User login
Most Potentially Hepatotoxic Meds Revealed: Real-World Data Analysis
TOPLINE:
An analysis of real-world evidence identified 17 medications, many not previously regarded as potentially hepatotoxic, that have high incidence rates of patient hospitalization for acute liver injury (ALI), offering insights on how to better determine which drugs carry the most significant risk and warrant liver monitoring.
METHODOLOGY:
- Without a systematic approach to classifying medications’ hepatotoxic risk, researchers have used case reports published on the National Institutes of Health’s LiverTox, which doesn’t account for the number of people exposed, to categorize drugs’ likelihood of causing ALI. The objective was to identify the most potentially hepatotoxic medications using real-world incidence rates of severe ALI.
- Researchers analyzed US Department of Veterans Affairs electronic health record data for almost 7.9 million individuals (mean age, 64.4 years; 92.5% men) without preexisting liver or biliary disease who were initiated in an outpatient setting on any one of 194 medications with four or more published reports of hepatotoxicity. Drugs delivered by injection or intravenously, prescribed for alcohol use disorder or liver disease treatment, or used as an anticoagulant were not included in the study.
- The primary outcome measured was hospitalization for severe ALI, defined by alanine aminotransferase levels > 120 U/L and total bilirubin levels > 2.0 mg/dL or the international normalized ratio ≥ 1.5 and total bilirubin levels > 2.0 mg/dL within the first 2 days of admission.
- Researchers organized the medications into groups on the basis of observed rates of severe ALI per 10,000 person-years and classified drugs with 10 or more hospitalizations (group 1) and 5-9.9 hospitalizations (group 2) as the most potentially hepatotoxic. The study period was October 2000 through September 2021.
TAKEAWAY:
- Among the study population, 1739 hospitalizations for severe ALI were identified. Incidence rates of severe ALI varied widely by medication, from 0 to 86.4 events per 10,000 person-years.
- Seventeen medications were classified as the most potentially hepatotoxic (groups 1 and 2). Seven of them (stavudine, erlotinib, lenalidomide or thalidomide, chlorpromazine, metronidazole, prochlorperazine, and isoniazid) had incidence rates of ≥ 10 events per 10,000 person-years. The other 10 medications (moxifloxacin, azathioprine, levofloxacin, clarithromycin, ketoconazole, fluconazole, captopril, amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and ciprofloxacin) showed incidence rates of 5-9.9 events per 10,000 person-years.
- Of the 17 most hepatotoxic medications, 11 (64%) were not classified as highly hepatotoxic in the published case reports, suggesting a discrepancy between real-world data and case report categorizations.
- Similarly, several medications, including some statins, identified as low-risk in this study were classified as among the most hepatotoxic in the published case reports.
IN PRACTICE:
“Categorization of hepatotoxicity based on the number of published case reports did not accurately reflect observed rates of severe ALI (acute liver injury),” the researchers wrote. “This study represents a systematic, reproducible approach to using real-world data to measure rates of severe ALI following medication initiation among patients without liver or biliary disease…Patients initiating a medication with a high rate of severe ALI might require closer monitoring of liver-related laboratory tests to detect evolving hepatic dysfunction earlier, which might improve prognosis.”
The study illustrates the potential to use electronic health record data to “revolutionize how we characterize drug-related toxic effects,” not just on the liver but other organs, Grace Y. Zhang, MD, and Jessica B. Rubin, MD, MPH, of the University of California, San Francisco, wrote in an accompanying editorial. “If curated and disseminated effectively…such evidence will undoubtedly improve clinical decision-making and allow for more informed patient counseling regarding the true risks of starting or discontinuing medications.
SOURCE:
The study, led by Jessie Torgersen, MD, MHS, MSCE, of the Division of Infectious Diseases, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, was published online in JAMA Internal Medicine.
LIMITATIONS:
The researchers listed several limitations, including the possibility that reliance on laboratory tests for ascertainment of acute liver injuries could introduce surveillance bias. The study focused on a population predominantly consisting of men without preexisting liver or biliary disease, so the findings may not be generalizable to women or individuals with liver disease. Additionally, researchers did not perform a causality assessment of all outcomes, did not study medications with fewer than four published case reports, and did not evaluate the influence of dosage.
DISCLOSURES:
This study was partly funded by several grants from the National Institutes of Health. Some authors declared receiving grants and personal fees from some of the funding agencies and other sources outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of real-world evidence identified 17 medications, many not previously regarded as potentially hepatotoxic, that have high incidence rates of patient hospitalization for acute liver injury (ALI), offering insights on how to better determine which drugs carry the most significant risk and warrant liver monitoring.
METHODOLOGY:
- Without a systematic approach to classifying medications’ hepatotoxic risk, researchers have used case reports published on the National Institutes of Health’s LiverTox, which doesn’t account for the number of people exposed, to categorize drugs’ likelihood of causing ALI. The objective was to identify the most potentially hepatotoxic medications using real-world incidence rates of severe ALI.
- Researchers analyzed US Department of Veterans Affairs electronic health record data for almost 7.9 million individuals (mean age, 64.4 years; 92.5% men) without preexisting liver or biliary disease who were initiated in an outpatient setting on any one of 194 medications with four or more published reports of hepatotoxicity. Drugs delivered by injection or intravenously, prescribed for alcohol use disorder or liver disease treatment, or used as an anticoagulant were not included in the study.
- The primary outcome measured was hospitalization for severe ALI, defined by alanine aminotransferase levels > 120 U/L and total bilirubin levels > 2.0 mg/dL or the international normalized ratio ≥ 1.5 and total bilirubin levels > 2.0 mg/dL within the first 2 days of admission.
- Researchers organized the medications into groups on the basis of observed rates of severe ALI per 10,000 person-years and classified drugs with 10 or more hospitalizations (group 1) and 5-9.9 hospitalizations (group 2) as the most potentially hepatotoxic. The study period was October 2000 through September 2021.
TAKEAWAY:
- Among the study population, 1739 hospitalizations for severe ALI were identified. Incidence rates of severe ALI varied widely by medication, from 0 to 86.4 events per 10,000 person-years.
- Seventeen medications were classified as the most potentially hepatotoxic (groups 1 and 2). Seven of them (stavudine, erlotinib, lenalidomide or thalidomide, chlorpromazine, metronidazole, prochlorperazine, and isoniazid) had incidence rates of ≥ 10 events per 10,000 person-years. The other 10 medications (moxifloxacin, azathioprine, levofloxacin, clarithromycin, ketoconazole, fluconazole, captopril, amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and ciprofloxacin) showed incidence rates of 5-9.9 events per 10,000 person-years.
- Of the 17 most hepatotoxic medications, 11 (64%) were not classified as highly hepatotoxic in the published case reports, suggesting a discrepancy between real-world data and case report categorizations.
- Similarly, several medications, including some statins, identified as low-risk in this study were classified as among the most hepatotoxic in the published case reports.
IN PRACTICE:
“Categorization of hepatotoxicity based on the number of published case reports did not accurately reflect observed rates of severe ALI (acute liver injury),” the researchers wrote. “This study represents a systematic, reproducible approach to using real-world data to measure rates of severe ALI following medication initiation among patients without liver or biliary disease…Patients initiating a medication with a high rate of severe ALI might require closer monitoring of liver-related laboratory tests to detect evolving hepatic dysfunction earlier, which might improve prognosis.”
The study illustrates the potential to use electronic health record data to “revolutionize how we characterize drug-related toxic effects,” not just on the liver but other organs, Grace Y. Zhang, MD, and Jessica B. Rubin, MD, MPH, of the University of California, San Francisco, wrote in an accompanying editorial. “If curated and disseminated effectively…such evidence will undoubtedly improve clinical decision-making and allow for more informed patient counseling regarding the true risks of starting or discontinuing medications.
SOURCE:
The study, led by Jessie Torgersen, MD, MHS, MSCE, of the Division of Infectious Diseases, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, was published online in JAMA Internal Medicine.
LIMITATIONS:
The researchers listed several limitations, including the possibility that reliance on laboratory tests for ascertainment of acute liver injuries could introduce surveillance bias. The study focused on a population predominantly consisting of men without preexisting liver or biliary disease, so the findings may not be generalizable to women or individuals with liver disease. Additionally, researchers did not perform a causality assessment of all outcomes, did not study medications with fewer than four published case reports, and did not evaluate the influence of dosage.
DISCLOSURES:
This study was partly funded by several grants from the National Institutes of Health. Some authors declared receiving grants and personal fees from some of the funding agencies and other sources outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of real-world evidence identified 17 medications, many not previously regarded as potentially hepatotoxic, that have high incidence rates of patient hospitalization for acute liver injury (ALI), offering insights on how to better determine which drugs carry the most significant risk and warrant liver monitoring.
METHODOLOGY:
- Without a systematic approach to classifying medications’ hepatotoxic risk, researchers have used case reports published on the National Institutes of Health’s LiverTox, which doesn’t account for the number of people exposed, to categorize drugs’ likelihood of causing ALI. The objective was to identify the most potentially hepatotoxic medications using real-world incidence rates of severe ALI.
- Researchers analyzed US Department of Veterans Affairs electronic health record data for almost 7.9 million individuals (mean age, 64.4 years; 92.5% men) without preexisting liver or biliary disease who were initiated in an outpatient setting on any one of 194 medications with four or more published reports of hepatotoxicity. Drugs delivered by injection or intravenously, prescribed for alcohol use disorder or liver disease treatment, or used as an anticoagulant were not included in the study.
- The primary outcome measured was hospitalization for severe ALI, defined by alanine aminotransferase levels > 120 U/L and total bilirubin levels > 2.0 mg/dL or the international normalized ratio ≥ 1.5 and total bilirubin levels > 2.0 mg/dL within the first 2 days of admission.
- Researchers organized the medications into groups on the basis of observed rates of severe ALI per 10,000 person-years and classified drugs with 10 or more hospitalizations (group 1) and 5-9.9 hospitalizations (group 2) as the most potentially hepatotoxic. The study period was October 2000 through September 2021.
TAKEAWAY:
- Among the study population, 1739 hospitalizations for severe ALI were identified. Incidence rates of severe ALI varied widely by medication, from 0 to 86.4 events per 10,000 person-years.
- Seventeen medications were classified as the most potentially hepatotoxic (groups 1 and 2). Seven of them (stavudine, erlotinib, lenalidomide or thalidomide, chlorpromazine, metronidazole, prochlorperazine, and isoniazid) had incidence rates of ≥ 10 events per 10,000 person-years. The other 10 medications (moxifloxacin, azathioprine, levofloxacin, clarithromycin, ketoconazole, fluconazole, captopril, amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and ciprofloxacin) showed incidence rates of 5-9.9 events per 10,000 person-years.
- Of the 17 most hepatotoxic medications, 11 (64%) were not classified as highly hepatotoxic in the published case reports, suggesting a discrepancy between real-world data and case report categorizations.
- Similarly, several medications, including some statins, identified as low-risk in this study were classified as among the most hepatotoxic in the published case reports.
IN PRACTICE:
“Categorization of hepatotoxicity based on the number of published case reports did not accurately reflect observed rates of severe ALI (acute liver injury),” the researchers wrote. “This study represents a systematic, reproducible approach to using real-world data to measure rates of severe ALI following medication initiation among patients without liver or biliary disease…Patients initiating a medication with a high rate of severe ALI might require closer monitoring of liver-related laboratory tests to detect evolving hepatic dysfunction earlier, which might improve prognosis.”
The study illustrates the potential to use electronic health record data to “revolutionize how we characterize drug-related toxic effects,” not just on the liver but other organs, Grace Y. Zhang, MD, and Jessica B. Rubin, MD, MPH, of the University of California, San Francisco, wrote in an accompanying editorial. “If curated and disseminated effectively…such evidence will undoubtedly improve clinical decision-making and allow for more informed patient counseling regarding the true risks of starting or discontinuing medications.
SOURCE:
The study, led by Jessie Torgersen, MD, MHS, MSCE, of the Division of Infectious Diseases, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, was published online in JAMA Internal Medicine.
LIMITATIONS:
The researchers listed several limitations, including the possibility that reliance on laboratory tests for ascertainment of acute liver injuries could introduce surveillance bias. The study focused on a population predominantly consisting of men without preexisting liver or biliary disease, so the findings may not be generalizable to women or individuals with liver disease. Additionally, researchers did not perform a causality assessment of all outcomes, did not study medications with fewer than four published case reports, and did not evaluate the influence of dosage.
