User login
Establishing a formalized mentorship program in a community practice
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
This insurance agent thinks disability insurance deserves a rebrand, and he's a doctor
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
Repeat endoscopy for deliberate foreign body ingestions
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
Experience with the AGA editorial fellowship for Gastroenterology
Joining Gastroenterology as an editorial fellow is an invaluable experience for the future scientific career. When I joined the fellowship, my main objectives were to learn important aspects about scientific publishing, while improving my editorial and writing skills. Importantly, participating in the fellowship would allow me to determine fields in gastroenterology and hepatology that need more intensive research, evaluate and review manuscripts submitted to a high-impact journal, learn which aspects are important for manuscripts to be considered for publication, and learn how to prepare concise summaries to share with readers. As an editorial fellow, I would not only work with experts on manuscript review and editing, but also be mentored by experts in the field to prepare for a career in scientific publishing.
The application process is easy and straightforward. A curriculum vitae, motivation letter, and conflict of interest form are needed to be considered for the position. Furthermore, a letter of recommendation has to be provided from senior faculty. For the latter, it is beneficial to have worked with someone who is currently active in scientific publishing.
After joining the editorial board, fellows are assigned to associate editors based on their previous experience. Fellows are expected to peer-review manuscripts and discuss their reviews with the associate editors during a brief call or by email, where it is also determined whether additional feedback from the board of editors is needed to move manuscripts forward. If so, fellows are given the opportunity to present manuscripts they reviewed to the editorial board and prepare the decision letter with comments to the authors and editors. This experience teaches which qualities manuscripts need to fulfill to be considered for publication, and how to communicate decisions to authors. During the meetings with the editorial board, fellows are also encouraged to provide feedback for additional manuscripts discussed.
Additionally, fellows have the chance to work on the “Covering the Cover” section of the journal under close mentorship of the responsible editors. Here, they learn to draft short synopses of submitted manuscripts that should be highlighted in the respective sections to give readers a brief overview of important pieces of research with potential clinical applicability. This experience teaches how to write concisely and rephrase the main message of manuscripts without overstating findings and conclusions. Additionally, fellows are invited to write commentaries on recent articles published in other journals and highlight these to Gastroenterology’s readership. This experience teaches how to critically comment on published literature and point out its strengths and limitations. Overall, these learning experiences improve not only editorial and writing skills, but also knowledge in the respective areas of research.
Finally, fellows have the opportunity to write a commentary about a topic of their choice. This experience allows fellows to deepen their expertise in an area of research on an emerging topic they would like to highlight to their readers. Since this type of article is peer reviewed, reviewers’ comments help identify weaknesses of the initially submitted manuscript and increase the awareness about factors that need to be addressed to finally provide a high-quality article that is of value to Gastroenterology’s readership.
Concluding, being an editorial fellow for Gastroenterology is an extremely valuable experience. From an editorial aspect, fellows learn to review, summarize, and comment on submitted manuscripts, as well as which factors need to be addressed by manuscripts to be considered for publication to a high-impact journal. Additionally, fellows network with leaders in the field and expand their knowledge on topics they had not worked on previously. Overall, the fellowship helps improve editorial and writing skills, while staying current in the literature, a skill set that can be applied broadly in the future medical and scientific career.
Dr. Kefalakes is a clinical fellow and research group leader in the department of gastroenterology, hepatology, and endocrinology at Hannover (Germany) Medical School. She has nothing to declare.
Joining Gastroenterology as an editorial fellow is an invaluable experience for the future scientific career. When I joined the fellowship, my main objectives were to learn important aspects about scientific publishing, while improving my editorial and writing skills. Importantly, participating in the fellowship would allow me to determine fields in gastroenterology and hepatology that need more intensive research, evaluate and review manuscripts submitted to a high-impact journal, learn which aspects are important for manuscripts to be considered for publication, and learn how to prepare concise summaries to share with readers. As an editorial fellow, I would not only work with experts on manuscript review and editing, but also be mentored by experts in the field to prepare for a career in scientific publishing.
The application process is easy and straightforward. A curriculum vitae, motivation letter, and conflict of interest form are needed to be considered for the position. Furthermore, a letter of recommendation has to be provided from senior faculty. For the latter, it is beneficial to have worked with someone who is currently active in scientific publishing.
After joining the editorial board, fellows are assigned to associate editors based on their previous experience. Fellows are expected to peer-review manuscripts and discuss their reviews with the associate editors during a brief call or by email, where it is also determined whether additional feedback from the board of editors is needed to move manuscripts forward. If so, fellows are given the opportunity to present manuscripts they reviewed to the editorial board and prepare the decision letter with comments to the authors and editors. This experience teaches which qualities manuscripts need to fulfill to be considered for publication, and how to communicate decisions to authors. During the meetings with the editorial board, fellows are also encouraged to provide feedback for additional manuscripts discussed.
Additionally, fellows have the chance to work on the “Covering the Cover” section of the journal under close mentorship of the responsible editors. Here, they learn to draft short synopses of submitted manuscripts that should be highlighted in the respective sections to give readers a brief overview of important pieces of research with potential clinical applicability. This experience teaches how to write concisely and rephrase the main message of manuscripts without overstating findings and conclusions. Additionally, fellows are invited to write commentaries on recent articles published in other journals and highlight these to Gastroenterology’s readership. This experience teaches how to critically comment on published literature and point out its strengths and limitations. Overall, these learning experiences improve not only editorial and writing skills, but also knowledge in the respective areas of research.
Finally, fellows have the opportunity to write a commentary about a topic of their choice. This experience allows fellows to deepen their expertise in an area of research on an emerging topic they would like to highlight to their readers. Since this type of article is peer reviewed, reviewers’ comments help identify weaknesses of the initially submitted manuscript and increase the awareness about factors that need to be addressed to finally provide a high-quality article that is of value to Gastroenterology’s readership.
Concluding, being an editorial fellow for Gastroenterology is an extremely valuable experience. From an editorial aspect, fellows learn to review, summarize, and comment on submitted manuscripts, as well as which factors need to be addressed by manuscripts to be considered for publication to a high-impact journal. Additionally, fellows network with leaders in the field and expand their knowledge on topics they had not worked on previously. Overall, the fellowship helps improve editorial and writing skills, while staying current in the literature, a skill set that can be applied broadly in the future medical and scientific career.
Dr. Kefalakes is a clinical fellow and research group leader in the department of gastroenterology, hepatology, and endocrinology at Hannover (Germany) Medical School. She has nothing to declare.
Joining Gastroenterology as an editorial fellow is an invaluable experience for the future scientific career. When I joined the fellowship, my main objectives were to learn important aspects about scientific publishing, while improving my editorial and writing skills. Importantly, participating in the fellowship would allow me to determine fields in gastroenterology and hepatology that need more intensive research, evaluate and review manuscripts submitted to a high-impact journal, learn which aspects are important for manuscripts to be considered for publication, and learn how to prepare concise summaries to share with readers. As an editorial fellow, I would not only work with experts on manuscript review and editing, but also be mentored by experts in the field to prepare for a career in scientific publishing.
The application process is easy and straightforward. A curriculum vitae, motivation letter, and conflict of interest form are needed to be considered for the position. Furthermore, a letter of recommendation has to be provided from senior faculty. For the latter, it is beneficial to have worked with someone who is currently active in scientific publishing.
After joining the editorial board, fellows are assigned to associate editors based on their previous experience. Fellows are expected to peer-review manuscripts and discuss their reviews with the associate editors during a brief call or by email, where it is also determined whether additional feedback from the board of editors is needed to move manuscripts forward. If so, fellows are given the opportunity to present manuscripts they reviewed to the editorial board and prepare the decision letter with comments to the authors and editors. This experience teaches which qualities manuscripts need to fulfill to be considered for publication, and how to communicate decisions to authors. During the meetings with the editorial board, fellows are also encouraged to provide feedback for additional manuscripts discussed.
