User login
Tips for getting involved with industry
Introduction
The professional activity of physicians has traditionally consisted of patient care, teaching/education, and research in varying proportions. These aims, especially education and research, have traditionally been achieved in academic health settings. However, involvement with industry can afford all physicians an opportunity to increase patient referrals, gain exposure to colleagues through a variety of educational opportunities, and participate in meaningful research projects they could not initiate independently.
How to initiate relationships with industry
Here are several ways to initiate a collaboration with industry. A few of the most common ways are to become a site investigator of a multicenter device or pharmaceutical trial, participate as a member of a speaker’s bureau, or obtain training on a new technology and subsequently incorporate it into your clinical practice. To find out what trials are enrolling and looking for additional sites or new studies that are being planned, I would suggest contacting the company’s local representative and have them put you in touch the appropriate personnel in the clinical trials division. For individuals who become involved in trials, this can be a great way to improve your understanding of how to design and conduct clinical trials as well as gain exposure to colleagues with similar clinical and research interests. Some of my closest long-term collaborators and friends have been individuals who I initially met as part of industry trials at investigator meetings. Another approach is to participate in a speaker’s bureau, which can be an excellent way to improve one’s presentation skills as well as gain knowledge with respect to a specific disease state. It is also a great way to network, meet colleagues, and develop a local and regional reputation as a content expert on a specific topic. Methods to find out about such opportunities include touring the exhibit halls during educational meetings and reading scientific journals to identify new products that are launching. I have found these sorts of opportunities can significantly increase topic-based referrals. Finally, obtaining training on a new diagnostic or therapeutic technology (usually through an industry-sponsored course) can allow individuals an opportunity to offer a unique or distinctive service to their community. In addition, as further clinical expertise is gained, the relationship can be expanded to offer local, regional, or even national training courses to colleagues via either on-site or virtual courses. Similarly, opportunities to speak about or demonstrate the technology/technique at educational courses may also follow.
Navigating and expanding the relationship
Once an individual establishes a relationship with a company or has established a reputation as a key opinion leader, additional opportunities for engagement may become available. These include serving as a consultant, becoming a member of an advisory board, participating or directing educational courses for trainees/practitioners, or serving as the principal investigator of a future clinical trial. Serving as a consultant can be quite rewarding as it can highlight clinical needs, identify where product improvement can be achieved, and focus where research and development funds should be directed. Serving on the advisory board can afford an even higher level of influence where corporate strategy can be influenced. Such input is particularly impactful with smaller companies looking to enter a new field or expand a limited market share. There are also a variety of educational opportunities offered by industry including local, regional, and national courses that focus on utilizing a new technology or education concerning a specific disease state. These courses can be held locally at the physician’s clinical site or off site to attract the desired target audience. Finally, being involved in research studies, especially early-stage projects, can be critical as many small companies have limited capital, and it is essential for them to design studies with appropriate endpoints that will ideally achieve both regulatory approval as well as payor coverage. Of note, in addition to relationships directly involving industry, the American Gastroenterological Association Center for GI Innovation and Technology (CGIT) also offers the opportunity to be part of key opinion leader meetings arranged and organized by the AGA. This may allow for some individuals to participate who may be restricted from direct relationships with industry partners. The industry services offered by the CGIT also include clinical trial design and registry management services.
Entrepreneurship/intellectual property
A less commonly explored opportunity with industry involves the development of one’s own intellectual property. Some of the most impactful technologies in my advanced endoscopy clinical practice have been developed from the ideas of gastroenterology colleagues that have been successfully commercialized. These include radiofrequency ablation technology to treat Barrett’s esophagus and the development of lumen-apposing stents. There are several options for physicians with an idea for an innovation. These can include working with a university technology transfer department if they are in an academic setting, creation of their own company, or collaborating with industry to develop the device through a licensing/royalty agreement. The AGA CGIT offers extensive resources to physicians with new ideas on how to secure their intellectual property as well as to evaluate the feasibility of the aforementioned options to choose which may be most appropriate for them.
Important caveats
It is important that physicians with industry relations be aware of their local institutional policies. Some institutions may prohibit such activities while others may limit the types of relationships or the amount of income that can be received. It is the physician’s responsibility to be aware of their institution’s guidelines prior to formalizing industry agreements. If intellectual property is involved, it is essential to know the specific rules regarding physician remuneration, especially pertaining to royalty or equity agreements. Furthermore, with regard to presentations and publications, it is required to acknowledge industry relations and potential conflicts of interest. Failure to do so may adversely affect an individual’s reputation as well as lead to additional consequences such as the potential for retraction of publications or restrictions regarding future educational speaking opportunities. In addition, key opinion leaders often consult for several companies that may be in competition with each other. Therefore, it is essential that there is no disclosure of confidential proprietary information among companies. Finally, the financial incentives resulting from industry collaboration should never influence physician judgment when interpreting or speaking about data regarding product efficacy or safety.
Conclusions
In summary, there are numerous opportunities for physicians to collaborate with industry. These relationships can be very rewarding and can serve to expedite the introduction of new diagnostic or treatment modalities and provide the opportunity to network and interact with colleagues as well as to participate in important research that improves clinical practice. The nature of these relationships should always be transparent, and it is the physician’s responsibility to ensure that the types of relationships that are engaged in are permitted by their employer. Over the course of my career, I have participated in nearly all forms of these relationships and have seen that participation lead to important publications, changes in corporate strategy, the fostering of acquisitions, and the rapid development and utilization of new endoscopic technologies. It is my personal belief than industry relationships can improve professional satisfaction, enhance one’s brand, and most importantly, expedite clinical innovation to improve patient care.
Dr. Muthusamy is professor of clinical medicine at the University of California, Los Angeles, and medical director of endoscopy, UCLA Health System. He disclosed ties with Medtronic, Boston Scientific, Motus GI, Endogastric Solutions, and Capsovision.
Introduction
The professional activity of physicians has traditionally consisted of patient care, teaching/education, and research in varying proportions. These aims, especially education and research, have traditionally been achieved in academic health settings. However, involvement with industry can afford all physicians an opportunity to increase patient referrals, gain exposure to colleagues through a variety of educational opportunities, and participate in meaningful research projects they could not initiate independently.
How to initiate relationships with industry
Here are several ways to initiate a collaboration with industry. A few of the most common ways are to become a site investigator of a multicenter device or pharmaceutical trial, participate as a member of a speaker’s bureau, or obtain training on a new technology and subsequently incorporate it into your clinical practice. To find out what trials are enrolling and looking for additional sites or new studies that are being planned, I would suggest contacting the company’s local representative and have them put you in touch the appropriate personnel in the clinical trials division. For individuals who become involved in trials, this can be a great way to improve your understanding of how to design and conduct clinical trials as well as gain exposure to colleagues with similar clinical and research interests. Some of my closest long-term collaborators and friends have been individuals who I initially met as part of industry trials at investigator meetings. Another approach is to participate in a speaker’s bureau, which can be an excellent way to improve one’s presentation skills as well as gain knowledge with respect to a specific disease state. It is also a great way to network, meet colleagues, and develop a local and regional reputation as a content expert on a specific topic. Methods to find out about such opportunities include touring the exhibit halls during educational meetings and reading scientific journals to identify new products that are launching. I have found these sorts of opportunities can significantly increase topic-based referrals. Finally, obtaining training on a new diagnostic or therapeutic technology (usually through an industry-sponsored course) can allow individuals an opportunity to offer a unique or distinctive service to their community. In addition, as further clinical expertise is gained, the relationship can be expanded to offer local, regional, or even national training courses to colleagues via either on-site or virtual courses. Similarly, opportunities to speak about or demonstrate the technology/technique at educational courses may also follow.
Navigating and expanding the relationship
Once an individual establishes a relationship with a company or has established a reputation as a key opinion leader, additional opportunities for engagement may become available. These include serving as a consultant, becoming a member of an advisory board, participating or directing educational courses for trainees/practitioners, or serving as the principal investigator of a future clinical trial. Serving as a consultant can be quite rewarding as it can highlight clinical needs, identify where product improvement can be achieved, and focus where research and development funds should be directed. Serving on the advisory board can afford an even higher level of influence where corporate strategy can be influenced. Such input is particularly impactful with smaller companies looking to enter a new field or expand a limited market share. There are also a variety of educational opportunities offered by industry including local, regional, and national courses that focus on utilizing a new technology or education concerning a specific disease state. These courses can be held locally at the physician’s clinical site or off site to attract the desired target audience. Finally, being involved in research studies, especially early-stage projects, can be critical as many small companies have limited capital, and it is essential for them to design studies with appropriate endpoints that will ideally achieve both regulatory approval as well as payor coverage. Of note, in addition to relationships directly involving industry, the American Gastroenterological Association Center for GI Innovation and Technology (CGIT) also offers the opportunity to be part of key opinion leader meetings arranged and organized by the AGA. This may allow for some individuals to participate who may be restricted from direct relationships with industry partners. The industry services offered by the CGIT also include clinical trial design and registry management services.
Entrepreneurship/intellectual property
A less commonly explored opportunity with industry involves the development of one’s own intellectual property. Some of the most impactful technologies in my advanced endoscopy clinical practice have been developed from the ideas of gastroenterology colleagues that have been successfully commercialized. These include radiofrequency ablation technology to treat Barrett’s esophagus and the development of lumen-apposing stents. There are several options for physicians with an idea for an innovation. These can include working with a university technology transfer department if they are in an academic setting, creation of their own company, or collaborating with industry to develop the device through a licensing/royalty agreement. The AGA CGIT offers extensive resources to physicians with new ideas on how to secure their intellectual property as well as to evaluate the feasibility of the aforementioned options to choose which may be most appropriate for them.
Important caveats
It is important that physicians with industry relations be aware of their local institutional policies. Some institutions may prohibit such activities while others may limit the types of relationships or the amount of income that can be received. It is the physician’s responsibility to be aware of their institution’s guidelines prior to formalizing industry agreements. If intellectual property is involved, it is essential to know the specific rules regarding physician remuneration, especially pertaining to royalty or equity agreements. Furthermore, with regard to presentations and publications, it is required to acknowledge industry relations and potential conflicts of interest. Failure to do so may adversely affect an individual’s reputation as well as lead to additional consequences such as the potential for retraction of publications or restrictions regarding future educational speaking opportunities. In addition, key opinion leaders often consult for several companies that may be in competition with each other. Therefore, it is essential that there is no disclosure of confidential proprietary information among companies. Finally, the financial incentives resulting from industry collaboration should never influence physician judgment when interpreting or speaking about data regarding product efficacy or safety.
Conclusions
In summary, there are numerous opportunities for physicians to collaborate with industry. These relationships can be very rewarding and can serve to expedite the introduction of new diagnostic or treatment modalities and provide the opportunity to network and interact with colleagues as well as to participate in important research that improves clinical practice. The nature of these relationships should always be transparent, and it is the physician’s responsibility to ensure that the types of relationships that are engaged in are permitted by their employer. Over the course of my career, I have participated in nearly all forms of these relationships and have seen that participation lead to important publications, changes in corporate strategy, the fostering of acquisitions, and the rapid development and utilization of new endoscopic technologies. It is my personal belief than industry relationships can improve professional satisfaction, enhance one’s brand, and most importantly, expedite clinical innovation to improve patient care.
Dr. Muthusamy is professor of clinical medicine at the University of California, Los Angeles, and medical director of endoscopy, UCLA Health System. He disclosed ties with Medtronic, Boston Scientific, Motus GI, Endogastric Solutions, and Capsovision.
Introduction
The professional activity of physicians has traditionally consisted of patient care, teaching/education, and research in varying proportions. These aims, especially education and research, have traditionally been achieved in academic health settings. However, involvement with industry can afford all physicians an opportunity to increase patient referrals, gain exposure to colleagues through a variety of educational opportunities, and participate in meaningful research projects they could not initiate independently.
How to initiate relationships with industry
Here are several ways to initiate a collaboration with industry. A few of the most common ways are to become a site investigator of a multicenter device or pharmaceutical trial, participate as a member of a speaker’s bureau, or obtain training on a new technology and subsequently incorporate it into your clinical practice. To find out what trials are enrolling and looking for additional sites or new studies that are being planned, I would suggest contacting the company’s local representative and have them put you in touch the appropriate personnel in the clinical trials division. For individuals who become involved in trials, this can be a great way to improve your understanding of how to design and conduct clinical trials as well as gain exposure to colleagues with similar clinical and research interests. Some of my closest long-term collaborators and friends have been individuals who I initially met as part of industry trials at investigator meetings. Another approach is to participate in a speaker’s bureau, which can be an excellent way to improve one’s presentation skills as well as gain knowledge with respect to a specific disease state. It is also a great way to network, meet colleagues, and develop a local and regional reputation as a content expert on a specific topic. Methods to find out about such opportunities include touring the exhibit halls during educational meetings and reading scientific journals to identify new products that are launching. I have found these sorts of opportunities can significantly increase topic-based referrals. Finally, obtaining training on a new diagnostic or therapeutic technology (usually through an industry-sponsored course) can allow individuals an opportunity to offer a unique or distinctive service to their community. In addition, as further clinical expertise is gained, the relationship can be expanded to offer local, regional, or even national training courses to colleagues via either on-site or virtual courses. Similarly, opportunities to speak about or demonstrate the technology/technique at educational courses may also follow.
