User login
Acute pancreatitis, dealing with difficult people, and more
I’m very excited about the first issue of The New Gastroenterologist in 2019, which has some fantastic articles that I hope you will find interesting and useful. The In Focus feature this month covers acute pancreatitis, which is an incredibly important topic for all in our field. Amar Mandalia and Matthew DiMagno (University of Michigan) provide a comprehensive overview of the management of acute pancreatitis, including a review of the recent AGA guideline on this topic. This article can be found online, as well as in print in the February issue of GI & Hepatology News.
Rhonda Cole (Michael E. DeBakey VAMC/Baylor) addresses the important topic of how to deal with difficult people, and she provides some useful tips for situations that many of us struggle with. Also in this issue, Rishi Naik (Vanderbilt) and current Associate Editor of Gastroenterology John Inadomi (University of Washington) provide some tips on how to write an effective cover letter for a journal submission. Anna Duloy and Sachin Wani (University of Colorado) provide an overview of the current state of training in advanced endoscopy, which will be very helpful for all those considering a fellowship or incorporation of these procedures into their practices.
For those looking to pick the right private practice position, David Ramsay (Digestive Health Specialists, Winston-Salem, N.C.) provides some useful tips to help you find the job that will be the best fit. In prior issues of The New Gastroenterologist, there have been several articles discussing saving for retirement, but how about how to effectively save for your children’s education? To address that topic, Michael Clancy (Drexel) provides an informative overview of 529 college savings accounts.
Finally, Gyanprakash Ketwaroo (Baylor), Peter Liang (NYU Langone), Carol Brown, and Celena NuQuay (AGA) provide an overview of one of the most important and impactful initiatives from the AGA for the early career community – the AGA Regional Practice Skills Workshops. These workshops are a tremendous resource for early career GIs, and I would recommend that you check one out if you have not already had the opportunity.
If you’re interested in browsing older articles from The New Gastroenterologist, articles from previous issues can be found on our webpage. Also, we are always looking for new ideas and new contributors. If you have suggestions or are interested, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected]
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
I’m very excited about the first issue of The New Gastroenterologist in 2019, which has some fantastic articles that I hope you will find interesting and useful. The In Focus feature this month covers acute pancreatitis, which is an incredibly important topic for all in our field. Amar Mandalia and Matthew DiMagno (University of Michigan) provide a comprehensive overview of the management of acute pancreatitis, including a review of the recent AGA guideline on this topic. This article can be found online, as well as in print in the February issue of GI & Hepatology News.
Rhonda Cole (Michael E. DeBakey VAMC/Baylor) addresses the important topic of how to deal with difficult people, and she provides some useful tips for situations that many of us struggle with. Also in this issue, Rishi Naik (Vanderbilt) and current Associate Editor of Gastroenterology John Inadomi (University of Washington) provide some tips on how to write an effective cover letter for a journal submission. Anna Duloy and Sachin Wani (University of Colorado) provide an overview of the current state of training in advanced endoscopy, which will be very helpful for all those considering a fellowship or incorporation of these procedures into their practices.
For those looking to pick the right private practice position, David Ramsay (Digestive Health Specialists, Winston-Salem, N.C.) provides some useful tips to help you find the job that will be the best fit. In prior issues of The New Gastroenterologist, there have been several articles discussing saving for retirement, but how about how to effectively save for your children’s education? To address that topic, Michael Clancy (Drexel) provides an informative overview of 529 college savings accounts.
Finally, Gyanprakash Ketwaroo (Baylor), Peter Liang (NYU Langone), Carol Brown, and Celena NuQuay (AGA) provide an overview of one of the most important and impactful initiatives from the AGA for the early career community – the AGA Regional Practice Skills Workshops. These workshops are a tremendous resource for early career GIs, and I would recommend that you check one out if you have not already had the opportunity.
If you’re interested in browsing older articles from The New Gastroenterologist, articles from previous issues can be found on our webpage. Also, we are always looking for new ideas and new contributors. If you have suggestions or are interested, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected]
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
I’m very excited about the first issue of The New Gastroenterologist in 2019, which has some fantastic articles that I hope you will find interesting and useful. The In Focus feature this month covers acute pancreatitis, which is an incredibly important topic for all in our field. Amar Mandalia and Matthew DiMagno (University of Michigan) provide a comprehensive overview of the management of acute pancreatitis, including a review of the recent AGA guideline on this topic. This article can be found online, as well as in print in the February issue of GI & Hepatology News.
Rhonda Cole (Michael E. DeBakey VAMC/Baylor) addresses the important topic of how to deal with difficult people, and she provides some useful tips for situations that many of us struggle with. Also in this issue, Rishi Naik (Vanderbilt) and current Associate Editor of Gastroenterology John Inadomi (University of Washington) provide some tips on how to write an effective cover letter for a journal submission. Anna Duloy and Sachin Wani (University of Colorado) provide an overview of the current state of training in advanced endoscopy, which will be very helpful for all those considering a fellowship or incorporation of these procedures into their practices.
For those looking to pick the right private practice position, David Ramsay (Digestive Health Specialists, Winston-Salem, N.C.) provides some useful tips to help you find the job that will be the best fit. In prior issues of The New Gastroenterologist, there have been several articles discussing saving for retirement, but how about how to effectively save for your children’s education? To address that topic, Michael Clancy (Drexel) provides an informative overview of 529 college savings accounts.
Finally, Gyanprakash Ketwaroo (Baylor), Peter Liang (NYU Langone), Carol Brown, and Celena NuQuay (AGA) provide an overview of one of the most important and impactful initiatives from the AGA for the early career community – the AGA Regional Practice Skills Workshops. These workshops are a tremendous resource for early career GIs, and I would recommend that you check one out if you have not already had the opportunity.
