User login
AGA patient and physician advocates visit Capitol Hill to push for prior authorization reform

In our first in-person Advocacy Day on Capitol Hill since 2019, AGA leaders and patient advocates from 22 total states met with House and Senate offices to educate members of Congress and their staff about policies affecting GI patient care such as prior authorization and step therapy. Federal research funding and Medicare reimbursement were also on the agenda.
In the meetings, the patient shared their stories of living with various gastrointestinal diseases, including ulcerative colitis and Crohn’s disease, and the struggles they’ve gone through to get treatments approved by their insurers. AGA physicians shared the provider perspective of how policies like prior authorization negatively impact practices. According to a 2023 AGA member survey, 95% of respondents say that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes and 84% described that the burden associated with prior authorization policies have increased “significantly” or “somewhat” over the last 5 years. AGA’s advocacy day came not long after UnitedHealthcare’s announcement of a new “Gold Card” prior authorization policy to be implemented in 2024, which will impact most colonoscopies and endoscopies for its 27 million commercial beneficiaries. The group expressed serious concerns about the proposed policy to lawmakers.

“It was a wonderful and empowering experience to share my personal story with my Representative/Senator and know that they were really listening to my concerns about insurer overreach,” said Aaron Blocker, a Crohn’s disease patient and advocate. “I hope Congress acts swiftly on passing prior authorization reform, so no more patients are forced to live in pain while they wait for treatments to be approved.” As gastroenterologists, too much administrative time is spent submitting onerous prior authorization requests on a near daily basis. We hope Congress takes our concerns seriously and comes together to rein in prior authorization.
AGA thanks the patient and physician advocates who participated in this year’s Advocacy Day and looks forward to continuing our work to ensure timely access to care.

In our first in-person Advocacy Day on Capitol Hill since 2019, AGA leaders and patient advocates from 22 total states met with House and Senate offices to educate members of Congress and their staff about policies affecting GI patient care such as prior authorization and step therapy. Federal research funding and Medicare reimbursement were also on the agenda.
In the meetings, the patient shared their stories of living with various gastrointestinal diseases, including ulcerative colitis and Crohn’s disease, and the struggles they’ve gone through to get treatments approved by their insurers. AGA physicians shared the provider perspective of how policies like prior authorization negatively impact practices. According to a 2023 AGA member survey, 95% of respondents say that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes and 84% described that the burden associated with prior authorization policies have increased “significantly” or “somewhat” over the last 5 years. AGA’s advocacy day came not long after UnitedHealthcare’s announcement of a new “Gold Card” prior authorization policy to be implemented in 2024, which will impact most colonoscopies and endoscopies for its 27 million commercial beneficiaries. The group expressed serious concerns about the proposed policy to lawmakers.

“It was a wonderful and empowering experience to share my personal story with my Representative/Senator and know that they were really listening to my concerns about insurer overreach,” said Aaron Blocker, a Crohn’s disease patient and advocate. “I hope Congress acts swiftly on passing prior authorization reform, so no more patients are forced to live in pain while they wait for treatments to be approved.” As gastroenterologists, too much administrative time is spent submitting onerous prior authorization requests on a near daily basis. We hope Congress takes our concerns seriously and comes together to rein in prior authorization.
AGA thanks the patient and physician advocates who participated in this year’s Advocacy Day and looks forward to continuing our work to ensure timely access to care.

In our first in-person Advocacy Day on Capitol Hill since 2019, AGA leaders and patient advocates from 22 total states met with House and Senate offices to educate members of Congress and their staff about policies affecting GI patient care such as prior authorization and step therapy. Federal research funding and Medicare reimbursement were also on the agenda.
In the meetings, the patient shared their stories of living with various gastrointestinal diseases, including ulcerative colitis and Crohn’s disease, and the struggles they’ve gone through to get treatments approved by their insurers. AGA physicians shared the provider perspective of how policies like prior authorization negatively impact practices. According to a 2023 AGA member survey, 95% of respondents say that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes and 84% described that the burden associated with prior authorization policies have increased “significantly” or “somewhat” over the last 5 years. AGA’s advocacy day came not long after UnitedHealthcare’s announcement of a new “Gold Card” prior authorization policy to be implemented in 2024, which will impact most colonoscopies and endoscopies for its 27 million commercial beneficiaries. The group expressed serious concerns about the proposed policy to lawmakers.

“It was a wonderful and empowering experience to share my personal story with my Representative/Senator and know that they were really listening to my concerns about insurer overreach,” said Aaron Blocker, a Crohn’s disease patient and advocate. “I hope Congress acts swiftly on passing prior authorization reform, so no more patients are forced to live in pain while they wait for treatments to be approved.” As gastroenterologists, too much administrative time is spent submitting onerous prior authorization requests on a near daily basis. We hope Congress takes our concerns seriously and comes together to rein in prior authorization.
AGA thanks the patient and physician advocates who participated in this year’s Advocacy Day and looks forward to continuing our work to ensure timely access to care.
Scrubs & Heels Summit 2023: Filling a void for women in GI
.1-3 This gender disparity arises from a multitude of factors including lack of effective mentoring, unequal leadership and career advancement opportunities, and pay inequity. In this context, The Scrubs & Heels Leadership Summit (S&H) was launched in 2022 focused on the professional and personal development of women in gastroenterology.
I had the great pleasure and honor of attending the 2023 summit which took place in February in Rancho Palos Verdes, Calif. There were nearly 200 attendees ranging from trainees to midcareer and senior gastroenterologists and other health care professionals from both academia and private practices across the nation. The weekend course was directed by S&H cofounders, Dr. Aline Charabaty and Dr. Anita Afzali, and cochaired by Dr. Amy Oxentenko and Dr. Aja McCutchen.