Navigating and expanding the relationship
Once an individual establishes a relationship with a company or has established a reputation as a key opinion leader, additional opportunities for engagement may become available. These include serving as a consultant, becoming a member of an advisory board, participating or directing educational courses for trainees/practitioners, or serving as the principal investigator of a future clinical trial. Serving as a consultant can be quite rewarding as it can highlight clinical needs, identify where product improvement can be achieved, and focus where research and development funds should be directed. Serving on the advisory board can afford an even higher level of influence where corporate strategy can be influenced. Such input is particularly impactful with smaller companies looking to enter a new field or expand a limited market share. There are also a variety of educational opportunities offered by industry including local, regional, and national courses that focus on utilizing a new technology or education concerning a specific disease state. These courses can be held locally at the physician’s clinical site or off site to attract the desired target audience. Finally, being involved in research studies, especially early-stage projects, can be critical as many small companies have limited capital, and it is essential for them to design studies with appropriate endpoints that will ideally achieve both regulatory approval as well as payor coverage. Of note, in addition to relationships directly involving industry, the American Gastroenterological Association Center for GI Innovation and Technology (CGIT) also offers the opportunity to be part of key opinion leader meetings arranged and organized by the AGA. This may allow for some individuals to participate who may be restricted from direct relationships with industry partners. The industry services offered by the CGIT also include clinical trial design and registry management services.
Entrepreneurship/intellectual property
A less commonly explored opportunity with industry involves the development of one’s own intellectual property. Some of the most impactful technologies in my advanced endoscopy clinical practice have been developed from the ideas of gastroenterology colleagues that have been successfully commercialized. These include radiofrequency ablation technology to treat Barrett’s esophagus and the development of lumen-apposing stents. There are several options for physicians with an idea for an innovation. These can include working with a university technology transfer department if they are in an academic setting, creation of their own company, or collaborating with industry to develop the device through a licensing/royalty agreement. The AGA CGIT offers extensive resources to physicians with new ideas on how to secure their intellectual property as well as to evaluate the feasibility of the aforementioned options to choose which may be most appropriate for them.
Important caveats
It is important that physicians with industry relations be aware of their local institutional policies. Some institutions may prohibit such activities while others may limit the types of relationships or the amount of income that can be received. It is the physician’s responsibility to be aware of their institution’s guidelines prior to formalizing industry agreements. If intellectual property is involved, it is essential to know the specific rules regarding physician remuneration, especially pertaining to royalty or equity agreements. Furthermore, with regard to presentations and publications, it is required to acknowledge industry relations and potential conflicts of interest. Failure to do so may adversely affect an individual’s reputation as well as lead to additional consequences such as the potential for retraction of publications or restrictions regarding future educational speaking opportunities. In addition, key opinion leaders often consult for several companies that may be in competition with each other. Therefore, it is essential that there is no disclosure of confidential proprietary information among companies. Finally, the financial incentives resulting from industry collaboration should never influence physician judgment when interpreting or speaking about data regarding product efficacy or safety.
Conclusions
In summary, there are numerous opportunities for physicians to collaborate with industry. These relationships can be very rewarding and can serve to expedite the introduction of new diagnostic or treatment modalities and provide the opportunity to network and interact with colleagues as well as to participate in important research that improves clinical practice. The nature of these relationships should always be transparent, and it is the physician’s responsibility to ensure that the types of relationships that are engaged in are permitted by their employer. Over the course of my career, I have participated in nearly all forms of these relationships and have seen that participation lead to important publications, changes in corporate strategy, the fostering of acquisitions, and the rapid development and utilization of new endoscopic technologies. It is my personal belief than industry relationships can improve professional satisfaction, enhance one’s brand, and most importantly, expedite clinical innovation to improve patient care.
Dr. Muthusamy is professor of clinical medicine at the University of California, Los Angeles, and medical director of endoscopy, UCLA Health System. He disclosed ties with Medtronic, Boston Scientific, Motus GI, Endogastric Solutions, and Capsovision.
Artificial intelligence applications in colonoscopy
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4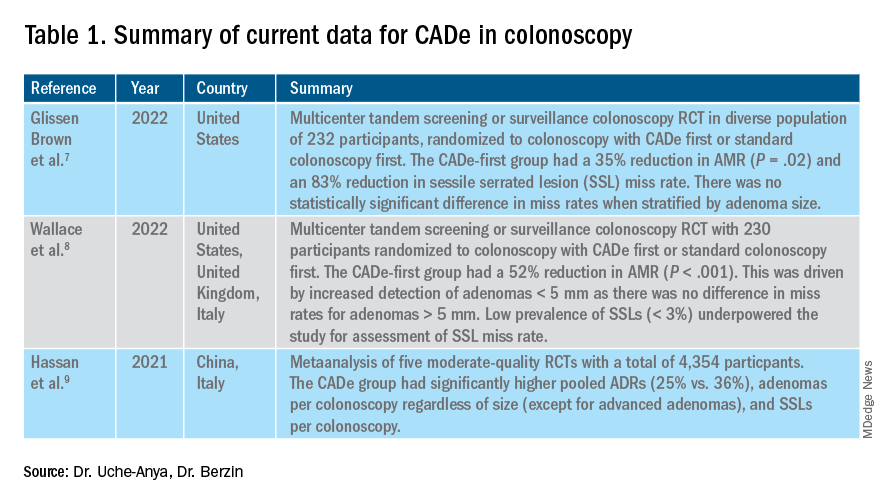
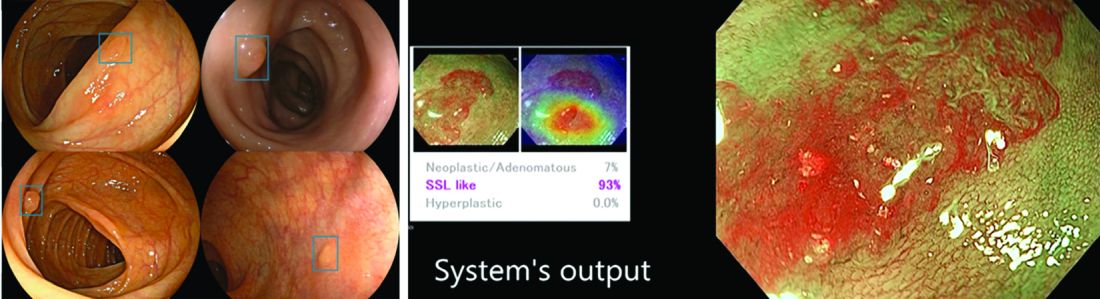
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.

Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.

Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
February 2023 - ICYMI
Gastroenterology
October 2022
Cryer B et al. Bridging the Racial, Ethnic, and Gender Gap in Gastroenterology. Gastroenterology. 2022 Oct;163(4):800-5. doi: 10.1053/j.gastro.2022.08.037. PMID: 36137708.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-51. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
November 2022
Grunvald E et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. 2022 Nov;163(5):1198-225. doi: 10.1053/j.gastro.2022.08.045. Epub 2022 Oct 20. PMID: 36273831.
December 2022
Blackett JW et al. Comparison of Anorectal Manometry, Rectal Balloon Expulsion Test, and Defecography for Diagnosing Defecatory Disorders. Gastroenterology. 2022 Dec;163(6):1582-92.e2. doi: 10.1053/j.gastro.2022.08.034. Epub 2022 Aug 19. PMID: 35995074; PMCID: PMC9691522.
de Voogd F et al. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients With Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology. 2022 Dec;163(6):1569-81. doi: 10.1053/j.gastro.2022.08.038. Epub 2022 Aug 24. PMID: 36030056.
Clinical Gastroenterology and Hepatology
October 2022
Bhavsar-Burke I et al. How to Promote Professional Identity Development and Support Fellows-In-Training Through Teaching, Coaching, Mentorship, and Sponsorship. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2166-9. doi: 10.1016/j.cgh.2022.05.043. Epub 2022 Aug 7. PMID: 35948073.
van Megen F et al. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms – A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2258-66.e3. doi: 10.1016/j.cgh.2022.01.011. Epub 2022 Jan 17. PMID: 35051648.
November 2022
Sharzehi K et al. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022 Nov;20(11):2435-43.e4. doi: 10.1016/j.cgh.2022.05.054. Epub 2022 Jul 13. PMID: 35842117.
December 2022
Kardashian A et al. Food Insecurity is Associated With Mortality Among U.S. Adults With Nonalcoholic Fatty Liver Disease and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2790-9.e4. doi: 10.1016/j.cgh.2021.11.029. Epub 2021 Dec 16. PMID: 34958747.
Schuitenmaker JM et al. Sleep Positional Therapy for Nocturnal Gastroesophageal Reflux: A Double-Blind, Randomized, Sham-Controlled Trial. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2753-62.e2. doi: 10.1016/j.cgh.2022.02.058. Epub 2022 Mar 14. PMID: 35301135.
Techniques and Innovations in Gastrointestinal Endoscopy
Azizian JM et al. Yield of Post-Acute Diverticulitis Colonoscopy for Ruling Out Colorectal Cancer. Tech Innov Gastrointest Endosc. 2022;24(3):254-61. doi: 10.1016/j.tige.2022.04.001. Epub 2022 Apr 18. PMID: 36540108; PMCID: PMC9762736.
Gastro Hep Advances
Kim RW et al. Timely Albumin Improves Survival in Patients With Cirrhosis on Diuretic Therapy Who Develop Acute Kidney Injury: Real-World Evidence in the United States. Gastro Hep Advances. 2023;2(2):252-60. doi: 10.1016/j.gastha.2022.10.008.
Gastroenterology
October 2022
Cryer B et al. Bridging the Racial, Ethnic, and Gender Gap in Gastroenterology. Gastroenterology. 2022 Oct;163(4):800-5. doi: 10.1053/j.gastro.2022.08.037. PMID: 36137708.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-51. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
November 2022
Grunvald E et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. 2022 Nov;163(5):1198-225. doi: 10.1053/j.gastro.2022.08.045. Epub 2022 Oct 20. PMID: 36273831.
December 2022
Blackett JW et al. Comparison of Anorectal Manometry, Rectal Balloon Expulsion Test, and Defecography for Diagnosing Defecatory Disorders. Gastroenterology. 2022 Dec;163(6):1582-92.e2. doi: 10.1053/j.gastro.2022.08.034. Epub 2022 Aug 19. PMID: 35995074; PMCID: PMC9691522.
de Voogd F et al. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients With Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology. 2022 Dec;163(6):1569-81. doi: 10.1053/j.gastro.2022.08.038. Epub 2022 Aug 24. PMID: 36030056.
Clinical Gastroenterology and Hepatology
October 2022
Bhavsar-Burke I et al. How to Promote Professional Identity Development and Support Fellows-In-Training Through Teaching, Coaching, Mentorship, and Sponsorship. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2166-9. doi: 10.1016/j.cgh.2022.05.043. Epub 2022 Aug 7. PMID: 35948073.
van Megen F et al. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms – A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2258-66.e3. doi: 10.1016/j.cgh.2022.01.011. Epub 2022 Jan 17. PMID: 35051648.
November 2022
Sharzehi K et al. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022 Nov;20(11):2435-43.e4. doi: 10.1016/j.cgh.2022.05.054. Epub 2022 Jul 13. PMID: 35842117.
December 2022
Kardashian A et al. Food Insecurity is Associated With Mortality Among U.S. Adults With Nonalcoholic Fatty Liver Disease and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2790-9.e4. doi: 10.1016/j.cgh.2021.11.029. Epub 2021 Dec 16. PMID: 34958747.
Schuitenmaker JM et al. Sleep Positional Therapy for Nocturnal Gastroesophageal Reflux: A Double-Blind, Randomized, Sham-Controlled Trial. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2753-62.e2. doi: 10.1016/j.cgh.2022.02.058. Epub 2022 Mar 14. PMID: 35301135.
Techniques and Innovations in Gastrointestinal Endoscopy
Azizian JM et al. Yield of Post-Acute Diverticulitis Colonoscopy for Ruling Out Colorectal Cancer. Tech Innov Gastrointest Endosc. 2022;24(3):254-61. doi: 10.1016/j.tige.2022.04.001. Epub 2022 Apr 18. PMID: 36540108; PMCID: PMC9762736.
Gastro Hep Advances
Kim RW et al. Timely Albumin Improves Survival in Patients With Cirrhosis on Diuretic Therapy Who Develop Acute Kidney Injury: Real-World Evidence in the United States. Gastro Hep Advances. 2023;2(2):252-60. doi: 10.1016/j.gastha.2022.10.008.
Gastroenterology
October 2022
Cryer B et al. Bridging the Racial, Ethnic, and Gender Gap in Gastroenterology. Gastroenterology. 2022 Oct;163(4):800-5. doi: 10.1053/j.gastro.2022.08.037. PMID: 36137708.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-51. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
November 2022
Grunvald E et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. 2022 Nov;163(5):1198-225. doi: 10.1053/j.gastro.2022.08.045. Epub 2022 Oct 20. PMID: 36273831.
December 2022
Blackett JW et al. Comparison of Anorectal Manometry, Rectal Balloon Expulsion Test, and Defecography for Diagnosing Defecatory Disorders. Gastroenterology. 2022 Dec;163(6):1582-92.e2. doi: 10.1053/j.gastro.2022.08.034. Epub 2022 Aug 19. PMID: 35995074; PMCID: PMC9691522.
de Voogd F et al. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients With Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology. 2022 Dec;163(6):1569-81. doi: 10.1053/j.gastro.2022.08.038. Epub 2022 Aug 24. PMID: 36030056.
Clinical Gastroenterology and Hepatology
October 2022
Bhavsar-Burke I et al. How to Promote Professional Identity Development and Support Fellows-In-Training Through Teaching, Coaching, Mentorship, and Sponsorship. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2166-9. doi: 10.1016/j.cgh.2022.05.043. Epub 2022 Aug 7. PMID: 35948073.
van Megen F et al. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms – A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2258-66.e3. doi: 10.1016/j.cgh.2022.01.011. Epub 2022 Jan 17. PMID: 35051648.
