User login
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
May 18-21, 2019
Digestive Disease Week® (DDW) 2018 – San Diego, CA
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is cosponsored by AGA, AASLD, ASGE, and SSAT.
AGA Trainee and Early Career GI Sessions at DDW 2019
Join your colleagues at special sessions to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
- May 18, 8:15 a.m.–5:30 p.m.; May 19, 8:30 a.m.–12:35 p.m.
2019 AGA Postgraduate Course
The AGA Institute’s flagship live educational offering and premier CME activity, the AGA Postgraduate Course, is a one-and-a-half-day multitopic course covering recent clinical advances that affect how care is delivered. This live CME activity is eligible for Maintenance of Certification (MOC) points. Saturday afternoon’s case-based breakout sessions incorporate new learning-style formats for greater audience-speaker engagement. AGA member trainees and early career GIs receive discounted pricing for this cour - May 19, 2–3 p.m.
AGA Networking Hour
This event is open to all DDW trainee and early career GI attendees and provides a casual atmosphere to network with peers. You can also meet the AGA Trainee & Early Career Committee, participate in an advocacy initiative, and learn more about all that AGA has to offer for fellows and early career GIs. This networking event will take place in the DDW Trainee and Early Career Lounge, Sails Pavilion (San Diego Convention Center). Light refreshments will be served. - May 20, 10–11:30 a.m.
Introduction to GI Practice: A Trainee Boot Camp
Trainees and early-career GIs interested in or going into private practice should attend this symposium for key tips and insights from experts for a successful career. Learn about the business of private practice GI, how to start and grow your practice, and most importantly, how to find the perfect fit for you. A “must-not-miss ‘insider tips’ session.” This activity has been approved for 1.5 AMA PRA Category 1 Credits™. - May 20, 1:30–5:30 p.m.
AGA Board Review Course
This session, designed around content from the newly released DDSEP® 9, serves as a primer for third-year fellows preparing for the board exam as well as a review course for others wanting to test their knowledge. Attendees will be provided with real-time feedback on their readiness to take the board exams and will also receive exclusive access to discounts on DDSEP to further prepare. This live CME activity is eligible for MOC points. This activity has been approved for 4 AMA PRA Category 1 Credits™. - May 20, 2-3:30 p.m.
Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects
This trainee-focused session will showcase selected abstracts from GI fellows based on quality improvement with a state-of-the-art lecture. Attendees will learn about practical examples of successful quality-improvement projects for integration into their day-to-day work lives. This session also will provide guidance on how to develop a quality-improvement project and will target a specific need in the GI training program curriculum of addressing the development of quality-improvement requirements within training programs. This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
- May 20, 4-5:30 p.m.
GI in the Digital Age
Social media is becoming a major part of our daily activities at both the social and professional levels. The session will provide attendees with a comprehensive review on the use of social media to build personal brand, interact with other colleagues and with patients. Experts presenting in the session also will discuss tips improve efficiency and safety when working with electronic health records (EHRs). This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
May 18, 2019, 6-8 p.m.
Digestive Health Physicians Association® and The New Gastroenterologist Networking Reception
After a full day of DDW sessions, join the Digestive Health Physicians Association (DHPA) and AGA’s The New Gastroenterologist to relax and network with independent GI physicians from across the nation. This event will take place in the Elevation Room at the Hilton San Diego Bayfront.
May 18-21, 2019, 7:30 a.m.–6 p.m.
Trainee and Early Career Lounge
This space is dedicated to trainee and young GI attendees. Come meet and network with peers from around the world over a cup of coffee. Information will be available from each of the sponsoring societies, in addition to trainee-specific programming tips at DDW.
May 21, 2019, 8-9:30 a.m.
2019 AGA Academy of Educators Plenary Session: Achieving Competence Through Learner-Engaged Teaching Methods
Join your peers to improve your teaching skills with systems and strategies from expert faculty leaders. This session is held in conjunction with DDW 2019. A portion of time will also be dedicated to endoscopy education tools and presentations from the 2018 academy grants recipients.
UPCOMING EVENTS
May 15, 2019
Coding and Reimbursement Solutions by McVey Associates Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Harrisburg, PA
May 15-16; June 19-20; September 18-19; October 9-10, 2019
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates Inc.
San Diego, CA (May 15-16); Nashville, TN (June 19-20); Atlanta, GA (Sept. 18-19); Las Vegas, NV (Oct. 9-10)
Aug. 9-10, 2019
2019 Freston Conference: Food at the Intersection of Gut Health and Disease
GI clinicians and allied health professionals are increasingly focused on how nutrients influence GI physiology and how diet can promote sound gut health. In response to this growing body of knowledge, the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease, will examine how nutrition management therapies can combat GI disorders such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and celiac disease and how diet supports improvement across the care continuum.
Chicago
Aug. 9-11, 2019
2019 Principles of GI for the NP and PA
The Principles of Gastroenterology for the Nurse Practitioner and Physician Assistant (NPPA) is the medical industry’s premiere course guiding and enabling nurse practitioners and physician assistants in the intricacies of identifying, treating, and managing GI disorders. Designed and taught by expert clinicians and advanced practice providers, NPPA provides the latest insights, knowledge, and research on how to improve GI patient care. Attendees will leave with stronger diagnostic and therapeutic skills, a more robust professional network, and an enhanced value for their practices.
Chicago
AWARDS APPLICATION DEADLINES
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for 3 years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application opens: June 3, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
May 18-21, 2019
Digestive Disease Week® (DDW) 2018 – San Diego, CA
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is cosponsored by AGA, AASLD, ASGE, and SSAT.
AGA Trainee and Early Career GI Sessions at DDW 2019
Join your colleagues at special sessions to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
- May 18, 8:15 a.m.–5:30 p.m.; May 19, 8:30 a.m.–12:35 p.m.
2019 AGA Postgraduate Course
The AGA Institute’s flagship live educational offering and premier CME activity, the AGA Postgraduate Course, is a one-and-a-half-day multitopic course covering recent clinical advances that affect how care is delivered. This live CME activity is eligible for Maintenance of Certification (MOC) points. Saturday afternoon’s case-based breakout sessions incorporate new learning-style formats for greater audience-speaker engagement. AGA member trainees and early career GIs receive discounted pricing for this cour - May 19, 2–3 p.m.
AGA Networking Hour
This event is open to all DDW trainee and early career GI attendees and provides a casual atmosphere to network with peers. You can also meet the AGA Trainee & Early Career Committee, participate in an advocacy initiative, and learn more about all that AGA has to offer for fellows and early career GIs. This networking event will take place in the DDW Trainee and Early Career Lounge, Sails Pavilion (San Diego Convention Center). Light refreshments will be served. - May 20, 10–11:30 a.m.
Introduction to GI Practice: A Trainee Boot Camp
Trainees and early-career GIs interested in or going into private practice should attend this symposium for key tips and insights from experts for a successful career. Learn about the business of private practice GI, how to start and grow your practice, and most importantly, how to find the perfect fit for you. A “must-not-miss ‘insider tips’ session.” This activity has been approved for 1.5 AMA PRA Category 1 Credits™. - May 20, 1:30–5:30 p.m.
AGA Board Review Course
This session, designed around content from the newly released DDSEP® 9, serves as a primer for third-year fellows preparing for the board exam as well as a review course for others wanting to test their knowledge. Attendees will be provided with real-time feedback on their readiness to take the board exams and will also receive exclusive access to discounts on DDSEP to further prepare. This live CME activity is eligible for MOC points. This activity has been approved for 4 AMA PRA Category 1 Credits™. - May 20, 2-3:30 p.m.
Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects
This trainee-focused session will showcase selected abstracts from GI fellows based on quality improvement with a state-of-the-art lecture. Attendees will learn about practical examples of successful quality-improvement projects for integration into their day-to-day work lives. This session also will provide guidance on how to develop a quality-improvement project and will target a specific need in the GI training program curriculum of addressing the development of quality-improvement requirements within training programs. This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
- May 20, 4-5:30 p.m.
GI in the Digital Age
Social media is becoming a major part of our daily activities at both the social and professional levels. The session will provide attendees with a comprehensive review on the use of social media to build personal brand, interact with other colleagues and with patients. Experts presenting in the session also will discuss tips improve efficiency and safety when working with electronic health records (EHRs). This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
May 18, 2019, 6-8 p.m.
Digestive Health Physicians Association® and The New Gastroenterologist Networking Reception
After a full day of DDW sessions, join the Digestive Health Physicians Association (DHPA) and AGA’s The New Gastroenterologist to relax and network with independent GI physicians from across the nation. This event will take place in the Elevation Room at the Hilton San Diego Bayfront.
May 18-21, 2019, 7:30 a.m.–6 p.m.
Trainee and Early Career Lounge
This space is dedicated to trainee and young GI attendees. Come meet and network with peers from around the world over a cup of coffee. Information will be available from each of the sponsoring societies, in addition to trainee-specific programming tips at DDW.
May 21, 2019, 8-9:30 a.m.
2019 AGA Academy of Educators Plenary Session: Achieving Competence Through Learner-Engaged Teaching Methods
Join your peers to improve your teaching skills with systems and strategies from expert faculty leaders. This session is held in conjunction with DDW 2019. A portion of time will also be dedicated to endoscopy education tools and presentations from the 2018 academy grants recipients.
UPCOMING EVENTS
May 15, 2019
Coding and Reimbursement Solutions by McVey Associates Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Harrisburg, PA
May 15-16; June 19-20; September 18-19; October 9-10, 2019
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates Inc.
San Diego, CA (May 15-16); Nashville, TN (June 19-20); Atlanta, GA (Sept. 18-19); Las Vegas, NV (Oct. 9-10)
Aug. 9-10, 2019
2019 Freston Conference: Food at the Intersection of Gut Health and Disease
GI clinicians and allied health professionals are increasingly focused on how nutrients influence GI physiology and how diet can promote sound gut health. In response to this growing body of knowledge, the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease, will examine how nutrition management therapies can combat GI disorders such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and celiac disease and how diet supports improvement across the care continuum.
Chicago
Aug. 9-11, 2019
2019 Principles of GI for the NP and PA
The Principles of Gastroenterology for the Nurse Practitioner and Physician Assistant (NPPA) is the medical industry’s premiere course guiding and enabling nurse practitioners and physician assistants in the intricacies of identifying, treating, and managing GI disorders. Designed and taught by expert clinicians and advanced practice providers, NPPA provides the latest insights, knowledge, and research on how to improve GI patient care. Attendees will leave with stronger diagnostic and therapeutic skills, a more robust professional network, and an enhanced value for their practices.
Chicago
AWARDS APPLICATION DEADLINES
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for 3 years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application opens: June 3, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
May 18-21, 2019
Digestive Disease Week® (DDW) 2018 – San Diego, CA
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is cosponsored by AGA, AASLD, ASGE, and SSAT.
AGA Trainee and Early Career GI Sessions at DDW 2019
Join your colleagues at special sessions to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
- May 18, 8:15 a.m.–5:30 p.m.; May 19, 8:30 a.m.–12:35 p.m.
2019 AGA Postgraduate Course
The AGA Institute’s flagship live educational offering and premier CME activity, the AGA Postgraduate Course, is a one-and-a-half-day multitopic course covering recent clinical advances that affect how care is delivered. This live CME activity is eligible for Maintenance of Certification (MOC) points. Saturday afternoon’s case-based breakout sessions incorporate new learning-style formats for greater audience-speaker engagement. AGA member trainees and early career GIs receive discounted pricing for this cour - May 19, 2–3 p.m.
AGA Networking Hour
This event is open to all DDW trainee and early career GI attendees and provides a casual atmosphere to network with peers. You can also meet the AGA Trainee & Early Career Committee, participate in an advocacy initiative, and learn more about all that AGA has to offer for fellows and early career GIs. This networking event will take place in the DDW Trainee and Early Career Lounge, Sails Pavilion (San Diego Convention Center). Light refreshments will be served. - May 20, 10–11:30 a.m.
Introduction to GI Practice: A Trainee Boot Camp
Trainees and early-career GIs interested in or going into private practice should attend this symposium for key tips and insights from experts for a successful career. Learn about the business of private practice GI, how to start and grow your practice, and most importantly, how to find the perfect fit for you. A “must-not-miss ‘insider tips’ session.” This activity has been approved for 1.5 AMA PRA Category 1 Credits™. - May 20, 1:30–5:30 p.m.
AGA Board Review Course
This session, designed around content from the newly released DDSEP® 9, serves as a primer for third-year fellows preparing for the board exam as well as a review course for others wanting to test their knowledge. Attendees will be provided with real-time feedback on their readiness to take the board exams and will also receive exclusive access to discounts on DDSEP to further prepare. This live CME activity is eligible for MOC points. This activity has been approved for 4 AMA PRA Category 1 Credits™. - May 20, 2-3:30 p.m.
Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects
This trainee-focused session will showcase selected abstracts from GI fellows based on quality improvement with a state-of-the-art lecture. Attendees will learn about practical examples of successful quality-improvement projects for integration into their day-to-day work lives. This session also will provide guidance on how to develop a quality-improvement project and will target a specific need in the GI training program curriculum of addressing the development of quality-improvement requirements within training programs. This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
- May 20, 4-5:30 p.m.
GI in the Digital Age
Social media is becoming a major part of our daily activities at both the social and professional levels. The session will provide attendees with a comprehensive review on the use of social media to build personal brand, interact with other colleagues and with patients. Experts presenting in the session also will discuss tips improve efficiency and safety when working with electronic health records (EHRs). This activity has been approved for 1.5 AMA PRA Category 1 Credits™.
May 18, 2019, 6-8 p.m.
Digestive Health Physicians Association® and The New Gastroenterologist Networking Reception
After a full day of DDW sessions, join the Digestive Health Physicians Association (DHPA) and AGA’s The New Gastroenterologist to relax and network with independent GI physicians from across the nation. This event will take place in the Elevation Room at the Hilton San Diego Bayfront.
May 18-21, 2019, 7:30 a.m.–6 p.m.
Trainee and Early Career Lounge
This space is dedicated to trainee and young GI attendees. Come meet and network with peers from around the world over a cup of coffee. Information will be available from each of the sponsoring societies, in addition to trainee-specific programming tips at DDW.
May 21, 2019, 8-9:30 a.m.