DISCLOSURES:
This study was partly funded by several grants from the National Institutes of Health. Some authors declared receiving grants and personal fees from some of the funding agencies and other sources outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Buprenorphine One of Many Options For Pain Relief In Oldest Adults
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Long-Term Assessment of Weight Loss Medications in a Veteran Population
The Centers for Disease Control and Prevention (CDC) classifies individuals with a body mass index (BMI) of 25 to 29.9 as overweight and those with a BMI > 30 as obese (obesity classes: I, BMI 30 to 34.9; II, BMI 35 to 39.9; and III, BMI ≥ 40).1 In 2011, the CDC estimated that 27.4% of adults in the United States were obese; less than a decade later, that number increased to 31.9%.1 In that same period, the percentage of adults in Indiana classified as obese increased from 30.8% to 36.8%.1 About 1 in 14 individuals in the US have class III obesity and 86% of veterans are either overweight or obese.2
High medical expenses can likely be attributed to the long-term health consequences of obesity. Compared to those with a healthy weight, individuals who are overweight or obese are at an increased risk for high blood pressure, high low-density lipoprotein cholesterol levels, low high-density lipoprotein cholesterol levels, high triglyceride levels, type 2 diabetes mellitus (T2DM), coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, cancer, mental health disorders, body pain, low quality of life, and death.3 Many of these conditions lead to increased health care needs, medication needs, hospitalizations, and overall health care system use.
Guidelines for the prevention and treatment of obesity have been produced by the American Heart Association, American College of Cardiology, and The Obesity Society; the Endocrine Society; the American Diabetes Association; and the US Departments of Veterans Affairs (VA) and Defense. Each follows a general algorithm to manage and prevent adverse effects (AEs) related to obesity. General practice is to assess a patient for elevated BMI (> 25), implement intense lifestyle modifications including calorie restriction and exercise, reassess for a maintained 5% to 10% weight loss for cardiovascular benefits, and potentially assess for pharmacological or surgical intervention to assist in weight loss.2,4-6
While some weight loss medications (eg, phentermine/topiramate, naltrexone/bupropion, orlistat, and lorcaserin) tend to have unfavorable AEs or mixed efficacy, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have provided new options.7-10 Lorcaserin, for example, was removed from the market in 2020 due to its association with cancer risks.11 The GLP-1RAs liraglutide and semaglutide received US Food and Drug Administration (FDA) approval for weight loss in 2014 and 2021, respectively.12,13 GLP-1RAs have shown the greatest efficacy and benefits in reducing hemoglobin A1c (HbA1c); they are the preferred agents for patients who qualify for pharmacologic intervention for weight loss, especially those with T2DM. However, these studies have not evaluated the long-term outcomes of using these medications for weight loss and may not reflect the veteran population.14,15
At Veteran Health Indiana (VHI), clinicians may use several weight loss medications for patients to achieve 5% to 10% weight loss. The medications most often used include liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone. However, more research is needed to determine which weight loss medication is the most beneficial for veterans, particularly following FDA approval of GLP-1RAs. At VHI, phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended. These are considered first-line due to their ease of use in pill form, lower cost, and comparable weight loss to the GLP-1 medication class.2 However, for patients with prediabetes, T2DM, BMI > 40, or BMI > 35 with specific comorbid conditions, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2
This study aimed to expand on the 2021 Hood and colleagues study that examined total weight loss and weight loss as a percentage of baseline weight in patients with obesity at 3, 6, 12, and > 12 months of pharmacologic therapy by extending the time frame to 48 months.16 This study excluded semaglutide because few patients were prescribed the medication for weight loss during the study.
METHODS
We conducted a single-center, retrospective chart review of patients prescribed weight loss medications at VHI. A patient list was generated based on prescription fills from June 1, 2017, to July 31, 2021. Data were obtained from the Computerized Patient Record System; patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and VHI Research and Development Committee.
At the time of this study, liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone were available at VHI for patients who met the clinical criteria for use. All patients must have been enrolled in dietary and lifestyle management programs, including the VA MOVE! program, to be approved for these medications. After the MOVE! orientation, patients could participate in group or individual 12-week programs that included weigh-ins, goal-setting strategies, meal planning, and habit modification support. If patients could not meet in person, phone and other telehealth opportunities were available.
Patients were included in the study if they were aged ≥ 18 years, received a prescription for any of the 5 available medications for weight loss during the enrollment period, and were on the medication for ≥ 6 consecutive months. Patients were excluded if they received a prescription, were treated outside the VA system, or were pregnant. The primary indication for the included medication was not weight loss; the primary indication for the GLP-1RA was T2DM, or the weight loss was attributed to another disease. Adherence was not a measured outcome of this study; if patients were filling the medication, it was assumed they were taking it. Data were collected for each instance of medication use; as a result, a few patients were included more than once. Data collection for a failed medication ended when failure was documented. New data points began when new medication was prescribed; all data were per medication, not per patient. This allowed us to account for medication failure and provide accurate weight loss results based on medication choice within VHI.
Primary outcomes included total weight loss and weight loss as a percentage ofbaseline weight during the study period at 3, 6, 12, 24, 36, and 48 months of therapy. Secondary outcomes included the percentage of patients who lost 5% to 10% of their body weight from baseline; the percentage of patients who maintained ≥ 5% weight loss from baseline to 12, 24, 36, and 48 months if maintained on medication for that duration; duration of medication treatment in weeks; medication discontinuation rate; reason for medication discontinuation; enrollment in the MOVE! clinic and the time enrolled; percentage of patients with a BMI of 18 to 24.9 at the end of the study; and change in HbA1c at 3, 6, 12, 24, 36, and 48 months.
Demographic data included race, age, sex, baseline weight, height, baseline BMI, and comorbid conditions (collected based on the most recent primary care clinical note before initiating medication). Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, maintenance dose of medication, duration of medication during the study period, the reason for medication discontinuation, or bariatric surgery intervention if applicable.
Basic descriptive statistics were used to characterize study participants. For continuous data, analysis of variance tests were used; if those results were not normal, then nonparametric tests were used, followed by pairwise tests between medication groups if the overall test was significant using the Fisher significant differences test. For nominal data, χ2 or Fisher exact tests were used. For comparisons of primary and secondary outcomes, if the analyses needed to include adjustment for confounding variables, analysis of covariance was used for continuous data. A 2-sided 5% significance level was used for all tests.
RESULTS
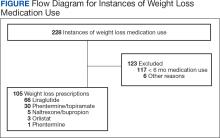
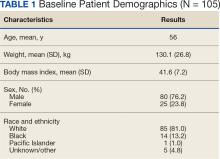
A total of 228 instances of medication use were identified based on prescription fills; 123 did not meet inclusion criteria (117 for < 6 consecutive months of medication use) (Figure). The study included 105 participants with a mean age of 56 years; 80 were male (76.2%), and 85 identified as White race (81.0%). Mean (SD) weight was 130.1 kg (26.8) and BMI was 41.6 (7.2). The most common comorbid disease states among patients included hypertension, dyslipidemia, obstructive sleep apnea, and T2DM (Table 1). The baseline characteristics were comparable to those of Hood and colleagues.16
Most patients at VHI started on liraglutide (63%) or phentermine/topiramate (28%). For primary and secondary outcomes, statistics were calculated to determine whether the results were statistically significant for comparing the liraglutide and phentermine/topiramate subgroups. Sample sizes were too small for statistical analysis for bupropion/naltrexone, phentermine, and orlistat.
Primary Outcomes
The mean (SD) weight of participants dropped 8.1% from 130.1 kg to 119.5 kg over the patient-specific duration of weight management medication therapy for an absolute difference of 10.6 kg (9.7). Duration of individual medication use varied from 6 to 48 months. Weight loss was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. Patient weight was not recorded after the medication was discontinued.
When classified by medication choice, the mean change in weight over the duration of the study was −23.9 kg for 2 patients using orlistat, −10.2 kg for 46 patients using liraglutide, −11.0 kg for 25 patients using phentermine/topiramate, -7.4 kg for 1 patient using phentermine, and -13.0 kg for 4 patients using naltrexone/bupropion. Patients without a weight documented at the end of their therapy or at the conclusion of the data collection period were not included in the total weight loss at the end of therapy. There were 78 documented instances of weight loss at the end of therapy (Table 2).
Body weight loss percentage was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. The mean (SD) body weight loss percentage over the duration of the study was 9.2% (11.2). When classified by medication choice, the mean percentage of body weight loss was 16.8% for 2 patients using orlistat, 9.4% for 46 patients using liraglutide, 8.2% for 25 patients using phentermine/topiramate, 6.0% for 1 patient using phentermine alone, and 10.6% for 4 patients using naltrexone/bupropion (Table 3).
Secondary Outcomes
While none of the secondary outcomes were statistically significant, the results of this study suggest that both medications may contribute to weight loss in many patients included in this study. Almost two-thirds of the included patients analyzed lost ≥ 5% of weight from baseline while taking weight management medication. Sixty-six patients (63%) lost ≥ 5% of body weight at any time during the data collection period. When stratified by liraglutide and phentermine/topiramate, 41 patients (63%) taking liraglutide and 20 patients (67%) taking phentermine/topiramate lost ≥ 5% of weight from baseline. Of the 66 patients who lost ≥ 5% of body weight from baseline, 36 (55%) lost ≥ 10% of body weight from baseline at any time during the data collection period.
The mean (SD) duration for weight management medication use was 23 months (14.9). Phentermine/topiramate was tolerated longer than liraglutide: 22.7 months vs 21.7 months, respectively (Table 4).
The average overall documented medication discontinuation rate was 35.2%. Reasons for discontinuation included 21 patient-elected discontinuations, 8 patients no longer met criteria for use, 4 medications were no longer indicated, and 4 patients experienced AEs. It is unknown whether weight management medication was discontinued or not in 18 patients (17.2%).
DISCUSSION
This study evaluated the use and outcomes of weight loss medications over a longer period (up to 48 months) than what was previously studied among patients at VHI (12 months). The study aimed to better understand the long-term effect of weight loss medications, determine which medication had better long-term outcomes, and examine the reasons for medication discontinuation.
The results of this study displayed some similarities and differences compared with the Hood and colleagues study.16 Both yielded similar results for 5% of body weight loss and 10% of body weight loss. The largest difference was mean weight loss over the study period. In this study, patients lost a mean 10.6 kg over the course of weight loss medication use compared to 15.8 kg found by Hood and colleagues.16 A reason patients in the current study lost less weight overall could be the difference in time frames. The current study encompassed the COVID-19 pandemic, meaning fewer overall in-person patient appointments, which led to patients being lost to follow-up, missing weigh-ins during the time period, and gaps in care. For some patients, the pandemic possibly contributed to depression, missed medication doses, and a more sedentary lifestyle, leading to more weight gain.17 Telemedicine services at VHI expanded during the pandemic in an attempt to increase patient monitoring and counseling. It is unclear whether this expansion was enough to replace the in-person contact necessary to promote a healthy lifestyle.
VA pharmacists now care for patients through telehealth and are more involved in weight loss management. Since the conclusion of the Hood and colleagues study and start of this research, 2 pharmacists at VHI have been assigned to follow patients for obesity management to help with adherence to medication and lifestyle changes, management of AEs, dispense logistics, interventions for medications that may cause weight gain, and case management of glycemic control and weight loss with GLP-1RAs. Care management by pharmacists at VHI helps improve the logistics of titratable orders and save money by improving the use of high-cost items like GLP-1RAs. VA clinical pharmacy practitioners already monitor GLP-1RAs for patients with T2DM, so they are prepared to educate and assist patients with these medications.
It is important to continue developing a standardized process for weight loss medication management across the VA to improve the quality of patient care and optimize prescription outcomes. VA facilities differ in how weight loss management care is delivered and the level at which pharmacists are involved. Given the high rate of obesity among patients at the VA, the advent of new prescription options for weight loss, and the high cost associated with these medications, there has been increased attention to obesity care. Some Veterans Integrated Service Networks are forming a weight management community of practice groups to create standard operating procedures and algorithms to standardize care. Developing consistent processes is necessary to improve weight loss and patient care for veterans regardless where they receive treatment.
Limitations
The data used in this study were dependent on clinician documentation. Because of a lack of documentation in many instances, it was difficult to determine the full efficacy of the medications studied due to missing weight recordings. The lack of documentation made it difficult to determine whether patients were enrolled and active in the MOVE! program. It is required that patients enroll in MOVE! to obtain medications, but many did not have any follow-up MOVE! visits after initially obtaining their weight loss medication.
In this study, differences in the outcomes of patients with and without T2DM were not compared. It is the VA standard of care to prefer liraglutide over phentermine/topiramate in patients with T2DM or prediabetes.2 This makes it difficult to assess whether phentermine/topiramate or liraglutide is more effective for weight loss in patients with T2DM. Weight gain after the discontinuation of weight loss medications was not assessed. Collecting this data may help determine whether a certain weight loss medication is less likely to cause rebound weight gain when discontinued.
Other limitations to this study consisted of excluding patients who discontinued therapy within 6 months, small sample sizes on some medications, and lack of data on adherence. Adherence was based on medication refills, which means that if a patient refilled the medication, it was assumed they were taking it. This is not always the case, and while accurate data on adherence is difficult to gather, it can impact how results may be interpreted. These additional limitations make it difficult to accurately determine the efficacy of the medications in this study.
