User login
My path: Challenges and decisions along the way
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
Management of gastroparesis in 2022
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.
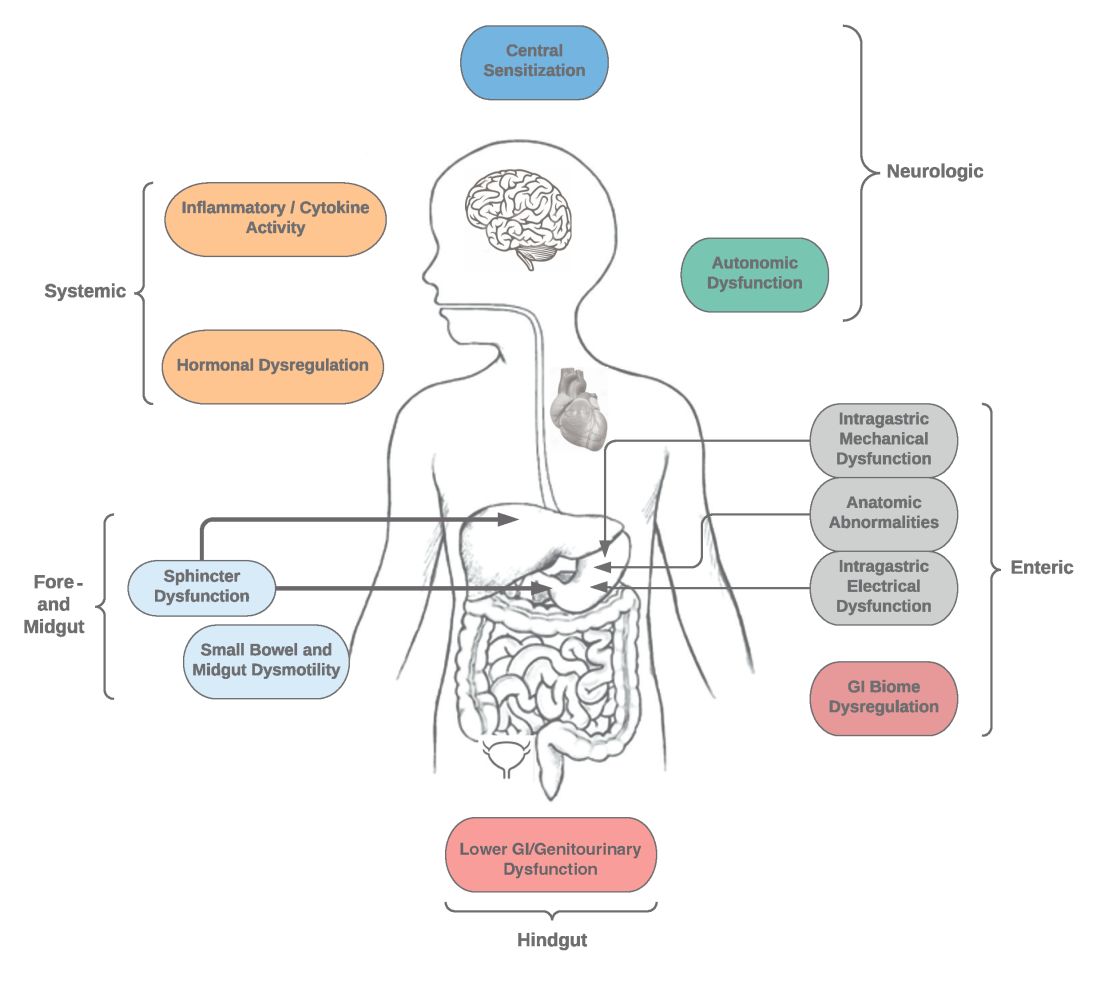
Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.
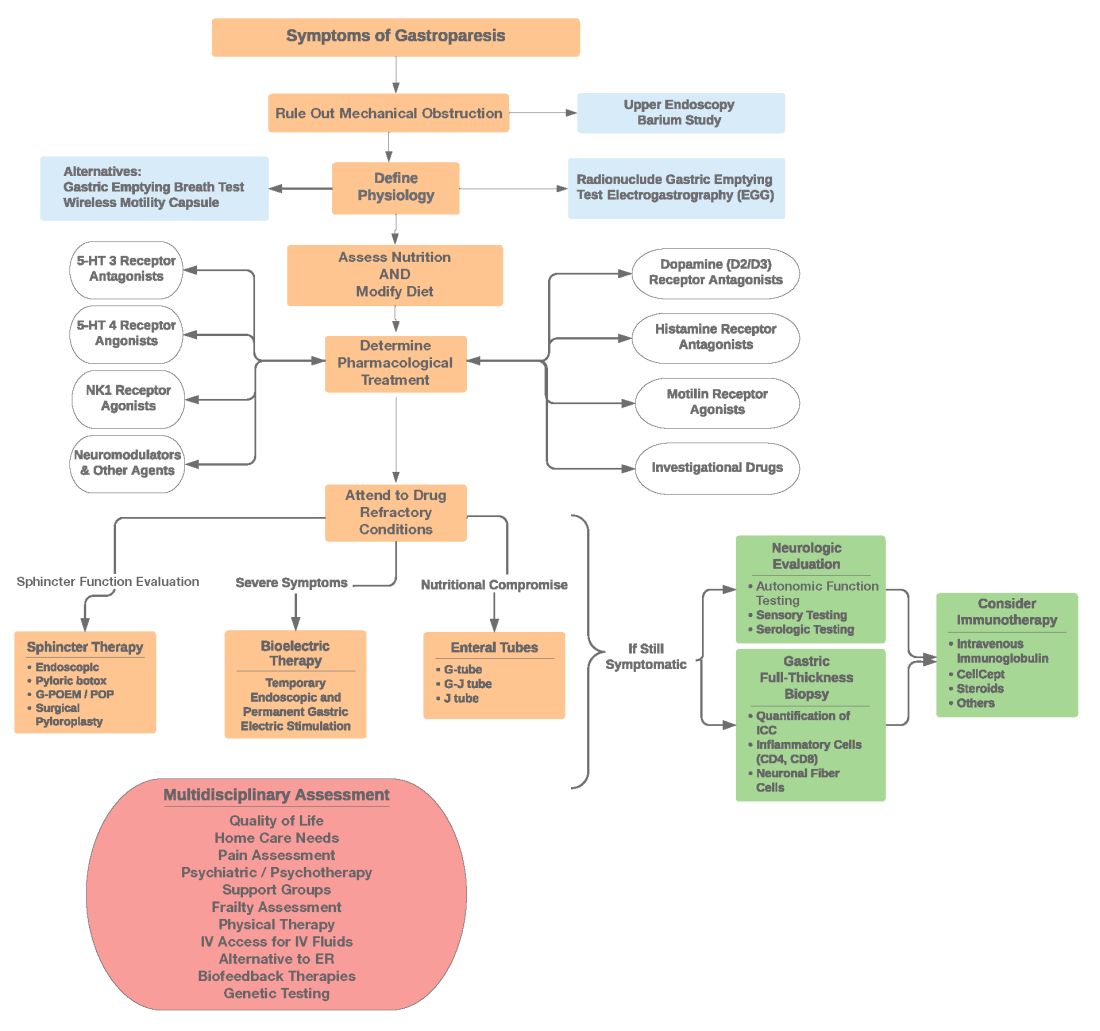
Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
AGA News - May 2022
AGA Fellow (AGAF) applications now open
AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release, and a digital badge to inform others of your accomplishment.
Learn more
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24, 2022.
If you have any questions, contact AGA Member Relations at [email protected] or 301-941-2651.
AGA Fellow (AGAF) applications now open
AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release, and a digital badge to inform others of your accomplishment.
Learn more
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24, 2022.
If you have any questions, contact AGA Member Relations at [email protected] or 301-941-2651.
AGA Fellow (AGAF) applications now open
AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release, and a digital badge to inform others of your accomplishment.
Learn more
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24, 2022.
If you have any questions, contact AGA Member Relations at [email protected] or 301-941-2651.
May 2022 - ICYMI
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
First-year fellows guide to gastroenterology
After the excitement and the well-deserved celebrations of matching in a gastroenterology fellowship program, a whole new set of unanswered questions and worries can start forming in a first-year fellow’s mind. “I made it, but now what? How do I learn a whole new career skill like endoscopy? Is my GI knowledge solid and wide enough to manage patients and answer the medical team consult? How will I keep up with my reading and learning with a busy fellowship schedule? How do I balance growth in clinical knowledge, endoscopy, and research? Can I integrate ‘life’ alongside a busy fellowship?” All of these questions and more can be overwhelming to answer in the beginning. The following guide is designed to help you through this transition and navigate the various aspects of first-year fellowship.
