User login
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
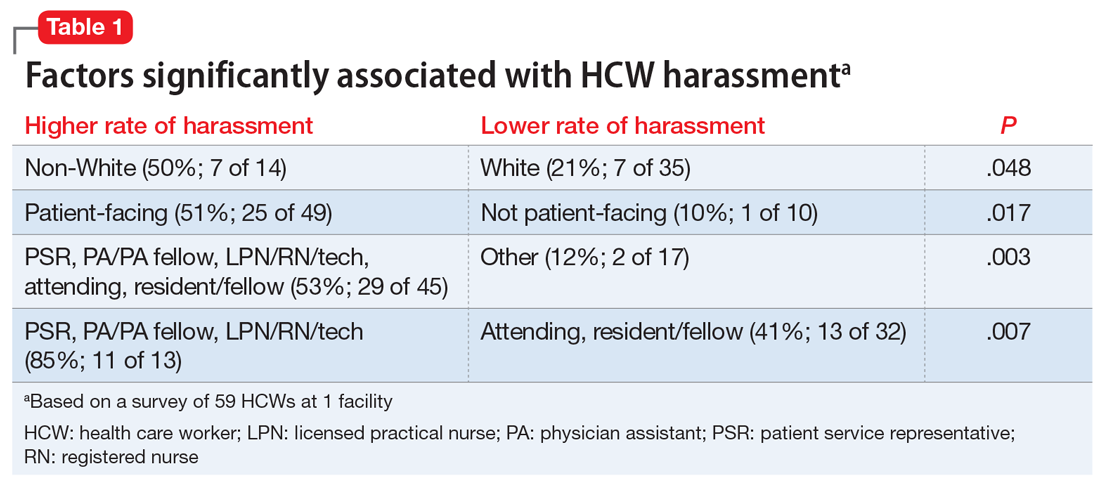
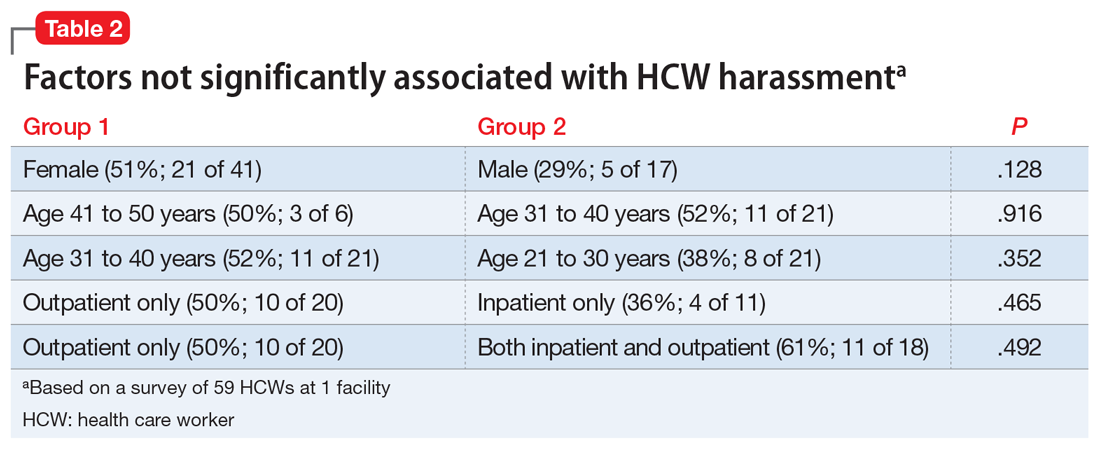
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
COVID-19: One Patient at a Time
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
The COVID-19 pandemic and changes in pediatric respiratory and nonrespiratory illnesses
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
Psychiatry is Neurology: White matter pathology permeates psychiatric disorders
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
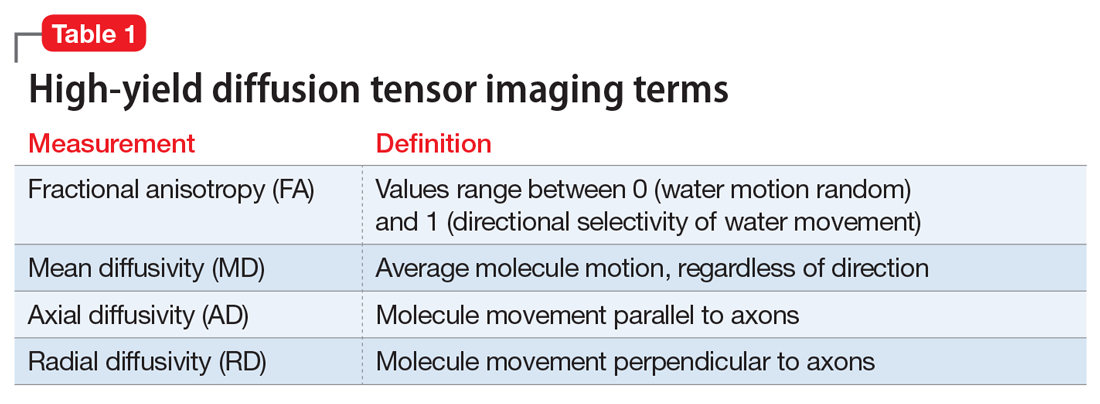
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
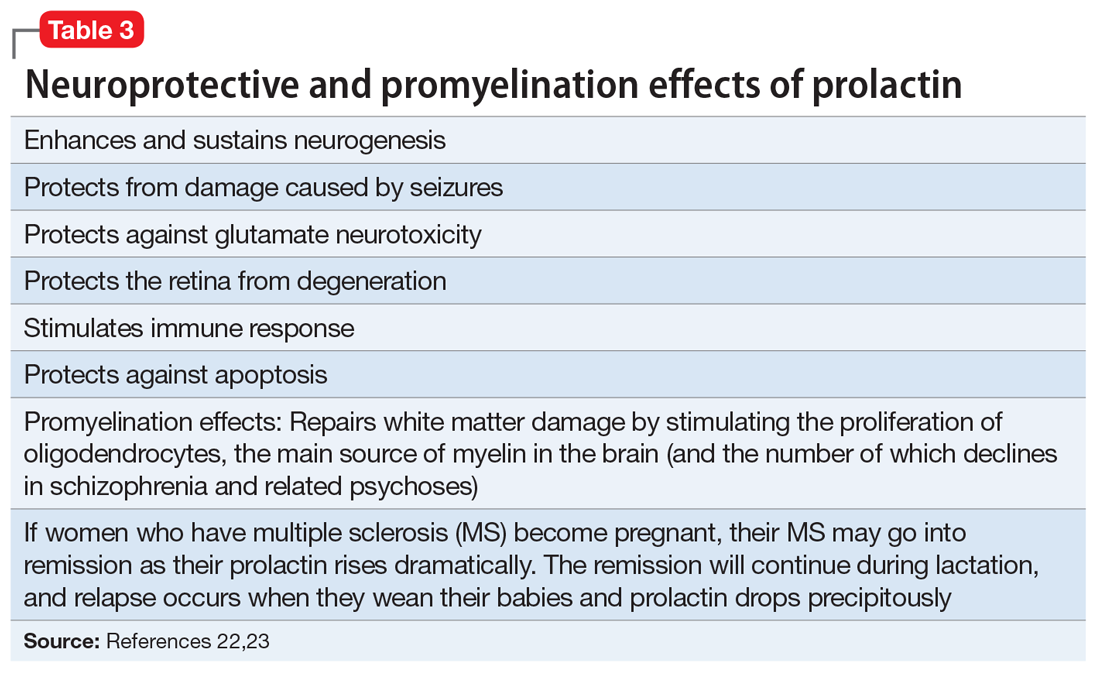
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Decision making regarding LEEP versus cone biopsy for excision of cervical dysplasia
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 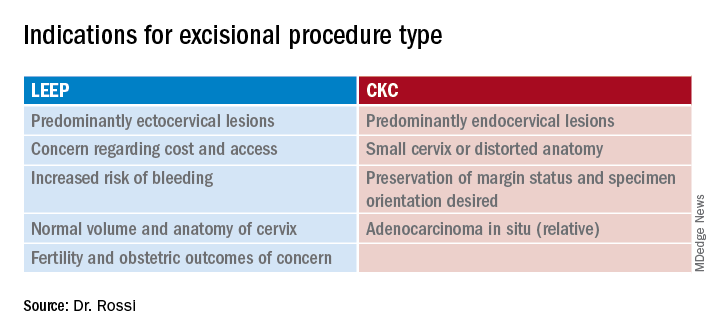
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Loop electrosurgical excision procedure (LEEP) or cold knife conization of the cervix (CKC) is the standard of care approach for women with cervical intra-epithelial neoplasia (CIN 3) because it achieves both disease control and diagnostic evaluation to rule out invasive carcinoma. While both techniques are associated with equivalent efficacy in disease control, each has its virtues and advantages, and clinical judgment is necessary when choosing a technique.1
LEEP, or large loop electrosurgical excision of the transformation zone (LLETZ) involves use of electrosurgical current directed through wire loops to excise pieces of cervical tissue. The equipment for this technique is widely available and this procedure can most often be performed safely and comfortably in an outpatient office setting, making it a cost-effective strategy. Its ease of access means that it can be employed in “see-and-treat” programs where there is concern regarding follow-up. The loop from the device has a tendency to take more shallow pieces of tissue, preserving more cervical stroma. This may be why LEEP has been associated with decreased risk for obstetric complications associated with cervical insufficiency when compared with CKC.2,3
The shallowness and standardized, preset shapes of the loops present challenges with this technique. It can be more difficult to tailor the shape of the excision for particular lesions, and surgeons may need to add a second “top hat” endocervical LEEP after the first ectocervical excision to adequately excise the endocervical canal. If the “coagulation” setting is used instead of “blend” or “cut,” excessive drag and resistance can develop during the procedure, which can result in the specimen’s being amputated, fragmented, or interrupted mid-sweep. This can severely limit pathologic interpretation of the specimen. Orienting these multiple fragments for pathology to specify margin status can be limited or impossible. Electrosurgical effect (“thermal effect”) at the margins of the specimen can limit accurate interpretation of adequacy of the excision.
CKC of the cervix is a procedure in which a narrow scalpel (typically an 11-blade) is used to excise the ecto- and endocervical tissues in a cone-shaped specimen that ensures maximal inclusion of ectocervical and endocervical mucosa but minimization of stromal excision. Absence of electrosurgery in the primary excision means that pathologists have clean edges to evaluate for margin status. Because the shape of the incision is unique for each patient, the surgeon can tailor the shape and extent of the cone to focus on known or suspected areas of disease. It is particularly useful when there is an endocervical lesion, such as in cases of adenocarcinoma in situ and in postmenopausal women whose transformation zone is frequently within the canal. In cases of a distorted, atrophic cervix, or one that is flush with the vagina, a conization procedure in the operating room affords surgeons greater control and precision. Major limitations of this procedure are that it is typically performed in an operating room setting because of the potential for intraoperative bleeding, and its increased risk for early and late complications. The conization procedure is associated with increased obstetric risk in later pregnancies, possibly because of more significant disturbance of cervical stroma.2,3
As mentioned earlier, both procedures are associated with equivalent outcomes with respect to control of disease.1 CKC procedures are associated with more complications, including bleeding (intraoperatively and postoperatively) than are LEEPs. Traditionally, adenocarcinoma in situ (AIS) has been preferentially treated with CKC because of the propensity of this lesion to reside within the endocervical canal, a region more readily and extensively sampled with the CKC. However, provided that the LEEP specimen achieves negative margin status, there is no specific benefit of CKC over LEEP. Guidelines recommend that AIS is excised as a single specimen (without a “top hat”) to achieve accurate pathology regarding margins in the endocervical canal.4 Considering that a specimen depth between 10 and 20 mm is ideal in the setting of AIS, it may be difficult to achieve this depth with a single-pass LEEP depending upon the dimensions of the cervix. It is due to these technical challenges associated with LEEP that CKC is typically preferred in the treatment of AIS.