Additionally, fellows have the chance to work on the “Covering the Cover” section of the journal under close mentorship of the responsible editors. Here, they learn to draft short synopses of submitted manuscripts that should be highlighted in the respective sections to give readers a brief overview of important pieces of research with potential clinical applicability. This experience teaches how to write concisely and rephrase the main message of manuscripts without overstating findings and conclusions. Additionally, fellows are invited to write commentaries on recent articles published in other journals and highlight these to Gastroenterology’s readership. This experience teaches how to critically comment on published literature and point out its strengths and limitations. Overall, these learning experiences improve not only editorial and writing skills, but also knowledge in the respective areas of research.
Finally, fellows have the opportunity to write a commentary about a topic of their choice. This experience allows fellows to deepen their expertise in an area of research on an emerging topic they would like to highlight to their readers. Since this type of article is peer reviewed, reviewers’ comments help identify weaknesses of the initially submitted manuscript and increase the awareness about factors that need to be addressed to finally provide a high-quality article that is of value to Gastroenterology’s readership.
Concluding, being an editorial fellow for Gastroenterology is an extremely valuable experience. From an editorial aspect, fellows learn to review, summarize, and comment on submitted manuscripts, as well as which factors need to be addressed by manuscripts to be considered for publication to a high-impact journal. Additionally, fellows network with leaders in the field and expand their knowledge on topics they had not worked on previously. Overall, the fellowship helps improve editorial and writing skills, while staying current in the literature, a skill set that can be applied broadly in the future medical and scientific career.
Dr. Kefalakes is a clinical fellow and research group leader in the department of gastroenterology, hepatology, and endocrinology at Hannover (Germany) Medical School. She has nothing to declare.
My time as an AGA editorial fellow for Clinical Gastroenterology and Hepatology
On the top left of my desktop is an electronic sticky note entitled, “Apply in Future.” This was where the AGA editorial fellowship was listed when I first started GI fellowship. My interest in the behind-the-scenes of scientific publishing developed early. I wanted to learn not only about the decision-making by the editorial board but also other aspects that impact the process from submission to publication. While waiting for my opportunity to apply, I became a reviewer for several journals and then an associate editor of ACG Case Reports Journal, which gave me some insight.
I applied to the editorial fellowship as a second-year fellow, to start as a third-year fellow. I figured this would give me several chances to apply again if I was not selected! I started off by talking to my program director to ensure that I would have enough time, given other responsibilities. The application consisted of a cover letter, where I expressed why I was interested, particularly focusing on my passion for research and my experience with scientific publishing, a letter of recommendation, and a CV. I was ecstatic when I was selected for the AGA editorial fellowship for Clinical Gastroenterology and Hepatology (CGH), whose editorial board’s mission best aligned with my interests.
Dr. Jonathan Buscaglia was the editorial fellows’ mentor for CGH – he met with us and provided an outline of the year. For the first 6 months, I was a reviewer for submitted manuscripts within my topic of interest – pancreaticobiliary diseases and advanced endoscopy and technology. Dr. Buscaglia gave me feedback on my reviews to improve my critical assessment of study designs and interpreted results. After 6 months as a reviewer, I took on the role of an associate editor. I selected reviewers, evaluated the reviews, and made the decision to accept or reject a manuscript. I presented my assigned manuscript at the weekly board of editors meeting, which was one of the biggest learning experiences. Leading researchers from all over the world gathered at a virtual table and discussed whether each study would have clinical impact on the medical community. Each editor contributed a unique perspective that facilitated a robust and thoughtful discussion of each manuscript brought to the table. I was in awe each week.
What I have learned so far during my year as an AGA editorial fellow with CGH has shaped my approach to personal research and will continue to do so as I develop my research career. It has greatly improved my assessment of the literature and I hope to continue to be involved in the critical review process, especially as a reviewer in my early career, and eventually, a part of an editorial board for a journal that truly impacts clinical medicine, such as Clinical Gastroenterology and Hepatology.
Dr. Trieu is a gastroenterology and hepatology fellow at Loyola University Medical Center, Maywood, Ill. She has no conflicts of interest to disclose.
On the top left of my desktop is an electronic sticky note entitled, “Apply in Future.” This was where the AGA editorial fellowship was listed when I first started GI fellowship. My interest in the behind-the-scenes of scientific publishing developed early. I wanted to learn not only about the decision-making by the editorial board but also other aspects that impact the process from submission to publication. While waiting for my opportunity to apply, I became a reviewer for several journals and then an associate editor of ACG Case Reports Journal, which gave me some insight.
I applied to the editorial fellowship as a second-year fellow, to start as a third-year fellow. I figured this would give me several chances to apply again if I was not selected! I started off by talking to my program director to ensure that I would have enough time, given other responsibilities. The application consisted of a cover letter, where I expressed why I was interested, particularly focusing on my passion for research and my experience with scientific publishing, a letter of recommendation, and a CV. I was ecstatic when I was selected for the AGA editorial fellowship for Clinical Gastroenterology and Hepatology (CGH), whose editorial board’s mission best aligned with my interests.
Dr. Jonathan Buscaglia was the editorial fellows’ mentor for CGH – he met with us and provided an outline of the year. For the first 6 months, I was a reviewer for submitted manuscripts within my topic of interest – pancreaticobiliary diseases and advanced endoscopy and technology. Dr. Buscaglia gave me feedback on my reviews to improve my critical assessment of study designs and interpreted results. After 6 months as a reviewer, I took on the role of an associate editor. I selected reviewers, evaluated the reviews, and made the decision to accept or reject a manuscript. I presented my assigned manuscript at the weekly board of editors meeting, which was one of the biggest learning experiences. Leading researchers from all over the world gathered at a virtual table and discussed whether each study would have clinical impact on the medical community. Each editor contributed a unique perspective that facilitated a robust and thoughtful discussion of each manuscript brought to the table. I was in awe each week.
What I have learned so far during my year as an AGA editorial fellow with CGH has shaped my approach to personal research and will continue to do so as I develop my research career. It has greatly improved my assessment of the literature and I hope to continue to be involved in the critical review process, especially as a reviewer in my early career, and eventually, a part of an editorial board for a journal that truly impacts clinical medicine, such as Clinical Gastroenterology and Hepatology.
Dr. Trieu is a gastroenterology and hepatology fellow at Loyola University Medical Center, Maywood, Ill. She has no conflicts of interest to disclose.
On the top left of my desktop is an electronic sticky note entitled, “Apply in Future.” This was where the AGA editorial fellowship was listed when I first started GI fellowship. My interest in the behind-the-scenes of scientific publishing developed early. I wanted to learn not only about the decision-making by the editorial board but also other aspects that impact the process from submission to publication. While waiting for my opportunity to apply, I became a reviewer for several journals and then an associate editor of ACG Case Reports Journal, which gave me some insight.
I applied to the editorial fellowship as a second-year fellow, to start as a third-year fellow. I figured this would give me several chances to apply again if I was not selected! I started off by talking to my program director to ensure that I would have enough time, given other responsibilities. The application consisted of a cover letter, where I expressed why I was interested, particularly focusing on my passion for research and my experience with scientific publishing, a letter of recommendation, and a CV. I was ecstatic when I was selected for the AGA editorial fellowship for Clinical Gastroenterology and Hepatology (CGH), whose editorial board’s mission best aligned with my interests.
Dr. Jonathan Buscaglia was the editorial fellows’ mentor for CGH – he met with us and provided an outline of the year. For the first 6 months, I was a reviewer for submitted manuscripts within my topic of interest – pancreaticobiliary diseases and advanced endoscopy and technology. Dr. Buscaglia gave me feedback on my reviews to improve my critical assessment of study designs and interpreted results. After 6 months as a reviewer, I took on the role of an associate editor. I selected reviewers, evaluated the reviews, and made the decision to accept or reject a manuscript. I presented my assigned manuscript at the weekly board of editors meeting, which was one of the biggest learning experiences. Leading researchers from all over the world gathered at a virtual table and discussed whether each study would have clinical impact on the medical community. Each editor contributed a unique perspective that facilitated a robust and thoughtful discussion of each manuscript brought to the table. I was in awe each week.