The 2-day summit opened with a presentation by Sally Helgesen, author of How Women Rise, describing the 12 common habits that often hold women back in career advancement, promotion, or opportunities. Dr. Aline Charabaty addressed the myth of women needing to fulfill the role of superwoman or have suprahuman abilities. Attendees were challenged to reframe this societal construct and begin to find balance and the reasonable choice to switch to part-time work and, as Dr. Aja McCutch emphasized, dial-down responsibilities to maintain wellness when life has competing priorities.
Dr. Amy Oxentenko shared her personal journey to success and instilled the importance of engaging with community and society at large. We then heard from Dr. Neena Abraham on how to gracefully embrace transitions in our professional lives, whether intentionally sought or natural progressions of a career. She encouraged attendees to control our own narrative and seek challenges that promote growth. We explored different practice models with Dr. Caroline Hwang and learned strategies of switching from academics to private practice or vice versa. We also heard from cofounder Dr. Anita Afzali on becoming a physician executive and the importance of staying connected to patient care when rising in ranks of leadership.
The second day opened with a keynote address delivered by Dr. Marla Dubinsky detailing her journey of becoming a CEO of a publicly-traded company while retaining her role as professor and chief of pediatric gastroenterology in a large academic institution. Attendees were provided with a master class on discovering ways to inspire our inner entrepreneur and highlighted the benefit of physicians, especially women, in being effective business leaders. This talk was followed by a talk by Phil Schoenfeld, MD, FACS, editor-in-chief of Evidence-Based GI for the American College of Gastroenterology. He spoke on the importance of male allyship for women in GI and shared his personal experiences and challenges with allyship.
The summit included a breakout session by Dr. Rashmi Advani designed for residents to hear tips on how to have a successful fellowship match and for fellows to embrace a steep learning curve when starting and included tips for efficiency. Additional breakout sessions included learning ergonomic strategies for positioning and scope-holding, vocal-cord exercises before giving oral presentations, and how to formulate a business plan and negotiate a contract.
We ended the summit with uplifting advice from executive coaches Sonia Narang and Dr. Dawn Sears who taught us the art of leaning into opportunities, mansizing aspirations, finding coconspirators for amplification of female GI leaders, and supporting our colleagues personally and professionally.
Three key takeaway messages:
- Recognize your self-worth and the contributions you bring to your patients and community as a whole.
- Lean into the importance of vocalizing your asks, advocating for yourself, building your brand, and showcasing your accomplishments.
- Be mindful of the balance between the time and energy you dedicate towards goals that bring you recognition and fuel your passion and your mental, physical, and emotional health.
As a trainee, I benefited tremendously from attending and expanding my professional network of mentors, sponsors and colleagues. I am encouraged by this programming and hope to see more of it in the future.
Contributors to this article included: Rashmi Advani, MD2; Anita Afzali, MD3; Aline Charabaty, MD.4
Neither Dr. Syed, nor the article contributors, had financial conflicts of interest associated with this article. The AGA was represented at the Scrubs and Heels Summit as a society partner committed to the advancement of women in GI. AGA is building on years of efforts to bolster leadership, mentorship, and sponsorship among women in GI through its annual women’s leadership conference and most recently with its 2022 regional women in GI workshops held around the country that led to the development of a comprehensive gender equity strategy designed to build an environment of gender equity in the field of GI so that all can thrive.
Institutions and social media handle
1. Santa Clara Valley Medical Center (San Jose, Calif), @noorannemd
2. Cedars Sinai (Los Angeles), @AdvaniRashmiMD
3. University of Cincinnati, @IBD_Afzali
4. Johns Hopkins Medicine (Washington), @DCharabaty
References
Advani R et al. Gender-specific attitudes of internal medicine residents toward gastroenterology. Dig Dis Sci. 2022 Nov;67(11):5044-52.
American Association of Medical Colleges. Diversity in Medicine: Facts and Figures (2019).
Elta GH. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol 2005;100:259-64
David, Yakira N. et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Journal of the American College of Gastroenterology, ACG 116.3 (2021):539-50.
Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93:1047-56.
Rabinowitz LG et al. Survey finds gender disparities impact both women mentors and mentees in gastroenterology. Journal of the American College of Gastroenterology, ACG 2021;116:1876-84.
.1-3 This gender disparity arises from a multitude of factors including lack of effective mentoring, unequal leadership and career advancement opportunities, and pay inequity. In this context, The Scrubs & Heels Leadership Summit (S&H) was launched in 2022 focused on the professional and personal development of women in gastroenterology.
I had the great pleasure and honor of attending the 2023 summit which took place in February in Rancho Palos Verdes, Calif. There were nearly 200 attendees ranging from trainees to midcareer and senior gastroenterologists and other health care professionals from both academia and private practices across the nation. The weekend course was directed by S&H cofounders, Dr. Aline Charabaty and Dr. Anita Afzali, and cochaired by Dr. Amy Oxentenko and Dr. Aja McCutchen.
The 2-day summit opened with a presentation by Sally Helgesen, author of How Women Rise, describing the 12 common habits that often hold women back in career advancement, promotion, or opportunities. Dr. Aline Charabaty addressed the myth of women needing to fulfill the role of superwoman or have suprahuman abilities. Attendees were challenged to reframe this societal construct and begin to find balance and the reasonable choice to switch to part-time work and, as Dr. Aja McCutch emphasized, dial-down responsibilities to maintain wellness when life has competing priorities.
Dr. Amy Oxentenko shared her personal journey to success and instilled the importance of engaging with community and society at large. We then heard from Dr. Neena Abraham on how to gracefully embrace transitions in our professional lives, whether intentionally sought or natural progressions of a career. She encouraged attendees to control our own narrative and seek challenges that promote growth. We explored different practice models with Dr. Caroline Hwang and learned strategies of switching from academics to private practice or vice versa. We also heard from cofounder Dr. Anita Afzali on becoming a physician executive and the importance of staying connected to patient care when rising in ranks of leadership.
