User login
Supporting career development for women in gastroenterology
When I was in fellowship in the late 1990s, it was rare to see women at many of the big gastroenterology conferences. And in terms of presentations, there was maybe one session led by or for women at lunchtime. These conferences were the only events I had ever been to where the line for the men’s room was longer than the line for the women’s room.
Over the years, the lines for the women’s room have gotten longer, and the sessions led by female gastroenterologists have grown exponentially. However, women are still underrepresented in our field. Two out of five GI fellows are women, but women constitute less than 18% of practicing gastroenterologists. And the number of women in leadership positions is even lower.
Women in medicine face many challenges
According to a report in JAMA Network Open, women have lower starting salaries more than 90% of the time, which can create income disparities in earning potential throughout our entire careers.
Other studies suggest that female physicians also spend more time with patients and answering messages from patients and colleagues as well. This extra time, although it is done in small increments, adds up quickly and could suggest the pay gap between women and men is wider than we think.
Of course, female physicians still spend more time parenting children and doing household labor. A study found that female physicians spent 8.5 hours more per week on activities that support the family and household.
We’ve been discussing equity for women in medicine, and in the workplace, for decades. But events over the past several years – such as the killing of George Floyd and the formation of the #MeToo movement in response to workplace sexual harassment – have accelerated a paradigm shift in how organizations are focusing on diversity, equity, and inclusion (DEI) and creating cultures that support leadership development for women.
The Gastro Health Women’s Network
In 2020, the leadership of Gastro Health reiterated its commitment to fight discrimination and support equity by sending out a company-wide correspondence that encouraged us to be good stewards within our communities during these turbulent times.
This led to the development of the Gastro Health DEI Council and the Gastro Health Women’s Network, led by Dr. Asma Khapra and based on the framework developed by Dr. Dawn Sears. The programs developed by Dr. Sears are focused on facilitating authentic and supportive relationships, and they helped us create a network for women focused on recruitment, mentorship and retention, networking and social events, and leadership development.
Our network started with a meet & greet, inviting all women in Gastro Health to join a virtual call and get to know each other in an informal setting. This was a great way to introduce people to each other in our natural elements. It was wonderful to see how people are when they are at home and not working.
Recruiting female gastroenterologists
Even though about half of gastroenterology fellows choose independent GI, most fellowship programs don’t educate students about private practice careers or promote that path. In addition, a lot of the national GI conferences are geared toward the academic experience.
It’s incumbent on those of us in private practice to educate students about the benefits and challenges we face as members of independent GI groups, and Gastro Health set out to hold networking and recruitment events at different national conferences with GI fellows and residents.
We’re also working to develop partnerships with fellowship programs. This past year, we’ve held several educational dinners for fellows and residents. Most recently, Dr. Khapra and others took a road trip to New York for dinners with fellows from Mount Sinai, Westchester Medical Center, and the Albert Einstein College of Medicine.
While it was beneficial for Gastro Health to provide information about life as private practice gastroenterologists, it was also helpful for us to hear how the GI leaders of tomorrow are navigating their career choices and what is impacting their decisions about the future.
Mentorship and retention are vital to practice sustainability
Once you’ve recruited physicians to join your practice, how do you ensure their success? Many practices are rightly concerned about their long-term sustainability and are exploring ways to help early-career physicians maintain the clinical skills they need to treat patients and learn the business skills they need to succeed in private practice.
Sometimes it’s as simple as reaching out to new associates on the first day to let them know you’re glad they’ve joined the practice and to let them know you’re available if they need anything. But there’s also growing recognition that implementing a formal mentorship program can help people feel included and supported.
The Women’s Network worked to pair its members with Gastro Health partners as mentors, and we’ve learned some things along the way. Initially, we tried to pair people with similar lifestyles and interests. What we found is that while this sometimes works, we may have overcomplicated the process. We learned that sometimes people would prefer mentors who have backgrounds that are different from their own. We were reminded that mentorship has many faces, and letting those relationships develop naturally can sometimes be more effective.
Networking and social events deter isolation and keep people engaged
Private practice can be different from working within a hospital because oftentimes your colleagues are working in different offices or facilities. In the case of our organization, those offices may be in different states hundreds of miles away. Within a hospital, there might be more potential to interact with your colleagues, whether in clinical conferences or through a chance encounter in the cafeteria.
In private practice, you may need to be more intentional about creating opportunities for people to network and get to know each other outside of work. This year, we developed an email and WhatsApp group so that women throughout the network can connect with each other. We have used it to disseminate information about upcoming events, fellowship opportunities with the national GI societies, interesting articles, and anything important that we think other women within Gastro Health would like to know.
In March, Gastro Health sponsored five women to attend the Scrubs & Heels Summit, which was developed by Dr. Anita Afzali and Dr. Aline Charabaty to create opportunities for women in GI at different stages of their GI careers and help them succeed and achieve their professional goals. There were 2 days of educational talks, but it also included plenty of events for our colleagues to get to know each other and network with other amazing female GI leaders from across the nation.
Where’s the boardroom?
A recent study found that the percentage of women on the boards of the 1,000 largest public companies in America is a little more than 28%, even though research shows that S&P 500 companies headquartered in California with 30% or more women on their boards had 29% higher revenue.
We’re working to develop opportunities for women to be in leadership positions, within our practices and on the national stage in terms of representation, within our national GI societies. It’s very exciting that we have women in leadership within AGA and ASGE, and that Dr. Latha Alaparthi has made increasing the focus on leadership and pipeline development one of her main priorities as the president and board chair of the Digestive Health Physicians Association (DHPA).
Another way private practices can support women who are leaders is by making recommendations for committees within our national societies and by recognizing that time spent developing presentations and speaking at national conferences is beneficial to the practice in terms of thought leadership, branding, and recruitment of the next generation of practice leaders.
While we have a long way to go, we’re also making strides in the board room at the practice level. I’m the first woman, and notably a woman of color, to join the Gastro Health board of directors under the guidance of support of CEO Joseph Garcia. Dr. Aja McCutchen, who serves as the chair of the DHPA Diversity, Equity, and Inclusion committee, is similarly the first woman and woman of color on the board of directors for United Digestive in Atlanta.
What to look for in joining a practice
When determining which practice you might join, ask how committed the leadership of the organization is to supporting career development for women. Does the practice have a network, a committee or other internal group that supports female physicians? What steps does the practice leadership take to support women who are interested in executive opportunities?
If the practice does have an internal organization, how does it measure progress? For example, we’ve implemented focus groups to measure what is working and where we face the most challenges. Gastro Health partnered with a consultant to hold three confidential sessions with 10 women at a time. This will allow for us to collect depersonalized data that can be compiled into a report for the Gastro Health Board and leadership.
If you’re a woman who is considering a career in independent GI, seek out women in private practice and ask about their experiences. Ask about their path and what opportunities they sought out when starting their careers. They may know of some great opportunities that are available to build your leadership skills.
By creating a network for women, Gastro Health is hoping to make it easier to develop relationships and create productive partnerships. We are certain that working to address the specific challenges that female physicians face in their careers will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Adams is a practicing gastroenterologist and partner at Gastro Health Fairfax in Virginia and serves on the Digestive Health Physicians Association’s Diversity, Equity, and Inclusion Committee. Dr. Adams has no conflicts to declare.
When I was in fellowship in the late 1990s, it was rare to see women at many of the big gastroenterology conferences. And in terms of presentations, there was maybe one session led by or for women at lunchtime. These conferences were the only events I had ever been to where the line for the men’s room was longer than the line for the women’s room.
Over the years, the lines for the women’s room have gotten longer, and the sessions led by female gastroenterologists have grown exponentially. However, women are still underrepresented in our field. Two out of five GI fellows are women, but women constitute less than 18% of practicing gastroenterologists. And the number of women in leadership positions is even lower.
Women in medicine face many challenges
According to a report in JAMA Network Open, women have lower starting salaries more than 90% of the time, which can create income disparities in earning potential throughout our entire careers.
Other studies suggest that female physicians also spend more time with patients and answering messages from patients and colleagues as well. This extra time, although it is done in small increments, adds up quickly and could suggest the pay gap between women and men is wider than we think.
Of course, female physicians still spend more time parenting children and doing household labor. A study found that female physicians spent 8.5 hours more per week on activities that support the family and household.
We’ve been discussing equity for women in medicine, and in the workplace, for decades. But events over the past several years – such as the killing of George Floyd and the formation of the #MeToo movement in response to workplace sexual harassment – have accelerated a paradigm shift in how organizations are focusing on diversity, equity, and inclusion (DEI) and creating cultures that support leadership development for women.
The Gastro Health Women’s Network
In 2020, the leadership of Gastro Health reiterated its commitment to fight discrimination and support equity by sending out a company-wide correspondence that encouraged us to be good stewards within our communities during these turbulent times.
This led to the development of the Gastro Health DEI Council and the Gastro Health Women’s Network, led by Dr. Asma Khapra and based on the framework developed by Dr. Dawn Sears. The programs developed by Dr. Sears are focused on facilitating authentic and supportive relationships, and they helped us create a network for women focused on recruitment, mentorship and retention, networking and social events, and leadership development.
Our network started with a meet & greet, inviting all women in Gastro Health to join a virtual call and get to know each other in an informal setting. This was a great way to introduce people to each other in our natural elements. It was wonderful to see how people are when they are at home and not working.
Recruiting female gastroenterologists
Even though about half of gastroenterology fellows choose independent GI, most fellowship programs don’t educate students about private practice careers or promote that path. In addition, a lot of the national GI conferences are geared toward the academic experience.
It’s incumbent on those of us in private practice to educate students about the benefits and challenges we face as members of independent GI groups, and Gastro Health set out to hold networking and recruitment events at different national conferences with GI fellows and residents.
We’re also working to develop partnerships with fellowship programs. This past year, we’ve held several educational dinners for fellows and residents. Most recently, Dr. Khapra and others took a road trip to New York for dinners with fellows from Mount Sinai, Westchester Medical Center, and the Albert Einstein College of Medicine.
