We attempt an athletic stance (the endo-athlete) during endoscopy: shoulders back, chest out, knees bent, and feet hip-width apart pointed at the endoscopy screen (Figure 1). These mats help prevent pelvic girdle twisting and turning that may lead to awkward positions and instead leave the endoscopist in an optimized position for the procedure. We encourage endoscopists to keep the scope in the most neutral position possible to reduce overuse of torque and the forces on the wrists and thumbs. When possible, we use two-piece lead aprons for procedures that require fluoroscopy, which transfers some of the weight of the apron from the shoulders to the hips and reduces upper-body strain. Optimization of the room for therapeutic procedures is even more important (with dual screens both fulfilling the criteria we have listed earlier) given the extra weight of the lead on the body. We suggest that, if procedures are performed in cramped endoscopy rooms, placement of additional monitors can help alleviate neck strain and rotation.
Working with our nurses was imperative. We first had our nurses watch videos on appropriate ergonomics in the endoscopy suite. Given that endoscopists usually are concentrating their attention on the screens in the suite, we tasked our nurses to not only monitor our patients, but also to observe the physical stance of the endoscopists. Our nurses are encouraged to help our endoscopists focus on their working stance: the nurses help with monitor positioning, and verbal cues when endoscopists are contorting their bodies unnaturally. This intervention requires open two-way communication in the endoscopy suite where safety of both the patient and staff is paramount. We are fortunate to be at an institution that trains fellows; we have two endoscopists in the suite at any time, which allows for additional two-way feedback between fellows and attendings to improve ergonomic positioning.
We also encourage some preventative exercises of the upper extremities to reduce pain and injuries. Stretches should emphasize finger, wrist, forearm, and shoulder flexion and extension. Even a minute of stretching between procedures allows for muscle relaxation and may lead to a decrease in overuse injuries. Adding these elements may seem inefficient and unnecessary if you have never had an injury, but we suggest the following paradigm: think of yourself as an endo-athlete. Similar to an athlete, you have worked years to gain the skills you possess. Taking a few moments to reduce your chances of a career-slowing (or career-ending) injury can pay long-term dividends.
Future remedies
Although there have been substantial advances in endoscopic imaging technology, the process of endoscope rotation and tip deflection has changed little since the development of flexible endoscopy. A freshman engineering student tasked with designing a device to navigate, examine, and provide therapy in the human colon likely would create a device that does not resemble the scope that we use daily to accomplish the task. Numerous investigators currently are working on novel devices designed to examine and deliver therapy to the digestive tract. These devices may diminish an endoscopist’s injury risk through the use of better ergonomic principles. This section is not intended to be a comprehensive review and is not an endorsement of any particular product. Rather, we hope it provides a glimpse into a possible future.
Reducing gravitational load
The concept of a mechanical device to hold some or all of the weight of the endoscope was first published in 197413. Since then, a number of products have been described for this purpose.14-17 In general, these consist of a simple metal tube with a hemicylindrical plastic clip, similar to a microphone stand, or a yoke/strap with a plastic scope holder in the front akin to what a percussionist in a marching band might wear. For a variety of reasons, including limited mobility and issues with disinfection, these devices have not gained traction.
Novel control mechanisms
Some of the largest forces on the endoscopist relate to moving the wheels on the scope head to effect tip deflection via a cable linkage. Because the wheels rotate only in one axis, the options for altering and adjusting load are few. One proposed solution is the use of a system with a fully detachable endoscope handle with a joystick style control deck (E210; Invendo Medical, Kissing, Germany). The control deck uses electromechanical assistance — as opposed to pure mechanical force — to transmit energy to the shaft of the instrument. Such assistive technologies have the potential to decrease injuries by decreased load, particularly on the carpometacarpal joint. Other devices seek to decrease the need for torque and high-load tip deflection though the use of self-propelled, disposable colonoscopes that use an aviation-style joystick (Aer-o-scope; GI View, Kissing, Germany). Although interesting and potentially useful, neither product is currently available for clinical use in the United States.
Robots and magnets
Magnetically controlled wireless capsules have been studied in vivo in human beings on several occasions in the United Kingdom and Asia. Wired colonic capsules are currently under development in the United States. These products use joystick-style controls to direct movement of the capsule. Optimal visualization often requires the patient to rotate through numerous positions and, at least in the stomach, to drink significant quantities of fluid to ensure adequate distention. At present, these devices provide only diagnostic capabilities.
Conclusions
The performance of endoscopy inherently places its practitioners at risk of biomechanical injury. Fortunately, there are numerous ways we can optimize our environment and ourselves. We should treat our bodies as professional athletes do: use good form, encourage colleagues to observe and provide feedback on our actions, optimize our practice facilities, and stretch our muscles. In the future, technological innovations, such as ergonomically designed endoscope handles and self-propelled colonoscopies, may reduce the inherent physical stresses of endoscopy. In doing so, hopefully we can preserve our own health and continue to better the health of our patients as well.
References
1. Rabin RC. Gastroenterologist shortage is forecast. (Available at: www.nytimes.com/2009/01/09/health/research/09gastro.html.) The New York Times, Jan. 9;2009.
2. Pedrosa MC, Farraye FA, Shergill AK. et al. Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc. 2010;72:227-35.
3. Ridtitid W, Coté GA, Leung W, et al. Prevalence and risk factors for musculoskeletal injuries related to endoscopy. Gastrointest Endosc. 2015;81:294-302.e294.
4. Geraghty J, George R, Babbs C. A questionnaire study assessing overuse injuries in United Kingdom endoscopists and any effect from the introduction of the National Bowel Cancer Screening Program on these injuries. Gastrointest Endos. 2011;73:1069-70.
5. Kuwabara T, Urabe Y, Hiyama T, et al. Prevalence and impact of musculoskeletal pain in Japanese gastrointestinal endoscopists: a controlled study. World J Gastroenterol. 2011;17:1488-93.
6. Rempel DM, Harrison RJ, Barnhart S. Work-related cumulative trauma disorders of the upper extremity. JAMA 1992;267:838-42.
7. O’Sullivan S, Bridge G, Ponich T. Musculoskeletal injuries among ERCP endoscopists in Canada. Can J Gastroenterol. 2002;16:369-74.
8. Moore B, vanSonnenberg E, Casola G. et al. The relationship between back pain and lead apron use in radiologists. AJR Am J Roentgenol. 1992;158:191-3.
9. Ross AM, Segal J, Borenstein D, et al. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79:68-70.
10. Buschbacher R. Overuse syndromes among endoscopists. Endoscopy. 1994;26:539-44.
11. Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-40.
12. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc. 2007;21:980-4.
13. Nivatvongs S, Goldberg SM. Holder for the fiberoptic colonoscope. Dis Colon Rectum. 1974;17:273-4.
14. Eusebio EB. A practical aid in colonoscopy. Dis Colon Rectum. 1989;32:996-7.
15. Rattan J, Rozen P. A new colonoscope holder. Dis Colon Rectum. 1987;30:639-40.
16. Marks G. A new technique of hand reversal using a harness-type endoscope holder for twin-knob colonoscopy and flexible fiberoptic sigmoidoscopy. Dis Colon Rectum. 1981;24:567-8.
17. Hayashi Y, Sunada K, Yamamoto H. Prototype holder adequately supports the overtube in balloon-assisted endoscopic submucosal dissection. Dig Endosc. 2014;26:682.
Dr. Young is professor of medicine, director, digestive disease division, Uniformed Services University, Bethesda, Md.; Dr. Commander Singla is assistant professor of medicine, Uniformed Services University, director, Inflammatory Bowel Diseases Services, Walter Reed National Military Medical Center, Bethesda, Md.; Dr. Major Kwok is assistant professor of medicine, Uniformed Services University, associate fellowship program director, gastroenterology, National Capital Consortium, Bethesda, Md.; and Dr. Deriban is associate professor of medicine, fellowship program director, gastroenterology, University Hospital Skopje, Macedonia.
As physicians, we work hard to take excellent care of our patients. Years of thoughtful practice and continuous learning allow us to deliver the best that medicine can provide. We often take poor care of ourselves, which can lead to burnout and physical injuries. As gastroenterologists, we spend substantial time performing endoscopic procedures that require repetitive motions such as flexion and extension of the wrist and fingers and torsional movements of the right hand, which may lead to overuse injuries. The volume of endoscopic procedures performed by a typical gastroenterologist has increased significantly in the past 20 years. Moreover, experts predict that by 2020 we will have too few endoscopists to meet clinical demands.1 It is imperative that we do whatever possible to ensure overuse injuries do not prematurely prevent us from providing much-needed care. One way to achieve this goal is to focus on ergonomics. The study of ergonomics, derived from the Greek words ergo (work) and nomos (law), seeks to optimize the interface between the worker, the equipment, and the work environment. This article reviews basic ergonomic principles that endoscopists can apply today and possible innovations that may improve endoscopic ergonomics in the future.
Breadth of the problem
Examinations of injuries related to endoscopy are limited to survey-based and small controlled studies with a 39%-89% overall prevalence of pain or musculoskeletal injuries reported.2 In a survey of 684 American Society for Gastrointestinal Endoscopy members examining injury prevalence and risk factors,3 53% experienced an injury believed to be definitely or probably related to endoscopy. Risk factors included higher procedure volume (more than 20 cases/wk), greater number of hours spent performing endoscopy (more than 16 h/wk), and total number of years spent performing endoscopy2,4. Community practitioners reported injuries at higher rates than those in an academic center. Other suggested but unproven risk factors include age5, sex, hand size, room design, and level of training in ergonomics and endoscopy2. Injuries can be severe and may lead to work load reduction, missed days of work3-5, reduction of activities outside of work, and long-term disability2.
Most surveys reflect symptoms localized to the back, neck, shoulder, elbow, hands/fingers, and thumbs likely from overuse causing strain and soft-tissue microtrauma6. Without time to heal, these injuries may lead to connective tissue weakening and permanent damage. Repetitive hand movements in endoscopy include left thumb abduction, flexion, and extension while manipulating dials and right wrist flexion, extension, and deviation from torqueing the insertion tube. The use of torque is a necessary part of successful colonoscopy; during scope reduction and maneuvering through the sigmoid colon, torque forces and forces applied against the wall of the colon are highest. When of sufficient magnitude and duration, these forces are associated with an increased risk of thumb and wrist injuries. These movements may result in “endoscopist’s thumb“ (i.e., de Quervain’s tenosynovitis) and carpal tunnel syndrome2. Prolonged standing and lead aprons are implicated in back and neck injuries;2,7-9 two-piece aprons,7,10 and antifatigue mats7 are recommended to decrease pressure on the lumbar and cervical disks as well as delay muscle fatigue.
Position of equipment
Endoscopist and patient positioning can be optimized. In the absence of direct data about ergonomics in endoscopy, we rely on surgical laparoscopy data.11,12These studies show that monitors placed directly in front of surgeons at eye-level (rather than off to the side or at the head of the bed) reduced neck and shoulder muscle activity. Monitors should be placed with a height 20 cm lower than the height of the surgeon (endoscopist), suggesting that optimized monitor height should be at eye-level or lower to prevent neck strain. Estimates based on computer simulation and laparoscopy practitioners show that the optimal distance between the endoscopist/surgeon and a 14” monitor is between 52 and 182 cm, which allows for the least amount of image degradation. Many modern monitors are larger (19”-26”), which allows for placement farther from the endoscopist without losing image quality. Bed height affects both spine and arm position; surgical data again suggest that optimal bed height is between elbow height and 10 cm below elbow height.
Immediate practice points
Since poor monitor placement was identified as a major risk factor for musculoskeletal injuries, the first steps in our endoscopy unit were to improve our sightlines. Our adjustable monitors previously were locked into a specific height, and those same monitors now easily are adjusted to heights appropriate to the endoscopist. Our practice has endoscopists from 61” to 77” tall, meaning we needed monitors that could adjust over a 16” height. When designing new endoscopy suites, monitors that adjust from 93 to 162 cm would accommodate the 5th percentile of female height to the 95th percentile of male height. We use adjustable-height beds; a bed that adjusts between 85 and 120 cm would accommodate the 5th percentile of female height to the 95th percentile of male height.
