User login
Caring for LGBTQ+ Patients with IBD
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.
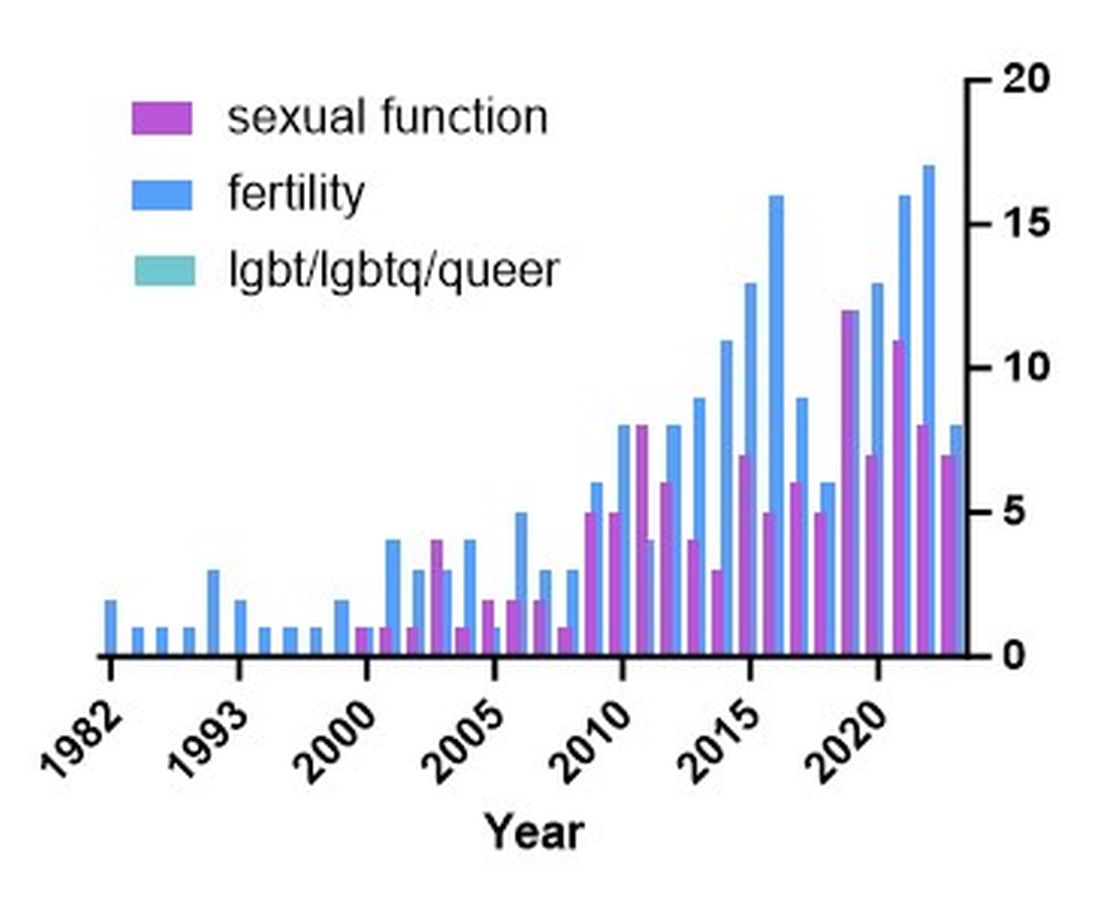
Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
One-year anniversary
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Resources to help new GI fellows thrive
The AGA Gastro Squad is excited you’re here and we’re here to help.
AGA has resources and programs specifically for fellows. Whether you’re embarking on your first year or your last one, these tools can be used at any point to help you during your training.
Start here:
Review and bookmark these guides to make the most out of fellowship.
10 tips for new GI fellows
First year fellows guide
Small talk, big topics
A podcast for trainees and early career GIs from fellow early career GIs. Join our hosts for conversations with leaders in gastroenterology. They offer guidance for training and dissect the changing landscape of GI and what it means for you. Check out recent episodes and download and subscribe wherever podcasts are available.
AGA university
Get your clinical questions answered or take a deeper dive into different topic areas with AGA University. Courses include Gastro Bites sessions about topics such as examining new guideline recommendations for managing chronic idiopathic constipation in adults. CME is available. Access AGA University here.
AGA young delegates program
Get plugged in with your fellow engaged early career GIs and learn about volunteer opportunities to represent AGA. The only criteria to participate is to be an AGA member. Join now.
Get more advice
Interested in receiving additional career guidance or professional development opportunities? Connect with the AGA Gastro Squad at @AmerGastroAssn on X and on Instagram.
The AGA Gastro Squad is excited you’re here and we’re here to help.
AGA has resources and programs specifically for fellows. Whether you’re embarking on your first year or your last one, these tools can be used at any point to help you during your training.
Start here:
Review and bookmark these guides to make the most out of fellowship.
10 tips for new GI fellows
First year fellows guide
Small talk, big topics
A podcast for trainees and early career GIs from fellow early career GIs. Join our hosts for conversations with leaders in gastroenterology. They offer guidance for training and dissect the changing landscape of GI and what it means for you. Check out recent episodes and download and subscribe wherever podcasts are available.
AGA university
Get your clinical questions answered or take a deeper dive into different topic areas with AGA University. Courses include Gastro Bites sessions about topics such as examining new guideline recommendations for managing chronic idiopathic constipation in adults. CME is available. Access AGA University here.
AGA young delegates program
Get plugged in with your fellow engaged early career GIs and learn about volunteer opportunities to represent AGA. The only criteria to participate is to be an AGA member. Join now.
