User login
Vancomycin AUC-Dosing Initiative at a Regional Antibiotic Stewardship Collaborative
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
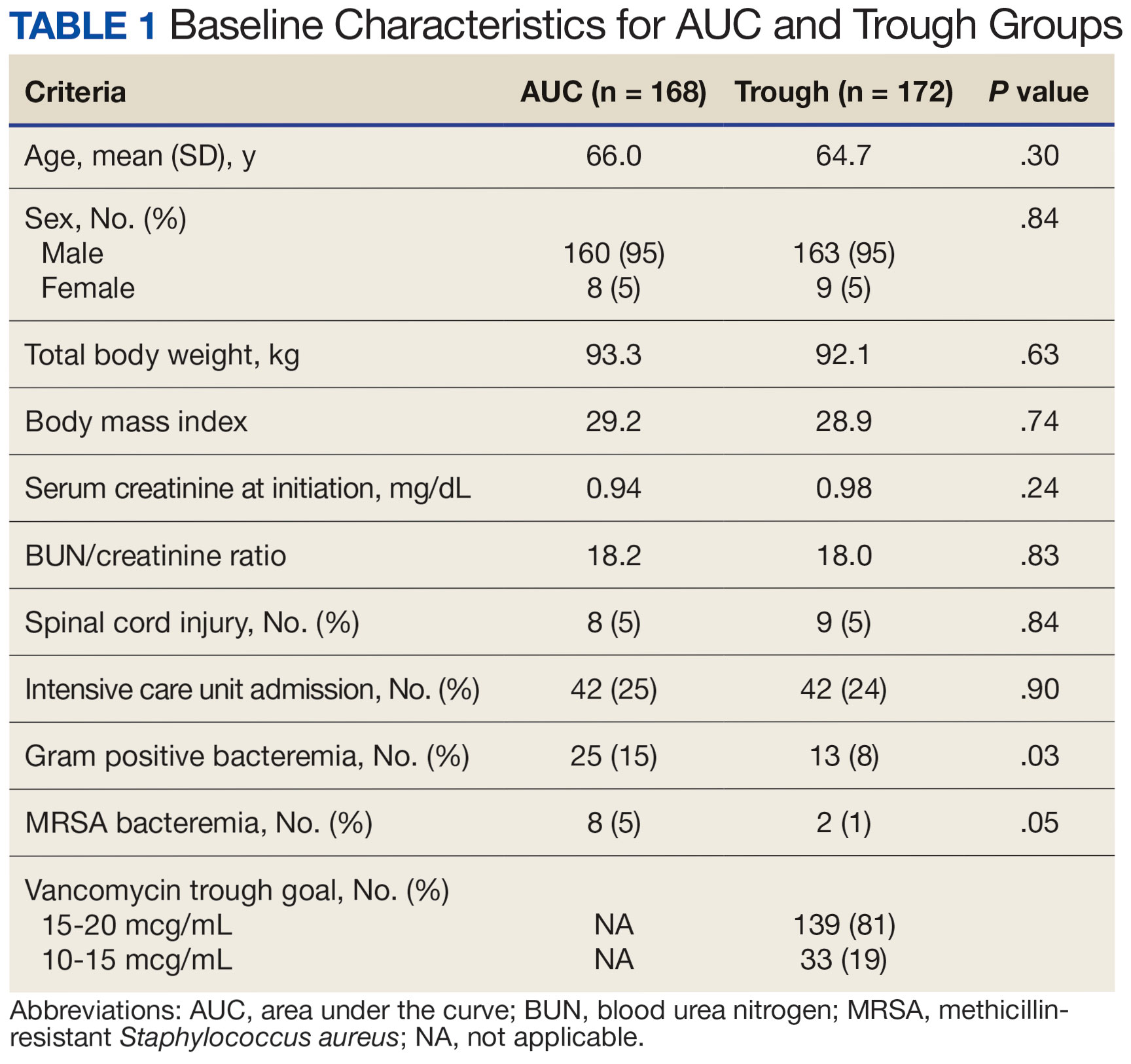
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.

We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
Which Medication Is Best? VA Genetic Tests May Have the Answer
The US Department of Veterans Affairs (VA) now has a permanent pharmacogenomics service that provides genetic tests to give clinicians insight into the best medication options for their patients.