While it was beneficial for Gastro Health to provide information about life as private practice gastroenterologists, it was also helpful for us to hear how the GI leaders of tomorrow are navigating their career choices and what is impacting their decisions about the future.
Mentorship and retention are vital to practice sustainability
Once you’ve recruited physicians to join your practice, how do you ensure their success? Many practices are rightly concerned about their long-term sustainability and are exploring ways to help early-career physicians maintain the clinical skills they need to treat patients and learn the business skills they need to succeed in private practice.
Sometimes it’s as simple as reaching out to new associates on the first day to let them know you’re glad they’ve joined the practice and to let them know you’re available if they need anything. But there’s also growing recognition that implementing a formal mentorship program can help people feel included and supported.
The Women’s Network worked to pair its members with Gastro Health partners as mentors, and we’ve learned some things along the way. Initially, we tried to pair people with similar lifestyles and interests. What we found is that while this sometimes works, we may have overcomplicated the process. We learned that sometimes people would prefer mentors who have backgrounds that are different from their own. We were reminded that mentorship has many faces, and letting those relationships develop naturally can sometimes be more effective.
Networking and social events deter isolation and keep people engaged
Private practice can be different from working within a hospital because oftentimes your colleagues are working in different offices or facilities. In the case of our organization, those offices may be in different states hundreds of miles away. Within a hospital, there might be more potential to interact with your colleagues, whether in clinical conferences or through a chance encounter in the cafeteria.
In private practice, you may need to be more intentional about creating opportunities for people to network and get to know each other outside of work. This year, we developed an email and WhatsApp group so that women throughout the network can connect with each other. We have used it to disseminate information about upcoming events, fellowship opportunities with the national GI societies, interesting articles, and anything important that we think other women within Gastro Health would like to know.
In March, Gastro Health sponsored five women to attend the Scrubs & Heels Summit, which was developed by Dr. Anita Afzali and Dr. Aline Charabaty to create opportunities for women in GI at different stages of their GI careers and help them succeed and achieve their professional goals. There were 2 days of educational talks, but it also included plenty of events for our colleagues to get to know each other and network with other amazing female GI leaders from across the nation.
Where’s the boardroom?
A recent study found that the percentage of women on the boards of the 1,000 largest public companies in America is a little more than 28%, even though research shows that S&P 500 companies headquartered in California with 30% or more women on their boards had 29% higher revenue.
We’re working to develop opportunities for women to be in leadership positions, within our practices and on the national stage in terms of representation, within our national GI societies. It’s very exciting that we have women in leadership within AGA and ASGE, and that Dr. Latha Alaparthi has made increasing the focus on leadership and pipeline development one of her main priorities as the president and board chair of the Digestive Health Physicians Association (DHPA).
Another way private practices can support women who are leaders is by making recommendations for committees within our national societies and by recognizing that time spent developing presentations and speaking at national conferences is beneficial to the practice in terms of thought leadership, branding, and recruitment of the next generation of practice leaders.
While we have a long way to go, we’re also making strides in the board room at the practice level. I’m the first woman, and notably a woman of color, to join the Gastro Health board of directors under the guidance of support of CEO Joseph Garcia. Dr. Aja McCutchen, who serves as the chair of the DHPA Diversity, Equity, and Inclusion committee, is similarly the first woman and woman of color on the board of directors for United Digestive in Atlanta.
What to look for in joining a practice
When determining which practice you might join, ask how committed the leadership of the organization is to supporting career development for women. Does the practice have a network, a committee or other internal group that supports female physicians? What steps does the practice leadership take to support women who are interested in executive opportunities?
If the practice does have an internal organization, how does it measure progress? For example, we’ve implemented focus groups to measure what is working and where we face the most challenges. Gastro Health partnered with a consultant to hold three confidential sessions with 10 women at a time. This will allow for us to collect depersonalized data that can be compiled into a report for the Gastro Health Board and leadership.
If you’re a woman who is considering a career in independent GI, seek out women in private practice and ask about their experiences. Ask about their path and what opportunities they sought out when starting their careers. They may know of some great opportunities that are available to build your leadership skills.
By creating a network for women, Gastro Health is hoping to make it easier to develop relationships and create productive partnerships. We are certain that working to address the specific challenges that female physicians face in their careers will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Adams is a practicing gastroenterologist and partner at Gastro Health Fairfax in Virginia and serves on the Digestive Health Physicians Association’s Diversity, Equity, and Inclusion Committee. Dr. Adams has no conflicts to declare.
When I was in fellowship in the late 1990s, it was rare to see women at many of the big gastroenterology conferences. And in terms of presentations, there was maybe one session led by or for women at lunchtime. These conferences were the only events I had ever been to where the line for the men’s room was longer than the line for the women’s room.
Over the years, the lines for the women’s room have gotten longer, and the sessions led by female gastroenterologists have grown exponentially. However, women are still underrepresented in our field. Two out of five GI fellows are women, but women constitute less than 18% of practicing gastroenterologists. And the number of women in leadership positions is even lower.
Women in medicine face many challenges
According to a report in JAMA Network Open, women have lower starting salaries more than 90% of the time, which can create income disparities in earning potential throughout our entire careers.
Other studies suggest that female physicians also spend more time with patients and answering messages from patients and colleagues as well. This extra time, although it is done in small increments, adds up quickly and could suggest the pay gap between women and men is wider than we think.
Of course, female physicians still spend more time parenting children and doing household labor. A study found that female physicians spent 8.5 hours more per week on activities that support the family and household.
We’ve been discussing equity for women in medicine, and in the workplace, for decades. But events over the past several years – such as the killing of George Floyd and the formation of the #MeToo movement in response to workplace sexual harassment – have accelerated a paradigm shift in how organizations are focusing on diversity, equity, and inclusion (DEI) and creating cultures that support leadership development for women.
The Gastro Health Women’s Network
In 2020, the leadership of Gastro Health reiterated its commitment to fight discrimination and support equity by sending out a company-wide correspondence that encouraged us to be good stewards within our communities during these turbulent times.
This led to the development of the Gastro Health DEI Council and the Gastro Health Women’s Network, led by Dr. Asma Khapra and based on the framework developed by Dr. Dawn Sears. The programs developed by Dr. Sears are focused on facilitating authentic and supportive relationships, and they helped us create a network for women focused on recruitment, mentorship and retention, networking and social events, and leadership development.
Our network started with a meet & greet, inviting all women in Gastro Health to join a virtual call and get to know each other in an informal setting. This was a great way to introduce people to each other in our natural elements. It was wonderful to see how people are when they are at home and not working.
Recruiting female gastroenterologists
Even though about half of gastroenterology fellows choose independent GI, most fellowship programs don’t educate students about private practice careers or promote that path. In addition, a lot of the national GI conferences are geared toward the academic experience.
It’s incumbent on those of us in private practice to educate students about the benefits and challenges we face as members of independent GI groups, and Gastro Health set out to hold networking and recruitment events at different national conferences with GI fellows and residents.
We’re also working to develop partnerships with fellowship programs. This past year, we’ve held several educational dinners for fellows and residents. Most recently, Dr. Khapra and others took a road trip to New York for dinners with fellows from Mount Sinai, Westchester Medical Center, and the Albert Einstein College of Medicine.
While it was beneficial for Gastro Health to provide information about life as private practice gastroenterologists, it was also helpful for us to hear how the GI leaders of tomorrow are navigating their career choices and what is impacting their decisions about the future.
Mentorship and retention are vital to practice sustainability
Once you’ve recruited physicians to join your practice, how do you ensure their success? Many practices are rightly concerned about their long-term sustainability and are exploring ways to help early-career physicians maintain the clinical skills they need to treat patients and learn the business skills they need to succeed in private practice.
Sometimes it’s as simple as reaching out to new associates on the first day to let them know you’re glad they’ve joined the practice and to let them know you’re available if they need anything. But there’s also growing recognition that implementing a formal mentorship program can help people feel included and supported.
The Women’s Network worked to pair its members with Gastro Health partners as mentors, and we’ve learned some things along the way. Initially, we tried to pair people with similar lifestyles and interests. What we found is that while this sometimes works, we may have overcomplicated the process. We learned that sometimes people would prefer mentors who have backgrounds that are different from their own. We were reminded that mentorship has many faces, and letting those relationships develop naturally can sometimes be more effective.
Networking and social events deter isolation and keep people engaged
Private practice can be different from working within a hospital because oftentimes your colleagues are working in different offices or facilities. In the case of our organization, those offices may be in different states hundreds of miles away. Within a hospital, there might be more potential to interact with your colleagues, whether in clinical conferences or through a chance encounter in the cafeteria.
In private practice, you may need to be more intentional about creating opportunities for people to network and get to know each other outside of work. This year, we developed an email and WhatsApp group so that women throughout the network can connect with each other. We have used it to disseminate information about upcoming events, fellowship opportunities with the national GI societies, interesting articles, and anything important that we think other women within Gastro Health would like to know.
In March, Gastro Health sponsored five women to attend the Scrubs & Heels Summit, which was developed by Dr. Anita Afzali and Dr. Aline Charabaty to create opportunities for women in GI at different stages of their GI careers and help them succeed and achieve their professional goals. There were 2 days of educational talks, but it also included plenty of events for our colleagues to get to know each other and network with other amazing female GI leaders from across the nation.
Where’s the boardroom?
A recent study found that the percentage of women on the boards of the 1,000 largest public companies in America is a little more than 28%, even though research shows that S&P 500 companies headquartered in California with 30% or more women on their boards had 29% higher revenue.
We’re working to develop opportunities for women to be in leadership positions, within our practices and on the national stage in terms of representation, within our national GI societies. It’s very exciting that we have women in leadership within AGA and ASGE, and that Dr. Latha Alaparthi has made increasing the focus on leadership and pipeline development one of her main priorities as the president and board chair of the Digestive Health Physicians Association (DHPA).
Another way private practices can support women who are leaders is by making recommendations for committees within our national societies and by recognizing that time spent developing presentations and speaking at national conferences is beneficial to the practice in terms of thought leadership, branding, and recruitment of the next generation of practice leaders.