2019 AGA Academy of Educators Plenary Session: Achieving Competence Through Learner-Engaged Teaching Methods
Join your peers to improve your teaching skills with systems and strategies from expert faculty leaders. This session is held in conjunction with DDW 2019. A portion of time will also be dedicated to endoscopy education tools and presentations from the 2018 academy grants recipients.
UPCOMING EVENTS
May 15, 2019
Coding and Reimbursement Solutions by McVey Associates Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Harrisburg, PA
May 15-16; June 19-20; September 18-19; October 9-10, 2019
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates Inc.
San Diego, CA (May 15-16); Nashville, TN (June 19-20); Atlanta, GA (Sept. 18-19); Las Vegas, NV (Oct. 9-10)
Aug. 9-10, 2019
2019 Freston Conference: Food at the Intersection of Gut Health and Disease
GI clinicians and allied health professionals are increasingly focused on how nutrients influence GI physiology and how diet can promote sound gut health. In response to this growing body of knowledge, the 2019 James W. Freston Conference: Food at the Intersection of Gut Health and Disease, will examine how nutrition management therapies can combat GI disorders such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and celiac disease and how diet supports improvement across the care continuum.
Chicago
Aug. 9-11, 2019
2019 Principles of GI for the NP and PA
The Principles of Gastroenterology for the Nurse Practitioner and Physician Assistant (NPPA) is the medical industry’s premiere course guiding and enabling nurse practitioners and physician assistants in the intricacies of identifying, treating, and managing GI disorders. Designed and taught by expert clinicians and advanced practice providers, NPPA provides the latest insights, knowledge, and research on how to improve GI patient care. Attendees will leave with stronger diagnostic and therapeutic skills, a more robust professional network, and an enhanced value for their practices.
Chicago
AWARDS APPLICATION DEADLINES
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for 3 years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application opens: June 3, 2019
Advanced degree programs to consider when changing careers
I have been in private practice as a gastroenterologist for 18 years. Many of us in gastroenterology and related fields have wondered how to navigate toward the next step in our careers. There are resources available to further our knowledge, add new skills, and fine tune personal talents to help position us for that next step.
Questions to ask at this stage are: What do I really want to do? Where do I see myself in 5-10 years? How do I go about achieving my target?
We come from different backgrounds including, broadly, academic clinical, academic research, basic science, clinical practice, and education. The next stage of these career paths can vary, and that should be kept in mind while choosing courses/programs. I reached out to two well-known gastroenterologists who have successfully changed their career paths after starting with different backgrounds.
Ronald Vender, MD, professor of medicine, associate dean of clinical affairs, chief medical officer, Yale University, New Haven, Conn.
Dr. Vender began in private practice gastroenterology after fellowship. His own trajectory has been one of “evolution” and has grown to the above titles through “incremental opportunity.” While reflecting on his career, Dr. Vender felt three main attributes were responsible: involvement in medical/GI societies, involvement in non-GI organizations, and engagement of needs for improvement at the hospital of practice. Opportunities became available by speaking up, raising issues, and demanding improvements. Dr. Vender’s involvement in both the private practice sector and hospital administration made his transition to hospital administration possible. This change was based on a “change in [him] and change in what [he] wanted to do.” His advice for all is to learn to say “yes” often in your early career and recognize when to say “no” later in your career.
John Allen, MD, MBA, clinical professor of medicine, University of Michigan, Ann Arbor
Dr. Allen started his career in the Veterans Affairs (VA) system, and during this time, he was exposed to research activities and learned research skills. His initial interest was in health care delivery, but this eventually changed to private practice gastroenterology. His exposure to information and the opportunity to learn about variations in practice and outcomes allowed him to maintain his interest in quality, which ultimately led to publications on colonoscopy quality. In his 40s he decided to obtain an executive master of business administration (EMBA), which he feels one should embark upon “when you have a problem to solve.” He has effectively moved from the VA system to private practice and now to academic medicine. Dr. Allen identified attending leadership conferences, engaging executive coaches, and participation in key committees as further opportunities to help you change careers. His prior work experience, education, and exposure enables him in his current position to help oversee a large department of medicine with 160 care sites, with quality and financials as key factors.
As we can see, there is no correct answer or set path for those of us wanting to change career directions. What was clear while speaking with both Dr. Vender and Dr. Allen was the importance of enthusiasm in solving issues, a willingness to commit to new projects, and an interest in exploring new areas.
Below is a brief overview of some degree programs that may help promote a change in your career path.
Masters in health care
This degree is aimed at those looking to advance their career in the field of health care in various locations, such as hospitals, clinics, and nonprofit organizations.1 Length of prior health care experience will vary based upon program. Programs are administered on a full-time and part-time basis, as well as online and study abroad. Numerous specialties are offered such as medicine, nutrition, psychiatry, nursing, veterinary medicine, physiotherapy, biomedical engineering, medical laboratory studies, radiology, alternative medicine, and health care management, administration, or leadership.
Health care MBA
Master of business administration (MBA) programs in health care administration management are offered by several universities. Given their aim of imparting essential information on a broad range of topics relevant to the health care industry, they are usually quite rigorous. It is recommended that you pursue an MBA only after a few years of working in your chosen field of practice. Many institutions require GMAT scores with the application.2-4
Executive MBA
EMBA programs are similar to health care MBAs in that they also include rigorous course work.5 EMBA programs are developed to meet the educational needs of managers and executives or physicians hoping to advance or change their career. Typically, students can earn an MBA in 2 years or less while working full-time. GMAT scores are required by most institutions offering EMBA.
Certification leadership programs
A benefit of leadership programs is that they help to develop a clear vision by creating a mission statement, goals, and action plans. Some notable programs include:
- American Gastroenterological Association leadership programs: Future Leaders Program, Women’s Leadership Conference, and Forward Program (new for 2019). These programs offer robust sessions which are well thought out and intended to advance the careers of traditionally underrepresented groups within the field of gastroenterology.6
- American Medical Association’s Women’s Leadership Certificate: This program was developed to promote knowledge and strong leadership, both within oneself and among others. Attendees are awarded with the “AMA Women’s Leadership Certificate” following the 2-day event.7
- Executive leadership training and development programs: As with many universities offer programs like these; for example, Harvard Medical School, Boston, offers a popular – and usually sold out – event for women titled “Career Advancement and Leadership Skills for Women in Health Care.”8
- American College of Healthcare Executives: Various conferences and educational meetings in addition to the annual ACHE Congress on Healthcare Leadership.9
- American Association of Physician Leadership: Physician executive accreditation along with career services, continued education, and publications.10
After reviewing the experiences of two well-known gastroenterologists and several of the available programs, the question to ask yourself is, “What’s next?” Most will likely have this question already in mind, so here are a few potential career directions/positions to consider:
Academic medicine: department chief, program director, director of endoscopy, chief medical officer
Private practice: managing director, director of endoscopy, finance director
Private sector: pharmaceutical industry, scientific advisor, medical director, medical insurance industry, malpractice insurance industry, medical informatics, public policy, private equity, entrepreneurial
Conclusion
In summary, there is no single answer nor a single program that fits everyone’s needs. Health care delivery and management/administration are complicated and will only continue to evolve. Consideration must be given to the fact that any change in one’s career direction needs time and commitment.
Here are some take-home points:
- You needs to be introspective about personal strengths and weaknesses and areas to focus on.
- Asking questions raised in the second paragraph will help you narrow options and choose the correct program.
- Enrolling in, and completing, your chosen program is crucial.
- Experience and exposure to issues are invaluable in building your skill set. As our featured leaders advised: “Put yourself out there.”
- Build your resume by listing any activity outside of clinical work that has contributed to enhancing your skills.
Good luck!
References
1. HealthcareAdministrationEDU.org. Master’s in Health Administration. https://www.healthcareadministrationedu.org.
2. Healthcare Management Degree Guide. https://www.healthcare-management-degree.net.
3. The Best Schools: The 15 Best Online MBA in Healthcare Management Degree Programs. https://thebestschools.org/rankings/best-online-mba-healthcare-management/.
4. US News. Best Executive MBA Programs. 2019. https://www.usnews.com/best-graduate-schools/top-business-schools/executive-rankings.
5. The Best Schools: The Best Executive MBA Programs Online & On-Campus. https://thebestschools.org/rankings/best-executive-mba-programs/.
6. AGA. https://www.gastro.org/.
7. AMA. https://www.ama-assn.org/about/leadership-development-institute.
8. Harvard Medical School. Career Advancement and Leadership Skills for Women in Healthcare. https://womensleadership.hmscme.com/.
9. American College of Healthcare Executives. https://www.ache.org/.
10. American Association for Physician Leadership. https://www.physicianleaders.org.
Dr. Alaparthi is in private practice in Hamden, Conn.; assistant clinical professor, Yale University, New Haven, Conn.; and assistant clinical professor, Quinnipiac University, Hamden. She is also an ex-officio member of the AGA Women’s Committee.
I have been in private practice as a gastroenterologist for 18 years. Many of us in gastroenterology and related fields have wondered how to navigate toward the next step in our careers. There are resources available to further our knowledge, add new skills, and fine tune personal talents to help position us for that next step.
Questions to ask at this stage are: What do I really want to do? Where do I see myself in 5-10 years? How do I go about achieving my target?
We come from different backgrounds including, broadly, academic clinical, academic research, basic science, clinical practice, and education. The next stage of these career paths can vary, and that should be kept in mind while choosing courses/programs. I reached out to two well-known gastroenterologists who have successfully changed their career paths after starting with different backgrounds.
Ronald Vender, MD, professor of medicine, associate dean of clinical affairs, chief medical officer, Yale University, New Haven, Conn.
Dr. Vender began in private practice gastroenterology after fellowship. His own trajectory has been one of “evolution” and has grown to the above titles through “incremental opportunity.” While reflecting on his career, Dr. Vender felt three main attributes were responsible: involvement in medical/GI societies, involvement in non-GI organizations, and engagement of needs for improvement at the hospital of practice. Opportunities became available by speaking up, raising issues, and demanding improvements. Dr. Vender’s involvement in both the private practice sector and hospital administration made his transition to hospital administration possible. This change was based on a “change in [him] and change in what [he] wanted to do.” His advice for all is to learn to say “yes” often in your early career and recognize when to say “no” later in your career.
John Allen, MD, MBA, clinical professor of medicine, University of Michigan, Ann Arbor
Dr. Allen started his career in the Veterans Affairs (VA) system, and during this time, he was exposed to research activities and learned research skills. His initial interest was in health care delivery, but this eventually changed to private practice gastroenterology. His exposure to information and the opportunity to learn about variations in practice and outcomes allowed him to maintain his interest in quality, which ultimately led to publications on colonoscopy quality. In his 40s he decided to obtain an executive master of business administration (EMBA), which he feels one should embark upon “when you have a problem to solve.” He has effectively moved from the VA system to private practice and now to academic medicine. Dr. Allen identified attending leadership conferences, engaging executive coaches, and participation in key committees as further opportunities to help you change careers. His prior work experience, education, and exposure enables him in his current position to help oversee a large department of medicine with 160 care sites, with quality and financials as key factors.
As we can see, there is no correct answer or set path for those of us wanting to change career directions. What was clear while speaking with both Dr. Vender and Dr. Allen was the importance of enthusiasm in solving issues, a willingness to commit to new projects, and an interest in exploring new areas.
Below is a brief overview of some degree programs that may help promote a change in your career path.
Masters in health care
This degree is aimed at those looking to advance their career in the field of health care in various locations, such as hospitals, clinics, and nonprofit organizations.1 Length of prior health care experience will vary based upon program. Programs are administered on a full-time and part-time basis, as well as online and study abroad. Numerous specialties are offered such as medicine, nutrition, psychiatry, nursing, veterinary medicine, physiotherapy, biomedical engineering, medical laboratory studies, radiology, alternative medicine, and health care management, administration, or leadership.
Health care MBA
Master of business administration (MBA) programs in health care administration management are offered by several universities. Given their aim of imparting essential information on a broad range of topics relevant to the health care industry, they are usually quite rigorous. It is recommended that you pursue an MBA only after a few years of working in your chosen field of practice. Many institutions require GMAT scores with the application.2-4
Executive MBA
EMBA programs are similar to health care MBAs in that they also include rigorous course work.5 EMBA programs are developed to meet the educational needs of managers and executives or physicians hoping to advance or change their career. Typically, students can earn an MBA in 2 years or less while working full-time. GMAT scores are required by most institutions offering EMBA.
Certification leadership programs
A benefit of leadership programs is that they help to develop a clear vision by creating a mission statement, goals, and action plans. Some notable programs include:
- American Gastroenterological Association leadership programs: Future Leaders Program, Women’s Leadership Conference, and Forward Program (new for 2019). These programs offer robust sessions which are well thought out and intended to advance the careers of traditionally underrepresented groups within the field of gastroenterology.6
- American Medical Association’s Women’s Leadership Certificate: This program was developed to promote knowledge and strong leadership, both within oneself and among others. Attendees are awarded with the “AMA Women’s Leadership Certificate” following the 2-day event.7
- Executive leadership training and development programs: As with many universities offer programs like these; for example, Harvard Medical School, Boston, offers a popular – and usually sold out – event for women titled “Career Advancement and Leadership Skills for Women in Health Care.”8
- American College of Healthcare Executives: Various conferences and educational meetings in addition to the annual ACHE Congress on Healthcare Leadership.9
- American Association of Physician Leadership: Physician executive accreditation along with career services, continued education, and publications.10
After reviewing the experiences of two well-known gastroenterologists and several of the available programs, the question to ask yourself is, “What’s next?” Most will likely have this question already in mind, so here are a few potential career directions/positions to consider:
Academic medicine: department chief, program director, director of endoscopy, chief medical officer
Private practice: managing director, director of endoscopy, finance director
Private sector: pharmaceutical industry, scientific advisor, medical director, medical insurance industry, malpractice insurance industry, medical informatics, public policy, private equity, entrepreneurial
Conclusion
In summary, there is no single answer nor a single program that fits everyone’s needs. Health care delivery and management/administration are complicated and will only continue to evolve. Consideration must be given to the fact that any change in one’s career direction needs time and commitment.
Here are some take-home points:
- You needs to be introspective about personal strengths and weaknesses and areas to focus on.