What I have learned so far during my year as an AGA editorial fellow with CGH has shaped my approach to personal research and will continue to do so as I develop my research career. It has greatly improved my assessment of the literature and I hope to continue to be involved in the critical review process, especially as a reviewer in my early career, and eventually, a part of an editorial board for a journal that truly impacts clinical medicine, such as Clinical Gastroenterology and Hepatology.
Dr. Trieu is a gastroenterology and hepatology fellow at Loyola University Medical Center, Maywood, Ill. She has no conflicts of interest to disclose.
Effect modification: An important, but often underappreciated, statistical concept
In previous articles, we explored the meaning of two statistical concepts: that of the P value1 and that of confounding.2 In today’s article, we will focus on another important, though often underrecognized, statistical concept: that of effect modification (sometimes also called “interaction,” although many authors draw a distinction between these terms).3-5 In brief, effect modification is the recognition that the relationship between two variables can be different based on the values of a third variable. Let me illustrate with an example. Suppose that you give a weight-loss drug to a group of people. After a predetermined time, say 3 months, you weigh them to measure the effects of the drug, and you record that on average each individual lost about 10 pounds. Suppose, however, that you are curious to see the effect of the drug in men and women separately. You perform an analysis stratified by sex, and you notice that men lost on average only about 1 pound each, whereas women lost on average about 30 pounds each. What do these results mean? It seems that the effect of the drug on weight loss is different depending on the value of a third variable, namely sex. That is, in this case, sex acts as an effect modifier. Based on this analysis, we may conclude that the drug has real effects on women but not on men.
Before reaching this conclusion, however, it is appropriate to ask whether we have made a mistake. The first obvious thing is to make sure that we have not made a mistake in our collection of data. Once we have excluded the presence of structural bias in our dataset, how can we ascertain that these results, which at eyeballing seem so different, have not been so as a result of chance? Fortunately, we do not have to guess. There is a way to formally test for this hypothesis. If sex is truly an effect modifier, then we can perform what is called in statistical terms an “interaction term” between the exposure (in this case the drug) and the potential effect modifier (in this case sex) in a multivariable model that includes both as exposures. If the P value for that interaction term is less than .05, then the interaction term is statistically significant, and therefore the variable (in this case sex) is confirmed to be an effect modifier. Hence, the results are not due to chance, and the different effects in men and women are plausibly attributable to different biological responses to the medication.
Difference between confounding and effect modification
At this point someone might ask, “What then is the difference between confounding and effect modification? In both cases, we do stratified analysis of the relationship between an exposure (in this case the weight-loss drug) and an outcome (in this case weight loss) based on strata of a third variable (in this case sex).” The difference is fundamental. Confounding, as we explored in a previous article,2 is something that we would like to get rid of. It is the effect of an outside variable on both the exposure and outcome that does not allow us to properly evaluate the real relationship between the two. As such, we try to adjust for this variable, so that its effect is eliminated and we can only observe the relationship between the exposure and the outcome. Effect modification, on the other side, is not something we would like to get rid of. On the contrary, effect modification is part of what we would like to explore and describe because it is part of the biological mechanism that explains the real relationship between the exposure and the outcome. In the example above, if effect modification by sex is confirmed, it implies that there is something in female biology that is not found in male biology (or vice-versa) that makes their response to the medication different and therefore something of interest to study further. Thus, differently from confounding, effect modification is part of the objective reality of the world which we would like to explore and evaluate.
Several questions then arise. How can we know whether a variable is a confounder, an effect modifier, or both? As a general rule of thumb, a confounder would be a variable which, when a stratified analysis is done (or when added to a multivariable model), will change the relationship between the exposure and outcome by 10% or more. However, the relationship between the exposure and the outcome in both strata will be similar. In the example above, it would mean that if sex was only a confounder, then stratifying by sex would show roughly a similar change in the effect (say 9 kg for men and 11 kg for women). An effect modifier on the other hand is one which, when a stratified analysis is done, the association between the exposure and the outcome is very different in the two strata, as illustrated in the example above (for simplicity I am only considering two-level effect modifiers in this article).
Can a variable be both a confounder and an effect modifier? Yes, that is possible. What can be done in this case? The most common approach is to behave the same as when only effect modification is present, namely to show with the interaction term that effect modification exists and present the results between the exposure and outcome separately by the level of the effect modifier (in this example, it means that we need to describe the effects of the weight-loss drug separately for men and women). The stratified analysis/presentation will, by definition, take care of confounding as well.2
Should we always look for effect modification? Not necessarily. As a general rule, we need to test for effect modification only if there is some biological rationale that would compel us to do so, and testing should be hypothesis-driven. A common mistake that some authors make is to perform too many interaction tests and then describe as “positive findings” any test for which P value happens to be less than .05. However, as we pointed out in the article on P value,1 if we perform multiple tests, this increases the probability of false positives, and therefore the probability of spurious findings. Thus, effect modification analysis (with interaction terms) should, generally speaking, be performed with a biological rationale and/or be hypothesis driven.
Conclusion
Effect modification is an essential statistical concept that describes an underlying biological reality in which the association between an exposure and an outcome is different based on the values of a third variable. Differently from confounding, which clouds the association between exposure and outcome, and therefore is something that we try to get rid of, effect modification serves to bring to light a more proper understanding of the biological reality underlying the true association between an exposure and an outcome and as such is something that needs to be explored and described.
Dr. Jovani is assistant professor of medicine, therapeutic endoscopy, digestive diseases, and nutrition at the University of Kentucky Albert B. Chandler Hospital, Lexington. He has no conflicts of interest.
References
1. “The P value: What to make of it? A simple guide for the uninitiated.” GI & Hepatology News. 2019 Sep 23. www.mdedge.com/gihepnews/article/208601/mixed-topics/p-value-what-make-it-simple-guide-uninitiated
2. “Evaluating a paper: Take care not to be confounded.” GI & Hepatology News. 2020 Sep 18. www.mdedge.com/gihepnews/article/228765/mixed-topics/evaluating-paper-take-care-not-be-confounded
3. Corraini P et al. Clin Epidemiol. 2017;9:331-8. doi: 10.2147/CLEP.S162236.
4. VanderWeele TJ. Epidemiology. 2009 Nov;20(6):863-71. doi: 10.1097/EDE.0b013e3181ba333c.
5. Shahar E and Shahar DJ. Epidemiology. 2010 Jul;21(4):587. doi: 10.1097/EDE.0b013e3181e0995c. Author reply 587-8.
In previous articles, we explored the meaning of two statistical concepts: that of the P value1 and that of confounding.2 In today’s article, we will focus on another important, though often underrecognized, statistical concept: that of effect modification (sometimes also called “interaction,” although many authors draw a distinction between these terms).3-5 In brief, effect modification is the recognition that the relationship between two variables can be different based on the values of a third variable. Let me illustrate with an example. Suppose that you give a weight-loss drug to a group of people. After a predetermined time, say 3 months, you weigh them to measure the effects of the drug, and you record that on average each individual lost about 10 pounds. Suppose, however, that you are curious to see the effect of the drug in men and women separately. You perform an analysis stratified by sex, and you notice that men lost on average only about 1 pound each, whereas women lost on average about 30 pounds each. What do these results mean? It seems that the effect of the drug on weight loss is different depending on the value of a third variable, namely sex. That is, in this case, sex acts as an effect modifier. Based on this analysis, we may conclude that the drug has real effects on women but not on men.
Before reaching this conclusion, however, it is appropriate to ask whether we have made a mistake. The first obvious thing is to make sure that we have not made a mistake in our collection of data. Once we have excluded the presence of structural bias in our dataset, how can we ascertain that these results, which at eyeballing seem so different, have not been so as a result of chance? Fortunately, we do not have to guess. There is a way to formally test for this hypothesis. If sex is truly an effect modifier, then we can perform what is called in statistical terms an “interaction term” between the exposure (in this case the drug) and the potential effect modifier (in this case sex) in a multivariable model that includes both as exposures. If the P value for that interaction term is less than .05, then the interaction term is statistically significant, and therefore the variable (in this case sex) is confirmed to be an effect modifier. Hence, the results are not due to chance, and the different effects in men and women are plausibly attributable to different biological responses to the medication.