While we have a long way to go, we’re also making strides in the board room at the practice level. I’m the first woman, and notably a woman of color, to join the Gastro Health board of directors under the guidance of support of CEO Joseph Garcia. Dr. Aja McCutchen, who serves as the chair of the DHPA Diversity, Equity, and Inclusion committee, is similarly the first woman and woman of color on the board of directors for United Digestive in Atlanta.
What to look for in joining a practice
When determining which practice you might join, ask how committed the leadership of the organization is to supporting career development for women. Does the practice have a network, a committee or other internal group that supports female physicians? What steps does the practice leadership take to support women who are interested in executive opportunities?
If the practice does have an internal organization, how does it measure progress? For example, we’ve implemented focus groups to measure what is working and where we face the most challenges. Gastro Health partnered with a consultant to hold three confidential sessions with 10 women at a time. This will allow for us to collect depersonalized data that can be compiled into a report for the Gastro Health Board and leadership.
If you’re a woman who is considering a career in independent GI, seek out women in private practice and ask about their experiences. Ask about their path and what opportunities they sought out when starting their careers. They may know of some great opportunities that are available to build your leadership skills.
By creating a network for women, Gastro Health is hoping to make it easier to develop relationships and create productive partnerships. We are certain that working to address the specific challenges that female physicians face in their careers will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Adams is a practicing gastroenterologist and partner at Gastro Health Fairfax in Virginia and serves on the Digestive Health Physicians Association’s Diversity, Equity, and Inclusion Committee. Dr. Adams has no conflicts to declare.
November 2022 - ICYMI
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
Lean and clean: Minimally invasive endoscopic and pharmacologic approaches to obesity
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
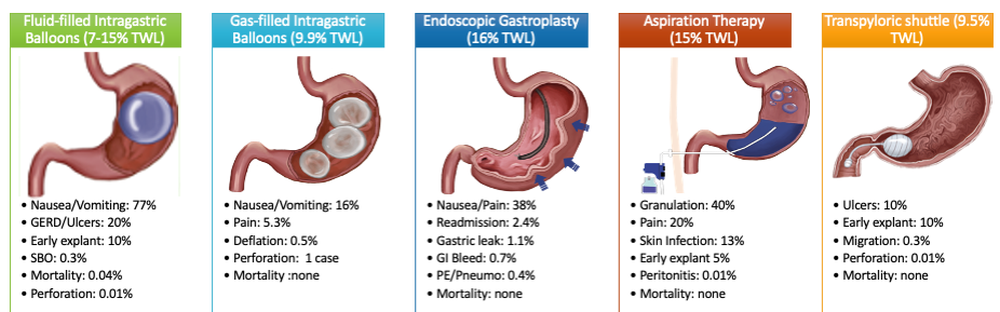
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29
Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.
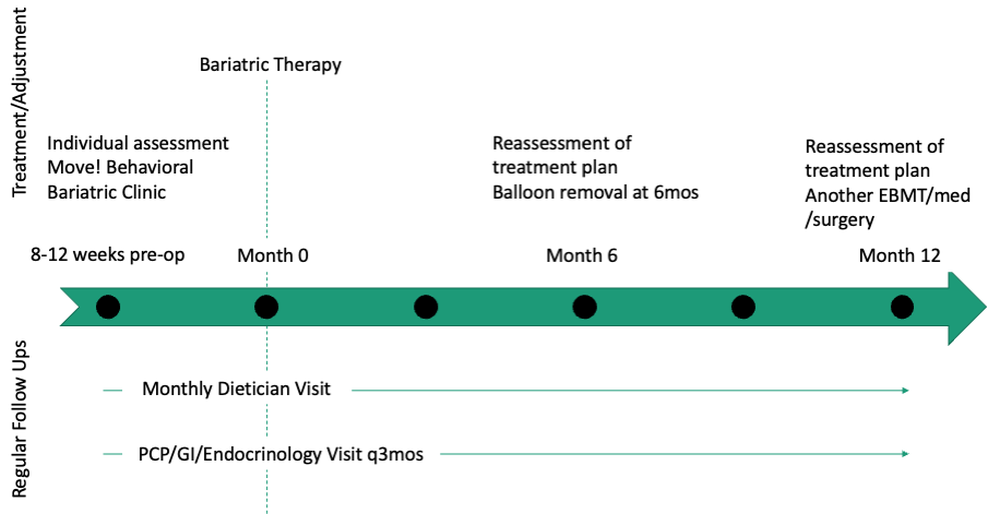
If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.
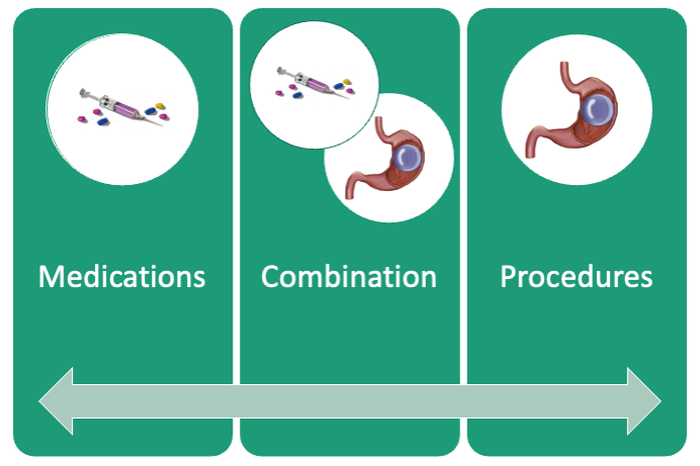
Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29

Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.

If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.

Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Obesity currently affects more than 40% of the U.S. population. It is the second-leading preventable cause of mortality behind smoking with an estimated 300,000 deaths per year.1,2 Weight loss can reduce the risk of metabolic comorbidities such as diabetes, heart disease, and stroke. However, 5%-10% total body weight loss (TBWL) is required for risk reduction.3 Sustained weight loss involves dietary alterations and physical activity, although it is difficult to maintain long term with lifestyle changes alone. Less than 10% of Americans with a BMI greater than 30 kg/m2 will achieve 5% TBWL each year, and nearly 80% of people will regain the weight within 5 years, a phenomenon known as “weight cycling.”4,5 Not only can these weight fluctuations make future weight-loss efforts more difficult, but they can also negatively impact cardiometabolic health in the long term.5 Thus, additional therapies are typically needed in conjunction with lifestyle interventions to treat obesity.
Current guidelines recommend bariatric surgery for patients unable to achieve or maintain weight loss through lifestyle changes.6 Surgeries like Roux-en-Y gastric bypass and sleeve gastrectomy lead to improvements in morbidity and mortality from metabolic diseases but are often only approved for select patients with a BMI of at least 40 or at least 35 with obesity-related comorbidities.7 These restrictions exclude patients at lower BMIs who may have early metabolic disease. Furthermore, only a small proportion of eligible patients are referred or willing to undergo surgery because of access issues, socioeconomic barriers, and concerns about adverse events.8,9 Endoscopic bariatric therapy and antiobesity medications (AOMs) have blossomed because of the need for other less-invasive options to stimulate weight loss.
Minimally invasive and noninvasive therapies in obesity
Endoscopic bariatric and metabolic therapies
Endoscopic bariatric and metabolic therapies (EBMTs) are used for the treatment of obesity in patients with a BMI of 30 kg/m2, a cohort that may be ineligible for bariatric surgery.10,11 EBMTs involve three categories: space-occupying devices (intragastric balloons [IGBs], transpyloric shuttle [TPS]), aspiration therapy, and gastric remodeling (endoscopic sleeve gastroplasty [ESG]).21,13 Presently, TPS and aspiration therapy are not commercially available in the United States. There are three types of IGB approved by the Food and Drug Administration, and Apollo ESGTM recently received de novo marketing authorization for the treatment of obesity. TBWL with EBMTs is promising at 12 months post procedure. Ranges include 7%-12% TBWL for IGBs and 15%-19% for ESG, with low rates of serious adverse events (AEs).13-18 Weight loss often reaches or exceeds the 10% TBWL needed to improve or completely reverse metabolic complications.
Obesity pharmacotherapy
Multiple professional societies support the use of obesity pharmacotherapy as an effective adjunct to lifestyle interventions.19 AOMs are classified as peripherally-acting to prevent nutrition absorption (e.g. orlistat), centrally acting to suppress appetite and/or cravings (e.g., phentermine/topiramate or naltrexone/bupropion), or incretin mimetics such as glucagonlike peptide–1 agonists (e.g., liraglutide, semaglutide).20 With the exception of orlistat, most agents have some effects on the hypothalamus to suppress appetite.21 Obesity medications tend to lead to a minimum weight loss of 3-10 kg after 12 months of treatment, and newer medications have even greater efficacy.22 Despite these results, discontinuation rates of the popular GLP-1 agonists can be as high as 47.7% and 70.1% at 12 and 24 months, respectively, because of the high cost of medications, gastrointestinal side effects, and poor tolerance.23,24
An ongoing challenge for patients is maintaining weight loss following cessation of pharmacotherapy when weight loss goals have been achieved. In this context, the combination of obesity pharmacotherapy and EBMTs can be utilized for long-term weight loss and weight maintenance given the chronic, relapsing, and complex nature of obesity.25
Advantages of less-invasive therapies in obesity management
The advantages of both pharmacologic and endoscopic weight-loss therapies are numerous. Pharmacotherapies are noninvasive, and their multiple mechanisms allow for combined use to synergistically promote weight reduction.26,27 Medications can be used in both the short- and long-term management of obesity, allowing for flexibility in use for patients pending fluctuations in weight. Furthermore, medications can improve markers of cardiovascular health including total cholesterol, LDL cholesterol, blood pressure, and glycemic control.28
As minimally invasive therapies, EBMTs have less morbidity and mortality, compared with bariatric surgeries.29 The most common side effects of IGBs or ESG include abdominal pain, nausea, and worsening of acid reflux symptoms, which can be medically managed unlike some of the AEs associated with surgery, such as bowel obstruction, anastomotic dehiscence, fistulization, and postoperative infections.30 Long-term AEs from surgery also include malabsorption, nutritional deficiencies, cholelithiasis, and anastomotic stenosis.31 Even with improvement in surgical techniques, the rate of perioperative and postoperative mortality in Roux-en-Y gastric bypass is estimated to be 0.4% and 0.7%, respectively, compared with only 0.08% with IGBs.30,32
In addition, EBMTs are also more cost effective than surgery, as they are often same-day outpatient procedures, leading to decreased length of stay (LOS) for patients. In ongoing research conducted by Sharaiha and colleagues, it was found that patients undergoing ESG had an average LOS of only 0.13 days, compared with 3.09 days for laparoscopic sleeve gastrectomy and 1.68 for laparoscopic gastric banding. The cost for ESG was approximately $12,000, compared with $15,000-$22,000 for laparoscopic bariatric surgeries.33 With their availability to patients with lower BMIs and their less-invasive nature, EBMTs and pharmacotherapy can be utilized on the spectrum of obesity care as bridge therapies both before and after surgery.