Ultimately, the decision regarding when to choose LEEP versus CKC is nuanced and should be tailored for each patient. Factors to consider include the patient’s ease of follow-up, financial limitations, preexisting distortion of anatomy, and the need to minimize obstetrics risks or achieve wider margins. For example, a young, nulliparous patient with an ectocervical lesion of squamous dysplasia would likely best be served by a LEEP, which preserves her cervical stroma and affords her easy access and affordability of the procedure. A patient with a bleeding diathesis including iatrogenic anticoagulant therapy may also benefit from a LEEP to achieve better hemostasis and lower risk of bleeding complications.
A postmenopausal woman with a narrow upper vagina and cervix flush with the vagina from prior excisional procedures may benefit from a conization in the operating room where adequate retraction and exposure can minimize the risk of damage to adjacent structures, and the shape and size of the excision can be tailored to the long, narrow segment that is indicated. The table highlights some of the factors to consider when choosing these options. 
In summary, LEEP and CKC are both highly effective excisional procedures that can be considered for all patients with cervical dysplasia. Decisions regarding which is preferred for patients are nuanced and should consider individualized anatomic, pathologic, functional and financial implications.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Contact her at [email protected].
References
1. Martin-Hirsch PL et al. Cochrane Database Syst Rev 2000;(2):CD001318.
2. Arbyn M et al. BMJ. 2008;337:a1284.
3. Jin G et al. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
4. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102.
Hospital at Home: Delivering hospital-level care without the hospital
How to implement a new model of care
The United States spends one-third of the nation’s health dollars on hospital care, amounting to $1.2 trillion in 2018.1 U.S. hospital beds are prevalent2, and expensive to build and operate, with most hospital services costs related to buildings, equipment, salaried labor, and overhead.3
Despite their mission to heal, hospitals can be harmful, especially for frail and elderly patients. A study completed by the Office of the Inspector General (OIG) found that 13.5% of hospitalized Medicare patients experienced an adverse event that resulted in a prolonged hospital stay, permanent harm, a life-sustaining intervention or death.4 In addition, there is growing concern about acquired post-hospitalization syndrome caused by the physiological stress that patients experience in the hospital, leaving them vulnerable to clinical adverse events such as falls and infections.5
In the mid-1990s, driven by a goal to “avoid the harm of inpatient care and honor the wishes of older adults who refused to go to the hospital”, Dr. Bruce Leff, director of the Center for Transformative Geriatric Research and professor of medicine at Johns Hopkins University in Baltimore, and his team set out to develop and test Hospital at Home (HaH) – an innovative model for delivering hospital-level care to selected patients in the safety of their homes.
More than 20 years later, despite extensive evidence supporting HaH safety and efficacy, and its successful rollout in other countries, the model has not been widely adopted in the U.S. However, the COVID-19 pandemic amplified interest in HaH by creating an urgent need for flexible hospital bed capacity and heightening concerns about hospital care safety, especially for vulnerable adults.
In this article, we will introduce HaH history and efficacy, and then discuss what it takes to successfully implement HaH.
Hospital at Home: History, efficacy, and early adoption
The earliest HaH study, a 17-patient pilot conducted by Dr. Leff’s team from 1996 to 1998, proved that HaH was feasible, safe, highly satisfactory and cost-effective for selected acutely ill older patients with community-acquired pneumonia, chronic heart failure, chronic obstructive pulmonary disease or cellulitis.6 In 2000 to 2002, a National Demonstration and Evaluation Study of 455 patients across three sites determined that patients treated in Hospital at Home had statistically significant shorter length of stay (3.2 vs 4.9 days), lower cost ($5,081 vs. $7,480) and complications.7 Equipped with evidence, Dr. Leff and his team focused on HaH dissemination and implementation across several health care systems.8
Presbyterian Healthcare Services in Albuquerque, N.M., was one of the earliest adopters of HaH and launched the program in 2008. The integrated system serves one-third of New Mexicans and includes nine hospitals, more than 100 clinics and the state’s largest health plan. According to Nancy Guinn, MD, a medical director of Presbyterian Healthcare at Home, “Innovation is key to survive in a lean environment like New Mexico, which has the lowest percentage of residents with insurance from their employer and a high rate of government payers.”
Presbyterian selected nine diagnoses for HaH focus: congestive heart failure, chronic obstructive pulmonary disease, community-acquired pneumonia, cellulitis, deep venous thrombosis, pulmonary embolism, complicated urinary tract infection or urosepsis, nausea and vomiting, and dehydration. The HaH care, including physician services, is reimbursed via a partial DRG (diagnosis-related group) payment that was negotiated internally between the health system and Presbyterian Health Plan.
The results demonstrated that, compared to hospitalized patients with similar conditions, patients in HaH had a lower rate of falls (0% vs. .8%), lower mortality (.93% vs. 3.4%), higher satisfaction (mean score 90.7 vs. 83.9) and 19% lower cost.9 According to Dr. Guinn, more recent results showed even larger cost savings of 42%.10 After starting the HaH model, Presbyterian has launched other programs that work closely with HaH to provide a seamless experience for patients. That includes the Complete Care Program, which offers home-based primary, urgent, and acute care to members covered through Presbyterian Health Plan and has a daily census of 600-700 patients.
Another important milestone came in 2014 when Icahn School of Medicine at Mount Sinai in New York was awarded $9.6 million by the Center for Medicare and Medicaid Innovation (CMMI) to test the HaH model during acute illness and for 30 days after admission. A case study of 507 patients enrolled in the program in 2014 through 2017 revealed that HaH patients had statistically significant shorter length of stay (3.2 days vs. 5.5 days), and lower rates of all-cause 30-day hospital readmissions (8.6% vs. 15.6%), 30-day ED revisits (5.8% vs. 11.7%), and SNF admissions (1.7% vs. 10.4%), and were also more likely to rate their hospital care highly (68.8% vs. 45.3%).11
In 2017, using data from their CMMI study, Mount Sinai submitted an application to the Physician-Focused Payment Model Technical Advisory Committee (PTAC) to implement Hospital at Home as an alternative payment model that bundles the acute episode with 30 days of post‐acute transitional care. The PTAC unanimously approved the proposal and submitted their recommendations to the Secretary of Health and Human Services (HHS) to implement HaH as an alternative payment model that included two parts:
1. A bundled payment equal to a percentage of the prospective DRG (diagnosis-related group) payment that would have been paid to a hospital.
2. A performance-based payment (shared savings/losses) based on (a) total spending during the acute care phase and 30 days afterward relative to a target price, and (b) performance on quality measures.12
In June 2018, the HHS secretary announced that he was not approving the proposal as written, citing, among other things, concerns about proposed payment methodology and patient safety.13
Hospital at Home: Present state
Despite additional evidence of HaH’s impact on lowering cost, decreasing 30-day readmissions, improving patient satisfaction and functional outcomes without an adverse effect on mortality,14, 15 the model has not been widely adopted, largely due to lack of fee-for-service reimbursement from the public payers (Medicare and Medicaid) and complex logistics to implement it.
However, the COVID-19 pandemic created an urgent need for flexible hospital bed capacity and amplified concerns about hospital care safety for vulnerable populations. In response, the Centers for Medicare and Medicaid Services (CMS) introduced its Hospitals without Walls initiative that allowed hospitals to provide services in other health care facilities and sites that are not part of the existing hospital.16 On November 25, 2020, CMS announced expansion of the Hospital without Walls initiatives to include a Hospital Care at Home program that allows eligible hospitals to treat eligible patients at home.17
With significant evidence supporting HaH’s safety and efficacy, and long overdue support from CMS, it’s now a matter of how to successfully implement it. Let’s explore what it takes to select and enroll patients, deliver acute care at home, and ensure a smooth post-acute transition within the HaH model.
Successfully implementing Hospital at Home
HaH implementation requires five key components – people, processes, technology, supply chain, and analytics – to select and enroll patients, deliver acute care at home, and ensure a smooth postacute transition. Let’s discuss each of them in more detail below.
Selecting and enrolling patients
Patients eligible for HaH are identified based on their insurance, as well as clinical and social criteria. Despite a lack of public payer support, several commercial payers embraced the model for selected patients who consented to receive acute hospital care at home. The patients must meet criteria for an inpatient admission, be medically stable and have a low level of diagnostic uncertainty. Advances in home monitoring technology expanded clinical criteria to include acutely ill patients with multiple comorbidities, including cancer. It is important that patients reside in a safe home environment and live within a reasonable distance from the hospital.
CareMore Health, an integrated health care delivery system serving more than 180,000 Medicare Advantage and Medicaid patients across nine states and Washington D.C., launched Hospital at Home in December 2018, and rapidly scaled from a few referrals to averaging more than 20 new patients per week.
Sashidaran Moodley, MD, medical director at CareMore Health and Aspire Health, in Cerritos, Calif., shared a valuable lesson regarding launching the program: “Do not presume that if you build it, they will come. This is a new model of care that requires physicians to change their behavior and health systems to modify their traditional admission work flows. Program designers should not limit their thinking around sourcing patients just from the emergency department.”
Dr. Moodley recommends moving upstream and bring awareness to the program to drive additional referrals from primary care providers, case managers, and remote patient monitoring programs (for example, heart failure).
Linda DeCherrie, MD, clinical director of Mount Sinai at Home, based in New York, says that “educating and involving hospitalists is key.” At Mount Sinai, patients eligible for HaH are initially evaluated by hospitalists in the ED who write initial orders and then transfer care to HaH hospitalists.
HaH also can enroll eligible patients who still require hospital-level care to complete the last few days of acute hospitalization at home. Early discharge programs have been implemented at CareMore, Presbyterian Healthcare Services in Albuquerque, N.M., and Mount Sinai. At Mount Sinai, a program called Completing Hospitalization at Home initially started with non-COVID patients and expanded to include COVID-19 early discharges, helping to free up much-needed hospital beds.
Delivering acute care at home
HaH requires a well-coordinated multidisciplinary team. Patient care is directed by a team of physicians and nurse practitioners who provide daily in-person or virtual visits. To enable provider work flow, an ambulatory version of electronic medical records (for example, Epic) must be customized to include specialized order sets that mimic inpatient orders and diagnoses-specific care delivery protocols. HaH physicians and nurse practitioners are available 24/7 to address acute patient issues.
In addition, patients receive at least daily visits from registered nurses (RNs) who carry out orders, administer medications, draw labs, and provide clinical assessment and patient education. Some organizations employ HaH nurses, while others contract with home health agencies.
Typically, patients are provided with a tablet to enable telehealth visits, as well as a blood pressure monitor, thermometer, pulse oximeter, and, if needed, scale and glucometer, that allow on-demand or continuous remote monitoring. Recent technology advances in home monitoring enhanced HaH’s capability to care for complex, high-acuity patients, and increased the potential volume of patients that can be safely treated at home.