The tests, which have no extra cost, are available to all veterans, said pharmacist Jill S. Bates, PharmD, MS, executive director of the VA National Pharmacogenomics Program, who spoke in an interview and a presentation at the annual meeting of the Association of VA Hematology/Oncology.
Genetic testing is “a tool that can help optimize care that we provide for veterans,” she said. “Pharmacogenomics is additional information to help the clinician make a decision. We know that most veterans—greater than 90%—carry a variant in a pharmacogenomics gene that is actionable.”
The genetic tests can provide insight into the optimal medication for multiple conditions such as mental illness, gastrointestinal disorders, cancer, pain, and heart disease. According to a 2019 analysis of over 6 years of data, more than half of the VA patient population used medications whose efficacy may have been affected by detectable genetic variants.
For instance, Bates said tests can let clinicians know whether patients are susceptible to statin-associated muscle adverse effects if they take simvastatin, the cholesterol medication. An estimated 25.6% of the VA population has this variant.
Elsewhere on the cardiac front, an estimated 58.3% of the VA population has a genetic variant that increases sensitivity to the blood thinner warfarin.
Testing could help psychiatrists determine whether certain medications should not be prescribed—or should be prescribed at lower doses—in patients who’ve had adverse reactions to antidepressants, Bates said.
In cancer, Bates said, genetic testing can identify patients who have a genetic variant that boosts toxicity from fluoropyrimidine chemotherapy treatments, which include capecitabine, floxuridine, and fluorouracil. Meanwhile, an estimated 0.9% will have no reaction or limited reaction to capecitabine and fluorouracil, and 4.8% will have hypersensitivity to carbamazepine and oxcarbazepine.
Tests can also identify a genetic variant that can lead to poor metabolism of the chemotherapy drug irinotecan, which is used to treat colon cancer. “In those patients, you’d want to reduce the dose by 20%,” Bates said. In other cases, alternate drugs may be the best strategy to address genetic variations.
Prior to 2019, clinicians had to order pharmacogenomic tests outside of the VA system, according to Bates. That year, a donation from Sanford Health brought VA pharmacogenomics to 40 pilot sites. Since then, more than 88,000 tests have been performed.
The VA has now made its pharmacogenomic program permanent, Bates said. As of early September, testing was available at 139 VA sites and is coming soon to 4 more. It’s not available at another 23 sites that are scattered across the country.
A tool in the VA electronic health record now reminds clinicians about the availability of genetic testing and allows them to order tests. However, testing isn’t available for patients who have had liver transplants or certain bone marrow transplants.
The VA is working on developing decision-making tools to help clinicians determine when the tests are appropriate, Bates said. It typically takes 2 to 3 weeks to get results, she said, adding that external laboratories provide results. “We eventually would like to bring in all pharmacogenomics testing to be conducted within the VA enterprise.”
Bates reported that she had no disclosures.
The US Department of Veterans Affairs (VA) now has a permanent pharmacogenomics service that provides genetic tests to give clinicians insight into the best medication options for their patients.
The tests, which have no extra cost, are available to all veterans, said pharmacist Jill S. Bates, PharmD, MS, executive director of the VA National Pharmacogenomics Program, who spoke in an interview and a presentation at the annual meeting of the Association of VA Hematology/Oncology.
Genetic testing is “a tool that can help optimize care that we provide for veterans,” she said. “Pharmacogenomics is additional information to help the clinician make a decision. We know that most veterans—greater than 90%—carry a variant in a pharmacogenomics gene that is actionable.”
The genetic tests can provide insight into the optimal medication for multiple conditions such as mental illness, gastrointestinal disorders, cancer, pain, and heart disease. According to a 2019 analysis of over 6 years of data, more than half of the VA patient population used medications whose efficacy may have been affected by detectable genetic variants.
For instance, Bates said tests can let clinicians know whether patients are susceptible to statin-associated muscle adverse effects if they take simvastatin, the cholesterol medication. An estimated 25.6% of the VA population has this variant.
Elsewhere on the cardiac front, an estimated 58.3% of the VA population has a genetic variant that increases sensitivity to the blood thinner warfarin.
Testing could help psychiatrists determine whether certain medications should not be prescribed—or should be prescribed at lower doses—in patients who’ve had adverse reactions to antidepressants, Bates said.
In cancer, Bates said, genetic testing can identify patients who have a genetic variant that boosts toxicity from fluoropyrimidine chemotherapy treatments, which include capecitabine, floxuridine, and fluorouracil. Meanwhile, an estimated 0.9% will have no reaction or limited reaction to capecitabine and fluorouracil, and 4.8% will have hypersensitivity to carbamazepine and oxcarbazepine.