We also moved our monitors to be closer to the opposite side of the bed to accommodate the 3’ to 6’ appropriate to our 16” screens. Our endoscopy suites have cushioned washable mats placed where endoscopists stand that allow for slight instability of the legs, leading to subtle movements of the legs and increased blood flow to reduce foot and leg injuries. We attempt an athletic stance (the endo-athlete) during endoscopy: shoulders back, chest out, knees bent, and feet hip-width apart pointed at the endoscopy screen (Figure 1). These mats help prevent pelvic girdle twisting and turning that may lead to awkward positions and instead leave the endoscopist in an optimized position for the procedure. We encourage endoscopists to keep the scope in the most neutral position possible to reduce overuse of torque and the forces on the wrists and thumbs. When possible, we use two-piece lead aprons for procedures that require fluoroscopy, which transfers some of the weight of the apron from the shoulders to the hips and reduces upper-body strain. Optimization of the room for therapeutic procedures is even more important (with dual screens both fulfilling the criteria we have listed earlier) given the extra weight of the lead on the body. We suggest that, if procedures are performed in cramped endoscopy rooms, placement of additional monitors can help alleviate neck strain and rotation.
Working with our nurses was imperative. We first had our nurses watch videos on appropriate ergonomics in the endoscopy suite. Given that endoscopists usually are concentrating their attention on the screens in the suite, we tasked our nurses to not only monitor our patients, but also to observe the physical stance of the endoscopists. Our nurses are encouraged to help our endoscopists focus on their working stance: the nurses help with monitor positioning, and verbal cues when endoscopists are contorting their bodies unnaturally. This intervention requires open two-way communication in the endoscopy suite where safety of both the patient and staff is paramount. We are fortunate to be at an institution that trains fellows; we have two endoscopists in the suite at any time, which allows for additional two-way feedback between fellows and attendings to improve ergonomic positioning.
We also encourage some preventative exercises of the upper extremities to reduce pain and injuries. Stretches should emphasize finger, wrist, forearm, and shoulder flexion and extension. Even a minute of stretching between procedures allows for muscle relaxation and may lead to a decrease in overuse injuries. Adding these elements may seem inefficient and unnecessary if you have never had an injury, but we suggest the following paradigm: think of yourself as an endo-athlete. Similar to an athlete, you have worked years to gain the skills you possess. Taking a few moments to reduce your chances of a career-slowing (or career-ending) injury can pay long-term dividends.
Future remedies
Although there have been substantial advances in endoscopic imaging technology, the process of endoscope rotation and tip deflection has changed little since the development of flexible endoscopy. A freshman engineering student tasked with designing a device to navigate, examine, and provide therapy in the human colon likely would create a device that does not resemble the scope that we use daily to accomplish the task. Numerous investigators currently are working on novel devices designed to examine and deliver therapy to the digestive tract. These devices may diminish an endoscopist’s injury risk through the use of better ergonomic principles. This section is not intended to be a comprehensive review and is not an endorsement of any particular product. Rather, we hope it provides a glimpse into a possible future.
Reducing gravitational load
The concept of a mechanical device to hold some or all of the weight of the endoscope was first published in 197413. Since then, a number of products have been described for this purpose.14-17 In general, these consist of a simple metal tube with a hemicylindrical plastic clip, similar to a microphone stand, or a yoke/strap with a plastic scope holder in the front akin to what a percussionist in a marching band might wear. For a variety of reasons, including limited mobility and issues with disinfection, these devices have not gained traction.
Novel control mechanisms
Some of the largest forces on the endoscopist relate to moving the wheels on the scope head to effect tip deflection via a cable linkage. Because the wheels rotate only in one axis, the options for altering and adjusting load are few. One proposed solution is the use of a system with a fully detachable endoscope handle with a joystick style control deck (E210; Invendo Medical, Kissing, Germany). The control deck uses electromechanical assistance — as opposed to pure mechanical force — to transmit energy to the shaft of the instrument. Such assistive technologies have the potential to decrease injuries by decreased load, particularly on the carpometacarpal joint. Other devices seek to decrease the need for torque and high-load tip deflection though the use of self-propelled, disposable colonoscopes that use an aviation-style joystick (Aer-o-scope; GI View, Kissing, Germany). Although interesting and potentially useful, neither product is currently available for clinical use in the United States.
Robots and magnets
Magnetically controlled wireless capsules have been studied in vivo in human beings on several occasions in the United Kingdom and Asia. Wired colonic capsules are currently under development in the United States. These products use joystick-style controls to direct movement of the capsule. Optimal visualization often requires the patient to rotate through numerous positions and, at least in the stomach, to drink significant quantities of fluid to ensure adequate distention. At present, these devices provide only diagnostic capabilities.
Conclusions
The performance of endoscopy inherently places its practitioners at risk of biomechanical injury. Fortunately, there are numerous ways we can optimize our environment and ourselves. We should treat our bodies as professional athletes do: use good form, encourage colleagues to observe and provide feedback on our actions, optimize our practice facilities, and stretch our muscles. In the future, technological innovations, such as ergonomically designed endoscope handles and self-propelled colonoscopies, may reduce the inherent physical stresses of endoscopy. In doing so, hopefully we can preserve our own health and continue to better the health of our patients as well.
References
1. Rabin RC. Gastroenterologist shortage is forecast. (Available at: www.nytimes.com/2009/01/09/health/research/09gastro.html.) The New York Times, Jan. 9;2009.
2. Pedrosa MC, Farraye FA, Shergill AK. et al. Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc. 2010;72:227-35.
3. Ridtitid W, Coté GA, Leung W, et al. Prevalence and risk factors for musculoskeletal injuries related to endoscopy. Gastrointest Endosc. 2015;81:294-302.e294.
4. Geraghty J, George R, Babbs C. A questionnaire study assessing overuse injuries in United Kingdom endoscopists and any effect from the introduction of the National Bowel Cancer Screening Program on these injuries. Gastrointest Endos. 2011;73:1069-70.
5. Kuwabara T, Urabe Y, Hiyama T, et al. Prevalence and impact of musculoskeletal pain in Japanese gastrointestinal endoscopists: a controlled study. World J Gastroenterol. 2011;17:1488-93.
6. Rempel DM, Harrison RJ, Barnhart S. Work-related cumulative trauma disorders of the upper extremity. JAMA 1992;267:838-42.
7. O’Sullivan S, Bridge G, Ponich T. Musculoskeletal injuries among ERCP endoscopists in Canada. Can J Gastroenterol. 2002;16:369-74.
8. Moore B, vanSonnenberg E, Casola G. et al. The relationship between back pain and lead apron use in radiologists. AJR Am J Roentgenol. 1992;158:191-3.
9. Ross AM, Segal J, Borenstein D, et al. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79:68-70.
10. Buschbacher R. Overuse syndromes among endoscopists. Endoscopy. 1994;26:539-44.
11. Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-40.
12. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc. 2007;21:980-4.
13. Nivatvongs S, Goldberg SM. Holder for the fiberoptic colonoscope. Dis Colon Rectum. 1974;17:273-4.
14. Eusebio EB. A practical aid in colonoscopy. Dis Colon Rectum. 1989;32:996-7.
15. Rattan J, Rozen P. A new colonoscope holder. Dis Colon Rectum. 1987;30:639-40.
16. Marks G. A new technique of hand reversal using a harness-type endoscope holder for twin-knob colonoscopy and flexible fiberoptic sigmoidoscopy. Dis Colon Rectum. 1981;24:567-8.
17. Hayashi Y, Sunada K, Yamamoto H. Prototype holder adequately supports the overtube in balloon-assisted endoscopic submucosal dissection. Dig Endosc. 2014;26:682.
Dr. Young is professor of medicine, director, digestive disease division, Uniformed Services University, Bethesda, Md.; Dr. Commander Singla is assistant professor of medicine, Uniformed Services University, director, Inflammatory Bowel Diseases Services, Walter Reed National Military Medical Center, Bethesda, Md.; Dr. Major Kwok is assistant professor of medicine, Uniformed Services University, associate fellowship program director, gastroenterology, National Capital Consortium, Bethesda, Md.; and Dr. Deriban is associate professor of medicine, fellowship program director, gastroenterology, University Hospital Skopje, Macedonia.
As physicians, we work hard to take excellent care of our patients. Years of thoughtful practice and continuous learning allow us to deliver the best that medicine can provide. We often take poor care of ourselves, which can lead to burnout and physical injuries. As gastroenterologists, we spend substantial time performing endoscopic procedures that require repetitive motions such as flexion and extension of the wrist and fingers and torsional movements of the right hand, which may lead to overuse injuries. The volume of endoscopic procedures performed by a typical gastroenterologist has increased significantly in the past 20 years. Moreover, experts predict that by 2020 we will have too few endoscopists to meet clinical demands.1 It is imperative that we do whatever possible to ensure overuse injuries do not prematurely prevent us from providing much-needed care. One way to achieve this goal is to focus on ergonomics. The study of ergonomics, derived from the Greek words ergo (work) and nomos (law), seeks to optimize the interface between the worker, the equipment, and the work environment. This article reviews basic ergonomic principles that endoscopists can apply today and possible innovations that may improve endoscopic ergonomics in the future.
Breadth of the problem
Examinations of injuries related to endoscopy are limited to survey-based and small controlled studies with a 39%-89% overall prevalence of pain or musculoskeletal injuries reported.2 In a survey of 684 American Society for Gastrointestinal Endoscopy members examining injury prevalence and risk factors,3 53% experienced an injury believed to be definitely or probably related to endoscopy. Risk factors included higher procedure volume (more than 20 cases/wk), greater number of hours spent performing endoscopy (more than 16 h/wk), and total number of years spent performing endoscopy2,4. Community practitioners reported injuries at higher rates than those in an academic center. Other suggested but unproven risk factors include age5, sex, hand size, room design, and level of training in ergonomics and endoscopy2. Injuries can be severe and may lead to work load reduction, missed days of work3-5, reduction of activities outside of work, and long-term disability2.
Most surveys reflect symptoms localized to the back, neck, shoulder, elbow, hands/fingers, and thumbs likely from overuse causing strain and soft-tissue microtrauma6. Without time to heal, these injuries may lead to connective tissue weakening and permanent damage. Repetitive hand movements in endoscopy include left thumb abduction, flexion, and extension while manipulating dials and right wrist flexion, extension, and deviation from torqueing the insertion tube. The use of torque is a necessary part of successful colonoscopy; during scope reduction and maneuvering through the sigmoid colon, torque forces and forces applied against the wall of the colon are highest. When of sufficient magnitude and duration, these forces are associated with an increased risk of thumb and wrist injuries. These movements may result in “endoscopist’s thumb“ (i.e., de Quervain’s tenosynovitis) and carpal tunnel syndrome2. Prolonged standing and lead aprons are implicated in back and neck injuries;2,7-9 two-piece aprons,7,10 and antifatigue mats7 are recommended to decrease pressure on the lumbar and cervical disks as well as delay muscle fatigue.
Position of equipment
Endoscopist and patient positioning can be optimized. In the absence of direct data about ergonomics in endoscopy, we rely on surgical laparoscopy data.11,12These studies show that monitors placed directly in front of surgeons at eye-level (rather than off to the side or at the head of the bed) reduced neck and shoulder muscle activity. Monitors should be placed with a height 20 cm lower than the height of the surgeon (endoscopist), suggesting that optimized monitor height should be at eye-level or lower to prevent neck strain. Estimates based on computer simulation and laparoscopy practitioners show that the optimal distance between the endoscopist/surgeon and a 14” monitor is between 52 and 182 cm, which allows for the least amount of image degradation. Many modern monitors are larger (19”-26”), which allows for placement farther from the endoscopist without losing image quality. Bed height affects both spine and arm position; surgical data again suggest that optimal bed height is between elbow height and 10 cm below elbow height.
