User login
Approach to dysphagia
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).
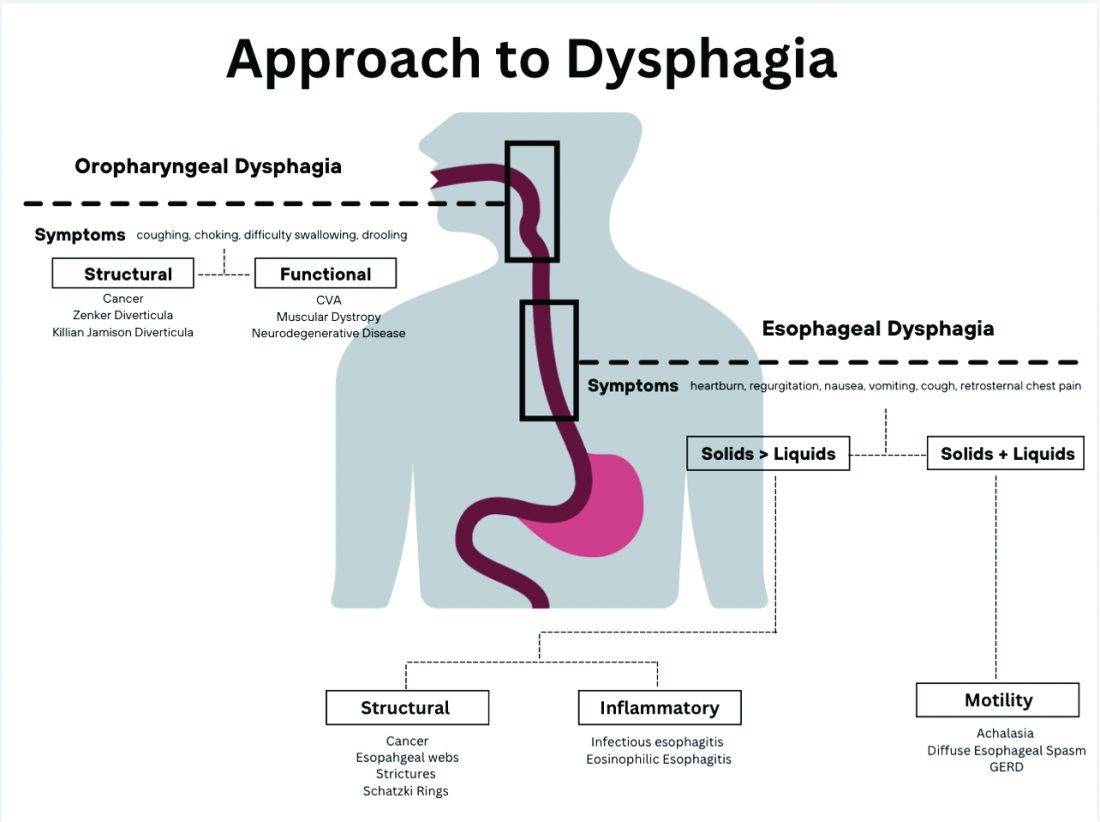
Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Introduction
Dysphagia is the sensation of difficulty swallowing food or liquid in the acute or chronic setting. The prevalence of dysphagia ranges based on the type and etiology but may impact up to one in six adults.1,2 Dysphagia can cause a significant impact on a patient’s health and overall quality of life. A recent study found that only 50% of symptomatic adults seek medical care despite modifying their eating habits by either eating slowly or changing to softer foods or liquids.1 The most common, serious complications of dysphagia include aspiration pneumonia, malnutrition, and dehydration.3 According to the Agency for Healthcare Research and Quality, dysphagia may be responsible for up to 60,000 deaths annually.3
The diagnosis of esophageal dysphagia can be challenging. An initial, thorough history is essential to delineate between oropharyngeal and esophageal dysphagia and guide subsequent diagnostic testing. In recent years, there have been a number of advances in the approach to diagnosing dysphagia, including novel diagnostic modalities. The goal of this review article is to discuss the current approach to esophageal dysphagia and future direction to allow for timely diagnosis and management.
History
The diagnosis of dysphagia begins with a thorough history. Questions about the timing, onset, progression, localization of symptoms, and types of food that are difficult to swallow are essential in differentiating oropharyngeal and esophageal dysphagia.3,4 Further history taking must include medication and allergy review, smoking history, and review of prior radiation or surgical therapies to the head and neck.
Briefly, oropharyngeal dysphagia is difficulty initiating a swallow or passing food from the mouth or throat and can be caused by structural or functional etiologies.5 Clinical presentations include a sensation of food stuck in the back of the throat, coughing or choking while eating, or drooling. Structural causes include head and neck cancer, Zenker diverticulum, Killian Jamieson diverticula, prolonged intubation, or changes secondary to prior surgery or radiation.3 Functional causes may include neurologic, rheumatologic, or muscular disorders.6
Esophageal dysphagia refers to difficulty transporting food or liquid down the esophagus and can be caused by structural, inflammatory, or functional disorders.5 Patients typically localize symptoms of heartburn, regurgitation, nausea, vomiting, cough, or chest pain along the sternum or epigastric region. Alarm signs concerning for malignancy include unintentional weight loss, fevers, or night sweats.3,7 Aside from symptoms, medication review is essential, as dysphagia is a common side effect of antipsychotics, anticholinergics, antimuscarinics, narcotics, and immunosuppressant drugs.8 Larger pills such as NSAIDs, antibiotics, bisphosphonates, potassium supplements, and methylxanthines can cause drug-induced esophagitis, which can initially present as dysphagia.8 Inflammatory causes can be elucidated by obtaining a history about allergies, tobacco use, and recent infections such as thrush or pneumonia. Patients with a history of recurrent pneumonias may be silently aspirating, a complication of dysphagia.3 Once esophageal dysphagia is clinically suspected based on history, workup can begin.
Differentiating etiologies of esophageal dysphagia
The next step in diagnosing esophageal dysphagia is differentiating between structural, inflammatory, or dysmotility etiology (Figure 1).

