User login
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
A Veteran With a Solitary Pulmonary Nodule
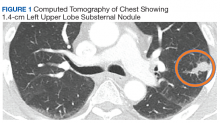
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
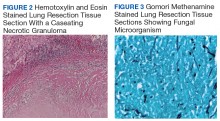
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Armed conflict disproportionately affects children
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
Patient poaching: High on practices’ dirty tricks list
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Caution about ‘miracle cures’; more
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
20 Reasons to celebrate our APA membership in 2020
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
SABCS research changes practice
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Withdrawal of candidacy for APA President-Elect
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
New evidence informs discussions on FL treatment and breast screening
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.