Providence St. Joseph Health, a not-for-profit health care system operating 51 hospitals and 1,085 clinics across seven states, launched their HaH program earlier this year. Per Danielsson, MD, executive medical director for hospital medicine at Swedish Medical Center in Seattle, describes it as a “high-touch, high-tech program anchored by hospitalists.” The Providence HaH team utilizes a wearable medical device for patients that enables at-home continuous monitoring of vital signs such as temperature, blood pressure, heart rate, respirations, and pulse oximetry. Single-lead EKG monitoring is available for selected patients. Individual patient data is transmitted to a central command center, where a team of nurses and physicians remotely monitor HaH patients. According to Todd Czartoski, MD, chief medical technology officer at Providence, “Hospital at Home improves quality and access, and can substitute for 20%-30% of hospital admissions.”
In addition to patient monitoring and 24/7 provider access, some HaH programs partner with community paramedics for emergency responses. At Mount Sinai, HaH providers can trigger paramedic response, if needed. Paramedics can set up a video link with a doctor and, under the direction of a physician, will provide treatment at home or transport patients to the hospital.
HaH would be impossible without a partnership with local ancillary service providers that can promptly deliver services and goods to patient homes. Raphael Rakowski, CEO of Medically Home, a Boston-based company that partners with health care providers to build virtual hospitals at home, calls it an “acute rapid response supply chain.” The services, both clinical and nonclinical, consist of infusions; x-rays; bedside ultrasound; laboratory; transportation; and skilled physical, occupational, and speech therapy. If patients require services that are not available at home (for example, a CT scan), patients can be transported to and from a diagnostic center. Medical and nonmedical goods include medications, oxygen, durable medical equipment, and even meals.
Delivery of hospital-level services at home requires a seamless coordination between clinical teams and suppliers that relies on nursing care coordinators and supporting nonclinical staff, and is enabled by a secure text messaging platform to communicate within the care team, with suppliers, and with other providers (for example, primary care providers and specialists).
Ensuring smooth postacute transition
Thirty days after hospital discharge is the most critical period, especially for elderly patients. According to one study, 19% of patients experienced adverse events within 3 weeks after hospital discharge.18 Adverse drug events were the most common postdischarge complication, followed by procedural complications and hospital-acquired infections. Furthermore, 30-day all-cause hospital readmissions is a common occurrence. Per the Healthcare Cost and Utilization Project database, 17.1% of Medicare and 13.9% of all-payers patients were readmitted to the hospital within 30 days in 2016.19
It is not surprising that some organizations offer ongoing home care during the postacute period. At Mount Sinai, patients discharged from HaH continue to have access to the HaH team around the clock for 30 days to address emergencies and health concerns. Recovery Care Coordinators and social workers monitor patient health status, develop a follow-up plan, coordinate care, and answer questions. Medically Home provides 24/7 care to HaH patients for the entire duration of the acute care episode (34 days) to ensure maximum access to care and no gaps in care and communication. At Presbyterian, most HaH patients are transitioned into a Home Health episode of care to ensure continued high-quality care.
In addition to people, processes, technology, and the supply chain, HaH implementation requires capabilities to collect and analyze quality and cost data to measure program efficacy and, in some arrangements with payers, to reconcile clams data to determine shared savings or losses.
Partnering with third parties
Considering the resources and capabilities required for HaH program development and implementation, it is not surprising that health care providers are choosing to partner with third parties. For example, Mount Sinai partnered with Contessa Health, a Nashville, Tenn.–based company that offers hospitals a turn-key Home Recovery Care program, to assist with supply chain contracting and management, and claims data reconciliation.
Medically Home has partnered with seven health care systems, including the Mayo Clinic, Tufts Medical Center in Boston, and Adventist Health in southern California, to create virtual beds, and is expected to launch the program with 15 health care systems by the end of 2020.
Medically Home offers the following services to its partners to enable care for high-acuity patients at home:
- Assistance with hiring and training of clinical staff.
- Proprietary EMR-integrated orders, notes, and clinical protocols.
- Technology for patient monitoring by the 24/7 central command center; tablets that provide health status updates and daily schedules, and enable televisits; a video platform for video communication; and secure texting.
- Selection, contracting and monitoring the performance of supply chain vendors.
- Analytics.
The future of Hospital at Home
There is no question that HaH can offer a safe, high-quality, and lower-cost alternative to hospitalizations for select patients, which is aligned with the Centers for Medicare and Medicaid Services’ triple aim of better care for individuals, better health for populations, and lower cost.20
The future of HaH depends on development of a common payment model that will be adopted beyond the pandemic by government and commercial payers. Current payment models vary and include capitated agreements, discounted diagnosis-related group payments for the acute episode, and discounted DRG payments plus shared losses or savings.
The COVID-19 pandemic has created, arguably, the biggest crisis that U.S. health care has ever experienced, and it is far from over. Short term, Hospital at Home offers a solution to create flexible hospital bed capacity and deliver safe hospital-level care for vulnerable populations. Long term, it may be the solution that helps achieve better care for individuals, better health for populations and lower health care costs.
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of the Hospitalist’s editorial advisory board.
References
1. Source: www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
2. Source: www.aha.org/statistics/fast-facts-us-hospitals
3. Roberts RR, et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999 Feb;281(7):644-9.
4. Levinson DR; US Department of Health and Human Services; HHS; Office of the Inspector General; OIG.
5. Krumholz HM. Post-Hospital Syndrome – An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013 Jan;368:100-102.
6. Leff B, et al. Home hospital program: a pilot study. J Am Geriatr Soc. 1999 Jun;47(6):697-702.
7. Leff B, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005 Dec;143(11):798-808.
8. Source: www.johnshopkinssolutions.com/solution/hospital-at-home/
9. Cryer L, et al. Costs for ‘Hospital at Home’ Patients Were 19 Percent Lower, with Equal or Better Outcomes Compared to Similar Inpatients. Health Affairs. 2012 Jun;31(6):1237–43.
10. Personal communication with Presbyterian Health Services. May 20, 2020.
11. Federman A, et al. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018 Aug;178(8):1033–40.
12. Source: aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf
13. Source: aspe.hhs.gov/system/files/pdf/255906/Secretarial_Responses_June_13_2018.508.pdf
14. Shepperd S, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9(9):CD007491. DOI:10.1002/14651858.CD007491.pub2.
15. Levine DM, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020 Jan;172(2);77-85.
16. Source: www.cms.gov/files/document/covid-hospitals.pdf
17. Centers for Medicare & Medicaid Services. CMS Announces Comprehensive Strategy to Enhance Hospital Capacity Amid COVID-19 Surge. 2020 Nov 20.
18. Forster AJ et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003 Mar;138(3):161-7. doi: 10.7326/0003-4819-138-3-200302040-00007.
19. Bailey MK et al. Characteristics of 30-Day All-Cause Hospital Readmissions, 2010-2016. Statistical Brief 248. Agency for Healthcare Research and Quality. 2019 Feb 12. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb248-Hospital-Readmissions-2010-2016.jsp.
20. Centers for Medicare & Medicaid Services. What are the value-based programs? 2020 Jan 6. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.
How to implement a new model of care
How to implement a new model of care
The United States spends one-third of the nation’s health dollars on hospital care, amounting to $1.2 trillion in 2018.1 U.S. hospital beds are prevalent2, and expensive to build and operate, with most hospital services costs related to buildings, equipment, salaried labor, and overhead.3
Despite their mission to heal, hospitals can be harmful, especially for frail and elderly patients. A study completed by the Office of the Inspector General (OIG) found that 13.5% of hospitalized Medicare patients experienced an adverse event that resulted in a prolonged hospital stay, permanent harm, a life-sustaining intervention or death.4 In addition, there is growing concern about acquired post-hospitalization syndrome caused by the physiological stress that patients experience in the hospital, leaving them vulnerable to clinical adverse events such as falls and infections.5
In the mid-1990s, driven by a goal to “avoid the harm of inpatient care and honor the wishes of older adults who refused to go to the hospital”, Dr. Bruce Leff, director of the Center for Transformative Geriatric Research and professor of medicine at Johns Hopkins University in Baltimore, and his team set out to develop and test Hospital at Home (HaH) – an innovative model for delivering hospital-level care to selected patients in the safety of their homes.
More than 20 years later, despite extensive evidence supporting HaH safety and efficacy, and its successful rollout in other countries, the model has not been widely adopted in the U.S. However, the COVID-19 pandemic amplified interest in HaH by creating an urgent need for flexible hospital bed capacity and heightening concerns about hospital care safety, especially for vulnerable adults.
In this article, we will introduce HaH history and efficacy, and then discuss what it takes to successfully implement HaH.
Hospital at Home: History, efficacy, and early adoption
The earliest HaH study, a 17-patient pilot conducted by Dr. Leff’s team from 1996 to 1998, proved that HaH was feasible, safe, highly satisfactory and cost-effective for selected acutely ill older patients with community-acquired pneumonia, chronic heart failure, chronic obstructive pulmonary disease or cellulitis.6 In 2000 to 2002, a National Demonstration and Evaluation Study of 455 patients across three sites determined that patients treated in Hospital at Home had statistically significant shorter length of stay (3.2 vs 4.9 days), lower cost ($5,081 vs. $7,480) and complications.7 Equipped with evidence, Dr. Leff and his team focused on HaH dissemination and implementation across several health care systems.8
Presbyterian Healthcare Services in Albuquerque, N.M., was one of the earliest adopters of HaH and launched the program in 2008. The integrated system serves one-third of New Mexicans and includes nine hospitals, more than 100 clinics and the state’s largest health plan. According to Nancy Guinn, MD, a medical director of Presbyterian Healthcare at Home, “Innovation is key to survive in a lean environment like New Mexico, which has the lowest percentage of residents with insurance from their employer and a high rate of government payers.”
Presbyterian selected nine diagnoses for HaH focus: congestive heart failure, chronic obstructive pulmonary disease, community-acquired pneumonia, cellulitis, deep venous thrombosis, pulmonary embolism, complicated urinary tract infection or urosepsis, nausea and vomiting, and dehydration. The HaH care, including physician services, is reimbursed via a partial DRG (diagnosis-related group) payment that was negotiated internally between the health system and Presbyterian Health Plan.
The results demonstrated that, compared to hospitalized patients with similar conditions, patients in HaH had a lower rate of falls (0% vs. .8%), lower mortality (.93% vs. 3.4%), higher satisfaction (mean score 90.7 vs. 83.9) and 19% lower cost.9 According to Dr. Guinn, more recent results showed even larger cost savings of 42%.10 After starting the HaH model, Presbyterian has launched other programs that work closely with HaH to provide a seamless experience for patients. That includes the Complete Care Program, which offers home-based primary, urgent, and acute care to members covered through Presbyterian Health Plan and has a daily census of 600-700 patients.
Another important milestone came in 2014 when Icahn School of Medicine at Mount Sinai in New York was awarded $9.6 million by the Center for Medicare and Medicaid Innovation (CMMI) to test the HaH model during acute illness and for 30 days after admission. A case study of 507 patients enrolled in the program in 2014 through 2017 revealed that HaH patients had statistically significant shorter length of stay (3.2 days vs. 5.5 days), and lower rates of all-cause 30-day hospital readmissions (8.6% vs. 15.6%), 30-day ED revisits (5.8% vs. 11.7%), and SNF admissions (1.7% vs. 10.4%), and were also more likely to rate their hospital care highly (68.8% vs. 45.3%).11
In 2017, using data from their CMMI study, Mount Sinai submitted an application to the Physician-Focused Payment Model Technical Advisory Committee (PTAC) to implement Hospital at Home as an alternative payment model that bundles the acute episode with 30 days of post‐acute transitional care. The PTAC unanimously approved the proposal and submitted their recommendations to the Secretary of Health and Human Services (HHS) to implement HaH as an alternative payment model that included two parts:
1. A bundled payment equal to a percentage of the prospective DRG (diagnosis-related group) payment that would have been paid to a hospital.