If you’re interested in browsing older articles from The New Gastroenterologist, articles from previous issues can be found on our webpage. Also, we are always looking for new ideas and new contributors. If you have suggestions or are interested, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected]
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
New concepts in the management of acute pancreatitis
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis
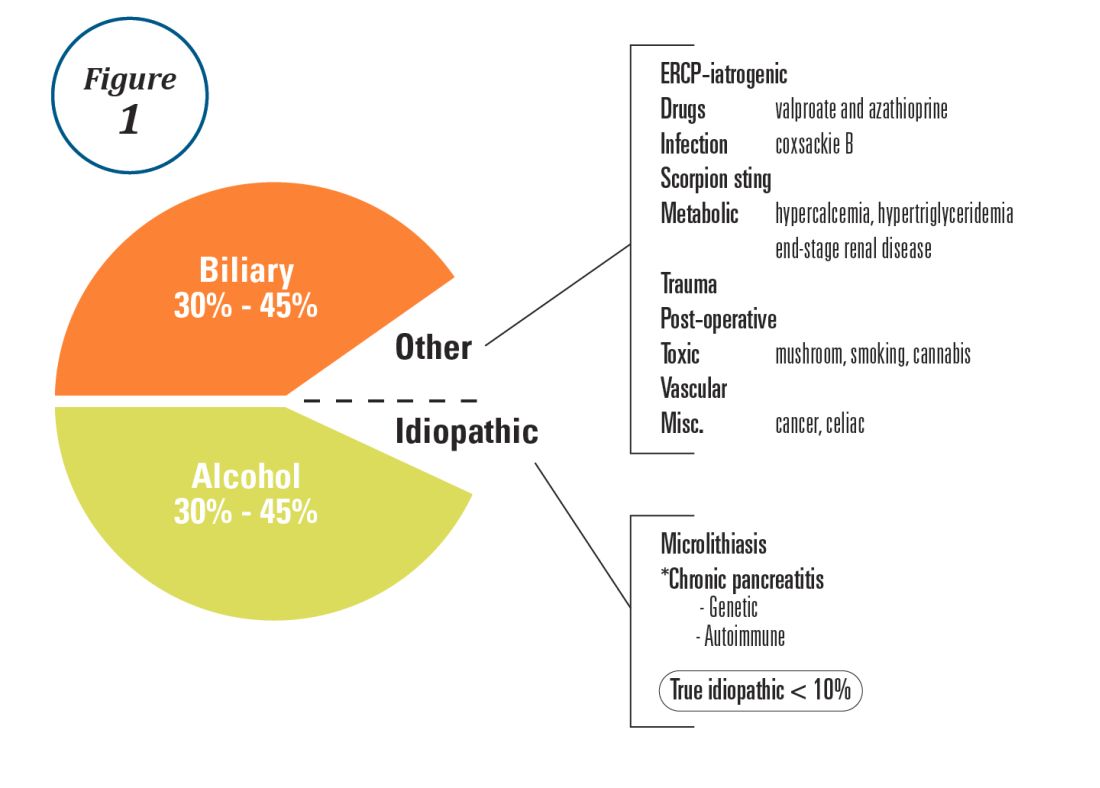
Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4
Recent advances in early treatment of AP
Literature review and definitions
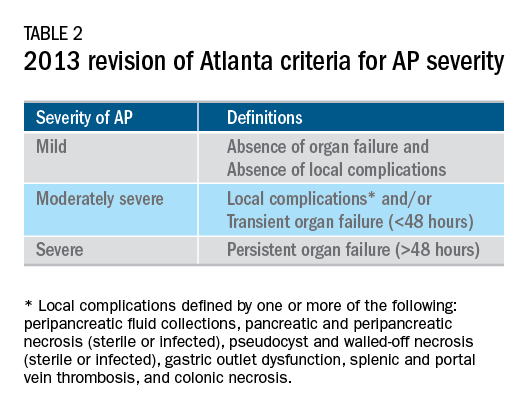
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration
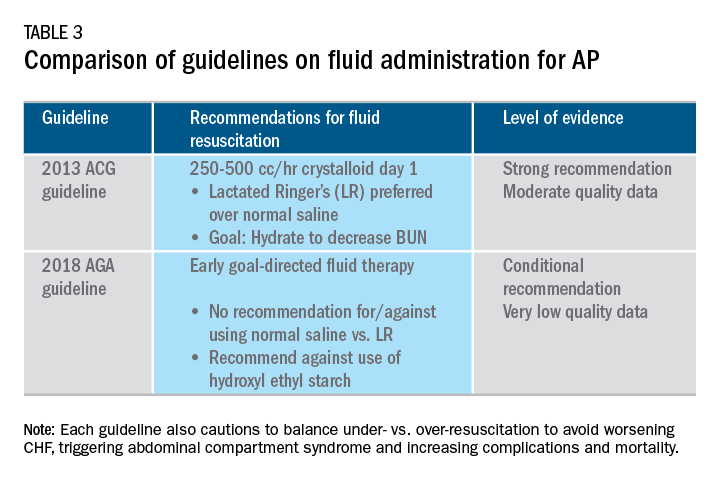
Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
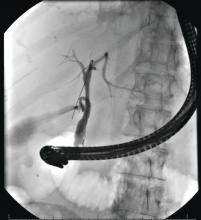
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Writing an effective cover letter
You have run your experiments, analyzed your data, and finished your manuscript, but now you are asked to write a cover letter for journal submission. How do you effectively convey your message in a cover letter? To understand how to gain the editors’ support for your paper, let us first discuss the role of this letter.

The cover letter is your first communication with the editors. As this serves as your first impression, you want to send a clear and concise message that highlights the novelty, validity, and significance of your manuscript. You also want to state why your manuscript would be a good fit for the journal. Keeping this letter concise and ideally to one page allows the editors to quickly review the highlights of your manuscript. Here are 10 steps we believe are important to follow when writing an effective cover letter (Table 1). We have listed examples to accompany each step; note that our examples are for illustrative purposes only.
1) Address the editor(s) formally by name in your cover letter
This information is found on the journal’s website and in the journal. Common mistakes we have seen is reporting the wrong editor(s) for submission or omitting an introduction. Though this cover letter is not published, it reflects poorly on the authors if there are incorrect editors listed or misspelled words.