Get more advice
Interested in receiving additional career guidance or professional development opportunities? Connect with the AGA Gastro Squad at @AmerGastroAssn on X and on Instagram.
The AGA Gastro Squad is excited you’re here and we’re here to help.
AGA has resources and programs specifically for fellows. Whether you’re embarking on your first year or your last one, these tools can be used at any point to help you during your training.
Start here:
Review and bookmark these guides to make the most out of fellowship.
10 tips for new GI fellows
First year fellows guide
Small talk, big topics
A podcast for trainees and early career GIs from fellow early career GIs. Join our hosts for conversations with leaders in gastroenterology. They offer guidance for training and dissect the changing landscape of GI and what it means for you. Check out recent episodes and download and subscribe wherever podcasts are available.
AGA university
Get your clinical questions answered or take a deeper dive into different topic areas with AGA University. Courses include Gastro Bites sessions about topics such as examining new guideline recommendations for managing chronic idiopathic constipation in adults. CME is available. Access AGA University here.
AGA young delegates program
Get plugged in with your fellow engaged early career GIs and learn about volunteer opportunities to represent AGA. The only criteria to participate is to be an AGA member. Join now.
Get more advice
Interested in receiving additional career guidance or professional development opportunities? Connect with the AGA Gastro Squad at @AmerGastroAssn on X and on Instagram.
Propel your academic research opportunities with AGA FORWARD
The FORWARD program supports and facilitates a unique pathway for physician-scientists from underrepresented populations to advance their careers and make a meaningful impact as leaders within AGA and in academic medicine. Here’s how the AGA FORWARD program creates a supportive environment that fosters career advancement, leadership development and a growing community:
- Learn important skills critical for a successful research career, expert grant writing training and coaching from esteemed GI mentors.
- Connect with a community of GI leaders through one-on-one mentorship and near-peer mentors in medicine from underrepresented populations.
- Develop leadership skills vital to directing your lab, your institution, the field, and AGA, partnering with an executive coach to assess, identify and work on key strengths and areas of improvement.