- Asking questions raised in the second paragraph will help you narrow options and choose the correct program.
- Enrolling in, and completing, your chosen program is crucial.
- Experience and exposure to issues are invaluable in building your skill set. As our featured leaders advised: “Put yourself out there.”
- Build your resume by listing any activity outside of clinical work that has contributed to enhancing your skills.
Good luck!
References
1. HealthcareAdministrationEDU.org. Master’s in Health Administration. https://www.healthcareadministrationedu.org.
2. Healthcare Management Degree Guide. https://www.healthcare-management-degree.net.
3. The Best Schools: The 15 Best Online MBA in Healthcare Management Degree Programs. https://thebestschools.org/rankings/best-online-mba-healthcare-management/.
4. US News. Best Executive MBA Programs. 2019. https://www.usnews.com/best-graduate-schools/top-business-schools/executive-rankings.
5. The Best Schools: The Best Executive MBA Programs Online & On-Campus. https://thebestschools.org/rankings/best-executive-mba-programs/.
6. AGA. https://www.gastro.org/.
7. AMA. https://www.ama-assn.org/about/leadership-development-institute.
8. Harvard Medical School. Career Advancement and Leadership Skills for Women in Healthcare. https://womensleadership.hmscme.com/.
9. American College of Healthcare Executives. https://www.ache.org/.
10. American Association for Physician Leadership. https://www.physicianleaders.org.
Dr. Alaparthi is in private practice in Hamden, Conn.; assistant clinical professor, Yale University, New Haven, Conn.; and assistant clinical professor, Quinnipiac University, Hamden. She is also an ex-officio member of the AGA Women’s Committee.
I have been in private practice as a gastroenterologist for 18 years. Many of us in gastroenterology and related fields have wondered how to navigate toward the next step in our careers. There are resources available to further our knowledge, add new skills, and fine tune personal talents to help position us for that next step.
Questions to ask at this stage are: What do I really want to do? Where do I see myself in 5-10 years? How do I go about achieving my target?
We come from different backgrounds including, broadly, academic clinical, academic research, basic science, clinical practice, and education. The next stage of these career paths can vary, and that should be kept in mind while choosing courses/programs. I reached out to two well-known gastroenterologists who have successfully changed their career paths after starting with different backgrounds.
Ronald Vender, MD, professor of medicine, associate dean of clinical affairs, chief medical officer, Yale University, New Haven, Conn.
Dr. Vender began in private practice gastroenterology after fellowship. His own trajectory has been one of “evolution” and has grown to the above titles through “incremental opportunity.” While reflecting on his career, Dr. Vender felt three main attributes were responsible: involvement in medical/GI societies, involvement in non-GI organizations, and engagement of needs for improvement at the hospital of practice. Opportunities became available by speaking up, raising issues, and demanding improvements. Dr. Vender’s involvement in both the private practice sector and hospital administration made his transition to hospital administration possible. This change was based on a “change in [him] and change in what [he] wanted to do.” His advice for all is to learn to say “yes” often in your early career and recognize when to say “no” later in your career.
John Allen, MD, MBA, clinical professor of medicine, University of Michigan, Ann Arbor
Dr. Allen started his career in the Veterans Affairs (VA) system, and during this time, he was exposed to research activities and learned research skills. His initial interest was in health care delivery, but this eventually changed to private practice gastroenterology. His exposure to information and the opportunity to learn about variations in practice and outcomes allowed him to maintain his interest in quality, which ultimately led to publications on colonoscopy quality. In his 40s he decided to obtain an executive master of business administration (EMBA), which he feels one should embark upon “when you have a problem to solve.” He has effectively moved from the VA system to private practice and now to academic medicine. Dr. Allen identified attending leadership conferences, engaging executive coaches, and participation in key committees as further opportunities to help you change careers. His prior work experience, education, and exposure enables him in his current position to help oversee a large department of medicine with 160 care sites, with quality and financials as key factors.
As we can see, there is no correct answer or set path for those of us wanting to change career directions. What was clear while speaking with both Dr. Vender and Dr. Allen was the importance of enthusiasm in solving issues, a willingness to commit to new projects, and an interest in exploring new areas.
Below is a brief overview of some degree programs that may help promote a change in your career path.
Masters in health care
This degree is aimed at those looking to advance their career in the field of health care in various locations, such as hospitals, clinics, and nonprofit organizations.1 Length of prior health care experience will vary based upon program. Programs are administered on a full-time and part-time basis, as well as online and study abroad. Numerous specialties are offered such as medicine, nutrition, psychiatry, nursing, veterinary medicine, physiotherapy, biomedical engineering, medical laboratory studies, radiology, alternative medicine, and health care management, administration, or leadership.
Health care MBA
Master of business administration (MBA) programs in health care administration management are offered by several universities. Given their aim of imparting essential information on a broad range of topics relevant to the health care industry, they are usually quite rigorous. It is recommended that you pursue an MBA only after a few years of working in your chosen field of practice. Many institutions require GMAT scores with the application.2-4
Executive MBA
EMBA programs are similar to health care MBAs in that they also include rigorous course work.5 EMBA programs are developed to meet the educational needs of managers and executives or physicians hoping to advance or change their career. Typically, students can earn an MBA in 2 years or less while working full-time. GMAT scores are required by most institutions offering EMBA.
Certification leadership programs
A benefit of leadership programs is that they help to develop a clear vision by creating a mission statement, goals, and action plans. Some notable programs include:
- American Gastroenterological Association leadership programs: Future Leaders Program, Women’s Leadership Conference, and Forward Program (new for 2019). These programs offer robust sessions which are well thought out and intended to advance the careers of traditionally underrepresented groups within the field of gastroenterology.6
- American Medical Association’s Women’s Leadership Certificate: This program was developed to promote knowledge and strong leadership, both within oneself and among others. Attendees are awarded with the “AMA Women’s Leadership Certificate” following the 2-day event.7
- Executive leadership training and development programs: As with many universities offer programs like these; for example, Harvard Medical School, Boston, offers a popular – and usually sold out – event for women titled “Career Advancement and Leadership Skills for Women in Health Care.”8
- American College of Healthcare Executives: Various conferences and educational meetings in addition to the annual ACHE Congress on Healthcare Leadership.9
- American Association of Physician Leadership: Physician executive accreditation along with career services, continued education, and publications.10
After reviewing the experiences of two well-known gastroenterologists and several of the available programs, the question to ask yourself is, “What’s next?” Most will likely have this question already in mind, so here are a few potential career directions/positions to consider:
Academic medicine: department chief, program director, director of endoscopy, chief medical officer
Private practice: managing director, director of endoscopy, finance director
Private sector: pharmaceutical industry, scientific advisor, medical director, medical insurance industry, malpractice insurance industry, medical informatics, public policy, private equity, entrepreneurial
Conclusion
In summary, there is no single answer nor a single program that fits everyone’s needs. Health care delivery and management/administration are complicated and will only continue to evolve. Consideration must be given to the fact that any change in one’s career direction needs time and commitment.
Here are some take-home points:
- You needs to be introspective about personal strengths and weaknesses and areas to focus on.
- Asking questions raised in the second paragraph will help you narrow options and choose the correct program.
- Enrolling in, and completing, your chosen program is crucial.
- Experience and exposure to issues are invaluable in building your skill set. As our featured leaders advised: “Put yourself out there.”
- Build your resume by listing any activity outside of clinical work that has contributed to enhancing your skills.
Good luck!
References
1. HealthcareAdministrationEDU.org. Master’s in Health Administration. https://www.healthcareadministrationedu.org.
2. Healthcare Management Degree Guide. https://www.healthcare-management-degree.net.
3. The Best Schools: The 15 Best Online MBA in Healthcare Management Degree Programs. https://thebestschools.org/rankings/best-online-mba-healthcare-management/.
4. US News. Best Executive MBA Programs. 2019. https://www.usnews.com/best-graduate-schools/top-business-schools/executive-rankings.
5. The Best Schools: The Best Executive MBA Programs Online & On-Campus. https://thebestschools.org/rankings/best-executive-mba-programs/.
6. AGA. https://www.gastro.org/.
7. AMA. https://www.ama-assn.org/about/leadership-development-institute.
8. Harvard Medical School. Career Advancement and Leadership Skills for Women in Healthcare. https://womensleadership.hmscme.com/.
9. American College of Healthcare Executives. https://www.ache.org/.
10. American Association for Physician Leadership. https://www.physicianleaders.org.
Dr. Alaparthi is in private practice in Hamden, Conn.; assistant clinical professor, Yale University, New Haven, Conn.; and assistant clinical professor, Quinnipiac University, Hamden. She is also an ex-officio member of the AGA Women’s Committee.
Keys to becoming an effective educator in gastroenterology
Introduction
For many young faculty, the transition from trainee to educator can be daunting. We may accrue valuable experiences as a senior resident or fellow on the floors, but nothing fully prepares you for the challenge of integrating education into your daily life as a new attending. This challenge is all the greater in a procedural field such as gastroenterology, in which educators need to turn their tangible skills into verbal instructions for a trainee.
The aim of this article is to ease that transition, whether it be on the wards, in the clinic, or in the endoscopy suite. Below are a few key tips on becoming an effective educator for the new gastroenterology attending.
In the clinic and on the wards
Don’t try to do too much: It is impossible to effectively teach every component of a single case. If you attempt to teach on multiple topics at once, the major points of the case may be missed. Choose a salient point from the specific case in front of you and explain how it changed your management. For example, “How did the ulcer stigmata change your management in the case of Mrs. B?” The clinical learning pearl in this case might be the bleeding risk of clean-based ulcers rather than the timing of endoscopy or PPI dosing. By focusing on one takeaway point per case, you can maximize the yield for the learner.
Make them commit: While reviewing a case with a trainee, you want to learn not just what they are thinking but also why they are thinking the way they are. By encouraging trainees to explain why they believe the diagnosis to be a particular disease or why a particular test should be the next step in a work-up, they are forced to explain their decision making. This allows you to truly understand their critical reasoning and ultimately correct any faulty logic along the way. In addition, trainees need practice in making clinical decisions. It is all too easy for them to let the attending drive clinical plans while on a busy service. Having them commit to a diagnosis or a plan will keep them engaged and is a key part of effective teaching frameworks such as the One-Minute Preceptor or SNAPPS.1
Correct mistakes: Trainee mistakes are a tremendous learning opportunity. A preceptor ought not gloss over these but rather address them directly. Clearly stating that something is wrong and then explaining why it is wrong and what the correct decision should be allows you to demonstrate clinical reasoning for your trainee. On a busy clinical service, it is easy to just say something is wrong, but the trainee will gain little from that experience.
In the endoscopy suite
Understand the learner’s objective: Depending on the trainee’s experience, the learning objective for a procedure may be different. A beginning endoscopist may hope to “reach the cecum,” while a more seasoned endoscopist may hope to effectively snare a flat polyp. The available procedural cognitive load for each trainee is different, and a beginning trainee may not be able to effectively integrate advanced techniques no matter how well you communicate with them.2 Establishment of the learner’s specific learning objectives for a procedure allows them to identify where they are and provides an opportunity for you to provide specific feedback and assistance to that individual.
Use specific language: Utilizing a common language between yourself and the trainee is very important. Phrases such as “Go right” or “Put your snare at the bottom” may not be specific enough for your learner. More exact language, such as “Little knob upward” or “Move your working channel to the 6 o’clock position,” will help the trainee comprehend your instruction and hopefully achieve the endoscopic objective at hand.3
Create an effective learning environment: Removing distractions from the endoscopy suite such as “multiple separate conversations” or “loud music” may be beneficial for trainees by minimizing extraneous load. Active engagement by the attending during a procedure has also been shown to be helpful in creating an effective learning environment.4 Examples of this include giving positive motivation or clear advice at a difficult junction of the case or just being engaged and watching the entire case rather than answering emails.
For all locations
Give feedback: Feedback should be given to the trainees on a regular basis in a comfortable, private setting away from the distractions of clinical responsibility. Feedback sandwiches – in which constructive comments are put between positive feedback – are no longer advised because trainees have been shown to not retain the topics they need to improve on but retain only the positive feedback from the end. Instead, utilize a format of soliciting self-reflection from the trainee, providing direct feedback on strengths and targets for improvement, and then concluding with an action plan for improvement.5
Get feedback: Do not be afraid of asking your trainees what you can do better. Don’t wait for the formal evaluations to be reviewed with your chairperson. Ask your trainees what you are doing well and what you can improve on. This feedback is a wealth of knowledge just waiting to be tapped.
Use your resources: There are many local, regional, and national resources available to educators. Senior faculty and fellowship directors at your institution can likely assist you. The office of graduate medical education in your institution likely has educational resources that are available for all faculty. Many institutions have some form of an institute for medical education that offers mentorship, online resources, and medical education journal clubs. The journal Gastroenterology includes a “Mentoring, Education, and Training” section in each issue that has many tips for educators. Lastly, there are national resources such as the AGA Academy of Educators that offer plenary sessions on medical education at Digestive Disease Week® and a collaborative network of faculty interested in medical education within gastroenterology.
References
1. Pascoe J et al. J Hosp Med. 2015 Feb;10(2):125-30.
2. Sewell JL et al. Acad Med. 2017 Nov;92(11):1622-31.
3. Dilly CK, Sewell JL. Gastroenterology 2017 Sept;153(3):632-6.
4. Pourmand K et al. J Surg Edu. 2018;75(5):1195-9.
5. Ramani S, Krackov SK. Med Teach. 2012;34(10):787-91.
Dr. Whitson is associate fellowship director, gastroenterology, assistant professor of medicine, The Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, New York. Twitter: @MJWhitsonMD
Introduction
For many young faculty, the transition from trainee to educator can be daunting. We may accrue valuable experiences as a senior resident or fellow on the floors, but nothing fully prepares you for the challenge of integrating education into your daily life as a new attending. This challenge is all the greater in a procedural field such as gastroenterology, in which educators need to turn their tangible skills into verbal instructions for a trainee.
The aim of this article is to ease that transition, whether it be on the wards, in the clinic, or in the endoscopy suite. Below are a few key tips on becoming an effective educator for the new gastroenterology attending.