Patients with a structural cause typically have difficulty swallowing solids but are able to swallow liquids unless the disease progresses. Symptoms can rapidly worsen and lead to odynophagia, weight loss, and vomiting. In comparison, patients with motility disorders typically have difficulty swallowing both solids and liquids initially, and symptoms can be constant or intermittent.5
Prior to diagnostic studies, a 4-week trial of a proton pump inhibitor (PPI) is appropriate for patients with reflux symptoms who are younger than 50 with no alarm features concerning for malignancy.7,9 If symptoms persist after a PPI trial, then an upper endoscopy (EGD) is indicated. An EGD allows for visualization of structural etiologies, obtaining biopsies to rule out inflammatory etiologies, and the option to therapeutically treat reduced luminal diameter with dilatation.10 The most common structural and inflammatory etiologies noted on EGD include strictures, webs, carcinomas, Schatzki rings, and gastroesophageal reflux or eosinophilic esophagitis.4
If upper endoscopy is normal and clinical suspicion for an obstructive cause remains high, barium esophagram can be utilized as an adjunctive study. Previously, barium esophagram was the initial test to distinguish between structural and motility disorders. The benefits of endoscopy over barium esophagram as the first diagnostic study include higher diagnostic yield, higher sensitivity and specificity, and lower costs.7 However, barium studies may be more sensitive for lower esophageal rings or extrinsic esophageal compression.3
Evaluation of esophageal motility disorder
If a structural or inflammatory etiology of dysphagia is not identified, investigation for an esophageal motility disorder (EMD) is warranted. Examples of motility disorders include achalasia, ineffective esophageal motility, hypercontractility, spasticity, or esophagogastric junction outflow obstruction (EGJOO).10,11 High-resolution esophageal manometry (HRM) remains the gold standard in diagnosis of EMD.12 An HRM catheter utilizes 36 sensors placed two centimeters apart and is placed in the esophagus to evaluate pressure and peristalsis between the upper and lower esophageal sphincters.13 In 2009, the Chicago Classification System was developed to provide a diagnostic algorithm that categorizes EMD based on HRM testing, with the most recent version (4.0) being published in 2020.12,14 Motility diagnoses are divided into two general classifications of disorders of body peristalsis and disorders of EGJ outflow. The most recent updates also include changes in swallow protocols, patient positioning, targeted symptoms, addition of impedance sensors, and consideration of supplemental testing when HRM is inconclusive based on the clinical context.12 There are some limitations of HRM to highlight. One of the main diagnostic values used with HRM is the integrated relaxation pressure (IRP). Despite standardization, IRP measurements vary based on the recorder and patient position. A minority of patients with achalasia may have IRP that does not approach the accepted cutoff and, therefore, the EGJ is not accurately assessed on HRM.15,16 In addition, some swallow protocols have lower sensitivity and specificity for certain motility disorders, and the test can result as inconclusive.14 In these scenarios, supplemental testing with timed barium esophagram or functional luminal imaging probe (EndoFLIP) is indicated.10,11
Over the past decade, EndoFLIP has emerged as a novel diagnostic tool in evaluating EMD. EndoFLIP is usually completed during an upper endoscopy and utilizes impedance planimetry to measure cross-sectional area and esophageal distensibility and evaluate contractile patterns.16 During the procedure, a small catheter with an inflatable balloon is inserted into the esophagus with the distal end in the stomach, traversing the esophagogastric junction (EGJ). The pressure transducer has electrodes every centimeter to allow for a three-dimensional construction of the esophagus and EGJ.17 EndoFLIP has been shown to accurately measure pyloric diameter, pressure, and distensibility at certain balloon volumes.18 In addition, FLIP is being used to further identify aspects of esophageal dysmotility in patients with eosinophilic esophagitis, thought primarily to be an inflammatory disorder.19 However, limitations include minimal accessibility of EndoFLIP within clinical practice and a specific computer program needed to generate the topographic plots.20
When used in conjunction with HRM, EndoFLIP provides complementary data that can be used to better detect major motility disorders.15,20,21 Each study adds unique information about the different physiologic events comprising the esophageal response to distention. Overall, the benefits of EndoFLIP include expediting workup during index endoscopy, patient comfort with sedation, and real-time diagnostic data that supplement results obtained during HRM.10,16,20,2223
Of note, if the diagnostic evaluation for structural, inflammatory, and motility disorders are unrevealing, investigating for atypical reflux symptoms can be pursued for patients with persistent dysphagia. Studies investigating pH, or acidity in the esophagus, in relation to symptoms, can be conducted wirelessly via a capsule fixed to the mucosa or with a nasal catheter.3
Normal workup – hypervigilance
In a subset of patients, all diagnostic testing for structural, inflammatory, or motility disorders is normal. These patients are classified as having a functional esophageal disorder. Despite normal testing, patients still have significant symptoms including epigastric pain, chest pain, globus sensation, or difficulty swallowing. It is theorized that a degree of visceral hypersensitivity between the brain-gut axis contributes to ongoing symptoms.24 Studies for effective treatments are ongoing but typically include cognitive-behavioral therapy, brain-gut behavioral therapy, swallow therapy antidepressants, or short courses of proton pump inhibitors.9
Conclusion
In this review article, we discussed the diagnostic approach for esophageal dysphagia. Initial assessment requires a thorough history, differentiation between oropharyngeal and esophageal dysphagia, and determination of who warrants an upper endoscopy. Upper endoscopy may reveal structural or inflammatory causes of dysphagia, including strictures, masses, or esophagitis, to name a few. If a structural or inflammatory cause is ruled out, this warrants investigation for esophageal motility disorders. The current gold standard for diagnosing EMD is manometry, and supplemental studies, including EndoFLIP, barium esophagram, and pH studies, may provide complimentary data. If workup for dysphagia is normal, evaluation for esophageal hypervigilance causing increased sensitivity to normal or mild sensations may be warranted. In conclusion, the diagnosis of dysphagia is challenging and requires investigation with a systematic approach to ensure timely diagnosis and treatment
Dr. Ronnie and Dr. Bloomberg are in the department of internal medicine at Loyola University Chicago, Maywood, Ill. Dr. Venu is in the division of gastroenterology at Loyola. He is on the speakers bureau at Medtronic.
References
1. Adkins C et al. Clin Gastroenterol Hepatol. 2020;18(9):1970-9.e2.
2. Bhattacharyya N. Otolaryngol Head Neck Surg. 2014;151(5):765-9.
3. McCarty EB and Chao TN. Med Clin North Am. 2021;105(5):939-54.
4. Thiyagalingam S et al. Mayo Clin Proc. 2021;96(2):488-97.
5. Malagelada JR et al. J Clin Gastroenterol. 2015;49(5):370-8.
6. Rommel, N and Hamdy S. Nat Rev Gastroenterol Hepatol. 2016;13(1):49-59.
7. Liu LWC et al. J Can Assoc Gastroenterol. 2018;1(1):5-19.
8. Schwemmle C et al. HNO. 2015;63(7):504-10.
9. Moayyedi P et al. Am J Gastroenterol. 2017;112(7):988-1013.
10. Triggs J and Pandolfino J. F1000Res. 2019 Aug 29. doi: 10.12688/f1000research.18900.1.
11. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14058.
12. Yadlapati R et al. Neurogastroenterol Motil. 2021;33(1):e14053.
13. Fox M et al. Neurogastroenterol Motil. 2004;16(5):533-42.
14. Sweis R and Fox M. Curr Gastroenterol Rep. 2020;22(10):49.
15. Carlson DA et al. Gastroenterology. 2015;149(7):1742-51.
16. Donnan EN and Pandolfino JE. Gastroenterol Clin North Am. 2020;49(3):427-35.
17. Carlson DA. Curr Opin Gastroenterol. 2016;32(4):310-8.
18. Zheng T et al. Neurogastroenterol Motil. 2022;34(10):e14386.
19. Carlson DA et al. Clin Gastroenterol Hepatol. 2022;20(8):1719-28.e3.
20. Carlson DA et al. Am J Gastroenterol. 2016;111(12):1726-35.
21. Carlson DA et al. Neurogastroenterol Motil. 2021;33(10):e14116.
22. Carlson DA et al. Gastrointest Endosc. 2019;90(6):915-923.e1.
23. Fox MR et al. Neurogastroenterol Motil. 2021;33(4):e14120.
24. Aziz Q et al. Gastroenterology. 2016 Feb 15. doi: 10.1053/j.gastro.2016.02.012.
Spring reflections
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Dear friends,
I celebrate my achievements (both personal and work related), try not to be too hard on myself with unaccomplished tasks, and plan goals for the upcoming year. Most importantly, it’s a time to be grateful for both opportunities and challenges. Thank you for your engagement with The New Gastroenterologist, and as you go through this issue, I hope you can find time for some spring reflections as well!
In this issue’s In Focus, Dr. Tanisha Ronnie, Dr. Lauren Bloomberg, and Dr. Mukund Venu break down the approach to a patient with dysphagia, a common and difficult encounter in GI practice. They emphasize the importance of a good clinical history as well as understanding the role of diagnostic testing. In our Short Clinical Review section, Dr. Noa Krugliak Cleveland and Dr. David Rubin review the rising role of intestinal ultrasound in inflammatory bowel disease, how to be trained, and how to incorporate it in clinical practice.
As early-career gastroenterologists, Dr. Samad Soudagar and Dr. Mohammad Bilal were tasked with establishing an advanced endoscopy practice, which may be overwhelming for many. They synthesized their experiences into 10 practical tips to build a successful practice. Our Post-fellowship Pathways article highlights Dr. Katie Hutchins’s journey from private practice to academic medicine; she provides insights into the life-changing decision and what she learned about herself to make that pivot.