Embark on a transformative journey toward a successful and fulfilling career in academic medicine.
Apply today.
The FORWARD program supports and facilitates a unique pathway for physician-scientists from underrepresented populations to advance their careers and make a meaningful impact as leaders within AGA and in academic medicine. Here’s how the AGA FORWARD program creates a supportive environment that fosters career advancement, leadership development and a growing community:
- Learn important skills critical for a successful research career, expert grant writing training and coaching from esteemed GI mentors.
- Connect with a community of GI leaders through one-on-one mentorship and near-peer mentors in medicine from underrepresented populations.
- Develop leadership skills vital to directing your lab, your institution, the field, and AGA, partnering with an executive coach to assess, identify and work on key strengths and areas of improvement.
Embark on a transformative journey toward a successful and fulfilling career in academic medicine.
Apply today.
The FORWARD program supports and facilitates a unique pathway for physician-scientists from underrepresented populations to advance their careers and make a meaningful impact as leaders within AGA and in academic medicine. Here’s how the AGA FORWARD program creates a supportive environment that fosters career advancement, leadership development and a growing community:
- Learn important skills critical for a successful research career, expert grant writing training and coaching from esteemed GI mentors.
- Connect with a community of GI leaders through one-on-one mentorship and near-peer mentors in medicine from underrepresented populations.
- Develop leadership skills vital to directing your lab, your institution, the field, and AGA, partnering with an executive coach to assess, identify and work on key strengths and areas of improvement.
Embark on a transformative journey toward a successful and fulfilling career in academic medicine.
Apply today.
November 2023 - ICYMI
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Five personal finance questions for the young GI
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
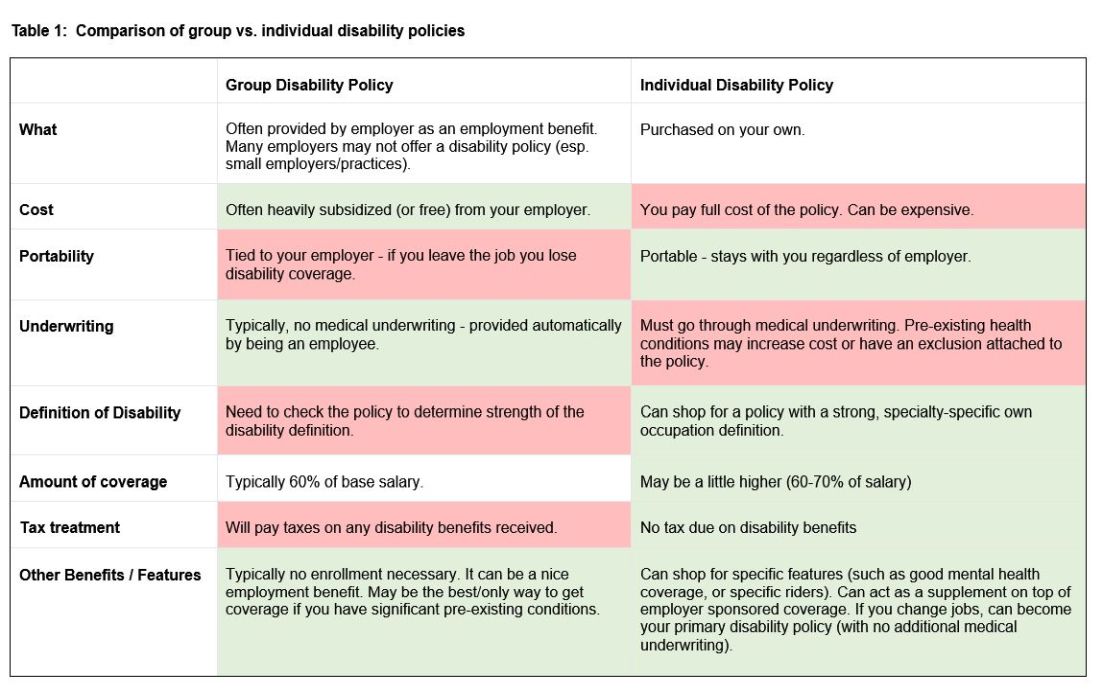
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
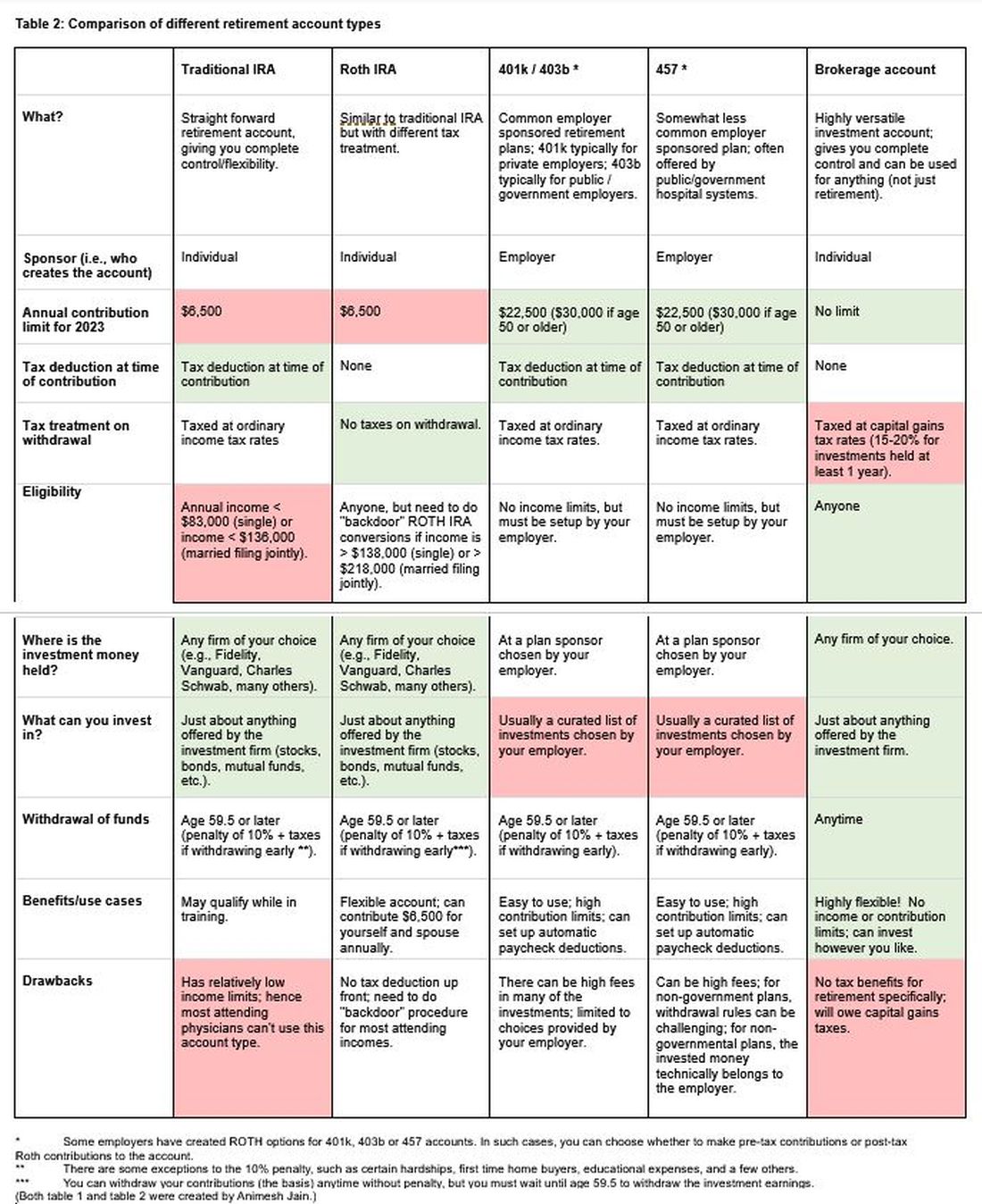
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.

Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.

Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.

Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.

Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
Advances in endoscopic therapies in inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16
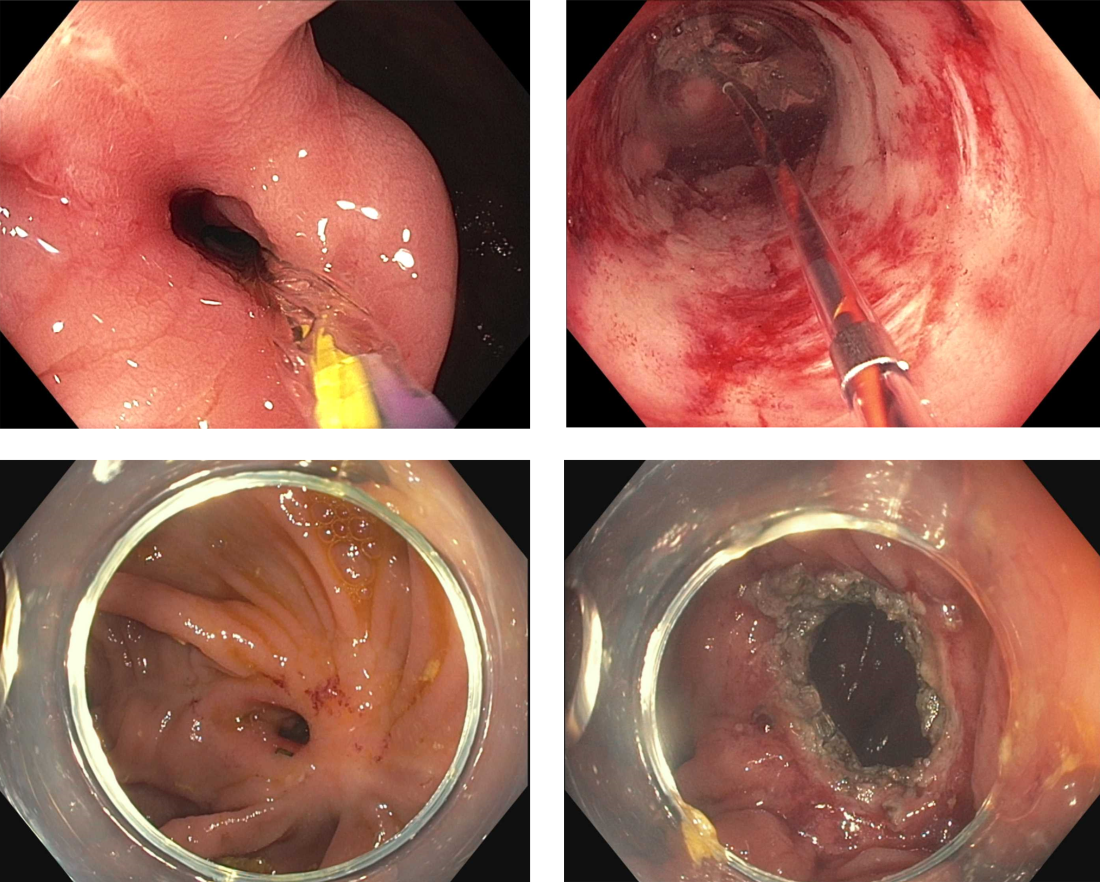
Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Early career considerations for gastroenterologists interested in diversity, equity, and inclusion roles

Highlighting the importance of DEI across all aspects of medicine is long overdue, and the field of gastroenterology is no exception. Diversity in the gastroenterology workforce still has significant room for improvement with only 12% of all gastroenterology fellows in 2018 identifying as Black, Latino/a/x, American Indian or Alaskan Native, or Native Hawaiian or Pacific Islander.1 Moreover, only 4.4% of practicing gastroenterologists identify as Black, 6.7% identify as Latino/a/x, 0.1% as American Indian or Alaskan Native, and 0.003% as Native Hawaiian or Pacific Islander.2
The intensified focus on diversity in GI is welcomed, but increasing physician workforce diversity is only one of the necessary steps. If our ultimate goal is to improve health outcomes and achieve health equity for historically marginalized racial, ethnic, and socioeconomically disadvantaged communities, we must critically evaluate the path beyond just enhancing workforce diversity.
Black and Latino/a/x physicians are more likely to care for historically marginalized communities,3 which has been shown to improve all-cause mortality and reduce racial disparities.4 Additionally, diverse work teams are more innovative and productive.5 Therefore, expanding diversity must include 1) providing equitable policies and access to opportunities and promotions; 2) building inclusive environments in our institutions and practices; and 3) providing space for all people to feel like they can belong, feel respected at work, and genuinely have their opinions and ideas valued. What diversity, equity, inclusion, and belonging provide for us and our patients are avenues to thrive, solve complex problems, and tackle prominent issues within our institutions, workplaces, and communities.
To this end, many academic centers, hospitals, and private practice entities have produced a flurry of new DEI initiatives coupled with titles and roles. Some of these roles have thankfully brought recognition and economic compensation to the people doing this work. Still, as an early career gastroenterologist, you may be offered or are considering taking on a DEI role during your early career. As two underrepresented minority women in medicine who took on DEI roles with their first jobs, we wanted to highlight a few aspects to think about during your early career:
Does the DEI role come with resources?
Historically, DEI efforts were treated as “extra work,” or an activity that was done using one’s own personal time. In addition, this work called upon the small number of physicians underrepresented in medicine, largely uncompensated and with an exorbitant minority tax during a critical moment in establishing their early careers. DEI should no longer be seen as an extracurricular activity but as a vital component of an institution’s success.
If you are considering a DEI role, the first question to ask is, “Does this role come with extra compensation or protected time?” We highly recommend not taking on the role if the answer is no. If your institution or employer is only offering increased minority tax, you are being set up to either fail, burn out, or both. Your employer or institution does not appear to value your time or effort in DEI, and you should interpret their lack of compensation or protected time as such.
If the answer is yes, then here are a few other things to consider: Is there institutional support for you to be successful in your new role? As DEI work challenges you to come up with solutions to combat years of historic marginalization for racial and ethnic minorities, this work can sometimes feel overwhelming and isolating. The importance of the DEI community and mentorship within and outside your institution is critical. You should consider joining DEI working groups or committees through GI national societies, the Association of American Medical Colleges, or the Accreditation Council for Graduate Medical Education. You can also connect with a fantastic network of people engaged in this work via social media and lean on friends and colleagues leading similar initiatives across the country.
Other critical logistical questions are if your role will come with administrative support, whether there is a budget for programs or events, and whether your institution/employer will support you in seeking continued professional development for your DEI role.6
Make sure to understand the “ask” from your division, department, or company.