November 2022
Sharzehi K et al. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022 Nov;20(11):2435-43.e4. doi: 10.1016/j.cgh.2022.05.054. Epub 2022 Jul 13. PMID: 35842117.
December 2022
Kardashian A et al. Food Insecurity is Associated With Mortality Among U.S. Adults With Nonalcoholic Fatty Liver Disease and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2790-9.e4. doi: 10.1016/j.cgh.2021.11.029. Epub 2021 Dec 16. PMID: 34958747.
Schuitenmaker JM et al. Sleep Positional Therapy for Nocturnal Gastroesophageal Reflux: A Double-Blind, Randomized, Sham-Controlled Trial. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2753-62.e2. doi: 10.1016/j.cgh.2022.02.058. Epub 2022 Mar 14. PMID: 35301135.
Techniques and Innovations in Gastrointestinal Endoscopy
Azizian JM et al. Yield of Post-Acute Diverticulitis Colonoscopy for Ruling Out Colorectal Cancer. Tech Innov Gastrointest Endosc. 2022;24(3):254-61. doi: 10.1016/j.tige.2022.04.001. Epub 2022 Apr 18. PMID: 36540108; PMCID: PMC9762736.
Gastro Hep Advances
Kim RW et al. Timely Albumin Improves Survival in Patients With Cirrhosis on Diuretic Therapy Who Develop Acute Kidney Injury: Real-World Evidence in the United States. Gastro Hep Advances. 2023;2(2):252-60. doi: 10.1016/j.gastha.2022.10.008.
Immune checkpoint inhibitor–related gastrointestinal adverse events
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10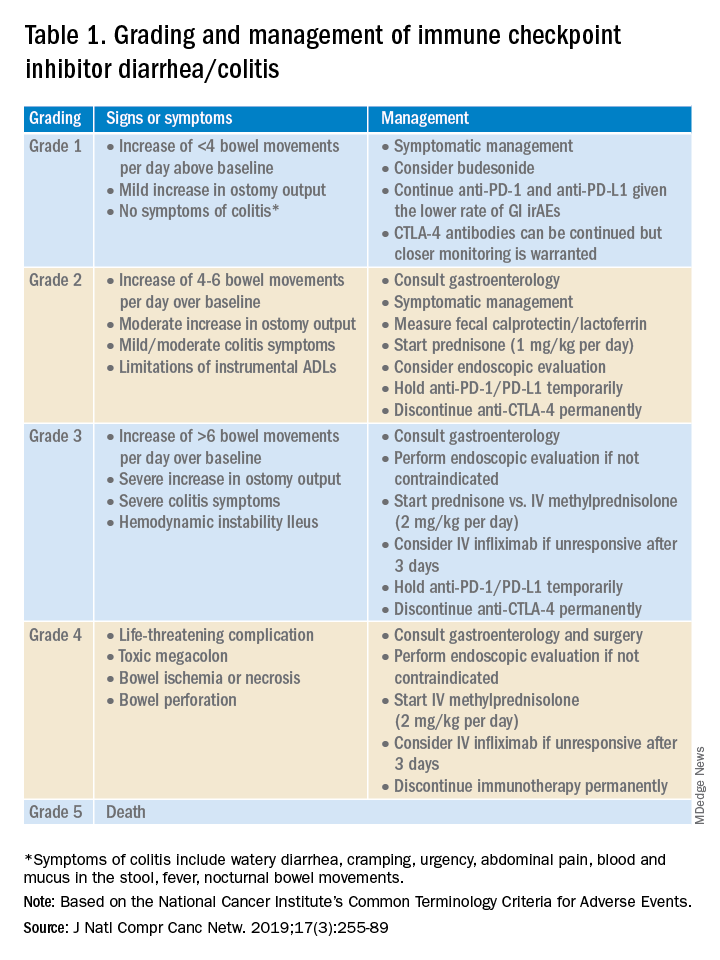
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.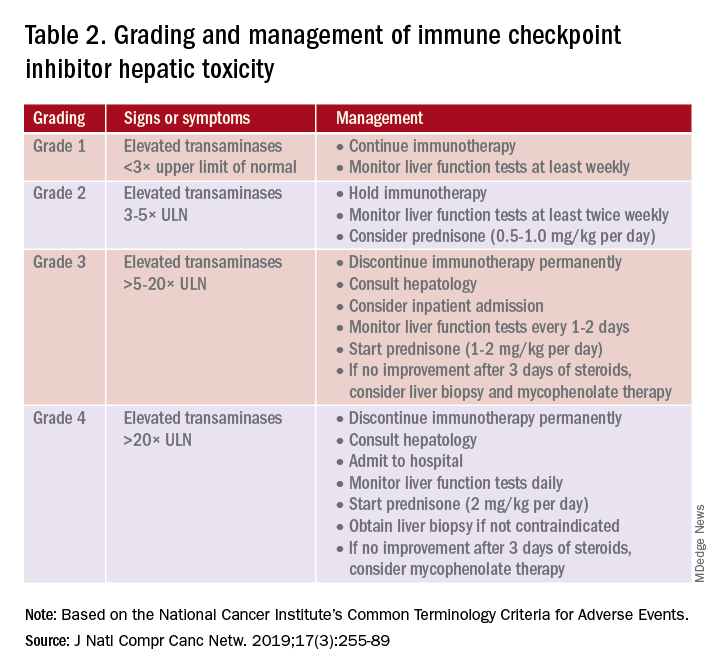
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14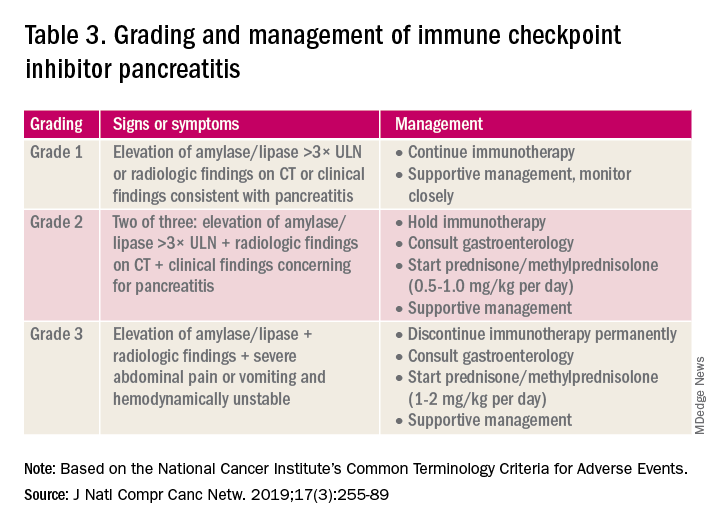
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Why I decided to get an MBA after becoming a private practice gastroenterologist
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
AGA News - February 2023
AGA members advocate for GI during Alliance of Specialty Medicine fly-in
In December, six AGA members joined more than 100 specialty doctors organized by the Alliance of Specialty Medicine, a coalition that represents specialty physicians, to meet with House and Senate offices and discuss pressing policy priorities.
Members spoke on behalf of GI and stressed the need for Congress to act immediately on the upcoming Medicare cuts, and discussed step therapy protocols, prior authorization reform, and the role of artificial intelligence in the specialty.
Our members represented GI well throughout the day’s meetings!
AGA PAC Board Member and Congressional Advocate Dr. Sadeea Abbasi spoke to House Leader Kevin McCarthy (R-CA) about challenges specialty doctors are facing and petitioned for Congress to mitigate the nearly 10 percent Medicare payment cuts before the end of the 117th Congress.
Additionally, AGA Government Affairs Committee Chair Dr. Rotonya Carr engaged with several members of Congress on issues impacting specialty care and discussed the future of AI in medicine with Rep. Mariannette Miller-Meeks (R-IA), who is an ophthalmologist.
Thank you to all our members who spoke on behalf of GI! We appreciate our AGA leaders who took time to participate!
- Sadeea Abbasi, MD, PhD
- Dawn B. Beaulieu, MD, AGAF
- Brent Burnette, MD
- Rotonya M. Carr, MD, FACP
- Peter S. Margolis, MD, AGAF
- Suzette Rivera MacMurray, MD
Learn how AGA is advocating for you. Visit gastro.org/advocacy.
AGA workshops champion women in GI
At AGA, we’re committed to supporting, empowering, and amplifying women in GI. As part of our ongoing efforts, we were thrilled to host nearly 250 women at four Women in GI regional workshops this fall. These workshops provided women across the country with opportunities to develop their personal and professional networks, participate in leadership development seminars, receive career mentoring, and engage in various wellness practices.
Following the conclusion of the regional workshops, we hosted the AGA Women’s Leadership Collaboration Conference at the AGA national office in Bethesda, Md.
I can’t stop talking about this weekend! What a profound experience! I continue to be inspired by this group of women, our mission, and the far-reaching impact of this collaboration. Each one of us was absolutely transformed this weekend. And the radius of that impact is immeasurable! – Dr. Bahar Adeli, TJUH–Albert Einstein Medical Center
During the two-day conference, 22 delegates discussed workplace experiences, reported on their region’s workshop, and participated in strategy development exercises to further gender equity in GI, which included identifying tactics and ideas for action.
This unique event brought together delegates from each regional workshop, including 2023 Dr. Maria Leo-Lieber scholarship winners Alyssa Parian and Alexandra Livanos, who attended the Northeast workshop. It was a great time of collaboration and connection with the AGA #WomenInGI community!
Thank you to everyone who participated and made the events a success. We look forward to uplifting all women in GI and identifying ways to support the community in the coming year.
AGA thanks Johnson & Johnson Health Care Systems, Inc. for their support of this program.
Help move innovation forward at the 2023 AGA Tech Summit
Innovative technologies for endoscopy, advanced imaging, and bariatric care are a few of the topics that will headline the 2023 AGA Tech Summit, March 9-10, 2023, at the Grand Hyatt San Francisco. Registration is now open. Visit techsummit.gastro.org to secure your spot.

The Tech Summit is where GI innovators, clinicians, medical device companies, venture capitalists and regulatory agencies meet to foster the development and adoption of GI technologies. It is the perfect venue for:
- All innovators, entrepreneurs and clinicians who want to build their professional networks in the GI space.
- Early-stage GI companies who want to showcase their technologies and find new ideas for further innovations that will improve patient care.
- GI fellows who want an exclusive and immersive behind-the-scenes look into the MedTech world.
Learn more by visiting techsummit.gastro.org.
AGA members advocate for GI during Alliance of Specialty Medicine fly-in
In December, six AGA members joined more than 100 specialty doctors organized by the Alliance of Specialty Medicine, a coalition that represents specialty physicians, to meet with House and Senate offices and discuss pressing policy priorities.
Members spoke on behalf of GI and stressed the need for Congress to act immediately on the upcoming Medicare cuts, and discussed step therapy protocols, prior authorization reform, and the role of artificial intelligence in the specialty.
Our members represented GI well throughout the day’s meetings!
AGA PAC Board Member and Congressional Advocate Dr. Sadeea Abbasi spoke to House Leader Kevin McCarthy (R-CA) about challenges specialty doctors are facing and petitioned for Congress to mitigate the nearly 10 percent Medicare payment cuts before the end of the 117th Congress.
Additionally, AGA Government Affairs Committee Chair Dr. Rotonya Carr engaged with several members of Congress on issues impacting specialty care and discussed the future of AI in medicine with Rep. Mariannette Miller-Meeks (R-IA), who is an ophthalmologist.
Thank you to all our members who spoke on behalf of GI! We appreciate our AGA leaders who took time to participate!
- Sadeea Abbasi, MD, PhD
- Dawn B. Beaulieu, MD, AGAF
- Brent Burnette, MD
- Rotonya M. Carr, MD, FACP
- Peter S. Margolis, MD, AGAF
- Suzette Rivera MacMurray, MD
Learn how AGA is advocating for you. Visit gastro.org/advocacy.
AGA workshops champion women in GI
At AGA, we’re committed to supporting, empowering, and amplifying women in GI. As part of our ongoing efforts, we were thrilled to host nearly 250 women at four Women in GI regional workshops this fall. These workshops provided women across the country with opportunities to develop their personal and professional networks, participate in leadership development seminars, receive career mentoring, and engage in various wellness practices.
Following the conclusion of the regional workshops, we hosted the AGA Women’s Leadership Collaboration Conference at the AGA national office in Bethesda, Md.
I can’t stop talking about this weekend! What a profound experience! I continue to be inspired by this group of women, our mission, and the far-reaching impact of this collaboration. Each one of us was absolutely transformed this weekend. And the radius of that impact is immeasurable! – Dr. Bahar Adeli, TJUH–Albert Einstein Medical Center
During the two-day conference, 22 delegates discussed workplace experiences, reported on their region’s workshop, and participated in strategy development exercises to further gender equity in GI, which included identifying tactics and ideas for action.
This unique event brought together delegates from each regional workshop, including 2023 Dr. Maria Leo-Lieber scholarship winners Alyssa Parian and Alexandra Livanos, who attended the Northeast workshop. It was a great time of collaboration and connection with the AGA #WomenInGI community!
Thank you to everyone who participated and made the events a success. We look forward to uplifting all women in GI and identifying ways to support the community in the coming year.
AGA thanks Johnson & Johnson Health Care Systems, Inc. for their support of this program.