The second day opened with a keynote address delivered by Dr. Marla Dubinsky detailing her journey of becoming a CEO of a publicly-traded company while retaining her role as professor and chief of pediatric gastroenterology in a large academic institution. Attendees were provided with a master class on discovering ways to inspire our inner entrepreneur and highlighted the benefit of physicians, especially women, in being effective business leaders. This talk was followed by a talk by Phil Schoenfeld, MD, FACS, editor-in-chief of Evidence-Based GI for the American College of Gastroenterology. He spoke on the importance of male allyship for women in GI and shared his personal experiences and challenges with allyship.
The summit included a breakout session by Dr. Rashmi Advani designed for residents to hear tips on how to have a successful fellowship match and for fellows to embrace a steep learning curve when starting and included tips for efficiency. Additional breakout sessions included learning ergonomic strategies for positioning and scope-holding, vocal-cord exercises before giving oral presentations, and how to formulate a business plan and negotiate a contract.
We ended the summit with uplifting advice from executive coaches Sonia Narang and Dr. Dawn Sears who taught us the art of leaning into opportunities, mansizing aspirations, finding coconspirators for amplification of female GI leaders, and supporting our colleagues personally and professionally.
Three key takeaway messages:
- Recognize your self-worth and the contributions you bring to your patients and community as a whole.
- Lean into the importance of vocalizing your asks, advocating for yourself, building your brand, and showcasing your accomplishments.
- Be mindful of the balance between the time and energy you dedicate towards goals that bring you recognition and fuel your passion and your mental, physical, and emotional health.
As a trainee, I benefited tremendously from attending and expanding my professional network of mentors, sponsors and colleagues. I am encouraged by this programming and hope to see more of it in the future.
Contributors to this article included: Rashmi Advani, MD2; Anita Afzali, MD3; Aline Charabaty, MD.4
Neither Dr. Syed, nor the article contributors, had financial conflicts of interest associated with this article. The AGA was represented at the Scrubs and Heels Summit as a society partner committed to the advancement of women in GI. AGA is building on years of efforts to bolster leadership, mentorship, and sponsorship among women in GI through its annual women’s leadership conference and most recently with its 2022 regional women in GI workshops held around the country that led to the development of a comprehensive gender equity strategy designed to build an environment of gender equity in the field of GI so that all can thrive.
Institutions and social media handle
1. Santa Clara Valley Medical Center (San Jose, Calif), @noorannemd
2. Cedars Sinai (Los Angeles), @AdvaniRashmiMD
3. University of Cincinnati, @IBD_Afzali
4. Johns Hopkins Medicine (Washington), @DCharabaty
References
Advani R et al. Gender-specific attitudes of internal medicine residents toward gastroenterology. Dig Dis Sci. 2022 Nov;67(11):5044-52.
American Association of Medical Colleges. Diversity in Medicine: Facts and Figures (2019).
Elta GH. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol 2005;100:259-64
David, Yakira N. et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Journal of the American College of Gastroenterology, ACG 116.3 (2021):539-50.
Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93:1047-56.
Rabinowitz LG et al. Survey finds gender disparities impact both women mentors and mentees in gastroenterology. Journal of the American College of Gastroenterology, ACG 2021;116:1876-84.
.1-3 This gender disparity arises from a multitude of factors including lack of effective mentoring, unequal leadership and career advancement opportunities, and pay inequity. In this context, The Scrubs & Heels Leadership Summit (S&H) was launched in 2022 focused on the professional and personal development of women in gastroenterology.
I had the great pleasure and honor of attending the 2023 summit which took place in February in Rancho Palos Verdes, Calif. There were nearly 200 attendees ranging from trainees to midcareer and senior gastroenterologists and other health care professionals from both academia and private practices across the nation. The weekend course was directed by S&H cofounders, Dr. Aline Charabaty and Dr. Anita Afzali, and cochaired by Dr. Amy Oxentenko and Dr. Aja McCutchen.
The 2-day summit opened with a presentation by Sally Helgesen, author of How Women Rise, describing the 12 common habits that often hold women back in career advancement, promotion, or opportunities. Dr. Aline Charabaty addressed the myth of women needing to fulfill the role of superwoman or have suprahuman abilities. Attendees were challenged to reframe this societal construct and begin to find balance and the reasonable choice to switch to part-time work and, as Dr. Aja McCutch emphasized, dial-down responsibilities to maintain wellness when life has competing priorities.
Dr. Amy Oxentenko shared her personal journey to success and instilled the importance of engaging with community and society at large. We then heard from Dr. Neena Abraham on how to gracefully embrace transitions in our professional lives, whether intentionally sought or natural progressions of a career. She encouraged attendees to control our own narrative and seek challenges that promote growth. We explored different practice models with Dr. Caroline Hwang and learned strategies of switching from academics to private practice or vice versa. We also heard from cofounder Dr. Anita Afzali on becoming a physician executive and the importance of staying connected to patient care when rising in ranks of leadership.
The second day opened with a keynote address delivered by Dr. Marla Dubinsky detailing her journey of becoming a CEO of a publicly-traded company while retaining her role as professor and chief of pediatric gastroenterology in a large academic institution. Attendees were provided with a master class on discovering ways to inspire our inner entrepreneur and highlighted the benefit of physicians, especially women, in being effective business leaders. This talk was followed by a talk by Phil Schoenfeld, MD, FACS, editor-in-chief of Evidence-Based GI for the American College of Gastroenterology. He spoke on the importance of male allyship for women in GI and shared his personal experiences and challenges with allyship.
The summit included a breakout session by Dr. Rashmi Advani designed for residents to hear tips on how to have a successful fellowship match and for fellows to embrace a steep learning curve when starting and included tips for efficiency. Additional breakout sessions included learning ergonomic strategies for positioning and scope-holding, vocal-cord exercises before giving oral presentations, and how to formulate a business plan and negotiate a contract.