First-year goals
It is important to keep in mind that you have 3 full years to become a well-rounded, highly skilled, and knowledgeable gastroenterologist and endoscopist. So, set realistic goals and expectations for your first year, but be mindful that this year also lays the solid foundations of who you will become as a clinician, educator, or researcher.
One of the main goals of fellowship is to learn and implement evidence-based medicine in the diagnosis and management of GI conditions, as well as to learn endoscopic skills and ethics, all while keeping the patient (as a whole person) at the center of what you do. According to a recently published article by Bollipo and colleagues,1 the overall growth as a gastroenterologist not only depends on acquisition of knowledge but also involves cultivating teamwork, communication, situational awareness, compassion, leadership, and situational awareness. Beyond your medical education, your professional growth is also dependent on intentionally working towards acquiring the following skills:
1. Manage your time efficiently and prioritize your daily tasks
2. Become a consultant: effectively communicate with others, teach, lead, and delegate as appropriate
3. Work as a team with colleagues, faculty, and endoscopy staff
4. Develop critical thinking, give and receive constructive feedback, and understand your skills, limitations, and growth potential
5. Identify mentors and potential niche area
6. Start building your professional network and your reputation
7. Get involved in national GI societies
Consults
Mindset
Shifting gear from residency to fellowship involves a shifting of your clinical mindset too, going from being part of a primary care team responsible for all aspects of a patient’s medical care, to that of a consulting team focused on a patient’s GI condition. It is important to find the right balance of refraining from micromanaging non-GI comorbidities while being fully aware of their impact on your diagnostic and therapeutic approach to the GI condition.
Let’s face it, you will not always get “exciting and interesting GI cases” consults, and on a busy day some consultations might feel unnecessary and frustrating to you. Remember that what seems obvious to you, based on your focused GI knowledge, might not be so simple to the primary team. In addition, every consult is an opportunity to improve your approach to patient care, as well as an opportunity to learn and teach others, from medical students to residents. So, always be professional and respectful when you pick up the phone, and build positive collaborative bridges between you and the medical or surgical consulting teams. Be the GI fellow others are not reluctant to call for help, and better, be the one who communicates GI pearls along the way, inspires others to join the field of gastroenterology, and positively represent the GI division.
Triage
When you answer your consult page, ask the primary team what specific question they have for you and/or what is the main GI complaint or test abnormality the patient has. This will help you assess the urgency and the complexity of the consult, and hence allow you to prioritize each consult (which one you need to see first and give the attending a heads-up), assign (or not) a rotating medical resident or student to the consult, tailor your preliminary recommendations to the primary team, and anticipate the need for a procedure. When you anticipate a procedure, assess its (semi-)urgency to get the process ready for same day or a bedside procedure by getting information on the patient’s vitals, basic labs, significant acute comorbidities, and supportive therapies in place. In other words, by judiciously obtaining key information from the primary team, you can efficiently triage the consults and keep your day organized and manageable (for the most part). Learn to divide and conquer the tasks of the day: split inpatient endoscopy and consults with your cofellows, assign appropriate consults and follow-ups to residents or students rotating on the GI service, and properly communicate with the primary team a plan of care (even a preliminary one) to avoid recurrent pages and interruptions. Some days the number or urgency of the consults and the required multitasking can be overwhelming: stop, breathe, and ask for help from your co-fellows and your attending. Remember, this is a fellowship, not a solo-ship and your program is here to support your work and growth.
Communication
Timely and efficient communication, between you and the different stakeholders, is crucial to provide optimal patient care and minimize the risk of “things falling through the cracks”. Convey to the primary team your recommendations and plan of care clearly, and use direct verbal communication (not just a note in the chart) when managing complex or urgent situations. Obtain information regarding current patient level of care (i.e., ICU), isolation precautions, and cardiac devices (i.e., left ventricular assist device). Keep the dialogue open with your attending about acutely ill patients and potentially urgent procedures. Inform the endoscopy suite early that you are adding a procedure on the same day, and communicate anticipated needs (such as intubation, fluoroscopy room, pediatric scope, stent). Using a “closed-loop” communication structure can ensure that your recommendations are received and implemented appropriately.2
Time management and structure
Having a structured routine to your day, in what seems to be a chaotic process of juggling different duties and being in different locations at once, will ensure that you efficiently complete your tasks in a timely manner. Find what works best for you, taking into account the challenges and resources available to you, such as the number of fellows and other trainees on the GI consult service, the average number of consults per day and their acuity, the availability of inpatient protocols for specific clinical situations (GI bleed, acute severe ulcerative colitis, etc.), and the time and style preferences of the rounding attending. We suggest the following schedule on a consult day: Round early in the morning and leave a note in the chart and/or communicate with the team key information, then review with the rotating trainees the patients they are following and personally reassess some patients as needed. Inform the endoscopy suite of the same day procedures and let your attending know of any issues that require immediate attention. Take your team and head to radiology and review the imaging studies on your patients. Learn and teach key points in diagnosis and therapeutic approach as you move through your day from the inpatient floors to the hallways. Divide consults during the day with your team and agree on a time to touch base. Review your patient list at the end of the day and assess which patients the GI service no longer need to follow and communicate that clearly to the primary team along with the appropriate outpatient GI care follow-up. Let the endoscopy suite know of any procedures you are adding for the next day along with their degree of urgency to allow the charge nurse to prioritize cases. When you leave the hospital, be intentional with your free time: Read about the GI conditions you have encountered, enjoy some fun relaxing time, and rest!
Call
Know your call environment and your emergency cart
Familiarize yourself with the locations where you could potentially perform an emergent case (i.e., the ICU, ED, operating room) and the relevant points of contact (such as the charge nurse, the anesthesia team, the on-call tech team) for overnight or weekend cases. Whether or not you have an endoscopy support team on call, learn to set up the emergency cart, find and check your equipment, and troubleshoot technical issues by soliciting an “in-service” from senior fellows or the endoscopy technical support staff. Before heading to an urgent case, double check that you have your “bleeder” or “food impaction” tools. For food impaction, consider obtaining rat tooth forceps, snares, Roth nets, and an overtube. For bleeding cases, obtain a therapeutic upper endoscope, hemostatic clips, clear caps, injection needles, epinephrine, HemosprayTM, banding kits, and the appropriate electrocautery/thermal set up.
What is an emergency?
Consults that require your immediate attention include food impactions, acute biliary conditions leading to septic shock, and hemodynamically unstable GI bleeds, especially variceal bleeds. Remember that patients who are hemodynamically unstable require adequate resuscitation before proceeding with any endoscopic intervention. Assess the need for intubation, the timing of the procedure, and the most optimal location to perform the procedure, depending on the time and acuity of the patients’ presentation, how they respond to resuscitation measures, and the resources and preferences of your institution.
The overnight ‘nonemergent’ call
Non-emergent consults can be addressed the next day, after reviewing the clinical information provided by the consulting team and the patient’s EMR to ensure no urgent measures are needed. Overnight call may include patient phone calls, from inquiries about colon prep (so familiarize yourself with the different prep instructions and how to troubleshoot prep difficulties) to GI symptoms that you will need to triage to either the ED or to an outpatient follow-up. Document all phone encounters in the EMR and route your note to the appropriate clinician and nurse or administrative assistant for follow-up.
The five E’s of endoscopy
Endoscopy training is a large component of a GI fellowship and can create achievement anxiety in many first-year fellows seeking the cecum! But there is more to endoscopy than technical skills: It is as important to adequately evaluate clinical situations, understand the indications and potential limitations and complications of the procedure, and assess how it will impact the management of the patient. And no, you don’t have to be a video gamer to be a good endoscopist; and yes, you will be able to regularly complete a colonoscopy before the end of your first year!
Evaluation
In order to improve your endoscopic skills, it is important to honestly assess your areas of proficiency and improvement and to welcome real-time constructive feedback from your teaching attending about your endoscopic skills range. Consider meeting regularly with your attending to discuss your short-term and long-term endoscopic goals and how to enhance your skills. This practice demonstrates responsibility, credibility, and accountability amongst your peers as well as a genuine commitment to your growth as an endoscopist.
Efficiency
In addition to focusing on the quality of your endoscopy, learn to be efficient in the pre- and postprocedure time flow. This entails any step from properly explaining the procedure to patients before they come to the endoscopy suite, making sure the needed endoscopy equipment and tools are available in your room, completing your personal setup (i.e., gowning up, setting up your bed/monitor height, testing your endoscope) even before time out, to discharging the patient and communicating key findings and plan of care to the primary team. Depending on the acuity of the procedure and patient’s comorbidities, certain procedures may need to be performed or completed by a more efficient and experienced senior fellow or attending; don’t let this situation trigger passive frustration in you, but rather use it as an active and engaging opportunity to learn.