Before confirming you are willing to take on this role, get a clear vision of what you are being asked to accomplish. There are so many opportunities to improve the DEI landscape. Therefore, knowing what you are specifically being asked to do will be critical to your success.
Are you being asked to work on diversity?
Does your institution want you to focus on and improve the recruitment and retention of trainees, physicians, or staff underrepresented in medicine? If so, you will need to have access to all the prior work and statistics. Capture the landscape before your interventions (% underrepresented in medicine [URiM] trainees, % URiM faculty at each level, % of URiM trainees retained as faculty, % of URiM faculty being promoted each year, etc.) This will allow you to determine the outcomes of your proposed improvements or programs.
Is your employer focused on equity?
Are you being asked to think about ways to operationalize improved patient health equity, or are you being asked to build equitable opportunities/programs for career advancement for URiMs at your institution? For either equity issue, you first need to understand the scope of the problem to ask for the necessary resources for a potential solution. Discuss timeline expectations, as equity work is a marathon and may take years to move the needle on any particular issue. This timeline is also critical for your employer to be aware of and support, as unrealistic timelines and expectations will also set you up for failure.
Or, are you being asked to concentrate on inclusion?
Does your institution need an assessment of how inclusive the climate is for trainees, staff, or physicians? Does this assessment align with your division or department’s impression, and how do you plan to work toward potential solutions for improvement?
Although diversity, equity, and inclusion are interconnected entities, they all have distinct objectives and solutions. It is essential to understand your vision and your employer’s vision for this role. If they are not aligned, having early and in-depth conversations about aligning your visions will set you on a path to success in your early career.
Know your why or more importantly, your who?
Early career physicians who are considering taking on DEI work do so for a reason. Being passionate about this type of work is usually born from a personal experience or your deep-rooted values. For us, experiencing and witnessing health disparities for our family members and people who look like us are what initially fueled our passion for this work. Additional experiences with trainees and patients keep us invigorated to continue highlighting the importance of DEI and encourage others to be passionate about DEI’s huge value added. As DEI work can come with challenges, remembering and re-centering on why you are passionate about this work or who you are engaging in this work for can keep you going.
There are several aspects to consider before taking on a DEI role, but overall, the work is rewarding and can be a great addition to the building blocks of your early career. In the short term, you build a DEI community network of peers, mentors, colleagues, and friends beyond your immediate institution and specialty. You also can demonstrate your leadership skills and potential early on in your career. In the long-term, engaging in these types of roles helps build a climate and culture that is conducive to enacting change for our patients and communities, including advancing healthcare equity and working toward recruitment, retention, and expansion efforts for our trainees and faculty. Overall, we think this type of work in your early career can be an integral part of your personal and professional development, while also having an impact that ripples beyond the walls of the endoscopy suite.
Dr. Fritz is an assistant professor of medicine in the division of gastroenterology at Washington University School of Medicine, St. Louis. Dr. Rodriguez is a gastroenterologist with Brigham and Women’s Hospital in Boston. Neither Dr. Rodriguez nor Dr. Fritz disclosed no conflicts of interest.
References
1. Santhosh L,Babik JM. Trends in racial and ethnic diversity in internal medicine subspecialty fellowships from 2006 to 2018. JAMA Network Open 2020;3:e1920482-e1920482.
2. Colleges AoAM. Physician Specialty Data Report/Active physicians who identified as Black or African-American, 2021. 2022.
3. Komaromy M et al. The role of black and Hispanic physicians in providing health care for underserved populations. New England Journal of Medicine 1996;334:1305-10.
4. Snyder JE et al. Black representation in the primary care physician workforce and its association with population life expectancy and mortality rates in the US. JAMA Network Open 2023;6:e236687-e236687.
5. Page S. Diversity bonuses and the business case. The Diversity Bonus: Princeton University Press, 2017:184-208.
6. Vela MB et al. Diversity, equity, and inclusion officer position available: Proceed with caution. Journal of Graduate Medical Education 2021;13:771-3.
Helpful resources
Diversity and Inclusion Toolkit Resources, AAMC
Blackinggastro.org, The Association of Black Gastroenterologists and Hepatologists (ABGH)
Podcast: Clinical Problem Solvers: Anti-Racism in Medicine

Highlighting the importance of DEI across all aspects of medicine is long overdue, and the field of gastroenterology is no exception. Diversity in the gastroenterology workforce still has significant room for improvement with only 12% of all gastroenterology fellows in 2018 identifying as Black, Latino/a/x, American Indian or Alaskan Native, or Native Hawaiian or Pacific Islander.1 Moreover, only 4.4% of practicing gastroenterologists identify as Black, 6.7% identify as Latino/a/x, 0.1% as American Indian or Alaskan Native, and 0.003% as Native Hawaiian or Pacific Islander.2
The intensified focus on diversity in GI is welcomed, but increasing physician workforce diversity is only one of the necessary steps. If our ultimate goal is to improve health outcomes and achieve health equity for historically marginalized racial, ethnic, and socioeconomically disadvantaged communities, we must critically evaluate the path beyond just enhancing workforce diversity.
Black and Latino/a/x physicians are more likely to care for historically marginalized communities,3 which has been shown to improve all-cause mortality and reduce racial disparities.4 Additionally, diverse work teams are more innovative and productive.5 Therefore, expanding diversity must include 1) providing equitable policies and access to opportunities and promotions; 2) building inclusive environments in our institutions and practices; and 3) providing space for all people to feel like they can belong, feel respected at work, and genuinely have their opinions and ideas valued. What diversity, equity, inclusion, and belonging provide for us and our patients are avenues to thrive, solve complex problems, and tackle prominent issues within our institutions, workplaces, and communities.