Immediate practice points
Since poor monitor placement was identified as a major risk factor for musculoskeletal injuries, the first steps in our endoscopy unit were to improve our sightlines. Our adjustable monitors previously were locked into a specific height, and those same monitors now easily are adjusted to heights appropriate to the endoscopist. Our practice has endoscopists from 61” to 77” tall, meaning we needed monitors that could adjust over a 16” height. When designing new endoscopy suites, monitors that adjust from 93 to 162 cm would accommodate the 5th percentile of female height to the 95th percentile of male height. We use adjustable-height beds; a bed that adjusts between 85 and 120 cm would accommodate the 5th percentile of female height to the 95th percentile of male height.
We also moved our monitors to be closer to the opposite side of the bed to accommodate the 3’ to 6’ appropriate to our 16” screens. Our endoscopy suites have cushioned washable mats placed where endoscopists stand that allow for slight instability of the legs, leading to subtle movements of the legs and increased blood flow to reduce foot and leg injuries. We attempt an athletic stance (the endo-athlete) during endoscopy: shoulders back, chest out, knees bent, and feet hip-width apart pointed at the endoscopy screen (Figure 1). These mats help prevent pelvic girdle twisting and turning that may lead to awkward positions and instead leave the endoscopist in an optimized position for the procedure. We encourage endoscopists to keep the scope in the most neutral position possible to reduce overuse of torque and the forces on the wrists and thumbs. When possible, we use two-piece lead aprons for procedures that require fluoroscopy, which transfers some of the weight of the apron from the shoulders to the hips and reduces upper-body strain. Optimization of the room for therapeutic procedures is even more important (with dual screens both fulfilling the criteria we have listed earlier) given the extra weight of the lead on the body. We suggest that, if procedures are performed in cramped endoscopy rooms, placement of additional monitors can help alleviate neck strain and rotation.
Working with our nurses was imperative. We first had our nurses watch videos on appropriate ergonomics in the endoscopy suite. Given that endoscopists usually are concentrating their attention on the screens in the suite, we tasked our nurses to not only monitor our patients, but also to observe the physical stance of the endoscopists. Our nurses are encouraged to help our endoscopists focus on their working stance: the nurses help with monitor positioning, and verbal cues when endoscopists are contorting their bodies unnaturally. This intervention requires open two-way communication in the endoscopy suite where safety of both the patient and staff is paramount. We are fortunate to be at an institution that trains fellows; we have two endoscopists in the suite at any time, which allows for additional two-way feedback between fellows and attendings to improve ergonomic positioning.
We also encourage some preventative exercises of the upper extremities to reduce pain and injuries. Stretches should emphasize finger, wrist, forearm, and shoulder flexion and extension. Even a minute of stretching between procedures allows for muscle relaxation and may lead to a decrease in overuse injuries. Adding these elements may seem inefficient and unnecessary if you have never had an injury, but we suggest the following paradigm: think of yourself as an endo-athlete. Similar to an athlete, you have worked years to gain the skills you possess. Taking a few moments to reduce your chances of a career-slowing (or career-ending) injury can pay long-term dividends.
Future remedies
Although there have been substantial advances in endoscopic imaging technology, the process of endoscope rotation and tip deflection has changed little since the development of flexible endoscopy. A freshman engineering student tasked with designing a device to navigate, examine, and provide therapy in the human colon likely would create a device that does not resemble the scope that we use daily to accomplish the task. Numerous investigators currently are working on novel devices designed to examine and deliver therapy to the digestive tract. These devices may diminish an endoscopist’s injury risk through the use of better ergonomic principles. This section is not intended to be a comprehensive review and is not an endorsement of any particular product. Rather, we hope it provides a glimpse into a possible future.
Reducing gravitational load
The concept of a mechanical device to hold some or all of the weight of the endoscope was first published in 197413. Since then, a number of products have been described for this purpose.14-17 In general, these consist of a simple metal tube with a hemicylindrical plastic clip, similar to a microphone stand, or a yoke/strap with a plastic scope holder in the front akin to what a percussionist in a marching band might wear. For a variety of reasons, including limited mobility and issues with disinfection, these devices have not gained traction.
Novel control mechanisms
Some of the largest forces on the endoscopist relate to moving the wheels on the scope head to effect tip deflection via a cable linkage. Because the wheels rotate only in one axis, the options for altering and adjusting load are few. One proposed solution is the use of a system with a fully detachable endoscope handle with a joystick style control deck (E210; Invendo Medical, Kissing, Germany). The control deck uses electromechanical assistance — as opposed to pure mechanical force — to transmit energy to the shaft of the instrument. Such assistive technologies have the potential to decrease injuries by decreased load, particularly on the carpometacarpal joint. Other devices seek to decrease the need for torque and high-load tip deflection though the use of self-propelled, disposable colonoscopes that use an aviation-style joystick (Aer-o-scope; GI View, Kissing, Germany). Although interesting and potentially useful, neither product is currently available for clinical use in the United States.
Robots and magnets
Magnetically controlled wireless capsules have been studied in vivo in human beings on several occasions in the United Kingdom and Asia. Wired colonic capsules are currently under development in the United States. These products use joystick-style controls to direct movement of the capsule. Optimal visualization often requires the patient to rotate through numerous positions and, at least in the stomach, to drink significant quantities of fluid to ensure adequate distention. At present, these devices provide only diagnostic capabilities.
Conclusions
The performance of endoscopy inherently places its practitioners at risk of biomechanical injury. Fortunately, there are numerous ways we can optimize our environment and ourselves. We should treat our bodies as professional athletes do: use good form, encourage colleagues to observe and provide feedback on our actions, optimize our practice facilities, and stretch our muscles. In the future, technological innovations, such as ergonomically designed endoscope handles and self-propelled colonoscopies, may reduce the inherent physical stresses of endoscopy. In doing so, hopefully we can preserve our own health and continue to better the health of our patients as well.
References
1. Rabin RC. Gastroenterologist shortage is forecast. (Available at: www.nytimes.com/2009/01/09/health/research/09gastro.html.) The New York Times, Jan. 9;2009.
2. Pedrosa MC, Farraye FA, Shergill AK. et al. Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc. 2010;72:227-35.
3. Ridtitid W, Coté GA, Leung W, et al. Prevalence and risk factors for musculoskeletal injuries related to endoscopy. Gastrointest Endosc. 2015;81:294-302.e294.
4. Geraghty J, George R, Babbs C. A questionnaire study assessing overuse injuries in United Kingdom endoscopists and any effect from the introduction of the National Bowel Cancer Screening Program on these injuries. Gastrointest Endos. 2011;73:1069-70.
5. Kuwabara T, Urabe Y, Hiyama T, et al. Prevalence and impact of musculoskeletal pain in Japanese gastrointestinal endoscopists: a controlled study. World J Gastroenterol. 2011;17:1488-93.
6. Rempel DM, Harrison RJ, Barnhart S. Work-related cumulative trauma disorders of the upper extremity. JAMA 1992;267:838-42.
7. O’Sullivan S, Bridge G, Ponich T. Musculoskeletal injuries among ERCP endoscopists in Canada. Can J Gastroenterol. 2002;16:369-74.
8. Moore B, vanSonnenberg E, Casola G. et al. The relationship between back pain and lead apron use in radiologists. AJR Am J Roentgenol. 1992;158:191-3.
9. Ross AM, Segal J, Borenstein D, et al. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79:68-70.
10. Buschbacher R. Overuse syndromes among endoscopists. Endoscopy. 1994;26:539-44.
11. Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-40.
12. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc. 2007;21:980-4.
13. Nivatvongs S, Goldberg SM. Holder for the fiberoptic colonoscope. Dis Colon Rectum. 1974;17:273-4.
14. Eusebio EB. A practical aid in colonoscopy. Dis Colon Rectum. 1989;32:996-7.
15. Rattan J, Rozen P. A new colonoscope holder. Dis Colon Rectum. 1987;30:639-40.
16. Marks G. A new technique of hand reversal using a harness-type endoscope holder for twin-knob colonoscopy and flexible fiberoptic sigmoidoscopy. Dis Colon Rectum. 1981;24:567-8.
17. Hayashi Y, Sunada K, Yamamoto H. Prototype holder adequately supports the overtube in balloon-assisted endoscopic submucosal dissection. Dig Endosc. 2014;26:682.
Dr. Young is professor of medicine, director, digestive disease division, Uniformed Services University, Bethesda, Md.; Dr. Commander Singla is assistant professor of medicine, Uniformed Services University, director, Inflammatory Bowel Diseases Services, Walter Reed National Military Medical Center, Bethesda, Md.; Dr. Major Kwok is assistant professor of medicine, Uniformed Services University, associate fellowship program director, gastroenterology, National Capital Consortium, Bethesda, Md.; and Dr. Deriban is associate professor of medicine, fellowship program director, gastroenterology, University Hospital Skopje, Macedonia.
Patient-reported outcomes for patients with chronic liver disease
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).
Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Making a case for patient-reported outcomes in clinical inflammatory bowel disease practice
Patients seek medical care when they perceive a deterioration in their health. Gastroenterologists and health care providers are trained to seek out clinical, laboratory, radiologic, and endoscopic evidence of pathology. Conventional endpoints in inflammatory bowel disease (IBD) clinical trials and clinical care may fail to capture the full health status and disease experience from the patient perspective. The Food and Drug Administration has called for the development of coprimary endpoints in research trials to include an objective measure of inflammation in conjunction with patient-reported outcomes (PROs). The objective is to support labeling claims and improve safety and effectiveness in the drug approval process.1,2 There is also growing recognition that high-value care includes management of biologic and psychosocial factors to enable patients with chronic diseases to regain their health. Clinicians might follow suit by incorporating valid, reliable PRO measures to usual IBD care in order better to achieve patient-centered care, inform decision making, and improve the care provided.
What are patient-reported outcomes?
The FDA defines a PRO as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” Two PROs are used to measure various aspects of health including physical, emotional, or social domains. PROs have emerged as tools that may foster a better understanding of the patient’s condition, which may go beyond disease activity or symptoms. In effect, incorporating PROs into clinical practice enables a model of “coproduction” of health care, and may contribute to a more reciprocal patient-provider interaction where the needs of the patient may be more fully understood and incorporated into decision-making that may lead to improved patient satisfaction and outcomes.3,4
There are hundreds of available PROs in gastroenterology,5 ranging from simple (characterizing pain with a basic numeric rating scale) to complex multidomain, multi-item instruments. PROs may cover symptom assessment, health-related quality of life, and adherence to and satisfaction with treatment, and may be generic or disease specific. Numerous PROs have been developed for patients with IBD. Commonly used PROs in IBD include severity scales for pain, defecatory urgency, and bloody stool, and several disease-specific and generic instruments assessing different health-related quality-of-life domains have been used in research studies for patients with IBD.