Tests can also identify a genetic variant that can lead to poor metabolism of the chemotherapy drug irinotecan, which is used to treat colon cancer. “In those patients, you’d want to reduce the dose by 20%,” Bates said. In other cases, alternate drugs may be the best strategy to address genetic variations.
Prior to 2019, clinicians had to order pharmacogenomic tests outside of the VA system, according to Bates. That year, a donation from Sanford Health brought VA pharmacogenomics to 40 pilot sites. Since then, more than 88,000 tests have been performed.
The VA has now made its pharmacogenomic program permanent, Bates said. As of early September, testing was available at 139 VA sites and is coming soon to 4 more. It’s not available at another 23 sites that are scattered across the country.
A tool in the VA electronic health record now reminds clinicians about the availability of genetic testing and allows them to order tests. However, testing isn’t available for patients who have had liver transplants or certain bone marrow transplants.
The VA is working on developing decision-making tools to help clinicians determine when the tests are appropriate, Bates said. It typically takes 2 to 3 weeks to get results, she said, adding that external laboratories provide results. “We eventually would like to bring in all pharmacogenomics testing to be conducted within the VA enterprise.”
Bates reported that she had no disclosures.
The US Department of Veterans Affairs (VA) now has a permanent pharmacogenomics service that provides genetic tests to give clinicians insight into the best medication options for their patients.
The tests, which have no extra cost, are available to all veterans, said pharmacist Jill S. Bates, PharmD, MS, executive director of the VA National Pharmacogenomics Program, who spoke in an interview and a presentation at the annual meeting of the Association of VA Hematology/Oncology.
Genetic testing is “a tool that can help optimize care that we provide for veterans,” she said. “Pharmacogenomics is additional information to help the clinician make a decision. We know that most veterans—greater than 90%—carry a variant in a pharmacogenomics gene that is actionable.”
The genetic tests can provide insight into the optimal medication for multiple conditions such as mental illness, gastrointestinal disorders, cancer, pain, and heart disease. According to a 2019 analysis of over 6 years of data, more than half of the VA patient population used medications whose efficacy may have been affected by detectable genetic variants.
For instance, Bates said tests can let clinicians know whether patients are susceptible to statin-associated muscle adverse effects if they take simvastatin, the cholesterol medication. An estimated 25.6% of the VA population has this variant.
Elsewhere on the cardiac front, an estimated 58.3% of the VA population has a genetic variant that increases sensitivity to the blood thinner warfarin.
Testing could help psychiatrists determine whether certain medications should not be prescribed—or should be prescribed at lower doses—in patients who’ve had adverse reactions to antidepressants, Bates said.
In cancer, Bates said, genetic testing can identify patients who have a genetic variant that boosts toxicity from fluoropyrimidine chemotherapy treatments, which include capecitabine, floxuridine, and fluorouracil. Meanwhile, an estimated 0.9% will have no reaction or limited reaction to capecitabine and fluorouracil, and 4.8% will have hypersensitivity to carbamazepine and oxcarbazepine.
Tests can also identify a genetic variant that can lead to poor metabolism of the chemotherapy drug irinotecan, which is used to treat colon cancer. “In those patients, you’d want to reduce the dose by 20%,” Bates said. In other cases, alternate drugs may be the best strategy to address genetic variations.
Prior to 2019, clinicians had to order pharmacogenomic tests outside of the VA system, according to Bates. That year, a donation from Sanford Health brought VA pharmacogenomics to 40 pilot sites. Since then, more than 88,000 tests have been performed.
The VA has now made its pharmacogenomic program permanent, Bates said. As of early September, testing was available at 139 VA sites and is coming soon to 4 more. It’s not available at another 23 sites that are scattered across the country.
A tool in the VA electronic health record now reminds clinicians about the availability of genetic testing and allows them to order tests. However, testing isn’t available for patients who have had liver transplants or certain bone marrow transplants.
The VA is working on developing decision-making tools to help clinicians determine when the tests are appropriate, Bates said. It typically takes 2 to 3 weeks to get results, she said, adding that external laboratories provide results. “We eventually would like to bring in all pharmacogenomics testing to be conducted within the VA enterprise.”
Bates reported that she had no disclosures.