CONCLUSIONS
This study found similar outcomes to what has been observed in larger clinical trials regarding weight loss medications. Nevertheless, there was a lack of accurate clinical documentation for most patients, which limits the conclusions. This lack of documentation potentially led to inaccurate results. It revealed that many patients at VHI did not uniformly receive consistent follow-up after starting a weight loss medication during the study period. With more standardized processes implemented at VA facilities, increased pharmacist involvement in weight loss medication management, and increased use of established telehealth services, patients could have the opportunity for closer follow-up that may lead to better weight loss outcomes. With these changes, there is more reason for additional studies to be conducted to assess follow-up, medication management, and weight loss overall.
1. Overweight & obesity. Centers for Disease Control and Prevention. Updated September 21, 2023. Accessed April 23, 2024. https://www.cdc.gov/obesity/index.html
2. US Department of Defense, US Department of Veterans Affairs. The Management of Adult Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for the Management of Adult Overweight and Obesity. Updated July 2020. Accessed April 23, 2024. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
3. Health effects of overweight and obesity. Centers for Disease Control and Prevention. Updated September 24, 2022. Accessed April 23, 2024. https://www.cdc.gov/healthyweight/effects/index.html
4. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
5. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415
6. American Diabetes Association Professional Practice Committee. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S39-S45. doi:10.2337/dc22-S003
7. Phentermine and topiramate extended-release. Package insert. Vivus, Inc; 2012. Accessed April 23, 2024. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf
8. Naltrexone and bupropion extended-release. Package insert. Orexigen Therapeutics, Inc; 2014. Accessed April 23, 2024. https://contrave.com/wp-content/uploads/2024/01/Contrave-label-113023.pdf
9. Orlistat. Package insert. Roche Laboratories, Inc; 2009. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf
10. Lorcaserin. Package insert. Arena Pharmaceuticals; 2012. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf
11. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. News release. US Food & Drug Administration. February 13, 2020. Accessed April 23, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
12. Saxenda Injection (Liraglutide [rDNA origin]). Novo Nordisk, Inc. October 1, 2015. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206321Orig1s000TOC.cfm
13. FDA approves new drug treatment for chronic weight management, first since 2014. News release. US Food & Drug Administration. June 4, 2021. Accessed April 23, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
14. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med. 2015;373:11-22. doi:10.1056/NEJMoa1411892
15. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. New Engl J Med 2021;384:989-1002. doi:10.1056/NEJMoa2032183
16. Hood SR, Berkeley AW, Moore EA. Evaluation of pharmacologic interventions for weight management in a veteran population. Fed Pract. 2021;38(5):220-226. doi:10.12788/fp.0117
17. Melamed OC, Selby P, Taylor VH. Mental health and obesity during the COVID-19 pandemic. Curr Obes Rep. 2022;11(1):23-31. doi:10.1007/s13679-021-00466-6
The Centers for Disease Control and Prevention (CDC) classifies individuals with a body mass index (BMI) of 25 to 29.9 as overweight and those with a BMI > 30 as obese (obesity classes: I, BMI 30 to 34.9; II, BMI 35 to 39.9; and III, BMI ≥ 40).1 In 2011, the CDC estimated that 27.4% of adults in the United States were obese; less than a decade later, that number increased to 31.9%.1 In that same period, the percentage of adults in Indiana classified as obese increased from 30.8% to 36.8%.1 About 1 in 14 individuals in the US have class III obesity and 86% of veterans are either overweight or obese.2
High medical expenses can likely be attributed to the long-term health consequences of obesity. Compared to those with a healthy weight, individuals who are overweight or obese are at an increased risk for high blood pressure, high low-density lipoprotein cholesterol levels, low high-density lipoprotein cholesterol levels, high triglyceride levels, type 2 diabetes mellitus (T2DM), coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, cancer, mental health disorders, body pain, low quality of life, and death.3 Many of these conditions lead to increased health care needs, medication needs, hospitalizations, and overall health care system use.
Guidelines for the prevention and treatment of obesity have been produced by the American Heart Association, American College of Cardiology, and The Obesity Society; the Endocrine Society; the American Diabetes Association; and the US Departments of Veterans Affairs (VA) and Defense. Each follows a general algorithm to manage and prevent adverse effects (AEs) related to obesity. General practice is to assess a patient for elevated BMI (> 25), implement intense lifestyle modifications including calorie restriction and exercise, reassess for a maintained 5% to 10% weight loss for cardiovascular benefits, and potentially assess for pharmacological or surgical intervention to assist in weight loss.2,4-6
While some weight loss medications (eg, phentermine/topiramate, naltrexone/bupropion, orlistat, and lorcaserin) tend to have unfavorable AEs or mixed efficacy, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have provided new options.7-10 Lorcaserin, for example, was removed from the market in 2020 due to its association with cancer risks.11 The GLP-1RAs liraglutide and semaglutide received US Food and Drug Administration (FDA) approval for weight loss in 2014 and 2021, respectively.12,13 GLP-1RAs have shown the greatest efficacy and benefits in reducing hemoglobin A1c (HbA1c); they are the preferred agents for patients who qualify for pharmacologic intervention for weight loss, especially those with T2DM. However, these studies have not evaluated the long-term outcomes of using these medications for weight loss and may not reflect the veteran population.14,15
At Veteran Health Indiana (VHI), clinicians may use several weight loss medications for patients to achieve 5% to 10% weight loss. The medications most often used include liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone. However, more research is needed to determine which weight loss medication is the most beneficial for veterans, particularly following FDA approval of GLP-1RAs. At VHI, phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended. These are considered first-line due to their ease of use in pill form, lower cost, and comparable weight loss to the GLP-1 medication class.2 However, for patients with prediabetes, T2DM, BMI > 40, or BMI > 35 with specific comorbid conditions, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2
This study aimed to expand on the 2021 Hood and colleagues study that examined total weight loss and weight loss as a percentage of baseline weight in patients with obesity at 3, 6, 12, and > 12 months of pharmacologic therapy by extending the time frame to 48 months.16 This study excluded semaglutide because few patients were prescribed the medication for weight loss during the study.
METHODS
We conducted a single-center, retrospective chart review of patients prescribed weight loss medications at VHI. A patient list was generated based on prescription fills from June 1, 2017, to July 31, 2021. Data were obtained from the Computerized Patient Record System; patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and VHI Research and Development Committee.
At the time of this study, liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone were available at VHI for patients who met the clinical criteria for use. All patients must have been enrolled in dietary and lifestyle management programs, including the VA MOVE! program, to be approved for these medications. After the MOVE! orientation, patients could participate in group or individual 12-week programs that included weigh-ins, goal-setting strategies, meal planning, and habit modification support. If patients could not meet in person, phone and other telehealth opportunities were available.
Patients were included in the study if they were aged ≥ 18 years, received a prescription for any of the 5 available medications for weight loss during the enrollment period, and were on the medication for ≥ 6 consecutive months. Patients were excluded if they received a prescription, were treated outside the VA system, or were pregnant. The primary indication for the included medication was not weight loss; the primary indication for the GLP-1RA was T2DM, or the weight loss was attributed to another disease. Adherence was not a measured outcome of this study; if patients were filling the medication, it was assumed they were taking it. Data were collected for each instance of medication use; as a result, a few patients were included more than once. Data collection for a failed medication ended when failure was documented. New data points began when new medication was prescribed; all data were per medication, not per patient. This allowed us to account for medication failure and provide accurate weight loss results based on medication choice within VHI.
Primary outcomes included total weight loss and weight loss as a percentage ofbaseline weight during the study period at 3, 6, 12, 24, 36, and 48 months of therapy. Secondary outcomes included the percentage of patients who lost 5% to 10% of their body weight from baseline; the percentage of patients who maintained ≥ 5% weight loss from baseline to 12, 24, 36, and 48 months if maintained on medication for that duration; duration of medication treatment in weeks; medication discontinuation rate; reason for medication discontinuation; enrollment in the MOVE! clinic and the time enrolled; percentage of patients with a BMI of 18 to 24.9 at the end of the study; and change in HbA1c at 3, 6, 12, 24, 36, and 48 months.
Demographic data included race, age, sex, baseline weight, height, baseline BMI, and comorbid conditions (collected based on the most recent primary care clinical note before initiating medication). Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, maintenance dose of medication, duration of medication during the study period, the reason for medication discontinuation, or bariatric surgery intervention if applicable.
Basic descriptive statistics were used to characterize study participants. For continuous data, analysis of variance tests were used; if those results were not normal, then nonparametric tests were used, followed by pairwise tests between medication groups if the overall test was significant using the Fisher significant differences test. For nominal data, χ2 or Fisher exact tests were used. For comparisons of primary and secondary outcomes, if the analyses needed to include adjustment for confounding variables, analysis of covariance was used for continuous data. A 2-sided 5% significance level was used for all tests.
RESULTS
A total of 228 instances of medication use were identified based on prescription fills; 123 did not meet inclusion criteria (117 for < 6 consecutive months of medication use) (Figure). The study included 105 participants with a mean age of 56 years; 80 were male (76.2%), and 85 identified as White race (81.0%). Mean (SD) weight was 130.1 kg (26.8) and BMI was 41.6 (7.2). The most common comorbid disease states among patients included hypertension, dyslipidemia, obstructive sleep apnea, and T2DM (Table 1). The baseline characteristics were comparable to those of Hood and colleagues.16
Most patients at VHI started on liraglutide (63%) or phentermine/topiramate (28%). For primary and secondary outcomes, statistics were calculated to determine whether the results were statistically significant for comparing the liraglutide and phentermine/topiramate subgroups. Sample sizes were too small for statistical analysis for bupropion/naltrexone, phentermine, and orlistat.
Primary Outcomes
The mean (SD) weight of participants dropped 8.1% from 130.1 kg to 119.5 kg over the patient-specific duration of weight management medication therapy for an absolute difference of 10.6 kg (9.7). Duration of individual medication use varied from 6 to 48 months. Weight loss was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. Patient weight was not recorded after the medication was discontinued.
When classified by medication choice, the mean change in weight over the duration of the study was −23.9 kg for 2 patients using orlistat, −10.2 kg for 46 patients using liraglutide, −11.0 kg for 25 patients using phentermine/topiramate, -7.4 kg for 1 patient using phentermine, and -13.0 kg for 4 patients using naltrexone/bupropion. Patients without a weight documented at the end of their therapy or at the conclusion of the data collection period were not included in the total weight loss at the end of therapy. There were 78 documented instances of weight loss at the end of therapy (Table 2).
Body weight loss percentage was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. The mean (SD) body weight loss percentage over the duration of the study was 9.2% (11.2). When classified by medication choice, the mean percentage of body weight loss was 16.8% for 2 patients using orlistat, 9.4% for 46 patients using liraglutide, 8.2% for 25 patients using phentermine/topiramate, 6.0% for 1 patient using phentermine alone, and 10.6% for 4 patients using naltrexone/bupropion (Table 3).
Secondary Outcomes
While none of the secondary outcomes were statistically significant, the results of this study suggest that both medications may contribute to weight loss in many patients included in this study. Almost two-thirds of the included patients analyzed lost ≥ 5% of weight from baseline while taking weight management medication. Sixty-six patients (63%) lost ≥ 5% of body weight at any time during the data collection period. When stratified by liraglutide and phentermine/topiramate, 41 patients (63%) taking liraglutide and 20 patients (67%) taking phentermine/topiramate lost ≥ 5% of weight from baseline. Of the 66 patients who lost ≥ 5% of body weight from baseline, 36 (55%) lost ≥ 10% of body weight from baseline at any time during the data collection period.
The mean (SD) duration for weight management medication use was 23 months (14.9). Phentermine/topiramate was tolerated longer than liraglutide: 22.7 months vs 21.7 months, respectively (Table 4).
The average overall documented medication discontinuation rate was 35.2%. Reasons for discontinuation included 21 patient-elected discontinuations, 8 patients no longer met criteria for use, 4 medications were no longer indicated, and 4 patients experienced AEs. It is unknown whether weight management medication was discontinued or not in 18 patients (17.2%).
DISCUSSION
This study evaluated the use and outcomes of weight loss medications over a longer period (up to 48 months) than what was previously studied among patients at VHI (12 months). The study aimed to better understand the long-term effect of weight loss medications, determine which medication had better long-term outcomes, and examine the reasons for medication discontinuation.
The results of this study displayed some similarities and differences compared with the Hood and colleagues study.16 Both yielded similar results for 5% of body weight loss and 10% of body weight loss. The largest difference was mean weight loss over the study period. In this study, patients lost a mean 10.6 kg over the course of weight loss medication use compared to 15.8 kg found by Hood and colleagues.16 A reason patients in the current study lost less weight overall could be the difference in time frames. The current study encompassed the COVID-19 pandemic, meaning fewer overall in-person patient appointments, which led to patients being lost to follow-up, missing weigh-ins during the time period, and gaps in care. For some patients, the pandemic possibly contributed to depression, missed medication doses, and a more sedentary lifestyle, leading to more weight gain.17 Telemedicine services at VHI expanded during the pandemic in an attempt to increase patient monitoring and counseling. It is unclear whether this expansion was enough to replace the in-person contact necessary to promote a healthy lifestyle.