Difference between confounding and effect modification
At this point someone might ask, “What then is the difference between confounding and effect modification? In both cases, we do stratified analysis of the relationship between an exposure (in this case the weight-loss drug) and an outcome (in this case weight loss) based on strata of a third variable (in this case sex).” The difference is fundamental. Confounding, as we explored in a previous article,2 is something that we would like to get rid of. It is the effect of an outside variable on both the exposure and outcome that does not allow us to properly evaluate the real relationship between the two. As such, we try to adjust for this variable, so that its effect is eliminated and we can only observe the relationship between the exposure and the outcome. Effect modification, on the other side, is not something we would like to get rid of. On the contrary, effect modification is part of what we would like to explore and describe because it is part of the biological mechanism that explains the real relationship between the exposure and the outcome. In the example above, if effect modification by sex is confirmed, it implies that there is something in female biology that is not found in male biology (or vice-versa) that makes their response to the medication different and therefore something of interest to study further. Thus, differently from confounding, effect modification is part of the objective reality of the world which we would like to explore and evaluate.
Several questions then arise. How can we know whether a variable is a confounder, an effect modifier, or both? As a general rule of thumb, a confounder would be a variable which, when a stratified analysis is done (or when added to a multivariable model), will change the relationship between the exposure and outcome by 10% or more. However, the relationship between the exposure and the outcome in both strata will be similar. In the example above, it would mean that if sex was only a confounder, then stratifying by sex would show roughly a similar change in the effect (say 9 kg for men and 11 kg for women). An effect modifier on the other hand is one which, when a stratified analysis is done, the association between the exposure and the outcome is very different in the two strata, as illustrated in the example above (for simplicity I am only considering two-level effect modifiers in this article).
Can a variable be both a confounder and an effect modifier? Yes, that is possible. What can be done in this case? The most common approach is to behave the same as when only effect modification is present, namely to show with the interaction term that effect modification exists and present the results between the exposure and outcome separately by the level of the effect modifier (in this example, it means that we need to describe the effects of the weight-loss drug separately for men and women). The stratified analysis/presentation will, by definition, take care of confounding as well.2
Should we always look for effect modification? Not necessarily. As a general rule, we need to test for effect modification only if there is some biological rationale that would compel us to do so, and testing should be hypothesis-driven. A common mistake that some authors make is to perform too many interaction tests and then describe as “positive findings” any test for which P value happens to be less than .05. However, as we pointed out in the article on P value,1 if we perform multiple tests, this increases the probability of false positives, and therefore the probability of spurious findings. Thus, effect modification analysis (with interaction terms) should, generally speaking, be performed with a biological rationale and/or be hypothesis driven.
Conclusion
Effect modification is an essential statistical concept that describes an underlying biological reality in which the association between an exposure and an outcome is different based on the values of a third variable. Differently from confounding, which clouds the association between exposure and outcome, and therefore is something that we try to get rid of, effect modification serves to bring to light a more proper understanding of the biological reality underlying the true association between an exposure and an outcome and as such is something that needs to be explored and described.
Dr. Jovani is assistant professor of medicine, therapeutic endoscopy, digestive diseases, and nutrition at the University of Kentucky Albert B. Chandler Hospital, Lexington. He has no conflicts of interest.
References
1. “The P value: What to make of it? A simple guide for the uninitiated.” GI & Hepatology News. 2019 Sep 23. www.mdedge.com/gihepnews/article/208601/mixed-topics/p-value-what-make-it-simple-guide-uninitiated
2. “Evaluating a paper: Take care not to be confounded.” GI & Hepatology News. 2020 Sep 18. www.mdedge.com/gihepnews/article/228765/mixed-topics/evaluating-paper-take-care-not-be-confounded
3. Corraini P et al. Clin Epidemiol. 2017;9:331-8. doi: 10.2147/CLEP.S162236.
4. VanderWeele TJ. Epidemiology. 2009 Nov;20(6):863-71. doi: 10.1097/EDE.0b013e3181ba333c.
5. Shahar E and Shahar DJ. Epidemiology. 2010 Jul;21(4):587. doi: 10.1097/EDE.0b013e3181e0995c. Author reply 587-8.
In previous articles, we explored the meaning of two statistical concepts: that of the P value1 and that of confounding.2 In today’s article, we will focus on another important, though often underrecognized, statistical concept: that of effect modification (sometimes also called “interaction,” although many authors draw a distinction between these terms).3-5 In brief, effect modification is the recognition that the relationship between two variables can be different based on the values of a third variable. Let me illustrate with an example. Suppose that you give a weight-loss drug to a group of people. After a predetermined time, say 3 months, you weigh them to measure the effects of the drug, and you record that on average each individual lost about 10 pounds. Suppose, however, that you are curious to see the effect of the drug in men and women separately. You perform an analysis stratified by sex, and you notice that men lost on average only about 1 pound each, whereas women lost on average about 30 pounds each. What do these results mean? It seems that the effect of the drug on weight loss is different depending on the value of a third variable, namely sex. That is, in this case, sex acts as an effect modifier. Based on this analysis, we may conclude that the drug has real effects on women but not on men.
Before reaching this conclusion, however, it is appropriate to ask whether we have made a mistake. The first obvious thing is to make sure that we have not made a mistake in our collection of data. Once we have excluded the presence of structural bias in our dataset, how can we ascertain that these results, which at eyeballing seem so different, have not been so as a result of chance? Fortunately, we do not have to guess. There is a way to formally test for this hypothesis. If sex is truly an effect modifier, then we can perform what is called in statistical terms an “interaction term” between the exposure (in this case the drug) and the potential effect modifier (in this case sex) in a multivariable model that includes both as exposures. If the P value for that interaction term is less than .05, then the interaction term is statistically significant, and therefore the variable (in this case sex) is confirmed to be an effect modifier. Hence, the results are not due to chance, and the different effects in men and women are plausibly attributable to different biological responses to the medication.
Difference between confounding and effect modification
At this point someone might ask, “What then is the difference between confounding and effect modification? In both cases, we do stratified analysis of the relationship between an exposure (in this case the weight-loss drug) and an outcome (in this case weight loss) based on strata of a third variable (in this case sex).” The difference is fundamental. Confounding, as we explored in a previous article,2 is something that we would like to get rid of. It is the effect of an outside variable on both the exposure and outcome that does not allow us to properly evaluate the real relationship between the two. As such, we try to adjust for this variable, so that its effect is eliminated and we can only observe the relationship between the exposure and the outcome. Effect modification, on the other side, is not something we would like to get rid of. On the contrary, effect modification is part of what we would like to explore and describe because it is part of the biological mechanism that explains the real relationship between the exposure and the outcome. In the example above, if effect modification by sex is confirmed, it implies that there is something in female biology that is not found in male biology (or vice-versa) that makes their response to the medication different and therefore something of interest to study further. Thus, differently from confounding, effect modification is part of the objective reality of the world which we would like to explore and evaluate.
Several questions then arise. How can we know whether a variable is a confounder, an effect modifier, or both? As a general rule of thumb, a confounder would be a variable which, when a stratified analysis is done (or when added to a multivariable model), will change the relationship between the exposure and outcome by 10% or more. However, the relationship between the exposure and the outcome in both strata will be similar. In the example above, it would mean that if sex was only a confounder, then stratifying by sex would show roughly a similar change in the effect (say 9 kg for men and 11 kg for women). An effect modifier on the other hand is one which, when a stratified analysis is done, the association between the exposure and the outcome is very different in the two strata, as illustrated in the example above (for simplicity I am only considering two-level effect modifiers in this article).