2. A performance-based payment (shared savings/losses) based on (a) total spending during the acute care phase and 30 days afterward relative to a target price, and (b) performance on quality measures.12
In June 2018, the HHS secretary announced that he was not approving the proposal as written, citing, among other things, concerns about proposed payment methodology and patient safety.13
Hospital at Home: Present state
Despite additional evidence of HaH’s impact on lowering cost, decreasing 30-day readmissions, improving patient satisfaction and functional outcomes without an adverse effect on mortality,14, 15 the model has not been widely adopted, largely due to lack of fee-for-service reimbursement from the public payers (Medicare and Medicaid) and complex logistics to implement it.
However, the COVID-19 pandemic created an urgent need for flexible hospital bed capacity and amplified concerns about hospital care safety for vulnerable populations. In response, the Centers for Medicare and Medicaid Services (CMS) introduced its Hospitals without Walls initiative that allowed hospitals to provide services in other health care facilities and sites that are not part of the existing hospital.16 On November 25, 2020, CMS announced expansion of the Hospital without Walls initiatives to include a Hospital Care at Home program that allows eligible hospitals to treat eligible patients at home.17
With significant evidence supporting HaH’s safety and efficacy, and long overdue support from CMS, it’s now a matter of how to successfully implement it. Let’s explore what it takes to select and enroll patients, deliver acute care at home, and ensure a smooth post-acute transition within the HaH model.
Successfully implementing Hospital at Home
HaH implementation requires five key components – people, processes, technology, supply chain, and analytics – to select and enroll patients, deliver acute care at home, and ensure a smooth postacute transition. Let’s discuss each of them in more detail below.
Selecting and enrolling patients
Patients eligible for HaH are identified based on their insurance, as well as clinical and social criteria. Despite a lack of public payer support, several commercial payers embraced the model for selected patients who consented to receive acute hospital care at home. The patients must meet criteria for an inpatient admission, be medically stable and have a low level of diagnostic uncertainty. Advances in home monitoring technology expanded clinical criteria to include acutely ill patients with multiple comorbidities, including cancer. It is important that patients reside in a safe home environment and live within a reasonable distance from the hospital.
CareMore Health, an integrated health care delivery system serving more than 180,000 Medicare Advantage and Medicaid patients across nine states and Washington D.C., launched Hospital at Home in December 2018, and rapidly scaled from a few referrals to averaging more than 20 new patients per week.
Sashidaran Moodley, MD, medical director at CareMore Health and Aspire Health, in Cerritos, Calif., shared a valuable lesson regarding launching the program: “Do not presume that if you build it, they will come. This is a new model of care that requires physicians to change their behavior and health systems to modify their traditional admission work flows. Program designers should not limit their thinking around sourcing patients just from the emergency department.”
Dr. Moodley recommends moving upstream and bring awareness to the program to drive additional referrals from primary care providers, case managers, and remote patient monitoring programs (for example, heart failure).
Linda DeCherrie, MD, clinical director of Mount Sinai at Home, based in New York, says that “educating and involving hospitalists is key.” At Mount Sinai, patients eligible for HaH are initially evaluated by hospitalists in the ED who write initial orders and then transfer care to HaH hospitalists.
HaH also can enroll eligible patients who still require hospital-level care to complete the last few days of acute hospitalization at home. Early discharge programs have been implemented at CareMore, Presbyterian Healthcare Services in Albuquerque, N.M., and Mount Sinai. At Mount Sinai, a program called Completing Hospitalization at Home initially started with non-COVID patients and expanded to include COVID-19 early discharges, helping to free up much-needed hospital beds.
Delivering acute care at home
HaH requires a well-coordinated multidisciplinary team. Patient care is directed by a team of physicians and nurse practitioners who provide daily in-person or virtual visits. To enable provider work flow, an ambulatory version of electronic medical records (for example, Epic) must be customized to include specialized order sets that mimic inpatient orders and diagnoses-specific care delivery protocols. HaH physicians and nurse practitioners are available 24/7 to address acute patient issues.
In addition, patients receive at least daily visits from registered nurses (RNs) who carry out orders, administer medications, draw labs, and provide clinical assessment and patient education. Some organizations employ HaH nurses, while others contract with home health agencies.
Typically, patients are provided with a tablet to enable telehealth visits, as well as a blood pressure monitor, thermometer, pulse oximeter, and, if needed, scale and glucometer, that allow on-demand or continuous remote monitoring. Recent technology advances in home monitoring enhanced HaH’s capability to care for complex, high-acuity patients, and increased the potential volume of patients that can be safely treated at home.
Providence St. Joseph Health, a not-for-profit health care system operating 51 hospitals and 1,085 clinics across seven states, launched their HaH program earlier this year. Per Danielsson, MD, executive medical director for hospital medicine at Swedish Medical Center in Seattle, describes it as a “high-touch, high-tech program anchored by hospitalists.” The Providence HaH team utilizes a wearable medical device for patients that enables at-home continuous monitoring of vital signs such as temperature, blood pressure, heart rate, respirations, and pulse oximetry. Single-lead EKG monitoring is available for selected patients. Individual patient data is transmitted to a central command center, where a team of nurses and physicians remotely monitor HaH patients. According to Todd Czartoski, MD, chief medical technology officer at Providence, “Hospital at Home improves quality and access, and can substitute for 20%-30% of hospital admissions.”
In addition to patient monitoring and 24/7 provider access, some HaH programs partner with community paramedics for emergency responses. At Mount Sinai, HaH providers can trigger paramedic response, if needed. Paramedics can set up a video link with a doctor and, under the direction of a physician, will provide treatment at home or transport patients to the hospital.
HaH would be impossible without a partnership with local ancillary service providers that can promptly deliver services and goods to patient homes. Raphael Rakowski, CEO of Medically Home, a Boston-based company that partners with health care providers to build virtual hospitals at home, calls it an “acute rapid response supply chain.” The services, both clinical and nonclinical, consist of infusions; x-rays; bedside ultrasound; laboratory; transportation; and skilled physical, occupational, and speech therapy. If patients require services that are not available at home (for example, a CT scan), patients can be transported to and from a diagnostic center. Medical and nonmedical goods include medications, oxygen, durable medical equipment, and even meals.
Delivery of hospital-level services at home requires a seamless coordination between clinical teams and suppliers that relies on nursing care coordinators and supporting nonclinical staff, and is enabled by a secure text messaging platform to communicate within the care team, with suppliers, and with other providers (for example, primary care providers and specialists).
Ensuring smooth postacute transition
Thirty days after hospital discharge is the most critical period, especially for elderly patients. According to one study, 19% of patients experienced adverse events within 3 weeks after hospital discharge.18 Adverse drug events were the most common postdischarge complication, followed by procedural complications and hospital-acquired infections. Furthermore, 30-day all-cause hospital readmissions is a common occurrence. Per the Healthcare Cost and Utilization Project database, 17.1% of Medicare and 13.9% of all-payers patients were readmitted to the hospital within 30 days in 2016.19
It is not surprising that some organizations offer ongoing home care during the postacute period. At Mount Sinai, patients discharged from HaH continue to have access to the HaH team around the clock for 30 days to address emergencies and health concerns. Recovery Care Coordinators and social workers monitor patient health status, develop a follow-up plan, coordinate care, and answer questions. Medically Home provides 24/7 care to HaH patients for the entire duration of the acute care episode (34 days) to ensure maximum access to care and no gaps in care and communication. At Presbyterian, most HaH patients are transitioned into a Home Health episode of care to ensure continued high-quality care.
In addition to people, processes, technology, and the supply chain, HaH implementation requires capabilities to collect and analyze quality and cost data to measure program efficacy and, in some arrangements with payers, to reconcile clams data to determine shared savings or losses.
Partnering with third parties
Considering the resources and capabilities required for HaH program development and implementation, it is not surprising that health care providers are choosing to partner with third parties. For example, Mount Sinai partnered with Contessa Health, a Nashville, Tenn.–based company that offers hospitals a turn-key Home Recovery Care program, to assist with supply chain contracting and management, and claims data reconciliation.
Medically Home has partnered with seven health care systems, including the Mayo Clinic, Tufts Medical Center in Boston, and Adventist Health in southern California, to create virtual beds, and is expected to launch the program with 15 health care systems by the end of 2020.
Medically Home offers the following services to its partners to enable care for high-acuity patients at home:
- Assistance with hiring and training of clinical staff.
- Proprietary EMR-integrated orders, notes, and clinical protocols.
- Technology for patient monitoring by the 24/7 central command center; tablets that provide health status updates and daily schedules, and enable televisits; a video platform for video communication; and secure texting.
- Selection, contracting and monitoring the performance of supply chain vendors.
- Analytics.
The future of Hospital at Home
There is no question that HaH can offer a safe, high-quality, and lower-cost alternative to hospitalizations for select patients, which is aligned with the Centers for Medicare and Medicaid Services’ triple aim of better care for individuals, better health for populations, and lower cost.20
The future of HaH depends on development of a common payment model that will be adopted beyond the pandemic by government and commercial payers. Current payment models vary and include capitated agreements, discounted diagnosis-related group payments for the acute episode, and discounted DRG payments plus shared losses or savings.
The COVID-19 pandemic has created, arguably, the biggest crisis that U.S. health care has ever experienced, and it is far from over. Short term, Hospital at Home offers a solution to create flexible hospital bed capacity and deliver safe hospital-level care for vulnerable populations. Long term, it may be the solution that helps achieve better care for individuals, better health for populations and lower health care costs.
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of the Hospitalist’s editorial advisory board.
References
1. Source: www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
2. Source: www.aha.org/statistics/fast-facts-us-hospitals
3. Roberts RR, et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999 Feb;281(7):644-9.
4. Levinson DR; US Department of Health and Human Services; HHS; Office of the Inspector General; OIG.
5. Krumholz HM. Post-Hospital Syndrome – An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013 Jan;368:100-102.
6. Leff B, et al. Home hospital program: a pilot study. J Am Geriatr Soc. 1999 Jun;47(6):697-702.
7. Leff B, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005 Dec;143(11):798-808.
8. Source: www.johnshopkinssolutions.com/solution/hospital-at-home/
9. Cryer L, et al. Costs for ‘Hospital at Home’ Patients Were 19 Percent Lower, with Equal or Better Outcomes Compared to Similar Inpatients. Health Affairs. 2012 Jun;31(6):1237–43.
10. Personal communication with Presbyterian Health Services. May 20, 2020.
11. Federman A, et al. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018 Aug;178(8):1033–40.
12. Source: aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf
13. Source: aspe.hhs.gov/system/files/pdf/255906/Secretarial_Responses_June_13_2018.508.pdf
14. Shepperd S, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9(9):CD007491. DOI:10.1002/14651858.CD007491.pub2.
15. Levine DM, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020 Jan;172(2);77-85.
16. Source: www.cms.gov/files/document/covid-hospitals.pdf
17. Centers for Medicare & Medicaid Services. CMS Announces Comprehensive Strategy to Enhance Hospital Capacity Amid COVID-19 Surge. 2020 Nov 20.
18. Forster AJ et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003 Mar;138(3):161-7. doi: 10.7326/0003-4819-138-3-200302040-00007.
19. Bailey MK et al. Characteristics of 30-Day All-Cause Hospital Readmissions, 2010-2016. Statistical Brief 248. Agency for Healthcare Research and Quality. 2019 Feb 12. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb248-Hospital-Readmissions-2010-2016.jsp.