In the clinic and on the wards
Don’t try to do too much: It is impossible to effectively teach every component of a single case. If you attempt to teach on multiple topics at once, the major points of the case may be missed. Choose a salient point from the specific case in front of you and explain how it changed your management. For example, “How did the ulcer stigmata change your management in the case of Mrs. B?” The clinical learning pearl in this case might be the bleeding risk of clean-based ulcers rather than the timing of endoscopy or PPI dosing. By focusing on one takeaway point per case, you can maximize the yield for the learner.
Make them commit: While reviewing a case with a trainee, you want to learn not just what they are thinking but also why they are thinking the way they are. By encouraging trainees to explain why they believe the diagnosis to be a particular disease or why a particular test should be the next step in a work-up, they are forced to explain their decision making. This allows you to truly understand their critical reasoning and ultimately correct any faulty logic along the way. In addition, trainees need practice in making clinical decisions. It is all too easy for them to let the attending drive clinical plans while on a busy service. Having them commit to a diagnosis or a plan will keep them engaged and is a key part of effective teaching frameworks such as the One-Minute Preceptor or SNAPPS.1
Correct mistakes: Trainee mistakes are a tremendous learning opportunity. A preceptor ought not gloss over these but rather address them directly. Clearly stating that something is wrong and then explaining why it is wrong and what the correct decision should be allows you to demonstrate clinical reasoning for your trainee. On a busy clinical service, it is easy to just say something is wrong, but the trainee will gain little from that experience.
In the endoscopy suite
Understand the learner’s objective: Depending on the trainee’s experience, the learning objective for a procedure may be different. A beginning endoscopist may hope to “reach the cecum,” while a more seasoned endoscopist may hope to effectively snare a flat polyp. The available procedural cognitive load for each trainee is different, and a beginning trainee may not be able to effectively integrate advanced techniques no matter how well you communicate with them.2 Establishment of the learner’s specific learning objectives for a procedure allows them to identify where they are and provides an opportunity for you to provide specific feedback and assistance to that individual.
Use specific language: Utilizing a common language between yourself and the trainee is very important. Phrases such as “Go right” or “Put your snare at the bottom” may not be specific enough for your learner. More exact language, such as “Little knob upward” or “Move your working channel to the 6 o’clock position,” will help the trainee comprehend your instruction and hopefully achieve the endoscopic objective at hand.3
Create an effective learning environment: Removing distractions from the endoscopy suite such as “multiple separate conversations” or “loud music” may be beneficial for trainees by minimizing extraneous load. Active engagement by the attending during a procedure has also been shown to be helpful in creating an effective learning environment.4 Examples of this include giving positive motivation or clear advice at a difficult junction of the case or just being engaged and watching the entire case rather than answering emails.
For all locations
Give feedback: Feedback should be given to the trainees on a regular basis in a comfortable, private setting away from the distractions of clinical responsibility. Feedback sandwiches – in which constructive comments are put between positive feedback – are no longer advised because trainees have been shown to not retain the topics they need to improve on but retain only the positive feedback from the end. Instead, utilize a format of soliciting self-reflection from the trainee, providing direct feedback on strengths and targets for improvement, and then concluding with an action plan for improvement.5
Get feedback: Do not be afraid of asking your trainees what you can do better. Don’t wait for the formal evaluations to be reviewed with your chairperson. Ask your trainees what you are doing well and what you can improve on. This feedback is a wealth of knowledge just waiting to be tapped.
Use your resources: There are many local, regional, and national resources available to educators. Senior faculty and fellowship directors at your institution can likely assist you. The office of graduate medical education in your institution likely has educational resources that are available for all faculty. Many institutions have some form of an institute for medical education that offers mentorship, online resources, and medical education journal clubs. The journal Gastroenterology includes a “Mentoring, Education, and Training” section in each issue that has many tips for educators. Lastly, there are national resources such as the AGA Academy of Educators that offer plenary sessions on medical education at Digestive Disease Week® and a collaborative network of faculty interested in medical education within gastroenterology.
References
1. Pascoe J et al. J Hosp Med. 2015 Feb;10(2):125-30.
2. Sewell JL et al. Acad Med. 2017 Nov;92(11):1622-31.
3. Dilly CK, Sewell JL. Gastroenterology 2017 Sept;153(3):632-6.
4. Pourmand K et al. J Surg Edu. 2018;75(5):1195-9.
5. Ramani S, Krackov SK. Med Teach. 2012;34(10):787-91.
Dr. Whitson is associate fellowship director, gastroenterology, assistant professor of medicine, The Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, New York. Twitter: @MJWhitsonMD
Introduction
For many young faculty, the transition from trainee to educator can be daunting. We may accrue valuable experiences as a senior resident or fellow on the floors, but nothing fully prepares you for the challenge of integrating education into your daily life as a new attending. This challenge is all the greater in a procedural field such as gastroenterology, in which educators need to turn their tangible skills into verbal instructions for a trainee.
The aim of this article is to ease that transition, whether it be on the wards, in the clinic, or in the endoscopy suite. Below are a few key tips on becoming an effective educator for the new gastroenterology attending.
In the clinic and on the wards
Don’t try to do too much: It is impossible to effectively teach every component of a single case. If you attempt to teach on multiple topics at once, the major points of the case may be missed. Choose a salient point from the specific case in front of you and explain how it changed your management. For example, “How did the ulcer stigmata change your management in the case of Mrs. B?” The clinical learning pearl in this case might be the bleeding risk of clean-based ulcers rather than the timing of endoscopy or PPI dosing. By focusing on one takeaway point per case, you can maximize the yield for the learner.
Make them commit: While reviewing a case with a trainee, you want to learn not just what they are thinking but also why they are thinking the way they are. By encouraging trainees to explain why they believe the diagnosis to be a particular disease or why a particular test should be the next step in a work-up, they are forced to explain their decision making. This allows you to truly understand their critical reasoning and ultimately correct any faulty logic along the way. In addition, trainees need practice in making clinical decisions. It is all too easy for them to let the attending drive clinical plans while on a busy service. Having them commit to a diagnosis or a plan will keep them engaged and is a key part of effective teaching frameworks such as the One-Minute Preceptor or SNAPPS.1
Correct mistakes: Trainee mistakes are a tremendous learning opportunity. A preceptor ought not gloss over these but rather address them directly. Clearly stating that something is wrong and then explaining why it is wrong and what the correct decision should be allows you to demonstrate clinical reasoning for your trainee. On a busy clinical service, it is easy to just say something is wrong, but the trainee will gain little from that experience.
In the endoscopy suite
Understand the learner’s objective: Depending on the trainee’s experience, the learning objective for a procedure may be different. A beginning endoscopist may hope to “reach the cecum,” while a more seasoned endoscopist may hope to effectively snare a flat polyp. The available procedural cognitive load for each trainee is different, and a beginning trainee may not be able to effectively integrate advanced techniques no matter how well you communicate with them.2 Establishment of the learner’s specific learning objectives for a procedure allows them to identify where they are and provides an opportunity for you to provide specific feedback and assistance to that individual.
Use specific language: Utilizing a common language between yourself and the trainee is very important. Phrases such as “Go right” or “Put your snare at the bottom” may not be specific enough for your learner. More exact language, such as “Little knob upward” or “Move your working channel to the 6 o’clock position,” will help the trainee comprehend your instruction and hopefully achieve the endoscopic objective at hand.3
Create an effective learning environment: Removing distractions from the endoscopy suite such as “multiple separate conversations” or “loud music” may be beneficial for trainees by minimizing extraneous load. Active engagement by the attending during a procedure has also been shown to be helpful in creating an effective learning environment.4 Examples of this include giving positive motivation or clear advice at a difficult junction of the case or just being engaged and watching the entire case rather than answering emails.
For all locations
Give feedback: Feedback should be given to the trainees on a regular basis in a comfortable, private setting away from the distractions of clinical responsibility. Feedback sandwiches – in which constructive comments are put between positive feedback – are no longer advised because trainees have been shown to not retain the topics they need to improve on but retain only the positive feedback from the end. Instead, utilize a format of soliciting self-reflection from the trainee, providing direct feedback on strengths and targets for improvement, and then concluding with an action plan for improvement.5
Get feedback: Do not be afraid of asking your trainees what you can do better. Don’t wait for the formal evaluations to be reviewed with your chairperson. Ask your trainees what you are doing well and what you can improve on. This feedback is a wealth of knowledge just waiting to be tapped.
Use your resources: There are many local, regional, and national resources available to educators. Senior faculty and fellowship directors at your institution can likely assist you. The office of graduate medical education in your institution likely has educational resources that are available for all faculty. Many institutions have some form of an institute for medical education that offers mentorship, online resources, and medical education journal clubs. The journal Gastroenterology includes a “Mentoring, Education, and Training” section in each issue that has many tips for educators. Lastly, there are national resources such as the AGA Academy of Educators that offer plenary sessions on medical education at Digestive Disease Week® and a collaborative network of faculty interested in medical education within gastroenterology.
References
1. Pascoe J et al. J Hosp Med. 2015 Feb;10(2):125-30.
2. Sewell JL et al. Acad Med. 2017 Nov;92(11):1622-31.
3. Dilly CK, Sewell JL. Gastroenterology 2017 Sept;153(3):632-6.
4. Pourmand K et al. J Surg Edu. 2018;75(5):1195-9.
5. Ramani S, Krackov SK. Med Teach. 2012;34(10):787-91.
Dr. Whitson is associate fellowship director, gastroenterology, assistant professor of medicine, The Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, New York. Twitter: @MJWhitsonMD
May 2019 – ICYMI
Gastroenterology
How to write an effective business plan in medicine. Jazayeri A; Park KT. 2019 April;156(5):1243-7.
doi.org/10.1053/j.gastro.2019.03.003 https://www.gastrojournal.org/article/S0016-5085(19)32513-2/fulltext
How to deliver safer and effective patient care: Tips for team leaders and educators. Shah BJ. 2019 March;156(4):852-5.
doi.org/10.1053/j.gastro.2019.02.017 https://www.gastrojournal.org/article/S0016-5085(19)30390-7/fulltext
AGA Clinical Practice Update on diagnosis and monitoring of celiac disease – changing utility of serology and histologic measures: expert review. Husby S; Murray JA; Kattzka DA. 2019 March;156(4):885-9.
doi.org/10.1053/j.gastro.2018.12.010 https://www.gastrojournal.org/article/S0016-5085(18)35408-8/fulltext
How to get involved in global health. Proctor DD. 2019 Feb;156(3):542-4.
doi.org/10.1053/j.gastro.2019.01.012 https://www.gastrojournal.org/article/S0016-5085(19)30033-2/fulltext
AGA Clinical Practice Guidelines on the management of mild-to-moderate ulcerative colitis. Ko CW; Singh S; Feuerstein JD; Falck-Yytter C; Falck-Ytter Y; Cross RK; on behalf of the American Gastroenterological Association Institute Clinical Guidelines Committee. 2019 Feb;156(3);748-64.
doi.org/10.1053/j.gastro.2018.12.009 https://www.gastrojournal.org/article/S0016-5085(18)35407-6/fulltext
Clin Gastro Hepatol
Translating best practices to meaningful quality measures: From measure conceptualization to implementation. Adams MA; Allen JI; Saini SD. 2019 April;17(5):805-8.
doi.org/10.1016/j.cgh.2018.10.027 https://www.cghjournal.org/article/S1542-3565(18)31149-2/fulltext
Switching between biologics and biosimilars in inflammatory bowel diseases. Raffals LE; Nguyen GC; Rubin DT. 2019 April;17(5):818-23.
doi.org/10.1016/j.cgh.2018.08.064 https://www.cghjournal.org/article/S1542-3565(18)30943-1/fulltext
Preventive medicine in inflammatory bowel disease. Weaver KN; Long MD. 2019 April;17(5):824-8.
doi.org/10.1016/j.cgh.2018.11.054 https://www.cghjournal.org/article/S1542-3565(18)31331-4/fulltext
Innovating in your practice: Overcoming barriers to create new opportunities. Muthusamy VR; Komanduri S. 2019 March;17(4):580-3.
doi.org/10.1016/j.cgh.2018.09.016 https://www.cghjournal.org/article/S1542-3565(18)30978-9/fulltext
Incorporating advanced practice providers into gastroenterology practice. Nandwani MDR; Clarke JO. 2019 Feb;17(3):365-9.
doi.org/10.1016/j.cgh.2018.09.015 https://www.cghjournal.org/article/S1542-3565(18)30977-7/fulltext
AGA Clinical Practice Update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Colombel J-F; Shin A; Gibson PR. 2019 Feb;17(3):380-90.
doi.org/10.1016/j.cgh.2018.08.001 https://www.cghjournal.org/article/S1542-3565(18)30810-3/fulltext
Gastroenterology
How to write an effective business plan in medicine. Jazayeri A; Park KT. 2019 April;156(5):1243-7.
doi.org/10.1053/j.gastro.2019.03.003 https://www.gastrojournal.org/article/S0016-5085(19)32513-2/fulltext
How to deliver safer and effective patient care: Tips for team leaders and educators. Shah BJ. 2019 March;156(4):852-5.
doi.org/10.1053/j.gastro.2019.02.017 https://www.gastrojournal.org/article/S0016-5085(19)30390-7/fulltext
AGA Clinical Practice Update on diagnosis and monitoring of celiac disease – changing utility of serology and histologic measures: expert review. Husby S; Murray JA; Kattzka DA. 2019 March;156(4):885-9.
doi.org/10.1053/j.gastro.2018.12.010 https://www.gastrojournal.org/article/S0016-5085(18)35408-8/fulltext
How to get involved in global health. Proctor DD. 2019 Feb;156(3):542-4.
doi.org/10.1053/j.gastro.2019.01.012 https://www.gastrojournal.org/article/S0016-5085(19)30033-2/fulltext
AGA Clinical Practice Guidelines on the management of mild-to-moderate ulcerative colitis. Ko CW; Singh S; Feuerstein JD; Falck-Yytter C; Falck-Ytter Y; Cross RK; on behalf of the American Gastroenterological Association Institute Clinical Guidelines Committee. 2019 Feb;156(3);748-64.
doi.org/10.1053/j.gastro.2018.12.009 https://www.gastrojournal.org/article/S0016-5085(18)35407-6/fulltext
Clin Gastro Hepatol
Translating best practices to meaningful quality measures: From measure conceptualization to implementation. Adams MA; Allen JI; Saini SD. 2019 April;17(5):805-8.