In our Finance section, Dr. Kelly Hathorn and Dr. David Creighton reflect on navigating as new parents while both working full time in medicine; their article weighs the pros and cons of various childcare options in the post–COVID pandemic world.
In an additional contribution this issue, gastroenterology and hepatology fellowship program leaders at the University of Florida, Gainesville, describe their experience with virtual recruitment, including feedback from their candidates, especially as we enter another cycle of GI Match.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are without appreciating where we were: The first formalized gastroenterology fellowship curriculum was a joint publication by four major GI and hepatology societies in 1996 – just 27 years ago!
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of gastroenterology & hepatology
University of North Carolina at Chapel Hill
Why my independent GI practice started a GI fellowship program
In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

In this video Naresh Gunaratnam, MD, discusses the gastroenterology fellowship program that Huron Gastroenterology developed with Trinity Health in Ann Arbor, Mich. Dr. Gunaratnam helped create the program because he and his colleagues felt that traditional fellowship programs don’t always provide information or guidance about non-academic career pathways in gastroenterology. Hear from Dr. Gunaratnam how the fellowship program at Huron Gastroenterology is training fellows to become excellent clinicians who care for patients in the community setting. He has no financial conflicts relative to the topics in this video.

AGA News – May 2023
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market.

The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.

Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”

In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market.

The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.

Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”

In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Season 2 of Small Talk, Big Topics is here!
AGA’s podcast for trainees and early career GIs, Small Talk, Big Topics, is back for season two. To kick off the new season, hosts Drs. Matthew Whitson, Nina Nandy, and CS Tse sit down with AGA President Dr. John Carethers in a two-part special to chat about his career and how his involvement with AGA has impacted him.
In episode one, Drs. Whitson, Nandy and Tse take a deep dive with Dr. Carethers to reflect on how he first got involved with AGA, his experience with different committees, and how those roles paved the way to leadership positions.
Now, as president, he says, “I am having so much fun. AGA has been with me for my entire GI career. It’s really the voice of the science and practice of gastroenterology.”
In episode two, Dr. Carethers examines the career advice he’s received, how it shaped his leadership style and provides guidance to early career GIs.
“What’s important about some of these higher-level [decisions] is to set a vision. You can’t be a leader if you have no followers, and people have to believe in something, that they’re moving toward something.”
Listen to more of Dr. Carethers’ insight in the first two episodes of Small Talk, Big Topics wherever you listen to podcasts and subscribe to stay up to date on new episodes.
Maximize your first day at DDW® 2023
Held during the first day of Digestive Disease Week®, this year’s AGA Postgraduate Course will be held live on Saturday, May 6, from 8:30 a.m. to 5 p.m. CT. This year’s theme – Advances in Gastroenterology: News You Can Use – will help you cut through the noise surrounding best practices for GI physicians.
Pricing is the same for both in-person and virtual attendees, giving you the flexibility to experience the course in-person or from the comfort of your home. All registrants will have on-demand access to the course for three months and the opportunity to earn up to 17.5 total credits when you complete all on-demand content.
What’s new this year?
General session format
Presentations will be given in an engaging format that will feel less didactic and more akin to a discussion among faculty, or a conversation with the experts! It’s also an exciting opportunity to mix junior and senior lecturers on the same platform.
Recent clinical practices
Session panelists will work together to select the key papers in their topic areas for discussion. Only the newest — within one year — and most important papers, clinical guidelines and pathways in the field will be selected.
Register to attend DDW and the Postgraduate Course today.
And the winner of this year’s Shark Tank is …
The 13th annual AGA Tech Summit took place in San Francisco, Calif., recently, bringing together GI entrepreneurs, clinicians, medical technology companies, venture capitalists, and regulatory agencies working to improve patient care in the field. A highlight of the event is the annual Shark Tank competition, where forward-thinking companies showcase and pitch their innovations to a panel of expert judges.
Congratulations to this year’s winner – Endiatx!
From devices providing rapid cancer detection to technology that makes endoscopy safer, the five companies selected for the 2023 AGA Shark Tank represented a glimpse of the future of GI patient care.
While each team offered a creative solution to modern-day GI challenges, only one could be declared the winner. Congratulations to our 2023 winner, Endiatx! Endiatx will represent AGA in the upcoming Shark Tank competition at DDW®.
Endiatx has developed a vitamin-sized intrabody robot
PillBot is a miniature robotic capsule endoscopy. Shipped to a patient’s home or picked up from a pharmacy, the standard size capsule is swallowed and then controlled by an external joystick-like device or a phone app by a physician in a physically separate location. Using real-time video transmissions visible to both operator and patient, the capsule navigates the entire stomach in a few minutes without anesthesia and ultimately is excreted outside the body without the need for recapture.
Future GI physician innovators
This year the AGA Center for GI Innovation and Technology (CGIT) welcomed 22 first-year to advanced endoscopy fellows to the AGA Innovation Fellows Program. The program provides a unique opportunity for the fellows to learn from GI clinicians, innovators, entrepreneurs, and medical technology executives on how new technologies are developed and brought to market.

The fellows received an exclusive behind-the-scenes tour of Medtronic’s R&D facility in Santa Clara, Calif., and got to experience hands-on demonstrations of GI GeniusTM, PillCamTM, EndoflipTM, NexpowderTM, BravoTM, BarrxTM and ProdiGITM technologies. The group was also hosted by Boston Scientific Corporation, Castle Biosciences and PENTAX Medical at a dinner that included an innovators panel discussion. The program will continue throughout the year with monthly educational sessions moderated by members of the AGA CGIT committee.
- Mohd Amer Alsamman, MD, Georgetown University
- Mohammad Arfeen, MD, Franciscan Health Olympia Fields
- Alexis Bayudan, MD, University of California, San Francisco
- Aileen Bui, MD, University of Southern California
- Divya Chalikonda, MD, Thomas Jefferson University Hospital
- Alec Faggen, MD, University of California, San Francisco
- Sweta Ghosh, PhD, University of Louisville School of Medicine
- Hemant Goyal, MD, University of Texas Houston
- Averill Guo, MD, Brown University
- Omar Jamil, MD, University of Chicago
- Christina Kratschmer, MD, Washington University in St. Louis
- Thi Khuc, MD, University of Maryland School of Medicine
- Anand Kumar, MD, Northwell Health – Lenox Hill Hospital
- Xing Li, MD, Massachusetts General Hospital
- Alana Persaud, MD, SUNY Downstate Medical Center
- Itegbemie Obaitan, MD, Indiana University School of Medicine
- Chethan Ramprasad, MD, University of Pennsylvania
- Abhishek Satishchandran, MD, University of Michigan
- Kevin Shah, MD, Emory University School of Medicine
- Shifa Umar, MD, University of Chicago
- Kornpong Vantanasiri, MD, Mayo Clinic Rochester
- Shaleen Vasavada, MD, Baylor College of Medicine
Highlights from social media
See what else attendees shared with #AGATech on Twitter.
The 2023 AGA Tech Summit was made possible by support from Castle Biosciences and Medtronic (Diamond Sponsors), AI Medical Services, Boston Scientific, Exact Sciences Corporation, FUJIFILM Medical Systems and Olympus Corporation (Gold Sponsors), Cook Medical Inc., and STERIS Endoscopy (Silver Sponsors), and Apollo Endosurgery and EvoEndo (Bronze Sponsors).
AGA takes CRC month to Capitol Hill
Participating in Colorectal Cancer Awareness Month in Washington, D.C., means one thing – taking the fight to save lives from CRC to Capitol Hill and advocating for increased access to screening and research to improve outcomes.