To this end, many academic centers, hospitals, and private practice entities have produced a flurry of new DEI initiatives coupled with titles and roles. Some of these roles have thankfully brought recognition and economic compensation to the people doing this work. Still, as an early career gastroenterologist, you may be offered or are considering taking on a DEI role during your early career. As two underrepresented minority women in medicine who took on DEI roles with their first jobs, we wanted to highlight a few aspects to think about during your early career:
Does the DEI role come with resources?
Historically, DEI efforts were treated as “extra work,” or an activity that was done using one’s own personal time. In addition, this work called upon the small number of physicians underrepresented in medicine, largely uncompensated and with an exorbitant minority tax during a critical moment in establishing their early careers. DEI should no longer be seen as an extracurricular activity but as a vital component of an institution’s success.
If you are considering a DEI role, the first question to ask is, “Does this role come with extra compensation or protected time?” We highly recommend not taking on the role if the answer is no. If your institution or employer is only offering increased minority tax, you are being set up to either fail, burn out, or both. Your employer or institution does not appear to value your time or effort in DEI, and you should interpret their lack of compensation or protected time as such.
If the answer is yes, then here are a few other things to consider: Is there institutional support for you to be successful in your new role? As DEI work challenges you to come up with solutions to combat years of historic marginalization for racial and ethnic minorities, this work can sometimes feel overwhelming and isolating. The importance of the DEI community and mentorship within and outside your institution is critical. You should consider joining DEI working groups or committees through GI national societies, the Association of American Medical Colleges, or the Accreditation Council for Graduate Medical Education. You can also connect with a fantastic network of people engaged in this work via social media and lean on friends and colleagues leading similar initiatives across the country.
Other critical logistical questions are if your role will come with administrative support, whether there is a budget for programs or events, and whether your institution/employer will support you in seeking continued professional development for your DEI role.6
Make sure to understand the “ask” from your division, department, or company.
Before confirming you are willing to take on this role, get a clear vision of what you are being asked to accomplish. There are so many opportunities to improve the DEI landscape. Therefore, knowing what you are specifically being asked to do will be critical to your success.
Are you being asked to work on diversity?
Does your institution want you to focus on and improve the recruitment and retention of trainees, physicians, or staff underrepresented in medicine? If so, you will need to have access to all the prior work and statistics. Capture the landscape before your interventions (% underrepresented in medicine [URiM] trainees, % URiM faculty at each level, % of URiM trainees retained as faculty, % of URiM faculty being promoted each year, etc.) This will allow you to determine the outcomes of your proposed improvements or programs.
Is your employer focused on equity?
Are you being asked to think about ways to operationalize improved patient health equity, or are you being asked to build equitable opportunities/programs for career advancement for URiMs at your institution? For either equity issue, you first need to understand the scope of the problem to ask for the necessary resources for a potential solution. Discuss timeline expectations, as equity work is a marathon and may take years to move the needle on any particular issue. This timeline is also critical for your employer to be aware of and support, as unrealistic timelines and expectations will also set you up for failure.
Or, are you being asked to concentrate on inclusion?
Does your institution need an assessment of how inclusive the climate is for trainees, staff, or physicians? Does this assessment align with your division or department’s impression, and how do you plan to work toward potential solutions for improvement?
Although diversity, equity, and inclusion are interconnected entities, they all have distinct objectives and solutions. It is essential to understand your vision and your employer’s vision for this role. If they are not aligned, having early and in-depth conversations about aligning your visions will set you on a path to success in your early career.
Know your why or more importantly, your who?
Early career physicians who are considering taking on DEI work do so for a reason. Being passionate about this type of work is usually born from a personal experience or your deep-rooted values. For us, experiencing and witnessing health disparities for our family members and people who look like us are what initially fueled our passion for this work. Additional experiences with trainees and patients keep us invigorated to continue highlighting the importance of DEI and encourage others to be passionate about DEI’s huge value added. As DEI work can come with challenges, remembering and re-centering on why you are passionate about this work or who you are engaging in this work for can keep you going.
There are several aspects to consider before taking on a DEI role, but overall, the work is rewarding and can be a great addition to the building blocks of your early career. In the short term, you build a DEI community network of peers, mentors, colleagues, and friends beyond your immediate institution and specialty. You also can demonstrate your leadership skills and potential early on in your career. In the long-term, engaging in these types of roles helps build a climate and culture that is conducive to enacting change for our patients and communities, including advancing healthcare equity and working toward recruitment, retention, and expansion efforts for our trainees and faculty. Overall, we think this type of work in your early career can be an integral part of your personal and professional development, while also having an impact that ripples beyond the walls of the endoscopy suite.
Dr. Fritz is an assistant professor of medicine in the division of gastroenterology at Washington University School of Medicine, St. Louis. Dr. Rodriguez is a gastroenterologist with Brigham and Women’s Hospital in Boston. Neither Dr. Rodriguez nor Dr. Fritz disclosed no conflicts of interest.
References
1. Santhosh L,Babik JM. Trends in racial and ethnic diversity in internal medicine subspecialty fellowships from 2006 to 2018. JAMA Network Open 2020;3:e1920482-e1920482.
2. Colleges AoAM. Physician Specialty Data Report/Active physicians who identified as Black or African-American, 2021. 2022.
3. Komaromy M et al. The role of black and Hispanic physicians in providing health care for underserved populations. New England Journal of Medicine 1996;334:1305-10.
4. Snyder JE et al. Black representation in the primary care physician workforce and its association with population life expectancy and mortality rates in the US. JAMA Network Open 2023;6:e236687-e236687.