Can a variable be both a confounder and an effect modifier? Yes, that is possible. What can be done in this case? The most common approach is to behave the same as when only effect modification is present, namely to show with the interaction term that effect modification exists and present the results between the exposure and outcome separately by the level of the effect modifier (in this example, it means that we need to describe the effects of the weight-loss drug separately for men and women). The stratified analysis/presentation will, by definition, take care of confounding as well.2
Should we always look for effect modification? Not necessarily. As a general rule, we need to test for effect modification only if there is some biological rationale that would compel us to do so, and testing should be hypothesis-driven. A common mistake that some authors make is to perform too many interaction tests and then describe as “positive findings” any test for which P value happens to be less than .05. However, as we pointed out in the article on P value,1 if we perform multiple tests, this increases the probability of false positives, and therefore the probability of spurious findings. Thus, effect modification analysis (with interaction terms) should, generally speaking, be performed with a biological rationale and/or be hypothesis driven.
Conclusion
Effect modification is an essential statistical concept that describes an underlying biological reality in which the association between an exposure and an outcome is different based on the values of a third variable. Differently from confounding, which clouds the association between exposure and outcome, and therefore is something that we try to get rid of, effect modification serves to bring to light a more proper understanding of the biological reality underlying the true association between an exposure and an outcome and as such is something that needs to be explored and described.
Dr. Jovani is assistant professor of medicine, therapeutic endoscopy, digestive diseases, and nutrition at the University of Kentucky Albert B. Chandler Hospital, Lexington. He has no conflicts of interest.
References
1. “The P value: What to make of it? A simple guide for the uninitiated.” GI & Hepatology News. 2019 Sep 23. www.mdedge.com/gihepnews/article/208601/mixed-topics/p-value-what-make-it-simple-guide-uninitiated
2. “Evaluating a paper: Take care not to be confounded.” GI & Hepatology News. 2020 Sep 18. www.mdedge.com/gihepnews/article/228765/mixed-topics/evaluating-paper-take-care-not-be-confounded
3. Corraini P et al. Clin Epidemiol. 2017;9:331-8. doi: 10.2147/CLEP.S162236.
4. VanderWeele TJ. Epidemiology. 2009 Nov;20(6):863-71. doi: 10.1097/EDE.0b013e3181ba333c.
5. Shahar E and Shahar DJ. Epidemiology. 2010 Jul;21(4):587. doi: 10.1097/EDE.0b013e3181e0995c. Author reply 587-8.
Endoscopic management of duodenal and ampullary adenomas
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.
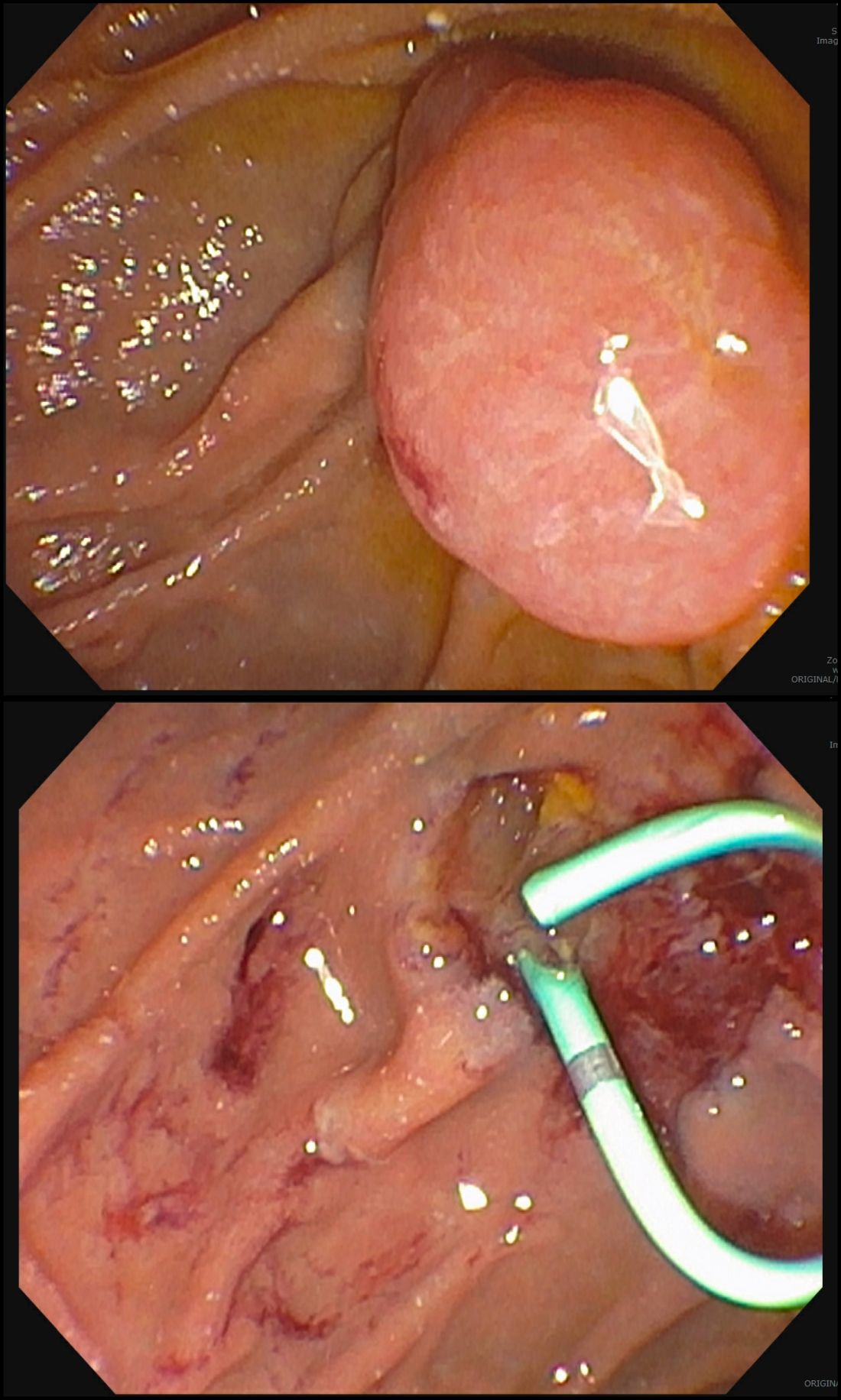
The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Become a highly effective endoscopy teacher, from start to finish
When I first became an attending, I was struck by how difficult it was to teach endoscopy effectively. As a fellow, I saw the various teaching styles of my attendings, and it was easy to pick out the best teachers from the group. But when the roles switched, and suddenly I was the supervising faculty member, it was hard to recall exactly what those teachers were doing to create an optimal learning environment in the endoscopy suite. Not only did I lack a framework on how to teach endoscopy, I also was still building confidence in my own endoscopic skills while feeling the pressure to keep my room running on time. All in all, although I loved the opportunity to teach, I found the experience to be quite stressful.
Hoping to find some guidance, I turned to the literature and was fortunate to find some great pieces on how to teach endoscopy effectively. I learned of cognitive load theory – the idea that short-term or “working memory” can manage only a few pieces of information at a time – and how excess feedback or other external distractions (e.g., pagers) during a procedure can overwhelm a learner and lead to declining performance.1 I also read about the pursuit of “conscious competence,” where an endoscopist can verbalize the steps of a maneuver so that a trainee can remain on the scope and maximize hands-on participation.2
Motivated to bring these key concepts together in an evidence-based framework, I helped lead a Delphi study of GI fellowship program directors and endoscopy education experts to reach consensus on the best practices of teaching endoscopy.3 After two rounds of surveys, the participants identified 10 essential endoscopy teaching practices, which I will summarize in the next sections. What I found most helpful was how these practices were distributed throughout the endoscopy learning experience. By breaking down the complicated task of teaching endoscopy to three discrete parts – prior to the procedure, during the procedure, and after the procedure – I now had a framework to take back to the endoscopy suite.
Prior to the procedure
With a busy endoscopy schedule and increasing clinical demands, it is tempting to use the time between cases to complete documentation, address patient messages, and review emails. While this is great for efficiency, make sure to also reserve time to set the stage for your fellow. One of the key practices during this phase is to assess your fellow’s current procedural competency. I start open-ended by asking my fellows how they have been doing with colonoscopy and then ask if they are working on a specific skill. With this information, I have a sense of how much hands-on assistance they will need, what realistic goals to set for them (e.g., navigate out of the sigmoid colon for an early learner vs. efficiently and independently completing the entire case for a later learner), and the areas to focus my observation to provide feedback after the procedure.