In March, AGA joined the national advocacy organization Fight Colorectal Cancer (Fight CRC) and partners in the colorectal cancer community for events in our nation’s capital. The goal was to destigmatize talking about gut health and CRC and to collaboratively develop solutions that will improve and increase access to CRC screening.

Fight CRC working lunch
Former AGA president Dr. David Lieberman and fellow AGA member and FORWARD graduate Dr. Fola May served as facilitators for the coalition of public and private leaders assembled by Fight CRC. The group is working to develop an action plan to further equitable CRC screening and lower the number of lives impacted by CRC. Among the participants were insurers, industry, federal agencies, healthcare providers, retail businesses, and patients.
White House Cancer Moonshot colorectal cancer forum
In partnership with President Biden’s reignited Cancer Moonshot initiative, we joined Fight CRC and other advocacy and industry leaders in the colorectal cancer community for the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House.
Dr. May participated as a panelist during the forum and discussed how we should address disparities in CRC. “Research dollars are essential in [combating CRC inequity]. We do not know how to effectively deliver care and preventive services to these populations unless we do deep dives into these particular settings to understand how to best deliver that care. This is not a “pick a model and apply broadly” approach. We need to go to the people, and we need to go to the people with the methods that work for that particular setting, and that’s going to be different in every community.”

In addition to Dr. Lieberman, who attended on behalf of AGA, fellow AGA members Drs. Austin Chiang, Swati Patel and AGA FORWARD Scholar Rachel Issaka were in attendance. We are appreciative of the opportunity to be included in these important discussions with the Administration and partners in the CRC community as we work together to reduce the burden of CRC and save lives.
Fight CRC United in Blue rally on the National Mall
It’s become an annual tradition for us to join Fight CRC’s United in Blue rally and blue flag installation on the National Mall, and this year was no different. We joined industry and patient advocacy groups in the CRC community to raise our voices about the need for screening, research, and advocacy to improve colon cancer outcomes.
The rally included inspiring calls to action and CRC testimonials from individuals who have been personally impacted by the disease, including Rep. Donald Payne Jr. (D-NJ), who lost his father to CRC and who personally underwent screening, which led to the discovery of 13 polyps.
Dr. Manish Singla from Capital Digestive Care spoke on behalf of AGA and provided encouragement and a reminder for patients and providers.
“What I keep hearing here is patients feel like they’re not being heard – so we’re listening. We’re trying and we’re here to fight the disease with you all. Everyone here knows somebody who is due for a colonoscopy and isn’t getting it, so use your persuasion – talk about it, convince, cajole, shame – use whatever you need so that everyone gets the screenings they need,” Dr. Singla said.
Our work is just beginning: Let’s work together to encourage screenings for colorectal cancer and save lives. Join us as we remind everyone that 45 is the new 50.
Establishing an advanced endoscopy practice: Tips for trainees and early faculty
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
Establishing an advanced endoscopy practice can appear challenging and overwhelming. It is often the culmination of more than a decade of education and training for advanced endoscopists and is usually their first foray into employment. all while creating a rewarding opportunity to provide a population with necessary services, which, more than likely, were not previously being offered at your institution or in your region.
Tip 1: Understand the current landscape
When joining a hospital-employed or private practice, it is important for the advanced endoscopist to gauge the current landscape of the job, beginning with gaining an understanding of the current services provided by your gastroenterology colleagues. This includes knowing the types of advanced endoscopy services previously provided, especially if you have partners or colleagues who perform these procedures, and their prior referral patterns, either within or outside their respective group. Also, it is important to understand the services that are provided locally at other institutions. This will allow you to develop a niche of the types of services you can provide that are not available in the current practice set-up.
Tip 2: Connect with peers, interspecialty collaborators, and referring physicians
It is important that you connect with your GI colleagues once you start a new job. This can differ in ease depending on the size of your group. For example, in a small group, it may be easier to familiarize yourself with your colleagues through regular interactions. If you are a part of a larger practice, however, it is necessary to be more proactive and set up introductory meetings/sessions. These interactions provide a great opportunity to share your goals and start building a relationship.
Efforts also should be made to reach out to primary care, hematology/oncology, surgical/radiation oncology, general surgery, and interventional radiology physicians, as these are the specialists with whom an advanced endoscopist typically has the most interaction. The relationship with these colleagues is bidirectional, as the majority of our patients need multidisciplinary decision-making and care. For example, the first time you speak to the colorectal surgeon at your institution should not be in the middle of a complication. The purpose of these introductions should not be solely to inform them of the services you are offering but to start developing a relationship in a true sense, because eventually those relationships will transform into excellent patient care.
Tip 3: Communication
Communication is a key principle in building a practice. Referring physicians often entrust you with managing a part of their patient’s medical problems. Patient/procedure outcomes should be relayed promptly to referring physicians, as this not only helps build the trust of the referring physician, but also enhances the patient’s trust in the health system, knowing that all physicians are communicating with the common goal of improving the patient’s disease course.
Communication with the referring physician is important not only after a procedure but also before it. Know that a consult is an “ask for help.” For example, even if you are not the correct specialist for a referral (for example, an inflammatory bowel disease patient was sent to an advanced endoscopist), it is good practice to take ownership of the patient and forward that person to the appropriate colleague.
Tip 4: Build a local reputation
Building upon this, it is also important to connect with other GI groups in the community, regardless of whether they have their own affiliated advanced endoscopists. This helps determine the advanced endoscopy services being offered regionally, which will further allow an understanding of the unmet needs of the region. In addition, building a relationship with local advanced endoscopists in the region can help establish a collaborative relationship going forward, rather than a contentious/competitive dynamic.
Tip 5: Advance your skills
As advanced endoscopy fellows are aware, completing an advanced endoscopy fellowship allows for building a strong foundation of skills, which will continue to refine and grow as you advance in your career.
Depending on your skill-set and training, the first year should focus on developing and establishing “your style” (since the training is tailored to follow the practice patterns of your mentors). The first few months are good to focus on refining endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, endoscopic mucosal resection, and luminal stenting techniques. As you start to build a reputation of being “safe, thoughtful, and skilled” and depending on your interests and goals, continued engagement in the advanced endoscopy community to understand new technologies/procedures is helpful. It is important to remember that new skills and procedures can be introduced in your practice, but this should be done in a timely and patient manner. You should appropriately educate and train yourself for such procedures through educational conferences/courses, shadowing and routine engagement with mentors, and collaboration with industry partners.
Tip 6: Team building
From a procedural standpoint, certain staff members should be recognized to be part of or lead an “advanced endoscopy team,” with a goal of dedicated exposure to a high volume of complex procedures. This builds camaraderie and trust within the team of advanced endoscopy nurses and technicians going forward, which is crucial to introducing and building a high-complexity procedural service. This is also an excellent opportunity to partner with our industry colleagues to ensure that they can train your team on the use of novel devices.
Tip 7: Offering new services to your patients
Advanced endoscopy is a rapidly evolving specialty, and new procedures, technology, and devices are allowing us to provide minimally invasive options to our patients. It is important that prior to introducing new services and programs, your hospital/practice administration should be informed about any such plans. Also, all potential collaborating services (surgery, interventional radiology, etc.) should be part of the decision-making to ensure patients receive the best possible multidisciplinary care.
Tip 8: Mentorship and peer-mentorship
Establishing a network of regional and national advanced endoscopy colleagues and mentors is critical. This may be harder to develop in community-based and private practice, where one may feel that they are on an “island.” Engagement with national organizations, use of social media, and other avenues are excellent ways to build this network. Advanced endoscopic procedures also are associated with higher rates of adverse events, so having a peer-support group to provide emotional and moral support when these adverse events occur also is important. Such a network also includes those collaborating specialties to which you would refer (surgical oncology, thoracic surgery, etc.). Being involved in local tumor boards and “gut clubs” is another way of remaining engaged and not feeling isolated.