Help move innovation forward at the 2023 AGA Tech Summit
Innovative technologies for endoscopy, advanced imaging, and bariatric care are a few of the topics that will headline the 2023 AGA Tech Summit, March 9-10, 2023, at the Grand Hyatt San Francisco. Registration is now open. Visit techsummit.gastro.org to secure your spot.

The Tech Summit is where GI innovators, clinicians, medical device companies, venture capitalists and regulatory agencies meet to foster the development and adoption of GI technologies. It is the perfect venue for:
- All innovators, entrepreneurs and clinicians who want to build their professional networks in the GI space.
- Early-stage GI companies who want to showcase their technologies and find new ideas for further innovations that will improve patient care.
- GI fellows who want an exclusive and immersive behind-the-scenes look into the MedTech world.
Learn more by visiting techsummit.gastro.org.
AGA members advocate for GI during Alliance of Specialty Medicine fly-in
In December, six AGA members joined more than 100 specialty doctors organized by the Alliance of Specialty Medicine, a coalition that represents specialty physicians, to meet with House and Senate offices and discuss pressing policy priorities.
Members spoke on behalf of GI and stressed the need for Congress to act immediately on the upcoming Medicare cuts, and discussed step therapy protocols, prior authorization reform, and the role of artificial intelligence in the specialty.
Our members represented GI well throughout the day’s meetings!
AGA PAC Board Member and Congressional Advocate Dr. Sadeea Abbasi spoke to House Leader Kevin McCarthy (R-CA) about challenges specialty doctors are facing and petitioned for Congress to mitigate the nearly 10 percent Medicare payment cuts before the end of the 117th Congress.
Additionally, AGA Government Affairs Committee Chair Dr. Rotonya Carr engaged with several members of Congress on issues impacting specialty care and discussed the future of AI in medicine with Rep. Mariannette Miller-Meeks (R-IA), who is an ophthalmologist.
Thank you to all our members who spoke on behalf of GI! We appreciate our AGA leaders who took time to participate!
- Sadeea Abbasi, MD, PhD
- Dawn B. Beaulieu, MD, AGAF
- Brent Burnette, MD
- Rotonya M. Carr, MD, FACP
- Peter S. Margolis, MD, AGAF
- Suzette Rivera MacMurray, MD
Learn how AGA is advocating for you. Visit gastro.org/advocacy.
AGA workshops champion women in GI
At AGA, we’re committed to supporting, empowering, and amplifying women in GI. As part of our ongoing efforts, we were thrilled to host nearly 250 women at four Women in GI regional workshops this fall. These workshops provided women across the country with opportunities to develop their personal and professional networks, participate in leadership development seminars, receive career mentoring, and engage in various wellness practices.
Following the conclusion of the regional workshops, we hosted the AGA Women’s Leadership Collaboration Conference at the AGA national office in Bethesda, Md.
I can’t stop talking about this weekend! What a profound experience! I continue to be inspired by this group of women, our mission, and the far-reaching impact of this collaboration. Each one of us was absolutely transformed this weekend. And the radius of that impact is immeasurable! – Dr. Bahar Adeli, TJUH–Albert Einstein Medical Center
During the two-day conference, 22 delegates discussed workplace experiences, reported on their region’s workshop, and participated in strategy development exercises to further gender equity in GI, which included identifying tactics and ideas for action.
This unique event brought together delegates from each regional workshop, including 2023 Dr. Maria Leo-Lieber scholarship winners Alyssa Parian and Alexandra Livanos, who attended the Northeast workshop. It was a great time of collaboration and connection with the AGA #WomenInGI community!
Thank you to everyone who participated and made the events a success. We look forward to uplifting all women in GI and identifying ways to support the community in the coming year.
AGA thanks Johnson & Johnson Health Care Systems, Inc. for their support of this program.
Help move innovation forward at the 2023 AGA Tech Summit
Innovative technologies for endoscopy, advanced imaging, and bariatric care are a few of the topics that will headline the 2023 AGA Tech Summit, March 9-10, 2023, at the Grand Hyatt San Francisco. Registration is now open. Visit techsummit.gastro.org to secure your spot.

The Tech Summit is where GI innovators, clinicians, medical device companies, venture capitalists and regulatory agencies meet to foster the development and adoption of GI technologies. It is the perfect venue for:
- All innovators, entrepreneurs and clinicians who want to build their professional networks in the GI space.
- Early-stage GI companies who want to showcase their technologies and find new ideas for further innovations that will improve patient care.
- GI fellows who want an exclusive and immersive behind-the-scenes look into the MedTech world.
Learn more by visiting techsummit.gastro.org.
Telemedicine increases access to care and optimizes practice revenue
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
A heartwarming welcome
Dear colleagues,
This November issue of The New Gastroenterologist marks my official transition as the new Editor in Chief! I am humbled with this opportunity to be a part of such a unique publication and have received immense support from Dr. Vijaya Rao, the TNG staff, as well as my mentors and colleagues. With its foundation built by Dr. Bryson Katona and then taken to the next level by Dr. Rao, TNG has grown over the years, and I hope that I can continue to extend its reach to more trainees and early faculty.
In this issue’s In Focus, Dr. Wenfei Wang and Dr. Neil Sengupta (both from University of Chicago) review the management of antithrombotic medications in elective endoscopic procedures and emphasize individualizing the approach while providing guideline recommendations on how to navigate the gastrointestinal bleeding risk and cardiovascular disease in this day and age.
With endoscopic bariatric therapy and antiobesity medications burgeoning within gastroenterology, Dr. Singrid Young (New York University), Dr. Cameron Zenger (New York University), Dr. Erik Holzwanger (Harvard Medical School in Boston), and Dr. Violeta Popov (New York University) review how their multidisciplinary approach has made their endoscopic bariatric program successful in treating patients struggling with obesity. In our Ethics section, Dr. David Ney (Thomas Jefferson University Hospital, Philadelphia) and Dr. Jason Karlawish (University of Pennsylvania, Philadelphia) delve into patient capacity, particularly when consenting for procedures.
Being involved with national society committees may seem daunting to a lot of trainees and early faculty, but Dr. Peter S. Liang (New York University Langone Health) and Dr. Stephanie D. Pointer (Tristar Hendersonville Medical Center in Tennessee) describe their journeys to becoming AGA committee chairs as early-career physicians. While you ponder whether to join a committee, it may be a good time to learn new ways to increase your financial portfolio through passive income, detailed by Dr. Latifat Alli-Akintade (Kaiser Permanente South Sacramento Medical Center in California).
Last but not least, I am excited to introduce a personal favorite in this newsletter – a piece on females supporting female gastroenterologists in career development and more. Dr. Tonya Adams outlines action items on how to create a culture that fosters professional and leadership development among females, using the Gastro Health Women’s Network as an example of how this network has succeeded in cultivating such an environment.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with an interesting historical fact: William Beaumont, the father of Gastroenterology, published the first findings on the digestive system after performing experiments on Alexis St. Martin when he developed a large gastrocutaneous fistula from an abdominal gunshot wound.
Yours truly,
Judy A. Trieu, MD, MPH
Editor in Chief
Advanced Endoscopy Fellow, University of North Carolina at Chapel Hill, Division of Gastroenterology & Hepatology
Dear colleagues,
This November issue of The New Gastroenterologist marks my official transition as the new Editor in Chief! I am humbled with this opportunity to be a part of such a unique publication and have received immense support from Dr. Vijaya Rao, the TNG staff, as well as my mentors and colleagues. With its foundation built by Dr. Bryson Katona and then taken to the next level by Dr. Rao, TNG has grown over the years, and I hope that I can continue to extend its reach to more trainees and early faculty.
In this issue’s In Focus, Dr. Wenfei Wang and Dr. Neil Sengupta (both from University of Chicago) review the management of antithrombotic medications in elective endoscopic procedures and emphasize individualizing the approach while providing guideline recommendations on how to navigate the gastrointestinal bleeding risk and cardiovascular disease in this day and age.
With endoscopic bariatric therapy and antiobesity medications burgeoning within gastroenterology, Dr. Singrid Young (New York University), Dr. Cameron Zenger (New York University), Dr. Erik Holzwanger (Harvard Medical School in Boston), and Dr. Violeta Popov (New York University) review how their multidisciplinary approach has made their endoscopic bariatric program successful in treating patients struggling with obesity. In our Ethics section, Dr. David Ney (Thomas Jefferson University Hospital, Philadelphia) and Dr. Jason Karlawish (University of Pennsylvania, Philadelphia) delve into patient capacity, particularly when consenting for procedures.
Being involved with national society committees may seem daunting to a lot of trainees and early faculty, but Dr. Peter S. Liang (New York University Langone Health) and Dr. Stephanie D. Pointer (Tristar Hendersonville Medical Center in Tennessee) describe their journeys to becoming AGA committee chairs as early-career physicians. While you ponder whether to join a committee, it may be a good time to learn new ways to increase your financial portfolio through passive income, detailed by Dr. Latifat Alli-Akintade (Kaiser Permanente South Sacramento Medical Center in California).
Last but not least, I am excited to introduce a personal favorite in this newsletter – a piece on females supporting female gastroenterologists in career development and more. Dr. Tonya Adams outlines action items on how to create a culture that fosters professional and leadership development among females, using the Gastro Health Women’s Network as an example of how this network has succeeded in cultivating such an environment.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with an interesting historical fact: William Beaumont, the father of Gastroenterology, published the first findings on the digestive system after performing experiments on Alexis St. Martin when he developed a large gastrocutaneous fistula from an abdominal gunshot wound.
Yours truly,
Judy A. Trieu, MD, MPH
Editor in Chief
Advanced Endoscopy Fellow, University of North Carolina at Chapel Hill, Division of Gastroenterology & Hepatology
Dear colleagues,
This November issue of The New Gastroenterologist marks my official transition as the new Editor in Chief! I am humbled with this opportunity to be a part of such a unique publication and have received immense support from Dr. Vijaya Rao, the TNG staff, as well as my mentors and colleagues. With its foundation built by Dr. Bryson Katona and then taken to the next level by Dr. Rao, TNG has grown over the years, and I hope that I can continue to extend its reach to more trainees and early faculty.
In this issue’s In Focus, Dr. Wenfei Wang and Dr. Neil Sengupta (both from University of Chicago) review the management of antithrombotic medications in elective endoscopic procedures and emphasize individualizing the approach while providing guideline recommendations on how to navigate the gastrointestinal bleeding risk and cardiovascular disease in this day and age.
With endoscopic bariatric therapy and antiobesity medications burgeoning within gastroenterology, Dr. Singrid Young (New York University), Dr. Cameron Zenger (New York University), Dr. Erik Holzwanger (Harvard Medical School in Boston), and Dr. Violeta Popov (New York University) review how their multidisciplinary approach has made their endoscopic bariatric program successful in treating patients struggling with obesity. In our Ethics section, Dr. David Ney (Thomas Jefferson University Hospital, Philadelphia) and Dr. Jason Karlawish (University of Pennsylvania, Philadelphia) delve into patient capacity, particularly when consenting for procedures.
Being involved with national society committees may seem daunting to a lot of trainees and early faculty, but Dr. Peter S. Liang (New York University Langone Health) and Dr. Stephanie D. Pointer (Tristar Hendersonville Medical Center in Tennessee) describe their journeys to becoming AGA committee chairs as early-career physicians. While you ponder whether to join a committee, it may be a good time to learn new ways to increase your financial portfolio through passive income, detailed by Dr. Latifat Alli-Akintade (Kaiser Permanente South Sacramento Medical Center in California).
Last but not least, I am excited to introduce a personal favorite in this newsletter – a piece on females supporting female gastroenterologists in career development and more. Dr. Tonya Adams outlines action items on how to create a culture that fosters professional and leadership development among females, using the Gastro Health Women’s Network as an example of how this network has succeeded in cultivating such an environment.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with an interesting historical fact: William Beaumont, the father of Gastroenterology, published the first findings on the digestive system after performing experiments on Alexis St. Martin when he developed a large gastrocutaneous fistula from an abdominal gunshot wound.
Yours truly,
Judy A. Trieu, MD, MPH
Editor in Chief
Advanced Endoscopy Fellow, University of North Carolina at Chapel Hill, Division of Gastroenterology & Hepatology
Management of antithrombotic medications in elective endoscopy
Antithrombotic therapy is increasingly used to either reduce the risk of or treat thromboembolic episodes in patients with various medical conditions such as ischemic and valvular heart disease, atrial fibrillation (AF), cerebrovascular disease, peripheral arterial disease, venous thromboembolism (VTE) and hypercoagulable diseases. Antithrombotics include medications classified as anticoagulants or antiplatelets. Anticoagulants work by interfering with the native clotting cascade and consist of four main classes: vitamin K antagonists (VKA), heparin derivatives, direct factor Xa inhibitors, and direct thrombin inhibitors. Direct oral anticoagulants (DOACs) refer to dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban).
Antiplatelets, on the other hand, work by decreasing platelet aggregation and thus preventing thrombus formation; they include P2Y12 receptor inhibitors, protease-activated receptor-1 inhibitors, glycoprotein IIb/IIIa receptor inhibitors, acetylsalicylic acid (ASA), and nonsteroidal anti-inflammatory drugs. All of these agents may directly cause or increase the risk of gastrointestinal (GI) bleeding from luminal sources such as ulcers or diverticula, as well as after endoscopic interventions such as polypectomy. However, there is also a risk of thromboembolic consequences if some of these agents are withheld. Thus, the management of patients receiving antithrombotic agents and undergoing GI endoscopy represents an important clinical challenge and something that every GI physician has to deal with routinely.