Example:
Richard M. Peek, Jr., MD, and Douglas A. Corley, MD, PhD, MPH
Editors in Chief, Gastroenterology
Dear Drs. Peek and Corley:
2) Manuscript title and authors
Bolding your manuscript and listing the authors are essential parts of the cover letter.
Example:
We are submitting our original article entitled “Endoscopic and medical management of acute food impaction: A double-blinded randomized control trial” by Naik R and Inadomi J.
3) Methods
We recommend dedicating one sentence to your methods. Take this time to highlight key features if appropriate, such as novel techniques, prospective enrollment, and randomized, controlled studies, which are all generally viewed as stronger study designs than retrospective studies. Make sure you are truthful in your claims. Mistakes we have seen include making claims the group is the “first” or the “largest,” but our review of the literature disproves these statements. These are false claims and raise flags regarding the integrity of the science.
In this pragmatic, randomized, double-blind placebo-controlled study, our primary aim was to calculate the efficacy of endoscopic dilation and oral steroids to reduce recurrent dysphagia in individuals aged 20-49 years with eosinophilic esophagitis admitted with acute food impaction.
4) Results and key findings
We recommend dedicating a sentence to the key finding of this study. This allows the editors in chief to identify which associate editor should handle the manuscript and highlights the importance of the study, which will determine whether the associate editor will send your paper for external review.
Example:
We found that combined endoscopic dilation and oral steroid therapy at index food impaction improved quality of life and need for repeat dilation measured at 1-year follow-up, compared with sham dilation and placebo.
5) Novelty
The editors want to know the degree to which your paper is unique among other publications in the field. We recommend emphasizing the novelty of your study as compared with other published work. The focus of this should be how your manuscript adds to the literature and serves to advance the science in this field.
Example:
Our study is unique because we enrolled patients in a pragmatic fashion at time of admission for food impaction to the emergency department. This design allows implementation of our intervention in most clinical settings, where we expect our findings to translate broadly into reduced hospital admissions and repeat endoscopic interventions, as well as improved quality of life as documented by patient-reported outcomes.
6) Submission to other journals
Certain manuscripts are only part of the entire study, which can be because of interim results or prespecified secondary endpoints. It is important to state whether this manuscript or any part has been published elsewhere or parts of the study are in submission elsewhere.
Example:
Neither the entire paper nor any part of its content has been published or has been accepted elsewhere. This work has not been submitted to any other journal, and in case of acceptance of the manuscript, the copyright is transferred to Gastroenterology.
7) Journal fit
Not all journals have the same readership or focus. When submitting to the journals, please highlight why this manuscript fits this journal and its readership. When able, also recommend what section of the journal this manuscript should go to for review. This allows for expedited review process and shows you understand the journal’s readership and categories.
Example:
We believe our study will be of great interest to readers of Gastroenterology and suggest the manuscript section: “Clinical: Alimentary Tract.”
8) Influence of sponsors and conflicts of interest
Given the concern for potential conflict of interests in study design, data collection, or analysis, we recommend listing any important conflicts of interest or study sponsors. The entire list of conflicts of interest is typically included elsewhere, but a sponsored study or direct conflict should be listed to prevent any perceived influence from sponsors that may limit data integrity.
Example:
The study was supported by RDN-Gastro, a nonprofit private organization aimed to promote clinical and translational research in eosinophilic esophagitis. The RDN-Gastro organization receives support from the National Institutes of Health and the Emergency Department Eosinophilic Esophagitis Association.
9) Suggestions for editors and reviewers
It is helpful to suggest potential associate editors to handle your manuscript. Similarly, suggesting potential reviewers (and providing their email addresses) who are experts in the field and understand your research topic can enhance the quality of reviews. Soliciting reviewers who you know may seem like a recipe for friendly reviews; however, this is a flawed assumption because sometimes these reviewers are more rigorous in their comments.
Example:
Preferred Associate Editor: John M. Inadomi.
Preferred Reviewer: Rishi Naik ([email protected]); expertise in esophageal motility.
10) Do Not Review list (optional)
Unfortunately, not all review processes for all journals are created equal. For particular topics, you may wish to ensure your data are not compromised through the review process. Some journals will have the option for selecting reviewers who you prefer not review your manuscript and this request should be respected in the review process.
Example:
Nonpreferred reviewers: Rishi D. Naik (Nashville, TN).
In summary, the cover letter should be viewed as an opportunity to highlight the significance of your manuscript and reasons why your manuscript should be published in the journal. Highlighting the innovation, validity, and importance of your manuscript using this systematic approach will allow the editors and reviewers to more effectively evaluate your paper. Being truthful about potential conflicts of interest and sponsorships will also be appreciated by the editors. Good luck with your future submissions!
Dr. Naik is a gastroenterology fellow, department of gastroenterology, Vanderbilt University, Nashville, Tenn., as well as fellow editor of Gastroenterology. Dr. Inadomi is the Cyrus E. Rubin Chair, professor of medicine and head, division of gastroenterology, University of Washington, Seattle, as well as associate editor of Gastroenterology. Dr. Naik and Dr. Inadomi have no conflicts of interest to disclose.
You have run your experiments, analyzed your data, and finished your manuscript, but now you are asked to write a cover letter for journal submission. How do you effectively convey your message in a cover letter? To understand how to gain the editors’ support for your paper, let us first discuss the role of this letter.

The cover letter is your first communication with the editors. As this serves as your first impression, you want to send a clear and concise message that highlights the novelty, validity, and significance of your manuscript. You also want to state why your manuscript would be a good fit for the journal. Keeping this letter concise and ideally to one page allows the editors to quickly review the highlights of your manuscript. Here are 10 steps we believe are important to follow when writing an effective cover letter (Table 1). We have listed examples to accompany each step; note that our examples are for illustrative purposes only.
1) Address the editor(s) formally by name in your cover letter
This information is found on the journal’s website and in the journal. Common mistakes we have seen is reporting the wrong editor(s) for submission or omitting an introduction. Though this cover letter is not published, it reflects poorly on the authors if there are incorrect editors listed or misspelled words.
Example:
Richard M. Peek, Jr., MD, and Douglas A. Corley, MD, PhD, MPH
Editors in Chief, Gastroenterology
Dear Drs. Peek and Corley:
2) Manuscript title and authors
Bolding your manuscript and listing the authors are essential parts of the cover letter.