Implementation of a Prior Authorization Drug Review Process for Care in the Community Oncology Prescriptions
Background
Veterans receiving care in the community (CITC) are prescribed oral oncology medications to be filled at VA pharmacies. Many of the outpatient prescriptions written for oncology medications require a prior authorization review by a pharmacist. A standardized workflow to obtain outside records to ensure patient safety, appropriate therapeutic selections, and maximize cost avoidance was established in March 2023. This quality improvement project evaluated the implementation of a clinical peer-to-peer prescription referral process between operational and oncology clinical pharmacists (CPS) to include a prior authorization drug request (PADR) review.
Methods
A retrospective chart review was completed to assess the effectiveness of the CITC Rx review process. Patients who had a CITC PADR consult entered between April 2023 and March 2024 were included. Metrics obtained included medication ordered, diagnosis, line of treatment, date prescription received, time to PADR completion, PADR outcome, FDA approval status, and conformity to VA National Oncology Program (NOP) disease pathway. Descriptive statistics were used to describe the data.
Results
Top reasons for referral for CITC included best medical interest and drive time. Fifty-one PADR requests were submitted for 41 patients. Forty-six PADR consults were completed. Approval rate was 85%. Consults involved 32 different oncolytics, 78% had VA Pharmacy Benefits Manager criteria for use. Thirty-seven percent of the PADR requests adhered to the NOP pathways. Approximately 30% of PADR requests did not have an associated NOP pathway. Seventy-four percent of drugs had an associated FDA approval. On average, two calls were made to CITC provider by the operational pharmacist to obtain necessary information for clinical review, resulting in a 5 day time to PADR entry. The average time to PADR consult completion was 9.5 hours. Four interventions addressed drug interactions or dosing adjustments.
Conclusions
This review demonstrated the feasibility and framework for implementing a standardized peer-to-peer PADR consult review process for CITC prescriptions requiring prior authorization. Having separate intake of CITC prescriptions by the operational pharmacist who is responsible for obtaining outside records, the CPS provided a timely clinical review of PADR consults, assuring appropriate therapeutic selections to maximize cost avoidance while maintaining patient safety.
Background
Veterans receiving care in the community (CITC) are prescribed oral oncology medications to be filled at VA pharmacies. Many of the outpatient prescriptions written for oncology medications require a prior authorization review by a pharmacist. A standardized workflow to obtain outside records to ensure patient safety, appropriate therapeutic selections, and maximize cost avoidance was established in March 2023. This quality improvement project evaluated the implementation of a clinical peer-to-peer prescription referral process between operational and oncology clinical pharmacists (CPS) to include a prior authorization drug request (PADR) review.
Methods
A retrospective chart review was completed to assess the effectiveness of the CITC Rx review process. Patients who had a CITC PADR consult entered between April 2023 and March 2024 were included. Metrics obtained included medication ordered, diagnosis, line of treatment, date prescription received, time to PADR completion, PADR outcome, FDA approval status, and conformity to VA National Oncology Program (NOP) disease pathway. Descriptive statistics were used to describe the data.
Results
Top reasons for referral for CITC included best medical interest and drive time. Fifty-one PADR requests were submitted for 41 patients. Forty-six PADR consults were completed. Approval rate was 85%. Consults involved 32 different oncolytics, 78% had VA Pharmacy Benefits Manager criteria for use. Thirty-seven percent of the PADR requests adhered to the NOP pathways. Approximately 30% of PADR requests did not have an associated NOP pathway. Seventy-four percent of drugs had an associated FDA approval. On average, two calls were made to CITC provider by the operational pharmacist to obtain necessary information for clinical review, resulting in a 5 day time to PADR entry. The average time to PADR consult completion was 9.5 hours. Four interventions addressed drug interactions or dosing adjustments.
Conclusions
This review demonstrated the feasibility and framework for implementing a standardized peer-to-peer PADR consult review process for CITC prescriptions requiring prior authorization. Having separate intake of CITC prescriptions by the operational pharmacist who is responsible for obtaining outside records, the CPS provided a timely clinical review of PADR consults, assuring appropriate therapeutic selections to maximize cost avoidance while maintaining patient safety.