During this preparatory time, faculty should also discuss the patient history and indications for the procedure. Reviewing information such as prior sedation requirements and confirming plans for the procedure (e.g., random colon biopsies in a patient with chronic diarrhea and concern for microscopic colitis) helps ensure a proper plan is in place for the patient while also presenting opportunities for learning. Faculty can take this time to review the steps of a more complicated procedure (e.g., PEG placement) and establish ground rules such as when the attending will take the scope from the trainee. Lastly, make sure that the patient understands the role of the fellow and the supervision you will be providing throughout the case.
During the procedure
Once the procedure starts, your most important task is to maintain attention throughout the case – if you do, the other best practices generally fall into place. I am most attentive when I am gowned and positioned next to the fellow. From this vantage point, I can see the patient, the fellow’s hands, and the endoscopy screen, which allows me to readily assist if needed while directly observing the fellow’s performance.
If I need to provide feedback in the moment, I often ask the fellows to pause what they are doing and first listen to my feedback. Taking this “timeout” helps manage their cognitive load such that they can actually hear the feedback. As a general rule, however, I try to reserve the bulk of my feedback for when the procedure is complete (see next section). Another way to manage your fellow’s cognitive load is by using standardized endoscopic language throughout the procedure. For example, rather than say “go to the left” during a colonoscopy, try saying “tip left” or “torque counterclockwise” to provide more clear instructions to the fellow. Holding your fellow’s pager during the procedure is a kind gesture that also helps minimize extraneous cognitive load so that the fellow can focus on the procedure.
If your fellows get to a point where they cannot complete the task despite your giving appropriate feedback, or if patient safety concerns arise, then it is time for you to take hands-on control of the scope. In my experience, most fellows welcome the hands-on assistance as they are overloaded by the difficulty of the procedure. Setting this expectation ahead of time, as noted above, makes for a smoother transition. While assuming control of the scope, try to narrate what you are doing differently so that the fellow can still learn while watching. Once you complete the difficult portion of the procedure (e.g., reducing a loop to reach the cecum), return the scope to the fellow to maximize the hands-on participation (if time permits).
After the procedure
In the third and final stage of the endoscopy teaching experience, faculty should take the time to confirm the findings of the procedure with the fellow and discuss next steps in management for the patient. Finding these teachable moments helps solidify the cognitive learning for the fellow while also ensuring the patient receives the appropriate postprocedure recommendations. As part of this process, make sure to review the procedure note drafted by the fellow, and if you need to make any substantive edits, review the changes with the fellow so that he or she can learn for future cases.
To wrap up the session, provide feedback to the fellow on performance based on your direct observation. Make sure to name this process aloud – “Let’s do some feedback” – and start by asking how the fellow felt about the performance, both in terms of what went well and what the fellow would like to improve. Then provide your feedback on the performance and be specific, such as, “I really like how you identified a loop and then reduced around the hepatic flexure.” Conclude by having the fellow set a plan for improvement and make sure to ask for feedback on your own teaching performance.
In conclusion, teaching endoscopy is hard – especially as a junior attending. By breaking down the endoscopy teaching experience into its three components, however, and committing to teaching from start to finish, you can provide high-quality endoscopy education to your fellows while ensuring the best care for your patients.
Dr. Kumar is associate medicine clerkship director at Harvard Medical School, and associate physician in the division of gastroenterology at Brigham and Women’s Hospital, both in Boston. He disclosed having no conflicts of interest. He is on Twitter @NavinKumarMD.
References
1. Dilly CK and Sewell JL. 2017 Sep;153(3):632-36.
2. Waschke KA et al. Best Pract Res Clin Gastroenterol. 2016 Jun;30(3):409-19.
3. Kumar NL et al. Clin Gastroenterol Hepatol. 2020 Mar;18(3):574-79.
When I first became an attending, I was struck by how difficult it was to teach endoscopy effectively. As a fellow, I saw the various teaching styles of my attendings, and it was easy to pick out the best teachers from the group. But when the roles switched, and suddenly I was the supervising faculty member, it was hard to recall exactly what those teachers were doing to create an optimal learning environment in the endoscopy suite. Not only did I lack a framework on how to teach endoscopy, I also was still building confidence in my own endoscopic skills while feeling the pressure to keep my room running on time. All in all, although I loved the opportunity to teach, I found the experience to be quite stressful.
Hoping to find some guidance, I turned to the literature and was fortunate to find some great pieces on how to teach endoscopy effectively. I learned of cognitive load theory – the idea that short-term or “working memory” can manage only a few pieces of information at a time – and how excess feedback or other external distractions (e.g., pagers) during a procedure can overwhelm a learner and lead to declining performance.1 I also read about the pursuit of “conscious competence,” where an endoscopist can verbalize the steps of a maneuver so that a trainee can remain on the scope and maximize hands-on participation.2
Motivated to bring these key concepts together in an evidence-based framework, I helped lead a Delphi study of GI fellowship program directors and endoscopy education experts to reach consensus on the best practices of teaching endoscopy.3 After two rounds of surveys, the participants identified 10 essential endoscopy teaching practices, which I will summarize in the next sections. What I found most helpful was how these practices were distributed throughout the endoscopy learning experience. By breaking down the complicated task of teaching endoscopy to three discrete parts – prior to the procedure, during the procedure, and after the procedure – I now had a framework to take back to the endoscopy suite.
Prior to the procedure
With a busy endoscopy schedule and increasing clinical demands, it is tempting to use the time between cases to complete documentation, address patient messages, and review emails. While this is great for efficiency, make sure to also reserve time to set the stage for your fellow. One of the key practices during this phase is to assess your fellow’s current procedural competency. I start open-ended by asking my fellows how they have been doing with colonoscopy and then ask if they are working on a specific skill. With this information, I have a sense of how much hands-on assistance they will need, what realistic goals to set for them (e.g., navigate out of the sigmoid colon for an early learner vs. efficiently and independently completing the entire case for a later learner), and the areas to focus my observation to provide feedback after the procedure.
During this preparatory time, faculty should also discuss the patient history and indications for the procedure. Reviewing information such as prior sedation requirements and confirming plans for the procedure (e.g., random colon biopsies in a patient with chronic diarrhea and concern for microscopic colitis) helps ensure a proper plan is in place for the patient while also presenting opportunities for learning. Faculty can take this time to review the steps of a more complicated procedure (e.g., PEG placement) and establish ground rules such as when the attending will take the scope from the trainee. Lastly, make sure that the patient understands the role of the fellow and the supervision you will be providing throughout the case.
During the procedure
Once the procedure starts, your most important task is to maintain attention throughout the case – if you do, the other best practices generally fall into place. I am most attentive when I am gowned and positioned next to the fellow. From this vantage point, I can see the patient, the fellow’s hands, and the endoscopy screen, which allows me to readily assist if needed while directly observing the fellow’s performance.
If I need to provide feedback in the moment, I often ask the fellows to pause what they are doing and first listen to my feedback. Taking this “timeout” helps manage their cognitive load such that they can actually hear the feedback. As a general rule, however, I try to reserve the bulk of my feedback for when the procedure is complete (see next section). Another way to manage your fellow’s cognitive load is by using standardized endoscopic language throughout the procedure. For example, rather than say “go to the left” during a colonoscopy, try saying “tip left” or “torque counterclockwise” to provide more clear instructions to the fellow. Holding your fellow’s pager during the procedure is a kind gesture that also helps minimize extraneous cognitive load so that the fellow can focus on the procedure.
If your fellows get to a point where they cannot complete the task despite your giving appropriate feedback, or if patient safety concerns arise, then it is time for you to take hands-on control of the scope. In my experience, most fellows welcome the hands-on assistance as they are overloaded by the difficulty of the procedure. Setting this expectation ahead of time, as noted above, makes for a smoother transition. While assuming control of the scope, try to narrate what you are doing differently so that the fellow can still learn while watching. Once you complete the difficult portion of the procedure (e.g., reducing a loop to reach the cecum), return the scope to the fellow to maximize the hands-on participation (if time permits).