Example:
We are submitting our original article entitled “Endoscopic and medical management of acute food impaction: A double-blinded randomized control trial” by Naik R and Inadomi J.
3) Methods
We recommend dedicating one sentence to your methods. Take this time to highlight key features if appropriate, such as novel techniques, prospective enrollment, and randomized, controlled studies, which are all generally viewed as stronger study designs than retrospective studies. Make sure you are truthful in your claims. Mistakes we have seen include making claims the group is the “first” or the “largest,” but our review of the literature disproves these statements. These are false claims and raise flags regarding the integrity of the science.
In this pragmatic, randomized, double-blind placebo-controlled study, our primary aim was to calculate the efficacy of endoscopic dilation and oral steroids to reduce recurrent dysphagia in individuals aged 20-49 years with eosinophilic esophagitis admitted with acute food impaction.
4) Results and key findings
We recommend dedicating a sentence to the key finding of this study. This allows the editors in chief to identify which associate editor should handle the manuscript and highlights the importance of the study, which will determine whether the associate editor will send your paper for external review.
Example:
We found that combined endoscopic dilation and oral steroid therapy at index food impaction improved quality of life and need for repeat dilation measured at 1-year follow-up, compared with sham dilation and placebo.
5) Novelty
The editors want to know the degree to which your paper is unique among other publications in the field. We recommend emphasizing the novelty of your study as compared with other published work. The focus of this should be how your manuscript adds to the literature and serves to advance the science in this field.
Example:
Our study is unique because we enrolled patients in a pragmatic fashion at time of admission for food impaction to the emergency department. This design allows implementation of our intervention in most clinical settings, where we expect our findings to translate broadly into reduced hospital admissions and repeat endoscopic interventions, as well as improved quality of life as documented by patient-reported outcomes.
6) Submission to other journals
Certain manuscripts are only part of the entire study, which can be because of interim results or prespecified secondary endpoints. It is important to state whether this manuscript or any part has been published elsewhere or parts of the study are in submission elsewhere.
Example:
Neither the entire paper nor any part of its content has been published or has been accepted elsewhere. This work has not been submitted to any other journal, and in case of acceptance of the manuscript, the copyright is transferred to Gastroenterology.
7) Journal fit
Not all journals have the same readership or focus. When submitting to the journals, please highlight why this manuscript fits this journal and its readership. When able, also recommend what section of the journal this manuscript should go to for review. This allows for expedited review process and shows you understand the journal’s readership and categories.
Example:
We believe our study will be of great interest to readers of Gastroenterology and suggest the manuscript section: “Clinical: Alimentary Tract.”
8) Influence of sponsors and conflicts of interest
Given the concern for potential conflict of interests in study design, data collection, or analysis, we recommend listing any important conflicts of interest or study sponsors. The entire list of conflicts of interest is typically included elsewhere, but a sponsored study or direct conflict should be listed to prevent any perceived influence from sponsors that may limit data integrity.
Example:
The study was supported by RDN-Gastro, a nonprofit private organization aimed to promote clinical and translational research in eosinophilic esophagitis. The RDN-Gastro organization receives support from the National Institutes of Health and the Emergency Department Eosinophilic Esophagitis Association.
9) Suggestions for editors and reviewers
It is helpful to suggest potential associate editors to handle your manuscript. Similarly, suggesting potential reviewers (and providing their email addresses) who are experts in the field and understand your research topic can enhance the quality of reviews. Soliciting reviewers who you know may seem like a recipe for friendly reviews; however, this is a flawed assumption because sometimes these reviewers are more rigorous in their comments.
Example:
Preferred Associate Editor: John M. Inadomi.
Preferred Reviewer: Rishi Naik ([email protected]); expertise in esophageal motility.
10) Do Not Review list (optional)
Unfortunately, not all review processes for all journals are created equal. For particular topics, you may wish to ensure your data are not compromised through the review process. Some journals will have the option for selecting reviewers who you prefer not review your manuscript and this request should be respected in the review process.
Example:
Nonpreferred reviewers: Rishi D. Naik (Nashville, TN).
In summary, the cover letter should be viewed as an opportunity to highlight the significance of your manuscript and reasons why your manuscript should be published in the journal. Highlighting the innovation, validity, and importance of your manuscript using this systematic approach will allow the editors and reviewers to more effectively evaluate your paper. Being truthful about potential conflicts of interest and sponsorships will also be appreciated by the editors. Good luck with your future submissions!
Dr. Naik is a gastroenterology fellow, department of gastroenterology, Vanderbilt University, Nashville, Tenn., as well as fellow editor of Gastroenterology. Dr. Inadomi is the Cyrus E. Rubin Chair, professor of medicine and head, division of gastroenterology, University of Washington, Seattle, as well as associate editor of Gastroenterology. Dr. Naik and Dr. Inadomi have no conflicts of interest to disclose.
You have run your experiments, analyzed your data, and finished your manuscript, but now you are asked to write a cover letter for journal submission. How do you effectively convey your message in a cover letter? To understand how to gain the editors’ support for your paper, let us first discuss the role of this letter.

The cover letter is your first communication with the editors. As this serves as your first impression, you want to send a clear and concise message that highlights the novelty, validity, and significance of your manuscript. You also want to state why your manuscript would be a good fit for the journal. Keeping this letter concise and ideally to one page allows the editors to quickly review the highlights of your manuscript. Here are 10 steps we believe are important to follow when writing an effective cover letter (Table 1). We have listed examples to accompany each step; note that our examples are for illustrative purposes only.
1) Address the editor(s) formally by name in your cover letter
This information is found on the journal’s website and in the journal. Common mistakes we have seen is reporting the wrong editor(s) for submission or omitting an introduction. Though this cover letter is not published, it reflects poorly on the authors if there are incorrect editors listed or misspelled words.
Example:
Richard M. Peek, Jr., MD, and Douglas A. Corley, MD, PhD, MPH
Editors in Chief, Gastroenterology
Dear Drs. Peek and Corley:
2) Manuscript title and authors
Bolding your manuscript and listing the authors are essential parts of the cover letter.