We ended the summit with uplifting advice from executive coaches Sonia Narang and Dr. Dawn Sears who taught us the art of leaning into opportunities, mansizing aspirations, finding coconspirators for amplification of female GI leaders, and supporting our colleagues personally and professionally.
Three key takeaway messages:
- Recognize your self-worth and the contributions you bring to your patients and community as a whole.
- Lean into the importance of vocalizing your asks, advocating for yourself, building your brand, and showcasing your accomplishments.
- Be mindful of the balance between the time and energy you dedicate towards goals that bring you recognition and fuel your passion and your mental, physical, and emotional health.
As a trainee, I benefited tremendously from attending and expanding my professional network of mentors, sponsors and colleagues. I am encouraged by this programming and hope to see more of it in the future.
Contributors to this article included: Rashmi Advani, MD2; Anita Afzali, MD3; Aline Charabaty, MD.4
Neither Dr. Syed, nor the article contributors, had financial conflicts of interest associated with this article. The AGA was represented at the Scrubs and Heels Summit as a society partner committed to the advancement of women in GI. AGA is building on years of efforts to bolster leadership, mentorship, and sponsorship among women in GI through its annual women’s leadership conference and most recently with its 2022 regional women in GI workshops held around the country that led to the development of a comprehensive gender equity strategy designed to build an environment of gender equity in the field of GI so that all can thrive.
Institutions and social media handle
1. Santa Clara Valley Medical Center (San Jose, Calif), @noorannemd
2. Cedars Sinai (Los Angeles), @AdvaniRashmiMD
3. University of Cincinnati, @IBD_Afzali
4. Johns Hopkins Medicine (Washington), @DCharabaty
References
Advani R et al. Gender-specific attitudes of internal medicine residents toward gastroenterology. Dig Dis Sci. 2022 Nov;67(11):5044-52.
American Association of Medical Colleges. Diversity in Medicine: Facts and Figures (2019).
Elta GH. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol 2005;100:259-64
David, Yakira N. et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Journal of the American College of Gastroenterology, ACG 116.3 (2021):539-50.
Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93:1047-56.
Rabinowitz LG et al. Survey finds gender disparities impact both women mentors and mentees in gastroenterology. Journal of the American College of Gastroenterology, ACG 2021;116:1876-84.
Navigating NAFLD: Unveiling the approach to mitigate the impact of NAFLD
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15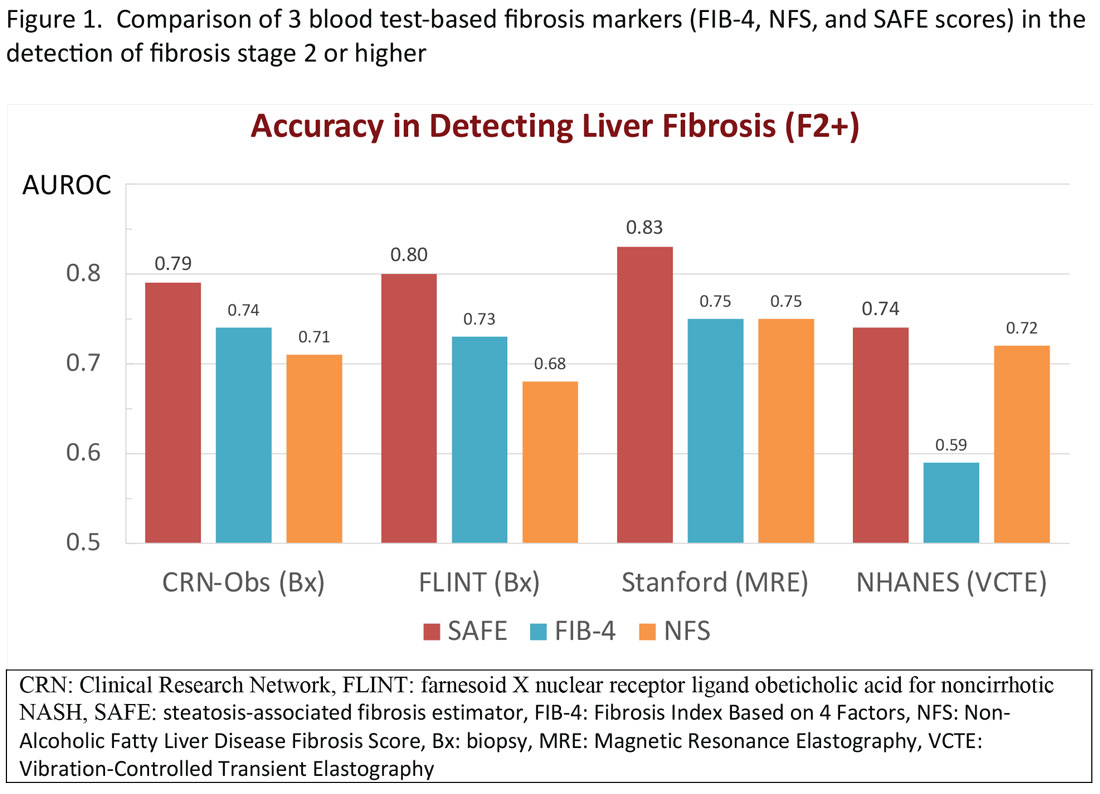
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16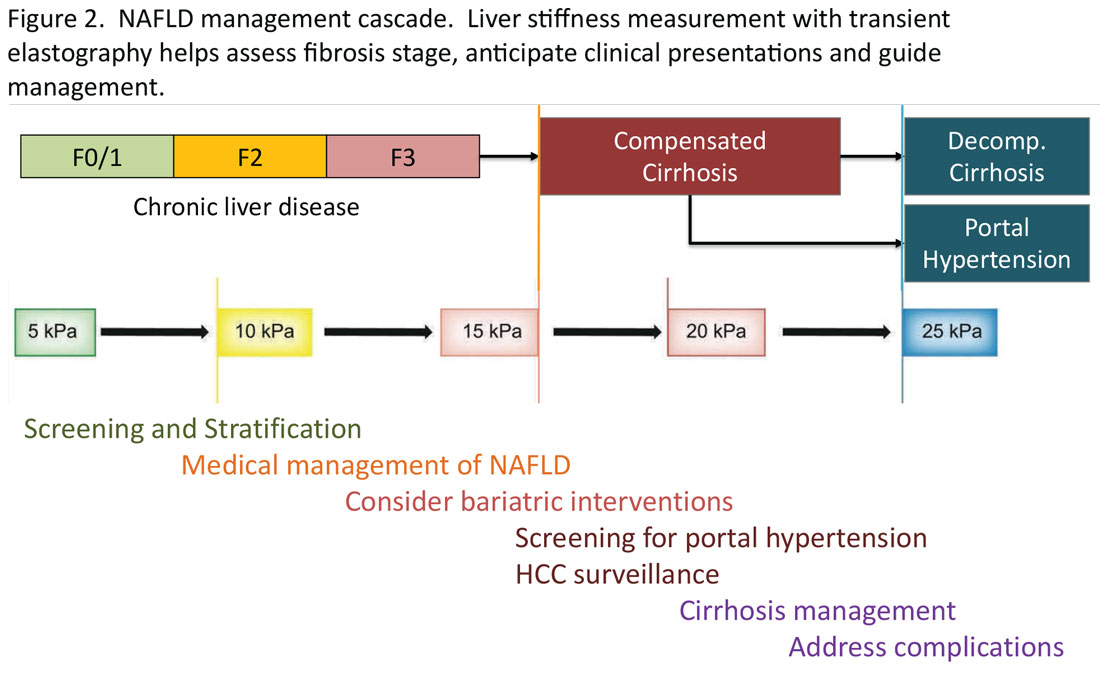
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: [email protected]. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: [email protected]. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: [email protected]. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Transitions and growth
Dear friends,
This fall, I will also be starting my first position out of fellowship. I look forward to many opportunities and challenges to come.
This month in In Focus, Dr. Mai Sedki and Dr. W. Ray Kim unpack the nuances of assessing and risk-stratifying patients with nonalcoholic fatty liver disease by using non-invasive testing in daily practice. Beyond daily practice, it is important to know where our field is advancing to offer patients more options. In Short Clinical Reviews, Dr. Aileen Bui and Dr. James Buxbaum review how the field of endohepatology is expanding into endoscopic ultrasound–guided liver biopsies, portal pressure measurements, and interventions of gastric varices.