VA pharmacists now care for patients through telehealth and are more involved in weight loss management. Since the conclusion of the Hood and colleagues study and start of this research, 2 pharmacists at VHI have been assigned to follow patients for obesity management to help with adherence to medication and lifestyle changes, management of AEs, dispense logistics, interventions for medications that may cause weight gain, and case management of glycemic control and weight loss with GLP-1RAs. Care management by pharmacists at VHI helps improve the logistics of titratable orders and save money by improving the use of high-cost items like GLP-1RAs. VA clinical pharmacy practitioners already monitor GLP-1RAs for patients with T2DM, so they are prepared to educate and assist patients with these medications.
It is important to continue developing a standardized process for weight loss medication management across the VA to improve the quality of patient care and optimize prescription outcomes. VA facilities differ in how weight loss management care is delivered and the level at which pharmacists are involved. Given the high rate of obesity among patients at the VA, the advent of new prescription options for weight loss, and the high cost associated with these medications, there has been increased attention to obesity care. Some Veterans Integrated Service Networks are forming a weight management community of practice groups to create standard operating procedures and algorithms to standardize care. Developing consistent processes is necessary to improve weight loss and patient care for veterans regardless where they receive treatment.
Limitations
The data used in this study were dependent on clinician documentation. Because of a lack of documentation in many instances, it was difficult to determine the full efficacy of the medications studied due to missing weight recordings. The lack of documentation made it difficult to determine whether patients were enrolled and active in the MOVE! program. It is required that patients enroll in MOVE! to obtain medications, but many did not have any follow-up MOVE! visits after initially obtaining their weight loss medication.
In this study, differences in the outcomes of patients with and without T2DM were not compared. It is the VA standard of care to prefer liraglutide over phentermine/topiramate in patients with T2DM or prediabetes.2 This makes it difficult to assess whether phentermine/topiramate or liraglutide is more effective for weight loss in patients with T2DM. Weight gain after the discontinuation of weight loss medications was not assessed. Collecting this data may help determine whether a certain weight loss medication is less likely to cause rebound weight gain when discontinued.
Other limitations to this study consisted of excluding patients who discontinued therapy within 6 months, small sample sizes on some medications, and lack of data on adherence. Adherence was based on medication refills, which means that if a patient refilled the medication, it was assumed they were taking it. This is not always the case, and while accurate data on adherence is difficult to gather, it can impact how results may be interpreted. These additional limitations make it difficult to accurately determine the efficacy of the medications in this study.
CONCLUSIONS
This study found similar outcomes to what has been observed in larger clinical trials regarding weight loss medications. Nevertheless, there was a lack of accurate clinical documentation for most patients, which limits the conclusions. This lack of documentation potentially led to inaccurate results. It revealed that many patients at VHI did not uniformly receive consistent follow-up after starting a weight loss medication during the study period. With more standardized processes implemented at VA facilities, increased pharmacist involvement in weight loss medication management, and increased use of established telehealth services, patients could have the opportunity for closer follow-up that may lead to better weight loss outcomes. With these changes, there is more reason for additional studies to be conducted to assess follow-up, medication management, and weight loss overall.
The Centers for Disease Control and Prevention (CDC) classifies individuals with a body mass index (BMI) of 25 to 29.9 as overweight and those with a BMI > 30 as obese (obesity classes: I, BMI 30 to 34.9; II, BMI 35 to 39.9; and III, BMI ≥ 40).1 In 2011, the CDC estimated that 27.4% of adults in the United States were obese; less than a decade later, that number increased to 31.9%.1 In that same period, the percentage of adults in Indiana classified as obese increased from 30.8% to 36.8%.1 About 1 in 14 individuals in the US have class III obesity and 86% of veterans are either overweight or obese.2
High medical expenses can likely be attributed to the long-term health consequences of obesity. Compared to those with a healthy weight, individuals who are overweight or obese are at an increased risk for high blood pressure, high low-density lipoprotein cholesterol levels, low high-density lipoprotein cholesterol levels, high triglyceride levels, type 2 diabetes mellitus (T2DM), coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, cancer, mental health disorders, body pain, low quality of life, and death.3 Many of these conditions lead to increased health care needs, medication needs, hospitalizations, and overall health care system use.
Guidelines for the prevention and treatment of obesity have been produced by the American Heart Association, American College of Cardiology, and The Obesity Society; the Endocrine Society; the American Diabetes Association; and the US Departments of Veterans Affairs (VA) and Defense. Each follows a general algorithm to manage and prevent adverse effects (AEs) related to obesity. General practice is to assess a patient for elevated BMI (> 25), implement intense lifestyle modifications including calorie restriction and exercise, reassess for a maintained 5% to 10% weight loss for cardiovascular benefits, and potentially assess for pharmacological or surgical intervention to assist in weight loss.2,4-6
While some weight loss medications (eg, phentermine/topiramate, naltrexone/bupropion, orlistat, and lorcaserin) tend to have unfavorable AEs or mixed efficacy, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have provided new options.7-10 Lorcaserin, for example, was removed from the market in 2020 due to its association with cancer risks.11 The GLP-1RAs liraglutide and semaglutide received US Food and Drug Administration (FDA) approval for weight loss in 2014 and 2021, respectively.12,13 GLP-1RAs have shown the greatest efficacy and benefits in reducing hemoglobin A1c (HbA1c); they are the preferred agents for patients who qualify for pharmacologic intervention for weight loss, especially those with T2DM. However, these studies have not evaluated the long-term outcomes of using these medications for weight loss and may not reflect the veteran population.14,15
At Veteran Health Indiana (VHI), clinicians may use several weight loss medications for patients to achieve 5% to 10% weight loss. The medications most often used include liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone. However, more research is needed to determine which weight loss medication is the most beneficial for veterans, particularly following FDA approval of GLP-1RAs. At VHI, phentermine/topiramate is the preferred first-line agent unless patients have contraindications for use, in which case naltrexone/bupropion is recommended. These are considered first-line due to their ease of use in pill form, lower cost, and comparable weight loss to the GLP-1 medication class.2 However, for patients with prediabetes, T2DM, BMI > 40, or BMI > 35 with specific comorbid conditions, liraglutide is preferred because of its beneficial effects for both weight loss and blood glucose control.2
This study aimed to expand on the 2021 Hood and colleagues study that examined total weight loss and weight loss as a percentage of baseline weight in patients with obesity at 3, 6, 12, and > 12 months of pharmacologic therapy by extending the time frame to 48 months.16 This study excluded semaglutide because few patients were prescribed the medication for weight loss during the study.
METHODS
We conducted a single-center, retrospective chart review of patients prescribed weight loss medications at VHI. A patient list was generated based on prescription fills from June 1, 2017, to July 31, 2021. Data were obtained from the Computerized Patient Record System; patients were not contacted. This study was approved by the Indiana University Health Institutional Review Board and VHI Research and Development Committee.
At the time of this study, liraglutide, phentermine/topiramate, naltrexone/bupropion, orlistat, and phentermine alone were available at VHI for patients who met the clinical criteria for use. All patients must have been enrolled in dietary and lifestyle management programs, including the VA MOVE! program, to be approved for these medications. After the MOVE! orientation, patients could participate in group or individual 12-week programs that included weigh-ins, goal-setting strategies, meal planning, and habit modification support. If patients could not meet in person, phone and other telehealth opportunities were available.
Patients were included in the study if they were aged ≥ 18 years, received a prescription for any of the 5 available medications for weight loss during the enrollment period, and were on the medication for ≥ 6 consecutive months. Patients were excluded if they received a prescription, were treated outside the VA system, or were pregnant. The primary indication for the included medication was not weight loss; the primary indication for the GLP-1RA was T2DM, or the weight loss was attributed to another disease. Adherence was not a measured outcome of this study; if patients were filling the medication, it was assumed they were taking it. Data were collected for each instance of medication use; as a result, a few patients were included more than once. Data collection for a failed medication ended when failure was documented. New data points began when new medication was prescribed; all data were per medication, not per patient. This allowed us to account for medication failure and provide accurate weight loss results based on medication choice within VHI.
Primary outcomes included total weight loss and weight loss as a percentage ofbaseline weight during the study period at 3, 6, 12, 24, 36, and 48 months of therapy. Secondary outcomes included the percentage of patients who lost 5% to 10% of their body weight from baseline; the percentage of patients who maintained ≥ 5% weight loss from baseline to 12, 24, 36, and 48 months if maintained on medication for that duration; duration of medication treatment in weeks; medication discontinuation rate; reason for medication discontinuation; enrollment in the MOVE! clinic and the time enrolled; percentage of patients with a BMI of 18 to 24.9 at the end of the study; and change in HbA1c at 3, 6, 12, 24, 36, and 48 months.
Demographic data included race, age, sex, baseline weight, height, baseline BMI, and comorbid conditions (collected based on the most recent primary care clinical note before initiating medication). Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, maintenance dose of medication, duration of medication during the study period, the reason for medication discontinuation, or bariatric surgery intervention if applicable.
Basic descriptive statistics were used to characterize study participants. For continuous data, analysis of variance tests were used; if those results were not normal, then nonparametric tests were used, followed by pairwise tests between medication groups if the overall test was significant using the Fisher significant differences test. For nominal data, χ2 or Fisher exact tests were used. For comparisons of primary and secondary outcomes, if the analyses needed to include adjustment for confounding variables, analysis of covariance was used for continuous data. A 2-sided 5% significance level was used for all tests.
RESULTS
A total of 228 instances of medication use were identified based on prescription fills; 123 did not meet inclusion criteria (117 for < 6 consecutive months of medication use) (Figure). The study included 105 participants with a mean age of 56 years; 80 were male (76.2%), and 85 identified as White race (81.0%). Mean (SD) weight was 130.1 kg (26.8) and BMI was 41.6 (7.2). The most common comorbid disease states among patients included hypertension, dyslipidemia, obstructive sleep apnea, and T2DM (Table 1). The baseline characteristics were comparable to those of Hood and colleagues.16
Most patients at VHI started on liraglutide (63%) or phentermine/topiramate (28%). For primary and secondary outcomes, statistics were calculated to determine whether the results were statistically significant for comparing the liraglutide and phentermine/topiramate subgroups. Sample sizes were too small for statistical analysis for bupropion/naltrexone, phentermine, and orlistat.
Primary Outcomes
The mean (SD) weight of participants dropped 8.1% from 130.1 kg to 119.5 kg over the patient-specific duration of weight management medication therapy for an absolute difference of 10.6 kg (9.7). Duration of individual medication use varied from 6 to 48 months. Weight loss was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. Patient weight was not recorded after the medication was discontinued.
When classified by medication choice, the mean change in weight over the duration of the study was −23.9 kg for 2 patients using orlistat, −10.2 kg for 46 patients using liraglutide, −11.0 kg for 25 patients using phentermine/topiramate, -7.4 kg for 1 patient using phentermine, and -13.0 kg for 4 patients using naltrexone/bupropion. Patients without a weight documented at the end of their therapy or at the conclusion of the data collection period were not included in the total weight loss at the end of therapy. There were 78 documented instances of weight loss at the end of therapy (Table 2).
Body weight loss percentage was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. The mean (SD) body weight loss percentage over the duration of the study was 9.2% (11.2). When classified by medication choice, the mean percentage of body weight loss was 16.8% for 2 patients using orlistat, 9.4% for 46 patients using liraglutide, 8.2% for 25 patients using phentermine/topiramate, 6.0% for 1 patient using phentermine alone, and 10.6% for 4 patients using naltrexone/bupropion (Table 3).
Secondary Outcomes
While none of the secondary outcomes were statistically significant, the results of this study suggest that both medications may contribute to weight loss in many patients included in this study. Almost two-thirds of the included patients analyzed lost ≥ 5% of weight from baseline while taking weight management medication. Sixty-six patients (63%) lost ≥ 5% of body weight at any time during the data collection period. When stratified by liraglutide and phentermine/topiramate, 41 patients (63%) taking liraglutide and 20 patients (67%) taking phentermine/topiramate lost ≥ 5% of weight from baseline. Of the 66 patients who lost ≥ 5% of body weight from baseline, 36 (55%) lost ≥ 10% of body weight from baseline at any time during the data collection period.
The mean (SD) duration for weight management medication use was 23 months (14.9). Phentermine/topiramate was tolerated longer than liraglutide: 22.7 months vs 21.7 months, respectively (Table 4).
The average overall documented medication discontinuation rate was 35.2%. Reasons for discontinuation included 21 patient-elected discontinuations, 8 patients no longer met criteria for use, 4 medications were no longer indicated, and 4 patients experienced AEs. It is unknown whether weight management medication was discontinued or not in 18 patients (17.2%).