Example:
We are submitting our original article entitled “Endoscopic and medical management of acute food impaction: A double-blinded randomized control trial” by Naik R and Inadomi J.
3) Methods
We recommend dedicating one sentence to your methods. Take this time to highlight key features if appropriate, such as novel techniques, prospective enrollment, and randomized, controlled studies, which are all generally viewed as stronger study designs than retrospective studies. Make sure you are truthful in your claims. Mistakes we have seen include making claims the group is the “first” or the “largest,” but our review of the literature disproves these statements. These are false claims and raise flags regarding the integrity of the science.
In this pragmatic, randomized, double-blind placebo-controlled study, our primary aim was to calculate the efficacy of endoscopic dilation and oral steroids to reduce recurrent dysphagia in individuals aged 20-49 years with eosinophilic esophagitis admitted with acute food impaction.
4) Results and key findings
We recommend dedicating a sentence to the key finding of this study. This allows the editors in chief to identify which associate editor should handle the manuscript and highlights the importance of the study, which will determine whether the associate editor will send your paper for external review.
Example:
We found that combined endoscopic dilation and oral steroid therapy at index food impaction improved quality of life and need for repeat dilation measured at 1-year follow-up, compared with sham dilation and placebo.
5) Novelty
The editors want to know the degree to which your paper is unique among other publications in the field. We recommend emphasizing the novelty of your study as compared with other published work. The focus of this should be how your manuscript adds to the literature and serves to advance the science in this field.
Example:
Our study is unique because we enrolled patients in a pragmatic fashion at time of admission for food impaction to the emergency department. This design allows implementation of our intervention in most clinical settings, where we expect our findings to translate broadly into reduced hospital admissions and repeat endoscopic interventions, as well as improved quality of life as documented by patient-reported outcomes.
6) Submission to other journals
Certain manuscripts are only part of the entire study, which can be because of interim results or prespecified secondary endpoints. It is important to state whether this manuscript or any part has been published elsewhere or parts of the study are in submission elsewhere.
Example:
Neither the entire paper nor any part of its content has been published or has been accepted elsewhere. This work has not been submitted to any other journal, and in case of acceptance of the manuscript, the copyright is transferred to Gastroenterology.
7) Journal fit
Not all journals have the same readership or focus. When submitting to the journals, please highlight why this manuscript fits this journal and its readership. When able, also recommend what section of the journal this manuscript should go to for review. This allows for expedited review process and shows you understand the journal’s readership and categories.
Example:
We believe our study will be of great interest to readers of Gastroenterology and suggest the manuscript section: “Clinical: Alimentary Tract.”
8) Influence of sponsors and conflicts of interest
Given the concern for potential conflict of interests in study design, data collection, or analysis, we recommend listing any important conflicts of interest or study sponsors. The entire list of conflicts of interest is typically included elsewhere, but a sponsored study or direct conflict should be listed to prevent any perceived influence from sponsors that may limit data integrity.
Example:
The study was supported by RDN-Gastro, a nonprofit private organization aimed to promote clinical and translational research in eosinophilic esophagitis. The RDN-Gastro organization receives support from the National Institutes of Health and the Emergency Department Eosinophilic Esophagitis Association.
9) Suggestions for editors and reviewers
It is helpful to suggest potential associate editors to handle your manuscript. Similarly, suggesting potential reviewers (and providing their email addresses) who are experts in the field and understand your research topic can enhance the quality of reviews. Soliciting reviewers who you know may seem like a recipe for friendly reviews; however, this is a flawed assumption because sometimes these reviewers are more rigorous in their comments.
Example:
Preferred Associate Editor: John M. Inadomi.
Preferred Reviewer: Rishi Naik ([email protected]); expertise in esophageal motility.
10) Do Not Review list (optional)
Unfortunately, not all review processes for all journals are created equal. For particular topics, you may wish to ensure your data are not compromised through the review process. Some journals will have the option for selecting reviewers who you prefer not review your manuscript and this request should be respected in the review process.
Example:
Nonpreferred reviewers: Rishi D. Naik (Nashville, TN).
In summary, the cover letter should be viewed as an opportunity to highlight the significance of your manuscript and reasons why your manuscript should be published in the journal. Highlighting the innovation, validity, and importance of your manuscript using this systematic approach will allow the editors and reviewers to more effectively evaluate your paper. Being truthful about potential conflicts of interest and sponsorships will also be appreciated by the editors. Good luck with your future submissions!
Dr. Naik is a gastroenterology fellow, department of gastroenterology, Vanderbilt University, Nashville, Tenn., as well as fellow editor of Gastroenterology. Dr. Inadomi is the Cyrus E. Rubin Chair, professor of medicine and head, division of gastroenterology, University of Washington, Seattle, as well as associate editor of Gastroenterology. Dr. Naik and Dr. Inadomi have no conflicts of interest to disclose.
AGA News
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb. 16, 2019
AGA Regional Practice Skills Workshop – Ohio
The workshop provides fellows with insights on life in private practice, life in academia, and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy, and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Columbus, OH
Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Hartford, CT
Feb. 21, 2019
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Richmond, VA
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth, and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching, and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World SummitThe 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
March 30, 2019
AGA Regional Practice Skills Workshop – Massachusetts
The workshop provides fellows with insights on life in private practice, life in academia and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Boston, MA
April 10-12, 2019
2019 AGA Tech Summit
By bringing together and fostering collaboration among innovators, entrepreneurs, clinicians, MedTech companies,regulatory agency representatives and venture capitalists, the Tech Summit helps ensure a pipeline of GI innovation that ultimately will improve delivery of care and patient outcomes.