In our Early Career feature, Dr. Corlan Eboh, Dr. Victoria Jaeger, and Dr. Dawn Sears describe how gastroenterologists are uniquely positioned for burnout and what can be done to prevent and treat it, particularly among new and transitioning gastroenterologists. In post-COVID era, practices have experienced an increase in portal messages and other non-face-to-face patient care, which may be contributing burnout.
In our Finance section this month, Dr. Luis Nieto and Dr. Jami Kinnucan review the types of patient encounters and billing options to optimize your compensation for time spent.
In Private Practice Perspectives, Dr. David Ramsey discusses why he joined a private practice and how understanding your own goals and values can guide you to a good fit in different practice models. Lastly, Dr. Dan Kroch describes his unique journey in becoming a third-space endoscopist without an advanced fellowship year and why dedicated training is the future of advanced endoscopic resection and third-space endoscopy.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: The first endoscopic retrograde cholangiopancreatography (ERCP) was first performed by an obstetrician, Dr. William McCune in 1968, and achieved by taping an external accessory channel to a duodenoscope.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
This fall, I will also be starting my first position out of fellowship. I look forward to many opportunities and challenges to come.
This month in In Focus, Dr. Mai Sedki and Dr. W. Ray Kim unpack the nuances of assessing and risk-stratifying patients with nonalcoholic fatty liver disease by using non-invasive testing in daily practice. Beyond daily practice, it is important to know where our field is advancing to offer patients more options. In Short Clinical Reviews, Dr. Aileen Bui and Dr. James Buxbaum review how the field of endohepatology is expanding into endoscopic ultrasound–guided liver biopsies, portal pressure measurements, and interventions of gastric varices.
In our Early Career feature, Dr. Corlan Eboh, Dr. Victoria Jaeger, and Dr. Dawn Sears describe how gastroenterologists are uniquely positioned for burnout and what can be done to prevent and treat it, particularly among new and transitioning gastroenterologists. In post-COVID era, practices have experienced an increase in portal messages and other non-face-to-face patient care, which may be contributing burnout.
In our Finance section this month, Dr. Luis Nieto and Dr. Jami Kinnucan review the types of patient encounters and billing options to optimize your compensation for time spent.
In Private Practice Perspectives, Dr. David Ramsey discusses why he joined a private practice and how understanding your own goals and values can guide you to a good fit in different practice models. Lastly, Dr. Dan Kroch describes his unique journey in becoming a third-space endoscopist without an advanced fellowship year and why dedicated training is the future of advanced endoscopic resection and third-space endoscopy.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: The first endoscopic retrograde cholangiopancreatography (ERCP) was first performed by an obstetrician, Dr. William McCune in 1968, and achieved by taping an external accessory channel to a duodenoscope.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
This fall, I will also be starting my first position out of fellowship. I look forward to many opportunities and challenges to come.