The current approach to patient-centered care for IBD is limited
IBD is a difficult disease to manage – in part because there is no known biomarker that accurately reflects the full spectrum of disease activity. Numerous indices have been developed to better quantify disease activity and measure response to treatment. Among the most frequently used indices in clinical trials are the Crohn’s Disease Activity Index (CDAI) and (for ulcerative colitis [UC]) the Mayo Clinic Score. These endpoints incorporate signs and symptoms, laboratory findings (in the CDAI), and endoscopic assessments. The CDAI is a suboptimal instrument because of a lack of correlation with endoscopic inflammation and potential confounding with concomitant gastrointestinal illnesses, such as irritable bowel syndrome.6 The Mayo Clinic Score is difficult to interpret because of some subjective elements (what is considered a normal number of stools per day?); vagueness (mostly bloody stools more than half the time?); and need for a physician assessment, which often does not correspond with the patient’s perception of their disease.7 From a research perspective, this disconnect can compromise the quality of trial data. Clinically, it can negatively impact patients’ satisfaction and impair the patient-provider relationship.8
To that end, regulatory agencies, scientific bodies, and health care payors are shifting toward a more “patient-centered” approach with an emphasis on PROs. However, although the FDA is incorporating the patient perspective in its trials, measuring meaningful outcomes in day-to-day clinical care is challenging. In the absence of active inflammation, more than 30% of patients with IBD still suffer from gastrointestinal symptoms.9 Furthermore, physicians frequently underestimate the effect of depression, anxiety, fatigue, and sleep on patient health. Likewise, some patients with active small-bowel Crohn’s disease (CD) may experience few gastrointestinal symptoms but have profound fatigue, weight loss, and impaired quality of life. A focused assessment for disease activity may fail to identify aspects of health most relevant or important to individual patient well-being. There is a need for effective, efficient, and standardized strategies to better understand the concerns of the individual seeking help.
Although there are several PROs that measure disease activity primarily for clinical research trials,10 their prevalence in gastroenterology practices has not been assessed. Most likely, few clinical practices currently integrate standardized PROs in routine patient care. This may be because of several reasons, including lack of awareness of newly developed PROs, administrative burden including time and resources to collect PROs, potentially complex interpretation of results, and perhaps a reluctance among physicians to alter traditional patient interview methods of obtaining information about the health status of their patients. For effective use in clinical care, PROs require simple and relevant interpretation to add value to the clinician’s practice, and must minimally impact clinical flow and resources. The use of Internet-enabled tablets has been shown to be a feasible, efficient, and effective means of PRO assessment in gastroenterology practices, with good levels of patient satisfaction.11
Reaping potential benefits of patient-reported outcomes
The National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) is an initiative developed to investigate and promote implementation of PRO measures among patients with chronic diseases. The collection of PROMIS measures has been shown to be feasible at a tertiary care IBD center, enabling a biopsychosocial model of care.12 Likewise, implementation of PROs in other clinical areas including oncology, orthopedics, and rheumatology has been robust.
In an innovative orthopedic study, PROMIS measures collected and linked to the electronic medical record predicted the likelihood of a clinically meaningful benefit from foot and ankle surgery.13 This facilitated tailored patient-specific preoperative discussions about the expected benefit of surgery. In a study at a rheumatology clinic patients with rheumatoid arthritis were asked to identify their highest priority treatment targets using PROMIS domains (fatigue, pain, depression, social function). The highest priority domain was tracked over time as a patient-centered marker of health, essentially personalizing measures of success for the individual patient.14
PROs have the unique potential to affect multiple levels of health care. At the patient level, PRO data can identify specific concerns, manage expectations of recovery, and tailor treatment decisions to personal preference. At the population level, PRO data can be used to standardize aspects of care to understand comparative health and disease among all patients in a practice or relative to outside practices, identify outliers, and drive improvement.
Optimizing PROs for use in clinical trials: CD–PROs and UC–PROs
Developing standardized, validated instruments according to FDA guidance is a complex process. The lack of an FDA-approved PRO has resulted in substantial variability in the definitions of clinical response or remission in clinical trials to date.15 As a result, IBD-specific PROs (CD-PRO and UC-PRO) are being developed under FDA guidance for use in clinical trials.16 With achievement of prequalification for open use, UC-PRO and CD-PRO will cover five IBD-specific outcomes domains or modules: 1) bowel signs and symptoms, 2) systemic symptoms, 3) emotional impact, 4) coping behaviors, and 5) IBD impact on daily life. The bowel signs and symptoms module may also incorporate a functional impact assessment. Each module includes numerous pertinent items (e.g., “I feel worried,” “I feel scared,” “I feel alone” in the emotional impact module) and are currently being tailored and scored for practicality and relevance. It is hoped that UC-PRO and CD-PRO in final form will be relevant and applicable for clinical trials and gastroenterology practices alike.
Because the development of the UC-PRO and the CD-PRO is still underway, interim PROs are being used in ongoing clinical trials. These interim measures were extracted from existing components of the CDAI, Mayo Clinic Score, and UC Disease Activity Index. The CD PRO-2 consists of two items: abdominal pain and stool frequency. The UC PRO-2 is composed of rectal bleeding and stool frequency. The PRO-3 adds an item regarding general well-being. The sensitivity of these PROs was tested in studies for CD and UC. Both PROs performed similarly to their respective parent instrument. Important limitations include the lack of validation, and the fact that these interim measures were derived from parent measures with acknowledged limitations as previously discussed. Current clinical trials are coupling these interim measures with endoscopic data as coprimary endpoints.
PROs in routine clinical practice: Are we ready for prime time?
Few instruments developed to date have been widely implemented into routine IBD clinical practice. Table 1 highlights commonly available or recently developed PROs for IBD care. As clinicians strive to more effectively integrate PROs into clinical practice, we propose a three-step process to getting started: 1) select and administer a PRO instrument, 2) identify areas of impairment and create a targeted treatment strategy to focus on those areas, and 3) repeat the same PRO at follow-up to assess for improvement. The instrument can be administered before the visit or in the clinic waiting room. Focus a portion of the patient’s visit on discussing the results and identifying one or more domains to target for improvement. For example, the patient may indicate diarrhea as his/her most important area to target, triggering a symptom-specific investigation and therapeutic approach. The PRO may also highlight social or emotional impairment that may require an ancillary referral. The benefits of this PRO-driven approach to IBD care are twofold. First, the patient’s primary concerns are positioned at the forefront of the clinical visit. Second, aligning the clinician’s focus with the patient input may actually help to streamline each visit and improve overall visit efficiency and patient satisfaction.
Conclusions
As therapies for IBD improve, so should standards of patient-centered care. Clinicians must actively seek and then listen to the concerns of patients and be able to address the multiple facets of living with a chronic disease. PROs empower patients, helping them identify important topics for discussion at the clinical visit. This affords clinicians a better understanding of primary patient concerns before the visit, and potentially improves the quality and value of care. At first, the process of incorporating PROs into a busy clinical practice may be challenging, but targeted treatment plans have the potential to foster a better patient – and physician – experience.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16[5]:603-7).
References
1. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
2. Burke, L.B., Kennedy, D.L., Miskala, P.H., et al. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther. 2008;84:281-3.
3. Batalden, M., Baltalden, P., Margolis, P., et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-17.
4. Johnson, L.C. Melmed, G.Y., Nelson, E.C., et al. Fostering collaboration through creation of an IBD learning health system. Am J Gastroenterol. 2017;112:406-8.
5. Khanna, P., Agarwal, N., Khanna, D., et al. Development of an online library of patient reported outcome measures in gastroenterology: the GI-PRO database. Am J Gastroenterol. 2014;109:234-48.
6. Bruining, D.H. Sandborn, W.J. Do not assume symptoms indicate failure of anti-tumor necrosis factor therapy in January 2015 Emerging Treatment Goals in IBD Trials and Practice 45 REVIEWS AND PERSPECTIVES Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:395-9.
7. Surti, B., Spiegel, B., Ippoliti, A., et al. Assessing health status in inflammatory bowel disease using a novel single-item numeric rating scale. Dig Dis Sci. 2013;58:1313-21.
8. Marshall, S., Haywood, K. Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559-68.
9. Simren, M., Axelsson, J., Gillberg, R., et al. Quality of life in inflammatory bowel disease in remission: the impact of IBD-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389-96.
10. De Jong, M.J., Huibregtse, R., Masclee, A.A.M., et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-63.
11. Atreja, A., Rizk, M. Capturing patient reported outcomes and quality of life in routine clinical practice: ready to prime time? Minerva Gastroenterol Dietol. 2012;58:19-24.
12. Ishak, W.W., Pan, D., Steiner, A.J., et al. Patient reported outcomes of quality of life, functioning, and GI/psychiatric symptom severity in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:798-803.
13. Ho, B., Houck, J.R., Flemister, A.S., et al. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37:911-8. 14. Bacalao, E., Greene, G.J., Beaumont, J.L., et al. Standardizing and personalizing the treat to target (T2T) approach for rheumatoid arthritis using the Patient-Reported Outcomes Measurement Information System (PROMIS): baseline findings on patient-centered treatment priorities. Clin Rheumatol. 2017;36:1729-36.
15. Ma, C., Panaccione, R., Fedorak, R.N., et al. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin Gastroenterol Hepatol. 2018;16:637-47.
16. Higgins P. Patient reported outcomes in IBD 2017. Available at: ibdctworkshop.files.wordpress.com/2017/01/patient-reported-outcomes-in-ibd___peter-higgins.pdf. Accessed Aug. 27, 2017.
17. Guyatt, G., Mitchell, A. Irvine, E.J., et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-10.
18. Love, J.R., Irvine, E.J., Fedorak, R.N. Quality of life in inflammatory bowel disease. J Clin Gastroenterol. 1992;14:15-9.
19. Irvine, E.J., Zhou, Q., Thompson, A.K. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571-8.
20. Fazio, V.W., O’Riordain, M.G., Lavery, I.C., et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230:575-84.
21. Gower-Rousseau, C., Sarter, H., Savoye, G., et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. 2017;66:588-96.
22. Gosh, S., Louis, E., Beaugerie, L., et al. Development of the IBD-Disk: a visual self-administered tool assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:333-40.
23. Khanna, R., Zou, G., D’Haens, G., et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41:77-86.
24. Walmsley, R.S., Ayres, R.C.S., Pounder, P.R., et al. A simple clinical colitis activity index. Gut. 1998;43:29-32.
25. Bodger, K., Ormerod, C., Shackcloth, D., et al. Development and validation of a rapid, general measure of disease control from the patient perspective: the IBD-Control questionnaire. Gut. 2014;63:1092-102.
26. Cleeland, C.S., Ryan, K.M. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-38.
27. Kroenke, K., Spitzer, R.L., Williams, J.B.W. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-13.
28. Zigmond, A.S., Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-70.
29. Spitzer, R.L., Korneke, K., Williams, J.B., et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-7.
30. Reilly, M.C., Zbrozek, A.S. Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmachoeconomics. 1993;4:353-65.
31. Smets, E.M., Garssen, B. Bonke, B., et al. The Multidimensional Fatigue Inventory psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315-25.
32. Czuber-Dochan, W., Norton, C., Bassettt, P., et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis. 2014;8:1398-406.
33. Drossman, D.A., Leserman, J., Li, Z.M., et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med. 1991;53:701-12. 34. Cohen, S., Kamarck, T., Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-96.
Dr. Cohen is in the division of digestive and liver diseases; Dr. Melmed is director, clinical inflammatory bowel disease, director, clinical research in the division of gastroenterology, and director, advanced inflammatory bowel disease fellowship program, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is a consultant for AbbVie, Boehringer Ingelheim, Celgene, Genentech, Janssen, Pfizer, Samsung Bioepis, Takeda, and UCB; and received support for research from Prometheus Labs. The remaining author discloses no conflicts.
Patients seek medical care when they perceive a deterioration in their health. Gastroenterologists and health care providers are trained to seek out clinical, laboratory, radiologic, and endoscopic evidence of pathology. Conventional endpoints in inflammatory bowel disease (IBD) clinical trials and clinical care may fail to capture the full health status and disease experience from the patient perspective. The Food and Drug Administration has called for the development of coprimary endpoints in research trials to include an objective measure of inflammation in conjunction with patient-reported outcomes (PROs). The objective is to support labeling claims and improve safety and effectiveness in the drug approval process.1,2 There is also growing recognition that high-value care includes management of biologic and psychosocial factors to enable patients with chronic diseases to regain their health. Clinicians might follow suit by incorporating valid, reliable PRO measures to usual IBD care in order better to achieve patient-centered care, inform decision making, and improve the care provided.