San Francisco, CA
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD, or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students, or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application Deadline: Dec. 16, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb. 16, 2019
AGA Regional Practice Skills Workshop – Ohio
The workshop provides fellows with insights on life in private practice, life in academia, and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy, and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Columbus, OH
Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Hartford, CT
Feb. 21, 2019
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Richmond, VA
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth, and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching, and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World SummitThe 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
March 30, 2019
AGA Regional Practice Skills Workshop – Massachusetts
The workshop provides fellows with insights on life in private practice, life in academia and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Boston, MA
April 10-12, 2019
2019 AGA Tech Summit
By bringing together and fostering collaboration among innovators, entrepreneurs, clinicians, MedTech companies,regulatory agency representatives and venture capitalists, the Tech Summit helps ensure a pipeline of GI innovation that ultimately will improve delivery of care and patient outcomes.
San Francisco, CA
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD, or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students, or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application Deadline: Dec. 16, 2019
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb. 16, 2019
AGA Regional Practice Skills Workshop – Ohio
The workshop provides fellows with insights on life in private practice, life in academia, and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy, and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Columbus, OH
Feb. 20-21, 2019
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Hartford, CT
Feb. 21, 2019
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Richmond, VA
March 8-9, 2019
2019 Women’s Leadership Conference
The conference is specifically designed for women looking to advance their careers, further professional goals, enhance personal growth, and effectively network.
Bethesda, MD
March 8-10, 2019
FORWARD Program
AGA’s Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) Program is a new initiative funded by NIH, supporting the career entry and development for underrepresented minority physician scientists in gastroenterology. The program provides concrete leadership and skill development that includes scientific manuscript and grant writing, research development, executive coaching, and more.
Bethesda, MD
March 8-10, 2019
Future Leaders Program
The Future Leaders Program provides a pathway within the organization to network, connect with mentors, develop leadership skills and learn about AGA’s governance and operations while advancing your career and supporting the profession.
Bethesda, MD
March 23–24, 2019
2019 Gut Microbiota for Health World SummitThe 2019 program will present the latest evidence on the interaction between diet, nutrition and the gut microbiome. Learn how diet and nutrition are being used in concert with traditional therapies to manage GI disorders.
Miami, FL
March 30, 2019
AGA Regional Practice Skills Workshop – Massachusetts
The workshop provides fellows with insights on life in private practice, life in academia and other career opportunities. Attendees will also gain practical knowledge on key topics such as contract negotiations, billing/coding, health care policy and other topics that can help to enhance their career. Work/life balance and financial management as an early career professional are also addressed.
Boston, MA
April 10-12, 2019
2019 AGA Tech Summit
By bringing together and fostering collaboration among innovators, entrepreneurs, clinicians, MedTech companies,regulatory agency representatives and venture capitalists, the Tech Summit helps ensure a pipeline of GI innovation that ultimately will improve delivery of care and patient outcomes.
San Francisco, CA
May 18-21, 2019
Digestive Disease Week (DDW)®
DDW® is the world’s leading educational forum for academicians, clinicians, researchers, students and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery and related fields. Whether you work in patient care, research, education or administration, the DDW program offers something for you. DDW is co-sponsored by AGA, AASLD, ASGE and SSAT.
San Diego, CA
AWARDS APPLICATION DEADLINES
AGA Fellow Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are MD, PhD, or equivalent fellows presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Moti L. & Kamla Rustgi International Travel Awards
This travel award provides two $750 prizes to recipients who are young basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA Student Abstract Award
This travel award provides nine $500 and one $1,000 prize to recipients who are high school, undergraduate, graduate, or medical students, or residents (residents up to year three postgraduate) presenting posters/oral sessions at Digestive Disease Week® (DDW).
Application Deadline: Feb. 15, 2019
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
This award provides $100,000 per year for three years (total $300,000) to early career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in gastric cancer research. Research involving precancerous lesions will be considered if relevance to gastric cancer is explicitly outlined.
Application Deadline: Dec. 16, 2019
February 2019 - ICYMI
Gastroenterology
How to approach a patient with refractory or recurrent benign esophageal stricture. Siersema PD. 2019 Jan;156(1):7-10. doi.org/10.1053/j.gastro.2018.11.040
How to approach a patient with ampullary lesion. Kandler J; Neuhaus H. 2018 Dec;155(6):1670-6. doi.org/10.1053/j.gastro.2018.11.010
How to promote the academic success of junior faculty physicians in gastroenterology. Shaheen NJ; Sandler RS. 2018 Nov;155(5):1293-7. doi.org/10.1053/j.gastro.2018.10.006
Clin Gastro Hepatol
Screening and surveillance of varices in patients with cirrhosis. Jakab SS; Garcia-Tsao G. 2019 Jan;17(1):26-9. doi.org/10.1016/j.cgh.2018.03.012
Common gastrostomy feeding tube complications and troubleshooting. Sealock RJ; Munot K. 2018 Dec;16(12):1864-9. doi.org/10.1016/j.cgh.2018.07.037
Endobariatrics: A primer. Storm AC; Abu Dayyeh BK; Topazian M. 2018 Nov;16(11):1701-4. doi.org/10.1016/j.cgh.2018.03.009
AGA Perspectives
My experiences during AGA’s Advocacy Day: Facilitating change
Published on 12/05/2018 by Yamini Natarajan, MD.
Lessons learned from the AGA Future Leaders Program
Published on 12/05/2018 by Jennifer Weiss, MD, MS, AGAF.
Gastroenterology
How to approach a patient with refractory or recurrent benign esophageal stricture. Siersema PD. 2019 Jan;156(1):7-10. doi.org/10.1053/j.gastro.2018.11.040
How to approach a patient with ampullary lesion. Kandler J; Neuhaus H. 2018 Dec;155(6):1670-6. doi.org/10.1053/j.gastro.2018.11.010
How to promote the academic success of junior faculty physicians in gastroenterology. Shaheen NJ; Sandler RS. 2018 Nov;155(5):1293-7. doi.org/10.1053/j.gastro.2018.10.006
Clin Gastro Hepatol
Screening and surveillance of varices in patients with cirrhosis. Jakab SS; Garcia-Tsao G. 2019 Jan;17(1):26-9. doi.org/10.1016/j.cgh.2018.03.012
Common gastrostomy feeding tube complications and troubleshooting. Sealock RJ; Munot K. 2018 Dec;16(12):1864-9. doi.org/10.1016/j.cgh.2018.07.037
Endobariatrics: A primer. Storm AC; Abu Dayyeh BK; Topazian M. 2018 Nov;16(11):1701-4. doi.org/10.1016/j.cgh.2018.03.009
AGA Perspectives
My experiences during AGA’s Advocacy Day: Facilitating change
Published on 12/05/2018 by Yamini Natarajan, MD.