This month in In Focus, Dr. Mai Sedki and Dr. W. Ray Kim unpack the nuances of assessing and risk-stratifying patients with nonalcoholic fatty liver disease by using non-invasive testing in daily practice. Beyond daily practice, it is important to know where our field is advancing to offer patients more options. In Short Clinical Reviews, Dr. Aileen Bui and Dr. James Buxbaum review how the field of endohepatology is expanding into endoscopic ultrasound–guided liver biopsies, portal pressure measurements, and interventions of gastric varices.
In our Early Career feature, Dr. Corlan Eboh, Dr. Victoria Jaeger, and Dr. Dawn Sears describe how gastroenterologists are uniquely positioned for burnout and what can be done to prevent and treat it, particularly among new and transitioning gastroenterologists. In post-COVID era, practices have experienced an increase in portal messages and other non-face-to-face patient care, which may be contributing burnout.
In our Finance section this month, Dr. Luis Nieto and Dr. Jami Kinnucan review the types of patient encounters and billing options to optimize your compensation for time spent.
In Private Practice Perspectives, Dr. David Ramsey discusses why he joined a private practice and how understanding your own goals and values can guide you to a good fit in different practice models. Lastly, Dr. Dan Kroch describes his unique journey in becoming a third-space endoscopist without an advanced fellowship year and why dedicated training is the future of advanced endoscopic resection and third-space endoscopy.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: The first endoscopic retrograde cholangiopancreatography (ERCP) was first performed by an obstetrician, Dr. William McCune in 1968, and achieved by taping an external accessory channel to a duodenoscope.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
New and transitioning gastroenterologists face burnout too
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
Developing training pathways in advanced endoscopic resection and third-space endoscopy in the U.S.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
Advances in endohepatology
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
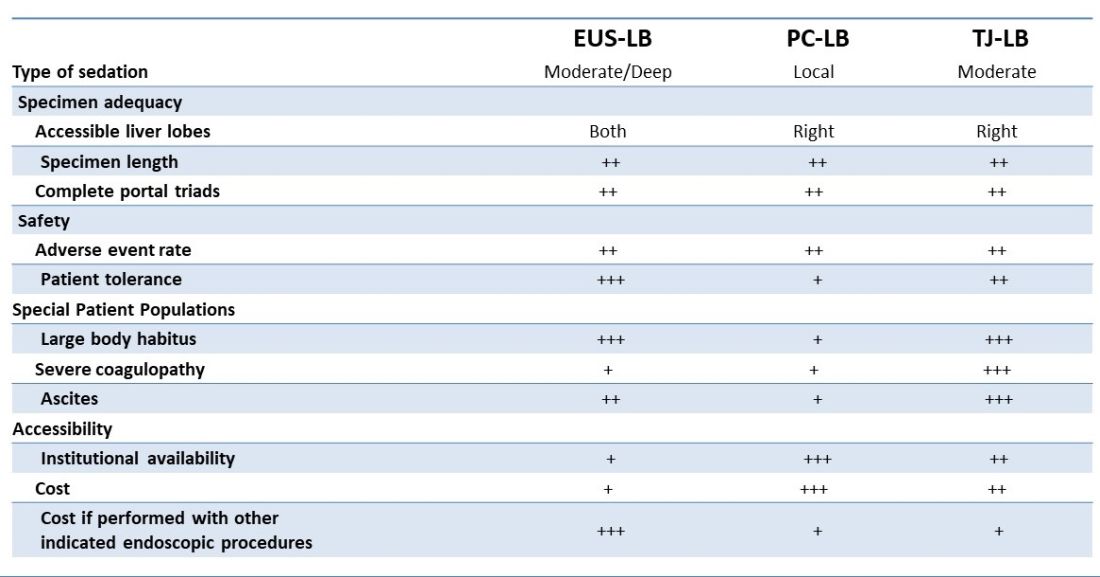
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
I selected a GI career path aligned with my goals
In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

In this video, Dr. David Ramsay of Digestive Health Specialists in Winston Salem, N.C., discusses the different career paths available to fellows and early-career physicians, and why he chose to become a private practice gastroenterologist. Dr. Ramsay shares his insights into different private practice models and what physicians should consider when beginning their careers, as well as what questions to ask when trying to determine if an organization will be a good fit for their future career plans. He has no financial conflicts relative to the topics in this video.

Increase in message volume begs the question: ‘Should we be compensated for our time?’
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.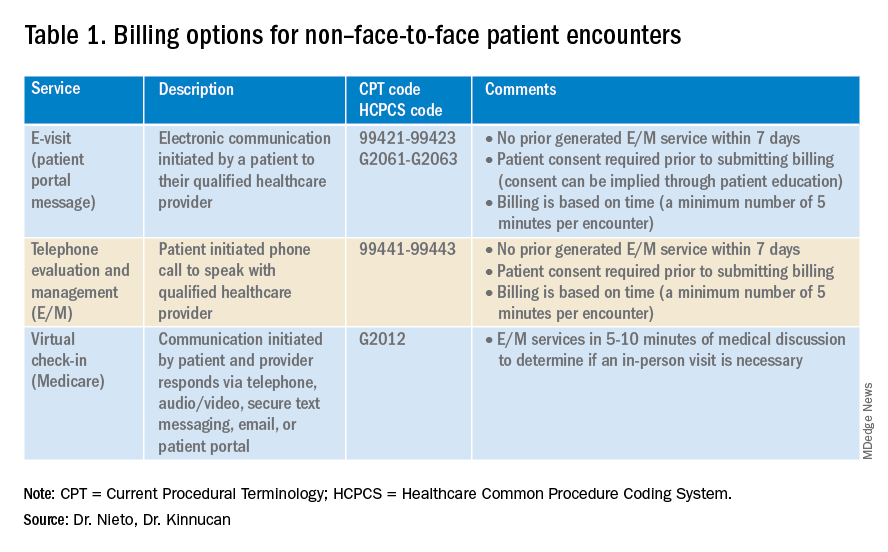
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
The American Gastroenterological Association and other gastrointestinal-specific organizations have excellent resources available to members that focus on optimizing reimbursement in your clinical and endoscopic practice.