Expectations
You (and all the other neighborhood kids) didn’t learn to ride a bike without falling, struggling, needing help, and practicing over and over again, and it goes the same when learning to scope as a first-year fellow. Keep this in mind to lessen frustration, set realistic expectations, and be patient with yourself and celebrate all the small victories. Set tangible goals with your attending prior to procedural days/rotations so they can help you hone in and perfect the desired endoscopy skills.
Ergonomics
In a recent study, endoscopy-related injury (ERI) was reported to occur in up to 75% of gastroenterologists.3 While your primary focus might be reaching the cecum, it is as crucial for you to learn how to prevent ERI to ensure your long term health and continued success in procedures.
Excellence over quantity
Your main focus as a trainee is to learn how to provide effective, efficient, and safe care to patients, including in endoscopy. The quality of the endoscopy you perform is much more important than the total number of procedures you do. Thus, it is key to take each procedure as a complete learning opportunity to perform a thorough evaluation, improve your technical skills, interpret the findings, and develop a therapeutic plan.
Work-life balance and burnout
Fellowship is a marathon and not a sprint, so you need to slow down after a busy workday and care for yourself and enjoy time with loved ones. The cognitive, physical, mental, and emotional demands for first-year fellows are arguably the highest during GI training and can lead to burnout. Signs of burnout include emotional exhaustion, loss of empathy, fatigue, depersonalization and detachment, and feelings of personal inadequacy.4 Antiburnout measures include respecting basic healthy life hygiene (eat and sleep well, regular physical activity), having a hobby, practicing meditation, avoiding taking work home, and having a healthy social network.5 Remember that your cofellows whom you share common experiences with are not only your colleagues but can also be your friends and your social support. If you are a parent juggling work and family, remember to ask for help from your peers if you need it and have an open discussion with your attending to find practical solutions to your schedule.
Professional growth in the field of gastroenterology
Becoming a successful gastroenterologist and endoscopist involves going above the “I” and into the inclusive “we.” Building collegial and professional relationships early on with different stakeholders will set you up for success during and beyond your fellowship.
Building relationships
Developing genuine collegial and collaborative relationships with cofellows and faculty will positively impact your wellness during fellowship but also build the foundation of your professional network necessary to your career growth. Be inclusive of your cofellows in your research projects and publications, and support and amplify their work as much as you amplify your own. Your cofellows or attendings are likely to be the ones to help you find the right job, invite you to speak at grand rounds, or sit on a GI committee and promote your postfellowship professional growth.
Mentorship, being an educator and role model
It is important to identify and seek out mentors, within or outside your fellowship program or institution, who can not only guide you in your career choices but also open doors for you and sponsor you to advance your career. On the other hand, you too can be a role model, mentor, and sponsor to medical residents and students interested in the field of GI. Teach others in didactic settings or on the consult service, include trainees in quality improvement projects and publications, and lead by example.
Research
Most academic GI programs have a baseline requirement of research. Choose and devise a project you can realistically complete despite your busy first-year schedule: expand on a residency research project, focus on a specific simple question triggered by a clinical situation you encountered, proceed with a retrospective chart review or quality improvement project, and include other fellows and trainees to divide tasks. Alternatively, devise a specific timeline with a research mentor to complete a larger research project during your three years of fellowship.
Involvement in GI societies/committees
Become a member of one (or all) of the national GI societies that align with your interests. Membership gives you access not only to peer-reviewed scientific articles and guidelines but also to fellow-focused programs, committees’ opportunities, early career research grants, and mentorship.6-10
Summary
The first year of a GI fellowship lays the foundation for your next 3 years: Be mindful of how you can optimize the opportunities at hand to learn, teach, build a solid reputation, and grow your professional network. But also remember you have 3 full years to accomplish all your goals, so be patient, pace yourself, and include others in your journey. Judiciously use the many resources within your program and GI societies to help you achieve your goals, reach out to others to overcome difficulties and barriers, and dedicate time to care for your personal health and growth. This is what a true comprehensive and healthy fellowship is all about!
Dr. Advani is with the division of gastroenterology and hepatology, Stony Brook (N.Y.) University Hospital. Dr. Saeed is with the division of gastroenterology and hepatology, University of Kentucky, Lexington. Dr. Charabaty is with the division of gastroenterology, Johns Hopkins University, Baltimore, and Johns Hopkins–Sibley Memorial Hospital, Washington. Dr. Advani and Dr. Saeed have no conflicts to disclose. Dr. Charabaty disclosed ties to AbbVie, Janssen, Takeda, Pfizer, and Bristol-Myers Squibb and is founder of @MondayNightIBD, and cofounder of Scrubs & Heels.
References
1. Bollipo S. Gastroenterology. 2020 Nov;159(5):1648-52.
2. Adams MA et al. Gastroenterology. 2014 Jan 1;146(1):5-9.
3. Pawa S et al. Am J Gastroenterol. 2021 Mar 1;116(3):530-8.
4. DeCross AJ. Gastroenterology. 2020 Jan 1;158(1):32-5.
5. Burke C et al. Am J Gastroenterol. 2017 Oct 1;112:S593-4.
6. Fellows Resources under Fellows & Early Career. American Gastroenterological Association. https://gastro.org/fellows-and-early-career/training-resources/fellows-resources/.
7. Trainee Courses and Events. American College of Gastroenterology. https://gi.org/trainees/trainee-courses-and-events/.
8. Trainee Resources. American Association for the Study of Liver Diseases. https://www.aasld.org/membership/hepatology-associates/trainee-resources.
9. First Year Fellows Courses under Education. American Society of Gastrointestinal Endoscopy. https://www.asge.org/home/education/advanced-education-training/first-year-fellow-(fyf)-courses.
10. Annual GI Fellow Summer Course Presentations. New York Society for Gastrointestinal Endoscopy. https://www.nysge.org/annual%20gi%20fellows%20summer%20course.
After the excitement and the well-deserved celebrations of matching in a gastroenterology fellowship program, a whole new set of unanswered questions and worries can start forming in a first-year fellow’s mind. “I made it, but now what? How do I learn a whole new career skill like endoscopy? Is my GI knowledge solid and wide enough to manage patients and answer the medical team consult? How will I keep up with my reading and learning with a busy fellowship schedule? How do I balance growth in clinical knowledge, endoscopy, and research? Can I integrate ‘life’ alongside a busy fellowship?” All of these questions and more can be overwhelming to answer in the beginning. The following guide is designed to help you through this transition and navigate the various aspects of first-year fellowship.
First-year goals
It is important to keep in mind that you have 3 full years to become a well-rounded, highly skilled, and knowledgeable gastroenterologist and endoscopist. So, set realistic goals and expectations for your first year, but be mindful that this year also lays the solid foundations of who you will become as a clinician, educator, or researcher.
One of the main goals of fellowship is to learn and implement evidence-based medicine in the diagnosis and management of GI conditions, as well as to learn endoscopic skills and ethics, all while keeping the patient (as a whole person) at the center of what you do. According to a recently published article by Bollipo and colleagues,1 the overall growth as a gastroenterologist not only depends on acquisition of knowledge but also involves cultivating teamwork, communication, situational awareness, compassion, leadership, and situational awareness. Beyond your medical education, your professional growth is also dependent on intentionally working towards acquiring the following skills:
1. Manage your time efficiently and prioritize your daily tasks
2. Become a consultant: effectively communicate with others, teach, lead, and delegate as appropriate
3. Work as a team with colleagues, faculty, and endoscopy staff
4. Develop critical thinking, give and receive constructive feedback, and understand your skills, limitations, and growth potential
5. Identify mentors and potential niche area
6. Start building your professional network and your reputation
7. Get involved in national GI societies
Consults
Mindset
Shifting gear from residency to fellowship involves a shifting of your clinical mindset too, going from being part of a primary care team responsible for all aspects of a patient’s medical care, to that of a consulting team focused on a patient’s GI condition. It is important to find the right balance of refraining from micromanaging non-GI comorbidities while being fully aware of their impact on your diagnostic and therapeutic approach to the GI condition.
Let’s face it, you will not always get “exciting and interesting GI cases” consults, and on a busy day some consultations might feel unnecessary and frustrating to you. Remember that what seems obvious to you, based on your focused GI knowledge, might not be so simple to the primary team. In addition, every consult is an opportunity to improve your approach to patient care, as well as an opportunity to learn and teach others, from medical students to residents. So, always be professional and respectful when you pick up the phone, and build positive collaborative bridges between you and the medical or surgical consulting teams. Be the GI fellow others are not reluctant to call for help, and better, be the one who communicates GI pearls along the way, inspires others to join the field of gastroenterology, and positively represent the GI division.