Lessons learned from the AGA Future Leaders Program
Published on 12/05/2018 by Jennifer Weiss, MD, MS, AGAF.
Gastroenterology
How to approach a patient with refractory or recurrent benign esophageal stricture. Siersema PD. 2019 Jan;156(1):7-10. doi.org/10.1053/j.gastro.2018.11.040
How to approach a patient with ampullary lesion. Kandler J; Neuhaus H. 2018 Dec;155(6):1670-6. doi.org/10.1053/j.gastro.2018.11.010
How to promote the academic success of junior faculty physicians in gastroenterology. Shaheen NJ; Sandler RS. 2018 Nov;155(5):1293-7. doi.org/10.1053/j.gastro.2018.10.006
Clin Gastro Hepatol
Screening and surveillance of varices in patients with cirrhosis. Jakab SS; Garcia-Tsao G. 2019 Jan;17(1):26-9. doi.org/10.1016/j.cgh.2018.03.012
Common gastrostomy feeding tube complications and troubleshooting. Sealock RJ; Munot K. 2018 Dec;16(12):1864-9. doi.org/10.1016/j.cgh.2018.07.037
Endobariatrics: A primer. Storm AC; Abu Dayyeh BK; Topazian M. 2018 Nov;16(11):1701-4. doi.org/10.1016/j.cgh.2018.03.009
AGA Perspectives
My experiences during AGA’s Advocacy Day: Facilitating change
Published on 12/05/2018 by Yamini Natarajan, MD.
Lessons learned from the AGA Future Leaders Program
Published on 12/05/2018 by Jennifer Weiss, MD, MS, AGAF.
Dealing with difficult people
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
AGA Regional Practice Skills Workshops
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
Finding your first job: Tips for picking the right practice
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.
Advanced endoscopy training in the United States
Introduction
Comprehensive training in endoscopic retrograde cholangioscopy (ERCP) and endoscopic ultrasound (EUS) is difficult to achieve within the curriculum of a standard 3-year Accreditation Council for Graduate Medical Education (ACGME)–accredited gastroenterology fellowship. ERCP and EUS are technically challenging, operator-dependent procedures that require specialized cognitive, technical, and integrative skills.1-4 A survey of physicians performing ERCP found that only 60% felt “very comfortable” performing the procedure after completion of a standard gastroenterology fellowship.5 Procedural volumes in ERCP and EUS tend to be low among general gastroenterology fellows; in a survey, only 9% and 4.5% of trainees in standard gastrointestinal fellowships had anticipated volumes of more than 200 ERCP and EUS procedures, respectively.6 The unique skills required to safely and effectively perform ERCP and EUS, along with the growing portfolio of therapeutic procedures such as endoscopic mucosal resection (EMR), endoluminal stent placement, deep enteroscopy, advanced closure techniques, bariatric endoscopy, therapeutic EUS, and submucosal endoscopy (including endoscopic submucosal dissection and peroral endoscopic myotomy), has led to the development of dedicated postgraduate advanced endoscopy training programs.7-9
Status of advanced endoscopy training in the United States
Advanced endoscopy fellowships are typically year-long training programs completed at tertiary care centers. Over the last 2 decades, there has been a dramatic increase in the number of advanced endoscopy training positions.9 In 2012, the American Society for Gastrointestinal Endoscopy established a match program to standardize the application process (www.asgematch.com).10 Since its inception, there have been approximately 100 applicants per year and 60 participating programs. In the 2018 match, there were 90 advanced endoscopy applicants for 69 positions. Each year, about 20% of graduating gastroenterology fellows apply for advanced endoscopy fellowship, and applicant match rates are approximately 60%.
The goal of advanced endoscopy fellowship is to teach trainees to safely and effectively perform high-risk endoscopic procedures.1,11,12 Without ACGME oversight, no defined curricular requirements exist, and programs can be quite variable. Stronger programs offer close mentorship, conferences, comprehensive didactics, research support, and regular feedback. All programs participating in this year’s match offered training in both ERCP and EUS with most offering training in EMR, ablation, and deep enteroscopy.10 Many programs also offered training in endoluminal stenting and advanced closure techniques, such as suturing. More than half offered training in endoscopic submucosal dissection, peroral endoscopic myotomy, and bariatric endoscopy, but trainee hands-on time is usually limited, and competence is not guaranteed. A recent, large, multicenter, prospective study found that the median number of ERCPs and EUSs performed by trainees during advancing endoscopy training was 350 (range 125-500) and 300 (range 155-650), respectively.2 Median number of ERCPs performed in patients with native papilla was 51 (range 32-79). Most ERCPs were performed for biliary indications, and most EUSs were performed for pancreaticobiliary indications. The study found that most advanced endoscopy trainees have limited exposure to interventional EUS procedures, ERCPs for pancreatic indications, and ERCPs requiring advanced cannulation techniques.