The goal of this review is to discuss the optimal strategy for managing antithrombotics in patients undergoing elective endoscopy based on current available evidence and published clinical guidelines.1-4 Much of our discussion will review recommendations from the recently published joint American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) guidelines on management of anticoagulants and antiplatelets in the periendoscopic period by Abraham et al.4
Factors that guide decision-making
The two most vital factors to consider prior to performing endoscopic procedures in patients receiving antithrombotic therapy are to assess the risk of bleeding associated with the procedure and to assess the risk of thromboembolism associated with the underlying medical condition for which the antithrombotic agents are being used. In addition, it is also important to keep in mind the individual characteristics of the antithrombotic agent(s) used when making these decisions.
Estimating procedure-related bleeding risk
Various endoscopic procedures have different risks of associated bleeding. Although guidelines from GI societies may differ when classifying procedures into low or high risk, it is important to know that most of the original data on postprocedural bleeding risks are from studies conducted in patients who are not on complex antithrombotic regimens and thus may not accurately reflect the bleeding risk of patients using newer antithrombotic therapies.1,4-7
Traditionally, some of the common low-risk procedures have included diagnostic EGD and colonoscopy with or without biopsy, ERCP without sphincterotomy, biliary stent placement, and push or balloon-assisted enteroscopy. On the other hand, endoscopic procedures associated with interventions are known to have higher bleeding risk, and other procedural factors can influence this risk as well.8 For example, polypectomy, one of the most common interventions during endoscopy, is associated with bleeding risk ranging from 0.3% to 10% depending on multiple factors including polyp size, location, morphology (nonpolypoid, sessile, pedunculated), resection technique (cold or hot forceps, cold or hot snare), and type of cautery used.9 For some procedures, such as routine screening colonoscopy, however, the preprocedure estimate of bleeding risk can be uncertain because it is unclear if a high risk intervention (e.g., polypectomy of large polyp) will be necessary. For example, in the most recent ACG/CAG guidelines, colonoscopy with polypectomy < 1cm is considered a low/moderate risk bleeding procedure, whereas polypectomy > 1cm is considered high risk for bleeding.4 In these situations, the management of antithrombotic medications may depend on the individual patient’s risk of thrombosis and the specific antithrombotic agent. In the example of a patient undergoing colonoscopy while on antithrombotic medications, the bleeding risk associated with polypectomy can potentially be reduced by procedural techniques such as preferential use of cold snare polypectomy. Further high-quality data on the optimal procedural technique to reduce postpolypectomy bleeding in patients on antithrombotic medications is needed to help guide management.
Estimating thromboembolic risk
The risk of thromboembolic events in patients who are withholding their antithrombotic therapy for an endoscopic procedure depends on their underlying condition and individual characteristics. In patients who are on antithrombotic therapy for stroke prevention in non-valvular AF, the risk of cerebral thromboembolism in these patients is predictable using the CHA2DS2Vasc index.10 This scoring index includes heart failure, hypertension, age 75 years or older, diabetes mellitus, prior stroke or transient ischemic attack (TIA), vascular disease, age 65-74 years, and sex categories.
Patients with previous VTE on anticoagulation or those who have mechanical heart valves may have different risk factors for thromboembolic episodes. Among patients with VTE, time from initial VTE, history of recurrent VTE with antithrombotic interruption, and presence of underlying thrombophilia are most predictive of future thromboembolic risk. And for patients with mechanical heart valves, presence of a mitral valve prosthesis, and the presence or absence of associated heart failure and AF determine the annual risk of thromboembolic events. Bioprosthetic valves are generally considered low risk.
In patients with coronary artery disease (CAD), high thrombosis risk scenarios with holding antiplatelets include patients within 3 months of an acute coronary syndrome (ACS) event, within 6 months of a drug-eluting stent (DES) placement, or within 1 month of a bare metal coronary stent (BMS) placement. In addition, patients with ACS that occurred within the past 12 months of DES placement or within 2 months of BMS placement are also considered high risk.11,12 Even beyond these periods, certain patients may still be at high risk of stent occlusion. In particular, patients with a prior history of stent occlusion, ACS or ST elevation myocardial infection, prior multivessel percutaneous coronary intervention, diabetes, renal failure, or diffuse CAD are at higher risk of stent occlusion or ACS events with alteration of antithrombotic therapy.13 Thus, modification of antithrombotic regimens in these patients should be cautiously approached.
Management of antithrombotics prior to elective endoscopy
In patients who need elective endoscopic procedures, if the indication for antithrombotic therapy is short-term, the procedure is probably best delayed until after that period.13 For patients on long-term or lifelong antithrombotic treatment, the decision to temporarily hold the treatment for endoscopy should occur after a discussion with the patient and all of the involved providers. In some high-risk patients, these agents cannot be interrupted; therefore, clinicians must carefully weigh the risks and benefits of the procedure before proceeding with endoscopy. For patients who are known to be undergoing an elective endoscopic procedure, antithrombotic medications may or may not need to be held prior to the procedure depending on the type of therapy. For example, according to the recent ACG/CAG guidelines, warfarin should be continued, whereas DOACs should be temporarily stopped for patients who are undergoing elective/planned endoscopic GI procedures.
Unfractionated heparin (UFH) administered as a continuous intravenous infusion can generally be held 3-4 hours before the procedure, given its short half-life. Low molecular weight heparin (LMWH), including enoxaparin and dalteparin, should be stopped 24 hours prior to the procedure.2,14 Fondaparinux is a synthetic X-a inhibitor that requires discontinuation at least 36 hours preceding a high risk procedure. For patients on warfarin who are undergoing elective endoscopic procedures that are low risk for inducing bleeding, warfarin can be continued, as opposed to temporarily interrupted, although the dose should be omitted the morning of the procedure.4 For those who are undergoing high-risk endoscopic procedures (including colonoscopy with possible polypectomy > 1 cm), 5 days of temporary interruption without periprocedural bridging is appropriate in most patients. This is contrary to previous guidelines, which had recommended bridging for patients with a CHA2DS2Vasc score ≥ 2. Recent impactful randomized trials (BRIDGE and PERIOP-2) have called into question the benefit of periprocedural bridging with LMWH. Avoiding bridging anticoagulation was generally found to be similar to bridging in regard to prevention of thromboembolic complications, but importantly was associated with a decreased risk of major bleeding.15,16 Of note, periprocedural bridging may still be appropriate in a small subset of patients, including those with mechanical valves, AF with CHADS2 score > 5, and previous thromboembolism during temporary interruption of VKAs. The decision to bridge or not should ideally be made in a multidisciplinary fashion.15-20
Data are lacking on the ideal strategy for periendoscopic DOAC management. As mentioned above, for patients on DOACs who are undergoing elective endoscopic GI procedures, temporarily interrupting DOACs rather than continuing them is recommended. Currently, there are no randomized controlled trials addressing the management of DOACs in the periendoscopic period. However, based on five cohort studies, the ideal duration of DOAC interruption before endoscopic procedures may be between 1 and 2 days, excluding the day of the procedure.21-25 This strategy allows for a short preprocedural duration of DOAC interruption and likely provides a balance between bleeding and thromboembolism risk. Importantly, there are no reliable laboratory assays to assess the anticoagulant effect of DOACs, and an individual patient’s degree of renal dysfunction may impact how long the DOAC should be held. In general, the anti-Xa drugs should be held for 1-2 days if the creatinine clearance (CrCl) is ≥ 60 mL/min, for 3 days if the CrCl is between 30 mL/min and 59 mL/min, and for 4 days if the CrCl is less than 30 mL/min.26 For edoxaban, the recommendation is to hold at least 24 hours before high-risk procedures. The recommendation for stopping dabigatran is 2-3 days before a high-risk procedure in patients with CrCl more than 80 mL/min, 3-4 days prior if between 30 and 49 mL/min, and 4-6 days prior if less than 30 mL/min respectively.27
In regard to antiplatelet management, ASA and the P2Y12 receptor inhibitors (e.g. clopidogrel, prasugrel, and ticagrelor) are the most commonly utilized antiplatelets in patients undergoing endoscopic procedures. For patients who are on ASA monotherapy, whether 81 mg or 325 mg daily, for secondary cardiovascular prevention, no interruption of ASA therapy is necessary for elective procedures. The benefit of ASA for secondary cardiovascular prevention and the possible reduction in thrombotic events seen in RCTs of nonendoscopic surgical procedures is well known. However, there may be certain exceptions in which aspirin should be temporarily held. For example, short-term interruption of ASA could be considered in high risk procedures such as biliary or pancreatic sphincterotomy, ampullectomy, and peroral endoscopic myotomy. For patients on single antiplatelet therapy with a P2Y12 receptor inhibitor who are undergoing elective endoscopic GI procedures, the recent CAG/ACG guidelines did not provide a clear recommendation for or against temporary interruption of the P2Y12 receptor inhibitor. Although interruption of a P2Y12 receptor inhibitor should theoretically decrease a patient’s risk of bleeding, the available evidence reported a nonsignificant increased bleeding risk in patients who stop a P2Y12 receptor inhibitor for an elective endoscopic procedure compared with those who continue the medication.28,29 Therefore, until further data are available, for patients on P2Y12 receptor monotherapy, a reasonable strategy would be to temporarily hold therapy prior to high risk endoscopic procedures, assuming the patients are not at high cardiovascular risk. Clopidogrel and prasugrel have to be stopped 5-7 days prior to allow normal platelet aggregation to resume as opposed to ticagrelor, a reversible P2Y12 receptor inhibitor that can be stopped 3-5 days prior.30
Lastly, for patients who are on dual antiplatelet therapy (DAPT) for secondary prevention, continuation of ASA and temporary interruption of the P2Y12 receptor inhibitor is recommended while undergoing elective endoscopy. Studies have shown that those who discontinued both had a much higher incidence of stent thrombosis compared with those who remained on aspirin alone.4,28,31
Resumption of antithrombotic therapy after endoscopy
In general, antithrombotic therapy should be resumed upon completion of the procedure unless there remains a persistent risk of major bleeding.1,14 This consensus is based on studies available on warfarin and heparin products, with minimal literature available regarding the resumption of DOACs. The benefits of immediate re-initiation of antithrombotic therapy for the prevention of thromboembolic events should be weighed against the risk of hemorrhage associated with the specific agent, the time to onset of the medication, and procedure-specific circumstances. For the small subset of patients on warfarin with a high risk of thromboembolism (e.g., mechanical heart valve), bridging with LMWH should be started at the earliest possible time when there is no risk of major bleeding and continued until the international normalized ratio (INR) reaches a therapeutic level with warfarin. For patients at a lower risk of thromboembolism, warfarin should be restarted within 24 hours of the procedure. In addition, because of the shorter duration of DOACs, if treatment with these agents cannot resume within 24 hours of a high-risk procedure, bridge therapy should be considered with UFH or LMWH in patients with a high risk of thrombosis.18 In patients receiving DOACs for stroke prophylaxis in AF, the DOACS can be safely resumed 1 day after low-risk procedures and 2-3 days after high-risk procedures without the need for bridging.25 All antiplatelet agents should be resumed as soon as hemostasis is achieved.
Conclusion
Antithrombotic therapy is increasingly used given the aging population, widespread burden of cardiovascular comorbidities, and expanding indications for classes of medications such as direct oral anticoagulants. Given the association with antithrombotic medications and gastrointestinal bleeding, it is essential for gastroenterologists to understand the importance, necessity, and timing when holding these medications for endoscopic procedures. Even with the practice guidelines available today to help clinicians navigate certain situations, each patient’s antithrombotic management may be different, and communication with the prescribing physicians and including patients in the decision-making process is essential before planned procedures.
Dr. Wang is a gastroenterology fellow at the University of Chicago. Dr. Sengupta is an associate professor at the University of Chicago. They reported no funding or conflicts of interest.
References
1. ASGE Standards of Practice Committee, Acosta RD et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83(1):3-16.
2. Veitch AM et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48(4):c1. doi: 10.1055/s-0042-122686.
3. Chan FKL et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: Joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67(3):405-17.
4. Abraham NS et al. American College of Gastroenterology – Canadian Association of Gastroenterology clinical practice guideline: Management of anticoagulants and antiplatelets during acute gastrointestinal bleeding and the periendoscopic period. Am J Gastroenterol. 2022;117(4):542-58.
5. Boustière C et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2011;43(5):445-61.
6. Fujimoto K et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26(1):1-14.
7. Wilke T et al. Patient preferences for oral anticoagulation therapy in atrial fibrillation: A systematic literature review. Patient 2017;10(1):17-37.
8. Gerson LB et al. Adverse events associated with anticoagulation therapy in the periendoscopic period. Gastrointest Endosc. 2010 Jun;71(7):1211-17.e2.
9. Horiuchi A et al. Removal of small colorectal polyps in anticoagulated patients: A prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc 2014;79(3):417-23.
10. Lip GYH et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-72.
11. 2012 Writing Committee Members, Jneid H et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (Updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126(7):875-910.
12. Douketis JD et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e326S-e350S.
13. Becker RC et al. Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. J Am Coll Cardiol. 2009;54(24):2261-76.
14. Kwok A and Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009;104(12):3085-97; quiz 3098.
15. Douketis JD et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-33.
16. Kovacs MJ et al. Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): Double blind randomised controlled trial. BMJ 2021;373:n1205.
17. Tafur A and Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart 2018;104(17):1461-7.
18. Kato M et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30(4):433-40.
19. Inoue T et al. Clinical features of postpolypectomy bleeding associated with heparin bridge therapy. Dig Endosc. 2014;26(2):243-9.
20. Takeuchi Y et al. Continuous anticoagulation and cold snare polypectomy versus heparin bridging and hot snare polypectomy in patients on anticoagulants with subcentimeter polyps: A randomized controlled trial. Ann Intern Med. 2019;171(4):229-37.