What are patient-reported outcomes?
The FDA defines a PRO as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” Two PROs are used to measure various aspects of health including physical, emotional, or social domains. PROs have emerged as tools that may foster a better understanding of the patient’s condition, which may go beyond disease activity or symptoms. In effect, incorporating PROs into clinical practice enables a model of “coproduction” of health care, and may contribute to a more reciprocal patient-provider interaction where the needs of the patient may be more fully understood and incorporated into decision-making that may lead to improved patient satisfaction and outcomes.3,4
There are hundreds of available PROs in gastroenterology,5 ranging from simple (characterizing pain with a basic numeric rating scale) to complex multidomain, multi-item instruments. PROs may cover symptom assessment, health-related quality of life, and adherence to and satisfaction with treatment, and may be generic or disease specific. Numerous PROs have been developed for patients with IBD. Commonly used PROs in IBD include severity scales for pain, defecatory urgency, and bloody stool, and several disease-specific and generic instruments assessing different health-related quality-of-life domains have been used in research studies for patients with IBD.
The current approach to patient-centered care for IBD is limited
IBD is a difficult disease to manage – in part because there is no known biomarker that accurately reflects the full spectrum of disease activity. Numerous indices have been developed to better quantify disease activity and measure response to treatment. Among the most frequently used indices in clinical trials are the Crohn’s Disease Activity Index (CDAI) and (for ulcerative colitis [UC]) the Mayo Clinic Score. These endpoints incorporate signs and symptoms, laboratory findings (in the CDAI), and endoscopic assessments. The CDAI is a suboptimal instrument because of a lack of correlation with endoscopic inflammation and potential confounding with concomitant gastrointestinal illnesses, such as irritable bowel syndrome.6 The Mayo Clinic Score is difficult to interpret because of some subjective elements (what is considered a normal number of stools per day?); vagueness (mostly bloody stools more than half the time?); and need for a physician assessment, which often does not correspond with the patient’s perception of their disease.7 From a research perspective, this disconnect can compromise the quality of trial data. Clinically, it can negatively impact patients’ satisfaction and impair the patient-provider relationship.8
To that end, regulatory agencies, scientific bodies, and health care payors are shifting toward a more “patient-centered” approach with an emphasis on PROs. However, although the FDA is incorporating the patient perspective in its trials, measuring meaningful outcomes in day-to-day clinical care is challenging. In the absence of active inflammation, more than 30% of patients with IBD still suffer from gastrointestinal symptoms.9 Furthermore, physicians frequently underestimate the effect of depression, anxiety, fatigue, and sleep on patient health. Likewise, some patients with active small-bowel Crohn’s disease (CD) may experience few gastrointestinal symptoms but have profound fatigue, weight loss, and impaired quality of life. A focused assessment for disease activity may fail to identify aspects of health most relevant or important to individual patient well-being. There is a need for effective, efficient, and standardized strategies to better understand the concerns of the individual seeking help.
Although there are several PROs that measure disease activity primarily for clinical research trials,10 their prevalence in gastroenterology practices has not been assessed. Most likely, few clinical practices currently integrate standardized PROs in routine patient care. This may be because of several reasons, including lack of awareness of newly developed PROs, administrative burden including time and resources to collect PROs, potentially complex interpretation of results, and perhaps a reluctance among physicians to alter traditional patient interview methods of obtaining information about the health status of their patients. For effective use in clinical care, PROs require simple and relevant interpretation to add value to the clinician’s practice, and must minimally impact clinical flow and resources. The use of Internet-enabled tablets has been shown to be a feasible, efficient, and effective means of PRO assessment in gastroenterology practices, with good levels of patient satisfaction.11
Reaping potential benefits of patient-reported outcomes
The National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) is an initiative developed to investigate and promote implementation of PRO measures among patients with chronic diseases. The collection of PROMIS measures has been shown to be feasible at a tertiary care IBD center, enabling a biopsychosocial model of care.12 Likewise, implementation of PROs in other clinical areas including oncology, orthopedics, and rheumatology has been robust.
In an innovative orthopedic study, PROMIS measures collected and linked to the electronic medical record predicted the likelihood of a clinically meaningful benefit from foot and ankle surgery.13 This facilitated tailored patient-specific preoperative discussions about the expected benefit of surgery. In a study at a rheumatology clinic patients with rheumatoid arthritis were asked to identify their highest priority treatment targets using PROMIS domains (fatigue, pain, depression, social function). The highest priority domain was tracked over time as a patient-centered marker of health, essentially personalizing measures of success for the individual patient.14
PROs have the unique potential to affect multiple levels of health care. At the patient level, PRO data can identify specific concerns, manage expectations of recovery, and tailor treatment decisions to personal preference. At the population level, PRO data can be used to standardize aspects of care to understand comparative health and disease among all patients in a practice or relative to outside practices, identify outliers, and drive improvement.
Optimizing PROs for use in clinical trials: CD–PROs and UC–PROs
Developing standardized, validated instruments according to FDA guidance is a complex process. The lack of an FDA-approved PRO has resulted in substantial variability in the definitions of clinical response or remission in clinical trials to date.15 As a result, IBD-specific PROs (CD-PRO and UC-PRO) are being developed under FDA guidance for use in clinical trials.16 With achievement of prequalification for open use, UC-PRO and CD-PRO will cover five IBD-specific outcomes domains or modules: 1) bowel signs and symptoms, 2) systemic symptoms, 3) emotional impact, 4) coping behaviors, and 5) IBD impact on daily life. The bowel signs and symptoms module may also incorporate a functional impact assessment. Each module includes numerous pertinent items (e.g., “I feel worried,” “I feel scared,” “I feel alone” in the emotional impact module) and are currently being tailored and scored for practicality and relevance. It is hoped that UC-PRO and CD-PRO in final form will be relevant and applicable for clinical trials and gastroenterology practices alike.
Because the development of the UC-PRO and the CD-PRO is still underway, interim PROs are being used in ongoing clinical trials. These interim measures were extracted from existing components of the CDAI, Mayo Clinic Score, and UC Disease Activity Index. The CD PRO-2 consists of two items: abdominal pain and stool frequency. The UC PRO-2 is composed of rectal bleeding and stool frequency. The PRO-3 adds an item regarding general well-being. The sensitivity of these PROs was tested in studies for CD and UC. Both PROs performed similarly to their respective parent instrument. Important limitations include the lack of validation, and the fact that these interim measures were derived from parent measures with acknowledged limitations as previously discussed. Current clinical trials are coupling these interim measures with endoscopic data as coprimary endpoints.
PROs in routine clinical practice: Are we ready for prime time?
Few instruments developed to date have been widely implemented into routine IBD clinical practice. Table 1 highlights commonly available or recently developed PROs for IBD care. As clinicians strive to more effectively integrate PROs into clinical practice, we propose a three-step process to getting started: 1) select and administer a PRO instrument, 2) identify areas of impairment and create a targeted treatment strategy to focus on those areas, and 3) repeat the same PRO at follow-up to assess for improvement. The instrument can be administered before the visit or in the clinic waiting room. Focus a portion of the patient’s visit on discussing the results and identifying one or more domains to target for improvement. For example, the patient may indicate diarrhea as his/her most important area to target, triggering a symptom-specific investigation and therapeutic approach. The PRO may also highlight social or emotional impairment that may require an ancillary referral. The benefits of this PRO-driven approach to IBD care are twofold. First, the patient’s primary concerns are positioned at the forefront of the clinical visit. Second, aligning the clinician’s focus with the patient input may actually help to streamline each visit and improve overall visit efficiency and patient satisfaction.

Conclusions
As therapies for IBD improve, so should standards of patient-centered care. Clinicians must actively seek and then listen to the concerns of patients and be able to address the multiple facets of living with a chronic disease. PROs empower patients, helping them identify important topics for discussion at the clinical visit. This affords clinicians a better understanding of primary patient concerns before the visit, and potentially improves the quality and value of care. At first, the process of incorporating PROs into a busy clinical practice may be challenging, but targeted treatment plans have the potential to foster a better patient – and physician – experience.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16[5]:603-7).
References
1. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
2. Burke, L.B., Kennedy, D.L., Miskala, P.H., et al. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther. 2008;84:281-3.
3. Batalden, M., Baltalden, P., Margolis, P., et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-17.
4. Johnson, L.C. Melmed, G.Y., Nelson, E.C., et al. Fostering collaboration through creation of an IBD learning health system. Am J Gastroenterol. 2017;112:406-8.
5. Khanna, P., Agarwal, N., Khanna, D., et al. Development of an online library of patient reported outcome measures in gastroenterology: the GI-PRO database. Am J Gastroenterol. 2014;109:234-48.
6. Bruining, D.H. Sandborn, W.J. Do not assume symptoms indicate failure of anti-tumor necrosis factor therapy in January 2015 Emerging Treatment Goals in IBD Trials and Practice 45 REVIEWS AND PERSPECTIVES Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:395-9.
7. Surti, B., Spiegel, B., Ippoliti, A., et al. Assessing health status in inflammatory bowel disease using a novel single-item numeric rating scale. Dig Dis Sci. 2013;58:1313-21.
8. Marshall, S., Haywood, K. Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559-68.
9. Simren, M., Axelsson, J., Gillberg, R., et al. Quality of life in inflammatory bowel disease in remission: the impact of IBD-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389-96.
10. De Jong, M.J., Huibregtse, R., Masclee, A.A.M., et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-63.
11. Atreja, A., Rizk, M. Capturing patient reported outcomes and quality of life in routine clinical practice: ready to prime time? Minerva Gastroenterol Dietol. 2012;58:19-24.
12. Ishak, W.W., Pan, D., Steiner, A.J., et al. Patient reported outcomes of quality of life, functioning, and GI/psychiatric symptom severity in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:798-803.
13. Ho, B., Houck, J.R., Flemister, A.S., et al. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37:911-8. 14. Bacalao, E., Greene, G.J., Beaumont, J.L., et al. Standardizing and personalizing the treat to target (T2T) approach for rheumatoid arthritis using the Patient-Reported Outcomes Measurement Information System (PROMIS): baseline findings on patient-centered treatment priorities. Clin Rheumatol. 2017;36:1729-36.
15. Ma, C., Panaccione, R., Fedorak, R.N., et al. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin Gastroenterol Hepatol. 2018;16:637-47.
16. Higgins P. Patient reported outcomes in IBD 2017. Available at: ibdctworkshop.files.wordpress.com/2017/01/patient-reported-outcomes-in-ibd___peter-higgins.pdf. Accessed Aug. 27, 2017.
17. Guyatt, G., Mitchell, A. Irvine, E.J., et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-10.
18. Love, J.R., Irvine, E.J., Fedorak, R.N. Quality of life in inflammatory bowel disease. J Clin Gastroenterol. 1992;14:15-9.
19. Irvine, E.J., Zhou, Q., Thompson, A.K. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571-8.
20. Fazio, V.W., O’Riordain, M.G., Lavery, I.C., et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230:575-84.
21. Gower-Rousseau, C., Sarter, H., Savoye, G., et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. 2017;66:588-96.
22. Gosh, S., Louis, E., Beaugerie, L., et al. Development of the IBD-Disk: a visual self-administered tool assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:333-40.
23. Khanna, R., Zou, G., D’Haens, G., et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41:77-86.
24. Walmsley, R.S., Ayres, R.C.S., Pounder, P.R., et al. A simple clinical colitis activity index. Gut. 1998;43:29-32.
25. Bodger, K., Ormerod, C., Shackcloth, D., et al. Development and validation of a rapid, general measure of disease control from the patient perspective: the IBD-Control questionnaire. Gut. 2014;63:1092-102.