Our clinical approach
In 2015, the first Veterans Affairs hospital-based endoscopic bariatric program was established at the VA New York Harbor Healthcare System utilizing IGBs and weight loss pharmacotherapy in conjunction with the VA MOVE! Program to treat obesity and metabolic comorbidities in veterans. Since then, EBMTs have expanded to include ESG and novel medications. Our treatment algorithm accounts for the chronic nature of obesity, the risk of weight regain after any intervention, and the need for longitudinal patient care.
Patients undergo work-up by a multidisciplinary team (MD team) with a nutritionist, psychologist, primary care physician, gastroenterologist, and endocrinologist to determine the optimal treatment plan (Fig. 1).29

Patients are required to attend multiple information sessions, where all weight-loss methods are presented, including surgery, bariatric endoscopy, and pharmacotherapy. Other specialists also help manage comorbid conditions. Prior to selecting an initial intervention, patients undergo intensive lifestyle and behavioral therapy (Fig. 2 and 3). Depending on the selected therapy, initial treatment lasts between 3 and 12 months with ongoing support from the MD team.

If patients do not achieve their targeted weight loss after initial treatment, a new strategy is selected. This includes a different EBMT such as ESG, alternate pharmacotherapy, or surgery until the weight and health goals of the patient are achieved and sustained (Fig. 3). From the start, patients are informed that our program is a long-term intervention and that active participation in the MOVE! Program, as well as follow-up with the MD team are keys to success. EBMTs and medications are presented as effective tools that only work to enhance the effects of lifestyle changes.

Our multidisciplinary approach provides flexibility for patients to trial different options depending on their progress. Research on long-term outcomes with weight loss and metabolic parameters is ongoing, though early results are promising. Thus far, we have observed that patients undergoing a combination therapy of EBMTs and AOMs have greater weight loss than patients on a single therapeutic approach with either EBMT or AOMs alone.34 Racial and socioeconomic disparities in referrals to bariatric surgery are yet another barrier for patients to access weight reduction and improvement in cardiovascular health.35 EBMTs and pharmacotherapy are no longer just on the horizon; they are here as accessible, effective, and long-term treatments for all patients with obesity. More expansive insurance coverage is needed for EBMTs and AOMs in order to prevent progression of obesity-related comorbidities, reduce high costs, and ensure more equitable access to these effective therapies.
Dr. Young and Dr. Zenger are resident physicians in the department of internal medicine at New York University. Dr. Holzwanger is an advanced endoscopy fellow in the division of gastroenterology at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. Dr. Popov is director of bariatric endoscopy at VA New York Harbor Healthcare System, and assistant professor of medicine at New York University. Dr. Popov reported relationships with Obalon, Microtech, and Spatz, but the remaining authors reported no competing interests.
References
1. Ward ZJ et al. N Engl J Med. 2019;381(25):2440-50.
2. Stein CJ and Colditz GA. J Clin Endocrinol Metab. 2004;89(6):2522-5.
3. Ryan DH and Yockey SR. Curr Obes Rep. 2017;6(2):187-94.
4. Fildes A et al. Am J Public Health. 2015;105(9):e54-9.
5. Rhee E-J. J Obes Metab Syndr. 2017;26(4):237-42.
6. American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Obesity (Silver Spring). 2014;22 Suppl 2:S5-39.
7. Adams TD et al. N Engl J Med. 2018;378(1):93-6.
8. Wharton S et al. Clin Obes. 2016;6(2):154-60.
9. Iuzzolino E and Kim Y. Obes Res Clin Pract. 2020;14(4):310-20.
10. Goyal D, Watson RR. Endoscopic Bariatric Therapies. Curr Gastroenterol Rep. 2016;18(6):26.
11. Ali MR et al. Surg Obes Relat Dis. 2016;12(3):462-467.
12. Turkeltaub JA, Edmundowicz SA. Curr Treat Options Gastroenterol. 2019;17(2):187-201.
13. Reja D et al. Transl Gastroenterol Hepatol. 2022;7:21.
14. Force ABET et al. Gastrointest Endosc. 2015;82(3):425-38e5.
15. Thompson CC et al. Am J Gastroenterol. 2017;112(3):447-57.
16. Nystrom M et al. Obes Surg. 2018;28(7):1860-8.
17. Abu Dayyeh BK et al. Surg Obes Relat Dis. 2019;15(8):1423-4.
18. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2017;15(4):504-10.
19. Apovian CM et al. J Clin Endocrinol Metab. 2015;100(2):342-62.
20. Son JW and Kim S. Diabetes Metab J. 2020;44(6):802-18.
21. Holst JJ. Int J Obes (Lond). Int J Obes (Lond). 2013;37(9):1161-8.
22. Joo JK and Lee KS. J Menopausal Med. 2014;20(3):90-6.
23. Weiss T et al. Patient Prefer Adherence. 2020;14:2337-45.
24. Sikirica MV et al. Diabetes Metab Syndr Obes. 2017;10:403-12.
25. Kahan S et al. Tech Innov Gastrointest Endosc. 2020;22(3):154-8.
26. Bhat SP and Sharma A. Curr Drug Targets. 2017;18(8):983-93.
27. Pendse J et al. Obesity (Silver Spring). 2021;29(2):308-16.
28. Rucker D et al. BMJ. 2007;335(7631):1194-9.
29. Jirapinyo P and Thompson CC. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
30. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81(5):1073-86.
31. Schulman AR and Thompson CC. Am J Gastroenterol. 2017;112(11):1640-55.
32. Ma IT and Madura JA, 2nd. Gastroenterol Hepatol (NY). 2015;11(8):526-35.
33. Sharaiha RZ. Endoscopic sleeve gastroplasty as a nonsurgical weight loss alternative. Digestive Disease Week, oral presentation. 2017.
34. Young S et al. Long-term efficacy of a multidisciplinary minimally invasive approach to weight management compared to single endoscopic therapy: A cohort study. P0865. American College of Gastroenterology Meeting, Abstract P0865. 2021.
35. Johnson-Mann C et al. Surg Obes Relat Dis. 2019;15(4):615-20.
Passive income for the astute gastroenterologist
I don’t think I heard the term “passive income” until I was already an attending gastroenterologist.
That was no surprise. Why would I as a gastroenterologist with a focus in inflammatory bowel diseases be even remotely interested in that term?
Like most physicians, I went into medicine to take care of patients. That was my entire dream. It was a pleasant surprise to hear that gastroenterologists were relatively well paid compared to many other internal medicine specialties.
That was a bonus. I was not practicing medicine for the money. I was here to do good, only. Money was the evil one. It’s no surprise money remained a taboo topic amongst physicians.
This is reflected in the lack of financial education in our training.
I went through all my medical training without getting any financial education. In my last year of training, I wondered how I was going to not end up being a burned out, overworked physician mom. I knew I was going to work in a large hospital-based practice or academic center. I was already aware that employed physicians had a higher burnout rate compared to independent physicians. My desperation to avoid what looked like the natural history of most physicians in medicine was what led me to my financial awakening, as you could call it.
I became curious about where my money was going as it hit my bank account. Where was I investing? How was I going to ensure that I wasn’t putting all my financial eggs in one basket by relying solely on my clinical income? This road led me into a world that I didn’t know existed. It was the world of physician entrepreneurs.
I began thinking more critically of how I was spending my time outside of the hospital. As a busy physician mom, there already were a lot of competing needs and demands on the 24 hours that I was limited to within a day. How could I get things done and increase my earnability without needing to exchange more time for money in a one-to-one ratio?
Passive income!
First of all, what exactly is passive income?
It refers to money earned that does not require you to physically and actively pump in time in order to get money out. For instance, seeing patients clinically is not passive. Performing procedures is not passive.
What are some examples of passive income?
• Dividend paying stocks or funds
• Investing through retirement accounts
• Passive real estate investment through syndications, crowdfunding, REITs
• Book writing
• Business partnership or ownership such as surgery center co-ownership
• Peer-to-peer lending
• Affiliate marketing
• House hacking
• Rent out your car
• Rent out your backyard/ swimming pool
• Invention with royalty payment
• Podcasting
There are some myths about passive income that are worth exploring
1. Passive income is completely passive: This is relative passivity, meaning that for every investment, there is a phase of learning, acquiring knowledge, vetting, and possibly researching that is not passive. After the initial phase of set up, most passive sources of income may require some monitoring or checking in. However, what makes an investment passive is the absence of that one-to-one ratio of input to output that would normally exist in a more active income source.
2. Making passive income is lazy: If you are a physician, you are probably not lazy. Yes, we have a high standard of expectation for ourselves, but anyone that is able to withstand the rigors of medical training, residency, and fellowship is not lazy in my books. Burnout can present in various ways, including apathy. Let’s not confuse that as lazy because, if we do, that would qualify as gaslighting and self-splaining. As someone that teaches physicians how to have money, here is my opinion: In order to make money ethically, there has to be exchange in value. One person gives value, the other gives money as a thank you. Value can be physical as seen in clinical work. Value can also be monetary. For example, I could give $100,000 to a start-up company that needs that money to execute their brilliant idea, and, in return for my investment, they could give me a 15% return per year. Is that lazy? Without this, their brilliant idea may not see daylight. Value exchange is the key. Giving value comes in different ways.