Tip 9: Have fun
Advanced endoscopy can be busy, as well as physically and mentally exhausting. It is important to maintain a good work-life balance. In addition, planning scheduled retreats or social events with your advanced endoscopy team (nurses, technicians, schedulers, colleagues) is important not only to show appreciation, but also to help build camaraderie and develop relationships.
Tip 10: Remember your ‘why’
Often times, there can be stressors associated with building a practice and increasing your volume, therefore, it is always important to remember why you became a medical professional and advanced endoscopist. This will get you through the days where you had a complication or when things didn’t go as planned.
Conclusion
Lastly, it is important to keep revisiting your skill sets and practice and evaluate what is working well and what can be improved. To all the advanced endoscopists starting their careers: Be patient and have a positive attitude! The leaders in our field did not become so overnight, and an advanced endoscopy–based career resembles a marathon rather than a sprint. Mistakes during procedures and practice building can be made, but how you grow and learn from those mistakes is what determines how likely you are to succeed going forward. Respect and acknowledge your staff, your collaborating physicians, and mentors. It takes time and effort to develop an advanced endoscopy practice. Being proud of your achievements and promoting procedural and patient care advances that you have made are beneficial and encouraged. We are fortunate to be in an ever-evolving specialty, and it is an exciting time to be practicing advanced endoscopy. Good luck!
Dr. Soudagar is a gastroenterologist at Northwestern Medical Group, Lake Forest, Ill. Dr. Bilal, assistant professor of medicine at the University of Minnesota, Minneapolis, is an advanced endoscopist and gastroenterologist at Minneapolis VA Medical Center. The authors have no conflicts of interest.
Navigating your childcare options in a post-COVID world
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.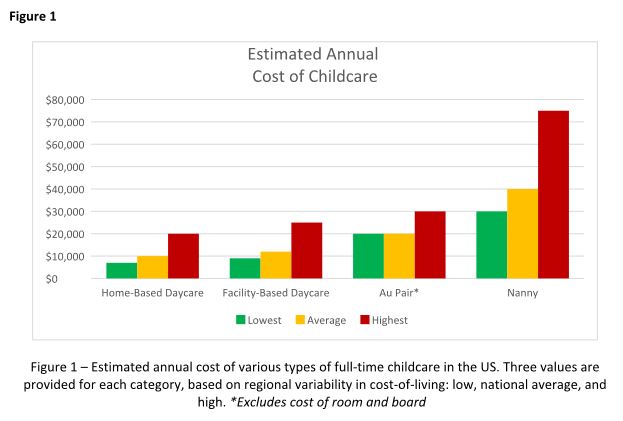
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
From private practice to academic medicine: My journey and lessons learned
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Integrating intestinal ultrasound into inflammatory bowel disease training and practice in the United States
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)
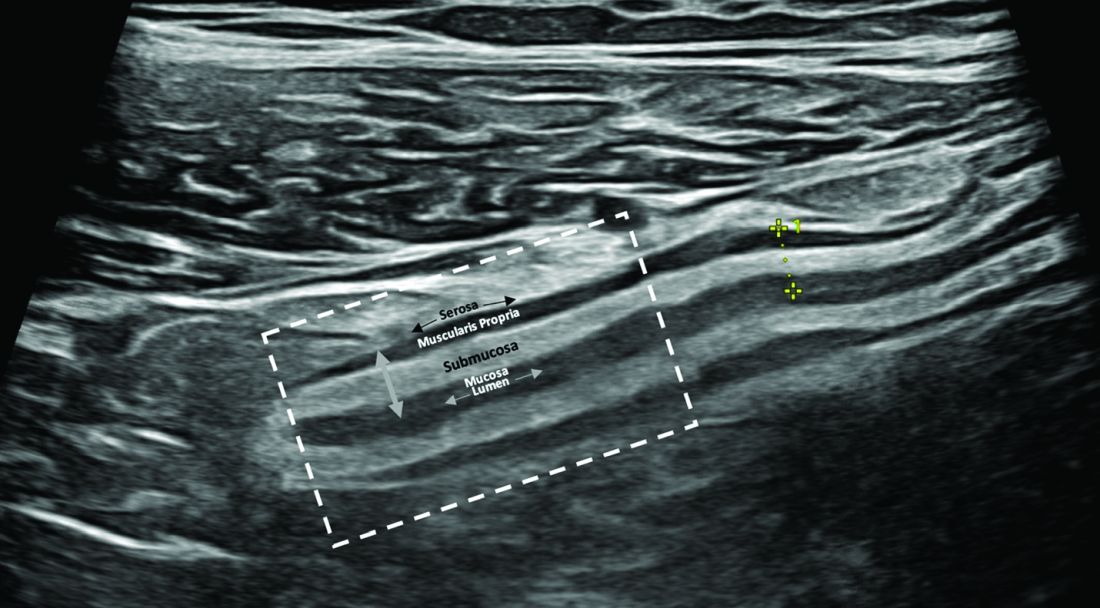
The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)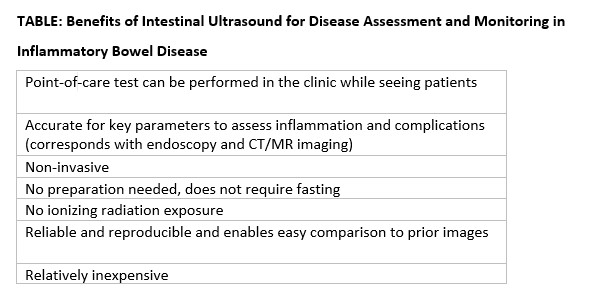
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Recruiting gastroenterology and hepatology fellows virtually - Should we continue after the pandemic?
Recruiting medical trainees is a major yearly step for all teaching hospitals in health care. The concept of interviewing residents and fellows virtually is not completely new and has been used in the past.1 With the coronavirus disease-19 (COVID-19) epidemic, the Association of American Medical Colleges (AAMC) recommended in May 2020 that all interviews be conducted virtually to ensure safety and prevent spread of the disease.2,3 Over the past few years, and with the gradual loosening of some restrictions, some programs have adopted a hybrid interview model for their recruitment plan, while others continue to use the virtual model exclusively.
, from the standpoint of the program director (Dr. Clark) and the associate program director (Dr. Dakhoul) of the fellowship program at the University of Florida in Gainesville. We have conducted our fellowship interviews for the academic year 2022-2023 completely virtually over multiple half-day sessions and fully matched all four positions. At the time of the interviews, we extended a general invitation to all candidates for an in-person visit for the purpose of touring the facilities and our community. Out of the 37 applicants interviewed, 2 made an in-person visit later.
After we concluded the interview season, we conducted a brief, anonymous survey to assess the overall experience of the interviewees with their virtual interviews. (See end of this article.) The survey contained a combination of single-choice questions and open-ended questions. The response rate was 35%. Most responders (92.3%) thought that they had a great understanding of the program from the information provided to them, and 84.6% were quite satisfied with their virtual interview experience. Regarding the likelihood of accepting the interview if it were offered in person, only one person answered that he/she would not have accepted the invitation. A total of 31% of participants might have changed the ranking of the program if they’d had an in-person interview instead.
When asked to choose between an invitation for an in-person vs. a virtual interview, the majority (77%) chose the virtual option. The stated pros of being interviewed virtually included convenience (not having to find coverage, etc.), time and cost savings, and a less stressful experience. Cons were focused mostly on not being able to see the hospital or the geographical area in person, as well as limited exposure to the facility and work environment for subjective assessment of “fitting” into the program. Additional comments included mostly positive feedback about the whole experience specific to the program. Finally, 77% of respondents recommended that the program should continue to conduct its interviews virtually.