21. Ara N et al. Prospective analysis of risk for bleeding after endoscopic biopsy without cessation of antithrombotics in Japan. Dig Endosc. 2015;27(4):458-64.
22. Yanagisawa N et al. Postpolypectomy bleeding and thromboembolism risks associated with warfarin vs. direct oral anticoagulants. World J Gastroenterol. 2018;24(14):1540-9.
23. Arimoto J et al. Safety of cold snare polypectomy in patients receiving treatment with antithrombotic agents. Dig Dis Sci. 2019;64(11):3247-55.
24. Heublein V et al. Gastrointestinal endoscopy in patients receiving novel direct oral anticoagulants: Results from the prospective Dresden NOAC registry. J Gastroenterol. 2018;53(2):236-46.
25. Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469-78.
26. Dubois V et al. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14.
27. Weitz JI et al. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation. 2012 Nov 13;126(20):2428-32.
28. Chan FKL et al. Risk of postpolypectomy bleeding with uninterrupted clopidogrel therapy in an industry-independent, double-blind, randomized trial. Gastroenterology. 2019;156(4):918-25.
29. Watanabe K et al. Effect of antiplatelet agent number, types, and pre-endoscopic management on postpolypectomy bleeding: Validation of endoscopy guidelines. Surg Endosc. 2021;35(1):317-25.
30. Gurbel PA et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation. 2009;120(25):2577-85.
31. Eisenberg MJ et al. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119(12):1634-42.
Antithrombotic therapy is increasingly used to either reduce the risk of or treat thromboembolic episodes in patients with various medical conditions such as ischemic and valvular heart disease, atrial fibrillation (AF), cerebrovascular disease, peripheral arterial disease, venous thromboembolism (VTE) and hypercoagulable diseases. Antithrombotics include medications classified as anticoagulants or antiplatelets. Anticoagulants work by interfering with the native clotting cascade and consist of four main classes: vitamin K antagonists (VKA), heparin derivatives, direct factor Xa inhibitors, and direct thrombin inhibitors. Direct oral anticoagulants (DOACs) refer to dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban).
Antiplatelets, on the other hand, work by decreasing platelet aggregation and thus preventing thrombus formation; they include P2Y12 receptor inhibitors, protease-activated receptor-1 inhibitors, glycoprotein IIb/IIIa receptor inhibitors, acetylsalicylic acid (ASA), and nonsteroidal anti-inflammatory drugs. All of these agents may directly cause or increase the risk of gastrointestinal (GI) bleeding from luminal sources such as ulcers or diverticula, as well as after endoscopic interventions such as polypectomy. However, there is also a risk of thromboembolic consequences if some of these agents are withheld. Thus, the management of patients receiving antithrombotic agents and undergoing GI endoscopy represents an important clinical challenge and something that every GI physician has to deal with routinely.
The goal of this review is to discuss the optimal strategy for managing antithrombotics in patients undergoing elective endoscopy based on current available evidence and published clinical guidelines.1-4 Much of our discussion will review recommendations from the recently published joint American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) guidelines on management of anticoagulants and antiplatelets in the periendoscopic period by Abraham et al.4
Factors that guide decision-making
The two most vital factors to consider prior to performing endoscopic procedures in patients receiving antithrombotic therapy are to assess the risk of bleeding associated with the procedure and to assess the risk of thromboembolism associated with the underlying medical condition for which the antithrombotic agents are being used. In addition, it is also important to keep in mind the individual characteristics of the antithrombotic agent(s) used when making these decisions.
Estimating procedure-related bleeding risk
Various endoscopic procedures have different risks of associated bleeding. Although guidelines from GI societies may differ when classifying procedures into low or high risk, it is important to know that most of the original data on postprocedural bleeding risks are from studies conducted in patients who are not on complex antithrombotic regimens and thus may not accurately reflect the bleeding risk of patients using newer antithrombotic therapies.1,4-7
Traditionally, some of the common low-risk procedures have included diagnostic EGD and colonoscopy with or without biopsy, ERCP without sphincterotomy, biliary stent placement, and push or balloon-assisted enteroscopy. On the other hand, endoscopic procedures associated with interventions are known to have higher bleeding risk, and other procedural factors can influence this risk as well.8 For example, polypectomy, one of the most common interventions during endoscopy, is associated with bleeding risk ranging from 0.3% to 10% depending on multiple factors including polyp size, location, morphology (nonpolypoid, sessile, pedunculated), resection technique (cold or hot forceps, cold or hot snare), and type of cautery used.9 For some procedures, such as routine screening colonoscopy, however, the preprocedure estimate of bleeding risk can be uncertain because it is unclear if a high risk intervention (e.g., polypectomy of large polyp) will be necessary. For example, in the most recent ACG/CAG guidelines, colonoscopy with polypectomy < 1cm is considered a low/moderate risk bleeding procedure, whereas polypectomy > 1cm is considered high risk for bleeding.4 In these situations, the management of antithrombotic medications may depend on the individual patient’s risk of thrombosis and the specific antithrombotic agent. In the example of a patient undergoing colonoscopy while on antithrombotic medications, the bleeding risk associated with polypectomy can potentially be reduced by procedural techniques such as preferential use of cold snare polypectomy. Further high-quality data on the optimal procedural technique to reduce postpolypectomy bleeding in patients on antithrombotic medications is needed to help guide management.
Estimating thromboembolic risk
The risk of thromboembolic events in patients who are withholding their antithrombotic therapy for an endoscopic procedure depends on their underlying condition and individual characteristics. In patients who are on antithrombotic therapy for stroke prevention in non-valvular AF, the risk of cerebral thromboembolism in these patients is predictable using the CHA2DS2Vasc index.10 This scoring index includes heart failure, hypertension, age 75 years or older, diabetes mellitus, prior stroke or transient ischemic attack (TIA), vascular disease, age 65-74 years, and sex categories.
Patients with previous VTE on anticoagulation or those who have mechanical heart valves may have different risk factors for thromboembolic episodes. Among patients with VTE, time from initial VTE, history of recurrent VTE with antithrombotic interruption, and presence of underlying thrombophilia are most predictive of future thromboembolic risk. And for patients with mechanical heart valves, presence of a mitral valve prosthesis, and the presence or absence of associated heart failure and AF determine the annual risk of thromboembolic events. Bioprosthetic valves are generally considered low risk.
In patients with coronary artery disease (CAD), high thrombosis risk scenarios with holding antiplatelets include patients within 3 months of an acute coronary syndrome (ACS) event, within 6 months of a drug-eluting stent (DES) placement, or within 1 month of a bare metal coronary stent (BMS) placement. In addition, patients with ACS that occurred within the past 12 months of DES placement or within 2 months of BMS placement are also considered high risk.11,12 Even beyond these periods, certain patients may still be at high risk of stent occlusion. In particular, patients with a prior history of stent occlusion, ACS or ST elevation myocardial infection, prior multivessel percutaneous coronary intervention, diabetes, renal failure, or diffuse CAD are at higher risk of stent occlusion or ACS events with alteration of antithrombotic therapy.13 Thus, modification of antithrombotic regimens in these patients should be cautiously approached.
Management of antithrombotics prior to elective endoscopy
In patients who need elective endoscopic procedures, if the indication for antithrombotic therapy is short-term, the procedure is probably best delayed until after that period.13 For patients on long-term or lifelong antithrombotic treatment, the decision to temporarily hold the treatment for endoscopy should occur after a discussion with the patient and all of the involved providers. In some high-risk patients, these agents cannot be interrupted; therefore, clinicians must carefully weigh the risks and benefits of the procedure before proceeding with endoscopy. For patients who are known to be undergoing an elective endoscopic procedure, antithrombotic medications may or may not need to be held prior to the procedure depending on the type of therapy. For example, according to the recent ACG/CAG guidelines, warfarin should be continued, whereas DOACs should be temporarily stopped for patients who are undergoing elective/planned endoscopic GI procedures.
Unfractionated heparin (UFH) administered as a continuous intravenous infusion can generally be held 3-4 hours before the procedure, given its short half-life. Low molecular weight heparin (LMWH), including enoxaparin and dalteparin, should be stopped 24 hours prior to the procedure.2,14 Fondaparinux is a synthetic X-a inhibitor that requires discontinuation at least 36 hours preceding a high risk procedure. For patients on warfarin who are undergoing elective endoscopic procedures that are low risk for inducing bleeding, warfarin can be continued, as opposed to temporarily interrupted, although the dose should be omitted the morning of the procedure.4 For those who are undergoing high-risk endoscopic procedures (including colonoscopy with possible polypectomy > 1 cm), 5 days of temporary interruption without periprocedural bridging is appropriate in most patients. This is contrary to previous guidelines, which had recommended bridging for patients with a CHA2DS2Vasc score ≥ 2. Recent impactful randomized trials (BRIDGE and PERIOP-2) have called into question the benefit of periprocedural bridging with LMWH. Avoiding bridging anticoagulation was generally found to be similar to bridging in regard to prevention of thromboembolic complications, but importantly was associated with a decreased risk of major bleeding.15,16 Of note, periprocedural bridging may still be appropriate in a small subset of patients, including those with mechanical valves, AF with CHADS2 score > 5, and previous thromboembolism during temporary interruption of VKAs. The decision to bridge or not should ideally be made in a multidisciplinary fashion.15-20
Data are lacking on the ideal strategy for periendoscopic DOAC management. As mentioned above, for patients on DOACs who are undergoing elective endoscopic GI procedures, temporarily interrupting DOACs rather than continuing them is recommended. Currently, there are no randomized controlled trials addressing the management of DOACs in the periendoscopic period. However, based on five cohort studies, the ideal duration of DOAC interruption before endoscopic procedures may be between 1 and 2 days, excluding the day of the procedure.21-25 This strategy allows for a short preprocedural duration of DOAC interruption and likely provides a balance between bleeding and thromboembolism risk. Importantly, there are no reliable laboratory assays to assess the anticoagulant effect of DOACs, and an individual patient’s degree of renal dysfunction may impact how long the DOAC should be held. In general, the anti-Xa drugs should be held for 1-2 days if the creatinine clearance (CrCl) is ≥ 60 mL/min, for 3 days if the CrCl is between 30 mL/min and 59 mL/min, and for 4 days if the CrCl is less than 30 mL/min.26 For edoxaban, the recommendation is to hold at least 24 hours before high-risk procedures. The recommendation for stopping dabigatran is 2-3 days before a high-risk procedure in patients with CrCl more than 80 mL/min, 3-4 days prior if between 30 and 49 mL/min, and 4-6 days prior if less than 30 mL/min respectively.27
In regard to antiplatelet management, ASA and the P2Y12 receptor inhibitors (e.g. clopidogrel, prasugrel, and ticagrelor) are the most commonly utilized antiplatelets in patients undergoing endoscopic procedures. For patients who are on ASA monotherapy, whether 81 mg or 325 mg daily, for secondary cardiovascular prevention, no interruption of ASA therapy is necessary for elective procedures. The benefit of ASA for secondary cardiovascular prevention and the possible reduction in thrombotic events seen in RCTs of nonendoscopic surgical procedures is well known. However, there may be certain exceptions in which aspirin should be temporarily held. For example, short-term interruption of ASA could be considered in high risk procedures such as biliary or pancreatic sphincterotomy, ampullectomy, and peroral endoscopic myotomy. For patients on single antiplatelet therapy with a P2Y12 receptor inhibitor who are undergoing elective endoscopic GI procedures, the recent CAG/ACG guidelines did not provide a clear recommendation for or against temporary interruption of the P2Y12 receptor inhibitor. Although interruption of a P2Y12 receptor inhibitor should theoretically decrease a patient’s risk of bleeding, the available evidence reported a nonsignificant increased bleeding risk in patients who stop a P2Y12 receptor inhibitor for an elective endoscopic procedure compared with those who continue the medication.28,29 Therefore, until further data are available, for patients on P2Y12 receptor monotherapy, a reasonable strategy would be to temporarily hold therapy prior to high risk endoscopic procedures, assuming the patients are not at high cardiovascular risk. Clopidogrel and prasugrel have to be stopped 5-7 days prior to allow normal platelet aggregation to resume as opposed to ticagrelor, a reversible P2Y12 receptor inhibitor that can be stopped 3-5 days prior.30
Lastly, for patients who are on dual antiplatelet therapy (DAPT) for secondary prevention, continuation of ASA and temporary interruption of the P2Y12 receptor inhibitor is recommended while undergoing elective endoscopy. Studies have shown that those who discontinued both had a much higher incidence of stent thrombosis compared with those who remained on aspirin alone.4,28,31
Resumption of antithrombotic therapy after endoscopy
In general, antithrombotic therapy should be resumed upon completion of the procedure unless there remains a persistent risk of major bleeding.1,14 This consensus is based on studies available on warfarin and heparin products, with minimal literature available regarding the resumption of DOACs. The benefits of immediate re-initiation of antithrombotic therapy for the prevention of thromboembolic events should be weighed against the risk of hemorrhage associated with the specific agent, the time to onset of the medication, and procedure-specific circumstances. For the small subset of patients on warfarin with a high risk of thromboembolism (e.g., mechanical heart valve), bridging with LMWH should be started at the earliest possible time when there is no risk of major bleeding and continued until the international normalized ratio (INR) reaches a therapeutic level with warfarin. For patients at a lower risk of thromboembolism, warfarin should be restarted within 24 hours of the procedure. In addition, because of the shorter duration of DOACs, if treatment with these agents cannot resume within 24 hours of a high-risk procedure, bridge therapy should be considered with UFH or LMWH in patients with a high risk of thrombosis.18 In patients receiving DOACs for stroke prophylaxis in AF, the DOACS can be safely resumed 1 day after low-risk procedures and 2-3 days after high-risk procedures without the need for bridging.25 All antiplatelet agents should be resumed as soon as hemostasis is achieved.