3. Finding ideas for passive income is hard: Many of us are invested in the stock market, most commonly through retirement accounts. This would qualify as passive income. Typically, we have simply elected that the growth in our investment or dividends be reinvested as we are choosing to use this money long term. In other words, if you have a retirement account, you already have passive income. The question now is how you can find additional passive ways to invest.
What are the benefits to passive income as a gastroenterologist?
1. Changing landscape of medicine: Over the last few decades, we have seen a growing shift in the landscape of medicine. There has been an increase in administrations surpassing the increase in physicians. There seem to be more and more growing bodies that are wedging between physicians and patients. This has led to increasing dissatisfaction for patients and physicians alike. In order to respond to these changes and create lasting changes, there is a need for a change in the leadership. It is fair to say that when you have a more diversified source of income, there is less pressure on a single source of income to provide “food and shelter” for your family. Physician leaders that are liberated have to have a sense of financial liberation.
2. Not putting eggs in one basket: At the beginning of the COVID-19 pandemic, there was significant fear of the unknown. Elective procedures were canceled, leading to financial strain for physicians. Gastroenterologists were not spared. When your income source is diverse, it provides more peace of mind.
3. Mental resourcefulness: This is an understated benefit of passive income and diversified income. As physicians, we went through a lot of hard work to get to where we are today. An average incoming medical student has had extensive demonstration of activity, volunteerism, and problem solving. Yet, as attending physicians, because of the burden of everyday clinical responsibilities and endless paperwork, as well as the platform and “warehouse” and “administrative-type involvement” in medicine, the average physician isn’t creating avenues to expend their cognitive abilities in a way that is diverse outside of the clinical setting. Having passive income opportunities creates a gym for mental resourcefulness that increases work satisfaction and may positively impact burnout.
4. Relationship building: As physicians, we tend to stick with our own. After working 60-80 hours per week, it is no surprise that most of your social network may end up being those that you work with. Passive income opportunities expose physicians to networking and social opportunities that may be critical for relationship building. This may improve mental wellness and overall sense of well-being.
5. Longevity in medicine: As more physicians elect to be employed by larger organizations outside of academics, sabbaticals are becoming less and less available. Having passive sources of income may permit a physician who would otherwise not be able to suffer loss of income the opportunity to take a leave of absence in the short term that may provide long-term longevity in medicine, while promoting wellness.
6. Wealth building: Wealth has had a negative reputation in the world. We seem to equate wealth as bad and being the source of evil. We forget that money is simply a tool that takes the shape of the container you place it in. If you are good, money becomes a tool for more good. Having passive income can help accelerate the journey to wealth building. This can be a great resource as physicians can support unique lifesaving, community-building, and environment-protecting initiatives, as well as support political candidates who will have a positive effect on patient care and the future of medicine.
I hope you are convinced that, Gastroenterologists have to do their due diligence to ensure that their finances are future proof to the best of their abilities.
Dr. Alli-Akintade, a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center, is founder of The MoneyFitMD and creator of The MoneyFitMD podcast (www.moneyfitmd.com).
I don’t think I heard the term “passive income” until I was already an attending gastroenterologist.
That was no surprise. Why would I as a gastroenterologist with a focus in inflammatory bowel diseases be even remotely interested in that term?
Like most physicians, I went into medicine to take care of patients. That was my entire dream. It was a pleasant surprise to hear that gastroenterologists were relatively well paid compared to many other internal medicine specialties.
That was a bonus. I was not practicing medicine for the money. I was here to do good, only. Money was the evil one. It’s no surprise money remained a taboo topic amongst physicians.
This is reflected in the lack of financial education in our training.
I went through all my medical training without getting any financial education. In my last year of training, I wondered how I was going to not end up being a burned out, overworked physician mom. I knew I was going to work in a large hospital-based practice or academic center. I was already aware that employed physicians had a higher burnout rate compared to independent physicians. My desperation to avoid what looked like the natural history of most physicians in medicine was what led me to my financial awakening, as you could call it.
I became curious about where my money was going as it hit my bank account. Where was I investing? How was I going to ensure that I wasn’t putting all my financial eggs in one basket by relying solely on my clinical income? This road led me into a world that I didn’t know existed. It was the world of physician entrepreneurs.
I began thinking more critically of how I was spending my time outside of the hospital. As a busy physician mom, there already were a lot of competing needs and demands on the 24 hours that I was limited to within a day. How could I get things done and increase my earnability without needing to exchange more time for money in a one-to-one ratio?
Passive income!
First of all, what exactly is passive income?
It refers to money earned that does not require you to physically and actively pump in time in order to get money out. For instance, seeing patients clinically is not passive. Performing procedures is not passive.
What are some examples of passive income?
• Dividend paying stocks or funds
• Investing through retirement accounts
• Passive real estate investment through syndications, crowdfunding, REITs
• Book writing
• Business partnership or ownership such as surgery center co-ownership
• Peer-to-peer lending
• Affiliate marketing
• House hacking
• Rent out your car
• Rent out your backyard/ swimming pool
• Invention with royalty payment
• Podcasting
There are some myths about passive income that are worth exploring
1. Passive income is completely passive: This is relative passivity, meaning that for every investment, there is a phase of learning, acquiring knowledge, vetting, and possibly researching that is not passive. After the initial phase of set up, most passive sources of income may require some monitoring or checking in. However, what makes an investment passive is the absence of that one-to-one ratio of input to output that would normally exist in a more active income source.
2. Making passive income is lazy: If you are a physician, you are probably not lazy. Yes, we have a high standard of expectation for ourselves, but anyone that is able to withstand the rigors of medical training, residency, and fellowship is not lazy in my books. Burnout can present in various ways, including apathy. Let’s not confuse that as lazy because, if we do, that would qualify as gaslighting and self-splaining. As someone that teaches physicians how to have money, here is my opinion: In order to make money ethically, there has to be exchange in value. One person gives value, the other gives money as a thank you. Value can be physical as seen in clinical work. Value can also be monetary. For example, I could give $100,000 to a start-up company that needs that money to execute their brilliant idea, and, in return for my investment, they could give me a 15% return per year. Is that lazy? Without this, their brilliant idea may not see daylight. Value exchange is the key. Giving value comes in different ways.
3. Finding ideas for passive income is hard: Many of us are invested in the stock market, most commonly through retirement accounts. This would qualify as passive income. Typically, we have simply elected that the growth in our investment or dividends be reinvested as we are choosing to use this money long term. In other words, if you have a retirement account, you already have passive income. The question now is how you can find additional passive ways to invest.
What are the benefits to passive income as a gastroenterologist?
1. Changing landscape of medicine: Over the last few decades, we have seen a growing shift in the landscape of medicine. There has been an increase in administrations surpassing the increase in physicians. There seem to be more and more growing bodies that are wedging between physicians and patients. This has led to increasing dissatisfaction for patients and physicians alike. In order to respond to these changes and create lasting changes, there is a need for a change in the leadership. It is fair to say that when you have a more diversified source of income, there is less pressure on a single source of income to provide “food and shelter” for your family. Physician leaders that are liberated have to have a sense of financial liberation.
2. Not putting eggs in one basket: At the beginning of the COVID-19 pandemic, there was significant fear of the unknown. Elective procedures were canceled, leading to financial strain for physicians. Gastroenterologists were not spared. When your income source is diverse, it provides more peace of mind.
3. Mental resourcefulness: This is an understated benefit of passive income and diversified income. As physicians, we went through a lot of hard work to get to where we are today. An average incoming medical student has had extensive demonstration of activity, volunteerism, and problem solving. Yet, as attending physicians, because of the burden of everyday clinical responsibilities and endless paperwork, as well as the platform and “warehouse” and “administrative-type involvement” in medicine, the average physician isn’t creating avenues to expend their cognitive abilities in a way that is diverse outside of the clinical setting. Having passive income opportunities creates a gym for mental resourcefulness that increases work satisfaction and may positively impact burnout.
4. Relationship building: As physicians, we tend to stick with our own. After working 60-80 hours per week, it is no surprise that most of your social network may end up being those that you work with. Passive income opportunities expose physicians to networking and social opportunities that may be critical for relationship building. This may improve mental wellness and overall sense of well-being.
5. Longevity in medicine: As more physicians elect to be employed by larger organizations outside of academics, sabbaticals are becoming less and less available. Having passive sources of income may permit a physician who would otherwise not be able to suffer loss of income the opportunity to take a leave of absence in the short term that may provide long-term longevity in medicine, while promoting wellness.
6. Wealth building: Wealth has had a negative reputation in the world. We seem to equate wealth as bad and being the source of evil. We forget that money is simply a tool that takes the shape of the container you place it in. If you are good, money becomes a tool for more good. Having passive income can help accelerate the journey to wealth building. This can be a great resource as physicians can support unique lifesaving, community-building, and environment-protecting initiatives, as well as support political candidates who will have a positive effect on patient care and the future of medicine.
I hope you are convinced that, Gastroenterologists have to do their due diligence to ensure that their finances are future proof to the best of their abilities.
Dr. Alli-Akintade, a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center, is founder of The MoneyFitMD and creator of The MoneyFitMD podcast (www.moneyfitmd.com).
I don’t think I heard the term “passive income” until I was already an attending gastroenterologist.
That was no surprise. Why would I as a gastroenterologist with a focus in inflammatory bowel diseases be even remotely interested in that term?
Like most physicians, I went into medicine to take care of patients. That was my entire dream. It was a pleasant surprise to hear that gastroenterologists were relatively well paid compared to many other internal medicine specialties.
That was a bonus. I was not practicing medicine for the money. I was here to do good, only. Money was the evil one. It’s no surprise money remained a taboo topic amongst physicians.
This is reflected in the lack of financial education in our training.