It seems that the general feedback from our survey was positive. Certainly, limitations exist, including but not limited to the response rate, the geographic locations of the invited candidates, the design of the interview day, and familiarity with the fellowship program and the surrounding area. Several studies have been published on the topic with variable results across centers and among specialties, but most of them reported an encouraging overall experience.4-9
While the virtual recruitment experience seems to be most appealing to candidates, fellowships program directors and faculty who are part of the selection committee do not seem to be completely satisfied with the process and/or the outcome. Although virtual recruitment was shown to reduce financial costs and use of institutional resources,6 the major drawbacks were a lack of perception of the communication skills of the candidates as well as an inability to properly assess the interpersonal interactions with fellows and other applicants, both major keys to ranking decisions.10
Furthermore, the number of candidates who applied to our program has been steadily on the rise since the virtual platform was introduced. This has been the case nationwide and in other specialties as well.11 Applicants invited for an interview rarely decline or cancel the invitation due to the convenience of the virtual setting.6 These factors can affect the choice of candidates and subsequently the results of the match, especially for smaller programs. These observations create a new dilemma of whether fellowship programs need to consider increasing the number of their interviewees to ascertain a full match. Although the number of gastroenterology fellowship positions is steadily increasing with new program openings every year, it might not match the speed of the up-trending number of applicants. This certainly creates concern for fairness and equity in the selection process in this very competitive subspecialty.
As most gastroenterology programs continue to recruit their fellows virtually, it is important to keep in mind a few key elements to enhance the virtual experience. These include: a) familiarity of the interviewers and interviewees with video conference software to avoid technical problems, b) inclusion of up-to-date information about the program on the institutional website as well as videos or live-stream tours to show the physical aspect of the training sites (mainly the endoscopy areas) as alternatives to in-person tours,12 and c) timing of the interview, taking into consideration the different time zones of the invited applicants. Despite optimizing the virtual experience, some interviewees might still choose to visit in person. While this decision is solely voluntary and remains optional (at least in our program), it does allow program directors to indirectly evaluate candidates with a strong interest in the program.
In conclusion, there is no clear-cut answer to whether conducting interviews virtually is the best way to continue to recruit gastroenterology and hepatology fellows beyond the pandemic. While our perspective might be somewhat biased by the positive experience we had in the past few years recruiting our fellows virtually, this should be an individualized decision for every program. It is highly dependent on the location and size of each fellowship program, faculty engagement in the interview process, and the historical matching rates of the program. On a positive note, the individualized approach by each fellowship program should highlight the best features of the program and have a positive impact on recruitment at the local level. We have to bear in mind that a nonstandardized approach to fellow recruitment may have disadvantages to both programs and applicants with fewer resources to successfully compete and may introduce another element of uncertainty to an already stressful process for applicants and programs alike. As we continue to understand the implications of using the virtual platform and to reflect on the previous match results through the performance and satisfaction of the fellows recruited virtually, this option does not seem to have completely replaced in-person meetings. Further follow-up to evaluate the impact of virtual interviews should be done by surveying program directors nationally on the impact of match results before and after implementation of virtual interviews.
Survey
A. Do you think you had a good understanding of the UF GI Fellowship program from the information provided to you during your virtual interview?
1. I was provided with all the information I needed to know, and I had a great understanding of the program
2. I was provided with some information, and I had a fair understanding of the program
3. I was not provided with enough information, and I don’t think I understand the program well
B. How likely were you to accept this interview if this had been an in-person interview?
1. I would have still accepted the invitation regardless
2. I would have thought about possibly not accepting the invitation
3. I wouldn’t have accepted the invitation
C. Do you think an in-person interview would have changed your program ranking?
1. Yes
2. Maybe, I am not sure
3. No
D. If you had a choice between conducting this interview virtually vs. in-person, which one would you have chosen?
1. Virtual
2. In-person
E. Overall, how satisfied were you with your virtual GI Fellowship interview experience at UF?
1. Quite satisfied
2. Somewhat satisfied
3. Not at all satisfied
F. If you chose “somewhat satisfied” or “not at all satisfied” in the previous question, please tell us why, and what are the things that we could have done better:G. Do you think the UF GI Fellowship program should continue to conduct its interviews virtually (regardless of COVID)?
1. Yes
2. No
H. Please list some of the pros and cons of being interviewed virtually, in your opinion:
I. Additional comments:
Dr. Dakhoul, Ms. Rhoden, and Dr. Clark are with the division of gastroenterology and hepatology, University of Florida, Gainesville. They have no disclosures or conflicts.
References
1. Shah SK et al. Randomized evaluation of a web based interview process for urology resident selection. J Urol. Apr 2012;187(4):1380-4.
2. AAMC Interview Guidance for the 2022-2023 Residency Cycle. May 16, 2022.
3. Bernstein SA et al. Graduate medical education virtual interviews and recruitment in the era of COVID-19. J Grad Med Educ. Oct 2020;12(5):557-60.
4. Gupta S et al. Is the changing landscape of fellowship recruitment during COVID-19 here to stay? J Pediatr Surg. Oct 2022;57(10):445-50.
5. Vining CC et al. Virtual surgical fellowship recruitment during COVID-19 and its implications for resident/fellow recruitment in the future. Ann Surg Oncol. 2020 Dec;27(Suppl 3):911-15.
6. Simmons RP et al. Virtual Recruitment: Experiences and Perspectives of Internal Medicine Program Directors. Am J Med. Feb 2022;135(2):258-63.e251.
7. Daram SR et al. Interview from anywhere: Feasibility and utility of web-based videoconference interviews in the gastroenterology fellowship selection process. Am J Gastroenterol. Feb 2014;109(2):155-9.
8. Ponterio JM et al. The virtual interview format for fellowship recruitment in obstetrics and gynecology: A nationwide survey of program directors. Med Educ Online. Dec 2022;27(1):2054304.
9. DiGiusto M et al. Impact of the COVID-19 pandemic on the 2020 pediatric anesthesiology fellowship application cycle: A survey of program directors. Paediatr Anaesth. Mar 2022;32(3):471-8.
10. Hamade N et al. Virtual Gastroenterology Fellowship Recruitment During COVID-19 and Its Implications for the Future. Dig Dis Sci. Jun 2022;67(6):2019-28.
11. AAMC: ERAS Statistics. Historical Specialty Specific Data.
12. Advani R et al. An Overview of the GI Fellowship Interview: Part II-Tips for Selection Committees and Interviewers. Dig Dis Sci. May 2022;67(5):1712-17.
Recruiting medical trainees is a major yearly step for all teaching hospitals in health care. The concept of interviewing residents and fellows virtually is not completely new and has been used in the past.1 With the coronavirus disease-19 (COVID-19) epidemic, the Association of American Medical Colleges (AAMC) recommended in May 2020 that all interviews be conducted virtually to ensure safety and prevent spread of the disease.2,3 Over the past few years, and with the gradual loosening of some restrictions, some programs have adopted a hybrid interview model for their recruitment plan, while others continue to use the virtual model exclusively.
, from the standpoint of the program director (Dr. Clark) and the associate program director (Dr. Dakhoul) of the fellowship program at the University of Florida in Gainesville. We have conducted our fellowship interviews for the academic year 2022-2023 completely virtually over multiple half-day sessions and fully matched all four positions. At the time of the interviews, we extended a general invitation to all candidates for an in-person visit for the purpose of touring the facilities and our community. Out of the 37 applicants interviewed, 2 made an in-person visit later.
After we concluded the interview season, we conducted a brief, anonymous survey to assess the overall experience of the interviewees with their virtual interviews. (See end of this article.) The survey contained a combination of single-choice questions and open-ended questions. The response rate was 35%. Most responders (92.3%) thought that they had a great understanding of the program from the information provided to them, and 84.6% were quite satisfied with their virtual interview experience. Regarding the likelihood of accepting the interview if it were offered in person, only one person answered that he/she would not have accepted the invitation. A total of 31% of participants might have changed the ranking of the program if they’d had an in-person interview instead.