After the procedure
In the third and final stage of the endoscopy teaching experience, faculty should take the time to confirm the findings of the procedure with the fellow and discuss next steps in management for the patient. Finding these teachable moments helps solidify the cognitive learning for the fellow while also ensuring the patient receives the appropriate postprocedure recommendations. As part of this process, make sure to review the procedure note drafted by the fellow, and if you need to make any substantive edits, review the changes with the fellow so that he or she can learn for future cases.
To wrap up the session, provide feedback to the fellow on performance based on your direct observation. Make sure to name this process aloud – “Let’s do some feedback” – and start by asking how the fellow felt about the performance, both in terms of what went well and what the fellow would like to improve. Then provide your feedback on the performance and be specific, such as, “I really like how you identified a loop and then reduced around the hepatic flexure.” Conclude by having the fellow set a plan for improvement and make sure to ask for feedback on your own teaching performance.
In conclusion, teaching endoscopy is hard – especially as a junior attending. By breaking down the endoscopy teaching experience into its three components, however, and committing to teaching from start to finish, you can provide high-quality endoscopy education to your fellows while ensuring the best care for your patients.
Dr. Kumar is associate medicine clerkship director at Harvard Medical School, and associate physician in the division of gastroenterology at Brigham and Women’s Hospital, both in Boston. He disclosed having no conflicts of interest. He is on Twitter @NavinKumarMD.
References
1. Dilly CK and Sewell JL. 2017 Sep;153(3):632-36.
2. Waschke KA et al. Best Pract Res Clin Gastroenterol. 2016 Jun;30(3):409-19.
3. Kumar NL et al. Clin Gastroenterol Hepatol. 2020 Mar;18(3):574-79.
When I first became an attending, I was struck by how difficult it was to teach endoscopy effectively. As a fellow, I saw the various teaching styles of my attendings, and it was easy to pick out the best teachers from the group. But when the roles switched, and suddenly I was the supervising faculty member, it was hard to recall exactly what those teachers were doing to create an optimal learning environment in the endoscopy suite. Not only did I lack a framework on how to teach endoscopy, I also was still building confidence in my own endoscopic skills while feeling the pressure to keep my room running on time. All in all, although I loved the opportunity to teach, I found the experience to be quite stressful.
Hoping to find some guidance, I turned to the literature and was fortunate to find some great pieces on how to teach endoscopy effectively. I learned of cognitive load theory – the idea that short-term or “working memory” can manage only a few pieces of information at a time – and how excess feedback or other external distractions (e.g., pagers) during a procedure can overwhelm a learner and lead to declining performance.1 I also read about the pursuit of “conscious competence,” where an endoscopist can verbalize the steps of a maneuver so that a trainee can remain on the scope and maximize hands-on participation.2
Motivated to bring these key concepts together in an evidence-based framework, I helped lead a Delphi study of GI fellowship program directors and endoscopy education experts to reach consensus on the best practices of teaching endoscopy.3 After two rounds of surveys, the participants identified 10 essential endoscopy teaching practices, which I will summarize in the next sections. What I found most helpful was how these practices were distributed throughout the endoscopy learning experience. By breaking down the complicated task of teaching endoscopy to three discrete parts – prior to the procedure, during the procedure, and after the procedure – I now had a framework to take back to the endoscopy suite.
Prior to the procedure
With a busy endoscopy schedule and increasing clinical demands, it is tempting to use the time between cases to complete documentation, address patient messages, and review emails. While this is great for efficiency, make sure to also reserve time to set the stage for your fellow. One of the key practices during this phase is to assess your fellow’s current procedural competency. I start open-ended by asking my fellows how they have been doing with colonoscopy and then ask if they are working on a specific skill. With this information, I have a sense of how much hands-on assistance they will need, what realistic goals to set for them (e.g., navigate out of the sigmoid colon for an early learner vs. efficiently and independently completing the entire case for a later learner), and the areas to focus my observation to provide feedback after the procedure.
During this preparatory time, faculty should also discuss the patient history and indications for the procedure. Reviewing information such as prior sedation requirements and confirming plans for the procedure (e.g., random colon biopsies in a patient with chronic diarrhea and concern for microscopic colitis) helps ensure a proper plan is in place for the patient while also presenting opportunities for learning. Faculty can take this time to review the steps of a more complicated procedure (e.g., PEG placement) and establish ground rules such as when the attending will take the scope from the trainee. Lastly, make sure that the patient understands the role of the fellow and the supervision you will be providing throughout the case.
During the procedure
Once the procedure starts, your most important task is to maintain attention throughout the case – if you do, the other best practices generally fall into place. I am most attentive when I am gowned and positioned next to the fellow. From this vantage point, I can see the patient, the fellow’s hands, and the endoscopy screen, which allows me to readily assist if needed while directly observing the fellow’s performance.
If I need to provide feedback in the moment, I often ask the fellows to pause what they are doing and first listen to my feedback. Taking this “timeout” helps manage their cognitive load such that they can actually hear the feedback. As a general rule, however, I try to reserve the bulk of my feedback for when the procedure is complete (see next section). Another way to manage your fellow’s cognitive load is by using standardized endoscopic language throughout the procedure. For example, rather than say “go to the left” during a colonoscopy, try saying “tip left” or “torque counterclockwise” to provide more clear instructions to the fellow. Holding your fellow’s pager during the procedure is a kind gesture that also helps minimize extraneous cognitive load so that the fellow can focus on the procedure.
If your fellows get to a point where they cannot complete the task despite your giving appropriate feedback, or if patient safety concerns arise, then it is time for you to take hands-on control of the scope. In my experience, most fellows welcome the hands-on assistance as they are overloaded by the difficulty of the procedure. Setting this expectation ahead of time, as noted above, makes for a smoother transition. While assuming control of the scope, try to narrate what you are doing differently so that the fellow can still learn while watching. Once you complete the difficult portion of the procedure (e.g., reducing a loop to reach the cecum), return the scope to the fellow to maximize the hands-on participation (if time permits).
After the procedure
In the third and final stage of the endoscopy teaching experience, faculty should take the time to confirm the findings of the procedure with the fellow and discuss next steps in management for the patient. Finding these teachable moments helps solidify the cognitive learning for the fellow while also ensuring the patient receives the appropriate postprocedure recommendations. As part of this process, make sure to review the procedure note drafted by the fellow, and if you need to make any substantive edits, review the changes with the fellow so that he or she can learn for future cases.
To wrap up the session, provide feedback to the fellow on performance based on your direct observation. Make sure to name this process aloud – “Let’s do some feedback” – and start by asking how the fellow felt about the performance, both in terms of what went well and what the fellow would like to improve. Then provide your feedback on the performance and be specific, such as, “I really like how you identified a loop and then reduced around the hepatic flexure.” Conclude by having the fellow set a plan for improvement and make sure to ask for feedback on your own teaching performance.
In conclusion, teaching endoscopy is hard – especially as a junior attending. By breaking down the endoscopy teaching experience into its three components, however, and committing to teaching from start to finish, you can provide high-quality endoscopy education to your fellows while ensuring the best care for your patients.
Dr. Kumar is associate medicine clerkship director at Harvard Medical School, and associate physician in the division of gastroenterology at Brigham and Women’s Hospital, both in Boston. He disclosed having no conflicts of interest. He is on Twitter @NavinKumarMD.
References
1. Dilly CK and Sewell JL. 2017 Sep;153(3):632-36.
2. Waschke KA et al. Best Pract Res Clin Gastroenterol. 2016 Jun;30(3):409-19.
3. Kumar NL et al. Clin Gastroenterol Hepatol. 2020 Mar;18(3):574-79.
Cautious optimism
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist! Digestive Disease Week® (DDW) is approaching quickly, which is our first since 2019 with an option to attend in person. This will give many an opportunity to reconnect in a way we have not been able to in so long – a welcome reprieve from the virtual platforms we have become so accustomed to. Cautious optimism is pervasive throughout the country that the acuity of the pandemic may be receding, and that we are perhaps better equipped for future surges should they occur.