I went through all my medical training without getting any financial education. In my last year of training, I wondered how I was going to not end up being a burned out, overworked physician mom. I knew I was going to work in a large hospital-based practice or academic center. I was already aware that employed physicians had a higher burnout rate compared to independent physicians. My desperation to avoid what looked like the natural history of most physicians in medicine was what led me to my financial awakening, as you could call it.
I became curious about where my money was going as it hit my bank account. Where was I investing? How was I going to ensure that I wasn’t putting all my financial eggs in one basket by relying solely on my clinical income? This road led me into a world that I didn’t know existed. It was the world of physician entrepreneurs.
I began thinking more critically of how I was spending my time outside of the hospital. As a busy physician mom, there already were a lot of competing needs and demands on the 24 hours that I was limited to within a day. How could I get things done and increase my earnability without needing to exchange more time for money in a one-to-one ratio?
Passive income!
First of all, what exactly is passive income?
It refers to money earned that does not require you to physically and actively pump in time in order to get money out. For instance, seeing patients clinically is not passive. Performing procedures is not passive.
What are some examples of passive income?
• Dividend paying stocks or funds
• Investing through retirement accounts
• Passive real estate investment through syndications, crowdfunding, REITs
• Book writing
• Business partnership or ownership such as surgery center co-ownership
• Peer-to-peer lending
• Affiliate marketing
• House hacking
• Rent out your car
• Rent out your backyard/ swimming pool
• Invention with royalty payment
• Podcasting
There are some myths about passive income that are worth exploring
1. Passive income is completely passive: This is relative passivity, meaning that for every investment, there is a phase of learning, acquiring knowledge, vetting, and possibly researching that is not passive. After the initial phase of set up, most passive sources of income may require some monitoring or checking in. However, what makes an investment passive is the absence of that one-to-one ratio of input to output that would normally exist in a more active income source.
2. Making passive income is lazy: If you are a physician, you are probably not lazy. Yes, we have a high standard of expectation for ourselves, but anyone that is able to withstand the rigors of medical training, residency, and fellowship is not lazy in my books. Burnout can present in various ways, including apathy. Let’s not confuse that as lazy because, if we do, that would qualify as gaslighting and self-splaining. As someone that teaches physicians how to have money, here is my opinion: In order to make money ethically, there has to be exchange in value. One person gives value, the other gives money as a thank you. Value can be physical as seen in clinical work. Value can also be monetary. For example, I could give $100,000 to a start-up company that needs that money to execute their brilliant idea, and, in return for my investment, they could give me a 15% return per year. Is that lazy? Without this, their brilliant idea may not see daylight. Value exchange is the key. Giving value comes in different ways.
3. Finding ideas for passive income is hard: Many of us are invested in the stock market, most commonly through retirement accounts. This would qualify as passive income. Typically, we have simply elected that the growth in our investment or dividends be reinvested as we are choosing to use this money long term. In other words, if you have a retirement account, you already have passive income. The question now is how you can find additional passive ways to invest.
What are the benefits to passive income as a gastroenterologist?
1. Changing landscape of medicine: Over the last few decades, we have seen a growing shift in the landscape of medicine. There has been an increase in administrations surpassing the increase in physicians. There seem to be more and more growing bodies that are wedging between physicians and patients. This has led to increasing dissatisfaction for patients and physicians alike. In order to respond to these changes and create lasting changes, there is a need for a change in the leadership. It is fair to say that when you have a more diversified source of income, there is less pressure on a single source of income to provide “food and shelter” for your family. Physician leaders that are liberated have to have a sense of financial liberation.
2. Not putting eggs in one basket: At the beginning of the COVID-19 pandemic, there was significant fear of the unknown. Elective procedures were canceled, leading to financial strain for physicians. Gastroenterologists were not spared. When your income source is diverse, it provides more peace of mind.
3. Mental resourcefulness: This is an understated benefit of passive income and diversified income. As physicians, we went through a lot of hard work to get to where we are today. An average incoming medical student has had extensive demonstration of activity, volunteerism, and problem solving. Yet, as attending physicians, because of the burden of everyday clinical responsibilities and endless paperwork, as well as the platform and “warehouse” and “administrative-type involvement” in medicine, the average physician isn’t creating avenues to expend their cognitive abilities in a way that is diverse outside of the clinical setting. Having passive income opportunities creates a gym for mental resourcefulness that increases work satisfaction and may positively impact burnout.
4. Relationship building: As physicians, we tend to stick with our own. After working 60-80 hours per week, it is no surprise that most of your social network may end up being those that you work with. Passive income opportunities expose physicians to networking and social opportunities that may be critical for relationship building. This may improve mental wellness and overall sense of well-being.
5. Longevity in medicine: As more physicians elect to be employed by larger organizations outside of academics, sabbaticals are becoming less and less available. Having passive sources of income may permit a physician who would otherwise not be able to suffer loss of income the opportunity to take a leave of absence in the short term that may provide long-term longevity in medicine, while promoting wellness.
6. Wealth building: Wealth has had a negative reputation in the world. We seem to equate wealth as bad and being the source of evil. We forget that money is simply a tool that takes the shape of the container you place it in. If you are good, money becomes a tool for more good. Having passive income can help accelerate the journey to wealth building. This can be a great resource as physicians can support unique lifesaving, community-building, and environment-protecting initiatives, as well as support political candidates who will have a positive effect on patient care and the future of medicine.
I hope you are convinced that, Gastroenterologists have to do their due diligence to ensure that their finances are future proof to the best of their abilities.
Dr. Alli-Akintade, a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center, is founder of The MoneyFitMD and creator of The MoneyFitMD podcast (www.moneyfitmd.com).
A case for when, how, and why to evaluate capacity
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
AGA News – August 2022
Huge win for patients: CRC screening coverage continuum is complete
In a huge win for patients, Medicare will begin covering colonoscopies after a positive noninvasive stool test starting next year. Medicare was previously the only insurer who did not cover this critical prevention procedure.
This change comes after a year of advocacy led by AGA – including multiple meetings with senior officials at Health & Human Services and legislative pressure by members across the country.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test,” says David Lieberman, MD, AGAF, who met with Centers for Medicare & Medicaid Services officials multiple times to push this policy forward. “AGA knows that increased participation in screening will further reduce the burden of colorectal cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the CMS proposed rule changes will lessen colorectal cancer disparities eliminating a financial burden for many patients,” says AGA president John Carethers, MD, AGAF, who met with CMS earlier this month to advocate for this change.
Thank you to everyone in the GI community who advocated for this important change!
CMS announced the coverage change as part of the 2023 Medicare proposed rule, which was released July 7, 2022. The rule must be finalized this Fall before taking effect Jan. 2, 2023.
What you need to know about proposed changes to Medicare payment policies
On July 7, CMS released the calendar year (CY) 2023 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The rule will be posted in the Federal Register no later than July 11.
Good news!
In a win for patients and thanks to collective advocacy efforts from AGA and partner societies, CMS is proposing to expand the regulatory definition of “colorectal cancer screening tests” and waive cost sharing for a necessary follow-up colonoscopy after a positive stool-based screening test.
Looming cuts
The rule proposes 4% cuts to Medicare physician reimbursement through required decreases in the conversion factor and expiration of temporary fixes passed by Congress. AGA will continue to work with a coalition of national and state medical societies in urging Congress to prevent these cuts before Jan. 1, 2023.
What to know
CMS expands CRC screening in a proposal to waive cost-sharing for a follow-up colonoscopy to a positive stool-based colorectal cancer screening test and to cover the service for individuals 45 years of age and above.
Medicare payment cuts are looming with cuts to the proposed CY 2023 conversion factor.
Split/shared visits policy delayed until CY 2024.
Payment rates for new bariatric device codes proposed.
Don’t let insurance policies burden GI practices
Join us at AGA Advocacy Day on Thursday, Sept. 22, 2022, to virtually meet with your members of Congress to urge them to rein in insurance policies like prior authorization and step therapy.
If GI providers don’t have a seat at the table and engage with lawmakers, these decisions will be influenced by payers and other parties that do not have you or your patients’ best interests at heart.
AGA Advocacy Day is held shortly before the end of the fiscal year – prime time to educate policymakers and their staff about your everyday challenges and the reality of GI patient care in your state. We will also discuss the need for robust federal funding for GI research and the devastating impact that Medicare cuts could have on your practice.
Register today and AGA will take care of the rest, including scheduling your meetings and providing comprehensive advocacy training. Now more than ever, your voice needs to be heard on Capitol Hill.
Huge win for patients: CRC screening coverage continuum is complete
In a huge win for patients, Medicare will begin covering colonoscopies after a positive noninvasive stool test starting next year. Medicare was previously the only insurer who did not cover this critical prevention procedure.
This change comes after a year of advocacy led by AGA – including multiple meetings with senior officials at Health & Human Services and legislative pressure by members across the country.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test,” says David Lieberman, MD, AGAF, who met with Centers for Medicare & Medicaid Services officials multiple times to push this policy forward. “AGA knows that increased participation in screening will further reduce the burden of colorectal cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the CMS proposed rule changes will lessen colorectal cancer disparities eliminating a financial burden for many patients,” says AGA president John Carethers, MD, AGAF, who met with CMS earlier this month to advocate for this change.
Thank you to everyone in the GI community who advocated for this important change!
CMS announced the coverage change as part of the 2023 Medicare proposed rule, which was released July 7, 2022. The rule must be finalized this Fall before taking effect Jan. 2, 2023.
What you need to know about proposed changes to Medicare payment policies
On July 7, CMS released the calendar year (CY) 2023 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The rule will be posted in the Federal Register no later than July 11.
Good news!
In a win for patients and thanks to collective advocacy efforts from AGA and partner societies, CMS is proposing to expand the regulatory definition of “colorectal cancer screening tests” and waive cost sharing for a necessary follow-up colonoscopy after a positive stool-based screening test.
Looming cuts
The rule proposes 4% cuts to Medicare physician reimbursement through required decreases in the conversion factor and expiration of temporary fixes passed by Congress. AGA will continue to work with a coalition of national and state medical societies in urging Congress to prevent these cuts before Jan. 1, 2023.