doi.org/10.1016/j.cgh.2018.10.027 https://www.cghjournal.org/article/S1542-3565(18)31149-2/fulltext
Switching between biologics and biosimilars in inflammatory bowel diseases. Raffals LE; Nguyen GC; Rubin DT. 2019 April;17(5):818-23.
doi.org/10.1016/j.cgh.2018.08.064 https://www.cghjournal.org/article/S1542-3565(18)30943-1/fulltext
Preventive medicine in inflammatory bowel disease. Weaver KN; Long MD. 2019 April;17(5):824-8.
doi.org/10.1016/j.cgh.2018.11.054 https://www.cghjournal.org/article/S1542-3565(18)31331-4/fulltext
Innovating in your practice: Overcoming barriers to create new opportunities. Muthusamy VR; Komanduri S. 2019 March;17(4):580-3.
doi.org/10.1016/j.cgh.2018.09.016 https://www.cghjournal.org/article/S1542-3565(18)30978-9/fulltext
Incorporating advanced practice providers into gastroenterology practice. Nandwani MDR; Clarke JO. 2019 Feb;17(3):365-9.
doi.org/10.1016/j.cgh.2018.09.015 https://www.cghjournal.org/article/S1542-3565(18)30977-7/fulltext
AGA Clinical Practice Update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Colombel J-F; Shin A; Gibson PR. 2019 Feb;17(3):380-90.
doi.org/10.1016/j.cgh.2018.08.001 https://www.cghjournal.org/article/S1542-3565(18)30810-3/fulltext
Gastroenterology
How to write an effective business plan in medicine. Jazayeri A; Park KT. 2019 April;156(5):1243-7.
doi.org/10.1053/j.gastro.2019.03.003 https://www.gastrojournal.org/article/S0016-5085(19)32513-2/fulltext
How to deliver safer and effective patient care: Tips for team leaders and educators. Shah BJ. 2019 March;156(4):852-5.
doi.org/10.1053/j.gastro.2019.02.017 https://www.gastrojournal.org/article/S0016-5085(19)30390-7/fulltext
AGA Clinical Practice Update on diagnosis and monitoring of celiac disease – changing utility of serology and histologic measures: expert review. Husby S; Murray JA; Kattzka DA. 2019 March;156(4):885-9.
doi.org/10.1053/j.gastro.2018.12.010 https://www.gastrojournal.org/article/S0016-5085(18)35408-8/fulltext
How to get involved in global health. Proctor DD. 2019 Feb;156(3):542-4.
doi.org/10.1053/j.gastro.2019.01.012 https://www.gastrojournal.org/article/S0016-5085(19)30033-2/fulltext
AGA Clinical Practice Guidelines on the management of mild-to-moderate ulcerative colitis. Ko CW; Singh S; Feuerstein JD; Falck-Yytter C; Falck-Ytter Y; Cross RK; on behalf of the American Gastroenterological Association Institute Clinical Guidelines Committee. 2019 Feb;156(3);748-64.
doi.org/10.1053/j.gastro.2018.12.009 https://www.gastrojournal.org/article/S0016-5085(18)35407-6/fulltext
Clin Gastro Hepatol
Translating best practices to meaningful quality measures: From measure conceptualization to implementation. Adams MA; Allen JI; Saini SD. 2019 April;17(5):805-8.
doi.org/10.1016/j.cgh.2018.10.027 https://www.cghjournal.org/article/S1542-3565(18)31149-2/fulltext
Switching between biologics and biosimilars in inflammatory bowel diseases. Raffals LE; Nguyen GC; Rubin DT. 2019 April;17(5):818-23.
doi.org/10.1016/j.cgh.2018.08.064 https://www.cghjournal.org/article/S1542-3565(18)30943-1/fulltext
Preventive medicine in inflammatory bowel disease. Weaver KN; Long MD. 2019 April;17(5):824-8.
doi.org/10.1016/j.cgh.2018.11.054 https://www.cghjournal.org/article/S1542-3565(18)31331-4/fulltext
Innovating in your practice: Overcoming barriers to create new opportunities. Muthusamy VR; Komanduri S. 2019 March;17(4):580-3.
doi.org/10.1016/j.cgh.2018.09.016 https://www.cghjournal.org/article/S1542-3565(18)30978-9/fulltext
Incorporating advanced practice providers into gastroenterology practice. Nandwani MDR; Clarke JO. 2019 Feb;17(3):365-9.
doi.org/10.1016/j.cgh.2018.09.015 https://www.cghjournal.org/article/S1542-3565(18)30977-7/fulltext
AGA Clinical Practice Update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Colombel J-F; Shin A; Gibson PR. 2019 Feb;17(3):380-90.
doi.org/10.1016/j.cgh.2018.08.001 https://www.cghjournal.org/article/S1542-3565(18)30810-3/fulltext
AGA News
Meet a rising star in fecal incontinence research
The AGA Research Foundation offers its flagship grant, the AGA Research Scholar Award, to the most promising early career investigators. Kyle Staller, MD, MPH, assistant professor of medicine at Harvard Medical School in Boston, is no exception. We’re thrilled to highlight Dr. Staller – a 2016 AGA Research Scholar Award winner – as our AGA Research Foundation researcher of the month.
The Staller lab’s AGA-funded project is specifically focused on the risk factors for fecal incontinence, which have not been well studied. One in 10 women over age 80 suffers from this debilitating condition. Dr. Staller looked at the lifestyles and dietary factors of female study participants in research databases to determine whether they were predisposed to developing fecal incontinence beyond the usual risk factors such as childbirth, which can cause injury to the pelvic floor, and diabetes. Dr. Staller believes that understanding and modifying risk factors could decrease the chance of women developing this condition, or could even prevent it.
With his AGA Research Foundation grant, Dr. Staller found that consumption of dietary fiber in higher quantities, and an increase of moderate exercise up to a point, lowered the risk of developing fecal incontinence. “This tells us that not only is fiber healthy but also preventative to fecal incontinence,” he said.
Dr. Staller says that he became interested in this area of study after patients, who were getting excited about their impending retirement or enjoying their retirement years, developed this life-altering condition. His compassion for his patients inspired him to study the factors leading to fecal incontinence, which will likely become more prevalent as the U.S. population ages.
Dr. Staller is using the baseline data from his AGA Research Foundation grant to support his application for a 5-year NIH grant designed to help young investigators learn new research skills to further their careers.
“This is the next step in my career,” he said. “If I didn’t have the AGA Research Foundation grant, I don’t know if the opportunity would be there for me to go on to the next level. The AGA grant gives you the opportunity to get that baseline data so you can become a competitive applicant for longer-term grants.”
Another benefit of Dr. Staller’s AGA Research Foundation grant: It got him involved with AGA. In March 2019, Dr. Staller joined the new class of AGA Future Leaders, AGA’s competitive leadership development program designed to prepare early career GIs for future leadership roles in AGA, at their home institutions, and within the field of digestive diseases. The program kicked off at AGA’s inaugural leadership development conference.
“It is a true honor to participate in the AGA Future Leaders Program. During the AGA Leadership Development Conference, we learned concrete tips about effective leadership strategies across the spectrum of GI practice from research to clinical practice. Among our mentors were prominent researchers, clinical innovators, and division and department heads from across the U.S. – there was no shortage of inspiration. Perhaps most importantly, I was able to form what I hope to be career-long connections with both my fellow future leaders program participants and our mentors,” he said.
Dr. Staller’s qualifications as a clinician and researcher of bowel issues are put to good use as a father of two boys, ages 4 and 6, who are at the peak of the potty humor stage.
“They’re interested in the GI tract as well,” Dr. Staller said with a laugh. “My mom likes to say I never got out of the potty phase and made it a career. It’s important to feel comfortable talking about these uncomfortable topics. That’s what people want from their physician. If you can talk about this and the physician doesn’t bat an eyelash, that’s a good setup to have a good therapeutic relationship.”
‘Put your own oxygen mask on first’
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence and remaining optimistic.
“Leadership 101: Put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”– Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an National Institutes of Health–funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” – Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” – Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” – Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” – ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably-Ellen Zimmerman, MD”
– Fazia Mir-Shaffi, MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” – Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face – a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
Dr. Vaibhav Wadhwa advocates for step therapy reform in Florida
Vaibhav Wadhwa, MD, met with Ms. Laurie Flink, deputy district director for Rep. Debbie Wasserman Schultz (FL-23), to discuss AGA’s legislative priorities.
Dr. Wadhwa thanked Ms. Flink for Rep. Wasserman Schultz’s support of the Removing Barriers to Colorectal Screening Act and NIH funding. Dr. Wadhwa also mentioned that Rep. Wasserman Schultz is not a cosponsor of the Restoring the Patient’s Voice Act and explained in detail about why this is an important resolution that needs to be passed.
Dr. Wadhwa gave examples of patients from his own practice and discussed the challenges they face. Ms. Flink was very interested in hearing about patients with chronic conditions such as inflammatory bowel disease (IBD) not being able to get the appropriate regimen because of the barriers created by step therapy. Ms. Flink was very appreciative of the visit and stated that these in-person visits along with personal stories about these issues go a long way in helping congressional offices understand the implications that these bills have.
Ms. Flink assured Dr. Wadhwa that she will raise these points with Rep. Wasserman Schultz and will discuss cosponsoring the Restoring the Patient’s Voice Act once it is reintroduced.
Dr. Wadhwa is a fellow at the Cleveland Clinic Florida in Weston, and is the AGA Congressional Advocates Program state leader for Florida. He is interested in therapeutic endoscopy and advocating for appropriate reimbursement for endoscopic procedures.
How to get involved in advocacy
Interested in advocacy but not sure how or whether you have time in your busy schedule? AGA has an array of options for how you can be active in advocacy. Some take as little as 5 minutes.
Letter writing. AGA uses GovPredict, an online advocacy platform that allows members to contact their representatives in Congress with just a few clicks. AGA develops messages on significant pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have a great impact on gastroenterology. AGA’s ongoing letter writing campaigns can always be found at gastro.org, but be sure to keep an eye out for advocacy emails, AGA eDigest, and social media, so you do not miss your opportunity to take action on timely issues. AGA encourages its members to share letter writing campaigns with their colleagues, as well as posting them on social media.
Meetings with members of Congress. In-person meetings are an excellent opportunity to share with your representatives in Congress, or their staff, how the issues that impact gastroenterology affect you, your patients, and your practice. AGA has a plethora of resources to help you set up such meetings, including up-to-date issue briefs, tips and tricks for productive meetings, and webinars on how to host an on-site visit. AGA staff is always more than happy to help you arrange a meeting either in Washington, D.C., or in your home state. If you are interested in arranging such a meeting, please contact AGA Public Policy Coordinator Jonathan Sollish, at [email protected] or 240-482-3228.
AGA PAC. AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA. It is the only political action committee supported by a national gastroenterology society, and its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections, and access to specialty care, and sustained federal funding of digestive disease research. If you are interested in learning more, contact AGA Government and Political Affairs Manager Navneet Buttar, at [email protected] or 240-482-3221.
Congressional Advocates Program. This grassroots program is aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities, ranging from creating educational posts on social media to meeting with members of Congress. Members of the Congressional Advocates Program are mentored and receive advocacy training by AGA leadership and staff. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
Introducing the AGA Future Leaders class of 2020
AGA has announced the 18 early career physicians and scientists selected to participate in its Future Leaders Program, which was created in 2015 to provide a pathway for leadership development within AGA for early career physicians and scientists who have the potential to make a significant impact on the specialty. These 18 participants will embark on an 18-month-long program designed to develop the skills necessary to become future leaders in the AGA, at their home institutions, and within the field of digestive diseases.
“The 2020 class of AGA Future Leaders represents the next generation of leaders in our field,” said Darrell S. Pardi, MD, MSc, AGAF, co–program chair for the AGA Future Leaders Program. “Along with my cochair, Sheryl Pfeil, MD, AGAF, and the esteemed mentors and faculty participating in this program, we look forward to cultivating these rising stars who stand out for their current achievements, commitment to advancing the field, and potential for future success.”
Class of 2020 Future Leaders
- Christen Klochan Dilly, MD, MEHP, Indiana University School of Medicine and Roudebush VA Medical Center
- Daniel Freedberg, MD, MS, Columbia University
- Wendy A. Henderson, PhD, National Institutes of Health
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Animesh Jain, MD, University of North Carolina at Chapel Hill
- Avinash Ketwaroo, MD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Bharati Kochar, MD, MSCR, University of North Carolina at Chapel Hill
- David Leiman, MD, MSHP, Duke University Medical Center
- James Lin, MD, City of Hope National Medical Center in Duarte
- Michelle Long, MD, Boston Medical Center
- Aimee Lucas, MD, MS, Icahn School of Medicine at Mount Sinai
- Miguel Malespin, MD, Tampa General Hospital
- Simon C. Mathews, MD, Johns Hopkins Medicine
- Karthik Ravi, MD, Mayo Clinic (Rochester, Minnesota)
- Florian Rieder, MD, Cleveland Clinic Foundation
- Kyle Staller, MD, MPH, Harvard Medical School
- Christina Twyman-Saint Victor, MD, University of Pennsylvania Perelman School of Medicine
- Ryan Ungaro, MD, MS, Icahn School of Medicine at Mount Sinai
View Future Leader Bios
The AGA Future Leaders Program will kick off with the AGA Leadership Development Conference March 8-10, 2019, at the Hilton Rockville Executive conference center in Rockville, Maryland, and will continue through Digestive Disease Week® (DDW) 2020 in Chicago, Illinois. Throughout the course of the program, participants will work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
Learn more about the AGA Future Leaders Program.
Sessions at DDW® 2019 designed for fellows and early career GIs
AGA has an agenda of special sessions at Digestive Disease Week® (DDW) 2019 to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
With the exception of the AGA Postgraduate Course, all of the sessions are free, but you must register for DDW to attend. Visit AGA University for a full list and additional details.
- AGA Postgraduate Course Saturday, May 18, and Sunday, May 19
- Introduction to GI Practice: A Trainee Boot Camp, Monday, May 20, 10-11:30 a.m.
- AGA Board Review CourseMonday, May 20, 1:30-5:30 p.m.
- Advancing Clinical Practice: GI Fellow-Directed Quality Improvement ProjectsMonday, May 20, 2-3:30 p.m.