20. Centers for Medicare & Medicaid Services. What are the value-based programs? 2020 Jan 6. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.
The United States spends one-third of the nation’s health dollars on hospital care, amounting to $1.2 trillion in 2018.1 U.S. hospital beds are prevalent2, and expensive to build and operate, with most hospital services costs related to buildings, equipment, salaried labor, and overhead.3
Despite their mission to heal, hospitals can be harmful, especially for frail and elderly patients. A study completed by the Office of the Inspector General (OIG) found that 13.5% of hospitalized Medicare patients experienced an adverse event that resulted in a prolonged hospital stay, permanent harm, a life-sustaining intervention or death.4 In addition, there is growing concern about acquired post-hospitalization syndrome caused by the physiological stress that patients experience in the hospital, leaving them vulnerable to clinical adverse events such as falls and infections.5
In the mid-1990s, driven by a goal to “avoid the harm of inpatient care and honor the wishes of older adults who refused to go to the hospital”, Dr. Bruce Leff, director of the Center for Transformative Geriatric Research and professor of medicine at Johns Hopkins University in Baltimore, and his team set out to develop and test Hospital at Home (HaH) – an innovative model for delivering hospital-level care to selected patients in the safety of their homes.
More than 20 years later, despite extensive evidence supporting HaH safety and efficacy, and its successful rollout in other countries, the model has not been widely adopted in the U.S. However, the COVID-19 pandemic amplified interest in HaH by creating an urgent need for flexible hospital bed capacity and heightening concerns about hospital care safety, especially for vulnerable adults.
In this article, we will introduce HaH history and efficacy, and then discuss what it takes to successfully implement HaH.
Hospital at Home: History, efficacy, and early adoption
The earliest HaH study, a 17-patient pilot conducted by Dr. Leff’s team from 1996 to 1998, proved that HaH was feasible, safe, highly satisfactory and cost-effective for selected acutely ill older patients with community-acquired pneumonia, chronic heart failure, chronic obstructive pulmonary disease or cellulitis.6 In 2000 to 2002, a National Demonstration and Evaluation Study of 455 patients across three sites determined that patients treated in Hospital at Home had statistically significant shorter length of stay (3.2 vs 4.9 days), lower cost ($5,081 vs. $7,480) and complications.7 Equipped with evidence, Dr. Leff and his team focused on HaH dissemination and implementation across several health care systems.8
Presbyterian Healthcare Services in Albuquerque, N.M., was one of the earliest adopters of HaH and launched the program in 2008. The integrated system serves one-third of New Mexicans and includes nine hospitals, more than 100 clinics and the state’s largest health plan. According to Nancy Guinn, MD, a medical director of Presbyterian Healthcare at Home, “Innovation is key to survive in a lean environment like New Mexico, which has the lowest percentage of residents with insurance from their employer and a high rate of government payers.”
Presbyterian selected nine diagnoses for HaH focus: congestive heart failure, chronic obstructive pulmonary disease, community-acquired pneumonia, cellulitis, deep venous thrombosis, pulmonary embolism, complicated urinary tract infection or urosepsis, nausea and vomiting, and dehydration. The HaH care, including physician services, is reimbursed via a partial DRG (diagnosis-related group) payment that was negotiated internally between the health system and Presbyterian Health Plan.
The results demonstrated that, compared to hospitalized patients with similar conditions, patients in HaH had a lower rate of falls (0% vs. .8%), lower mortality (.93% vs. 3.4%), higher satisfaction (mean score 90.7 vs. 83.9) and 19% lower cost.9 According to Dr. Guinn, more recent results showed even larger cost savings of 42%.10 After starting the HaH model, Presbyterian has launched other programs that work closely with HaH to provide a seamless experience for patients. That includes the Complete Care Program, which offers home-based primary, urgent, and acute care to members covered through Presbyterian Health Plan and has a daily census of 600-700 patients.
Another important milestone came in 2014 when Icahn School of Medicine at Mount Sinai in New York was awarded $9.6 million by the Center for Medicare and Medicaid Innovation (CMMI) to test the HaH model during acute illness and for 30 days after admission. A case study of 507 patients enrolled in the program in 2014 through 2017 revealed that HaH patients had statistically significant shorter length of stay (3.2 days vs. 5.5 days), and lower rates of all-cause 30-day hospital readmissions (8.6% vs. 15.6%), 30-day ED revisits (5.8% vs. 11.7%), and SNF admissions (1.7% vs. 10.4%), and were also more likely to rate their hospital care highly (68.8% vs. 45.3%).11
In 2017, using data from their CMMI study, Mount Sinai submitted an application to the Physician-Focused Payment Model Technical Advisory Committee (PTAC) to implement Hospital at Home as an alternative payment model that bundles the acute episode with 30 days of post‐acute transitional care. The PTAC unanimously approved the proposal and submitted their recommendations to the Secretary of Health and Human Services (HHS) to implement HaH as an alternative payment model that included two parts:
1. A bundled payment equal to a percentage of the prospective DRG (diagnosis-related group) payment that would have been paid to a hospital.
2. A performance-based payment (shared savings/losses) based on (a) total spending during the acute care phase and 30 days afterward relative to a target price, and (b) performance on quality measures.12
In June 2018, the HHS secretary announced that he was not approving the proposal as written, citing, among other things, concerns about proposed payment methodology and patient safety.13
Hospital at Home: Present state
Despite additional evidence of HaH’s impact on lowering cost, decreasing 30-day readmissions, improving patient satisfaction and functional outcomes without an adverse effect on mortality,14, 15 the model has not been widely adopted, largely due to lack of fee-for-service reimbursement from the public payers (Medicare and Medicaid) and complex logistics to implement it.
However, the COVID-19 pandemic created an urgent need for flexible hospital bed capacity and amplified concerns about hospital care safety for vulnerable populations. In response, the Centers for Medicare and Medicaid Services (CMS) introduced its Hospitals without Walls initiative that allowed hospitals to provide services in other health care facilities and sites that are not part of the existing hospital.16 On November 25, 2020, CMS announced expansion of the Hospital without Walls initiatives to include a Hospital Care at Home program that allows eligible hospitals to treat eligible patients at home.17
With significant evidence supporting HaH’s safety and efficacy, and long overdue support from CMS, it’s now a matter of how to successfully implement it. Let’s explore what it takes to select and enroll patients, deliver acute care at home, and ensure a smooth post-acute transition within the HaH model.
Successfully implementing Hospital at Home
HaH implementation requires five key components – people, processes, technology, supply chain, and analytics – to select and enroll patients, deliver acute care at home, and ensure a smooth postacute transition. Let’s discuss each of them in more detail below.
Selecting and enrolling patients
Patients eligible for HaH are identified based on their insurance, as well as clinical and social criteria. Despite a lack of public payer support, several commercial payers embraced the model for selected patients who consented to receive acute hospital care at home. The patients must meet criteria for an inpatient admission, be medically stable and have a low level of diagnostic uncertainty. Advances in home monitoring technology expanded clinical criteria to include acutely ill patients with multiple comorbidities, including cancer. It is important that patients reside in a safe home environment and live within a reasonable distance from the hospital.
CareMore Health, an integrated health care delivery system serving more than 180,000 Medicare Advantage and Medicaid patients across nine states and Washington D.C., launched Hospital at Home in December 2018, and rapidly scaled from a few referrals to averaging more than 20 new patients per week.
Sashidaran Moodley, MD, medical director at CareMore Health and Aspire Health, in Cerritos, Calif., shared a valuable lesson regarding launching the program: “Do not presume that if you build it, they will come. This is a new model of care that requires physicians to change their behavior and health systems to modify their traditional admission work flows. Program designers should not limit their thinking around sourcing patients just from the emergency department.”
Dr. Moodley recommends moving upstream and bring awareness to the program to drive additional referrals from primary care providers, case managers, and remote patient monitoring programs (for example, heart failure).
Linda DeCherrie, MD, clinical director of Mount Sinai at Home, based in New York, says that “educating and involving hospitalists is key.” At Mount Sinai, patients eligible for HaH are initially evaluated by hospitalists in the ED who write initial orders and then transfer care to HaH hospitalists.
HaH also can enroll eligible patients who still require hospital-level care to complete the last few days of acute hospitalization at home. Early discharge programs have been implemented at CareMore, Presbyterian Healthcare Services in Albuquerque, N.M., and Mount Sinai. At Mount Sinai, a program called Completing Hospitalization at Home initially started with non-COVID patients and expanded to include COVID-19 early discharges, helping to free up much-needed hospital beds.
Delivering acute care at home
HaH requires a well-coordinated multidisciplinary team. Patient care is directed by a team of physicians and nurse practitioners who provide daily in-person or virtual visits. To enable provider work flow, an ambulatory version of electronic medical records (for example, Epic) must be customized to include specialized order sets that mimic inpatient orders and diagnoses-specific care delivery protocols. HaH physicians and nurse practitioners are available 24/7 to address acute patient issues.
In addition, patients receive at least daily visits from registered nurses (RNs) who carry out orders, administer medications, draw labs, and provide clinical assessment and patient education. Some organizations employ HaH nurses, while others contract with home health agencies.
Typically, patients are provided with a tablet to enable telehealth visits, as well as a blood pressure monitor, thermometer, pulse oximeter, and, if needed, scale and glucometer, that allow on-demand or continuous remote monitoring. Recent technology advances in home monitoring enhanced HaH’s capability to care for complex, high-acuity patients, and increased the potential volume of patients that can be safely treated at home.
Providence St. Joseph Health, a not-for-profit health care system operating 51 hospitals and 1,085 clinics across seven states, launched their HaH program earlier this year. Per Danielsson, MD, executive medical director for hospital medicine at Swedish Medical Center in Seattle, describes it as a “high-touch, high-tech program anchored by hospitalists.” The Providence HaH team utilizes a wearable medical device for patients that enables at-home continuous monitoring of vital signs such as temperature, blood pressure, heart rate, respirations, and pulse oximetry. Single-lead EKG monitoring is available for selected patients. Individual patient data is transmitted to a central command center, where a team of nurses and physicians remotely monitor HaH patients. According to Todd Czartoski, MD, chief medical technology officer at Providence, “Hospital at Home improves quality and access, and can substitute for 20%-30% of hospital admissions.”
In addition to patient monitoring and 24/7 provider access, some HaH programs partner with community paramedics for emergency responses. At Mount Sinai, HaH providers can trigger paramedic response, if needed. Paramedics can set up a video link with a doctor and, under the direction of a physician, will provide treatment at home or transport patients to the hospital.
HaH would be impossible without a partnership with local ancillary service providers that can promptly deliver services and goods to patient homes. Raphael Rakowski, CEO of Medically Home, a Boston-based company that partners with health care providers to build virtual hospitals at home, calls it an “acute rapid response supply chain.” The services, both clinical and nonclinical, consist of infusions; x-rays; bedside ultrasound; laboratory; transportation; and skilled physical, occupational, and speech therapy. If patients require services that are not available at home (for example, a CT scan), patients can be transported to and from a diagnostic center. Medical and nonmedical goods include medications, oxygen, durable medical equipment, and even meals.
Delivery of hospital-level services at home requires a seamless coordination between clinical teams and suppliers that relies on nursing care coordinators and supporting nonclinical staff, and is enabled by a secure text messaging platform to communicate within the care team, with suppliers, and with other providers (for example, primary care providers and specialists).