5. Page S. Diversity bonuses and the business case. The Diversity Bonus: Princeton University Press, 2017:184-208.
6. Vela MB et al. Diversity, equity, and inclusion officer position available: Proceed with caution. Journal of Graduate Medical Education 2021;13:771-3.
Helpful resources
Diversity and Inclusion Toolkit Resources, AAMC
Blackinggastro.org, The Association of Black Gastroenterologists and Hepatologists (ABGH)
Podcast: Clinical Problem Solvers: Anti-Racism in Medicine

Highlighting the importance of DEI across all aspects of medicine is long overdue, and the field of gastroenterology is no exception. Diversity in the gastroenterology workforce still has significant room for improvement with only 12% of all gastroenterology fellows in 2018 identifying as Black, Latino/a/x, American Indian or Alaskan Native, or Native Hawaiian or Pacific Islander.1 Moreover, only 4.4% of practicing gastroenterologists identify as Black, 6.7% identify as Latino/a/x, 0.1% as American Indian or Alaskan Native, and 0.003% as Native Hawaiian or Pacific Islander.2
The intensified focus on diversity in GI is welcomed, but increasing physician workforce diversity is only one of the necessary steps. If our ultimate goal is to improve health outcomes and achieve health equity for historically marginalized racial, ethnic, and socioeconomically disadvantaged communities, we must critically evaluate the path beyond just enhancing workforce diversity.
Black and Latino/a/x physicians are more likely to care for historically marginalized communities,3 which has been shown to improve all-cause mortality and reduce racial disparities.4 Additionally, diverse work teams are more innovative and productive.5 Therefore, expanding diversity must include 1) providing equitable policies and access to opportunities and promotions; 2) building inclusive environments in our institutions and practices; and 3) providing space for all people to feel like they can belong, feel respected at work, and genuinely have their opinions and ideas valued. What diversity, equity, inclusion, and belonging provide for us and our patients are avenues to thrive, solve complex problems, and tackle prominent issues within our institutions, workplaces, and communities.
To this end, many academic centers, hospitals, and private practice entities have produced a flurry of new DEI initiatives coupled with titles and roles. Some of these roles have thankfully brought recognition and economic compensation to the people doing this work. Still, as an early career gastroenterologist, you may be offered or are considering taking on a DEI role during your early career. As two underrepresented minority women in medicine who took on DEI roles with their first jobs, we wanted to highlight a few aspects to think about during your early career:
Does the DEI role come with resources?
Historically, DEI efforts were treated as “extra work,” or an activity that was done using one’s own personal time. In addition, this work called upon the small number of physicians underrepresented in medicine, largely uncompensated and with an exorbitant minority tax during a critical moment in establishing their early careers. DEI should no longer be seen as an extracurricular activity but as a vital component of an institution’s success.
If you are considering a DEI role, the first question to ask is, “Does this role come with extra compensation or protected time?” We highly recommend not taking on the role if the answer is no. If your institution or employer is only offering increased minority tax, you are being set up to either fail, burn out, or both. Your employer or institution does not appear to value your time or effort in DEI, and you should interpret their lack of compensation or protected time as such.
If the answer is yes, then here are a few other things to consider: Is there institutional support for you to be successful in your new role? As DEI work challenges you to come up with solutions to combat years of historic marginalization for racial and ethnic minorities, this work can sometimes feel overwhelming and isolating. The importance of the DEI community and mentorship within and outside your institution is critical. You should consider joining DEI working groups or committees through GI national societies, the Association of American Medical Colleges, or the Accreditation Council for Graduate Medical Education. You can also connect with a fantastic network of people engaged in this work via social media and lean on friends and colleagues leading similar initiatives across the country.
Other critical logistical questions are if your role will come with administrative support, whether there is a budget for programs or events, and whether your institution/employer will support you in seeking continued professional development for your DEI role.6
Make sure to understand the “ask” from your division, department, or company.
Before confirming you are willing to take on this role, get a clear vision of what you are being asked to accomplish. There are so many opportunities to improve the DEI landscape. Therefore, knowing what you are specifically being asked to do will be critical to your success.
Are you being asked to work on diversity?
Does your institution want you to focus on and improve the recruitment and retention of trainees, physicians, or staff underrepresented in medicine? If so, you will need to have access to all the prior work and statistics. Capture the landscape before your interventions (% underrepresented in medicine [URiM] trainees, % URiM faculty at each level, % of URiM trainees retained as faculty, % of URiM faculty being promoted each year, etc.) This will allow you to determine the outcomes of your proposed improvements or programs.
Is your employer focused on equity?
Are you being asked to think about ways to operationalize improved patient health equity, or are you being asked to build equitable opportunities/programs for career advancement for URiMs at your institution? For either equity issue, you first need to understand the scope of the problem to ask for the necessary resources for a potential solution. Discuss timeline expectations, as equity work is a marathon and may take years to move the needle on any particular issue. This timeline is also critical for your employer to be aware of and support, as unrealistic timelines and expectations will also set you up for failure.
Or, are you being asked to concentrate on inclusion?
Does your institution need an assessment of how inclusive the climate is for trainees, staff, or physicians? Does this assessment align with your division or department’s impression, and how do you plan to work toward potential solutions for improvement?
Although diversity, equity, and inclusion are interconnected entities, they all have distinct objectives and solutions. It is essential to understand your vision and your employer’s vision for this role. If they are not aligned, having early and in-depth conversations about aligning your visions will set you on a path to success in your early career.
Know your why or more importantly, your who?