What to know
CMS expands CRC screening in a proposal to waive cost-sharing for a follow-up colonoscopy to a positive stool-based colorectal cancer screening test and to cover the service for individuals 45 years of age and above.
Medicare payment cuts are looming with cuts to the proposed CY 2023 conversion factor.
Split/shared visits policy delayed until CY 2024.
Payment rates for new bariatric device codes proposed.
Don’t let insurance policies burden GI practices
Join us at AGA Advocacy Day on Thursday, Sept. 22, 2022, to virtually meet with your members of Congress to urge them to rein in insurance policies like prior authorization and step therapy.
If GI providers don’t have a seat at the table and engage with lawmakers, these decisions will be influenced by payers and other parties that do not have you or your patients’ best interests at heart.
AGA Advocacy Day is held shortly before the end of the fiscal year – prime time to educate policymakers and their staff about your everyday challenges and the reality of GI patient care in your state. We will also discuss the need for robust federal funding for GI research and the devastating impact that Medicare cuts could have on your practice.
Register today and AGA will take care of the rest, including scheduling your meetings and providing comprehensive advocacy training. Now more than ever, your voice needs to be heard on Capitol Hill.
Huge win for patients: CRC screening coverage continuum is complete
In a huge win for patients, Medicare will begin covering colonoscopies after a positive noninvasive stool test starting next year. Medicare was previously the only insurer who did not cover this critical prevention procedure.
This change comes after a year of advocacy led by AGA – including multiple meetings with senior officials at Health & Human Services and legislative pressure by members across the country.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test,” says David Lieberman, MD, AGAF, who met with Centers for Medicare & Medicaid Services officials multiple times to push this policy forward. “AGA knows that increased participation in screening will further reduce the burden of colorectal cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the CMS proposed rule changes will lessen colorectal cancer disparities eliminating a financial burden for many patients,” says AGA president John Carethers, MD, AGAF, who met with CMS earlier this month to advocate for this change.
Thank you to everyone in the GI community who advocated for this important change!
CMS announced the coverage change as part of the 2023 Medicare proposed rule, which was released July 7, 2022. The rule must be finalized this Fall before taking effect Jan. 2, 2023.
What you need to know about proposed changes to Medicare payment policies
On July 7, CMS released the calendar year (CY) 2023 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The rule will be posted in the Federal Register no later than July 11.
Good news!
In a win for patients and thanks to collective advocacy efforts from AGA and partner societies, CMS is proposing to expand the regulatory definition of “colorectal cancer screening tests” and waive cost sharing for a necessary follow-up colonoscopy after a positive stool-based screening test.
Looming cuts
The rule proposes 4% cuts to Medicare physician reimbursement through required decreases in the conversion factor and expiration of temporary fixes passed by Congress. AGA will continue to work with a coalition of national and state medical societies in urging Congress to prevent these cuts before Jan. 1, 2023.
What to know
CMS expands CRC screening in a proposal to waive cost-sharing for a follow-up colonoscopy to a positive stool-based colorectal cancer screening test and to cover the service for individuals 45 years of age and above.
Medicare payment cuts are looming with cuts to the proposed CY 2023 conversion factor.
Split/shared visits policy delayed until CY 2024.
Payment rates for new bariatric device codes proposed.
Don’t let insurance policies burden GI practices
Join us at AGA Advocacy Day on Thursday, Sept. 22, 2022, to virtually meet with your members of Congress to urge them to rein in insurance policies like prior authorization and step therapy.
If GI providers don’t have a seat at the table and engage with lawmakers, these decisions will be influenced by payers and other parties that do not have you or your patients’ best interests at heart.
AGA Advocacy Day is held shortly before the end of the fiscal year – prime time to educate policymakers and their staff about your everyday challenges and the reality of GI patient care in your state. We will also discuss the need for robust federal funding for GI research and the devastating impact that Medicare cuts could have on your practice.
Register today and AGA will take care of the rest, including scheduling your meetings and providing comprehensive advocacy training. Now more than ever, your voice needs to be heard on Capitol Hill.
A bittersweet farewell
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Therapeutic management of NAFLD
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.
Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45
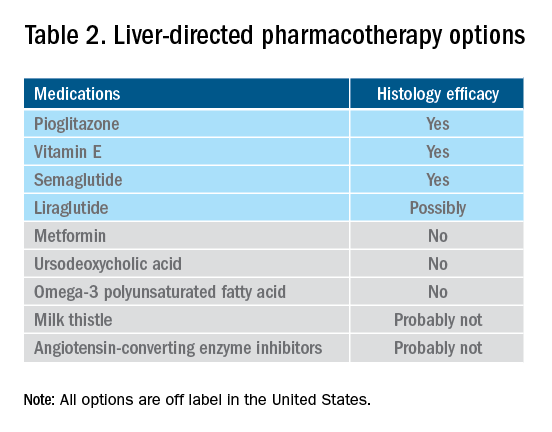
Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Becoming an AGA committee chair as an early-career physician
One of the cornerstones of member engagement within the American Gastroenterological Association is its committees, which provide a platform for AGA members to network, effect change at the institutional level, and obtain leadership positions. For many within the AGA, exposure to these committees occurs during training. Both of us were first introduced to the possibility of serving on an AGA committee by faculty members at our institution. Each year, applications for available committee positions open in the fall and are due on Nov. 1. While you can be nominated by other members, self-nomination is common and encouraged. Truthfully, neither of us was quite certain what committee membership would entail. However, we both applied to several committees because we knew that it would be an excellent opportunity to network with leading gastroenterologists across the country and to have the ability to become involved in key AGA programs.
We were selected to serve 2-year terms as trainee members on the Government Affairs and Publication Committees, respectively, which gave us a deeper understanding of how an organization with both a full-time professional staff and group of volunteer members functions. A unique feature of the AGA is its Trainee and Early Career (TEC) Committee, which mainly comprises trainee members who serve on other committees. By virtue of our roles with the other committees, we also became full-fledged members of the TEC committee, which is dedicated to enhancing the experience for trainees and those who are within 5 years of graduation.
One of the most innovative programs developed by the TEC committee is Career Development Workshops, which is a webinar series focused on important topics not covered in fellowship, such as different career paths, personal finance, and how to increase the number of underrepresented individuals in the field. The predecessor of the Career Development Workshops was the in-person Regional Practice Skills Workshop, and we both took on the responsibility of planning and organizing separate workshops. For one of us (Stephanie), that involved enlisting our fellowship program to host the event. For the other (Peter), it meant collaborating with our local gastroenterology society to cosponsor the workshop. It was extremely rewarding to organize the workshops, which allowed us to work closely with AGA staff and local gastroenterology faculty, as well as our peers, to bring the events to fruition. For both of us, the success of the Regional Practice Skills Workshop was one of the highlights of our tenure on the TEC committee.
Although we were not aware of it at the time, volunteering to plan a workshop and assisting with other projects and subcommittees were signs of enthusiasm and leadership that the AGA recognized and valued. Our advice on becoming a committee chair is to not only show an interest in committee projects but also to turn that interest into action. A committee member who is strongly interested in a leadership position cannot expect to transition into that role by being a “silent but present” member. You need to do more than just show up. You should actively participate in projects, engage in discussions, and devote your time and energy to ensure the success of committee programs. However, you should also make sure to have sufficient bandwidth to make meaningful contributions to each project and not commit to tasks that you cannot complete. To set yourself apart on a committee, it is important to be actively engaged and committed to a project (or two) that allows for professional growth and visibility. Ideally, you will become an integral part of a committee that sparks your drive to serve.
Applying to become a committee chair follows the same process and timeline as for any other committee position, and you can be nominated or self-nominate. Although previous experience on that specific committee is not a prerequisite for most chair positions, having previously served on any AGA committee or task force is generally required. Successful applicants serve for 1 year as chair-elect, during which they work closely with the outgoing chair and staff to ensure a smooth transition when their 3-year term as chair officially begins in June.
Each committee has a guiding mission statement and a staff liaison who provides institutional knowledge and logistical support. However, the committee members, and especially the chair, have considerable latitude to develop and implement new initiatives or retire old ones. The entire committee meets twice per year, once in September in Washington, D.C., and once at DDW. Between the meetings, working groups are formed to move the various programs forward. In addition to the Career Development Workshops, the TEC committee organizes the Young Delegates program (which allows any AGA member to volunteer on small, time-limited projects), a symposium at DDW focused on trainee and early career issues, and a networking event at DDW. Moreover, we collaborate with other committees and provide input from the perspective of younger members on larger initiatives such as the AGA Equity Project and Career Compass.
As chair, we lead the twice-yearly meetings as well as the working groups. We strongly encourage all committee members to participate on at least one working group, which develops leadership skills and provides the opportunity to moderate sessions for the Career Development Workshops and DDW symposium. Moreover, we solicit feedback on ways to improve current programming, start new initiatives, and work with other committees that the TEC committee members are part of. Trainees and early career members are seen as a key constituency group within the AGA, and we take the responsibility of increasing the value of membership for this group seriously.
As early-career physicians ourselves, we also view the chance to serve as a committee chair as a great career development opportunity. It allows us to expand our professional networks, help shape an organization that is a leading voice and advocate for digestive health, and meet the needs of young members who are the future of the AGA.
There is no doubt that all of you have achieved amazing things on the way to becoming a trainee or early career professional in the competitive fields of gastroenterology and hepatology. The AGA is constantly looking for bright, motivated individuals to serve as volunteers and future leaders. Our experience shows that with a bit of persistence to get in the door – through Young Delegates or a committee – along with lots of hard work along the way, you will be in a great position to rise through the ranks and help lead an organization at the vanguard of our field.
Dr. Liang is assistant professor of medicine and population health, New York University Langone Health, and a staff physician at VA New York Harbor Health Care System. Dr. Pointer is a founder and managing partner of Digestive and Liver Health Specialists. She is on staff as a clinical gastroenterologist at Tristar Hendersonville (Tenn.) Medical Center. They have no conflicts of interest.