Ensuring smooth postacute transition
Thirty days after hospital discharge is the most critical period, especially for elderly patients. According to one study, 19% of patients experienced adverse events within 3 weeks after hospital discharge.18 Adverse drug events were the most common postdischarge complication, followed by procedural complications and hospital-acquired infections. Furthermore, 30-day all-cause hospital readmissions is a common occurrence. Per the Healthcare Cost and Utilization Project database, 17.1% of Medicare and 13.9% of all-payers patients were readmitted to the hospital within 30 days in 2016.19
It is not surprising that some organizations offer ongoing home care during the postacute period. At Mount Sinai, patients discharged from HaH continue to have access to the HaH team around the clock for 30 days to address emergencies and health concerns. Recovery Care Coordinators and social workers monitor patient health status, develop a follow-up plan, coordinate care, and answer questions. Medically Home provides 24/7 care to HaH patients for the entire duration of the acute care episode (34 days) to ensure maximum access to care and no gaps in care and communication. At Presbyterian, most HaH patients are transitioned into a Home Health episode of care to ensure continued high-quality care.
In addition to people, processes, technology, and the supply chain, HaH implementation requires capabilities to collect and analyze quality and cost data to measure program efficacy and, in some arrangements with payers, to reconcile clams data to determine shared savings or losses.
Partnering with third parties
Considering the resources and capabilities required for HaH program development and implementation, it is not surprising that health care providers are choosing to partner with third parties. For example, Mount Sinai partnered with Contessa Health, a Nashville, Tenn.–based company that offers hospitals a turn-key Home Recovery Care program, to assist with supply chain contracting and management, and claims data reconciliation.
Medically Home has partnered with seven health care systems, including the Mayo Clinic, Tufts Medical Center in Boston, and Adventist Health in southern California, to create virtual beds, and is expected to launch the program with 15 health care systems by the end of 2020.
Medically Home offers the following services to its partners to enable care for high-acuity patients at home:
- Assistance with hiring and training of clinical staff.
- Proprietary EMR-integrated orders, notes, and clinical protocols.
- Technology for patient monitoring by the 24/7 central command center; tablets that provide health status updates and daily schedules, and enable televisits; a video platform for video communication; and secure texting.
- Selection, contracting and monitoring the performance of supply chain vendors.
- Analytics.
The future of Hospital at Home
There is no question that HaH can offer a safe, high-quality, and lower-cost alternative to hospitalizations for select patients, which is aligned with the Centers for Medicare and Medicaid Services’ triple aim of better care for individuals, better health for populations, and lower cost.20
The future of HaH depends on development of a common payment model that will be adopted beyond the pandemic by government and commercial payers. Current payment models vary and include capitated agreements, discounted diagnosis-related group payments for the acute episode, and discounted DRG payments plus shared losses or savings.
The COVID-19 pandemic has created, arguably, the biggest crisis that U.S. health care has ever experienced, and it is far from over. Short term, Hospital at Home offers a solution to create flexible hospital bed capacity and deliver safe hospital-level care for vulnerable populations. Long term, it may be the solution that helps achieve better care for individuals, better health for populations and lower health care costs.
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of the Hospitalist’s editorial advisory board.
References
1. Source: www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
2. Source: www.aha.org/statistics/fast-facts-us-hospitals
3. Roberts RR, et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999 Feb;281(7):644-9.
4. Levinson DR; US Department of Health and Human Services; HHS; Office of the Inspector General; OIG.
5. Krumholz HM. Post-Hospital Syndrome – An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013 Jan;368:100-102.
6. Leff B, et al. Home hospital program: a pilot study. J Am Geriatr Soc. 1999 Jun;47(6):697-702.
7. Leff B, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005 Dec;143(11):798-808.
8. Source: www.johnshopkinssolutions.com/solution/hospital-at-home/
9. Cryer L, et al. Costs for ‘Hospital at Home’ Patients Were 19 Percent Lower, with Equal or Better Outcomes Compared to Similar Inpatients. Health Affairs. 2012 Jun;31(6):1237–43.
10. Personal communication with Presbyterian Health Services. May 20, 2020.
11. Federman A, et al. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018 Aug;178(8):1033–40.
12. Source: aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf
13. Source: aspe.hhs.gov/system/files/pdf/255906/Secretarial_Responses_June_13_2018.508.pdf
14. Shepperd S, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9(9):CD007491. DOI:10.1002/14651858.CD007491.pub2.
15. Levine DM, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020 Jan;172(2);77-85.
16. Source: www.cms.gov/files/document/covid-hospitals.pdf
17. Centers for Medicare & Medicaid Services. CMS Announces Comprehensive Strategy to Enhance Hospital Capacity Amid COVID-19 Surge. 2020 Nov 20.
18. Forster AJ et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003 Mar;138(3):161-7. doi: 10.7326/0003-4819-138-3-200302040-00007.
19. Bailey MK et al. Characteristics of 30-Day All-Cause Hospital Readmissions, 2010-2016. Statistical Brief 248. Agency for Healthcare Research and Quality. 2019 Feb 12. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb248-Hospital-Readmissions-2010-2016.jsp.
20. Centers for Medicare & Medicaid Services. What are the value-based programs? 2020 Jan 6. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.
Novel drug approvals of 2020
In 2020, the Food and Drug Administration approved 53 new drugs for humans. One of these agents, Annovera (segesterone and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. Orgovyx (relugolix) is used for prostate cancer and Lampit (nifurtimox) is drug used in children – neither of these two agents will be covered. The remaining 49 are covered below. The agents with molecular weights less than 1,000 probably cross the placenta in the first half of pregnancy, but nearly all, regardless of MW, will cross in the second half of pregnancy.
No human pregnancy data for these agents has been found, but there are five drugs included in pregnancy registries. It will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in an article that I coauthored, “Should pregnant women be included in phase 4 clinical drug trials?”. The article makes a strong argument for including some pregnant women in these trials.
Anti-infectives
Artesunate (384)
The drug appears low risk when used in the second and third trimesters. There is inadequate information regarding its use in the first trimester, so the safest course for the embryo appears to be avoiding its use during this period. A single intravenous dose given to rats early in gestation resulted in embryolethality.
Ebanga (ansuvimab) (147,000)
Studies on its use in pregnant animals have not been conducted.
Inmazeb (atoltivimab, maftivimab, odesivimab) (144,000-146,000)
Inmazeb is a combination of the three agents. Studies on its use in pregnant animals have not been conducted.
Veklury (remdesivir) (603)
Veklury is indicated for the treatment of pregnant women hospitalized with COVID-19 who are at risk for serious morbidity and mortality. The drug should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus.
Antineoplastics
Ayvakit (avapritinib) (499)
The drug may cause fetal harm. The drug was teratogenic in animals.
Blenrep (belantamab mafodotin-blmf) (152,000)
A B-cell maturation antigen, it is indicated for the treatment of multiple myeloma. No human or animal pregnancy data have been located.
Danyelza (naxitamab-gqgk) (144,000)
This agent is used for the treatment of neuroblastoma. Based on its mechanism of action it may cause fetal harm if used in pregnancy.
Gavreto (pralsetinib) (534)
Gavreto is indicated for the treatment of small cell lung cancer. It may cause embryo-fetal harm if used in pregnancy.
Inqovi (cedazuridine + decitabine) (268,228)
The drug combination can cause fetal harm in human pregnancy. It is toxic in pregnant animals.
Margenza (margetuximab-cmkb) (149,000)
Although there are no data on the use of this drug in human pregnancy, the findings in animals and mechanism of action suggest that it will cause fetal harm.
Monjuvi (tafasitamab-cxix) (150,000)
This drug is a cytolytic antibody that is indicated in combination with lenalidomide. The combination may cause fetal harm.
Pemazyre (pemigatinib) (488)
It is indicated for the treatment of cholangiocarcinoma. In an animal study, the drug caused fetal defects, fetal growth retardation, and embryo-fetal death at maternal exposures lower than the human exposure.
Qinlock (ripretinib) (510)
This drug is used for the treatment of patients with advanced gastrointestinal stromal tumor. The drug was teratogenic in pregnant animals.
Retevmo (selpercatinib) (526)
This is a kinase inhibitor used for the treatment of small cell lung cancer. The drug is teratogenic in animals.
Sarclisa (isatuximab-irfc) (148,000)This drug is used in combination with pomalidomide and dexamethasone. The combination would probably cause major toxicity in an embryo or fetus.
Tabrecta (capmatinib) (412 – free base)Capmatinib is a kinase inhibitor used for the treatment of metastatic non–small cell lung cancer. It is teratogenic in animals.
Tazverik (tazemetostat) (654)Tazemetostat is indicated for the treatment of epithelioid sarcoma and follicular lymphoma, The drug is teratogenic in animals.
Trodelvy (sacituzumab govitecan-hziy) (1,602)This agent is used for the treatment of breast cancer. The drug has not been tested in pregnant animals. However, according to the manufacturer, there is a high possibility of human teratogenicity if it is given to a pregnant woman.
Tukysa (tucatinib) (481)
Tukysa is a tyrosine kinase inhibitor that is used in combination with trastuzumab and capecitabine for the treatment of breast cancer. The drug is teratogenic in animals.
Zeposia (ozanimod) (441)
Zeposia is indicated for the treatment of multiple sclerosis. The drug takes about 3 months to eliminate from the body. The drug is teratogenic in animals.
Zepzelca (lurbinectedin) (785)
This agent is used for the treatment of metastatic small cell lung cancer. The drug is teratogenic in animals.
Antiemetics
Barhemsys (amisulpride) (369)
This agent is Indicated to prevent nausea and vomiting. Animal data suggest low risk of embryo/fetal birth defects.
Antimigraine
Nurtec (rimegepant) (611)
Nurtec is indicated for acute treatment of migraine. Development toxicity was not observed in animals given doses similar to those used in humans.
Vyepti (eptinezumab-jjmr) (143,000)
A humanized monoclonal antibody that is given every 3 months to prevent migraine. There was no embryo-fetal harm in animals given the drug.
CNS
Byfavo (remimazolam) (493 – free base)
This drug is indicated for procedural sedation in adults undergoing procedures lasting 30 minutes or less. No defects were observed in animals.
Diagnostics
Cerianna (fluoroestradiol F 18) (289)
It is indicated for use with PET for characterization of estrogen receptor status in patients with ER-positive breast cancer. It has the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. There are no data on its use in pregnant women or animals.
Detectnet (copper CU-64 dotatate) (1,497)
All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. There are no pregnancy data in humans or animals
Miscellaneous
Dojolvi (triheptanoin) (429)
This agent is indicated as a source of calories and fatty acids for the treatment of pediatric and adult patients with molecularly confirmed long-chain fatty acid oxidation disorders. Advise patients that there is a pregnancy safety study that collects pregnancy outcome data in women taking Dojolvi during pregnancy. Pregnant patients can enroll in the study by calling 1-888-756-8657.
Enspryng (satralizumab-mwge) (143,000)
It is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti–aquaporin-4 (AQP4) antibody positive. No information is available on the risks, if any, in pregnancy. No adverse effects on maternal or fetal development were observed in pregnant monkeys and their offspring.
Evrysdi (risdiplam) (401)
This is a prescription medicine used to treat spinal muscular atrophy in adults and children aged 2 months and older. In pregnant animals the drug caused adverse effects on fetal development.
Gemtesa (vibegron) (445)
Gemtesa is used in adults to treat the symptoms of overactive bladder. The drug had no adverse effects on pregnant animals.
Imcivree (setmelanotide) (1,117)
This drug is indicated for chronic weight management in adult and pediatric patients aged 6 years and older with obesity because of proopiomelanocortin, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency. The drug was not embryo toxic in animals.