Triage
When you answer your consult page, ask the primary team what specific question they have for you and/or what is the main GI complaint or test abnormality the patient has. This will help you assess the urgency and the complexity of the consult, and hence allow you to prioritize each consult (which one you need to see first and give the attending a heads-up), assign (or not) a rotating medical resident or student to the consult, tailor your preliminary recommendations to the primary team, and anticipate the need for a procedure. When you anticipate a procedure, assess its (semi-)urgency to get the process ready for same day or a bedside procedure by getting information on the patient’s vitals, basic labs, significant acute comorbidities, and supportive therapies in place. In other words, by judiciously obtaining key information from the primary team, you can efficiently triage the consults and keep your day organized and manageable (for the most part). Learn to divide and conquer the tasks of the day: split inpatient endoscopy and consults with your cofellows, assign appropriate consults and follow-ups to residents or students rotating on the GI service, and properly communicate with the primary team a plan of care (even a preliminary one) to avoid recurrent pages and interruptions. Some days the number or urgency of the consults and the required multitasking can be overwhelming: stop, breathe, and ask for help from your co-fellows and your attending. Remember, this is a fellowship, not a solo-ship and your program is here to support your work and growth.
Communication
Timely and efficient communication, between you and the different stakeholders, is crucial to provide optimal patient care and minimize the risk of “things falling through the cracks”. Convey to the primary team your recommendations and plan of care clearly, and use direct verbal communication (not just a note in the chart) when managing complex or urgent situations. Obtain information regarding current patient level of care (i.e., ICU), isolation precautions, and cardiac devices (i.e., left ventricular assist device). Keep the dialogue open with your attending about acutely ill patients and potentially urgent procedures. Inform the endoscopy suite early that you are adding a procedure on the same day, and communicate anticipated needs (such as intubation, fluoroscopy room, pediatric scope, stent). Using a “closed-loop” communication structure can ensure that your recommendations are received and implemented appropriately.2
Time management and structure
Having a structured routine to your day, in what seems to be a chaotic process of juggling different duties and being in different locations at once, will ensure that you efficiently complete your tasks in a timely manner. Find what works best for you, taking into account the challenges and resources available to you, such as the number of fellows and other trainees on the GI consult service, the average number of consults per day and their acuity, the availability of inpatient protocols for specific clinical situations (GI bleed, acute severe ulcerative colitis, etc.), and the time and style preferences of the rounding attending. We suggest the following schedule on a consult day: Round early in the morning and leave a note in the chart and/or communicate with the team key information, then review with the rotating trainees the patients they are following and personally reassess some patients as needed. Inform the endoscopy suite of the same day procedures and let your attending know of any issues that require immediate attention. Take your team and head to radiology and review the imaging studies on your patients. Learn and teach key points in diagnosis and therapeutic approach as you move through your day from the inpatient floors to the hallways. Divide consults during the day with your team and agree on a time to touch base. Review your patient list at the end of the day and assess which patients the GI service no longer need to follow and communicate that clearly to the primary team along with the appropriate outpatient GI care follow-up. Let the endoscopy suite know of any procedures you are adding for the next day along with their degree of urgency to allow the charge nurse to prioritize cases. When you leave the hospital, be intentional with your free time: Read about the GI conditions you have encountered, enjoy some fun relaxing time, and rest!
Call
Know your call environment and your emergency cart
Familiarize yourself with the locations where you could potentially perform an emergent case (i.e., the ICU, ED, operating room) and the relevant points of contact (such as the charge nurse, the anesthesia team, the on-call tech team) for overnight or weekend cases. Whether or not you have an endoscopy support team on call, learn to set up the emergency cart, find and check your equipment, and troubleshoot technical issues by soliciting an “in-service” from senior fellows or the endoscopy technical support staff. Before heading to an urgent case, double check that you have your “bleeder” or “food impaction” tools. For food impaction, consider obtaining rat tooth forceps, snares, Roth nets, and an overtube. For bleeding cases, obtain a therapeutic upper endoscope, hemostatic clips, clear caps, injection needles, epinephrine, HemosprayTM, banding kits, and the appropriate electrocautery/thermal set up.
What is an emergency?
Consults that require your immediate attention include food impactions, acute biliary conditions leading to septic shock, and hemodynamically unstable GI bleeds, especially variceal bleeds. Remember that patients who are hemodynamically unstable require adequate resuscitation before proceeding with any endoscopic intervention. Assess the need for intubation, the timing of the procedure, and the most optimal location to perform the procedure, depending on the time and acuity of the patients’ presentation, how they respond to resuscitation measures, and the resources and preferences of your institution.
The overnight ‘nonemergent’ call
Non-emergent consults can be addressed the next day, after reviewing the clinical information provided by the consulting team and the patient’s EMR to ensure no urgent measures are needed. Overnight call may include patient phone calls, from inquiries about colon prep (so familiarize yourself with the different prep instructions and how to troubleshoot prep difficulties) to GI symptoms that you will need to triage to either the ED or to an outpatient follow-up. Document all phone encounters in the EMR and route your note to the appropriate clinician and nurse or administrative assistant for follow-up.
The five E’s of endoscopy
Endoscopy training is a large component of a GI fellowship and can create achievement anxiety in many first-year fellows seeking the cecum! But there is more to endoscopy than technical skills: It is as important to adequately evaluate clinical situations, understand the indications and potential limitations and complications of the procedure, and assess how it will impact the management of the patient. And no, you don’t have to be a video gamer to be a good endoscopist; and yes, you will be able to regularly complete a colonoscopy before the end of your first year!
Evaluation
In order to improve your endoscopic skills, it is important to honestly assess your areas of proficiency and improvement and to welcome real-time constructive feedback from your teaching attending about your endoscopic skills range. Consider meeting regularly with your attending to discuss your short-term and long-term endoscopic goals and how to enhance your skills. This practice demonstrates responsibility, credibility, and accountability amongst your peers as well as a genuine commitment to your growth as an endoscopist.
Efficiency
In addition to focusing on the quality of your endoscopy, learn to be efficient in the pre- and postprocedure time flow. This entails any step from properly explaining the procedure to patients before they come to the endoscopy suite, making sure the needed endoscopy equipment and tools are available in your room, completing your personal setup (i.e., gowning up, setting up your bed/monitor height, testing your endoscope) even before time out, to discharging the patient and communicating key findings and plan of care to the primary team. Depending on the acuity of the procedure and patient’s comorbidities, certain procedures may need to be performed or completed by a more efficient and experienced senior fellow or attending; don’t let this situation trigger passive frustration in you, but rather use it as an active and engaging opportunity to learn.
Expectations
You (and all the other neighborhood kids) didn’t learn to ride a bike without falling, struggling, needing help, and practicing over and over again, and it goes the same when learning to scope as a first-year fellow. Keep this in mind to lessen frustration, set realistic expectations, and be patient with yourself and celebrate all the small victories. Set tangible goals with your attending prior to procedural days/rotations so they can help you hone in and perfect the desired endoscopy skills.
Ergonomics
In a recent study, endoscopy-related injury (ERI) was reported to occur in up to 75% of gastroenterologists.3 While your primary focus might be reaching the cecum, it is as crucial for you to learn how to prevent ERI to ensure your long term health and continued success in procedures.
Excellence over quantity
Your main focus as a trainee is to learn how to provide effective, efficient, and safe care to patients, including in endoscopy. The quality of the endoscopy you perform is much more important than the total number of procedures you do. Thus, it is key to take each procedure as a complete learning opportunity to perform a thorough evaluation, improve your technical skills, interpret the findings, and develop a therapeutic plan.
Work-life balance and burnout
Fellowship is a marathon and not a sprint, so you need to slow down after a busy workday and care for yourself and enjoy time with loved ones. The cognitive, physical, mental, and emotional demands for first-year fellows are arguably the highest during GI training and can lead to burnout. Signs of burnout include emotional exhaustion, loss of empathy, fatigue, depersonalization and detachment, and feelings of personal inadequacy.4 Antiburnout measures include respecting basic healthy life hygiene (eat and sleep well, regular physical activity), having a hobby, practicing meditation, avoiding taking work home, and having a healthy social network.5 Remember that your cofellows whom you share common experiences with are not only your colleagues but can also be your friends and your social support. If you are a parent juggling work and family, remember to ask for help from your peers if you need it and have an open discussion with your attending to find practical solutions to your schedule.
Professional growth in the field of gastroenterology
Becoming a successful gastroenterologist and endoscopist involves going above the “I” and into the inclusive “we.” Building collegial and professional relationships early on with different stakeholders will set you up for success during and beyond your fellowship.
Building relationships
Developing genuine collegial and collaborative relationships with cofellows and faculty will positively impact your wellness during fellowship but also build the foundation of your professional network necessary to your career growth. Be inclusive of your cofellows in your research projects and publications, and support and amplify their work as much as you amplify your own. Your cofellows or attendings are likely to be the ones to help you find the right job, invite you to speak at grand rounds, or sit on a GI committee and promote your postfellowship professional growth.