DISCUSSION
This study evaluated the use and outcomes of weight loss medications over a longer period (up to 48 months) than what was previously studied among patients at VHI (12 months). The study aimed to better understand the long-term effect of weight loss medications, determine which medication had better long-term outcomes, and examine the reasons for medication discontinuation.
The results of this study displayed some similarities and differences compared with the Hood and colleagues study.16 Both yielded similar results for 5% of body weight loss and 10% of body weight loss. The largest difference was mean weight loss over the study period. In this study, patients lost a mean 10.6 kg over the course of weight loss medication use compared to 15.8 kg found by Hood and colleagues.16 A reason patients in the current study lost less weight overall could be the difference in time frames. The current study encompassed the COVID-19 pandemic, meaning fewer overall in-person patient appointments, which led to patients being lost to follow-up, missing weigh-ins during the time period, and gaps in care. For some patients, the pandemic possibly contributed to depression, missed medication doses, and a more sedentary lifestyle, leading to more weight gain.17 Telemedicine services at VHI expanded during the pandemic in an attempt to increase patient monitoring and counseling. It is unclear whether this expansion was enough to replace the in-person contact necessary to promote a healthy lifestyle.
VA pharmacists now care for patients through telehealth and are more involved in weight loss management. Since the conclusion of the Hood and colleagues study and start of this research, 2 pharmacists at VHI have been assigned to follow patients for obesity management to help with adherence to medication and lifestyle changes, management of AEs, dispense logistics, interventions for medications that may cause weight gain, and case management of glycemic control and weight loss with GLP-1RAs. Care management by pharmacists at VHI helps improve the logistics of titratable orders and save money by improving the use of high-cost items like GLP-1RAs. VA clinical pharmacy practitioners already monitor GLP-1RAs for patients with T2DM, so they are prepared to educate and assist patients with these medications.
It is important to continue developing a standardized process for weight loss medication management across the VA to improve the quality of patient care and optimize prescription outcomes. VA facilities differ in how weight loss management care is delivered and the level at which pharmacists are involved. Given the high rate of obesity among patients at the VA, the advent of new prescription options for weight loss, and the high cost associated with these medications, there has been increased attention to obesity care. Some Veterans Integrated Service Networks are forming a weight management community of practice groups to create standard operating procedures and algorithms to standardize care. Developing consistent processes is necessary to improve weight loss and patient care for veterans regardless where they receive treatment.
Limitations
The data used in this study were dependent on clinician documentation. Because of a lack of documentation in many instances, it was difficult to determine the full efficacy of the medications studied due to missing weight recordings. The lack of documentation made it difficult to determine whether patients were enrolled and active in the MOVE! program. It is required that patients enroll in MOVE! to obtain medications, but many did not have any follow-up MOVE! visits after initially obtaining their weight loss medication.
In this study, differences in the outcomes of patients with and without T2DM were not compared. It is the VA standard of care to prefer liraglutide over phentermine/topiramate in patients with T2DM or prediabetes.2 This makes it difficult to assess whether phentermine/topiramate or liraglutide is more effective for weight loss in patients with T2DM. Weight gain after the discontinuation of weight loss medications was not assessed. Collecting this data may help determine whether a certain weight loss medication is less likely to cause rebound weight gain when discontinued.
Other limitations to this study consisted of excluding patients who discontinued therapy within 6 months, small sample sizes on some medications, and lack of data on adherence. Adherence was based on medication refills, which means that if a patient refilled the medication, it was assumed they were taking it. This is not always the case, and while accurate data on adherence is difficult to gather, it can impact how results may be interpreted. These additional limitations make it difficult to accurately determine the efficacy of the medications in this study.
CONCLUSIONS
This study found similar outcomes to what has been observed in larger clinical trials regarding weight loss medications. Nevertheless, there was a lack of accurate clinical documentation for most patients, which limits the conclusions. This lack of documentation potentially led to inaccurate results. It revealed that many patients at VHI did not uniformly receive consistent follow-up after starting a weight loss medication during the study period. With more standardized processes implemented at VA facilities, increased pharmacist involvement in weight loss medication management, and increased use of established telehealth services, patients could have the opportunity for closer follow-up that may lead to better weight loss outcomes. With these changes, there is more reason for additional studies to be conducted to assess follow-up, medication management, and weight loss overall.
1. Overweight & obesity. Centers for Disease Control and Prevention. Updated September 21, 2023. Accessed April 23, 2024. https://www.cdc.gov/obesity/index.html
2. US Department of Defense, US Department of Veterans Affairs. The Management of Adult Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for the Management of Adult Overweight and Obesity. Updated July 2020. Accessed April 23, 2024. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
3. Health effects of overweight and obesity. Centers for Disease Control and Prevention. Updated September 24, 2022. Accessed April 23, 2024. https://www.cdc.gov/healthyweight/effects/index.html
4. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
5. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415
6. American Diabetes Association Professional Practice Committee. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S39-S45. doi:10.2337/dc22-S003
7. Phentermine and topiramate extended-release. Package insert. Vivus, Inc; 2012. Accessed April 23, 2024. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf
8. Naltrexone and bupropion extended-release. Package insert. Orexigen Therapeutics, Inc; 2014. Accessed April 23, 2024. https://contrave.com/wp-content/uploads/2024/01/Contrave-label-113023.pdf
9. Orlistat. Package insert. Roche Laboratories, Inc; 2009. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf
10. Lorcaserin. Package insert. Arena Pharmaceuticals; 2012. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf
11. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. News release. US Food & Drug Administration. February 13, 2020. Accessed April 23, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
12. Saxenda Injection (Liraglutide [rDNA origin]). Novo Nordisk, Inc. October 1, 2015. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206321Orig1s000TOC.cfm
13. FDA approves new drug treatment for chronic weight management, first since 2014. News release. US Food & Drug Administration. June 4, 2021. Accessed April 23, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
14. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med. 2015;373:11-22. doi:10.1056/NEJMoa1411892
15. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. New Engl J Med 2021;384:989-1002. doi:10.1056/NEJMoa2032183
16. Hood SR, Berkeley AW, Moore EA. Evaluation of pharmacologic interventions for weight management in a veteran population. Fed Pract. 2021;38(5):220-226. doi:10.12788/fp.0117
17. Melamed OC, Selby P, Taylor VH. Mental health and obesity during the COVID-19 pandemic. Curr Obes Rep. 2022;11(1):23-31. doi:10.1007/s13679-021-00466-6
1. Overweight & obesity. Centers for Disease Control and Prevention. Updated September 21, 2023. Accessed April 23, 2024. https://www.cdc.gov/obesity/index.html
2. US Department of Defense, US Department of Veterans Affairs. The Management of Adult Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for the Management of Adult Overweight and Obesity. Updated July 2020. Accessed April 23, 2024. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
3. Health effects of overweight and obesity. Centers for Disease Control and Prevention. Updated September 24, 2022. Accessed April 23, 2024. https://www.cdc.gov/healthyweight/effects/index.html
4. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
5. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415
6. American Diabetes Association Professional Practice Committee. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S39-S45. doi:10.2337/dc22-S003
7. Phentermine and topiramate extended-release. Package insert. Vivus, Inc; 2012. Accessed April 23, 2024. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf
8. Naltrexone and bupropion extended-release. Package insert. Orexigen Therapeutics, Inc; 2014. Accessed April 23, 2024. https://contrave.com/wp-content/uploads/2024/01/Contrave-label-113023.pdf
9. Orlistat. Package insert. Roche Laboratories, Inc; 2009. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf
10. Lorcaserin. Package insert. Arena Pharmaceuticals; 2012. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf
11. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. News release. US Food & Drug Administration. February 13, 2020. Accessed April 23, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
12. Saxenda Injection (Liraglutide [rDNA origin]). Novo Nordisk, Inc. October 1, 2015. Accessed April 23, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206321Orig1s000TOC.cfm
13. FDA approves new drug treatment for chronic weight management, first since 2014. News release. US Food & Drug Administration. June 4, 2021. Accessed April 23, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
14. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med. 2015;373:11-22. doi:10.1056/NEJMoa1411892
15. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. New Engl J Med 2021;384:989-1002. doi:10.1056/NEJMoa2032183
16. Hood SR, Berkeley AW, Moore EA. Evaluation of pharmacologic interventions for weight management in a veteran population. Fed Pract. 2021;38(5):220-226. doi:10.12788/fp.0117
17. Melamed OC, Selby P, Taylor VH. Mental health and obesity during the COVID-19 pandemic. Curr Obes Rep. 2022;11(1):23-31. doi:10.1007/s13679-021-00466-6
OTC Solution for Erectile Dysfunction?
Up to 60% of men with erectile dysfunction who were not candidates for phosphodiesterase 5 (PDE5) inhibitors achieved erections in less than 10 minutes after a single application of a first-on-the-market nonprescription gel to the glans, a new study found.
Wayne Hellstrom, MD, chief of andrology at Tulane School of Medicine in New Orleans, who presented the study of MED3000 [Eroxon] on May 5 at the 2024 annual meeting of the American Urological Association in San Antonio, Texas, said that the gel is considered to be a device by the US Food and Drug Administration (FDA). The agency approved the product in June 2023.
A spokesman for Futura, which makes MED3000, said that the gel will be on the market 2025. No price for the United States has been announced, but a four-pack of single-use tubes sells for the equivalent of roughly $31 in the United Kingdom.
Dr. Hellstrom, a former adviser to Futura, he said he expects MED3000 will be “a potential first-line therapy in addition to PDE5 inhibitors,” which are vasodilating drugs that stimulate the corpora cavernosa of the penis, facilitating erection with sexual stimulation.
He noted that PDE5s are contraindicated for many men; are not tolerated in others; are not completely effective; or work too slowly, taking 1-2 hours to work. As a result, up to 50% of patients cease using a PDE5 inhibitor within 1 year, he said.
Futura said the gel contains a combination of volatile solvents which, when applied to the head of the penis, evaporate rapidly, stimulating nerve endings through an initial cooling effect followed by a warming sensation. This reaction releases nitric oxide, relaxing the smooth muscle tissue inside the penis and increasing blood flow that is needed to obtain an erection.
Dr. Hellstrom noted that MED3000 is noninvasive and causes no side effects and is slightly more effective if applied by a partner.
The new findings come from two studies of 250 men with erectile dysfunction (FM57) who used MED3000 over 12 weeks and a randomly assigned arm (FM71) with two groups of 48 men who used either MED3000 or 5 mg of tadalafil over 24 weeks.
Erections were achieved in less than 10 minutes in 60.1% of men in the FM57 group and 44.9% of those in the FM71 group.
Overall, less than 2% of the men who usedMED3000 and 4% of those who took tadalafil reported adverse effects. These events included headaches in 3% of the combined MED3000 group and 19.1% of the tadalafil group. Roughly 1% of men who used MED3000 reported penile burning sensation compared with none in the group taking tadalafil.
Problematic Design?
Kevin McVary, MD, a professor of urology at Stritch School of Medicine of Loyola University, outside of Chicago, and director of the Center for Male Health, criticized the study design and added that he did not believe MED3000 had been proven beneficial.
“Are they expecting the Cialis 5 mg to work within 10 minutes? Because it doesn’t,” Dr. McVary said. “It doesn’t get absorbed into the bloodstream for about 2.5 hours.”
Dr. McVary said that men with erectile dysfunction will probably do anything to avoid seeing a physician about the condition, which could make MED3000 highly marketable.
However, he said, examinations would be important to detect unrecognized underlying cardiac disease, especially in younger men. “ED can function as the classic canary in a coal mine where it tells you who’s at risk for unexpected early death,” he said.
Dr. Hellstrom is a former adviser to Futura Medical Developments, which funded the research. Dr. McVary reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com .
Up to 60% of men with erectile dysfunction who were not candidates for phosphodiesterase 5 (PDE5) inhibitors achieved erections in less than 10 minutes after a single application of a first-on-the-market nonprescription gel to the glans, a new study found.
Wayne Hellstrom, MD, chief of andrology at Tulane School of Medicine in New Orleans, who presented the study of MED3000 [Eroxon] on May 5 at the 2024 annual meeting of the American Urological Association in San Antonio, Texas, said that the gel is considered to be a device by the US Food and Drug Administration (FDA). The agency approved the product in June 2023.
A spokesman for Futura, which makes MED3000, said that the gel will be on the market 2025. No price for the United States has been announced, but a four-pack of single-use tubes sells for the equivalent of roughly $31 in the United Kingdom.
Dr. Hellstrom, a former adviser to Futura, he said he expects MED3000 will be “a potential first-line therapy in addition to PDE5 inhibitors,” which are vasodilating drugs that stimulate the corpora cavernosa of the penis, facilitating erection with sexual stimulation.