Isturisa (osilodrostat) (325)
Isturisa is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease. No adverse fetal effects were observed in pregnant animals.
Klisyri (tirbanibulin) (431)
Tirbanibulin ointment is a microtubule inhibitor that is used to treat actinic keratosis. Information on its effects in pregnancy is not available.
Koselugo (selumetinib) (556)
This is a kinase inhibitor indicated for the treatment of pediatric patients aged 2 years and older. The drug is toxic in pregnant animals but its effects in human pregnancy are not known.
Nexletol (bempedoic acid) (344)
Nexletol is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL cholesterol. The drug was not teratogenic in animals. Discontinue Nexletol when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
Olinvyk (oliceridine) (503)
Olinvyk injection is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic. Prolonged use of Olinvyk during pregnancy can result in neonatal opioid withdrawal syndrome. The drug was not teratogenic in animals.
Ongentys (opicapone) (413)
Ongentys is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. The drug was teratogenic in rabbits but not in rats.
Orladeyo (berotralstat) (635)
This drug is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema. It was not teratogenic in animals.
Oxlumo (lumasiran) (17,286)
Oxlumo is a HAO1-directed small interfering ribonucleic acid indicated for the treatment of primary hyperoxaluria type 1 to lower urinary oxalate levels. No adverse effects on pregnancy or embryo-fetal development related to the drug were observed in animals.
Pizensy (lactitol) (344)
Lactitol is minimally absorbed systemically following oral administration. It is unknown whether maternal use will result in fetal exposure to the drug. No effects on embryo-fetal development were observed in animals at doses much higher than the maximum recommended human dosage.
Rukobia (fostemsavir) (705; 584 for free acid)
This drug is an HIV-1–directed attachment inhibitor, in combination with other antiretrovirals. There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to the drug during pregnancy. Health care providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.
Sogroya (somapacitan-beco) (23,305)
This is a human growth hormone analog indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency. The drug was not teratogenic in animals.
Tepezza (teprotumumab-trbw) (148,000)
Drug is indicated for the treatment of thyroid eye disease. The drug was teratogenic in cynomolgus monkeys. The manufacturer states that because of the risk, the drug should not be used in pregnancy.
Tauvid (flortaucipir F-18) (262)
This drug is indicated for use with PET imaging of the brain to evaluate for Alzheimer’s disease. It is a radioactive drug and should not be used in pregnant women.
Uplizna (inebilizumab-cdon) (149,000)
Uplizna is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti-AQP4 antibody positive. It is a humanized IgG1 monoclonal antibody and immunoglobulins are known to cross the placental barrier. Based on animal data, the drug can cause fetal harm because of B-cell lymphopenia and reduce antibody response in offspring exposed to the drug. Women of childbearing potential should use contraception while receiving Uplizna and for 6 months after the last dose.
Winlevi (clascoterone) (403)
This cream is an androgen receptor inhibitor that is indicated for the topical treatment of acne vulgaris in patients aged 12 years and older. Subcutaneous use in animals was associated with fetal defects.
Xeglyze (abametapir) (1,840)
Xeglyze is indicated for the topical treatment of head lice infestation in patients aged 6 months and older. The drug was not teratogenic in animals.
Zokinvy (lonafarnib) (639)
Zokinvy is indicated in patients 12 months or older to reduce the risk of mortality in several conditions. Animal studies have found embryo-fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2020, the Food and Drug Administration approved 53 new drugs for humans. One of these agents, Annovera (segesterone and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. Orgovyx (relugolix) is used for prostate cancer and Lampit (nifurtimox) is drug used in children – neither of these two agents will be covered. The remaining 49 are covered below. The agents with molecular weights less than 1,000 probably cross the placenta in the first half of pregnancy, but nearly all, regardless of MW, will cross in the second half of pregnancy.
No human pregnancy data for these agents has been found, but there are five drugs included in pregnancy registries. It will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in an article that I coauthored, “Should pregnant women be included in phase 4 clinical drug trials?”. The article makes a strong argument for including some pregnant women in these trials.
Anti-infectives
Artesunate (384)
The drug appears low risk when used in the second and third trimesters. There is inadequate information regarding its use in the first trimester, so the safest course for the embryo appears to be avoiding its use during this period. A single intravenous dose given to rats early in gestation resulted in embryolethality.
Ebanga (ansuvimab) (147,000)
Studies on its use in pregnant animals have not been conducted.
Inmazeb (atoltivimab, maftivimab, odesivimab) (144,000-146,000)
Inmazeb is a combination of the three agents. Studies on its use in pregnant animals have not been conducted.
Veklury (remdesivir) (603)
Veklury is indicated for the treatment of pregnant women hospitalized with COVID-19 who are at risk for serious morbidity and mortality. The drug should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus.
Antineoplastics
Ayvakit (avapritinib) (499)
The drug may cause fetal harm. The drug was teratogenic in animals.
Blenrep (belantamab mafodotin-blmf) (152,000)
A B-cell maturation antigen, it is indicated for the treatment of multiple myeloma. No human or animal pregnancy data have been located.
Danyelza (naxitamab-gqgk) (144,000)
This agent is used for the treatment of neuroblastoma. Based on its mechanism of action it may cause fetal harm if used in pregnancy.
Gavreto (pralsetinib) (534)
Gavreto is indicated for the treatment of small cell lung cancer. It may cause embryo-fetal harm if used in pregnancy.
Inqovi (cedazuridine + decitabine) (268,228)
The drug combination can cause fetal harm in human pregnancy. It is toxic in pregnant animals.
Margenza (margetuximab-cmkb) (149,000)
Although there are no data on the use of this drug in human pregnancy, the findings in animals and mechanism of action suggest that it will cause fetal harm.
Monjuvi (tafasitamab-cxix) (150,000)
This drug is a cytolytic antibody that is indicated in combination with lenalidomide. The combination may cause fetal harm.
Pemazyre (pemigatinib) (488)
It is indicated for the treatment of cholangiocarcinoma. In an animal study, the drug caused fetal defects, fetal growth retardation, and embryo-fetal death at maternal exposures lower than the human exposure.
Qinlock (ripretinib) (510)
This drug is used for the treatment of patients with advanced gastrointestinal stromal tumor. The drug was teratogenic in pregnant animals.
Retevmo (selpercatinib) (526)
This is a kinase inhibitor used for the treatment of small cell lung cancer. The drug is teratogenic in animals.
Sarclisa (isatuximab-irfc) (148,000)This drug is used in combination with pomalidomide and dexamethasone. The combination would probably cause major toxicity in an embryo or fetus.
Tabrecta (capmatinib) (412 – free base)Capmatinib is a kinase inhibitor used for the treatment of metastatic non–small cell lung cancer. It is teratogenic in animals.
Tazverik (tazemetostat) (654)Tazemetostat is indicated for the treatment of epithelioid sarcoma and follicular lymphoma, The drug is teratogenic in animals.
Trodelvy (sacituzumab govitecan-hziy) (1,602)This agent is used for the treatment of breast cancer. The drug has not been tested in pregnant animals. However, according to the manufacturer, there is a high possibility of human teratogenicity if it is given to a pregnant woman.
Tukysa (tucatinib) (481)
Tukysa is a tyrosine kinase inhibitor that is used in combination with trastuzumab and capecitabine for the treatment of breast cancer. The drug is teratogenic in animals.
Zeposia (ozanimod) (441)
Zeposia is indicated for the treatment of multiple sclerosis. The drug takes about 3 months to eliminate from the body. The drug is teratogenic in animals.
Zepzelca (lurbinectedin) (785)
This agent is used for the treatment of metastatic small cell lung cancer. The drug is teratogenic in animals.
Antiemetics
Barhemsys (amisulpride) (369)
This agent is Indicated to prevent nausea and vomiting. Animal data suggest low risk of embryo/fetal birth defects.
Antimigraine
Nurtec (rimegepant) (611)
Nurtec is indicated for acute treatment of migraine. Development toxicity was not observed in animals given doses similar to those used in humans.
Vyepti (eptinezumab-jjmr) (143,000)
A humanized monoclonal antibody that is given every 3 months to prevent migraine. There was no embryo-fetal harm in animals given the drug.
CNS
Byfavo (remimazolam) (493 – free base)
This drug is indicated for procedural sedation in adults undergoing procedures lasting 30 minutes or less. No defects were observed in animals.
Diagnostics
Cerianna (fluoroestradiol F 18) (289)
It is indicated for use with PET for characterization of estrogen receptor status in patients with ER-positive breast cancer. It has the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. There are no data on its use in pregnant women or animals.
Detectnet (copper CU-64 dotatate) (1,497)
All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. There are no pregnancy data in humans or animals
Miscellaneous
Dojolvi (triheptanoin) (429)
This agent is indicated as a source of calories and fatty acids for the treatment of pediatric and adult patients with molecularly confirmed long-chain fatty acid oxidation disorders. Advise patients that there is a pregnancy safety study that collects pregnancy outcome data in women taking Dojolvi during pregnancy. Pregnant patients can enroll in the study by calling 1-888-756-8657.
Enspryng (satralizumab-mwge) (143,000)
It is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti–aquaporin-4 (AQP4) antibody positive. No information is available on the risks, if any, in pregnancy. No adverse effects on maternal or fetal development were observed in pregnant monkeys and their offspring.
Evrysdi (risdiplam) (401)
This is a prescription medicine used to treat spinal muscular atrophy in adults and children aged 2 months and older. In pregnant animals the drug caused adverse effects on fetal development.
Gemtesa (vibegron) (445)
Gemtesa is used in adults to treat the symptoms of overactive bladder. The drug had no adverse effects on pregnant animals.
Imcivree (setmelanotide) (1,117)
This drug is indicated for chronic weight management in adult and pediatric patients aged 6 years and older with obesity because of proopiomelanocortin, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency. The drug was not embryo toxic in animals.
Isturisa (osilodrostat) (325)
Isturisa is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease. No adverse fetal effects were observed in pregnant animals.
Klisyri (tirbanibulin) (431)
Tirbanibulin ointment is a microtubule inhibitor that is used to treat actinic keratosis. Information on its effects in pregnancy is not available.
Koselugo (selumetinib) (556)
This is a kinase inhibitor indicated for the treatment of pediatric patients aged 2 years and older. The drug is toxic in pregnant animals but its effects in human pregnancy are not known.
Nexletol (bempedoic acid) (344)
Nexletol is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL cholesterol. The drug was not teratogenic in animals. Discontinue Nexletol when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
Olinvyk (oliceridine) (503)
Olinvyk injection is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic. Prolonged use of Olinvyk during pregnancy can result in neonatal opioid withdrawal syndrome. The drug was not teratogenic in animals.
Ongentys (opicapone) (413)
Ongentys is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. The drug was teratogenic in rabbits but not in rats.
Orladeyo (berotralstat) (635)
This drug is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema. It was not teratogenic in animals.
Oxlumo (lumasiran) (17,286)
Oxlumo is a HAO1-directed small interfering ribonucleic acid indicated for the treatment of primary hyperoxaluria type 1 to lower urinary oxalate levels. No adverse effects on pregnancy or embryo-fetal development related to the drug were observed in animals.
Pizensy (lactitol) (344)
Lactitol is minimally absorbed systemically following oral administration. It is unknown whether maternal use will result in fetal exposure to the drug. No effects on embryo-fetal development were observed in animals at doses much higher than the maximum recommended human dosage.
Rukobia (fostemsavir) (705; 584 for free acid)
This drug is an HIV-1–directed attachment inhibitor, in combination with other antiretrovirals. There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to the drug during pregnancy. Health care providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.