Mentorship, being an educator and role model
It is important to identify and seek out mentors, within or outside your fellowship program or institution, who can not only guide you in your career choices but also open doors for you and sponsor you to advance your career. On the other hand, you too can be a role model, mentor, and sponsor to medical residents and students interested in the field of GI. Teach others in didactic settings or on the consult service, include trainees in quality improvement projects and publications, and lead by example.
Research
Most academic GI programs have a baseline requirement of research. Choose and devise a project you can realistically complete despite your busy first-year schedule: expand on a residency research project, focus on a specific simple question triggered by a clinical situation you encountered, proceed with a retrospective chart review or quality improvement project, and include other fellows and trainees to divide tasks. Alternatively, devise a specific timeline with a research mentor to complete a larger research project during your three years of fellowship.
Involvement in GI societies/committees
Become a member of one (or all) of the national GI societies that align with your interests. Membership gives you access not only to peer-reviewed scientific articles and guidelines but also to fellow-focused programs, committees’ opportunities, early career research grants, and mentorship.6-10
Summary
The first year of a GI fellowship lays the foundation for your next 3 years: Be mindful of how you can optimize the opportunities at hand to learn, teach, build a solid reputation, and grow your professional network. But also remember you have 3 full years to accomplish all your goals, so be patient, pace yourself, and include others in your journey. Judiciously use the many resources within your program and GI societies to help you achieve your goals, reach out to others to overcome difficulties and barriers, and dedicate time to care for your personal health and growth. This is what a true comprehensive and healthy fellowship is all about!
Dr. Advani is with the division of gastroenterology and hepatology, Stony Brook (N.Y.) University Hospital. Dr. Saeed is with the division of gastroenterology and hepatology, University of Kentucky, Lexington. Dr. Charabaty is with the division of gastroenterology, Johns Hopkins University, Baltimore, and Johns Hopkins–Sibley Memorial Hospital, Washington. Dr. Advani and Dr. Saeed have no conflicts to disclose. Dr. Charabaty disclosed ties to AbbVie, Janssen, Takeda, Pfizer, and Bristol-Myers Squibb and is founder of @MondayNightIBD, and cofounder of Scrubs & Heels.
References
1. Bollipo S. Gastroenterology. 2020 Nov;159(5):1648-52.
2. Adams MA et al. Gastroenterology. 2014 Jan 1;146(1):5-9.
3. Pawa S et al. Am J Gastroenterol. 2021 Mar 1;116(3):530-8.
4. DeCross AJ. Gastroenterology. 2020 Jan 1;158(1):32-5.
5. Burke C et al. Am J Gastroenterol. 2017 Oct 1;112:S593-4.
6. Fellows Resources under Fellows & Early Career. American Gastroenterological Association. https://gastro.org/fellows-and-early-career/training-resources/fellows-resources/.
7. Trainee Courses and Events. American College of Gastroenterology. https://gi.org/trainees/trainee-courses-and-events/.
8. Trainee Resources. American Association for the Study of Liver Diseases. https://www.aasld.org/membership/hepatology-associates/trainee-resources.
9. First Year Fellows Courses under Education. American Society of Gastrointestinal Endoscopy. https://www.asge.org/home/education/advanced-education-training/first-year-fellow-(fyf)-courses.
10. Annual GI Fellow Summer Course Presentations. New York Society for Gastrointestinal Endoscopy. https://www.nysge.org/annual%20gi%20fellows%20summer%20course.
After the excitement and the well-deserved celebrations of matching in a gastroenterology fellowship program, a whole new set of unanswered questions and worries can start forming in a first-year fellow’s mind. “I made it, but now what? How do I learn a whole new career skill like endoscopy? Is my GI knowledge solid and wide enough to manage patients and answer the medical team consult? How will I keep up with my reading and learning with a busy fellowship schedule? How do I balance growth in clinical knowledge, endoscopy, and research? Can I integrate ‘life’ alongside a busy fellowship?” All of these questions and more can be overwhelming to answer in the beginning. The following guide is designed to help you through this transition and navigate the various aspects of first-year fellowship.
First-year goals
It is important to keep in mind that you have 3 full years to become a well-rounded, highly skilled, and knowledgeable gastroenterologist and endoscopist. So, set realistic goals and expectations for your first year, but be mindful that this year also lays the solid foundations of who you will become as a clinician, educator, or researcher.
One of the main goals of fellowship is to learn and implement evidence-based medicine in the diagnosis and management of GI conditions, as well as to learn endoscopic skills and ethics, all while keeping the patient (as a whole person) at the center of what you do. According to a recently published article by Bollipo and colleagues,1 the overall growth as a gastroenterologist not only depends on acquisition of knowledge but also involves cultivating teamwork, communication, situational awareness, compassion, leadership, and situational awareness. Beyond your medical education, your professional growth is also dependent on intentionally working towards acquiring the following skills:
1. Manage your time efficiently and prioritize your daily tasks
2. Become a consultant: effectively communicate with others, teach, lead, and delegate as appropriate
3. Work as a team with colleagues, faculty, and endoscopy staff
4. Develop critical thinking, give and receive constructive feedback, and understand your skills, limitations, and growth potential
5. Identify mentors and potential niche area
6. Start building your professional network and your reputation
7. Get involved in national GI societies
Consults
Mindset
Shifting gear from residency to fellowship involves a shifting of your clinical mindset too, going from being part of a primary care team responsible for all aspects of a patient’s medical care, to that of a consulting team focused on a patient’s GI condition. It is important to find the right balance of refraining from micromanaging non-GI comorbidities while being fully aware of their impact on your diagnostic and therapeutic approach to the GI condition.
Let’s face it, you will not always get “exciting and interesting GI cases” consults, and on a busy day some consultations might feel unnecessary and frustrating to you. Remember that what seems obvious to you, based on your focused GI knowledge, might not be so simple to the primary team. In addition, every consult is an opportunity to improve your approach to patient care, as well as an opportunity to learn and teach others, from medical students to residents. So, always be professional and respectful when you pick up the phone, and build positive collaborative bridges between you and the medical or surgical consulting teams. Be the GI fellow others are not reluctant to call for help, and better, be the one who communicates GI pearls along the way, inspires others to join the field of gastroenterology, and positively represent the GI division.
Triage
When you answer your consult page, ask the primary team what specific question they have for you and/or what is the main GI complaint or test abnormality the patient has. This will help you assess the urgency and the complexity of the consult, and hence allow you to prioritize each consult (which one you need to see first and give the attending a heads-up), assign (or not) a rotating medical resident or student to the consult, tailor your preliminary recommendations to the primary team, and anticipate the need for a procedure. When you anticipate a procedure, assess its (semi-)urgency to get the process ready for same day or a bedside procedure by getting information on the patient’s vitals, basic labs, significant acute comorbidities, and supportive therapies in place. In other words, by judiciously obtaining key information from the primary team, you can efficiently triage the consults and keep your day organized and manageable (for the most part). Learn to divide and conquer the tasks of the day: split inpatient endoscopy and consults with your cofellows, assign appropriate consults and follow-ups to residents or students rotating on the GI service, and properly communicate with the primary team a plan of care (even a preliminary one) to avoid recurrent pages and interruptions. Some days the number or urgency of the consults and the required multitasking can be overwhelming: stop, breathe, and ask for help from your co-fellows and your attending. Remember, this is a fellowship, not a solo-ship and your program is here to support your work and growth.
Communication
Timely and efficient communication, between you and the different stakeholders, is crucial to provide optimal patient care and minimize the risk of “things falling through the cracks”. Convey to the primary team your recommendations and plan of care clearly, and use direct verbal communication (not just a note in the chart) when managing complex or urgent situations. Obtain information regarding current patient level of care (i.e., ICU), isolation precautions, and cardiac devices (i.e., left ventricular assist device). Keep the dialogue open with your attending about acutely ill patients and potentially urgent procedures. Inform the endoscopy suite early that you are adding a procedure on the same day, and communicate anticipated needs (such as intubation, fluoroscopy room, pediatric scope, stent). Using a “closed-loop” communication structure can ensure that your recommendations are received and implemented appropriately.2
Time management and structure
Having a structured routine to your day, in what seems to be a chaotic process of juggling different duties and being in different locations at once, will ensure that you efficiently complete your tasks in a timely manner. Find what works best for you, taking into account the challenges and resources available to you, such as the number of fellows and other trainees on the GI consult service, the average number of consults per day and their acuity, the availability of inpatient protocols for specific clinical situations (GI bleed, acute severe ulcerative colitis, etc.), and the time and style preferences of the rounding attending. We suggest the following schedule on a consult day: Round early in the morning and leave a note in the chart and/or communicate with the team key information, then review with the rotating trainees the patients they are following and personally reassess some patients as needed. Inform the endoscopy suite of the same day procedures and let your attending know of any issues that require immediate attention. Take your team and head to radiology and review the imaging studies on your patients. Learn and teach key points in diagnosis and therapeutic approach as you move through your day from the inpatient floors to the hallways. Divide consults during the day with your team and agree on a time to touch base. Review your patient list at the end of the day and assess which patients the GI service no longer need to follow and communicate that clearly to the primary team along with the appropriate outpatient GI care follow-up. Let the endoscopy suite know of any procedures you are adding for the next day along with their degree of urgency to allow the charge nurse to prioritize cases. When you leave the hospital, be intentional with your free time: Read about the GI conditions you have encountered, enjoy some fun relaxing time, and rest!