One of the cornerstones of member engagement within the American Gastroenterological Association is its committees, which provide a platform for AGA members to network, effect change at the institutional level, and obtain leadership positions. For many within the AGA, exposure to these committees occurs during training. Both of us were first introduced to the possibility of serving on an AGA committee by faculty members at our institution. Each year, applications for available committee positions open in the fall and are due on Nov. 1. While you can be nominated by other members, self-nomination is common and encouraged. Truthfully, neither of us was quite certain what committee membership would entail. However, we both applied to several committees because we knew that it would be an excellent opportunity to network with leading gastroenterologists across the country and to have the ability to become involved in key AGA programs.
We were selected to serve 2-year terms as trainee members on the Government Affairs and Publication Committees, respectively, which gave us a deeper understanding of how an organization with both a full-time professional staff and group of volunteer members functions. A unique feature of the AGA is its Trainee and Early Career (TEC) Committee, which mainly comprises trainee members who serve on other committees. By virtue of our roles with the other committees, we also became full-fledged members of the TEC committee, which is dedicated to enhancing the experience for trainees and those who are within 5 years of graduation.
One of the most innovative programs developed by the TEC committee is Career Development Workshops, which is a webinar series focused on important topics not covered in fellowship, such as different career paths, personal finance, and how to increase the number of underrepresented individuals in the field. The predecessor of the Career Development Workshops was the in-person Regional Practice Skills Workshop, and we both took on the responsibility of planning and organizing separate workshops. For one of us (Stephanie), that involved enlisting our fellowship program to host the event. For the other (Peter), it meant collaborating with our local gastroenterology society to cosponsor the workshop. It was extremely rewarding to organize the workshops, which allowed us to work closely with AGA staff and local gastroenterology faculty, as well as our peers, to bring the events to fruition. For both of us, the success of the Regional Practice Skills Workshop was one of the highlights of our tenure on the TEC committee.
Although we were not aware of it at the time, volunteering to plan a workshop and assisting with other projects and subcommittees were signs of enthusiasm and leadership that the AGA recognized and valued. Our advice on becoming a committee chair is to not only show an interest in committee projects but also to turn that interest into action. A committee member who is strongly interested in a leadership position cannot expect to transition into that role by being a “silent but present” member. You need to do more than just show up. You should actively participate in projects, engage in discussions, and devote your time and energy to ensure the success of committee programs. However, you should also make sure to have sufficient bandwidth to make meaningful contributions to each project and not commit to tasks that you cannot complete. To set yourself apart on a committee, it is important to be actively engaged and committed to a project (or two) that allows for professional growth and visibility. Ideally, you will become an integral part of a committee that sparks your drive to serve.
Applying to become a committee chair follows the same process and timeline as for any other committee position, and you can be nominated or self-nominate. Although previous experience on that specific committee is not a prerequisite for most chair positions, having previously served on any AGA committee or task force is generally required. Successful applicants serve for 1 year as chair-elect, during which they work closely with the outgoing chair and staff to ensure a smooth transition when their 3-year term as chair officially begins in June.
Each committee has a guiding mission statement and a staff liaison who provides institutional knowledge and logistical support. However, the committee members, and especially the chair, have considerable latitude to develop and implement new initiatives or retire old ones. The entire committee meets twice per year, once in September in Washington, D.C., and once at DDW. Between the meetings, working groups are formed to move the various programs forward. In addition to the Career Development Workshops, the TEC committee organizes the Young Delegates program (which allows any AGA member to volunteer on small, time-limited projects), a symposium at DDW focused on trainee and early career issues, and a networking event at DDW. Moreover, we collaborate with other committees and provide input from the perspective of younger members on larger initiatives such as the AGA Equity Project and Career Compass.
As chair, we lead the twice-yearly meetings as well as the working groups. We strongly encourage all committee members to participate on at least one working group, which develops leadership skills and provides the opportunity to moderate sessions for the Career Development Workshops and DDW symposium. Moreover, we solicit feedback on ways to improve current programming, start new initiatives, and work with other committees that the TEC committee members are part of. Trainees and early career members are seen as a key constituency group within the AGA, and we take the responsibility of increasing the value of membership for this group seriously.
As early-career physicians ourselves, we also view the chance to serve as a committee chair as a great career development opportunity. It allows us to expand our professional networks, help shape an organization that is a leading voice and advocate for digestive health, and meet the needs of young members who are the future of the AGA.
There is no doubt that all of you have achieved amazing things on the way to becoming a trainee or early career professional in the competitive fields of gastroenterology and hepatology. The AGA is constantly looking for bright, motivated individuals to serve as volunteers and future leaders. Our experience shows that with a bit of persistence to get in the door – through Young Delegates or a committee – along with lots of hard work along the way, you will be in a great position to rise through the ranks and help lead an organization at the vanguard of our field.
Dr. Liang is assistant professor of medicine and population health, New York University Langone Health, and a staff physician at VA New York Harbor Health Care System. Dr. Pointer is a founder and managing partner of Digestive and Liver Health Specialists. She is on staff as a clinical gastroenterologist at Tristar Hendersonville (Tenn.) Medical Center. They have no conflicts of interest.
One of the cornerstones of member engagement within the American Gastroenterological Association is its committees, which provide a platform for AGA members to network, effect change at the institutional level, and obtain leadership positions. For many within the AGA, exposure to these committees occurs during training. Both of us were first introduced to the possibility of serving on an AGA committee by faculty members at our institution. Each year, applications for available committee positions open in the fall and are due on Nov. 1. While you can be nominated by other members, self-nomination is common and encouraged. Truthfully, neither of us was quite certain what committee membership would entail. However, we both applied to several committees because we knew that it would be an excellent opportunity to network with leading gastroenterologists across the country and to have the ability to become involved in key AGA programs.
We were selected to serve 2-year terms as trainee members on the Government Affairs and Publication Committees, respectively, which gave us a deeper understanding of how an organization with both a full-time professional staff and group of volunteer members functions. A unique feature of the AGA is its Trainee and Early Career (TEC) Committee, which mainly comprises trainee members who serve on other committees. By virtue of our roles with the other committees, we also became full-fledged members of the TEC committee, which is dedicated to enhancing the experience for trainees and those who are within 5 years of graduation.
One of the most innovative programs developed by the TEC committee is Career Development Workshops, which is a webinar series focused on important topics not covered in fellowship, such as different career paths, personal finance, and how to increase the number of underrepresented individuals in the field. The predecessor of the Career Development Workshops was the in-person Regional Practice Skills Workshop, and we both took on the responsibility of planning and organizing separate workshops. For one of us (Stephanie), that involved enlisting our fellowship program to host the event. For the other (Peter), it meant collaborating with our local gastroenterology society to cosponsor the workshop. It was extremely rewarding to organize the workshops, which allowed us to work closely with AGA staff and local gastroenterology faculty, as well as our peers, to bring the events to fruition. For both of us, the success of the Regional Practice Skills Workshop was one of the highlights of our tenure on the TEC committee.
Although we were not aware of it at the time, volunteering to plan a workshop and assisting with other projects and subcommittees were signs of enthusiasm and leadership that the AGA recognized and valued. Our advice on becoming a committee chair is to not only show an interest in committee projects but also to turn that interest into action. A committee member who is strongly interested in a leadership position cannot expect to transition into that role by being a “silent but present” member. You need to do more than just show up. You should actively participate in projects, engage in discussions, and devote your time and energy to ensure the success of committee programs. However, you should also make sure to have sufficient bandwidth to make meaningful contributions to each project and not commit to tasks that you cannot complete. To set yourself apart on a committee, it is important to be actively engaged and committed to a project (or two) that allows for professional growth and visibility. Ideally, you will become an integral part of a committee that sparks your drive to serve.
Applying to become a committee chair follows the same process and timeline as for any other committee position, and you can be nominated or self-nominate. Although previous experience on that specific committee is not a prerequisite for most chair positions, having previously served on any AGA committee or task force is generally required. Successful applicants serve for 1 year as chair-elect, during which they work closely with the outgoing chair and staff to ensure a smooth transition when their 3-year term as chair officially begins in June.
Each committee has a guiding mission statement and a staff liaison who provides institutional knowledge and logistical support. However, the committee members, and especially the chair, have considerable latitude to develop and implement new initiatives or retire old ones. The entire committee meets twice per year, once in September in Washington, D.C., and once at DDW. Between the meetings, working groups are formed to move the various programs forward. In addition to the Career Development Workshops, the TEC committee organizes the Young Delegates program (which allows any AGA member to volunteer on small, time-limited projects), a symposium at DDW focused on trainee and early career issues, and a networking event at DDW. Moreover, we collaborate with other committees and provide input from the perspective of younger members on larger initiatives such as the AGA Equity Project and Career Compass.
As chair, we lead the twice-yearly meetings as well as the working groups. We strongly encourage all committee members to participate on at least one working group, which develops leadership skills and provides the opportunity to moderate sessions for the Career Development Workshops and DDW symposium. Moreover, we solicit feedback on ways to improve current programming, start new initiatives, and work with other committees that the TEC committee members are part of. Trainees and early career members are seen as a key constituency group within the AGA, and we take the responsibility of increasing the value of membership for this group seriously.
As early-career physicians ourselves, we also view the chance to serve as a committee chair as a great career development opportunity. It allows us to expand our professional networks, help shape an organization that is a leading voice and advocate for digestive health, and meet the needs of young members who are the future of the AGA.
There is no doubt that all of you have achieved amazing things on the way to becoming a trainee or early career professional in the competitive fields of gastroenterology and hepatology. The AGA is constantly looking for bright, motivated individuals to serve as volunteers and future leaders. Our experience shows that with a bit of persistence to get in the door – through Young Delegates or a committee – along with lots of hard work along the way, you will be in a great position to rise through the ranks and help lead an organization at the vanguard of our field.
Dr. Liang is assistant professor of medicine and population health, New York University Langone Health, and a staff physician at VA New York Harbor Health Care System. Dr. Pointer is a founder and managing partner of Digestive and Liver Health Specialists. She is on staff as a clinical gastroenterologist at Tristar Hendersonville (Tenn.) Medical Center. They have no conflicts of interest.
August 2022 - ICYMI
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.


