Conclusion
Antithrombotic therapy is increasingly used given the aging population, widespread burden of cardiovascular comorbidities, and expanding indications for classes of medications such as direct oral anticoagulants. Given the association with antithrombotic medications and gastrointestinal bleeding, it is essential for gastroenterologists to understand the importance, necessity, and timing when holding these medications for endoscopic procedures. Even with the practice guidelines available today to help clinicians navigate certain situations, each patient’s antithrombotic management may be different, and communication with the prescribing physicians and including patients in the decision-making process is essential before planned procedures.
Dr. Wang is a gastroenterology fellow at the University of Chicago. Dr. Sengupta is an associate professor at the University of Chicago. They reported no funding or conflicts of interest.
References
1. ASGE Standards of Practice Committee, Acosta RD et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83(1):3-16.
2. Veitch AM et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48(4):c1. doi: 10.1055/s-0042-122686.
3. Chan FKL et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: Joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67(3):405-17.
4. Abraham NS et al. American College of Gastroenterology – Canadian Association of Gastroenterology clinical practice guideline: Management of anticoagulants and antiplatelets during acute gastrointestinal bleeding and the periendoscopic period. Am J Gastroenterol. 2022;117(4):542-58.
5. Boustière C et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2011;43(5):445-61.
6. Fujimoto K et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26(1):1-14.
7. Wilke T et al. Patient preferences for oral anticoagulation therapy in atrial fibrillation: A systematic literature review. Patient 2017;10(1):17-37.
8. Gerson LB et al. Adverse events associated with anticoagulation therapy in the periendoscopic period. Gastrointest Endosc. 2010 Jun;71(7):1211-17.e2.
9. Horiuchi A et al. Removal of small colorectal polyps in anticoagulated patients: A prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc 2014;79(3):417-23.
10. Lip GYH et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-72.
11. 2012 Writing Committee Members, Jneid H et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (Updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126(7):875-910.
12. Douketis JD et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e326S-e350S.
13. Becker RC et al. Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. J Am Coll Cardiol. 2009;54(24):2261-76.
14. Kwok A and Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009;104(12):3085-97; quiz 3098.
15. Douketis JD et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-33.
16. Kovacs MJ et al. Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): Double blind randomised controlled trial. BMJ 2021;373:n1205.
17. Tafur A and Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart 2018;104(17):1461-7.
18. Kato M et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30(4):433-40.
19. Inoue T et al. Clinical features of postpolypectomy bleeding associated with heparin bridge therapy. Dig Endosc. 2014;26(2):243-9.
20. Takeuchi Y et al. Continuous anticoagulation and cold snare polypectomy versus heparin bridging and hot snare polypectomy in patients on anticoagulants with subcentimeter polyps: A randomized controlled trial. Ann Intern Med. 2019;171(4):229-37.
21. Ara N et al. Prospective analysis of risk for bleeding after endoscopic biopsy without cessation of antithrombotics in Japan. Dig Endosc. 2015;27(4):458-64.
22. Yanagisawa N et al. Postpolypectomy bleeding and thromboembolism risks associated with warfarin vs. direct oral anticoagulants. World J Gastroenterol. 2018;24(14):1540-9.
23. Arimoto J et al. Safety of cold snare polypectomy in patients receiving treatment with antithrombotic agents. Dig Dis Sci. 2019;64(11):3247-55.
24. Heublein V et al. Gastrointestinal endoscopy in patients receiving novel direct oral anticoagulants: Results from the prospective Dresden NOAC registry. J Gastroenterol. 2018;53(2):236-46.
25. Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469-78.
26. Dubois V et al. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14.
27. Weitz JI et al. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation. 2012 Nov 13;126(20):2428-32.
28. Chan FKL et al. Risk of postpolypectomy bleeding with uninterrupted clopidogrel therapy in an industry-independent, double-blind, randomized trial. Gastroenterology. 2019;156(4):918-25.
29. Watanabe K et al. Effect of antiplatelet agent number, types, and pre-endoscopic management on postpolypectomy bleeding: Validation of endoscopy guidelines. Surg Endosc. 2021;35(1):317-25.
30. Gurbel PA et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation. 2009;120(25):2577-85.
31. Eisenberg MJ et al. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119(12):1634-42.
Antithrombotic therapy is increasingly used to either reduce the risk of or treat thromboembolic episodes in patients with various medical conditions such as ischemic and valvular heart disease, atrial fibrillation (AF), cerebrovascular disease, peripheral arterial disease, venous thromboembolism (VTE) and hypercoagulable diseases. Antithrombotics include medications classified as anticoagulants or antiplatelets. Anticoagulants work by interfering with the native clotting cascade and consist of four main classes: vitamin K antagonists (VKA), heparin derivatives, direct factor Xa inhibitors, and direct thrombin inhibitors. Direct oral anticoagulants (DOACs) refer to dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban).
Antiplatelets, on the other hand, work by decreasing platelet aggregation and thus preventing thrombus formation; they include P2Y12 receptor inhibitors, protease-activated receptor-1 inhibitors, glycoprotein IIb/IIIa receptor inhibitors, acetylsalicylic acid (ASA), and nonsteroidal anti-inflammatory drugs. All of these agents may directly cause or increase the risk of gastrointestinal (GI) bleeding from luminal sources such as ulcers or diverticula, as well as after endoscopic interventions such as polypectomy. However, there is also a risk of thromboembolic consequences if some of these agents are withheld. Thus, the management of patients receiving antithrombotic agents and undergoing GI endoscopy represents an important clinical challenge and something that every GI physician has to deal with routinely.
The goal of this review is to discuss the optimal strategy for managing antithrombotics in patients undergoing elective endoscopy based on current available evidence and published clinical guidelines.1-4 Much of our discussion will review recommendations from the recently published joint American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) guidelines on management of anticoagulants and antiplatelets in the periendoscopic period by Abraham et al.4
Factors that guide decision-making
The two most vital factors to consider prior to performing endoscopic procedures in patients receiving antithrombotic therapy are to assess the risk of bleeding associated with the procedure and to assess the risk of thromboembolism associated with the underlying medical condition for which the antithrombotic agents are being used. In addition, it is also important to keep in mind the individual characteristics of the antithrombotic agent(s) used when making these decisions.
Estimating procedure-related bleeding risk
Various endoscopic procedures have different risks of associated bleeding. Although guidelines from GI societies may differ when classifying procedures into low or high risk, it is important to know that most of the original data on postprocedural bleeding risks are from studies conducted in patients who are not on complex antithrombotic regimens and thus may not accurately reflect the bleeding risk of patients using newer antithrombotic therapies.1,4-7
Traditionally, some of the common low-risk procedures have included diagnostic EGD and colonoscopy with or without biopsy, ERCP without sphincterotomy, biliary stent placement, and push or balloon-assisted enteroscopy. On the other hand, endoscopic procedures associated with interventions are known to have higher bleeding risk, and other procedural factors can influence this risk as well.8 For example, polypectomy, one of the most common interventions during endoscopy, is associated with bleeding risk ranging from 0.3% to 10% depending on multiple factors including polyp size, location, morphology (nonpolypoid, sessile, pedunculated), resection technique (cold or hot forceps, cold or hot snare), and type of cautery used.9 For some procedures, such as routine screening colonoscopy, however, the preprocedure estimate of bleeding risk can be uncertain because it is unclear if a high risk intervention (e.g., polypectomy of large polyp) will be necessary. For example, in the most recent ACG/CAG guidelines, colonoscopy with polypectomy < 1cm is considered a low/moderate risk bleeding procedure, whereas polypectomy > 1cm is considered high risk for bleeding.4 In these situations, the management of antithrombotic medications may depend on the individual patient’s risk of thrombosis and the specific antithrombotic agent. In the example of a patient undergoing colonoscopy while on antithrombotic medications, the bleeding risk associated with polypectomy can potentially be reduced by procedural techniques such as preferential use of cold snare polypectomy. Further high-quality data on the optimal procedural technique to reduce postpolypectomy bleeding in patients on antithrombotic medications is needed to help guide management.
Estimating thromboembolic risk
The risk of thromboembolic events in patients who are withholding their antithrombotic therapy for an endoscopic procedure depends on their underlying condition and individual characteristics. In patients who are on antithrombotic therapy for stroke prevention in non-valvular AF, the risk of cerebral thromboembolism in these patients is predictable using the CHA2DS2Vasc index.10 This scoring index includes heart failure, hypertension, age 75 years or older, diabetes mellitus, prior stroke or transient ischemic attack (TIA), vascular disease, age 65-74 years, and sex categories.
Patients with previous VTE on anticoagulation or those who have mechanical heart valves may have different risk factors for thromboembolic episodes. Among patients with VTE, time from initial VTE, history of recurrent VTE with antithrombotic interruption, and presence of underlying thrombophilia are most predictive of future thromboembolic risk. And for patients with mechanical heart valves, presence of a mitral valve prosthesis, and the presence or absence of associated heart failure and AF determine the annual risk of thromboembolic events. Bioprosthetic valves are generally considered low risk.
In patients with coronary artery disease (CAD), high thrombosis risk scenarios with holding antiplatelets include patients within 3 months of an acute coronary syndrome (ACS) event, within 6 months of a drug-eluting stent (DES) placement, or within 1 month of a bare metal coronary stent (BMS) placement. In addition, patients with ACS that occurred within the past 12 months of DES placement or within 2 months of BMS placement are also considered high risk.11,12 Even beyond these periods, certain patients may still be at high risk of stent occlusion. In particular, patients with a prior history of stent occlusion, ACS or ST elevation myocardial infection, prior multivessel percutaneous coronary intervention, diabetes, renal failure, or diffuse CAD are at higher risk of stent occlusion or ACS events with alteration of antithrombotic therapy.13 Thus, modification of antithrombotic regimens in these patients should be cautiously approached.
Management of antithrombotics prior to elective endoscopy
In patients who need elective endoscopic procedures, if the indication for antithrombotic therapy is short-term, the procedure is probably best delayed until after that period.13 For patients on long-term or lifelong antithrombotic treatment, the decision to temporarily hold the treatment for endoscopy should occur after a discussion with the patient and all of the involved providers. In some high-risk patients, these agents cannot be interrupted; therefore, clinicians must carefully weigh the risks and benefits of the procedure before proceeding with endoscopy. For patients who are known to be undergoing an elective endoscopic procedure, antithrombotic medications may or may not need to be held prior to the procedure depending on the type of therapy. For example, according to the recent ACG/CAG guidelines, warfarin should be continued, whereas DOACs should be temporarily stopped for patients who are undergoing elective/planned endoscopic GI procedures.