26. Cleeland, C.S., Ryan, K.M. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-38.
27. Kroenke, K., Spitzer, R.L., Williams, J.B.W. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-13.
28. Zigmond, A.S., Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-70.
29. Spitzer, R.L., Korneke, K., Williams, J.B., et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-7.
30. Reilly, M.C., Zbrozek, A.S. Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmachoeconomics. 1993;4:353-65.
31. Smets, E.M., Garssen, B. Bonke, B., et al. The Multidimensional Fatigue Inventory psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315-25.
32. Czuber-Dochan, W., Norton, C., Bassettt, P., et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis. 2014;8:1398-406.
33. Drossman, D.A., Leserman, J., Li, Z.M., et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med. 1991;53:701-12. 34. Cohen, S., Kamarck, T., Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-96.
Dr. Cohen is in the division of digestive and liver diseases; Dr. Melmed is director, clinical inflammatory bowel disease, director, clinical research in the division of gastroenterology, and director, advanced inflammatory bowel disease fellowship program, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is a consultant for AbbVie, Boehringer Ingelheim, Celgene, Genentech, Janssen, Pfizer, Samsung Bioepis, Takeda, and UCB; and received support for research from Prometheus Labs. The remaining author discloses no conflicts.
Patients seek medical care when they perceive a deterioration in their health. Gastroenterologists and health care providers are trained to seek out clinical, laboratory, radiologic, and endoscopic evidence of pathology. Conventional endpoints in inflammatory bowel disease (IBD) clinical trials and clinical care may fail to capture the full health status and disease experience from the patient perspective. The Food and Drug Administration has called for the development of coprimary endpoints in research trials to include an objective measure of inflammation in conjunction with patient-reported outcomes (PROs). The objective is to support labeling claims and improve safety and effectiveness in the drug approval process.1,2 There is also growing recognition that high-value care includes management of biologic and psychosocial factors to enable patients with chronic diseases to regain their health. Clinicians might follow suit by incorporating valid, reliable PRO measures to usual IBD care in order better to achieve patient-centered care, inform decision making, and improve the care provided.
What are patient-reported outcomes?
The FDA defines a PRO as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” Two PROs are used to measure various aspects of health including physical, emotional, or social domains. PROs have emerged as tools that may foster a better understanding of the patient’s condition, which may go beyond disease activity or symptoms. In effect, incorporating PROs into clinical practice enables a model of “coproduction” of health care, and may contribute to a more reciprocal patient-provider interaction where the needs of the patient may be more fully understood and incorporated into decision-making that may lead to improved patient satisfaction and outcomes.3,4
There are hundreds of available PROs in gastroenterology,5 ranging from simple (characterizing pain with a basic numeric rating scale) to complex multidomain, multi-item instruments. PROs may cover symptom assessment, health-related quality of life, and adherence to and satisfaction with treatment, and may be generic or disease specific. Numerous PROs have been developed for patients with IBD. Commonly used PROs in IBD include severity scales for pain, defecatory urgency, and bloody stool, and several disease-specific and generic instruments assessing different health-related quality-of-life domains have been used in research studies for patients with IBD.
The current approach to patient-centered care for IBD is limited
IBD is a difficult disease to manage – in part because there is no known biomarker that accurately reflects the full spectrum of disease activity. Numerous indices have been developed to better quantify disease activity and measure response to treatment. Among the most frequently used indices in clinical trials are the Crohn’s Disease Activity Index (CDAI) and (for ulcerative colitis [UC]) the Mayo Clinic Score. These endpoints incorporate signs and symptoms, laboratory findings (in the CDAI), and endoscopic assessments. The CDAI is a suboptimal instrument because of a lack of correlation with endoscopic inflammation and potential confounding with concomitant gastrointestinal illnesses, such as irritable bowel syndrome.6 The Mayo Clinic Score is difficult to interpret because of some subjective elements (what is considered a normal number of stools per day?); vagueness (mostly bloody stools more than half the time?); and need for a physician assessment, which often does not correspond with the patient’s perception of their disease.7 From a research perspective, this disconnect can compromise the quality of trial data. Clinically, it can negatively impact patients’ satisfaction and impair the patient-provider relationship.8
To that end, regulatory agencies, scientific bodies, and health care payors are shifting toward a more “patient-centered” approach with an emphasis on PROs. However, although the FDA is incorporating the patient perspective in its trials, measuring meaningful outcomes in day-to-day clinical care is challenging. In the absence of active inflammation, more than 30% of patients with IBD still suffer from gastrointestinal symptoms.9 Furthermore, physicians frequently underestimate the effect of depression, anxiety, fatigue, and sleep on patient health. Likewise, some patients with active small-bowel Crohn’s disease (CD) may experience few gastrointestinal symptoms but have profound fatigue, weight loss, and impaired quality of life. A focused assessment for disease activity may fail to identify aspects of health most relevant or important to individual patient well-being. There is a need for effective, efficient, and standardized strategies to better understand the concerns of the individual seeking help.
Although there are several PROs that measure disease activity primarily for clinical research trials,10 their prevalence in gastroenterology practices has not been assessed. Most likely, few clinical practices currently integrate standardized PROs in routine patient care. This may be because of several reasons, including lack of awareness of newly developed PROs, administrative burden including time and resources to collect PROs, potentially complex interpretation of results, and perhaps a reluctance among physicians to alter traditional patient interview methods of obtaining information about the health status of their patients. For effective use in clinical care, PROs require simple and relevant interpretation to add value to the clinician’s practice, and must minimally impact clinical flow and resources. The use of Internet-enabled tablets has been shown to be a feasible, efficient, and effective means of PRO assessment in gastroenterology practices, with good levels of patient satisfaction.11
Reaping potential benefits of patient-reported outcomes
The National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) is an initiative developed to investigate and promote implementation of PRO measures among patients with chronic diseases. The collection of PROMIS measures has been shown to be feasible at a tertiary care IBD center, enabling a biopsychosocial model of care.12 Likewise, implementation of PROs in other clinical areas including oncology, orthopedics, and rheumatology has been robust.
In an innovative orthopedic study, PROMIS measures collected and linked to the electronic medical record predicted the likelihood of a clinically meaningful benefit from foot and ankle surgery.13 This facilitated tailored patient-specific preoperative discussions about the expected benefit of surgery. In a study at a rheumatology clinic patients with rheumatoid arthritis were asked to identify their highest priority treatment targets using PROMIS domains (fatigue, pain, depression, social function). The highest priority domain was tracked over time as a patient-centered marker of health, essentially personalizing measures of success for the individual patient.14
PROs have the unique potential to affect multiple levels of health care. At the patient level, PRO data can identify specific concerns, manage expectations of recovery, and tailor treatment decisions to personal preference. At the population level, PRO data can be used to standardize aspects of care to understand comparative health and disease among all patients in a practice or relative to outside practices, identify outliers, and drive improvement.
Optimizing PROs for use in clinical trials: CD–PROs and UC–PROs
Developing standardized, validated instruments according to FDA guidance is a complex process. The lack of an FDA-approved PRO has resulted in substantial variability in the definitions of clinical response or remission in clinical trials to date.15 As a result, IBD-specific PROs (CD-PRO and UC-PRO) are being developed under FDA guidance for use in clinical trials.16 With achievement of prequalification for open use, UC-PRO and CD-PRO will cover five IBD-specific outcomes domains or modules: 1) bowel signs and symptoms, 2) systemic symptoms, 3) emotional impact, 4) coping behaviors, and 5) IBD impact on daily life. The bowel signs and symptoms module may also incorporate a functional impact assessment. Each module includes numerous pertinent items (e.g., “I feel worried,” “I feel scared,” “I feel alone” in the emotional impact module) and are currently being tailored and scored for practicality and relevance. It is hoped that UC-PRO and CD-PRO in final form will be relevant and applicable for clinical trials and gastroenterology practices alike.
Because the development of the UC-PRO and the CD-PRO is still underway, interim PROs are being used in ongoing clinical trials. These interim measures were extracted from existing components of the CDAI, Mayo Clinic Score, and UC Disease Activity Index. The CD PRO-2 consists of two items: abdominal pain and stool frequency. The UC PRO-2 is composed of rectal bleeding and stool frequency. The PRO-3 adds an item regarding general well-being. The sensitivity of these PROs was tested in studies for CD and UC. Both PROs performed similarly to their respective parent instrument. Important limitations include the lack of validation, and the fact that these interim measures were derived from parent measures with acknowledged limitations as previously discussed. Current clinical trials are coupling these interim measures with endoscopic data as coprimary endpoints.
PROs in routine clinical practice: Are we ready for prime time?
Few instruments developed to date have been widely implemented into routine IBD clinical practice. Table 1 highlights commonly available or recently developed PROs for IBD care. As clinicians strive to more effectively integrate PROs into clinical practice, we propose a three-step process to getting started: 1) select and administer a PRO instrument, 2) identify areas of impairment and create a targeted treatment strategy to focus on those areas, and 3) repeat the same PRO at follow-up to assess for improvement. The instrument can be administered before the visit or in the clinic waiting room. Focus a portion of the patient’s visit on discussing the results and identifying one or more domains to target for improvement. For example, the patient may indicate diarrhea as his/her most important area to target, triggering a symptom-specific investigation and therapeutic approach. The PRO may also highlight social or emotional impairment that may require an ancillary referral. The benefits of this PRO-driven approach to IBD care are twofold. First, the patient’s primary concerns are positioned at the forefront of the clinical visit. Second, aligning the clinician’s focus with the patient input may actually help to streamline each visit and improve overall visit efficiency and patient satisfaction.

Conclusions
As therapies for IBD improve, so should standards of patient-centered care. Clinicians must actively seek and then listen to the concerns of patients and be able to address the multiple facets of living with a chronic disease. PROs empower patients, helping them identify important topics for discussion at the clinical visit. This affords clinicians a better understanding of primary patient concerns before the visit, and potentially improves the quality and value of care. At first, the process of incorporating PROs into a busy clinical practice may be challenging, but targeted treatment plans have the potential to foster a better patient – and physician – experience.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16[5]:603-7).
References
1. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
2. Burke, L.B., Kennedy, D.L., Miskala, P.H., et al. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther. 2008;84:281-3.
3. Batalden, M., Baltalden, P., Margolis, P., et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-17.
4. Johnson, L.C. Melmed, G.Y., Nelson, E.C., et al. Fostering collaboration through creation of an IBD learning health system. Am J Gastroenterol. 2017;112:406-8.
5. Khanna, P., Agarwal, N., Khanna, D., et al. Development of an online library of patient reported outcome measures in gastroenterology: the GI-PRO database. Am J Gastroenterol. 2014;109:234-48.
6. Bruining, D.H. Sandborn, W.J. Do not assume symptoms indicate failure of anti-tumor necrosis factor therapy in January 2015 Emerging Treatment Goals in IBD Trials and Practice 45 REVIEWS AND PERSPECTIVES Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:395-9.
7. Surti, B., Spiegel, B., Ippoliti, A., et al. Assessing health status in inflammatory bowel disease using a novel single-item numeric rating scale. Dig Dis Sci. 2013;58:1313-21.
8. Marshall, S., Haywood, K. Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559-68.
9. Simren, M., Axelsson, J., Gillberg, R., et al. Quality of life in inflammatory bowel disease in remission: the impact of IBD-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389-96.
10. De Jong, M.J., Huibregtse, R., Masclee, A.A.M., et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-63.
11. Atreja, A., Rizk, M. Capturing patient reported outcomes and quality of life in routine clinical practice: ready to prime time? Minerva Gastroenterol Dietol. 2012;58:19-24.
12. Ishak, W.W., Pan, D., Steiner, A.J., et al. Patient reported outcomes of quality of life, functioning, and GI/psychiatric symptom severity in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:798-803.
13. Ho, B., Houck, J.R., Flemister, A.S., et al. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37:911-8. 14. Bacalao, E., Greene, G.J., Beaumont, J.L., et al. Standardizing and personalizing the treat to target (T2T) approach for rheumatoid arthritis using the Patient-Reported Outcomes Measurement Information System (PROMIS): baseline findings on patient-centered treatment priorities. Clin Rheumatol. 2017;36:1729-36.