Call
Know your call environment and your emergency cart
Familiarize yourself with the locations where you could potentially perform an emergent case (i.e., the ICU, ED, operating room) and the relevant points of contact (such as the charge nurse, the anesthesia team, the on-call tech team) for overnight or weekend cases. Whether or not you have an endoscopy support team on call, learn to set up the emergency cart, find and check your equipment, and troubleshoot technical issues by soliciting an “in-service” from senior fellows or the endoscopy technical support staff. Before heading to an urgent case, double check that you have your “bleeder” or “food impaction” tools. For food impaction, consider obtaining rat tooth forceps, snares, Roth nets, and an overtube. For bleeding cases, obtain a therapeutic upper endoscope, hemostatic clips, clear caps, injection needles, epinephrine, HemosprayTM, banding kits, and the appropriate electrocautery/thermal set up.
What is an emergency?
Consults that require your immediate attention include food impactions, acute biliary conditions leading to septic shock, and hemodynamically unstable GI bleeds, especially variceal bleeds. Remember that patients who are hemodynamically unstable require adequate resuscitation before proceeding with any endoscopic intervention. Assess the need for intubation, the timing of the procedure, and the most optimal location to perform the procedure, depending on the time and acuity of the patients’ presentation, how they respond to resuscitation measures, and the resources and preferences of your institution.
The overnight ‘nonemergent’ call
Non-emergent consults can be addressed the next day, after reviewing the clinical information provided by the consulting team and the patient’s EMR to ensure no urgent measures are needed. Overnight call may include patient phone calls, from inquiries about colon prep (so familiarize yourself with the different prep instructions and how to troubleshoot prep difficulties) to GI symptoms that you will need to triage to either the ED or to an outpatient follow-up. Document all phone encounters in the EMR and route your note to the appropriate clinician and nurse or administrative assistant for follow-up.
The five E’s of endoscopy
Endoscopy training is a large component of a GI fellowship and can create achievement anxiety in many first-year fellows seeking the cecum! But there is more to endoscopy than technical skills: It is as important to adequately evaluate clinical situations, understand the indications and potential limitations and complications of the procedure, and assess how it will impact the management of the patient. And no, you don’t have to be a video gamer to be a good endoscopist; and yes, you will be able to regularly complete a colonoscopy before the end of your first year!
Evaluation
In order to improve your endoscopic skills, it is important to honestly assess your areas of proficiency and improvement and to welcome real-time constructive feedback from your teaching attending about your endoscopic skills range. Consider meeting regularly with your attending to discuss your short-term and long-term endoscopic goals and how to enhance your skills. This practice demonstrates responsibility, credibility, and accountability amongst your peers as well as a genuine commitment to your growth as an endoscopist.
Efficiency
In addition to focusing on the quality of your endoscopy, learn to be efficient in the pre- and postprocedure time flow. This entails any step from properly explaining the procedure to patients before they come to the endoscopy suite, making sure the needed endoscopy equipment and tools are available in your room, completing your personal setup (i.e., gowning up, setting up your bed/monitor height, testing your endoscope) even before time out, to discharging the patient and communicating key findings and plan of care to the primary team. Depending on the acuity of the procedure and patient’s comorbidities, certain procedures may need to be performed or completed by a more efficient and experienced senior fellow or attending; don’t let this situation trigger passive frustration in you, but rather use it as an active and engaging opportunity to learn.
Expectations
You (and all the other neighborhood kids) didn’t learn to ride a bike without falling, struggling, needing help, and practicing over and over again, and it goes the same when learning to scope as a first-year fellow. Keep this in mind to lessen frustration, set realistic expectations, and be patient with yourself and celebrate all the small victories. Set tangible goals with your attending prior to procedural days/rotations so they can help you hone in and perfect the desired endoscopy skills.
Ergonomics
In a recent study, endoscopy-related injury (ERI) was reported to occur in up to 75% of gastroenterologists.3 While your primary focus might be reaching the cecum, it is as crucial for you to learn how to prevent ERI to ensure your long term health and continued success in procedures.
Excellence over quantity
Your main focus as a trainee is to learn how to provide effective, efficient, and safe care to patients, including in endoscopy. The quality of the endoscopy you perform is much more important than the total number of procedures you do. Thus, it is key to take each procedure as a complete learning opportunity to perform a thorough evaluation, improve your technical skills, interpret the findings, and develop a therapeutic plan.
Work-life balance and burnout
Fellowship is a marathon and not a sprint, so you need to slow down after a busy workday and care for yourself and enjoy time with loved ones. The cognitive, physical, mental, and emotional demands for first-year fellows are arguably the highest during GI training and can lead to burnout. Signs of burnout include emotional exhaustion, loss of empathy, fatigue, depersonalization and detachment, and feelings of personal inadequacy.4 Antiburnout measures include respecting basic healthy life hygiene (eat and sleep well, regular physical activity), having a hobby, practicing meditation, avoiding taking work home, and having a healthy social network.5 Remember that your cofellows whom you share common experiences with are not only your colleagues but can also be your friends and your social support. If you are a parent juggling work and family, remember to ask for help from your peers if you need it and have an open discussion with your attending to find practical solutions to your schedule.
Professional growth in the field of gastroenterology
Becoming a successful gastroenterologist and endoscopist involves going above the “I” and into the inclusive “we.” Building collegial and professional relationships early on with different stakeholders will set you up for success during and beyond your fellowship.
Building relationships
Developing genuine collegial and collaborative relationships with cofellows and faculty will positively impact your wellness during fellowship but also build the foundation of your professional network necessary to your career growth. Be inclusive of your cofellows in your research projects and publications, and support and amplify their work as much as you amplify your own. Your cofellows or attendings are likely to be the ones to help you find the right job, invite you to speak at grand rounds, or sit on a GI committee and promote your postfellowship professional growth.
Mentorship, being an educator and role model
It is important to identify and seek out mentors, within or outside your fellowship program or institution, who can not only guide you in your career choices but also open doors for you and sponsor you to advance your career. On the other hand, you too can be a role model, mentor, and sponsor to medical residents and students interested in the field of GI. Teach others in didactic settings or on the consult service, include trainees in quality improvement projects and publications, and lead by example.
Research
Most academic GI programs have a baseline requirement of research. Choose and devise a project you can realistically complete despite your busy first-year schedule: expand on a residency research project, focus on a specific simple question triggered by a clinical situation you encountered, proceed with a retrospective chart review or quality improvement project, and include other fellows and trainees to divide tasks. Alternatively, devise a specific timeline with a research mentor to complete a larger research project during your three years of fellowship.
Involvement in GI societies/committees
Become a member of one (or all) of the national GI societies that align with your interests. Membership gives you access not only to peer-reviewed scientific articles and guidelines but also to fellow-focused programs, committees’ opportunities, early career research grants, and mentorship.6-10
Summary
The first year of a GI fellowship lays the foundation for your next 3 years: Be mindful of how you can optimize the opportunities at hand to learn, teach, build a solid reputation, and grow your professional network. But also remember you have 3 full years to accomplish all your goals, so be patient, pace yourself, and include others in your journey. Judiciously use the many resources within your program and GI societies to help you achieve your goals, reach out to others to overcome difficulties and barriers, and dedicate time to care for your personal health and growth. This is what a true comprehensive and healthy fellowship is all about!
Dr. Advani is with the division of gastroenterology and hepatology, Stony Brook (N.Y.) University Hospital. Dr. Saeed is with the division of gastroenterology and hepatology, University of Kentucky, Lexington. Dr. Charabaty is with the division of gastroenterology, Johns Hopkins University, Baltimore, and Johns Hopkins–Sibley Memorial Hospital, Washington. Dr. Advani and Dr. Saeed have no conflicts to disclose. Dr. Charabaty disclosed ties to AbbVie, Janssen, Takeda, Pfizer, and Bristol-Myers Squibb and is founder of @MondayNightIBD, and cofounder of Scrubs & Heels.
References
1. Bollipo S. Gastroenterology. 2020 Nov;159(5):1648-52.
2. Adams MA et al. Gastroenterology. 2014 Jan 1;146(1):5-9.
3. Pawa S et al. Am J Gastroenterol. 2021 Mar 1;116(3):530-8.
4. DeCross AJ. Gastroenterology. 2020 Jan 1;158(1):32-5.
5. Burke C et al. Am J Gastroenterol. 2017 Oct 1;112:S593-4.
6. Fellows Resources under Fellows & Early Career. American Gastroenterological Association. https://gastro.org/fellows-and-early-career/training-resources/fellows-resources/.