He noted that PDE5s are contraindicated for many men; are not tolerated in others; are not completely effective; or work too slowly, taking 1-2 hours to work. As a result, up to 50% of patients cease using a PDE5 inhibitor within 1 year, he said.
Futura said the gel contains a combination of volatile solvents which, when applied to the head of the penis, evaporate rapidly, stimulating nerve endings through an initial cooling effect followed by a warming sensation. This reaction releases nitric oxide, relaxing the smooth muscle tissue inside the penis and increasing blood flow that is needed to obtain an erection.
Dr. Hellstrom noted that MED3000 is noninvasive and causes no side effects and is slightly more effective if applied by a partner.
The new findings come from two studies of 250 men with erectile dysfunction (FM57) who used MED3000 over 12 weeks and a randomly assigned arm (FM71) with two groups of 48 men who used either MED3000 or 5 mg of tadalafil over 24 weeks.
Erections were achieved in less than 10 minutes in 60.1% of men in the FM57 group and 44.9% of those in the FM71 group.
Overall, less than 2% of the men who usedMED3000 and 4% of those who took tadalafil reported adverse effects. These events included headaches in 3% of the combined MED3000 group and 19.1% of the tadalafil group. Roughly 1% of men who used MED3000 reported penile burning sensation compared with none in the group taking tadalafil.
Problematic Design?
Kevin McVary, MD, a professor of urology at Stritch School of Medicine of Loyola University, outside of Chicago, and director of the Center for Male Health, criticized the study design and added that he did not believe MED3000 had been proven beneficial.
“Are they expecting the Cialis 5 mg to work within 10 minutes? Because it doesn’t,” Dr. McVary said. “It doesn’t get absorbed into the bloodstream for about 2.5 hours.”
Dr. McVary said that men with erectile dysfunction will probably do anything to avoid seeing a physician about the condition, which could make MED3000 highly marketable.
However, he said, examinations would be important to detect unrecognized underlying cardiac disease, especially in younger men. “ED can function as the classic canary in a coal mine where it tells you who’s at risk for unexpected early death,” he said.
Dr. Hellstrom is a former adviser to Futura Medical Developments, which funded the research. Dr. McVary reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com .
Up to 60% of men with erectile dysfunction who were not candidates for phosphodiesterase 5 (PDE5) inhibitors achieved erections in less than 10 minutes after a single application of a first-on-the-market nonprescription gel to the glans, a new study found.
Wayne Hellstrom, MD, chief of andrology at Tulane School of Medicine in New Orleans, who presented the study of MED3000 [Eroxon] on May 5 at the 2024 annual meeting of the American Urological Association in San Antonio, Texas, said that the gel is considered to be a device by the US Food and Drug Administration (FDA). The agency approved the product in June 2023.
A spokesman for Futura, which makes MED3000, said that the gel will be on the market 2025. No price for the United States has been announced, but a four-pack of single-use tubes sells for the equivalent of roughly $31 in the United Kingdom.
Dr. Hellstrom, a former adviser to Futura, he said he expects MED3000 will be “a potential first-line therapy in addition to PDE5 inhibitors,” which are vasodilating drugs that stimulate the corpora cavernosa of the penis, facilitating erection with sexual stimulation.
He noted that PDE5s are contraindicated for many men; are not tolerated in others; are not completely effective; or work too slowly, taking 1-2 hours to work. As a result, up to 50% of patients cease using a PDE5 inhibitor within 1 year, he said.
Futura said the gel contains a combination of volatile solvents which, when applied to the head of the penis, evaporate rapidly, stimulating nerve endings through an initial cooling effect followed by a warming sensation. This reaction releases nitric oxide, relaxing the smooth muscle tissue inside the penis and increasing blood flow that is needed to obtain an erection.
Dr. Hellstrom noted that MED3000 is noninvasive and causes no side effects and is slightly more effective if applied by a partner.
The new findings come from two studies of 250 men with erectile dysfunction (FM57) who used MED3000 over 12 weeks and a randomly assigned arm (FM71) with two groups of 48 men who used either MED3000 or 5 mg of tadalafil over 24 weeks.
Erections were achieved in less than 10 minutes in 60.1% of men in the FM57 group and 44.9% of those in the FM71 group.
Overall, less than 2% of the men who usedMED3000 and 4% of those who took tadalafil reported adverse effects. These events included headaches in 3% of the combined MED3000 group and 19.1% of the tadalafil group. Roughly 1% of men who used MED3000 reported penile burning sensation compared with none in the group taking tadalafil.
Problematic Design?
Kevin McVary, MD, a professor of urology at Stritch School of Medicine of Loyola University, outside of Chicago, and director of the Center for Male Health, criticized the study design and added that he did not believe MED3000 had been proven beneficial.
“Are they expecting the Cialis 5 mg to work within 10 minutes? Because it doesn’t,” Dr. McVary said. “It doesn’t get absorbed into the bloodstream for about 2.5 hours.”
Dr. McVary said that men with erectile dysfunction will probably do anything to avoid seeing a physician about the condition, which could make MED3000 highly marketable.
However, he said, examinations would be important to detect unrecognized underlying cardiac disease, especially in younger men. “ED can function as the classic canary in a coal mine where it tells you who’s at risk for unexpected early death,” he said.
Dr. Hellstrom is a former adviser to Futura Medical Developments, which funded the research. Dr. McVary reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com .
Knee Osteoarthritis Trials Show Promising Results for Several Novel Injectables
VIENNA — Encouraging primary or secondary analyses of trial data for the use of several novel injectables and gene therapy for knee osteoarthritis (OA) were reported at the OARSI 2024 World Congress.
Of all the approaches discussed during the News in Therapies session at OARSI 2024, the most intriguing was the use of the placental extract PTP-001 (MOTYS, Bioventus), session chair Nancy E. Lane, MD, of the University of California Davis School of Medicine, Sacramento, California, told this news organization.
Other notable presentations of data from trials of investigational agents for knee OA included an update from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate; a phase 2 trial of pentosan polysulfate sodium (PPS), a non-opioid, semi-synthetic xylose-based polysaccharide; and an update on phase 2 study results for XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant of interleukin 10 (IL-10).
PTP-001 (MOTYS)
Indeed, promising results were seen in a phase 2 trial testing a single intra-articular (IA) injection of PTP-001 vs an IA saline placebo in just over 200 individuals with symptomatic knee OA. Results of this dose-finding study were presented by Alessandra Pavesio, senior vice president and the chief science officer of Bioventus/Doron Therapeutics, Durham, North Carolina.
Ms. Pavesio reported there were decreases in knee pain and improvements in knee function, as measured using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). These changes were seen after 26 weeks of treatment with PTP-001 given at either a low (100 mg, n = 74) or high (200 mg, n = 40) dose.
Although the changes were only numerically and not statistically different from placebo (n = 71) when looking at the total study population, Ms. Pavesio noted that a key objective of the trial had been to identify populations of patients that may benefit.
When they looked at the effects of PTP-001 solely in those with unilateral knee OA, WOMAC pain scores were decreased to a significantly greater extent with both the high and low doses of PTP-001 vs placebo. Decreases in the least squares mean (LSM) change in WOMAC pain from baseline to week 26 were 26.8 with 100-mg PTP-001, 36.1 with 200-mg PTP-001, and 24.0 with placebo (P = .072). A similarly greater effect for PTP-001 was also seen for LSM change in WOMAC function (26.4, 36.0, and 20.0, respectively; P = .023).
Ms. Pavesio noted that the only real side effect seen during the trial was an initial inflammatory reaction within the first 2 days of IA injection, which resolved within a few days without further problems.
The results are promising enough for Ms. Pavesio and her team to consider a phase 3 trial.
Dr. Lane asked Ms. Pavesio: “So, what’s in the secret sauce? You said it was ground-up placentas?” To which Ms. Pavesio replied that it contained about 300 different molecules which came from amnion, chorion, and umbilical cord tissue obtained from consented placental donation.
Dr. Lane subsequently told this news organization: “It’s probably a bunch of growth factors and cytokines, but if it’s not toxic, and they can standardize it, then it might be good. We remain open minded because we haven’t figured it out.”
Novel Fluticasone Delivery
In the same session, James A. Helliwell, MD, cofounder, director, and chief executive officer of Eupraxia Pharmaceuticals in Victoria, British Columbia, Canada, presented updated data from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate.
Dr. Helliwell, a cardiothoracic anesthesiologist, explained that EP-104IAR uses proprietary technology to form fluticasone into a crystal that can then be injected directly into the joint. This then slowly diffuses out to provide a highly localized treatment.
The SPRINGBOARD trial recruited just over 300 individuals with moderate knee OA and moderate to severe WOMAC pain and randomly allocated 164 to a single IA injection of EP-104IAR and 164 to a matching vehicle injection as a placebo. The latter was a slightly viscous substance that behaved like hyaluronic acid, Dr. Helliwell said.
The LSM change in total WOMAC score from baseline to week 12 showed a greater improvement with EP-104IAR than with placebo in a per protocol analysis (−2.79 vs −2.07; P = .002). Similar results were seen for the WOMAC subscales of pain (−2.97 vs −2.24; P = .003), function (−2.64 vs −1.99; P = .005), and stiffness (−2.85 vs −2.05; P = .001).
These differences persisted, Dr. Helliwell reported, out to a 20-week assessment for total WOMAC score, function, and stiffness and out to a 15-week assessment for WOMAC pain.
It’s probably no surprise that a steroid works, Dr. Helliwell said, noting that the safety profile of EP-104IAR may be better than that of regular IA steroid injection because it has “few off-target” effects. He reported that there were “minimal, clinically insignificant, and transient effects” of EP-104IAR on serum cortisol. There was no effect on glucose metabolism, even in patients with diabetes, he said.
“There is a group of our patients that we give long-acting steroids to in the joint, so it looked like [the EP-104IAR] safety profile was really good,” Dr. Lane told this news organization. However, she added: “I’m worried about the price tag associated with it.”
PPS
Although it perhaps can’t be described as a novel injectable per se, Mukesh Ahuja, MBBS, global clinical head of osteoarthritis at Paradigm Biopharmaceuticals, presented results of the novel use of PPS.
“PPS is a non-opioid, semi-synthetic xylose-based polysaccharide that is derived from beechwood trees,” Dr. Ahuja said. “It has a long-track record for treating pain, inflammation, and thrombosis in humans.”
There are currently two approved formulations: Oral capsules used for the treatment of interstitial cystitis in the European Union, United States, and Australia and an injectable form used in Italy for thromboprophylaxis.
Dr. Ahuja presented data from a phase 2 trial that looked at the effect of once- or twice-weekly subcutaneous injections of PPS vs placebo in 61 people with knee OA pain. Assessments were made after 56, 168, and 365 days of treatment.
Results showed PPS injections resulted in significant improvements in total WOMAC score, WOMAC pain, and WOMAC function, with more PPS- than placebo-treated individuals achieving and then maintaining at least a 30% or greater improvement in pain and a 56% improvement in function.
Rescue medication use was lower in the PPS-treated patients, and Patient Global Impression of Change were significantly higher, Dr. Ahuja said.
Exploratory analyses of synovial fluid biomarkers showed PPS could be having a direct inflammatory effect, with reductions in several proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha.
An assessment of OA disease progression using MRI analysis suggested that there may be an effect on cartilage thickness and volume, as well as bone marrow lesions and overall joint inflammation.
Gene Therapy
Elsewhere at OARSI 2024, updated data were reported on XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant (v) of IL-10.
Howard Rutman, MD, MBA, chief medical officer of Xalud Therapeutics, reported data from a patient subgroup analysis of a phase 2 trial, which evaluated the effects of single and repeat IA injections of XT-150.
Previously, it was found that a single dose of XT-150 (0.15 mg/mL or 0.45 mg/mL) given as a 1-mL IA injection did not meet its primary endpoint of a greater proportion of patients achieving a 30% or more improvement in WOMAC pain at 180 days vs a matching placebo.
However, it was noted that 17% of the patients in the trial had a baseline WOMAC pain score of less than 8, so the new analysis focused on a modified intention-to-treat population of 210 patients who had baseline WOMAC pain scores of 9 or higher.
Two injections of XT-150 at a dose of 0.45 mg were found to produce the best effect on WOMAC pain, with a LSM change from baseline of −4.09 vs −2.74 for a single 0.45-mg injection (P = .044).
Dr. Rutman reported that the 0.45-mg dose would be the one moving forward into future studies as this had the best effect when they looked at various patient demographics, including baseline age, gender, body mass index, Kellgren-Lawrence grade, and use of concomitant medications.
XT-150 acts locally, does not integrate into the host genome, and “has a very favorable safety profile,” Dr. Rutman said. As it is not a protein, there is no antibody response, and this gives it the possibility for repeat dosing, with no drug-drug serious adverse events so far reported.
The Best Is Yet to Come?
“There’s a lot of things cooking that haven’t been presented here [at OARSI],” Dr. Lane observed.