When asked to choose between an invitation for an in-person vs. a virtual interview, the majority (77%) chose the virtual option. The stated pros of being interviewed virtually included convenience (not having to find coverage, etc.), time and cost savings, and a less stressful experience. Cons were focused mostly on not being able to see the hospital or the geographical area in person, as well as limited exposure to the facility and work environment for subjective assessment of “fitting” into the program. Additional comments included mostly positive feedback about the whole experience specific to the program. Finally, 77% of respondents recommended that the program should continue to conduct its interviews virtually.
It seems that the general feedback from our survey was positive. Certainly, limitations exist, including but not limited to the response rate, the geographic locations of the invited candidates, the design of the interview day, and familiarity with the fellowship program and the surrounding area. Several studies have been published on the topic with variable results across centers and among specialties, but most of them reported an encouraging overall experience.4-9
While the virtual recruitment experience seems to be most appealing to candidates, fellowships program directors and faculty who are part of the selection committee do not seem to be completely satisfied with the process and/or the outcome. Although virtual recruitment was shown to reduce financial costs and use of institutional resources,6 the major drawbacks were a lack of perception of the communication skills of the candidates as well as an inability to properly assess the interpersonal interactions with fellows and other applicants, both major keys to ranking decisions.10
Furthermore, the number of candidates who applied to our program has been steadily on the rise since the virtual platform was introduced. This has been the case nationwide and in other specialties as well.11 Applicants invited for an interview rarely decline or cancel the invitation due to the convenience of the virtual setting.6 These factors can affect the choice of candidates and subsequently the results of the match, especially for smaller programs. These observations create a new dilemma of whether fellowship programs need to consider increasing the number of their interviewees to ascertain a full match. Although the number of gastroenterology fellowship positions is steadily increasing with new program openings every year, it might not match the speed of the up-trending number of applicants. This certainly creates concern for fairness and equity in the selection process in this very competitive subspecialty.
As most gastroenterology programs continue to recruit their fellows virtually, it is important to keep in mind a few key elements to enhance the virtual experience. These include: a) familiarity of the interviewers and interviewees with video conference software to avoid technical problems, b) inclusion of up-to-date information about the program on the institutional website as well as videos or live-stream tours to show the physical aspect of the training sites (mainly the endoscopy areas) as alternatives to in-person tours,12 and c) timing of the interview, taking into consideration the different time zones of the invited applicants. Despite optimizing the virtual experience, some interviewees might still choose to visit in person. While this decision is solely voluntary and remains optional (at least in our program), it does allow program directors to indirectly evaluate candidates with a strong interest in the program.
In conclusion, there is no clear-cut answer to whether conducting interviews virtually is the best way to continue to recruit gastroenterology and hepatology fellows beyond the pandemic. While our perspective might be somewhat biased by the positive experience we had in the past few years recruiting our fellows virtually, this should be an individualized decision for every program. It is highly dependent on the location and size of each fellowship program, faculty engagement in the interview process, and the historical matching rates of the program. On a positive note, the individualized approach by each fellowship program should highlight the best features of the program and have a positive impact on recruitment at the local level. We have to bear in mind that a nonstandardized approach to fellow recruitment may have disadvantages to both programs and applicants with fewer resources to successfully compete and may introduce another element of uncertainty to an already stressful process for applicants and programs alike. As we continue to understand the implications of using the virtual platform and to reflect on the previous match results through the performance and satisfaction of the fellows recruited virtually, this option does not seem to have completely replaced in-person meetings. Further follow-up to evaluate the impact of virtual interviews should be done by surveying program directors nationally on the impact of match results before and after implementation of virtual interviews.
Survey
A. Do you think you had a good understanding of the UF GI Fellowship program from the information provided to you during your virtual interview?
1. I was provided with all the information I needed to know, and I had a great understanding of the program
2. I was provided with some information, and I had a fair understanding of the program
3. I was not provided with enough information, and I don’t think I understand the program well
B. How likely were you to accept this interview if this had been an in-person interview?
1. I would have still accepted the invitation regardless
2. I would have thought about possibly not accepting the invitation
3. I wouldn’t have accepted the invitation
C. Do you think an in-person interview would have changed your program ranking?
1. Yes
2. Maybe, I am not sure
3. No
D. If you had a choice between conducting this interview virtually vs. in-person, which one would you have chosen?
1. Virtual
2. In-person
E. Overall, how satisfied were you with your virtual GI Fellowship interview experience at UF?
1. Quite satisfied
2. Somewhat satisfied
3. Not at all satisfied
F. If you chose “somewhat satisfied” or “not at all satisfied” in the previous question, please tell us why, and what are the things that we could have done better:G. Do you think the UF GI Fellowship program should continue to conduct its interviews virtually (regardless of COVID)?
1. Yes
2. No
H. Please list some of the pros and cons of being interviewed virtually, in your opinion:
I. Additional comments:
Dr. Dakhoul, Ms. Rhoden, and Dr. Clark are with the division of gastroenterology and hepatology, University of Florida, Gainesville. They have no disclosures or conflicts.
References
1. Shah SK et al. Randomized evaluation of a web based interview process for urology resident selection. J Urol. Apr 2012;187(4):1380-4.
2. AAMC Interview Guidance for the 2022-2023 Residency Cycle. May 16, 2022.
3. Bernstein SA et al. Graduate medical education virtual interviews and recruitment in the era of COVID-19. J Grad Med Educ. Oct 2020;12(5):557-60.
4. Gupta S et al. Is the changing landscape of fellowship recruitment during COVID-19 here to stay? J Pediatr Surg. Oct 2022;57(10):445-50.
5. Vining CC et al. Virtual surgical fellowship recruitment during COVID-19 and its implications for resident/fellow recruitment in the future. Ann Surg Oncol. 2020 Dec;27(Suppl 3):911-15.
6. Simmons RP et al. Virtual Recruitment: Experiences and Perspectives of Internal Medicine Program Directors. Am J Med. Feb 2022;135(2):258-63.e251.
7. Daram SR et al. Interview from anywhere: Feasibility and utility of web-based videoconference interviews in the gastroenterology fellowship selection process. Am J Gastroenterol. Feb 2014;109(2):155-9.
8. Ponterio JM et al. The virtual interview format for fellowship recruitment in obstetrics and gynecology: A nationwide survey of program directors. Med Educ Online. Dec 2022;27(1):2054304.
9. DiGiusto M et al. Impact of the COVID-19 pandemic on the 2020 pediatric anesthesiology fellowship application cycle: A survey of program directors. Paediatr Anaesth. Mar 2022;32(3):471-8.
10. Hamade N et al. Virtual Gastroenterology Fellowship Recruitment During COVID-19 and Its Implications for the Future. Dig Dis Sci. Jun 2022;67(6):2019-28.
11. AAMC: ERAS Statistics. Historical Specialty Specific Data.
12. Advani R et al. An Overview of the GI Fellowship Interview: Part II-Tips for Selection Committees and Interviewers. Dig Dis Sci. May 2022;67(5):1712-17.
Recruiting medical trainees is a major yearly step for all teaching hospitals in health care. The concept of interviewing residents and fellows virtually is not completely new and has been used in the past.1 With the coronavirus disease-19 (COVID-19) epidemic, the Association of American Medical Colleges (AAMC) recommended in May 2020 that all interviews be conducted virtually to ensure safety and prevent spread of the disease.2,3 Over the past few years, and with the gradual loosening of some restrictions, some programs have adopted a hybrid interview model for their recruitment plan, while others continue to use the virtual model exclusively.
, from the standpoint of the program director (Dr. Clark) and the associate program director (Dr. Dakhoul) of the fellowship program at the University of Florida in Gainesville. We have conducted our fellowship interviews for the academic year 2022-2023 completely virtually over multiple half-day sessions and fully matched all four positions. At the time of the interviews, we extended a general invitation to all candidates for an in-person visit for the purpose of touring the facilities and our community. Out of the 37 applicants interviewed, 2 made an in-person visit later.