7. Trainee Courses and Events. American College of Gastroenterology. https://gi.org/trainees/trainee-courses-and-events/.
8. Trainee Resources. American Association for the Study of Liver Diseases. https://www.aasld.org/membership/hepatology-associates/trainee-resources.
9. First Year Fellows Courses under Education. American Society of Gastrointestinal Endoscopy. https://www.asge.org/home/education/advanced-education-training/first-year-fellow-(fyf)-courses.
10. Annual GI Fellow Summer Course Presentations. New York Society for Gastrointestinal Endoscopy. https://www.nysge.org/annual%20gi%20fellows%20summer%20course.
The central role of informed consent in novel procedures
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Integrating psychogastroenterology into GI care
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
The New Gastroenterologist seeks its next editor-in-chief
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2022 – Sept. 30, 2027, with a transition period starting July 2022.

About TNG
TNG content covers highly relevant clinical topics, such as diverticular hemorrhage as well as microscopic colitis and diarrhea. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, brief reviews on clinically-relevant topics, issues in clinical medical ethics, and other topics that are relevant to early career GIs. Each issue also contains an introductory letter from the editor as well as a curated list of relevant articles from the AGA Journals.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
- AGA member, between second year of fellowship and five years post-fellowship.
- Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
- Experience in medical, scientific or news-related publishing is preferred, but not required.
- Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
- The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for applications. If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2022 – Sept. 30, 2027, with a transition period starting July 2022.

About TNG
TNG content covers highly relevant clinical topics, such as diverticular hemorrhage as well as microscopic colitis and diarrhea. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, brief reviews on clinically-relevant topics, issues in clinical medical ethics, and other topics that are relevant to early career GIs. Each issue also contains an introductory letter from the editor as well as a curated list of relevant articles from the AGA Journals.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
- AGA member, between second year of fellowship and five years post-fellowship.
- Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
- Experience in medical, scientific or news-related publishing is preferred, but not required.
- Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
- The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for applications. If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2022 – Sept. 30, 2027, with a transition period starting July 2022.

About TNG
TNG content covers highly relevant clinical topics, such as diverticular hemorrhage as well as microscopic colitis and diarrhea. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, brief reviews on clinically-relevant topics, issues in clinical medical ethics, and other topics that are relevant to early career GIs. Each issue also contains an introductory letter from the editor as well as a curated list of relevant articles from the AGA Journals.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
- AGA member, between second year of fellowship and five years post-fellowship.
- Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
- Experience in medical, scientific or news-related publishing is preferred, but not required.
- Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
- The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for applications. If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
The management of inflammatory bowel disease in pregnancy
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).
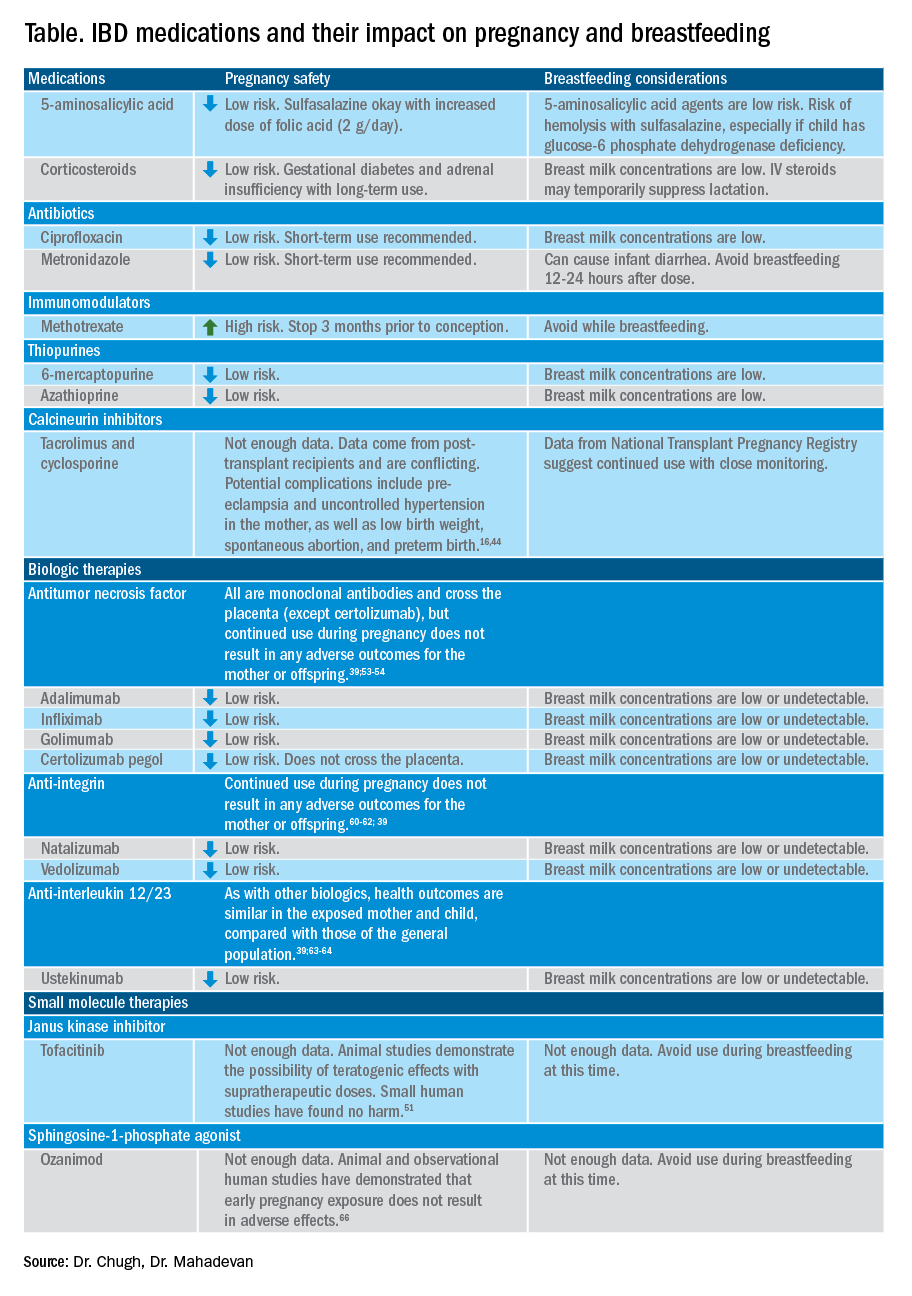
Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34
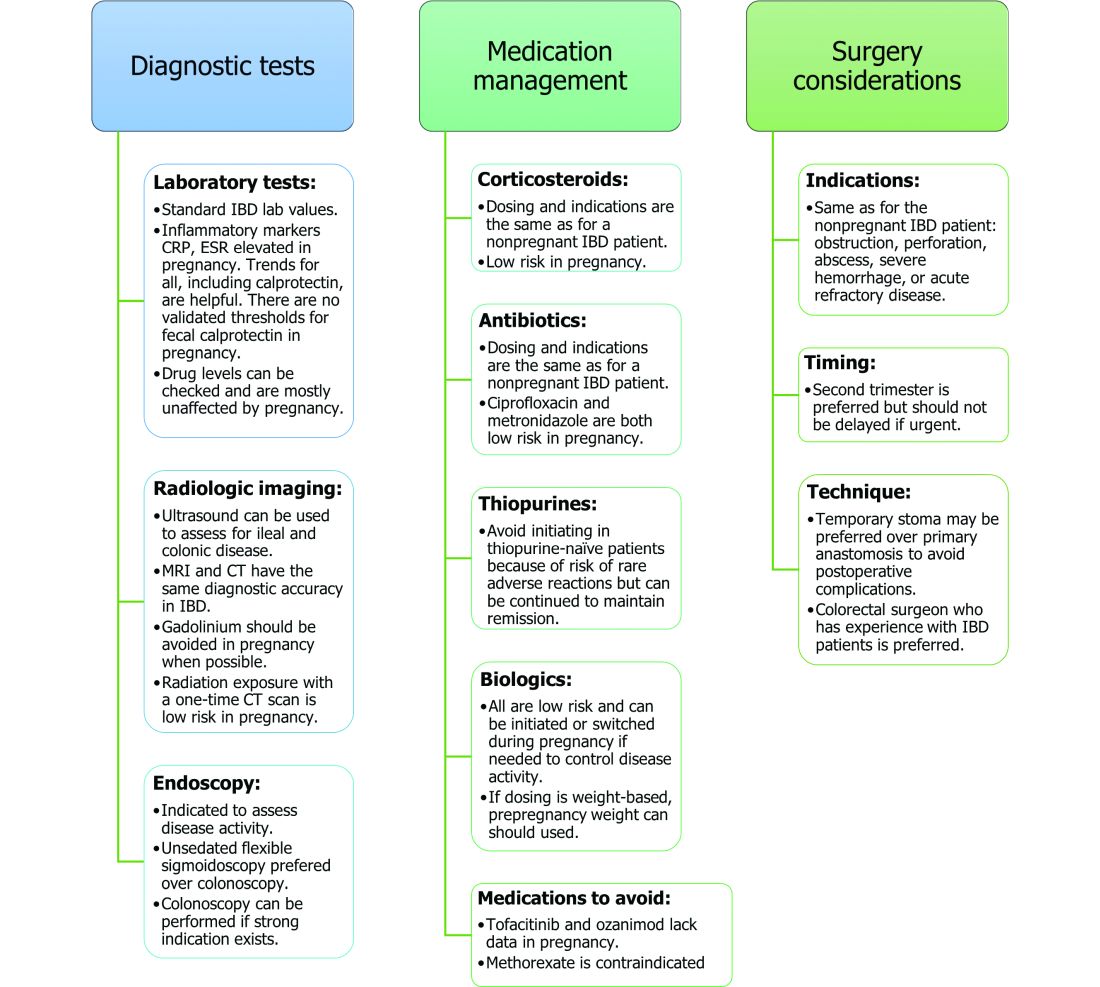
An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Looking for glimpses of normalcy
Dear colleagues,
I’m thrilled to introduce the first edition of The New Gastroenterologist in 2022! The onslaught of the COVID-19 pandemic continues, and as physicians, we are exhausted. The past year brought glimpses of normalcy, but these were ultimately eclipsed by the precipitous surge of the very contagious Omicron variant, once again overwhelming health systems and threatening our daily routines. We will keep on, doing our best to protect our patients and our families, with the hope for an eventual transition ‘from pandemic to endemic.’