Unfractionated heparin (UFH) administered as a continuous intravenous infusion can generally be held 3-4 hours before the procedure, given its short half-life. Low molecular weight heparin (LMWH), including enoxaparin and dalteparin, should be stopped 24 hours prior to the procedure.2,14 Fondaparinux is a synthetic X-a inhibitor that requires discontinuation at least 36 hours preceding a high risk procedure. For patients on warfarin who are undergoing elective endoscopic procedures that are low risk for inducing bleeding, warfarin can be continued, as opposed to temporarily interrupted, although the dose should be omitted the morning of the procedure.4 For those who are undergoing high-risk endoscopic procedures (including colonoscopy with possible polypectomy > 1 cm), 5 days of temporary interruption without periprocedural bridging is appropriate in most patients. This is contrary to previous guidelines, which had recommended bridging for patients with a CHA2DS2Vasc score ≥ 2. Recent impactful randomized trials (BRIDGE and PERIOP-2) have called into question the benefit of periprocedural bridging with LMWH. Avoiding bridging anticoagulation was generally found to be similar to bridging in regard to prevention of thromboembolic complications, but importantly was associated with a decreased risk of major bleeding.15,16 Of note, periprocedural bridging may still be appropriate in a small subset of patients, including those with mechanical valves, AF with CHADS2 score > 5, and previous thromboembolism during temporary interruption of VKAs. The decision to bridge or not should ideally be made in a multidisciplinary fashion.15-20
Data are lacking on the ideal strategy for periendoscopic DOAC management. As mentioned above, for patients on DOACs who are undergoing elective endoscopic GI procedures, temporarily interrupting DOACs rather than continuing them is recommended. Currently, there are no randomized controlled trials addressing the management of DOACs in the periendoscopic period. However, based on five cohort studies, the ideal duration of DOAC interruption before endoscopic procedures may be between 1 and 2 days, excluding the day of the procedure.21-25 This strategy allows for a short preprocedural duration of DOAC interruption and likely provides a balance between bleeding and thromboembolism risk. Importantly, there are no reliable laboratory assays to assess the anticoagulant effect of DOACs, and an individual patient’s degree of renal dysfunction may impact how long the DOAC should be held. In general, the anti-Xa drugs should be held for 1-2 days if the creatinine clearance (CrCl) is ≥ 60 mL/min, for 3 days if the CrCl is between 30 mL/min and 59 mL/min, and for 4 days if the CrCl is less than 30 mL/min.26 For edoxaban, the recommendation is to hold at least 24 hours before high-risk procedures. The recommendation for stopping dabigatran is 2-3 days before a high-risk procedure in patients with CrCl more than 80 mL/min, 3-4 days prior if between 30 and 49 mL/min, and 4-6 days prior if less than 30 mL/min respectively.27
In regard to antiplatelet management, ASA and the P2Y12 receptor inhibitors (e.g. clopidogrel, prasugrel, and ticagrelor) are the most commonly utilized antiplatelets in patients undergoing endoscopic procedures. For patients who are on ASA monotherapy, whether 81 mg or 325 mg daily, for secondary cardiovascular prevention, no interruption of ASA therapy is necessary for elective procedures. The benefit of ASA for secondary cardiovascular prevention and the possible reduction in thrombotic events seen in RCTs of nonendoscopic surgical procedures is well known. However, there may be certain exceptions in which aspirin should be temporarily held. For example, short-term interruption of ASA could be considered in high risk procedures such as biliary or pancreatic sphincterotomy, ampullectomy, and peroral endoscopic myotomy. For patients on single antiplatelet therapy with a P2Y12 receptor inhibitor who are undergoing elective endoscopic GI procedures, the recent CAG/ACG guidelines did not provide a clear recommendation for or against temporary interruption of the P2Y12 receptor inhibitor. Although interruption of a P2Y12 receptor inhibitor should theoretically decrease a patient’s risk of bleeding, the available evidence reported a nonsignificant increased bleeding risk in patients who stop a P2Y12 receptor inhibitor for an elective endoscopic procedure compared with those who continue the medication.28,29 Therefore, until further data are available, for patients on P2Y12 receptor monotherapy, a reasonable strategy would be to temporarily hold therapy prior to high risk endoscopic procedures, assuming the patients are not at high cardiovascular risk. Clopidogrel and prasugrel have to be stopped 5-7 days prior to allow normal platelet aggregation to resume as opposed to ticagrelor, a reversible P2Y12 receptor inhibitor that can be stopped 3-5 days prior.30
Lastly, for patients who are on dual antiplatelet therapy (DAPT) for secondary prevention, continuation of ASA and temporary interruption of the P2Y12 receptor inhibitor is recommended while undergoing elective endoscopy. Studies have shown that those who discontinued both had a much higher incidence of stent thrombosis compared with those who remained on aspirin alone.4,28,31
Resumption of antithrombotic therapy after endoscopy
In general, antithrombotic therapy should be resumed upon completion of the procedure unless there remains a persistent risk of major bleeding.1,14 This consensus is based on studies available on warfarin and heparin products, with minimal literature available regarding the resumption of DOACs. The benefits of immediate re-initiation of antithrombotic therapy for the prevention of thromboembolic events should be weighed against the risk of hemorrhage associated with the specific agent, the time to onset of the medication, and procedure-specific circumstances. For the small subset of patients on warfarin with a high risk of thromboembolism (e.g., mechanical heart valve), bridging with LMWH should be started at the earliest possible time when there is no risk of major bleeding and continued until the international normalized ratio (INR) reaches a therapeutic level with warfarin. For patients at a lower risk of thromboembolism, warfarin should be restarted within 24 hours of the procedure. In addition, because of the shorter duration of DOACs, if treatment with these agents cannot resume within 24 hours of a high-risk procedure, bridge therapy should be considered with UFH or LMWH in patients with a high risk of thrombosis.18 In patients receiving DOACs for stroke prophylaxis in AF, the DOACS can be safely resumed 1 day after low-risk procedures and 2-3 days after high-risk procedures without the need for bridging.25 All antiplatelet agents should be resumed as soon as hemostasis is achieved.
Conclusion
Antithrombotic therapy is increasingly used given the aging population, widespread burden of cardiovascular comorbidities, and expanding indications for classes of medications such as direct oral anticoagulants. Given the association with antithrombotic medications and gastrointestinal bleeding, it is essential for gastroenterologists to understand the importance, necessity, and timing when holding these medications for endoscopic procedures. Even with the practice guidelines available today to help clinicians navigate certain situations, each patient’s antithrombotic management may be different, and communication with the prescribing physicians and including patients in the decision-making process is essential before planned procedures.
Dr. Wang is a gastroenterology fellow at the University of Chicago. Dr. Sengupta is an associate professor at the University of Chicago. They reported no funding or conflicts of interest.
References
1. ASGE Standards of Practice Committee, Acosta RD et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83(1):3-16.
2. Veitch AM et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48(4):c1. doi: 10.1055/s-0042-122686.
3. Chan FKL et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: Joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67(3):405-17.
4. Abraham NS et al. American College of Gastroenterology – Canadian Association of Gastroenterology clinical practice guideline: Management of anticoagulants and antiplatelets during acute gastrointestinal bleeding and the periendoscopic period. Am J Gastroenterol. 2022;117(4):542-58.
5. Boustière C et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2011;43(5):445-61.
6. Fujimoto K et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26(1):1-14.
7. Wilke T et al. Patient preferences for oral anticoagulation therapy in atrial fibrillation: A systematic literature review. Patient 2017;10(1):17-37.
8. Gerson LB et al. Adverse events associated with anticoagulation therapy in the periendoscopic period. Gastrointest Endosc. 2010 Jun;71(7):1211-17.e2.
9. Horiuchi A et al. Removal of small colorectal polyps in anticoagulated patients: A prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc 2014;79(3):417-23.
10. Lip GYH et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-72.
11. 2012 Writing Committee Members, Jneid H et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (Updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126(7):875-910.
12. Douketis JD et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e326S-e350S.
13. Becker RC et al. Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. J Am Coll Cardiol. 2009;54(24):2261-76.
14. Kwok A and Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009;104(12):3085-97; quiz 3098.
15. Douketis JD et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-33.
16. Kovacs MJ et al. Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): Double blind randomised controlled trial. BMJ 2021;373:n1205.
17. Tafur A and Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart 2018;104(17):1461-7.
18. Kato M et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30(4):433-40.
19. Inoue T et al. Clinical features of postpolypectomy bleeding associated with heparin bridge therapy. Dig Endosc. 2014;26(2):243-9.
20. Takeuchi Y et al. Continuous anticoagulation and cold snare polypectomy versus heparin bridging and hot snare polypectomy in patients on anticoagulants with subcentimeter polyps: A randomized controlled trial. Ann Intern Med. 2019;171(4):229-37.
21. Ara N et al. Prospective analysis of risk for bleeding after endoscopic biopsy without cessation of antithrombotics in Japan. Dig Endosc. 2015;27(4):458-64.
22. Yanagisawa N et al. Postpolypectomy bleeding and thromboembolism risks associated with warfarin vs. direct oral anticoagulants. World J Gastroenterol. 2018;24(14):1540-9.
23. Arimoto J et al. Safety of cold snare polypectomy in patients receiving treatment with antithrombotic agents. Dig Dis Sci. 2019;64(11):3247-55.
24. Heublein V et al. Gastrointestinal endoscopy in patients receiving novel direct oral anticoagulants: Results from the prospective Dresden NOAC registry. J Gastroenterol. 2018;53(2):236-46.
25. Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469-78.
26. Dubois V et al. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14.
27. Weitz JI et al. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation. 2012 Nov 13;126(20):2428-32.
28. Chan FKL et al. Risk of postpolypectomy bleeding with uninterrupted clopidogrel therapy in an industry-independent, double-blind, randomized trial. Gastroenterology. 2019;156(4):918-25.
29. Watanabe K et al. Effect of antiplatelet agent number, types, and pre-endoscopic management on postpolypectomy bleeding: Validation of endoscopy guidelines. Surg Endosc. 2021;35(1):317-25.
30. Gurbel PA et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation. 2009;120(25):2577-85.
31. Eisenberg MJ et al. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119(12):1634-42.
AGA News – November 2022
Vaccine recommendations for your patients with IBD
It’s cold and flu season – are your patients with inflammatory bowel disease (IBD) properly informed about increased risk of infections?
It is especially important for patients with IBD to receive the flu vaccine every year. With IBD, you can only get the shot (and not the spray in the nose).
The AGA GI Patient Center provides additional recommendations about vaccines in adults with IBD. Talk to your patients with IBD about what vaccines are needed for their treatment regimen, age and sex.
Review vaccines and vaccine-preventable diseases the patient had when first diagnosed with IBD, no matter the age and continue discussing vaccines during regular health care visits.
Give patients vaccine(s) for infections they are not immune as soon as possible.
Make sure patients are up to date or receive any live vaccines prior to starting some immunosuppressive treatments, such as biologics.
Help drive HCV testing, treatment, and eradication
AGA has been working with the Centers for Medicare & Medicaid Services and the Centers for Disease Control and Prevention to help drive the national priority for hepatitis C virus (HCV) testing, treatment and eradication.
Updates to QPP measure 400
In July 2022, CMS contacted AGA to modify measure 400 – to update the coverage for one-time screening for HCV to include a referral for treatment for patients with positive antibodies. CMS also advised that confirmation of eradication is a priority and we have started working with the CDC to modify AGA’s sustained virologic response measure for future consideration in the Quality Payment Program.
The modifications to measure 400 have been drafted and given the substantial changes to the measure specification, the measure needs to be retested and submitted to the National Quality Forum for consideration by the Measures Application Partnership. The CMS contractor for this project, Mathematica, will be leading this testing initiative and selected test sites will qualify for up to $2,000 to participate.
Additionally, there will be a second phase of testing to use deidentified patient-level data to assess the measure’s validity and reliability, which will be contracted separately. Our hope is that testing sites recruited for the first phase of testing will stay on through the second phase.
Participants in the second phase of testing are expected to have at least 2 years of patient data available containing data elements needed to calculate the measure. Ideally, each of the participating clinicians at the prospective testing sites would see at least 40 confirmed HCV-positive cases annually.
Vaccine recommendations for your patients with IBD
It’s cold and flu season – are your patients with inflammatory bowel disease (IBD) properly informed about increased risk of infections?
It is especially important for patients with IBD to receive the flu vaccine every year. With IBD, you can only get the shot (and not the spray in the nose).
The AGA GI Patient Center provides additional recommendations about vaccines in adults with IBD. Talk to your patients with IBD about what vaccines are needed for their treatment regimen, age and sex.
Review vaccines and vaccine-preventable diseases the patient had when first diagnosed with IBD, no matter the age and continue discussing vaccines during regular health care visits.
Give patients vaccine(s) for infections they are not immune as soon as possible.
Make sure patients are up to date or receive any live vaccines prior to starting some immunosuppressive treatments, such as biologics.
Help drive HCV testing, treatment, and eradication
AGA has been working with the Centers for Medicare & Medicaid Services and the Centers for Disease Control and Prevention to help drive the national priority for hepatitis C virus (HCV) testing, treatment and eradication.
Updates to QPP measure 400
In July 2022, CMS contacted AGA to modify measure 400 – to update the coverage for one-time screening for HCV to include a referral for treatment for patients with positive antibodies. CMS also advised that confirmation of eradication is a priority and we have started working with the CDC to modify AGA’s sustained virologic response measure for future consideration in the Quality Payment Program.
The modifications to measure 400 have been drafted and given the substantial changes to the measure specification, the measure needs to be retested and submitted to the National Quality Forum for consideration by the Measures Application Partnership. The CMS contractor for this project, Mathematica, will be leading this testing initiative and selected test sites will qualify for up to $2,000 to participate.
Additionally, there will be a second phase of testing to use deidentified patient-level data to assess the measure’s validity and reliability, which will be contracted separately. Our hope is that testing sites recruited for the first phase of testing will stay on through the second phase.
Participants in the second phase of testing are expected to have at least 2 years of patient data available containing data elements needed to calculate the measure. Ideally, each of the participating clinicians at the prospective testing sites would see at least 40 confirmed HCV-positive cases annually.
Vaccine recommendations for your patients with IBD
It’s cold and flu season – are your patients with inflammatory bowel disease (IBD) properly informed about increased risk of infections?
It is especially important for patients with IBD to receive the flu vaccine every year. With IBD, you can only get the shot (and not the spray in the nose).
The AGA GI Patient Center provides additional recommendations about vaccines in adults with IBD. Talk to your patients with IBD about what vaccines are needed for their treatment regimen, age and sex.
Review vaccines and vaccine-preventable diseases the patient had when first diagnosed with IBD, no matter the age and continue discussing vaccines during regular health care visits.
Give patients vaccine(s) for infections they are not immune as soon as possible.
Make sure patients are up to date or receive any live vaccines prior to starting some immunosuppressive treatments, such as biologics.
Help drive HCV testing, treatment, and eradication
AGA has been working with the Centers for Medicare & Medicaid Services and the Centers for Disease Control and Prevention to help drive the national priority for hepatitis C virus (HCV) testing, treatment and eradication.
Updates to QPP measure 400
In July 2022, CMS contacted AGA to modify measure 400 – to update the coverage for one-time screening for HCV to include a referral for treatment for patients with positive antibodies. CMS also advised that confirmation of eradication is a priority and we have started working with the CDC to modify AGA’s sustained virologic response measure for future consideration in the Quality Payment Program.
The modifications to measure 400 have been drafted and given the substantial changes to the measure specification, the measure needs to be retested and submitted to the National Quality Forum for consideration by the Measures Application Partnership. The CMS contractor for this project, Mathematica, will be leading this testing initiative and selected test sites will qualify for up to $2,000 to participate.
Additionally, there will be a second phase of testing to use deidentified patient-level data to assess the measure’s validity and reliability, which will be contracted separately. Our hope is that testing sites recruited for the first phase of testing will stay on through the second phase.
Participants in the second phase of testing are expected to have at least 2 years of patient data available containing data elements needed to calculate the measure. Ideally, each of the participating clinicians at the prospective testing sites would see at least 40 confirmed HCV-positive cases annually.

