- GI in the Digital Age, Monday, May 20, 4-5:30 p.m.
DDW Trainee and Early Career Lounge
Included with the cost of DDW registration, trainee and early career GI attendees have access to this lounge in the Sails Pavilion. It’s a great way to meet and network with peers from around the world over a cup of coffee and will feature new programming in 2019. Meet with experts to have your questions answered about practical issues of career choice, contracting, or how to write a manuscript.
Meet a rising star in fecal incontinence research
The AGA Research Foundation offers its flagship grant, the AGA Research Scholar Award, to the most promising early career investigators. Kyle Staller, MD, MPH, assistant professor of medicine at Harvard Medical School in Boston, is no exception. We’re thrilled to highlight Dr. Staller – a 2016 AGA Research Scholar Award winner – as our AGA Research Foundation researcher of the month.
The Staller lab’s AGA-funded project is specifically focused on the risk factors for fecal incontinence, which have not been well studied. One in 10 women over age 80 suffers from this debilitating condition. Dr. Staller looked at the lifestyles and dietary factors of female study participants in research databases to determine whether they were predisposed to developing fecal incontinence beyond the usual risk factors such as childbirth, which can cause injury to the pelvic floor, and diabetes. Dr. Staller believes that understanding and modifying risk factors could decrease the chance of women developing this condition, or could even prevent it.
With his AGA Research Foundation grant, Dr. Staller found that consumption of dietary fiber in higher quantities, and an increase of moderate exercise up to a point, lowered the risk of developing fecal incontinence. “This tells us that not only is fiber healthy but also preventative to fecal incontinence,” he said.
Dr. Staller says that he became interested in this area of study after patients, who were getting excited about their impending retirement or enjoying their retirement years, developed this life-altering condition. His compassion for his patients inspired him to study the factors leading to fecal incontinence, which will likely become more prevalent as the U.S. population ages.
Dr. Staller is using the baseline data from his AGA Research Foundation grant to support his application for a 5-year NIH grant designed to help young investigators learn new research skills to further their careers.
“This is the next step in my career,” he said. “If I didn’t have the AGA Research Foundation grant, I don’t know if the opportunity would be there for me to go on to the next level. The AGA grant gives you the opportunity to get that baseline data so you can become a competitive applicant for longer-term grants.”
Another benefit of Dr. Staller’s AGA Research Foundation grant: It got him involved with AGA. In March 2019, Dr. Staller joined the new class of AGA Future Leaders, AGA’s competitive leadership development program designed to prepare early career GIs for future leadership roles in AGA, at their home institutions, and within the field of digestive diseases. The program kicked off at AGA’s inaugural leadership development conference.
“It is a true honor to participate in the AGA Future Leaders Program. During the AGA Leadership Development Conference, we learned concrete tips about effective leadership strategies across the spectrum of GI practice from research to clinical practice. Among our mentors were prominent researchers, clinical innovators, and division and department heads from across the U.S. – there was no shortage of inspiration. Perhaps most importantly, I was able to form what I hope to be career-long connections with both my fellow future leaders program participants and our mentors,” he said.
Dr. Staller’s qualifications as a clinician and researcher of bowel issues are put to good use as a father of two boys, ages 4 and 6, who are at the peak of the potty humor stage.
“They’re interested in the GI tract as well,” Dr. Staller said with a laugh. “My mom likes to say I never got out of the potty phase and made it a career. It’s important to feel comfortable talking about these uncomfortable topics. That’s what people want from their physician. If you can talk about this and the physician doesn’t bat an eyelash, that’s a good setup to have a good therapeutic relationship.”
‘Put your own oxygen mask on first’
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence and remaining optimistic.
“Leadership 101: Put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”– Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an National Institutes of Health–funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” – Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” – Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” – Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” – ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably-Ellen Zimmerman, MD”
– Fazia Mir-Shaffi, MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” – Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face – a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
Dr. Vaibhav Wadhwa advocates for step therapy reform in Florida
Vaibhav Wadhwa, MD, met with Ms. Laurie Flink, deputy district director for Rep. Debbie Wasserman Schultz (FL-23), to discuss AGA’s legislative priorities.
Dr. Wadhwa thanked Ms. Flink for Rep. Wasserman Schultz’s support of the Removing Barriers to Colorectal Screening Act and NIH funding. Dr. Wadhwa also mentioned that Rep. Wasserman Schultz is not a cosponsor of the Restoring the Patient’s Voice Act and explained in detail about why this is an important resolution that needs to be passed.
Dr. Wadhwa gave examples of patients from his own practice and discussed the challenges they face. Ms. Flink was very interested in hearing about patients with chronic conditions such as inflammatory bowel disease (IBD) not being able to get the appropriate regimen because of the barriers created by step therapy. Ms. Flink was very appreciative of the visit and stated that these in-person visits along with personal stories about these issues go a long way in helping congressional offices understand the implications that these bills have.
Ms. Flink assured Dr. Wadhwa that she will raise these points with Rep. Wasserman Schultz and will discuss cosponsoring the Restoring the Patient’s Voice Act once it is reintroduced.
Dr. Wadhwa is a fellow at the Cleveland Clinic Florida in Weston, and is the AGA Congressional Advocates Program state leader for Florida. He is interested in therapeutic endoscopy and advocating for appropriate reimbursement for endoscopic procedures.
How to get involved in advocacy
Interested in advocacy but not sure how or whether you have time in your busy schedule? AGA has an array of options for how you can be active in advocacy. Some take as little as 5 minutes.
Letter writing. AGA uses GovPredict, an online advocacy platform that allows members to contact their representatives in Congress with just a few clicks. AGA develops messages on significant pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have a great impact on gastroenterology. AGA’s ongoing letter writing campaigns can always be found at gastro.org, but be sure to keep an eye out for advocacy emails, AGA eDigest, and social media, so you do not miss your opportunity to take action on timely issues. AGA encourages its members to share letter writing campaigns with their colleagues, as well as posting them on social media.
Meetings with members of Congress. In-person meetings are an excellent opportunity to share with your representatives in Congress, or their staff, how the issues that impact gastroenterology affect you, your patients, and your practice. AGA has a plethora of resources to help you set up such meetings, including up-to-date issue briefs, tips and tricks for productive meetings, and webinars on how to host an on-site visit. AGA staff is always more than happy to help you arrange a meeting either in Washington, D.C., or in your home state. If you are interested in arranging such a meeting, please contact AGA Public Policy Coordinator Jonathan Sollish, at [email protected] or 240-482-3228.
AGA PAC. AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA. It is the only political action committee supported by a national gastroenterology society, and its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections, and access to specialty care, and sustained federal funding of digestive disease research. If you are interested in learning more, contact AGA Government and Political Affairs Manager Navneet Buttar, at [email protected] or 240-482-3221.
Congressional Advocates Program. This grassroots program is aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities, ranging from creating educational posts on social media to meeting with members of Congress. Members of the Congressional Advocates Program are mentored and receive advocacy training by AGA leadership and staff. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
Introducing the AGA Future Leaders class of 2020
AGA has announced the 18 early career physicians and scientists selected to participate in its Future Leaders Program, which was created in 2015 to provide a pathway for leadership development within AGA for early career physicians and scientists who have the potential to make a significant impact on the specialty. These 18 participants will embark on an 18-month-long program designed to develop the skills necessary to become future leaders in the AGA, at their home institutions, and within the field of digestive diseases.
“The 2020 class of AGA Future Leaders represents the next generation of leaders in our field,” said Darrell S. Pardi, MD, MSc, AGAF, co–program chair for the AGA Future Leaders Program. “Along with my cochair, Sheryl Pfeil, MD, AGAF, and the esteemed mentors and faculty participating in this program, we look forward to cultivating these rising stars who stand out for their current achievements, commitment to advancing the field, and potential for future success.”
Class of 2020 Future Leaders
- Christen Klochan Dilly, MD, MEHP, Indiana University School of Medicine and Roudebush VA Medical Center
- Daniel Freedberg, MD, MS, Columbia University
- Wendy A. Henderson, PhD, National Institutes of Health
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Animesh Jain, MD, University of North Carolina at Chapel Hill
- Avinash Ketwaroo, MD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Bharati Kochar, MD, MSCR, University of North Carolina at Chapel Hill
- David Leiman, MD, MSHP, Duke University Medical Center
- James Lin, MD, City of Hope National Medical Center in Duarte
- Michelle Long, MD, Boston Medical Center
- Aimee Lucas, MD, MS, Icahn School of Medicine at Mount Sinai
- Miguel Malespin, MD, Tampa General Hospital
- Simon C. Mathews, MD, Johns Hopkins Medicine
- Karthik Ravi, MD, Mayo Clinic (Rochester, Minnesota)
- Florian Rieder, MD, Cleveland Clinic Foundation
- Kyle Staller, MD, MPH, Harvard Medical School
- Christina Twyman-Saint Victor, MD, University of Pennsylvania Perelman School of Medicine
- Ryan Ungaro, MD, MS, Icahn School of Medicine at Mount Sinai
View Future Leader Bios
The AGA Future Leaders Program will kick off with the AGA Leadership Development Conference March 8-10, 2019, at the Hilton Rockville Executive conference center in Rockville, Maryland, and will continue through Digestive Disease Week® (DDW) 2020 in Chicago, Illinois. Throughout the course of the program, participants will work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
Learn more about the AGA Future Leaders Program.
Sessions at DDW® 2019 designed for fellows and early career GIs
AGA has an agenda of special sessions at Digestive Disease Week® (DDW) 2019 to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
With the exception of the AGA Postgraduate Course, all of the sessions are free, but you must register for DDW to attend. Visit AGA University for a full list and additional details.
- AGA Postgraduate Course Saturday, May 18, and Sunday, May 19
- Introduction to GI Practice: A Trainee Boot Camp, Monday, May 20, 10-11:30 a.m.
- AGA Board Review CourseMonday, May 20, 1:30-5:30 p.m.
- Advancing Clinical Practice: GI Fellow-Directed Quality Improvement ProjectsMonday, May 20, 2-3:30 p.m.
- GI in the Digital Age, Monday, May 20, 4-5:30 p.m.
DDW Trainee and Early Career Lounge
Included with the cost of DDW registration, trainee and early career GI attendees have access to this lounge in the Sails Pavilion. It’s a great way to meet and network with peers from around the world over a cup of coffee and will feature new programming in 2019. Meet with experts to have your questions answered about practical issues of career choice, contracting, or how to write a manuscript.
Meet a rising star in fecal incontinence research
The AGA Research Foundation offers its flagship grant, the AGA Research Scholar Award, to the most promising early career investigators. Kyle Staller, MD, MPH, assistant professor of medicine at Harvard Medical School in Boston, is no exception. We’re thrilled to highlight Dr. Staller – a 2016 AGA Research Scholar Award winner – as our AGA Research Foundation researcher of the month.
The Staller lab’s AGA-funded project is specifically focused on the risk factors for fecal incontinence, which have not been well studied. One in 10 women over age 80 suffers from this debilitating condition. Dr. Staller looked at the lifestyles and dietary factors of female study participants in research databases to determine whether they were predisposed to developing fecal incontinence beyond the usual risk factors such as childbirth, which can cause injury to the pelvic floor, and diabetes. Dr. Staller believes that understanding and modifying risk factors could decrease the chance of women developing this condition, or could even prevent it.
With his AGA Research Foundation grant, Dr. Staller found that consumption of dietary fiber in higher quantities, and an increase of moderate exercise up to a point, lowered the risk of developing fecal incontinence. “This tells us that not only is fiber healthy but also preventative to fecal incontinence,” he said.
Dr. Staller says that he became interested in this area of study after patients, who were getting excited about their impending retirement or enjoying their retirement years, developed this life-altering condition. His compassion for his patients inspired him to study the factors leading to fecal incontinence, which will likely become more prevalent as the U.S. population ages.
Dr. Staller is using the baseline data from his AGA Research Foundation grant to support his application for a 5-year NIH grant designed to help young investigators learn new research skills to further their careers.
“This is the next step in my career,” he said. “If I didn’t have the AGA Research Foundation grant, I don’t know if the opportunity would be there for me to go on to the next level. The AGA grant gives you the opportunity to get that baseline data so you can become a competitive applicant for longer-term grants.”
Another benefit of Dr. Staller’s AGA Research Foundation grant: It got him involved with AGA. In March 2019, Dr. Staller joined the new class of AGA Future Leaders, AGA’s competitive leadership development program designed to prepare early career GIs for future leadership roles in AGA, at their home institutions, and within the field of digestive diseases. The program kicked off at AGA’s inaugural leadership development conference.
“It is a true honor to participate in the AGA Future Leaders Program. During the AGA Leadership Development Conference, we learned concrete tips about effective leadership strategies across the spectrum of GI practice from research to clinical practice. Among our mentors were prominent researchers, clinical innovators, and division and department heads from across the U.S. – there was no shortage of inspiration. Perhaps most importantly, I was able to form what I hope to be career-long connections with both my fellow future leaders program participants and our mentors,” he said.
Dr. Staller’s qualifications as a clinician and researcher of bowel issues are put to good use as a father of two boys, ages 4 and 6, who are at the peak of the potty humor stage.
“They’re interested in the GI tract as well,” Dr. Staller said with a laugh. “My mom likes to say I never got out of the potty phase and made it a career. It’s important to feel comfortable talking about these uncomfortable topics. That’s what people want from their physician. If you can talk about this and the physician doesn’t bat an eyelash, that’s a good setup to have a good therapeutic relationship.”
‘Put your own oxygen mask on first’
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence and remaining optimistic.
“Leadership 101: Put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”– Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an National Institutes of Health–funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” – Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” – Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” – Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” – ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably-Ellen Zimmerman, MD”
– Fazia Mir-Shaffi, MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” – Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face – a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
Dr. Vaibhav Wadhwa advocates for step therapy reform in Florida
Vaibhav Wadhwa, MD, met with Ms. Laurie Flink, deputy district director for Rep. Debbie Wasserman Schultz (FL-23), to discuss AGA’s legislative priorities.
Dr. Wadhwa thanked Ms. Flink for Rep. Wasserman Schultz’s support of the Removing Barriers to Colorectal Screening Act and NIH funding. Dr. Wadhwa also mentioned that Rep. Wasserman Schultz is not a cosponsor of the Restoring the Patient’s Voice Act and explained in detail about why this is an important resolution that needs to be passed.