Background
Veterans receiving care in the community (CITC) are prescribed oral oncology medications to be filled at VA pharmacies. Many of the outpatient prescriptions written for oncology medications require a prior authorization review by a pharmacist. A standardized workflow to obtain outside records to ensure patient safety, appropriate therapeutic selections, and maximize cost avoidance was established in March 2023. This quality improvement project evaluated the implementation of a clinical peer-to-peer prescription referral process between operational and oncology clinical pharmacists (CPS) to include a prior authorization drug request (PADR) review.
Methods
A retrospective chart review was completed to assess the effectiveness of the CITC Rx review process. Patients who had a CITC PADR consult entered between April 2023 and March 2024 were included. Metrics obtained included medication ordered, diagnosis, line of treatment, date prescription received, time to PADR completion, PADR outcome, FDA approval status, and conformity to VA National Oncology Program (NOP) disease pathway. Descriptive statistics were used to describe the data.
Results
Top reasons for referral for CITC included best medical interest and drive time. Fifty-one PADR requests were submitted for 41 patients. Forty-six PADR consults were completed. Approval rate was 85%. Consults involved 32 different oncolytics, 78% had VA Pharmacy Benefits Manager criteria for use. Thirty-seven percent of the PADR requests adhered to the NOP pathways. Approximately 30% of PADR requests did not have an associated NOP pathway. Seventy-four percent of drugs had an associated FDA approval. On average, two calls were made to CITC provider by the operational pharmacist to obtain necessary information for clinical review, resulting in a 5 day time to PADR entry. The average time to PADR consult completion was 9.5 hours. Four interventions addressed drug interactions or dosing adjustments.
Conclusions
This review demonstrated the feasibility and framework for implementing a standardized peer-to-peer PADR consult review process for CITC prescriptions requiring prior authorization. Having separate intake of CITC prescriptions by the operational pharmacist who is responsible for obtaining outside records, the CPS provided a timely clinical review of PADR consults, assuring appropriate therapeutic selections to maximize cost avoidance while maintaining patient safety.
Posterior Reversible Encephalopathy Syndrome (PRES) Following Bevacizumab and Atezolizumab Therapy in Hepatocellular Carcinoma (HCC)
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Carboplatin as a Radiosensitizing Agent in Locally Advanced Head and Neck Cancer: Friendly to an Older Veteran Population
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
How to Make Keeping Up With the Drugs as Easy as Keeping Up With the Kardashians: Implementing a Local Oncology Drug Review Committee
Background
From 2000-2022 there were over 200 new drug and over 500 indication approvals specific to oncology. The rate of approvals has increased exponentially, making it difficult to maintain an up-to-date, standardized practice. Nationally, Veterans Affairs (VA) formulary decisions can take time given a lengthy approval process. Locally, the need was identified to incorporate new drugs and data into practice more rapidly. When bringing requests to the facility Pharmacy and Therapeutics (P&T) Committee, it was recognized that the membership consisting of non-oncology practitioners did not allow for meaningful discussion of utilization. In 2017, a dedicated oncology drug review committee (DRC) comprised of oncology practitioners and a facility formulary representative was created as a P&T workgroup. Purpose: Evaluate and describe the utility of forming a local oncology DRC to incorporate new drugs and data into practice.
Methods
DRC minutes from December 2017 to May 2023 were reviewed. Discussion items were categorized into type of review. Date of local review was compared to national formulary criteria for use publication dates, and date of FDA approval for new drugs or publication date for new data, where applicable. Items were excluded if crucial information was missing from minutes. Descriptive statistics were used.
Results
Over 65 months, 38 meetings were held. Thirty total members include: pharmacists, physicians, fellows, and advanced practice providers. Items reviewed included: 36 new drugs (ND), 36 new indications/data (NI), 14 institutional preferences, 10 new dosage form/biosimilars, 4 drug shortages and 2 others. The median time from ND approval to discussion was 3 months (n= 36, IQR 3-6) and NI from publication was 3 months (n=30, IQR 1-8). Nearly all (34/36, 94%) ND were reviewed prior to national review. Local review was a median of 7 months before national, with 11 drugs currently having no published national criteria for use (n=25, IQR 2-12).
Conclusions
DRC formation has enabled faster incorporation of new drugs/indications into practice. It has also created an appropriate forum for in-depth utilization discussions, pharmacoeconomic stewardship, and sharing of formulary and medication related information. VA Health Systems could consider implementing similar committees to review and implement up-to-date oncology practices.