Early career physicians who are considering taking on DEI work do so for a reason. Being passionate about this type of work is usually born from a personal experience or your deep-rooted values. For us, experiencing and witnessing health disparities for our family members and people who look like us are what initially fueled our passion for this work. Additional experiences with trainees and patients keep us invigorated to continue highlighting the importance of DEI and encourage others to be passionate about DEI’s huge value added. As DEI work can come with challenges, remembering and re-centering on why you are passionate about this work or who you are engaging in this work for can keep you going.
There are several aspects to consider before taking on a DEI role, but overall, the work is rewarding and can be a great addition to the building blocks of your early career. In the short term, you build a DEI community network of peers, mentors, colleagues, and friends beyond your immediate institution and specialty. You also can demonstrate your leadership skills and potential early on in your career. In the long-term, engaging in these types of roles helps build a climate and culture that is conducive to enacting change for our patients and communities, including advancing healthcare equity and working toward recruitment, retention, and expansion efforts for our trainees and faculty. Overall, we think this type of work in your early career can be an integral part of your personal and professional development, while also having an impact that ripples beyond the walls of the endoscopy suite.
Dr. Fritz is an assistant professor of medicine in the division of gastroenterology at Washington University School of Medicine, St. Louis. Dr. Rodriguez is a gastroenterologist with Brigham and Women’s Hospital in Boston. Neither Dr. Rodriguez nor Dr. Fritz disclosed no conflicts of interest.
References
1. Santhosh L,Babik JM. Trends in racial and ethnic diversity in internal medicine subspecialty fellowships from 2006 to 2018. JAMA Network Open 2020;3:e1920482-e1920482.
2. Colleges AoAM. Physician Specialty Data Report/Active physicians who identified as Black or African-American, 2021. 2022.
3. Komaromy M et al. The role of black and Hispanic physicians in providing health care for underserved populations. New England Journal of Medicine 1996;334:1305-10.
4. Snyder JE et al. Black representation in the primary care physician workforce and its association with population life expectancy and mortality rates in the US. JAMA Network Open 2023;6:e236687-e236687.
5. Page S. Diversity bonuses and the business case. The Diversity Bonus: Princeton University Press, 2017:184-208.
6. Vela MB et al. Diversity, equity, and inclusion officer position available: Proceed with caution. Journal of Graduate Medical Education 2021;13:771-3.
Helpful resources
Diversity and Inclusion Toolkit Resources, AAMC
Blackinggastro.org, The Association of Black Gastroenterologists and Hepatologists (ABGH)
Podcast: Clinical Problem Solvers: Anti-Racism in Medicine
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
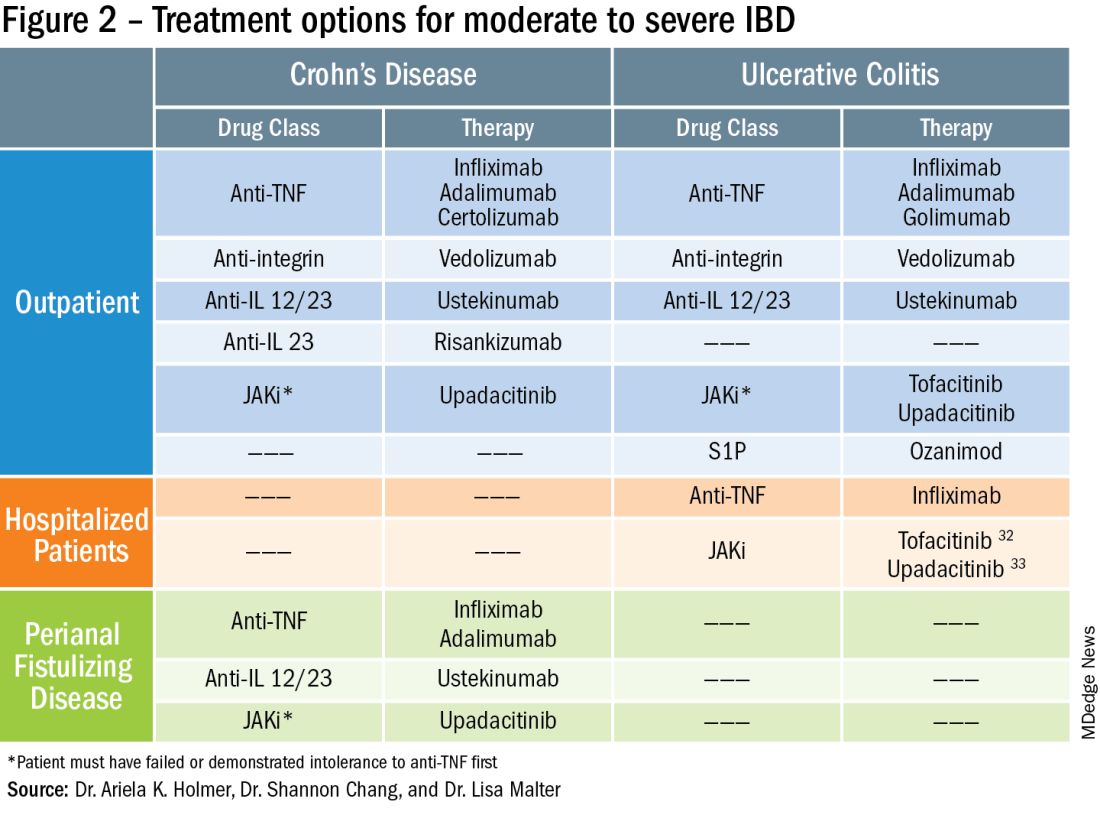
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Working with industry in private practice gastroenterology
In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.

In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.

In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.
