Dr. Wadhwa gave examples of patients from his own practice and discussed the challenges they face. Ms. Flink was very interested in hearing about patients with chronic conditions such as inflammatory bowel disease (IBD) not being able to get the appropriate regimen because of the barriers created by step therapy. Ms. Flink was very appreciative of the visit and stated that these in-person visits along with personal stories about these issues go a long way in helping congressional offices understand the implications that these bills have.
Ms. Flink assured Dr. Wadhwa that she will raise these points with Rep. Wasserman Schultz and will discuss cosponsoring the Restoring the Patient’s Voice Act once it is reintroduced.
Dr. Wadhwa is a fellow at the Cleveland Clinic Florida in Weston, and is the AGA Congressional Advocates Program state leader for Florida. He is interested in therapeutic endoscopy and advocating for appropriate reimbursement for endoscopic procedures.
How to get involved in advocacy
Interested in advocacy but not sure how or whether you have time in your busy schedule? AGA has an array of options for how you can be active in advocacy. Some take as little as 5 minutes.
Letter writing. AGA uses GovPredict, an online advocacy platform that allows members to contact their representatives in Congress with just a few clicks. AGA develops messages on significant pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have a great impact on gastroenterology. AGA’s ongoing letter writing campaigns can always be found at gastro.org, but be sure to keep an eye out for advocacy emails, AGA eDigest, and social media, so you do not miss your opportunity to take action on timely issues. AGA encourages its members to share letter writing campaigns with their colleagues, as well as posting them on social media.
Meetings with members of Congress. In-person meetings are an excellent opportunity to share with your representatives in Congress, or their staff, how the issues that impact gastroenterology affect you, your patients, and your practice. AGA has a plethora of resources to help you set up such meetings, including up-to-date issue briefs, tips and tricks for productive meetings, and webinars on how to host an on-site visit. AGA staff is always more than happy to help you arrange a meeting either in Washington, D.C., or in your home state. If you are interested in arranging such a meeting, please contact AGA Public Policy Coordinator Jonathan Sollish, at [email protected] or 240-482-3228.
AGA PAC. AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA. It is the only political action committee supported by a national gastroenterology society, and its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections, and access to specialty care, and sustained federal funding of digestive disease research. If you are interested in learning more, contact AGA Government and Political Affairs Manager Navneet Buttar, at [email protected] or 240-482-3221.
Congressional Advocates Program. This grassroots program is aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities, ranging from creating educational posts on social media to meeting with members of Congress. Members of the Congressional Advocates Program are mentored and receive advocacy training by AGA leadership and staff. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
Introducing the AGA Future Leaders class of 2020
AGA has announced the 18 early career physicians and scientists selected to participate in its Future Leaders Program, which was created in 2015 to provide a pathway for leadership development within AGA for early career physicians and scientists who have the potential to make a significant impact on the specialty. These 18 participants will embark on an 18-month-long program designed to develop the skills necessary to become future leaders in the AGA, at their home institutions, and within the field of digestive diseases.
“The 2020 class of AGA Future Leaders represents the next generation of leaders in our field,” said Darrell S. Pardi, MD, MSc, AGAF, co–program chair for the AGA Future Leaders Program. “Along with my cochair, Sheryl Pfeil, MD, AGAF, and the esteemed mentors and faculty participating in this program, we look forward to cultivating these rising stars who stand out for their current achievements, commitment to advancing the field, and potential for future success.”
Class of 2020 Future Leaders
- Christen Klochan Dilly, MD, MEHP, Indiana University School of Medicine and Roudebush VA Medical Center
- Daniel Freedberg, MD, MS, Columbia University
- Wendy A. Henderson, PhD, National Institutes of Health
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Animesh Jain, MD, University of North Carolina at Chapel Hill
- Avinash Ketwaroo, MD, Baylor College of Medicine and Michael E. DeBakey VA Medical Center
- Bharati Kochar, MD, MSCR, University of North Carolina at Chapel Hill
- David Leiman, MD, MSHP, Duke University Medical Center
- James Lin, MD, City of Hope National Medical Center in Duarte
- Michelle Long, MD, Boston Medical Center
- Aimee Lucas, MD, MS, Icahn School of Medicine at Mount Sinai
- Miguel Malespin, MD, Tampa General Hospital
- Simon C. Mathews, MD, Johns Hopkins Medicine
- Karthik Ravi, MD, Mayo Clinic (Rochester, Minnesota)
- Florian Rieder, MD, Cleveland Clinic Foundation
- Kyle Staller, MD, MPH, Harvard Medical School
- Christina Twyman-Saint Victor, MD, University of Pennsylvania Perelman School of Medicine
- Ryan Ungaro, MD, MS, Icahn School of Medicine at Mount Sinai
View Future Leader Bios
The AGA Future Leaders Program will kick off with the AGA Leadership Development Conference March 8-10, 2019, at the Hilton Rockville Executive conference center in Rockville, Maryland, and will continue through Digestive Disease Week® (DDW) 2020 in Chicago, Illinois. Throughout the course of the program, participants will work closely with AGA mentors on projects linked to AGA’s Strategic Plan.
Learn more about the AGA Future Leaders Program.
Sessions at DDW® 2019 designed for fellows and early career GIs
AGA has an agenda of special sessions at Digestive Disease Week® (DDW) 2019 to meet the unique needs of physicians who are new to the field. Participants will learn about all aspects of starting a career in clinical practice or research, have the opportunity to network with mentors and peers, and review board material.
With the exception of the AGA Postgraduate Course, all of the sessions are free, but you must register for DDW to attend. Visit AGA University for a full list and additional details.
- AGA Postgraduate Course Saturday, May 18, and Sunday, May 19
- Introduction to GI Practice: A Trainee Boot Camp, Monday, May 20, 10-11:30 a.m.
- AGA Board Review CourseMonday, May 20, 1:30-5:30 p.m.
- Advancing Clinical Practice: GI Fellow-Directed Quality Improvement ProjectsMonday, May 20, 2-3:30 p.m.
- GI in the Digital Age, Monday, May 20, 4-5:30 p.m.
DDW Trainee and Early Career Lounge
Included with the cost of DDW registration, trainee and early career GI attendees have access to this lounge in the Sails Pavilion. It’s a great way to meet and network with peers from around the world over a cup of coffee and will feature new programming in 2019. Meet with experts to have your questions answered about practical issues of career choice, contracting, or how to write a manuscript.
Gastroenterology billing and coding: Just the basics
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Understanding the business side of medicine helps physicians run a successful practice. However, the business side of medicine is not part of the normal curriculum in training and fellowship programs. Physicians come out of training with the knowledge to treat patients but with little or no knowledge of how to get reimbursed for their services. Gastroenterologists provide both medical and surgical services.
Listed below are some of the basic principles for both documentation and reimbursement policies. All reimbursement is based upon Relative Value Units (RVUs) assigned to every service provided. The services are based upon three factors: physician work value, malpractice cost, and practice expense. Those factors added together and multiplied by a conversion factor assigned by the Centers for Medicare & Medicaid Services (CMS) creates the national physician fee schedule. Each Medicare carrier has localities, and there is another percentage that is multiplied based upon geographic location, which will finalize the approved amount for each service. Your Medicare carrier has the actual approved amounts available on their websites with an effective date of Jan. 1. Commercial payers most commonly base contracts on the Medicare Fee Schedule, but each practice and payer relationship is different. For a better understanding, please contact your practice manager for more specific information based on your payer contracts.
Medical necessity is the key to success. If medical necessity is not demonstrated, payers can deny a claim, deny authorization for a lab test and/or diagnostic study, or recoup previously paid claims. Medicare and commercial payers will often have local coverage determinations (LCDs) for procedures and testing that include indications and restrictions along with approved diagnosis codes. Listed below are the four primary services that GI providers perform and provide interpretation for:
1. Evaluation and management (E&M) services: There are three criteria that have to be met to support any initial visit with patients: the history obtained, the examination performed, and the development of the treatment plan. There are five levels of service for office visits and three levels for inpatient visits, respectively. The levels are chosen based on the decision-making element of the visit, provided the documentation requirements are met for the level chosen. This is often not an easy selection unless the providers are educated on the E&M criteria. Auditors often see that visits are chosen by “guessing” the level, which leads to choosing either a lower or higher level of service than what was actually provided. Some providers have been instructed that E&M services are not that important since procedures are the major source of revenue for the practice. However, GI practices are visit-driven practices, and the initial visits are often worth more RVUs than some procedures. The E&M visit is truly vital and often the backbone for the medical necessity of any additional procedures and diagnostic services required in order for successful treatment of the patient.
2. Endoscopy and procedural billing: Here, medical necessity must be documented in order to submit charges for what was done. Gastroenterologists will often use multiple techniques when treating different areas within the gastrointestinal tract. Documentation has to include the location of lesions/abnormalities, method of treatment/removal, and the reason(s)/indication(s) for those procedures. There may be different instruments used in the colon (for example, snare in the sigmoid colon or biopsy forceps in the transverse colon). These may be separately reported with an appropriate modifier to indicate that these services were performed to different lesions/abnormalities. However, in order to bill for each of the procedures, all of this has to be documented in the endoscopy report. The physician is responsible for accurate and specific documentation and bringing charges back to the billing staff for claim submission. For a successful practice, a team approach is vital. Physicians and coding staff need to have an open line of communication to make sure that everything is submitted appropriately according to payer policies. Billing staff need to communicate any significant changes to the physicians/providers as these changes occur. Ignorance of payer policy is not considered an appropriate excuse when a payer investigates a claim and potential recoupment of moneys paid.
3. Diagnostic studies: Medical necessity/indication for the testing must be documented in order to submit charges for diagnostic studies. The terms “rule out” and “suspect” don’t completely give coders the reason why a physician suspects the patient might have a condition. Usually, abnormal lab tests, signs, and symptoms will often warrant the need for further investigation, and these are the most crucial indications for testing. Not only is this important for diagnostic studies but also for procedures. Make sure that the interpretation of the test results is clear along with a plan/recommendation(s).
4. Diagnosis codes: Assignment of codes per the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) is the next and most important step after a visit, diagnostic study, and/or procedure. These codes support medical necessity for the services provided, and specificity of the diagnosis code is vital to successful submission and payment of a claim. Signs and symptoms are valid code choices when ruling out a more significant disease/diagnosis because these support medical necessity for a work-up to determine etiology. Comorbidities that impact the provider’s decision making should also be added as additional diagnoses to support the higher level of decision making. Up to 12 diagnosis codes can be assigned to any type of service provided. This also applies to preauthorization of all services, such as lab tests, radiology studies, GI diagnostic studies, and procedures. If specific information is not in the documentation for your staff to access, payers will often deny certain lab and radiology studies, as well as some procedures. There are 71,932 ICD-10-CM codes to choose from, and it is often difficult to find the “specific” code when doing a search in the electronic health record and billing system. Education and training are essential during the orientation sessions prior to active employment, as well as any time the system is upgraded. The providers should be willing to work with the IT representative(s) in the practice to help make the information easier to access. In other words, what “buzz” words would they like included in the description of the ICD-10-CM code in the practice’s list of favorites? For example, Crohn’s disease and ulcerative colitis have over a hundred choices. The choices are based on the location of the disease and whether the disease is without or with complications. If you are going to choose to provide a higher level of E&M service for a patient with Crohn’s disease of the large intestine because of exacerbation of the disease with bleeding, then the appropriate diagnosis code would be one of the following:
- K50.10 Crohn’s disease of large intestine without complications.
- K50.111 Crohn’s disease of large intestine with rectal bleeding.
- K50.112 Crohn’s disease of large intestine with intestinal obstruction.
- K50.113 Crohn’s disease of large intestine with fistula.
- K50.114 Crohn’s disease of large intestine with abscess.
- K50.118 Crohn’s disease of large intestine with other complication.
- K50.119 Crohn’s disease of large intestine with unspecified complications.
Getting paid for your provided services requires attention to detail and communication with your entire staffing team, including all providers. Make sure that your team is educated on all current issues and services pertaining to gastroenterology practices. If there is ever a question when reviewing a procedure note or any service, ask the provider who performed that service. Often, there will have to be legal corrections to the note before services can be billed. Making sure that the claim you are submitting is “clean” is essential for prompt payment. There are multiple resources available through the AGA that will help guide you with coding and billing. There are webinars, training sessions, and onsite services available via http://agau.gastro.org/diweb/catalog that can be provided for all providers, coding and billing staff, administrators, and clinical staff. Everyone needs to take an active role.
Ms. Mueller is a health care consultant with more than 35 years of experience in health care, including ICU/CCU nursing, physician office administration, GI claims submission and adjudication, and seminar instruction. She is president and owner of AskMueller Consulting in Lenzburg, Ill., which provides consulting services for physicians nationwide. Ms. Mueller is a nationally known speaker and the author of many multispecialty medical and surgical coding workbooks. She has a great amount of experience in gastroenterology, surgical subspecialties, and pediatric subspecialties. Her presentations have had audiences with the American Gastroenterological Association (AGA), North American Society for Pediatric Gastroenterology and Nutrition (NASPGAN), Society of Gastroenterology Nurses and Associates, Digestive Disease Week, American Pediatric Surgical Associations, and Decision Health and the Coding Institute. Ms. Mueller has written coding columns for ASGE, NASPGHAN, and AGA. She is the coeditor of the ASGE Coding Primer and also answers the coding hotline for the ASGE.
Better late than later: Lessons learned from an investigator-led clinical trial
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
As a second-year gastroenterology fellow, I designed a prospective, double-blind randomized, controlled trial for vitamin D repletion in patients with Crohn’s disease at a referral inflammatory bowel disease (IBD) center. I had the support of a dedicated research team, several mentors, and a 2-year time frame in which to complete this study. Intellectually curious and academically eager, I labored over a grant application that I did not receive. Under generous financial support from my department, I forged on and opened the trial for enrollment at the start of my advanced IBD fellowship year. However, we experienced recruitment challenges that ultimately led to the study’s premature termination. Through this journey, I gained invaluable experience that will continue to serve me – and, I hope, the reader – as I progress in my career. Below are some important insights gleaned from this experience that may benefit others interested in clinical trial design.