Sogroya (somapacitan-beco) (23,305)
This is a human growth hormone analog indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency. The drug was not teratogenic in animals.
Tepezza (teprotumumab-trbw) (148,000)
Drug is indicated for the treatment of thyroid eye disease. The drug was teratogenic in cynomolgus monkeys. The manufacturer states that because of the risk, the drug should not be used in pregnancy.
Tauvid (flortaucipir F-18) (262)
This drug is indicated for use with PET imaging of the brain to evaluate for Alzheimer’s disease. It is a radioactive drug and should not be used in pregnant women.
Uplizna (inebilizumab-cdon) (149,000)
Uplizna is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti-AQP4 antibody positive. It is a humanized IgG1 monoclonal antibody and immunoglobulins are known to cross the placental barrier. Based on animal data, the drug can cause fetal harm because of B-cell lymphopenia and reduce antibody response in offspring exposed to the drug. Women of childbearing potential should use contraception while receiving Uplizna and for 6 months after the last dose.
Winlevi (clascoterone) (403)
This cream is an androgen receptor inhibitor that is indicated for the topical treatment of acne vulgaris in patients aged 12 years and older. Subcutaneous use in animals was associated with fetal defects.
Xeglyze (abametapir) (1,840)
Xeglyze is indicated for the topical treatment of head lice infestation in patients aged 6 months and older. The drug was not teratogenic in animals.
Zokinvy (lonafarnib) (639)
Zokinvy is indicated in patients 12 months or older to reduce the risk of mortality in several conditions. Animal studies have found embryo-fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
In 2020, the Food and Drug Administration approved 53 new drugs for humans. One of these agents, Annovera (segesterone and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. Orgovyx (relugolix) is used for prostate cancer and Lampit (nifurtimox) is drug used in children – neither of these two agents will be covered. The remaining 49 are covered below. The agents with molecular weights less than 1,000 probably cross the placenta in the first half of pregnancy, but nearly all, regardless of MW, will cross in the second half of pregnancy.
No human pregnancy data for these agents has been found, but there are five drugs included in pregnancy registries. It will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in an article that I coauthored, “Should pregnant women be included in phase 4 clinical drug trials?”. The article makes a strong argument for including some pregnant women in these trials.
Anti-infectives
Artesunate (384)
The drug appears low risk when used in the second and third trimesters. There is inadequate information regarding its use in the first trimester, so the safest course for the embryo appears to be avoiding its use during this period. A single intravenous dose given to rats early in gestation resulted in embryolethality.
Ebanga (ansuvimab) (147,000)
Studies on its use in pregnant animals have not been conducted.
Inmazeb (atoltivimab, maftivimab, odesivimab) (144,000-146,000)
Inmazeb is a combination of the three agents. Studies on its use in pregnant animals have not been conducted.
Veklury (remdesivir) (603)
Veklury is indicated for the treatment of pregnant women hospitalized with COVID-19 who are at risk for serious morbidity and mortality. The drug should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus.
Antineoplastics
Ayvakit (avapritinib) (499)
The drug may cause fetal harm. The drug was teratogenic in animals.
Blenrep (belantamab mafodotin-blmf) (152,000)
A B-cell maturation antigen, it is indicated for the treatment of multiple myeloma. No human or animal pregnancy data have been located.
Danyelza (naxitamab-gqgk) (144,000)
This agent is used for the treatment of neuroblastoma. Based on its mechanism of action it may cause fetal harm if used in pregnancy.
Gavreto (pralsetinib) (534)
Gavreto is indicated for the treatment of small cell lung cancer. It may cause embryo-fetal harm if used in pregnancy.
Inqovi (cedazuridine + decitabine) (268,228)
The drug combination can cause fetal harm in human pregnancy. It is toxic in pregnant animals.
Margenza (margetuximab-cmkb) (149,000)
Although there are no data on the use of this drug in human pregnancy, the findings in animals and mechanism of action suggest that it will cause fetal harm.
Monjuvi (tafasitamab-cxix) (150,000)
This drug is a cytolytic antibody that is indicated in combination with lenalidomide. The combination may cause fetal harm.
Pemazyre (pemigatinib) (488)
It is indicated for the treatment of cholangiocarcinoma. In an animal study, the drug caused fetal defects, fetal growth retardation, and embryo-fetal death at maternal exposures lower than the human exposure.
Qinlock (ripretinib) (510)
This drug is used for the treatment of patients with advanced gastrointestinal stromal tumor. The drug was teratogenic in pregnant animals.
Retevmo (selpercatinib) (526)
This is a kinase inhibitor used for the treatment of small cell lung cancer. The drug is teratogenic in animals.
Sarclisa (isatuximab-irfc) (148,000)This drug is used in combination with pomalidomide and dexamethasone. The combination would probably cause major toxicity in an embryo or fetus.
Tabrecta (capmatinib) (412 – free base)Capmatinib is a kinase inhibitor used for the treatment of metastatic non–small cell lung cancer. It is teratogenic in animals.
Tazverik (tazemetostat) (654)Tazemetostat is indicated for the treatment of epithelioid sarcoma and follicular lymphoma, The drug is teratogenic in animals.
Trodelvy (sacituzumab govitecan-hziy) (1,602)This agent is used for the treatment of breast cancer. The drug has not been tested in pregnant animals. However, according to the manufacturer, there is a high possibility of human teratogenicity if it is given to a pregnant woman.
Tukysa (tucatinib) (481)
Tukysa is a tyrosine kinase inhibitor that is used in combination with trastuzumab and capecitabine for the treatment of breast cancer. The drug is teratogenic in animals.
Zeposia (ozanimod) (441)
Zeposia is indicated for the treatment of multiple sclerosis. The drug takes about 3 months to eliminate from the body. The drug is teratogenic in animals.
Zepzelca (lurbinectedin) (785)
This agent is used for the treatment of metastatic small cell lung cancer. The drug is teratogenic in animals.
Antiemetics
Barhemsys (amisulpride) (369)
This agent is Indicated to prevent nausea and vomiting. Animal data suggest low risk of embryo/fetal birth defects.
Antimigraine
Nurtec (rimegepant) (611)
Nurtec is indicated for acute treatment of migraine. Development toxicity was not observed in animals given doses similar to those used in humans.
Vyepti (eptinezumab-jjmr) (143,000)
A humanized monoclonal antibody that is given every 3 months to prevent migraine. There was no embryo-fetal harm in animals given the drug.
CNS
Byfavo (remimazolam) (493 – free base)
This drug is indicated for procedural sedation in adults undergoing procedures lasting 30 minutes or less. No defects were observed in animals.
Diagnostics
Cerianna (fluoroestradiol F 18) (289)
It is indicated for use with PET for characterization of estrogen receptor status in patients with ER-positive breast cancer. It has the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. There are no data on its use in pregnant women or animals.
Detectnet (copper CU-64 dotatate) (1,497)
All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. There are no pregnancy data in humans or animals
Miscellaneous
Dojolvi (triheptanoin) (429)
This agent is indicated as a source of calories and fatty acids for the treatment of pediatric and adult patients with molecularly confirmed long-chain fatty acid oxidation disorders. Advise patients that there is a pregnancy safety study that collects pregnancy outcome data in women taking Dojolvi during pregnancy. Pregnant patients can enroll in the study by calling 1-888-756-8657.
Enspryng (satralizumab-mwge) (143,000)
It is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti–aquaporin-4 (AQP4) antibody positive. No information is available on the risks, if any, in pregnancy. No adverse effects on maternal or fetal development were observed in pregnant monkeys and their offspring.
Evrysdi (risdiplam) (401)
This is a prescription medicine used to treat spinal muscular atrophy in adults and children aged 2 months and older. In pregnant animals the drug caused adverse effects on fetal development.
Gemtesa (vibegron) (445)
Gemtesa is used in adults to treat the symptoms of overactive bladder. The drug had no adverse effects on pregnant animals.
Imcivree (setmelanotide) (1,117)
This drug is indicated for chronic weight management in adult and pediatric patients aged 6 years and older with obesity because of proopiomelanocortin, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency. The drug was not embryo toxic in animals.
Isturisa (osilodrostat) (325)
Isturisa is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease. No adverse fetal effects were observed in pregnant animals.
Klisyri (tirbanibulin) (431)
Tirbanibulin ointment is a microtubule inhibitor that is used to treat actinic keratosis. Information on its effects in pregnancy is not available.
Koselugo (selumetinib) (556)
This is a kinase inhibitor indicated for the treatment of pediatric patients aged 2 years and older. The drug is toxic in pregnant animals but its effects in human pregnancy are not known.
Nexletol (bempedoic acid) (344)
Nexletol is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL cholesterol. The drug was not teratogenic in animals. Discontinue Nexletol when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
Olinvyk (oliceridine) (503)
Olinvyk injection is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic. Prolonged use of Olinvyk during pregnancy can result in neonatal opioid withdrawal syndrome. The drug was not teratogenic in animals.
Ongentys (opicapone) (413)
Ongentys is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. The drug was teratogenic in rabbits but not in rats.
Orladeyo (berotralstat) (635)
This drug is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema. It was not teratogenic in animals.
Oxlumo (lumasiran) (17,286)
Oxlumo is a HAO1-directed small interfering ribonucleic acid indicated for the treatment of primary hyperoxaluria type 1 to lower urinary oxalate levels. No adverse effects on pregnancy or embryo-fetal development related to the drug were observed in animals.
Pizensy (lactitol) (344)
Lactitol is minimally absorbed systemically following oral administration. It is unknown whether maternal use will result in fetal exposure to the drug. No effects on embryo-fetal development were observed in animals at doses much higher than the maximum recommended human dosage.
Rukobia (fostemsavir) (705; 584 for free acid)
This drug is an HIV-1–directed attachment inhibitor, in combination with other antiretrovirals. There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to the drug during pregnancy. Health care providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.
Sogroya (somapacitan-beco) (23,305)
This is a human growth hormone analog indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency. The drug was not teratogenic in animals.
Tepezza (teprotumumab-trbw) (148,000)
Drug is indicated for the treatment of thyroid eye disease. The drug was teratogenic in cynomolgus monkeys. The manufacturer states that because of the risk, the drug should not be used in pregnancy.
Tauvid (flortaucipir F-18) (262)
This drug is indicated for use with PET imaging of the brain to evaluate for Alzheimer’s disease. It is a radioactive drug and should not be used in pregnant women.
Uplizna (inebilizumab-cdon) (149,000)
Uplizna is indicated for the treatment of neuromyelitis optica spectrum disorder in adult patients who are anti-AQP4 antibody positive. It is a humanized IgG1 monoclonal antibody and immunoglobulins are known to cross the placental barrier. Based on animal data, the drug can cause fetal harm because of B-cell lymphopenia and reduce antibody response in offspring exposed to the drug. Women of childbearing potential should use contraception while receiving Uplizna and for 6 months after the last dose.
Winlevi (clascoterone) (403)
This cream is an androgen receptor inhibitor that is indicated for the topical treatment of acne vulgaris in patients aged 12 years and older. Subcutaneous use in animals was associated with fetal defects.
Xeglyze (abametapir) (1,840)
Xeglyze is indicated for the topical treatment of head lice infestation in patients aged 6 months and older. The drug was not teratogenic in animals.
Zokinvy (lonafarnib) (639)
Zokinvy is indicated in patients 12 months or older to reduce the risk of mortality in several conditions. Animal studies have found embryo-fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Cultivating emotional awareness
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
A path to resilience and joy in the hospital
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.