“We are figuring out how to regenerate cartilage, and it’s a little different than throwing some stem cells in there. There’s some ground-breaking stuff [coming], it just takes us a while.”
Dr. Lane also noted that researchers were “really figuring out” how joints become painful, which will be a major step in figuring out how to make them less painful for patients.
“We’re making a lot of progress in ways that I don’t think we previously thought of, for example, the weight loss drugs. They probably have a central pain reduction effect, I think there’s a little overlap with the opioid receptors, so that’s pretty exciting. So, we’re getting there,” Dr. Lane said.
The congress was sponsored by the Osteoarthritis Research Society International.
Dr. Lane had no relevant conflicts to declare. The trial of PTP-001 (MOTYS) was funded by Bioventus. Ms. Pavesio is an employee of Doron Therapeutics, a subsidiary of Bioventus. The SPRINGBOARD trial with EP-104IAR was funded by Eupraxia Pharmaceuticals. Dr. Helliwell is an employee and stockholder of Eupraxia Pharmaceuticals. The trial of PPS was funded by Paradigm Biopharmaceuticals. Dr. Ahuja is an employee and stockholder of Paradigm Biopharmaceuticals and holds stock in ChitogenX. The trial of XT-150 was funded by Xalud Therapeutics. Dr. Rutman is an employee and equity holder of the company.
A version of this article appeared on Medscape.com.
VIENNA — Encouraging primary or secondary analyses of trial data for the use of several novel injectables and gene therapy for knee osteoarthritis (OA) were reported at the OARSI 2024 World Congress.
Of all the approaches discussed during the News in Therapies session at OARSI 2024, the most intriguing was the use of the placental extract PTP-001 (MOTYS, Bioventus), session chair Nancy E. Lane, MD, of the University of California Davis School of Medicine, Sacramento, California, told this news organization.
Other notable presentations of data from trials of investigational agents for knee OA included an update from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate; a phase 2 trial of pentosan polysulfate sodium (PPS), a non-opioid, semi-synthetic xylose-based polysaccharide; and an update on phase 2 study results for XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant of interleukin 10 (IL-10).
PTP-001 (MOTYS)
Indeed, promising results were seen in a phase 2 trial testing a single intra-articular (IA) injection of PTP-001 vs an IA saline placebo in just over 200 individuals with symptomatic knee OA. Results of this dose-finding study were presented by Alessandra Pavesio, senior vice president and the chief science officer of Bioventus/Doron Therapeutics, Durham, North Carolina.
Ms. Pavesio reported there were decreases in knee pain and improvements in knee function, as measured using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). These changes were seen after 26 weeks of treatment with PTP-001 given at either a low (100 mg, n = 74) or high (200 mg, n = 40) dose.
Although the changes were only numerically and not statistically different from placebo (n = 71) when looking at the total study population, Ms. Pavesio noted that a key objective of the trial had been to identify populations of patients that may benefit.
When they looked at the effects of PTP-001 solely in those with unilateral knee OA, WOMAC pain scores were decreased to a significantly greater extent with both the high and low doses of PTP-001 vs placebo. Decreases in the least squares mean (LSM) change in WOMAC pain from baseline to week 26 were 26.8 with 100-mg PTP-001, 36.1 with 200-mg PTP-001, and 24.0 with placebo (P = .072). A similarly greater effect for PTP-001 was also seen for LSM change in WOMAC function (26.4, 36.0, and 20.0, respectively; P = .023).
Ms. Pavesio noted that the only real side effect seen during the trial was an initial inflammatory reaction within the first 2 days of IA injection, which resolved within a few days without further problems.
The results are promising enough for Ms. Pavesio and her team to consider a phase 3 trial.
Dr. Lane asked Ms. Pavesio: “So, what’s in the secret sauce? You said it was ground-up placentas?” To which Ms. Pavesio replied that it contained about 300 different molecules which came from amnion, chorion, and umbilical cord tissue obtained from consented placental donation.
Dr. Lane subsequently told this news organization: “It’s probably a bunch of growth factors and cytokines, but if it’s not toxic, and they can standardize it, then it might be good. We remain open minded because we haven’t figured it out.”
Novel Fluticasone Delivery
In the same session, James A. Helliwell, MD, cofounder, director, and chief executive officer of Eupraxia Pharmaceuticals in Victoria, British Columbia, Canada, presented updated data from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate.
Dr. Helliwell, a cardiothoracic anesthesiologist, explained that EP-104IAR uses proprietary technology to form fluticasone into a crystal that can then be injected directly into the joint. This then slowly diffuses out to provide a highly localized treatment.
The SPRINGBOARD trial recruited just over 300 individuals with moderate knee OA and moderate to severe WOMAC pain and randomly allocated 164 to a single IA injection of EP-104IAR and 164 to a matching vehicle injection as a placebo. The latter was a slightly viscous substance that behaved like hyaluronic acid, Dr. Helliwell said.
The LSM change in total WOMAC score from baseline to week 12 showed a greater improvement with EP-104IAR than with placebo in a per protocol analysis (−2.79 vs −2.07; P = .002). Similar results were seen for the WOMAC subscales of pain (−2.97 vs −2.24; P = .003), function (−2.64 vs −1.99; P = .005), and stiffness (−2.85 vs −2.05; P = .001).
These differences persisted, Dr. Helliwell reported, out to a 20-week assessment for total WOMAC score, function, and stiffness and out to a 15-week assessment for WOMAC pain.
It’s probably no surprise that a steroid works, Dr. Helliwell said, noting that the safety profile of EP-104IAR may be better than that of regular IA steroid injection because it has “few off-target” effects. He reported that there were “minimal, clinically insignificant, and transient effects” of EP-104IAR on serum cortisol. There was no effect on glucose metabolism, even in patients with diabetes, he said.
“There is a group of our patients that we give long-acting steroids to in the joint, so it looked like [the EP-104IAR] safety profile was really good,” Dr. Lane told this news organization. However, she added: “I’m worried about the price tag associated with it.”
PPS
Although it perhaps can’t be described as a novel injectable per se, Mukesh Ahuja, MBBS, global clinical head of osteoarthritis at Paradigm Biopharmaceuticals, presented results of the novel use of PPS.
“PPS is a non-opioid, semi-synthetic xylose-based polysaccharide that is derived from beechwood trees,” Dr. Ahuja said. “It has a long-track record for treating pain, inflammation, and thrombosis in humans.”
There are currently two approved formulations: Oral capsules used for the treatment of interstitial cystitis in the European Union, United States, and Australia and an injectable form used in Italy for thromboprophylaxis.
Dr. Ahuja presented data from a phase 2 trial that looked at the effect of once- or twice-weekly subcutaneous injections of PPS vs placebo in 61 people with knee OA pain. Assessments were made after 56, 168, and 365 days of treatment.
Results showed PPS injections resulted in significant improvements in total WOMAC score, WOMAC pain, and WOMAC function, with more PPS- than placebo-treated individuals achieving and then maintaining at least a 30% or greater improvement in pain and a 56% improvement in function.
Rescue medication use was lower in the PPS-treated patients, and Patient Global Impression of Change were significantly higher, Dr. Ahuja said.
Exploratory analyses of synovial fluid biomarkers showed PPS could be having a direct inflammatory effect, with reductions in several proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha.
An assessment of OA disease progression using MRI analysis suggested that there may be an effect on cartilage thickness and volume, as well as bone marrow lesions and overall joint inflammation.
Gene Therapy
Elsewhere at OARSI 2024, updated data were reported on XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant (v) of IL-10.
Howard Rutman, MD, MBA, chief medical officer of Xalud Therapeutics, reported data from a patient subgroup analysis of a phase 2 trial, which evaluated the effects of single and repeat IA injections of XT-150.
Previously, it was found that a single dose of XT-150 (0.15 mg/mL or 0.45 mg/mL) given as a 1-mL IA injection did not meet its primary endpoint of a greater proportion of patients achieving a 30% or more improvement in WOMAC pain at 180 days vs a matching placebo.
However, it was noted that 17% of the patients in the trial had a baseline WOMAC pain score of less than 8, so the new analysis focused on a modified intention-to-treat population of 210 patients who had baseline WOMAC pain scores of 9 or higher.
Two injections of XT-150 at a dose of 0.45 mg were found to produce the best effect on WOMAC pain, with a LSM change from baseline of −4.09 vs −2.74 for a single 0.45-mg injection (P = .044).
Dr. Rutman reported that the 0.45-mg dose would be the one moving forward into future studies as this had the best effect when they looked at various patient demographics, including baseline age, gender, body mass index, Kellgren-Lawrence grade, and use of concomitant medications.
XT-150 acts locally, does not integrate into the host genome, and “has a very favorable safety profile,” Dr. Rutman said. As it is not a protein, there is no antibody response, and this gives it the possibility for repeat dosing, with no drug-drug serious adverse events so far reported.
The Best Is Yet to Come?
“There’s a lot of things cooking that haven’t been presented here [at OARSI],” Dr. Lane observed.
“We are figuring out how to regenerate cartilage, and it’s a little different than throwing some stem cells in there. There’s some ground-breaking stuff [coming], it just takes us a while.”
Dr. Lane also noted that researchers were “really figuring out” how joints become painful, which will be a major step in figuring out how to make them less painful for patients.
“We’re making a lot of progress in ways that I don’t think we previously thought of, for example, the weight loss drugs. They probably have a central pain reduction effect, I think there’s a little overlap with the opioid receptors, so that’s pretty exciting. So, we’re getting there,” Dr. Lane said.
The congress was sponsored by the Osteoarthritis Research Society International.
Dr. Lane had no relevant conflicts to declare. The trial of PTP-001 (MOTYS) was funded by Bioventus. Ms. Pavesio is an employee of Doron Therapeutics, a subsidiary of Bioventus. The SPRINGBOARD trial with EP-104IAR was funded by Eupraxia Pharmaceuticals. Dr. Helliwell is an employee and stockholder of Eupraxia Pharmaceuticals. The trial of PPS was funded by Paradigm Biopharmaceuticals. Dr. Ahuja is an employee and stockholder of Paradigm Biopharmaceuticals and holds stock in ChitogenX. The trial of XT-150 was funded by Xalud Therapeutics. Dr. Rutman is an employee and equity holder of the company.
A version of this article appeared on Medscape.com.
VIENNA — Encouraging primary or secondary analyses of trial data for the use of several novel injectables and gene therapy for knee osteoarthritis (OA) were reported at the OARSI 2024 World Congress.
Of all the approaches discussed during the News in Therapies session at OARSI 2024, the most intriguing was the use of the placental extract PTP-001 (MOTYS, Bioventus), session chair Nancy E. Lane, MD, of the University of California Davis School of Medicine, Sacramento, California, told this news organization.
Other notable presentations of data from trials of investigational agents for knee OA included an update from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate; a phase 2 trial of pentosan polysulfate sodium (PPS), a non-opioid, semi-synthetic xylose-based polysaccharide; and an update on phase 2 study results for XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant of interleukin 10 (IL-10).
PTP-001 (MOTYS)
Indeed, promising results were seen in a phase 2 trial testing a single intra-articular (IA) injection of PTP-001 vs an IA saline placebo in just over 200 individuals with symptomatic knee OA. Results of this dose-finding study were presented by Alessandra Pavesio, senior vice president and the chief science officer of Bioventus/Doron Therapeutics, Durham, North Carolina.
Ms. Pavesio reported there were decreases in knee pain and improvements in knee function, as measured using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). These changes were seen after 26 weeks of treatment with PTP-001 given at either a low (100 mg, n = 74) or high (200 mg, n = 40) dose.
Although the changes were only numerically and not statistically different from placebo (n = 71) when looking at the total study population, Ms. Pavesio noted that a key objective of the trial had been to identify populations of patients that may benefit.
When they looked at the effects of PTP-001 solely in those with unilateral knee OA, WOMAC pain scores were decreased to a significantly greater extent with both the high and low doses of PTP-001 vs placebo. Decreases in the least squares mean (LSM) change in WOMAC pain from baseline to week 26 were 26.8 with 100-mg PTP-001, 36.1 with 200-mg PTP-001, and 24.0 with placebo (P = .072). A similarly greater effect for PTP-001 was also seen for LSM change in WOMAC function (26.4, 36.0, and 20.0, respectively; P = .023).
Ms. Pavesio noted that the only real side effect seen during the trial was an initial inflammatory reaction within the first 2 days of IA injection, which resolved within a few days without further problems.
The results are promising enough for Ms. Pavesio and her team to consider a phase 3 trial.
Dr. Lane asked Ms. Pavesio: “So, what’s in the secret sauce? You said it was ground-up placentas?” To which Ms. Pavesio replied that it contained about 300 different molecules which came from amnion, chorion, and umbilical cord tissue obtained from consented placental donation.