Know your “why” (personally and clinically)
Asked to reflect on their career path, experts tend to recount their “being in the right place at the right time” and having “good mentors.” While luck and good mentorship are necessary, I propose that doing your homework is equally as important. Before ambling down the path of an investigator-led trial, I urge a hard pause to reflect on your “why.” The personal “why” of “getting into fellowship,” “advancement in the department,” or “learning more about the principles of research,” are all valid. But I suggest a deeper dive is in order. Successful clinical trials require resources, a substantial time commitment, lots of sweat and maybe a few tears. In the ideal setting, your trial experience will serve as the foundation for a compelling personal narrative and might help launch a productive clinical research career. With stakes that high, asking the tough questions is critical.
The clinical “why” is just as critical. We press our attendings on why they used this drug or that clip, so don’t be afraid to ask whether this is a space in which others have succeeded. Or conversely, why is there such a large gap in the literature? I remember emailing the world’s expert about my topic because the published data were so murky. He had more questions than answers which, in retrospect, should have raised red flags about the ability to design a sound study. It is equally important to determine if patients are vested in the research question. A successful clinical trial hinges on subject participation, often outside their clinic visit. Patients with complex chronic diseases spend a lot of time navigating the health care system. Participating in a clinical trial needs to be meaningful to them if you want your patients to fully engage. Thoughtfully answering these questions on the front end – for yourself and the study in question – will improve both your experience and the ultimate outcome exponentially.
Identify your village (and listen to them)
Designing a clinical trial truly takes a village, and you need to identify the villagers early. As trainees, the value of mentorship is frequently underscored. But the importance of, and the nuance involved in managing, collaborating, and support cannot be overstated. Meet with a biostatistician to ensure your sample size calculations are correct. Work closely with the research pharmacist to ensure the medication formulation is available; decide on the manner of distribution so as not to inconvenience subjects; create a budget with an experienced manager. Seek out research coordinators often for assistance in creating case report forms, learning appropriate documentation, and crafting responses to Institutional Review Board concerns. Ask clinic personnel about arranging consent or follow-up visits around subjects’ clinic appointments. Present a draft of the protocol to your colleagues, as you will ultimately need them on board to recruit patients. And most importantly, listen to them ... all of them. Get your biases in check and write down all constructive criticism. Thoughtfully address each concern encountered to your satisfaction (and your mentor’s) and present to your village again. Rinse and repeat. Throw nothing under the rug, because if you do, it will eventually rear its head while you are in the throes of the study.
In retrospect, there were concerns raised by faculty and grant reviewers that I did not adequately address. First was the feasibility of screening, recruiting, and enrolling 80 patients during a busy clinical fellowship. While I took this criticism as a reflection of my personal commitment to the project, it was actually a call to consider the impact on clinical (and familial) responsibilities. But as I started enrolling patients, I realized there were logistic issues implied in this suggestion. I could not recruit subjects in Clinic A if I was assigned to see patients at the same time in Clinic B. Patients were not likely to come back another day for study-related discussions. Second, I designed eligibility criteria to make the data as clean as possible. Limiting the study to subjects with Crohn’s disease, in clinical and biochemical remission, without complications of their disease, who also have vitamin deficiency, may be an unrealistic recipe to recruit 80 people in a limited period of time. Finally, I designed laboratory follow-up schedules based on the pharmacokinetics of the drug alone, failing to consider the clinical milieu from which study subjects were recruited. Neglecting the fact that many patients obtain labs with their infusions, my study increased lab draw burden, heaped more patient reminders onto my plate – and more concerning – decreased overall study compliance. In short, trial design cannot be done in a vacuum or by just poring over published data. There are logistical and patient-related considerations that require early input from physicians and clinical staff in order for all the moving parts of a clinical trial to successfully work in harmony.
Create a timetable and follow it
Make a recruitment timetable very early on in the enrollment period. Set up biweekly meetings with mentors to discuss enrollment numbers and reflect on any unforeseen challenges. And be sure to celebrate the wins as well – not matter how small. In our study, falling behind on recruitment goals forced me to amend the stringent eligibility criteria and add additional manpower to help with reminders for laboratory follow-up and patient screening. These pivots caused study delays and cost resources. Ultimately, having a timetable forced me to take pause when it became clear I could not finish the study in the allotted time.
Know when to fold ’em
Knowing when to close a study is far easier said than done. The sunk cost fallacy says it is much harder to abandon a project after investing so many resources into it. For us, it was the recruitment timetable that gave us pause. Finishing trial accrual by the end of my advanced fellowship year was wholly unfeasible. When it became clear that nobody in the department could see it through to completion, I was propelled to terminate the study. If there is concern about termination, I suggest sending the protocol, recruitment numbers, and timeline to an outside colleague for a second, unbiased opinion. Review the already compiled data for any notable findings worthy of a smaller publication. It is said, we often learn more from our failures than our successes. The experience described herein – largely in part to my mentors, collaborators, and the patients who put their faith in me – translates to a lasting, invaluable win.
Mentor’s note
Clinical research is hard. Many trainees meet with me to “get involved” in clinical research, and the challenge as a mentor is to identify a project appropriate to the level of training and provide the infrastructure and resources to facilitate success for the motivated trainee. Trainees have various goals of their involvement in research – to foster a relationship in the hopes of receiving a strong letter of support, to facilitate getting into a competitive training program, and/or to publish. My goal as a mentor is to help my trainees reach their goals, but as a clinical researcher, I look for the trainee’s desire to engage with and learn the research process, with the ancillary potential for a letter, for acceptance to a program, or for publication.
This particular study, a randomized, controlled trial of vitamin D in patients with Crohn’s disease, involved an enormous undertaking by a very motivated trainee who took the project from its inception; to putting a thoughtful grant proposal together; to developing a full clinical trial protocol with its ancillary regulatory documents; and obtaining institutional review board approval, statistician input, pharmacy support, and buy-in from faculty and ancillary staff stakeholders. The study ultimately failed because of low enrollment – patients did not want to participate (for reasons elucidated above) – not because of poor design or execution of the myriad components of a prospective clinical trial. Low enrollment has led to the failure of many otherwise excellent studies, including several in our field of IBD.1,2 As a mentor, it is rational to accept blame for the failure of a trainee project; how could I have better foreseen the outcome of this study? Could this have been prevented with more support, more oversight, or more “micromanagement,” to the potential detriment of fostering independence?
Ultimately, the value of clinical research to trainees is multifaceted. If the goal was a first-author publication with high clinical impact, this trial failed. But if the goal was to learn about the clinical trial process, this study was a resounding success. Ultimately, it behooves trainees and their mentors to engage in early, upfront conversations about research. What are the goals? What does success look like? What if the trial fails? By shifting the focus from the success of the project to the success of the mentorship and educational process, even failed projects are resounding successes, upon which future careers can be further developed.
References
1. Kan S et al. When subjects violate the research covenant: Lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508-10.
2. Dassopoulos T et al. Randomised clinical trial: Individualised vs.weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014 Jan;39(2):163-75.
Dr. Cohen is an inflammatory bowel disease fellow, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles. Dr. Melmed is director, inflammatory bowel disease clinical research and codirector, clinical inflammatory bowel disease, inflammatory bowel disease center, division of gastroenterology and hepatology, department of medicine, Cedars-Sinai Medical Center, Los Angeles.
A career in industry: Is it right for me?
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.
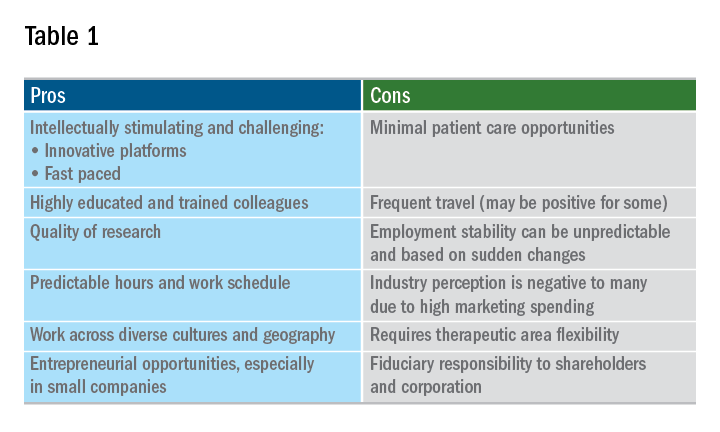
A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
How to create your specialized niche in a private practice
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
Let’s imagine you landed your first job in a private gastroenterology practice or are trying to find the perfect job that allows you to put your energy toward your passions. And, like many GI doctors, you spent additional time in your fellowship training focusing on a specific interest – whether inflammatory bowel disease, advanced endoscopy, motility, hepatology, or maybe the lesser-traveled paths of weight management, geriatrics, or public policy.
Perhaps you haven’t taken an extra year of training, but you have a desire to specialize. What steps should you take to create your own niche in a private practice? How do you go about growing a practice that allows you to utilize your training?
Why specialize? Know your market!
Without a focus, unless you plan to work in an underserved area or to take over a retiring physician’s practice, a generalist position can be challenging because the demand for your skills may not be met with the supply of patients. Much like in any business, the more focused you are, the more you have a differentiator that separates you from your colleagues, increasing your chances of success.
With specialization, however, comes the importance of understanding your patient catchment area. If your focus is highly specialized and serves a less-diagnosed entity, you’ll need a larger catchment area or you won’t have the volume of patients. Also, be mindful about an oversupply of subspecialists in your given area. If you are the third or fourth subspecialist in your group, the only way you will get patients is if you are far superior in talent or personality (sorry – not typical!) or your more senior colleagues are looking to turn over work to you.
Economic considerations for subspecialties
Compensation for subspecializing is often a major factor. Understanding the economics of your specialty are important, as providers can become disappointed and disenchanted when they realize that their desire for income, especially when compared to other colleagues, differs from what their subspecialty can provide.
For instance, in GI, a physician pursuing a procedurally focused subspecialty like advanced endoscopy is likely to be compensated more highly than one who focuses on a more office-based, evaluation and management (E/M) billing-driven specialty, like motility, geriatric gastroenterology, or even hepatology. These office-based specialties are no less important, but the reality is that they create less revenue for a private practice.
Negotiating a fair contract at the beginning is critical, as you may need your income to be supplemented by your higher revenue-producing colleagues and partners for long-term success. Academic centers are often able to provide the supplement through endowments, grants, or better payer reimbursement for E/M codes, compared with a private practice.
Remember, everyone’s in sales
From my vantage point as a partner at Atlanta Gastroenterology Associates, there are several ways new GI physicians can set about a path toward specialization.
One of the first things that you should ask yourself during training is whether you want to spend another year beyond the typical 3 years. Best-case scenario would be figuring out a way to get the necessary training during the 3 years, possibly spending the third year dedicated to the specialty. Another possibility is to simply get on-the-job training during your first few years in practice without the extra year.
Whichever path you choose, building up a specialized niche within a private practice won’t come overnight. You have to create a plan and navigate a course. Here are a few ways to do that:
Take the case, especially the hard ones!
Have the mentality: “I will take care of it.” One of the best ways to specialize is to offer to help with all cases, but especially the most challenging ones. Be open to helping take on any patient. In the beginning, if you develop a reputation that you enjoy caring for all patients, even when the case requires more time and effort, this will translate into future referrals. Naturally, it may be slower in the beginning, as there may not be enough patients to treat within your specialty. Being willing to do everything will expedite the growth of your practice. No consult should be rebuffed, even when it appears unnecessary (i.e., heme-positive stool in an elderly, septic ICU patient – we all have gotten them); think of it as your opportunity to show off your skills and share your interests.
Market yourself.
This is perhaps one of the most important steps you can take. Get out in the community! This includes:
- Attend your hospital grand rounds and offer to be a presenter. There is no better way to show your enthusiasm and knowledge on a topic than to teach it. Many state GI societies have meetings, which provide opportunities to introduce yourself to physicians in other practices that can act as a good referral source if you are a local expert.
- Remember, as a subspecialist, always communicate back with the primary gastroenterologist. In doing so, feel out whether the referring doctor wants you to take over the patient’s management or send the patient back.
- Reach out to foundations, pharmaceutical companies, and advocacy groups in the area. Understand each specialty has an ecosystem beyond just a doctor-patient relationship. Participating in events that support the patient outside of the office will provide goodwill. Further, many patients rely on foundations for referrals.
- Consider research studies. Many pharmaceutical companies have the opportunity for you to register patients in investigational drug studies. By being a part of these studies, you will be included in publications, which will build your brand.
- Many disease processes need a multidisciplinary approach to treating them. Attending multidisciplinary conferences will allow you to lend your expertise. Also, presenting interesting cases and asking for help from more experienced physicians will show humility and leads to more referrals; it won’t be viewed as a weakness.
- Be creative. Develop relationships with providers who are not often considered to be a primary referral source. Motility experts may want to work closely with the local speech pathologists. An IBD specialist should develop a network of specialists for patients with extraintestinal manifestations. Advanced endoscopists and oncologists work closely together.
- Get involved in social media. Engage with other specialists and become part of the online community. Follow the subspecialty organizations or key thought leaders in your space on Twitter, Facebook, and LinkedIn. You should share relevant articles or interesting cases.
There are so many aspects of gastroenterology that present great opportunities to specialize. Following your passions will lead to long-term happiness and prevent burnout. Remember that, even once you’ve built your practice, you must continue to stay involved and nurture what you’ve built. Go to the conferences. Make connections. Continue your education. Your career will thank you.
Dr. Sonenshine joined Atlanta Gastroenterology Associates in 2012. An Atlanta native, he graduated magna cum laude from the University of Georgia in Athens where he received a bachelor’s degree in microbiology and was selected to the Phi Beta Kappa Academic Honor Society. He received his medical degree from the Medical College of Georgia in Augusta, where he was named to the Alpha Omega Alpha Medical Honor Society. He completed both his internship and residency through the Osler Housestaff Training Program at Johns Hopkins Hospital in Baltimore. Following his residency, Dr. Sonenshine completed a fellowship in digestive diseases at Emory University in Atlanta while earning a master of business administration degree from the Terry College of Business at the University of Georgia. He is a partner in United Digestive and the chairman of medicine at Northside Hospital.
The New Gastroenterologist seeks its next editor-in-chief
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].