During the COVID-19 pandemic and public health emergency (PHE), many previously noncovered services were now covered under rules of the Centers for Medicare & Medicaid Services. During the pandemic, patient portal messages increased by 157%, meaning more work for health care teams, negatively impacting physician satisfaction, and increasing burnout.1 Medical burnout has been associated with increased time spent on electronic health records, with some subspeciality gastroenterology (GI) groups having a high EHR burden, according to a recently published article in the American Journal of Gastroenterology.2
This topic is a timely discussion as several large health systems have implemented processes to bill for non–face-to-face services (termed “asynchronous care”), some of which have not been well received in the lay media. It is important to note that despite these implementations, studies have shown only 1% of all incoming portal messages would meet criteria to be submitted for reimbursement. This impact might be slightly higher in chronic care management practices.
Providers and practices have several options when considering billing for non–face-to-face encounters, which we outline in Table 1.3
The focus of this article will be to review the more common non–face-to-face evaluation and management services, such as telephone E/M (patient phone call) and e-visits (patient portal messages) as these have recently generated the most interest and discussion amongst health care providers.
Telemedicine after COVID-19 pandemic
During the beginning of the pandemic, a web-based survey study found that almost all providers in GI practices implemented some form of telemedicine to continue to provide care for patients, compared to 32% prior to the pandemic.4,5 The high demand and essential requirement for telehealth evaluation facilitated its reimbursement, eliminating the primary barrier to previous use.6
One of the new covered benefits by CMS was asynchronous telehealth care.7 The PHE ended in May 2023, and since then a qualified health care provider (QHCP) does not have the full flexibility to deliver telemedicine services across state lines. The U.S. Department of Health and Human Services has considered some telehealth policy changes after the COVID-19 PHE and many of those will be extended, at least through 2024.8 As during the pandemic, where the U.S. national payer network (CMS, state Medicaid, and private payers) and state health agencies assisted to ensure patients get the care they need by authorizing providers to be compensated for non–face-to-face services, we believe this service will continue to be part of our clinical practice.
We recommend you stay informed about local and federal laws, regulations, and alternatives for reimbursement as they may be modified at the beginning of a new calendar year. Remember, you can always talk with your revenue cycle team to clarify any query.
Telephone evaluation and management services
The patient requests to speak with you.
Telephone evaluation and management services became more widely used after the pandemic and were recognized by CMS as a covered medical service under PHE. As outlined in Table 1, there are associated codes with this service and it can only apply to an established patient in your practice. The cumulative time spent over a 7-day period without generating an immediate follow-up visit could qualify for this CPT code. However, for a patient with a high-complexity diagnosis and/or decisions being made about care, it might be better to consider a virtual office visit as this would value the complex care at a higher level than the time spent during the telephone E/M encounter.
A common question comes up: Can my nurse or support team bill for telephone care? No, only QHCP can, which means physicians and advanced practice providers can bill for this E/M service, and it does not include time spent by other members of clinical staff in patient care. However, there are CPT codes for chronic care management, which is not covered in this article.
Virtual evaluation and management services
You respond to a patient-initiated portal message.
Patient portal messages increased exponentially during the pandemic with 2.5 more minutes spent per message, resulting in more EHR work by practitioners, compared with prior to the pandemic. One study showed an immediate postpandemic increase in EHR patient-initiated messages with no return to prepandemic baseline.1
Although studies evaluating postpandemic telemedicine services are needed, we believe that this trend will continue, and for this reason, it is important to create sustainable workflows to continue to provide this patient driven avenue of care.9
E-visits are asynchronous patient or guardian portal messages that require a minimum of 5 minutes to provide medical decision-making without prior E/M services in the last 7 days. To obtain reimbursement for this service, it cannot be initiated by the provider, and patient consent must be obtained. Documentation should include this information and the time spent in the encounter. The associated CPT codes with this e-service are outlined in Table 1.
A common question is, “Are there additional codes I should use if a portal message E/M visit lasts more than 30 minutes?” No. If an e-visit lasts more than 30 minutes, the QHCP should bill the CPT code 99423. However, we would advise that, if this care requires more than 30 minutes, then either virtual or face-to-face E/M be considered for the optimal reimbursement for provider time spent. Another common question is around consent for services, and we advise providers to review this requirement with their compliance colleagues as each institution has different policies.
Virtual check-in
Medicare also covers brief communication technology–based services also known as virtual check-ins, where patients can communicate with their provider after having established care. During this brief conversation that can be via telephone, audio/video, secure text messaging, email, or patient portal, providers will determine if an in-person visit is necessary. CMS has designed G codes for these virtual check-ins that are from the Healthcare Common Procedure Coding System (HCPCS). Two codes are available for this E/M service: G2012, which is outlined in Table 1, and G2010, which covers the evaluation of images and/or recorded videos. In order to be reimbursed for a G2010 code, providers need at least a 5-minute response to make a clinical determination or give the patient a medical impression.
Patient satisfaction, physician well-being and quality of care outcomes
Large health care systems like Kaiser Permanente implemented secure message patient-physician communication (the patient portal) even before the pandemic, showing promising results in 2010 with reduction in office visits, improvement in measurable quality outcomes, and high level of patient satisfaction.10 Post pandemic, several large health care centers opted to announce the billing implementation for patient-initiated portal messages.11 A focus was placed on educating their patients about when a message will and will not be billed. Using this type of strategy can help to improve patient awareness about potential billing without affecting patient satisfaction and care outcomes. Studies have shown the EHR has contributed to physician burnout and some physicians reducing their clinical time or leaving medicine; a reduction in messaging might have a positive impact on physician well-being.