After we concluded the interview season, we conducted a brief, anonymous survey to assess the overall experience of the interviewees with their virtual interviews. (See end of this article.) The survey contained a combination of single-choice questions and open-ended questions. The response rate was 35%. Most responders (92.3%) thought that they had a great understanding of the program from the information provided to them, and 84.6% were quite satisfied with their virtual interview experience. Regarding the likelihood of accepting the interview if it were offered in person, only one person answered that he/she would not have accepted the invitation. A total of 31% of participants might have changed the ranking of the program if they’d had an in-person interview instead.
When asked to choose between an invitation for an in-person vs. a virtual interview, the majority (77%) chose the virtual option. The stated pros of being interviewed virtually included convenience (not having to find coverage, etc.), time and cost savings, and a less stressful experience. Cons were focused mostly on not being able to see the hospital or the geographical area in person, as well as limited exposure to the facility and work environment for subjective assessment of “fitting” into the program. Additional comments included mostly positive feedback about the whole experience specific to the program. Finally, 77% of respondents recommended that the program should continue to conduct its interviews virtually.
It seems that the general feedback from our survey was positive. Certainly, limitations exist, including but not limited to the response rate, the geographic locations of the invited candidates, the design of the interview day, and familiarity with the fellowship program and the surrounding area. Several studies have been published on the topic with variable results across centers and among specialties, but most of them reported an encouraging overall experience.4-9
While the virtual recruitment experience seems to be most appealing to candidates, fellowships program directors and faculty who are part of the selection committee do not seem to be completely satisfied with the process and/or the outcome. Although virtual recruitment was shown to reduce financial costs and use of institutional resources,6 the major drawbacks were a lack of perception of the communication skills of the candidates as well as an inability to properly assess the interpersonal interactions with fellows and other applicants, both major keys to ranking decisions.10
Furthermore, the number of candidates who applied to our program has been steadily on the rise since the virtual platform was introduced. This has been the case nationwide and in other specialties as well.11 Applicants invited for an interview rarely decline or cancel the invitation due to the convenience of the virtual setting.6 These factors can affect the choice of candidates and subsequently the results of the match, especially for smaller programs. These observations create a new dilemma of whether fellowship programs need to consider increasing the number of their interviewees to ascertain a full match. Although the number of gastroenterology fellowship positions is steadily increasing with new program openings every year, it might not match the speed of the up-trending number of applicants. This certainly creates concern for fairness and equity in the selection process in this very competitive subspecialty.
As most gastroenterology programs continue to recruit their fellows virtually, it is important to keep in mind a few key elements to enhance the virtual experience. These include: a) familiarity of the interviewers and interviewees with video conference software to avoid technical problems, b) inclusion of up-to-date information about the program on the institutional website as well as videos or live-stream tours to show the physical aspect of the training sites (mainly the endoscopy areas) as alternatives to in-person tours,12 and c) timing of the interview, taking into consideration the different time zones of the invited applicants. Despite optimizing the virtual experience, some interviewees might still choose to visit in person. While this decision is solely voluntary and remains optional (at least in our program), it does allow program directors to indirectly evaluate candidates with a strong interest in the program.
In conclusion, there is no clear-cut answer to whether conducting interviews virtually is the best way to continue to recruit gastroenterology and hepatology fellows beyond the pandemic. While our perspective might be somewhat biased by the positive experience we had in the past few years recruiting our fellows virtually, this should be an individualized decision for every program. It is highly dependent on the location and size of each fellowship program, faculty engagement in the interview process, and the historical matching rates of the program. On a positive note, the individualized approach by each fellowship program should highlight the best features of the program and have a positive impact on recruitment at the local level. We have to bear in mind that a nonstandardized approach to fellow recruitment may have disadvantages to both programs and applicants with fewer resources to successfully compete and may introduce another element of uncertainty to an already stressful process for applicants and programs alike. As we continue to understand the implications of using the virtual platform and to reflect on the previous match results through the performance and satisfaction of the fellows recruited virtually, this option does not seem to have completely replaced in-person meetings. Further follow-up to evaluate the impact of virtual interviews should be done by surveying program directors nationally on the impact of match results before and after implementation of virtual interviews.
Survey
A. Do you think you had a good understanding of the UF GI Fellowship program from the information provided to you during your virtual interview?
1. I was provided with all the information I needed to know, and I had a great understanding of the program
2. I was provided with some information, and I had a fair understanding of the program
3. I was not provided with enough information, and I don’t think I understand the program well
B. How likely were you to accept this interview if this had been an in-person interview?
1. I would have still accepted the invitation regardless
2. I would have thought about possibly not accepting the invitation
3. I wouldn’t have accepted the invitation
C. Do you think an in-person interview would have changed your program ranking?
1. Yes
2. Maybe, I am not sure
3. No
D. If you had a choice between conducting this interview virtually vs. in-person, which one would you have chosen?
1. Virtual
2. In-person
E. Overall, how satisfied were you with your virtual GI Fellowship interview experience at UF?
1. Quite satisfied
2. Somewhat satisfied
3. Not at all satisfied
F. If you chose “somewhat satisfied” or “not at all satisfied” in the previous question, please tell us why, and what are the things that we could have done better:G. Do you think the UF GI Fellowship program should continue to conduct its interviews virtually (regardless of COVID)?
1. Yes
2. No
H. Please list some of the pros and cons of being interviewed virtually, in your opinion:
I. Additional comments:
Dr. Dakhoul, Ms. Rhoden, and Dr. Clark are with the division of gastroenterology and hepatology, University of Florida, Gainesville. They have no disclosures or conflicts.
References
1. Shah SK et al. Randomized evaluation of a web based interview process for urology resident selection. J Urol. Apr 2012;187(4):1380-4.
2. AAMC Interview Guidance for the 2022-2023 Residency Cycle. May 16, 2022.
3. Bernstein SA et al. Graduate medical education virtual interviews and recruitment in the era of COVID-19. J Grad Med Educ. Oct 2020;12(5):557-60.
4. Gupta S et al. Is the changing landscape of fellowship recruitment during COVID-19 here to stay? J Pediatr Surg. Oct 2022;57(10):445-50.
5. Vining CC et al. Virtual surgical fellowship recruitment during COVID-19 and its implications for resident/fellow recruitment in the future. Ann Surg Oncol. 2020 Dec;27(Suppl 3):911-15.
6. Simmons RP et al. Virtual Recruitment: Experiences and Perspectives of Internal Medicine Program Directors. Am J Med. Feb 2022;135(2):258-63.e251.
7. Daram SR et al. Interview from anywhere: Feasibility and utility of web-based videoconference interviews in the gastroenterology fellowship selection process. Am J Gastroenterol. Feb 2014;109(2):155-9.
8. Ponterio JM et al. The virtual interview format for fellowship recruitment in obstetrics and gynecology: A nationwide survey of program directors. Med Educ Online. Dec 2022;27(1):2054304.
9. DiGiusto M et al. Impact of the COVID-19 pandemic on the 2020 pediatric anesthesiology fellowship application cycle: A survey of program directors. Paediatr Anaesth. Mar 2022;32(3):471-8.
10. Hamade N et al. Virtual Gastroenterology Fellowship Recruitment During COVID-19 and Its Implications for the Future. Dig Dis Sci. Jun 2022;67(6):2019-28.
11. AAMC: ERAS Statistics. Historical Specialty Specific Data.
12. Advani R et al. An Overview of the GI Fellowship Interview: Part II-Tips for Selection Committees and Interviewers. Dig Dis Sci. May 2022;67(5):1712-17.
The future of GI
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill




