I’m excited to introduce this quarter’s content – beginning with our feature clinical “In Focus” piece. Gastroparesis often poses a therapeutic challenge to gastroenterologists; Dr. Thomas Abell and Dr. Prateek Mathur (University of Louisville) provide an excellent, comprehensive discussion of the utility and efficacy of dietary modifications, pharmacotherapy, pylorus-directed therapies, bioelectric therapy, and other novel approaches to the treatment of gastroparesis.
The role of a gastrointestinal psychologist within a gastroenterology practice is invaluable. The gut-brain axis is a key feature of any gastroenterological disorder and one of the hallmarks of therapy is behavioral symptom management. Dr. Alyse Bedell (University of Chicago) educates us on how to effectively integrate psychogastroenterology into our treatment plans and discusses which patients are poised to benefit the most from referral.
In just 2 short months, gastroenterology fellowship programs across the country will welcome their newest trainees. Dr. Rashmi Advani (Stony Brook University), Dr. Naba Saeed (University of Kentucky) and Dr. Aline Charabaty (Johns Hopkins University) offer detailed, practical advice to incoming fellows on how to make the most of (and survive!) the first year of gastroenterology fellowship, which can be one of the most challenging years of medical training.
In our Postfellowship Pathways section, we are fortunate to have Dr. Barbara Jung, chair of the department of medicine at the University of Washington and future AGA president, share her story. Her journey is inspirational as she discusses her path to success: How her roots in basic science led to building clinical programs and her transition from chief of a gastroenterology division to chair of a large department at one of the most prolific academic centers in the country.
One of the hallmarks of any heavily procedural field such as gastroenterology is innovation, namely the continuous evolution of procedural technique and utilization of novel technology. It can be difficult, however, to reconcile this innovation in the informed consent process when there are limited data on safety and efficacy. Dr. Peter Angelos and Dr. Jelani Williams (University of Chicago) share a riveting perspective on how to approach these scenarios in a wonderful addition to our medical ethics case series.
Finally, the DHPA Private Practice Perspectives article this quarter, written by Dr. Paul Feuerstadt (PACT-Gastroenterology Center, Hamden, Conn.) and Dr. Louis Korman (Capital Digestive Care, Maryland), reviews the benefits of performing clinical research in private practice and what early career physicians who would like to explore clinical research should look for when evaluating job opportunities.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist! Digestive Disease Week® (DDW) is approaching quickly, which is our first since 2019 with an option to attend in person. This will give many an opportunity to reconnect in a way we have not been able to in so long – a welcome reprieve from the virtual platforms we have become so accustomed to. Cautious optimism is pervasive throughout the country that the acuity of the pandemic may be receding, and that we are perhaps better equipped for future surges should they occur.
I’m excited to introduce this quarter’s content – beginning with our feature clinical “In Focus” piece. Gastroparesis often poses a therapeutic challenge to gastroenterologists; Dr. Thomas Abell and Dr. Prateek Mathur (University of Louisville) provide an excellent, comprehensive discussion of the utility and efficacy of dietary modifications, pharmacotherapy, pylorus-directed therapies, bioelectric therapy, and other novel approaches to the treatment of gastroparesis.
The role of a gastrointestinal psychologist within a gastroenterology practice is invaluable. The gut-brain axis is a key feature of any gastroenterological disorder and one of the hallmarks of therapy is behavioral symptom management. Dr. Alyse Bedell (University of Chicago) educates us on how to effectively integrate psychogastroenterology into our treatment plans and discusses which patients are poised to benefit the most from referral.
In just 2 short months, gastroenterology fellowship programs across the country will welcome their newest trainees. Dr. Rashmi Advani (Stony Brook University), Dr. Naba Saeed (University of Kentucky) and Dr. Aline Charabaty (Johns Hopkins University) offer detailed, practical advice to incoming fellows on how to make the most of (and survive!) the first year of gastroenterology fellowship, which can be one of the most challenging years of medical training.
In our Postfellowship Pathways section, we are fortunate to have Dr. Barbara Jung, chair of the department of medicine at the University of Washington and future AGA president, share her story. Her journey is inspirational as she discusses her path to success: How her roots in basic science led to building clinical programs and her transition from chief of a gastroenterology division to chair of a large department at one of the most prolific academic centers in the country.
One of the hallmarks of any heavily procedural field such as gastroenterology is innovation, namely the continuous evolution of procedural technique and utilization of novel technology. It can be difficult, however, to reconcile this innovation in the informed consent process when there are limited data on safety and efficacy. Dr. Peter Angelos and Dr. Jelani Williams (University of Chicago) share a riveting perspective on how to approach these scenarios in a wonderful addition to our medical ethics case series.
Finally, the DHPA Private Practice Perspectives article this quarter, written by Dr. Paul Feuerstadt (PACT-Gastroenterology Center, Hamden, Conn.) and Dr. Louis Korman (Capital Digestive Care, Maryland), reviews the benefits of performing clinical research in private practice and what early career physicians who would like to explore clinical research should look for when evaluating job opportunities.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist! Digestive Disease Week® (DDW) is approaching quickly, which is our first since 2019 with an option to attend in person. This will give many an opportunity to reconnect in a way we have not been able to in so long – a welcome reprieve from the virtual platforms we have become so accustomed to. Cautious optimism is pervasive throughout the country that the acuity of the pandemic may be receding, and that we are perhaps better equipped for future surges should they occur.
I’m excited to introduce this quarter’s content – beginning with our feature clinical “In Focus” piece. Gastroparesis often poses a therapeutic challenge to gastroenterologists; Dr. Thomas Abell and Dr. Prateek Mathur (University of Louisville) provide an excellent, comprehensive discussion of the utility and efficacy of dietary modifications, pharmacotherapy, pylorus-directed therapies, bioelectric therapy, and other novel approaches to the treatment of gastroparesis.
The role of a gastrointestinal psychologist within a gastroenterology practice is invaluable. The gut-brain axis is a key feature of any gastroenterological disorder and one of the hallmarks of therapy is behavioral symptom management. Dr. Alyse Bedell (University of Chicago) educates us on how to effectively integrate psychogastroenterology into our treatment plans and discusses which patients are poised to benefit the most from referral.
In just 2 short months, gastroenterology fellowship programs across the country will welcome their newest trainees. Dr. Rashmi Advani (Stony Brook University), Dr. Naba Saeed (University of Kentucky) and Dr. Aline Charabaty (Johns Hopkins University) offer detailed, practical advice to incoming fellows on how to make the most of (and survive!) the first year of gastroenterology fellowship, which can be one of the most challenging years of medical training.
In our Postfellowship Pathways section, we are fortunate to have Dr. Barbara Jung, chair of the department of medicine at the University of Washington and future AGA president, share her story. Her journey is inspirational as she discusses her path to success: How her roots in basic science led to building clinical programs and her transition from chief of a gastroenterology division to chair of a large department at one of the most prolific academic centers in the country.
One of the hallmarks of any heavily procedural field such as gastroenterology is innovation, namely the continuous evolution of procedural technique and utilization of novel technology. It can be difficult, however, to reconcile this innovation in the informed consent process when there are limited data on safety and efficacy. Dr. Peter Angelos and Dr. Jelani Williams (University of Chicago) share a riveting perspective on how to approach these scenarios in a wonderful addition to our medical ethics case series.
Finally, the DHPA Private Practice Perspectives article this quarter, written by Dr. Paul Feuerstadt (PACT-Gastroenterology Center, Hamden, Conn.) and Dr. Louis Korman (Capital Digestive Care, Maryland), reviews the benefits of performing clinical research in private practice and what early career physicians who would like to explore clinical research should look for when evaluating job opportunities.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
The benefits of conducting clinical research in private practice
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.





