The challenge is that medical billing is not routinely included as a curriculum topic in many residency and fellowship programs; however, trainees are part of E/M services and have limited knowledge of billing processes. Unfortunately, at this time, trainees cannot submit for reimbursement for asynchronous care as described above. We hope that this brief article will help junior gastroenterologists optimize their outpatient billing practices.
Dr. Nieto is an internal medicine chief resident with WellStar Cobb Medical Center, Austell, Ga. Dr. Kinnucan is a gastroenterologist with Mayo Clinic, Jacksonville, Fla. The authors have no conflicts of interest to disclose for this article. The authors certify that no financial and grant support has been received for this article.
References
1. Holmgren AJ et al. J Am Med Inform Assoc. 2021 Dec 9. doi: 10.1093/jamia/ocab268.
2. Bali AS et al. Am J Gastroenterol. 2023 Apr 24. doi: 10.14309/ajg.0000000000002254.
3. AAFP. Family Physician. “Coding Scenario: Coding for Virtual-Digital Visits”
4. Keihanian T. et al. Telehealth Utilization in Gastroenterology Clinics Amid the COVID-19 Pandemic: Impact on Clinical Practice and Gastroenterology Training. Gastroenterology. 2020 Jun 20. doi: 10.1053/j.gastro.2020.06.040.
5. Lewin S et al. J Crohns Colitis. 2020 Oct 21. doi: 10.1093/ecco-jcc/jjaa140.
6. Perisetti A and H Goyal. Dig Dis Sci. 2021 Mar 3. doi: 10.1007/s10620-021-06874-x.
7. Telehealth.HHS.gov. Medicaid and Medicare billing for asynchronous telehealth. Updated: 2022 May 4.
8. Telehealth.HHS.gov. Telehealth policy changes after the COVID-19 public health emergency. Last updated: 2023 Jan 23.
9. Fox B and Sizemore JO. Telehealth: Fad or the future. Epic Health Research Network. 2020 Aug 18.
10. Baer D. Patient-physician e-mail communication: the kaiser permanente experience. J Oncol Pract. 2011 Jul. doi: 10.1200/JOP.2011.000323.
11. Myclevelandclinic.org. MyChart Messaging.
12. Sinsky CA et al. J Gen Intern Med. 2022 Aug 29. doi: 10.1007/s11606-022-07766-0.
May 2023 - ICYMI
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: [email protected]. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: [email protected]. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.
Gastroenterology
January 2023
Yardeni D et al. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. PMID: 36243037; PMCID: PMC9772068.
Laine L et al. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023 Jan;164(1):61-71. doi: 10.1053/j.gastro.2022.09.041. Epub 2022 Oct 10. PMID: 36228734.
February 2023
Ufere NN et al. Promoting Prognostic Understanding and Health Equity for Patients With Advanced Liver Disease: Using “Best Case/Worst Case.” Gastroenterology. 2023 Feb;164(2):171-6. doi: 10.1053/j.gastro.2022.12.005. PMID: 36702571.
March 2023
Heath JK et al. Training Generations of Clinician Educators: Applying the Novel Clinician Educator Milestones to Faculty Development. Gastroenterology. 2023 Mar;164(3):325-8.e1. doi: 10.1053/j.gastro.2022.12.003. Epub 2022 Dec 9. PMID: 36509156.
Singh S et al. AGA Clinical Guidelines Committee. Electronic address: [email protected]. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023 Mar;164(3):344-72. doi: 10.1053/j.gastro.2022.12.007. PMID: 36822736.
Clinical Gastroenterology and Hepatology
January 2023
Speicher LL and Francis D. Improving Employee Experience: Reducing Burnout, Decreasing Turnover and Building Well-being. Clin Gastroenterol Hepatol. 2023 Jan;21(1):11-4. doi: 10.1016/j.cgh.2022.09.020. Epub 2022 Sep 22. PMID: 36155248; PMCID: PMC9547273.
Penagini R et al. Rapid Drink Challenge During High-resolution Manometry for Evaluation of Esophageal Emptying in Treated Achalasia. Clin Gastroenterol Hepatol. 2023 Jan;21(1):55-63. doi: 10.1016/j.cgh.2022.02.047. Epub 2022 Feb 28. PMID: 35240328.
February 2023
Zaki TA et al. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023 Feb;21(2):497-506.e3. doi: 10.1016/j.cgh.2022.05.035. Epub 2022 Jun 16. PMID: 35716905; PMCID: PMC9835097.
Brenner DM et al. Rare, Overlooked, or Underappreciated Causes of Recurrent Abdominal Pain: A Primer for Gastroenterologists. Clin Gastroenterol Hepatol. 2023 Feb;21(2):264-79. doi: 10.1016/j.cgh.2022.09.022. Epub 2022 Sep 27. PMID: 36180010.
March 2023
Hanna M et al. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023 Mar;21(3):604-16. doi: 10.1016/j.cgh.2022.12.008. Epub 2022 Dec 17. PMID: 36539002; PMCID: PMC9974876.
Ormsby EL et al. Association of Standardized Radiology Reporting and Management of Abdominal CT and MRI With Diagnosis of Pancreatic Cancer. Clin Gastroenterol Hepatol. 2023 Mar;21(3):644-52.e2. doi: 10.1016/j.cgh.2022.03.047. Epub 2022 Apr 15. PMID: 35436626.
Techniques and Innovations in Gastrointestinal Endoscopy
Mohapatra S et al. (Accepted/In press). Outcomes of Endoscopic Resection for Colorectal Polyps with High-Grade Dysplasia or Intramucosal Cancer. Tech Innov Gastrointest Endosc. 2023 Jan 22. doi: 10.1016/j.tige.2023.01.003.
Holzwanger EA et al. Improving Dysplasia Detection in Barrett’s Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy. Tech Innov Gastrointest Endosc. 2023;25(2):157-66. doi: 10.1016/j.tige.2023.01.002.

