Dr. Lane subsequently told this news organization: “It’s probably a bunch of growth factors and cytokines, but if it’s not toxic, and they can standardize it, then it might be good. We remain open minded because we haven’t figured it out.”
Novel Fluticasone Delivery
In the same session, James A. Helliwell, MD, cofounder, director, and chief executive officer of Eupraxia Pharmaceuticals in Victoria, British Columbia, Canada, presented updated data from the SPRINGBOARD phase 2B trial of EP-104IAR, a novel long-acting formulation of the corticosteroid fluticasone propionate.
Dr. Helliwell, a cardiothoracic anesthesiologist, explained that EP-104IAR uses proprietary technology to form fluticasone into a crystal that can then be injected directly into the joint. This then slowly diffuses out to provide a highly localized treatment.
The SPRINGBOARD trial recruited just over 300 individuals with moderate knee OA and moderate to severe WOMAC pain and randomly allocated 164 to a single IA injection of EP-104IAR and 164 to a matching vehicle injection as a placebo. The latter was a slightly viscous substance that behaved like hyaluronic acid, Dr. Helliwell said.
The LSM change in total WOMAC score from baseline to week 12 showed a greater improvement with EP-104IAR than with placebo in a per protocol analysis (−2.79 vs −2.07; P = .002). Similar results were seen for the WOMAC subscales of pain (−2.97 vs −2.24; P = .003), function (−2.64 vs −1.99; P = .005), and stiffness (−2.85 vs −2.05; P = .001).
These differences persisted, Dr. Helliwell reported, out to a 20-week assessment for total WOMAC score, function, and stiffness and out to a 15-week assessment for WOMAC pain.
It’s probably no surprise that a steroid works, Dr. Helliwell said, noting that the safety profile of EP-104IAR may be better than that of regular IA steroid injection because it has “few off-target” effects. He reported that there were “minimal, clinically insignificant, and transient effects” of EP-104IAR on serum cortisol. There was no effect on glucose metabolism, even in patients with diabetes, he said.
“There is a group of our patients that we give long-acting steroids to in the joint, so it looked like [the EP-104IAR] safety profile was really good,” Dr. Lane told this news organization. However, she added: “I’m worried about the price tag associated with it.”
PPS
Although it perhaps can’t be described as a novel injectable per se, Mukesh Ahuja, MBBS, global clinical head of osteoarthritis at Paradigm Biopharmaceuticals, presented results of the novel use of PPS.
“PPS is a non-opioid, semi-synthetic xylose-based polysaccharide that is derived from beechwood trees,” Dr. Ahuja said. “It has a long-track record for treating pain, inflammation, and thrombosis in humans.”
There are currently two approved formulations: Oral capsules used for the treatment of interstitial cystitis in the European Union, United States, and Australia and an injectable form used in Italy for thromboprophylaxis.
Dr. Ahuja presented data from a phase 2 trial that looked at the effect of once- or twice-weekly subcutaneous injections of PPS vs placebo in 61 people with knee OA pain. Assessments were made after 56, 168, and 365 days of treatment.
Results showed PPS injections resulted in significant improvements in total WOMAC score, WOMAC pain, and WOMAC function, with more PPS- than placebo-treated individuals achieving and then maintaining at least a 30% or greater improvement in pain and a 56% improvement in function.
Rescue medication use was lower in the PPS-treated patients, and Patient Global Impression of Change were significantly higher, Dr. Ahuja said.
Exploratory analyses of synovial fluid biomarkers showed PPS could be having a direct inflammatory effect, with reductions in several proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha.
An assessment of OA disease progression using MRI analysis suggested that there may be an effect on cartilage thickness and volume, as well as bone marrow lesions and overall joint inflammation.
Gene Therapy
Elsewhere at OARSI 2024, updated data were reported on XT-150, a non-viral, plasmid-based gene therapy designed to express a proprietary variant (v) of IL-10.
Howard Rutman, MD, MBA, chief medical officer of Xalud Therapeutics, reported data from a patient subgroup analysis of a phase 2 trial, which evaluated the effects of single and repeat IA injections of XT-150.
Previously, it was found that a single dose of XT-150 (0.15 mg/mL or 0.45 mg/mL) given as a 1-mL IA injection did not meet its primary endpoint of a greater proportion of patients achieving a 30% or more improvement in WOMAC pain at 180 days vs a matching placebo.
However, it was noted that 17% of the patients in the trial had a baseline WOMAC pain score of less than 8, so the new analysis focused on a modified intention-to-treat population of 210 patients who had baseline WOMAC pain scores of 9 or higher.
Two injections of XT-150 at a dose of 0.45 mg were found to produce the best effect on WOMAC pain, with a LSM change from baseline of −4.09 vs −2.74 for a single 0.45-mg injection (P = .044).
Dr. Rutman reported that the 0.45-mg dose would be the one moving forward into future studies as this had the best effect when they looked at various patient demographics, including baseline age, gender, body mass index, Kellgren-Lawrence grade, and use of concomitant medications.
XT-150 acts locally, does not integrate into the host genome, and “has a very favorable safety profile,” Dr. Rutman said. As it is not a protein, there is no antibody response, and this gives it the possibility for repeat dosing, with no drug-drug serious adverse events so far reported.
The Best Is Yet to Come?
“There’s a lot of things cooking that haven’t been presented here [at OARSI],” Dr. Lane observed.
“We are figuring out how to regenerate cartilage, and it’s a little different than throwing some stem cells in there. There’s some ground-breaking stuff [coming], it just takes us a while.”
Dr. Lane also noted that researchers were “really figuring out” how joints become painful, which will be a major step in figuring out how to make them less painful for patients.
“We’re making a lot of progress in ways that I don’t think we previously thought of, for example, the weight loss drugs. They probably have a central pain reduction effect, I think there’s a little overlap with the opioid receptors, so that’s pretty exciting. So, we’re getting there,” Dr. Lane said.
The congress was sponsored by the Osteoarthritis Research Society International.
Dr. Lane had no relevant conflicts to declare. The trial of PTP-001 (MOTYS) was funded by Bioventus. Ms. Pavesio is an employee of Doron Therapeutics, a subsidiary of Bioventus. The SPRINGBOARD trial with EP-104IAR was funded by Eupraxia Pharmaceuticals. Dr. Helliwell is an employee and stockholder of Eupraxia Pharmaceuticals. The trial of PPS was funded by Paradigm Biopharmaceuticals. Dr. Ahuja is an employee and stockholder of Paradigm Biopharmaceuticals and holds stock in ChitogenX. The trial of XT-150 was funded by Xalud Therapeutics. Dr. Rutman is an employee and equity holder of the company.
A version of this article appeared on Medscape.com.
FROM OARSI 2024
Asthma, COPD inhaler price caps set for summer
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
In addition to warmer weather, June will usher in changes in asthma and COPD inhaler costs for many patients, potentially reducing barriers to those seeing high prescription prices. Price ceilings have been set by some companies, likely following action earlier this year by a Senate Committee which pointed to higher costs of US inhalers compared with other countries.
Senator Sanders stated: “In my view, Americans who have asthma and COPD should not be forced to pay, in many cases, 10-70 times more for the same exact inhalers as patients in Europe and other parts of the world.”
Starting June 1, Boehringer Ingelheim will cap out-of-pocket costs for the company’s inhaler products for chronic lung disease and asthma at $35 per month, according to a March 7, 2024, press release from the German drugmaker’s US headquarters in Ridgefield, Conn. The reductions cover the full range of the company’s inhaler products for asthma and chronic obstructive pulmonary disease (COPD) including Atrovent, Combivent Respimat and Spiriva HandiHaler and Respimat, Stiolto Respimat and Striverdi Respimat. In the release, Boehringer Ingelheim USA Corporation’s President and CEO Jean-Michel Boers stated, “The US health care system is complex and often doesn’t work for patients, especially the most vulnerable. While we can’t fix the entire system alone, we are bringing forward a solution to make it fairer. We want to do our part to help patients living with COPD or asthma who struggle to pay for their medications.”
Similar announcements were made by AstraZeneca and GSK. GSK’s cap will go into effect on January 1, 2025, and includes Advair Diskus, Advair HFA, Anoro Ellipta, Arnuity Ellipta, Breo Ellipta, Incruse Ellipta, Serevent Diskus, Trelegy Ellipta, and Ventolin HFA. The AstraZeneca cap, which covers Airsupra, Bevespi Aerosphere, Breztri Aeroshpere, and Symbicort, goes into effect on June 1, 2024.
Senate statement on pricing
These companies plus Teva had received letters sent on January 8, 2024, by the members of the Senate Committee on Health, Education, Labor, and Pensions: senators Sanders, Baldwin, Luján and Markey. The letters cited enormous inhaler price discrepancies, for example $489 for Combivent Respimat in the United States but just $7 in France, and announced the conduct of an investigation into efforts by these companies to artificially inflate and manipulate prices of asthma inhalers that have been on the market for decades. A statement from Sen. Sanders’ office noted that AstraZeneca, GSK, and Teva made more than $25 billion in revenue from inhalers alone in the past 5 years (Boehringer Ingelheim does not provide public US inhaler revenue information).
Suit claims generic delay
A federal lawsuit filed in Boston on March 6, according to a Reuters brief from March 7, cited Boehringer for improperly submitting patents to the US Food and Drug Administration (FDA). The purpose of those patents, the suit charges, was to delay generic competition and inflate Combivent Respimat and Spiriva Respimat inhaler prices.
Inhaler prices soared in the United States, according to a March 10 U.S. News & World Report commentary by The Conversation, a nonprofit news organization, after the 2008 FDA ban on chlorofluorocarbon (CFC)-propellants led to the phase-out of CFC-containing inhalers and their replacement with hydrofluoroalkane-propellant inhalers. For the insured that meant an average out-of-pocket inhaler cost increase from $13.60 per prescription in 2004 to $25 in 2015. The current rate for the now nongeneric HFA-propelled but otherwise identical albuterol inhaler is $98. Competition from a more recently FDA-approved (2020) generic version has not been robust enough to effect meaningful price reductions, the report stated. While good insurance generally covers most of inhaler costs, the more than 25 million uninsured in 2023 faced steep market prices that put strain even on some insured, the CDC found, driving many in the United States to purchase from Mexican, Canadian, or other foreign pharmacies. The Teva QVAR REdiHaler corticosteroid inhaler, costing $9 in Germany, costs $286 in the US. Dosages, however, may not be identical. A first FDA-authorization of drug importing this past January applied only to agents for a limited number of disease states and pertained only to Florida, but may serve as a model for other states, according to the commentary.
“The announced price cap from Boehringer Ingelheim,” stated Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA) in a press release, “is a step toward improving access to essential asthma medicine and demonstrates that the voice of the asthma patient community is being heard.” The AAFA release noted further that asthma death rates, while declining overall, are triple in Blacks compared with Whites. Death rates, asthma rates, and rates of being uninsured or underinsured are much higher in Black and Puerto Rican populations than in Whites. The complex layers of the current US system, composed of pharmaceutical manufacturers, pharmacy benefit managers, insurance companies, employers, and federal policies often conspire against those people who need asthma drugs the most. AAFA research has shown that when drug prices become a barrier to treatment, people with asthma ration or simply discontinue their essential asthma medications. Beyond saved lives, access to asthma medications can reduce hospitalizations and lower the more than $82 billion in annual asthma costs to the US economy.
Sen. Sanders, on March 20, applauded the GSK announcement: “As Chairman of the Senate Health, Education, Labor, and Pensions Committee, I very much appreciate GlaxoSmithKline’s announcement today that Americans throughout the country with asthma and COPD will pay no more than $35 for the brand name inhalers they manufacture. I look forward to working with GSK to make sure that this decision reaches as many patients as possible.”
“Inhaled medications continue to be an essential part of the therapy for patients with asthma, COPD, and other respiratory conditions,” said Diego J. Maselli, professor and chief, Division of Pulmonary Diseases & Critical Care, UT Health at San Antonio, San Antonio, Texas, in an interview with CHEST Physician. He added, “Unfortunately, with increasing cost of these and other treatments, access has been challenging for many patients. Patients, families, and providers constantly experience frustration with the difficulties of obtaining these lifesaving medications, and cost is the main barrier. Even those with ample insurance coverage face difficult challenges, as the high prices of these medications motivate insurance carriers to constantly adjust what is the ‘preferred’ option among inhalers. Regrettably, noncompliance and nonadherence to inhaled therapies has been linked to poor patient outcomes and increased health care utilization in both asthma and COPD. Because of the high prevalence of these diseases in the US and worldwide, efforts to increase the access of these vital medications has been a priority. With the leveling of the prices of these medications across the world, we hope that there will be both improved access and, as a consequence, better patient outcomes.”
New Contraindications to Coadministration of Atazanavir
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
Vaccine Against Urinary Tract Infections in Development
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Probiotics Emerge as Promising Intervention in Cirrhosis
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
FDA Approves New Antibiotic for Uncomplicated UTIs
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.