15. Ma, C., Panaccione, R., Fedorak, R.N., et al. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin Gastroenterol Hepatol. 2018;16:637-47.
16. Higgins P. Patient reported outcomes in IBD 2017. Available at: ibdctworkshop.files.wordpress.com/2017/01/patient-reported-outcomes-in-ibd___peter-higgins.pdf. Accessed Aug. 27, 2017.
17. Guyatt, G., Mitchell, A. Irvine, E.J., et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-10.
18. Love, J.R., Irvine, E.J., Fedorak, R.N. Quality of life in inflammatory bowel disease. J Clin Gastroenterol. 1992;14:15-9.
19. Irvine, E.J., Zhou, Q., Thompson, A.K. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571-8.
20. Fazio, V.W., O’Riordain, M.G., Lavery, I.C., et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230:575-84.
21. Gower-Rousseau, C., Sarter, H., Savoye, G., et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. 2017;66:588-96.
22. Gosh, S., Louis, E., Beaugerie, L., et al. Development of the IBD-Disk: a visual self-administered tool assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:333-40.
23. Khanna, R., Zou, G., D’Haens, G., et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41:77-86.
24. Walmsley, R.S., Ayres, R.C.S., Pounder, P.R., et al. A simple clinical colitis activity index. Gut. 1998;43:29-32.
25. Bodger, K., Ormerod, C., Shackcloth, D., et al. Development and validation of a rapid, general measure of disease control from the patient perspective: the IBD-Control questionnaire. Gut. 2014;63:1092-102.
26. Cleeland, C.S., Ryan, K.M. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-38.
27. Kroenke, K., Spitzer, R.L., Williams, J.B.W. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-13.
28. Zigmond, A.S., Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-70.
29. Spitzer, R.L., Korneke, K., Williams, J.B., et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-7.
30. Reilly, M.C., Zbrozek, A.S. Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmachoeconomics. 1993;4:353-65.
31. Smets, E.M., Garssen, B. Bonke, B., et al. The Multidimensional Fatigue Inventory psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315-25.
32. Czuber-Dochan, W., Norton, C., Bassettt, P., et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis. 2014;8:1398-406.
33. Drossman, D.A., Leserman, J., Li, Z.M., et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med. 1991;53:701-12. 34. Cohen, S., Kamarck, T., Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-96.
Dr. Cohen is in the division of digestive and liver diseases; Dr. Melmed is director, clinical inflammatory bowel disease, director, clinical research in the division of gastroenterology, and director, advanced inflammatory bowel disease fellowship program, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is a consultant for AbbVie, Boehringer Ingelheim, Celgene, Genentech, Janssen, Pfizer, Samsung Bioepis, Takeda, and UCB; and received support for research from Prometheus Labs. The remaining author discloses no conflicts.
Employing irritable bowel syndrome patient-reported outcomes in the clinical trenches
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.
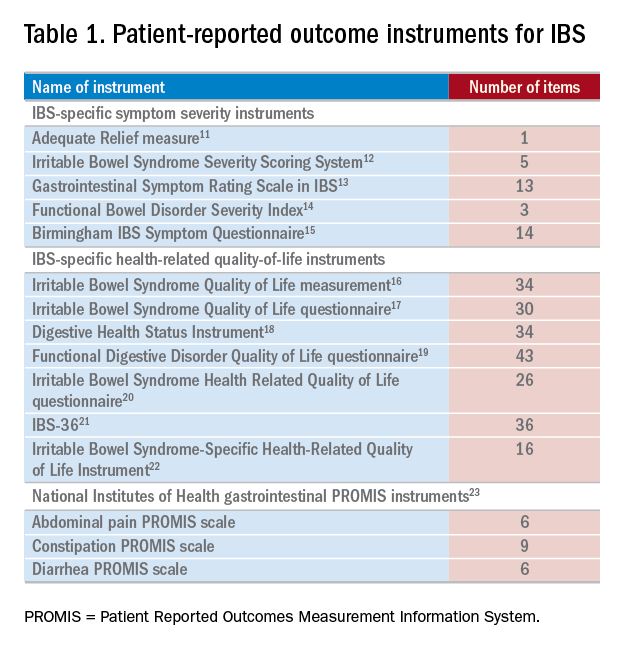
Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patient-reported outcomes in esophageal diseases
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).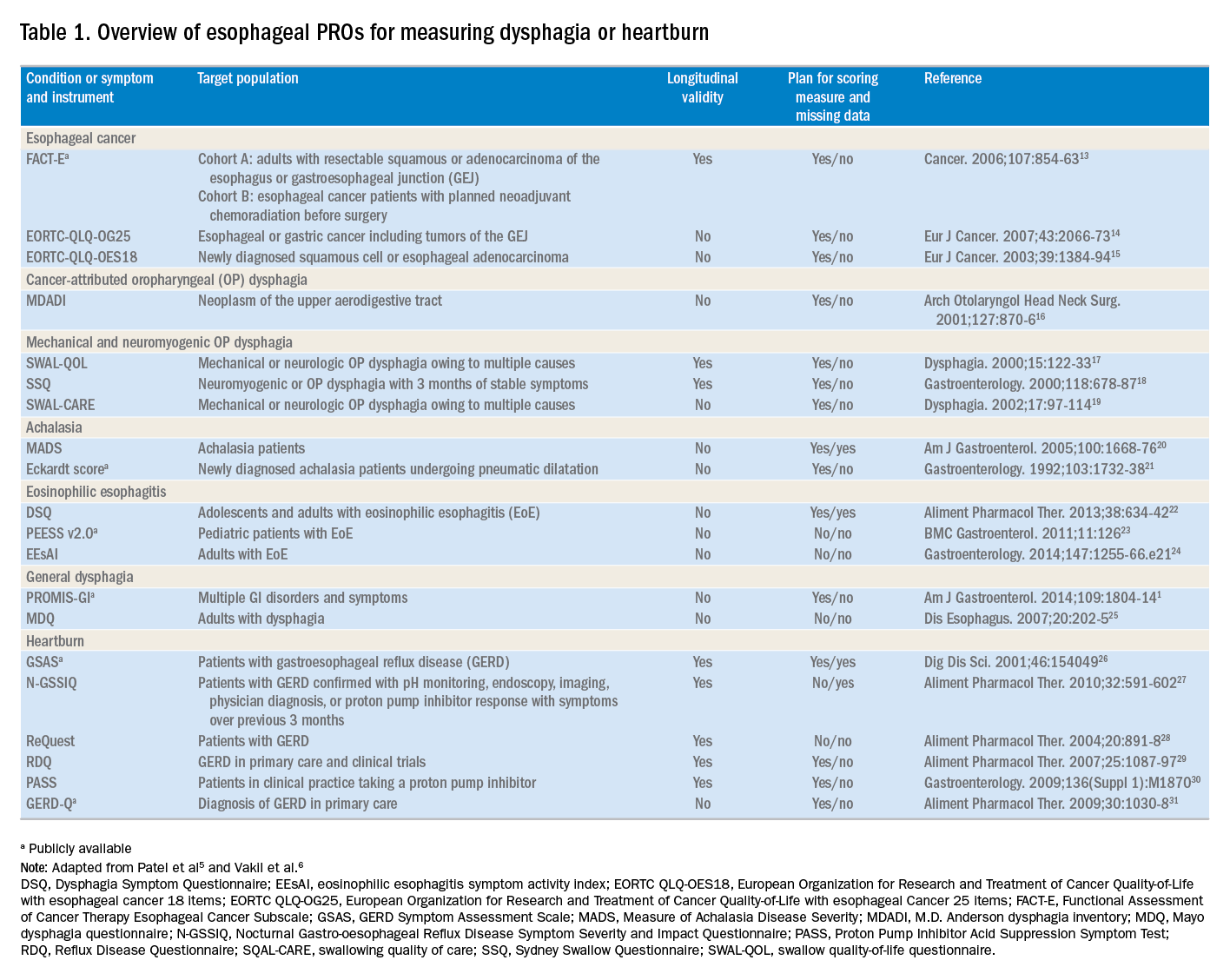
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
Current and future applications of telemedicine to optimize the delivery of care in chronic liver disease
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.
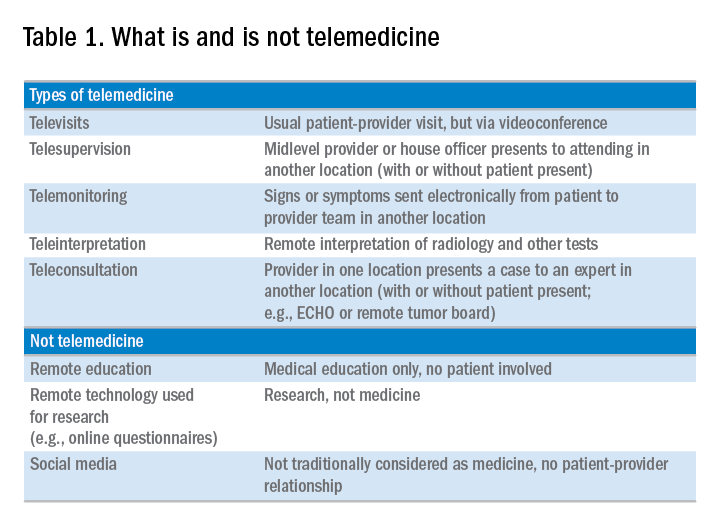
In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.

In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.
Telemedicine is defined broadly by the World Health Organization as the delivery of health care services at a distance using electronic means for “the diagnosis of treatment, and prevention of disease and injuries, research and evaluation, education of health care providers”1 to improve health. Although no single accepted definition exists, telehealth often is used as the umbrella term to encompass telemedicine (health care delivery) in addition to other activities such as education, research, health surveillance, and public health promotion.2 These various terms often are used interchangeably throughout the literature, leading to confusion.1,3 For the purpose of this review, we will use the term telemedicine to describe any care delivery model whereby patient care is provided at a distance using information technology such as cellphones, computers, or other electronic devices.

In the United States, the use of telemedicine is increasing. According to a 2017 survey of 184 health care executives conducted by the American Telemedicine Association, 88% believed that they would invest in telehealth in the near future, 98% believed that it offered a competitive advantage, with the caveat that 71% believed that lack of coverage and payments were barriers to implementation. Recent studies have shown that telehealth interventions are effective at improving clinical outcomes and decreasing inpatient utilization, with good patient satisfaction in the areas of mental health and chronic disease management. The Veterans Administration has emerged as an early telehealth adopter in chronic disease settings such as mental health, dermatology, hypertension, heart failure, and, as of 2016, has provided care to nearly 700,000 (12%) veterans since its inception.4-6 Despite the increased uptake, significant infrastructure and legal barriers to telemedicine remain and the literature regarding its utility in clinical practice continues to emerge.
Compared with other chronic diseases (e.g., heart failure, diabetes, mental illness) there is a dearth of literature on the use of telemedicine in liver disease. The first portion of this review synthesizes currently published literature of telemedicine/telehealth interventions to improve health care delivery and health outcomes in chronic liver disease including published peer-reviewed articles, abstracts, and ongoing clinical trials. The second portion discusses a framework for the future development of telemedicine and its integration into clinical practice by citing examples currently used throughout the country as well as ways to overcome implementation barriers.
Use of telemedicine in chronic liver disease: A literature review
We performed a systematic review of telemedicine in chronic liver disease. In consultation with a biomedical librarian, we searched for English-language articles for relevant studies with adult participants from July 1984 to May 2017 in PubMed, OVID Medline, American Association for the Study of Liver Disease, EMBASE, Web of Science, ClinicalTrials.gov, Elsevier/Science Direct, and the Cochrane Library (the search strategy is shown in the Supplementary Material at https://doi.org/10.1016/j.cgh.2017.10.004). The references of original publications and of review articles additionally were screened for potentially relevant studies. Abstracts that later resulted in no publications and studies in which telemedicine was used to deliver care, but was neither an exposure nor outcome, were excluded. Social media studies were not considered telemedicine if no patient care was involved. Studies of purely medical education interventions or those that evaluated the accuracy of technology to aid in diagnosis also were excluded.