Background
From 2000-2022 there were over 200 new drug and over 500 indication approvals specific to oncology. The rate of approvals has increased exponentially, making it difficult to maintain an up-to-date, standardized practice. Nationally, Veterans Affairs (VA) formulary decisions can take time given a lengthy approval process. Locally, the need was identified to incorporate new drugs and data into practice more rapidly. When bringing requests to the facility Pharmacy and Therapeutics (P&T) Committee, it was recognized that the membership consisting of non-oncology practitioners did not allow for meaningful discussion of utilization. In 2017, a dedicated oncology drug review committee (DRC) comprised of oncology practitioners and a facility formulary representative was created as a P&T workgroup. Purpose: Evaluate and describe the utility of forming a local oncology DRC to incorporate new drugs and data into practice.
Methods
DRC minutes from December 2017 to May 2023 were reviewed. Discussion items were categorized into type of review. Date of local review was compared to national formulary criteria for use publication dates, and date of FDA approval for new drugs or publication date for new data, where applicable. Items were excluded if crucial information was missing from minutes. Descriptive statistics were used.
Results
Over 65 months, 38 meetings were held. Thirty total members include: pharmacists, physicians, fellows, and advanced practice providers. Items reviewed included: 36 new drugs (ND), 36 new indications/data (NI), 14 institutional preferences, 10 new dosage form/biosimilars, 4 drug shortages and 2 others. The median time from ND approval to discussion was 3 months (n= 36, IQR 3-6) and NI from publication was 3 months (n=30, IQR 1-8). Nearly all (34/36, 94%) ND were reviewed prior to national review. Local review was a median of 7 months before national, with 11 drugs currently having no published national criteria for use (n=25, IQR 2-12).
Conclusions
DRC formation has enabled faster incorporation of new drugs/indications into practice. It has also created an appropriate forum for in-depth utilization discussions, pharmacoeconomic stewardship, and sharing of formulary and medication related information. VA Health Systems could consider implementing similar committees to review and implement up-to-date oncology practices.
Background
From 2000-2022 there were over 200 new drug and over 500 indication approvals specific to oncology. The rate of approvals has increased exponentially, making it difficult to maintain an up-to-date, standardized practice. Nationally, Veterans Affairs (VA) formulary decisions can take time given a lengthy approval process. Locally, the need was identified to incorporate new drugs and data into practice more rapidly. When bringing requests to the facility Pharmacy and Therapeutics (P&T) Committee, it was recognized that the membership consisting of non-oncology practitioners did not allow for meaningful discussion of utilization. In 2017, a dedicated oncology drug review committee (DRC) comprised of oncology practitioners and a facility formulary representative was created as a P&T workgroup. Purpose: Evaluate and describe the utility of forming a local oncology DRC to incorporate new drugs and data into practice.
Methods
DRC minutes from December 2017 to May 2023 were reviewed. Discussion items were categorized into type of review. Date of local review was compared to national formulary criteria for use publication dates, and date of FDA approval for new drugs or publication date for new data, where applicable. Items were excluded if crucial information was missing from minutes. Descriptive statistics were used.
Results
Over 65 months, 38 meetings were held. Thirty total members include: pharmacists, physicians, fellows, and advanced practice providers. Items reviewed included: 36 new drugs (ND), 36 new indications/data (NI), 14 institutional preferences, 10 new dosage form/biosimilars, 4 drug shortages and 2 others. The median time from ND approval to discussion was 3 months (n= 36, IQR 3-6) and NI from publication was 3 months (n=30, IQR 1-8). Nearly all (34/36, 94%) ND were reviewed prior to national review. Local review was a median of 7 months before national, with 11 drugs currently having no published national criteria for use (n=25, IQR 2-12).
Conclusions
DRC formation has enabled faster incorporation of new drugs/indications into practice. It has also created an appropriate forum for in-depth utilization discussions, pharmacoeconomic stewardship, and sharing of formulary and medication related information. VA Health Systems could consider implementing similar committees to review and implement up-to-date oncology practices.
Variation in Cardiovascular Risk Assessment Status in Patients Receiving Oral Anti-Cancer Therapies: A Focus on Equity throughout VISN (Veteran Integrated Service Network) 12
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Impact of a Pharmacist-Led Emergency Department Urinary Tract Infection Aftercare Program
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
Paclitaxel Drug-Drug Interactions in the Military Health System
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Impact of VA Hematology/Oncology Clinical Pharmacy Practitioners in the Review of Community Prescriptions for Specialty Medications
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047