Due to the pandemic, telemedicine has now been firmly cemented as a cornerstone of clinical care, which Dr. Naresh Gunaratnam (Huron Gastroenterology, Ann Arbor, Mich.) discusses in our DHPA Private Practice Perspectives article for the quarter. Telemedicine boasts many benefits and while it will never be adopted entirely in lieu of in-person visits, it is a tool that should remain an option for years to come in the appropriate subset of patients.
Similarly, progress is needed for pregnant and post-partum gastroenterologists, especially trainees. Dr. Lauren Feld (University of Washington, Seattle) and Dr. Loren Galler Rabinowitz (Beth Israel Deaconess Medical Center, Boston) present valuable perspectives on challenges faced by early career gastroenterologists and trainees; specifically how important changes to parental leave policies can facilitate the transition of new parents returning to work.
The lack of financial knowledge is common among physicians. Our finance piece for the quarter is written by Dr. Latifat Alli-Akintade (Kaiser Permanente, South Sacramento (Calif.) Medical Center), a gastroenterologist who is passionate about educating others on money management. She discusses how financial independence is one of the keys to mitigating long term burnout as a physician.
The management of inflammatory bowel disease (IBD) in pregnancy can be difficult to navigate with the litany of therapeutic options. Our “In Focus” feature for February is a fantastic piece written by Dr. Rishika Chugh and Dr. Uma Mahadevan (UCSF), who provide a comprehensive multifaceted approach, discussing the importance of health care maintenance and disease control and how to choose the right therapeutic regimen for pregnant patients.
Our post-fellowship pathways section is written by Dr. Adam Mikolajczyk, hepatologist and associate program director of the internal medicine program at the University of Illinois Chicago. He describes his journey throughout training and into his years as junior faculty, offering advice to those interested in a career in medical education.
Lastly, in October 2021, the AGA and EndoscopyNow hosted an online fellows forum entitled “Navigating New Frontiers of Training in Gastroenterology.” Dr. Joy Liu (Northwestern University, Chicago) attended and offers an excellent summary of the course for those who may have missed it.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the first edition of The New Gastroenterologist in 2022! The onslaught of the COVID-19 pandemic continues, and as physicians, we are exhausted. The past year brought glimpses of normalcy, but these were ultimately eclipsed by the precipitous surge of the very contagious Omicron variant, once again overwhelming health systems and threatening our daily routines. We will keep on, doing our best to protect our patients and our families, with the hope for an eventual transition ‘from pandemic to endemic.’
Due to the pandemic, telemedicine has now been firmly cemented as a cornerstone of clinical care, which Dr. Naresh Gunaratnam (Huron Gastroenterology, Ann Arbor, Mich.) discusses in our DHPA Private Practice Perspectives article for the quarter. Telemedicine boasts many benefits and while it will never be adopted entirely in lieu of in-person visits, it is a tool that should remain an option for years to come in the appropriate subset of patients.
Similarly, progress is needed for pregnant and post-partum gastroenterologists, especially trainees. Dr. Lauren Feld (University of Washington, Seattle) and Dr. Loren Galler Rabinowitz (Beth Israel Deaconess Medical Center, Boston) present valuable perspectives on challenges faced by early career gastroenterologists and trainees; specifically how important changes to parental leave policies can facilitate the transition of new parents returning to work.
The lack of financial knowledge is common among physicians. Our finance piece for the quarter is written by Dr. Latifat Alli-Akintade (Kaiser Permanente, South Sacramento (Calif.) Medical Center), a gastroenterologist who is passionate about educating others on money management. She discusses how financial independence is one of the keys to mitigating long term burnout as a physician.
The management of inflammatory bowel disease (IBD) in pregnancy can be difficult to navigate with the litany of therapeutic options. Our “In Focus” feature for February is a fantastic piece written by Dr. Rishika Chugh and Dr. Uma Mahadevan (UCSF), who provide a comprehensive multifaceted approach, discussing the importance of health care maintenance and disease control and how to choose the right therapeutic regimen for pregnant patients.
Our post-fellowship pathways section is written by Dr. Adam Mikolajczyk, hepatologist and associate program director of the internal medicine program at the University of Illinois Chicago. He describes his journey throughout training and into his years as junior faculty, offering advice to those interested in a career in medical education.
Lastly, in October 2021, the AGA and EndoscopyNow hosted an online fellows forum entitled “Navigating New Frontiers of Training in Gastroenterology.” Dr. Joy Liu (Northwestern University, Chicago) attended and offers an excellent summary of the course for those who may have missed it.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the first edition of The New Gastroenterologist in 2022! The onslaught of the COVID-19 pandemic continues, and as physicians, we are exhausted. The past year brought glimpses of normalcy, but these were ultimately eclipsed by the precipitous surge of the very contagious Omicron variant, once again overwhelming health systems and threatening our daily routines. We will keep on, doing our best to protect our patients and our families, with the hope for an eventual transition ‘from pandemic to endemic.’
Due to the pandemic, telemedicine has now been firmly cemented as a cornerstone of clinical care, which Dr. Naresh Gunaratnam (Huron Gastroenterology, Ann Arbor, Mich.) discusses in our DHPA Private Practice Perspectives article for the quarter. Telemedicine boasts many benefits and while it will never be adopted entirely in lieu of in-person visits, it is a tool that should remain an option for years to come in the appropriate subset of patients.
Similarly, progress is needed for pregnant and post-partum gastroenterologists, especially trainees. Dr. Lauren Feld (University of Washington, Seattle) and Dr. Loren Galler Rabinowitz (Beth Israel Deaconess Medical Center, Boston) present valuable perspectives on challenges faced by early career gastroenterologists and trainees; specifically how important changes to parental leave policies can facilitate the transition of new parents returning to work.
The lack of financial knowledge is common among physicians. Our finance piece for the quarter is written by Dr. Latifat Alli-Akintade (Kaiser Permanente, South Sacramento (Calif.) Medical Center), a gastroenterologist who is passionate about educating others on money management. She discusses how financial independence is one of the keys to mitigating long term burnout as a physician.
The management of inflammatory bowel disease (IBD) in pregnancy can be difficult to navigate with the litany of therapeutic options. Our “In Focus” feature for February is a fantastic piece written by Dr. Rishika Chugh and Dr. Uma Mahadevan (UCSF), who provide a comprehensive multifaceted approach, discussing the importance of health care maintenance and disease control and how to choose the right therapeutic regimen for pregnant patients.
Our post-fellowship pathways section is written by Dr. Adam Mikolajczyk, hepatologist and associate program director of the internal medicine program at the University of Illinois Chicago. He describes his journey throughout training and into his years as junior faculty, offering advice to those interested in a career in medical education.
Lastly, in October 2021, the AGA and EndoscopyNow hosted an online fellows forum entitled “Navigating New Frontiers of Training in Gastroenterology.” Dr. Joy Liu (Northwestern University, Chicago) attended and offers an excellent summary of the course for those who may have missed it.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition


