Supplementary Table 1 (https://doi.org/10.1016/j.cgh.2017.10.004) shows the 20 published articles of telemedicine studies. Among these, there were 9 prospective trials, 3 retrospective studies, 2 case reports, and 6 small case series. One of the studies was randomized prospectively and 10 were uncontrolled.
Telemedicine in hepatitis C treatment
Telemedicine to aid in procedural/surgical management
A few reports have been published in the use of synchronous video and digital technology to aid in periprocedural management in liver disease. A case report highlighted a successful example of gastroenterologist-led teleproctoring using basic video technology to enable a surgeon to perform sclerotherapy for hemostasis in the setting of a variceal bleed.9 Another case report described the transmission of smart phone images from surgical trainees to an attending physician to make a real-time decision regarding a possibly questionable liver procurement, which took place 545 km away from the university hospital.10 A retrospective case series described the feasibility and successful use of high-resolution digital macroscopic photography and electronic transmission between liver transplant centers in the United Kingdom to increase the utilization of split liver transplantation, a setting in which detailed knowledge of vessel anatomy is needed for advanced surgical planning.11 Similarly, an uncontrolled case series from Greece reported on the feasibility and reliability of macroscopic image transmission to aid in the evaluation of liver grafts for transplantation.12
Telemedicine to support evaluation and management of hepatocellular carcinoma
One recent abstract reported on the use of asynchronous store-and-forward telemedicine for screening and management of hepatocellular carcinoma and evaluated process outcomes of specialty care access for newly diagnosed patients.13 A multifaceted approach included live video teleconferencing and centralized radiology review, which was conducted by a multidisciplinary tumor board at an expert hub site, which provided expert opinion and subsequent care (e.g., locoregional therapy, liver transplant evaluation) to spoke sites. As a result of the initiative, the time to specialty evaluation and receipt of hepatocellular carcinoma therapy decreased by 23 and 25 days, respectively.
Remote monitoring interventions
Proposed framework for advancing telemedicine in liver disease: The case for more research and policy changes
Telemedicine can serve two main goals in liver disease: improve access to specialty care, and improve care between visits. For the first goal, the technology is straightforward and limited research is required; the main barriers are regulatory and reimbursement. As an example, one of the authors (M.L.V.) uses telemedicine to perform liver transplant evaluations in Las Vegas, N.V., a state without a liver transplant program. Patients are seen initially by a nurse practitioner who resides in Las Vegas, and those patients needing transplant evaluation are scheduled for a video visit with the attending physician who is physically in California. This works well and patients love it; however, the business model is dependent on the downstream financial incentive of transplantation. In addition, various regulatory requirements must be satisfied such as monthly in-person visits. For the second goal, a number of exciting possibilities exist such as remote monitoring and patient disease management, but more research is needed.
Research
According to the Pew Research Center, 95% of American adults own a cellphone and 77% own a smartphone. These devices passively gather an extraordinary amount of data that could be harnessed to identify early warning signs of complications (remote monitoring). Another potentially fruitful area of research is patient disease management. This includes using technology (e.g., reminder texts) to effect behavior change such as with medication adherence, lifestyle modification, education, or peer mentoring. As an example, the coauthor (M.S.) is leading a study to promote physical activity among liver transplant recipients by using an online web portal developed by researchers at the University of Pennsylvania (Way to Health), which interfaces with patient cell phones and digital accelerometer devices. Participants receive daily feedback through text messages with their step counts, and small financial incentives are provided for adequate levels of physical activity. Technology also can facilitate the development of disease management platforms, which could improve both access and in-between visit monitoring, especially in remote areas. One of the authors (M.L.V.) currently is leading the development of a remote disease management program with funding from the American Association for the Study of Liver Diseases.
Despite the tremendous promise, traditional research methods in telemedicine may be challenging given the rapid and increasing uptake of health technology among patients and health systems. As such, the classic paradigm of randomized controlled trials to evaluate the success of an intervention or change in care delivery often is not feasible. We believe there is a need to recalibrate the definition of what constitutes a high-quality telemedicine study. For example, pragmatic trials and those designed within an implementation science framework that evaluate feasibility, scalability, and cost, in parallel with traditional clinical outcomes, may be better suited and should be accepted more widely.17
Policy
Even when the technology is available and research shows efficacy, the implementation of telemedicine in clinical practice faces regulatory and reimbursement barriers. The first regulatory question is whether a patient–provider relationship is being established (with the exception of limited provider–provider curbside consultation, the answer usually is yes). If so, the practice then is subject to all the usual regulatory concerns. The provider needs to be licensed at the site of origin (where the patient is located) and hold malpractice coverage for that location, and the video and medical record transmission should be compliant with the Health Insurance Portability and Accountability Act. The next challenge is reimbursement. Medicare only pays for video consultation if the patient lives in a designated rural Health Professional Shortage Area (www.cms.gov), and reimbursement by private payers varies. Even this is dependent on ever-changing state laws. Reimbursement for remote patient monitoring is even more limited (the National Telehealth Policy Research Center publishes a useful handbook: http://www.cchpca.org/sites/default/files/resources/50%20State%20FINAL%20April%202016.pdf). Absent a bipartisan Congressional effort to remedy this situation, the best hope for removing reimbursement barriers lies with payment reform. The Medicare Access and CHIP Reauthorization Act of 2015 mandates that the Centers for Medicare and Medicaid Services shift from fee-for-service to alternative payment models in the coming years. In these alternate payment models, providers are responsible for the overall quality and total cost of care for a population of patients. In this scenario, there may be a financial incentive for telemedicine, especially remote monitoring, to keep patients out of the hospital. Until then, under current payment models, reimbursement is limited and the barriers to widespread implementation are high.
Conclusions
Telemedicine has continued to increase in uptake and shows tremendous promise in expanding access to health care, promoting patient disease management, and facilitating in-between health care visit monitoring. Although the future is bright, more research is needed to determine optimal ways to integrate telemedicine — especially remote monitoring — into routine clinical care. We call on our specialty societies to send a clear political advocacy message that policy changes are needed to overcome regulatory and reimbursement challenges.
Acknowledgments
The authors would like to thank Lauren Jones and Mackenzie McDougal for their assistance with the literature review.
Supplementary materials and methods
The telemedicine interventions PubMed literature search strategy was as follows: ((“liver diseases”[MeSH Terms] OR (“liver”[All Fields] AND “diseases”[All Fields]) OR “liver diseases”[All Fields] OR (“liver”[All Fields] AND “disease”[All Fields]) OR “liver disease”[All Fields] OR liver dysfunction OR liver dysfunctions)) OR “liver transplantation”[MeSH Terms] OR “liver transplantation” [All Fields] AND (((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR mobile health OR mhealth OR telehealth OR mhealth)) OR (videoconferencing OR videoconference)).
References
1. Kirsh S., Su G.L., Sales A., et al. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30:88-90.
2. Wilson L.S., Maeder A.J. recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21:213-22.
3. Cross R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
4. Darkins A., Ryan P., Kobb R., et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118-26.
5. Tuerk P.W., Fortney J., Bosworth H.B., et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16:115-7.
6. VA Press Release. Available: https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Accessed: July 27, 2017.
7. Arora S., Kalishman S., Thornton K., et al. Expanding access to hepatitis C virus treatment-extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124-33.
8. Mitruka K., Thornton K., Cusick S., et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model–Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-8.
9. Ahmed A., Slosberg E., Prasad P., et al. The successful use of telemedicine in acute variceal hemorrhage. J Clin Gastroenterol. 1999;29:212-3.
10. Croome K.P., Shum J., Al-Basheer M.A., et al. The benefit of smart phone usage in liver organ procurement. J Telemed Telecare. 2011;17:158-60.
11. Bhati C.S., Wigmore S.J., Reddy S., et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010;24:98-103.
12. Mammas C.S., Geropoulos S., Saatsakis G., et al. Telepathology as a method to optimize quality in organ transplantation: a feasibility and reliability study of the virtual benching of liver graft. Stud Health Technol Inform. 2013;190:276-8.
13. Egert E.M., et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. Hepatology. 2015;62:469A
14. Thomson M., Volk M., Kim H.M., et al. An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci. 2015;60:3563-9.
15. Ertel A.E., Kaiser T.E., Abbott D.E., et al. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869-76.
16. Thygesen G.B., Andersen H., Damsgaard B.S. et al. The effect of nurse performed telemedical video consultations for patients suffering from alcohol-related liver cirrhosis. J Hepatol. 2017;66:S349
17. Proctor E., Silmere H., Raghavan R. et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76.
Dr. Serper is in the division of gastroenterology, University of Pennsylvania Perelman School of Medicine, Philadelphia, and the department of medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia; Dr. Volk is in the division of gastroenterology and Transplantation Institute, Loma Linda University, Loma Linda, Calif. The authors disclose no conflicts.
New models of gastroenterology practice
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).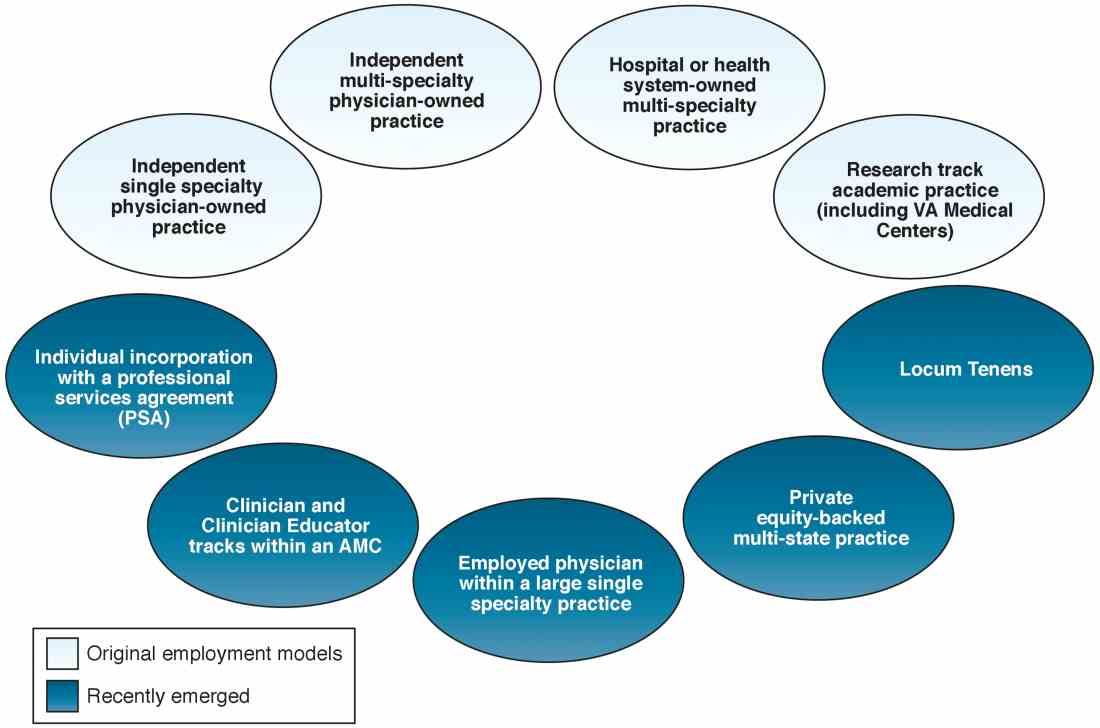
Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).
Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).
Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.