Competency assessment
Advanced endoscopy fellowship programs must ensure trainees have achieved technical and cognitive competence and are safe for independent practice. Methods to assess trainee competence in advanced procedures have changed significantly over the last several years.1 Historically, endoscopic training was based on an apprenticeship model. Procedural volume and subjective assessments from trainers were used as surrogates for competence. Most current societal guidelines now recommend competency thresholds – a minimum number of supervised procedures that a trainee should complete before competency can be assessed – instead of absolute procedure volume requirements.4,13,14 The ASGE recommends that at least 200 supervised independent ERCPs, including 80 independent sphincterotomies and 60 biliary stent placements, should be performed before assessing competence.4 Similarly, 225 supervised independent EUS cases are recommended before assessing competence. Importantly, these guidelines are not validated and do not account for the inherent variability in which different trainees acquire endoscopic skills.15-18
Because of the limitations of volume-based assessments of competence, a greater emphasis has been placed on developing comprehensive, standardized competency assessments. With the ACGME’s adoption of the Next Accreditation System (NAS), a greater emphasis has been placed on competency-based medical education throughout the United States. The goal of the Next Accreditation System is to ensure that specific milestones are achieved by trainees and that trainee progress is clearly reported. Similarly, within advanced endoscopic training, it is now accepted that a minimum procedural volume is a necessary, but insufficient, marker of competence.1 Therefore, recent work has focused on defining milestones, developing assessment tools with strong validity, establishing trainee learning curves, and providing trainees with continuous feedback that allows for targeted improvement. Although the data are limited, a few studies have assessed learning curves among trainees. A prospective study of 15 trainees from the Netherlands found that trainees acquire competence in ERCP skills at variable rates; specifically, trainees achieved competence in native papilla cannulation later than other ERCP skills.18 Similarly, a recent prospective multicenter study of advanced endoscopy trainees using a standardized assessment tool and cumulative sum analysis found significant variability in the learning curves for cognitive and technical aspects of ERCP.15
The EUS and ERCP Skills Assessment Tool (TEESAT) is a competence assessment tool for EUS and ERCP with strong validity evidence.2,15,19-21 The tool assesses several individual technical and cognitive skills, in addition to a global assessment of competence, and should be used in a continuous fashion throughout fellowship training. A prospective, multicenter study using the TEESAT showed substantial variability in EUS and ERCP learning curves among trainees and demonstrated the feasibility of creating a national, centralized database that allows for continuous monitoring and reporting of individualized learning curves for EUS and ERCP among advanced endoscopy trainees.2 Such a database is an important step in evolving with the ACGME/NAS reporting requirement and would allow for fellowship program directors and trainers to identify specific trainee deficiencies in order to deliver targeted remediation.
The impact of individualized feedback on trainee learning curves and EUS and ERCP quality indicators was addressed in a recently published prospective multicenter cohort study.22 In phase 1 of the study, 24 advanced endoscopy trainees from 20 programs were assessed using the TEESAT and given quarterly feedback. By the end of training, 92% and 74% of fellows had achieved overall technical competence in EUS and ERCP, respectively. In phase 2, trainees were assessed in their first year of independent practice to determine whether participation in competency-based fellowship programs results in high-quality care in independent practice. The study found that most trainees met performance thresholds for quality indicators in EUS (94% diagnostic rate of adequate samples and 84% diagnostic yield of malignancy in pancreatic masses) and ERCP (95% overall cannulation rate). While competence could not be confirmed for all trainees after fellowship completion, most met quality indicator thresholds for EUS and ERCP during the first year of independent practice. These data provide construct validity evidence for TEESAT and the data collection and reporting system that provides periodic feedback using learning curves and ultimately affirm the effectiveness of current training programs.
Establishing minimal standards for training programs
Although the ASGE offers rudimentary metrics to characterize fellowships through the match program, a more comprehensive evaluation of advanced endoscopy training programs would be of value to potential trainees. It is in this context that we offered the minimum ERCP (~250 cases for Grade 1 ERCP and ~300 cases for Grade 2 ERCP) and EUS (~225 cases) volumes that should serve as a basis for a more rigorous assessment of advanced endoscopy training programs. We also recently proposed structure, process, and outcomes measures that should be defined along with associated benchmarks (Table 1). These quality metrics could then be utilized to guide trainees in the selection of a program.
Conclusion
Advanced endoscopy training is a critical first step to ensuring endoscopists have the procedural and cognitive skills necessary to safely and effectively perform these high-risk procedures. As the portfolio of new procedures grows longer and more complex, it will become even more important for training programs to establish a standardized curriculum, adopt universal competency assessment tools, and provide continuous and targeted feedback to their trainees.
References
1. Wani S et al. Gastrointest Endosc. 2018;87:1371-82.
2. Wani S et al. Clin Gastroenterol Hepatol. 2017;15:1758-67 e11.
3. Patel SG et al. Am J Gastroenterol. 2015;110:956-62.
4. Committee ASoP et al. Gastrointest Endosc. 2017;85:273-81.
5. Cote GA et al. Gastrointest Endosc. 2011;74:65-73 e12.
6. Cotton PB et al. Gastrointest Endosc 2017;86:866-9.
7. Moffatt DC et al. Gastrointest Endosc. 2014;79:615-22.
8. Training and Education Committee of the American Gastroenterological Association. Gastroenterology 1988;94:1083-6.
9. Elta GH et al. Gastroenterology 2015;148:488-90.
10. www.asgematch.com. (Accessed June 21, 2018)
11. Jowell PS et al. Ann Intern Med 1996;125:983-9.
12. Eisen GM et al. Gastrointest Endosc 2002;55:780-3.
13. Polkowski M et al. Endoscopy 2012;44:190-206.
14. Committee AT et al. Gastrointest Endosc 2016;83:279-89.
15. Wani S et al. Gastrointest Endosc 2016;83:711-9 e11.
16. Northup PG et al. Gastroenterology 2013;144:677-80.
17. Eisen GM et al. Gastrointest Endosc 2001;53:846-8.
18. Ekkelenkamp VE et al. Endoscopy 2014;46:949-55.
19. Wani S et al. Clin Gastroenterol Hepatol 2015;13:1318-25 e2.
20. Wani S et al. Gastrointest Endosc 2013;77:558-65.
Dr. Duloy is a therapeutic gastroenterology fellow; Dr. Wani is an associate professor of medicine, University of Colorado Anschutz Medical Campus, Aurora, Colo.
Introduction
Comprehensive training in endoscopic retrograde cholangioscopy (ERCP) and endoscopic ultrasound (EUS) is difficult to achieve within the curriculum of a standard 3-year Accreditation Council for Graduate Medical Education (ACGME)–accredited gastroenterology fellowship. ERCP and EUS are technically challenging, operator-dependent procedures that require specialized cognitive, technical, and integrative skills.1-4 A survey of physicians performing ERCP found that only 60% felt “very comfortable” performing the procedure after completion of a standard gastroenterology fellowship.5 Procedural volumes in ERCP and EUS tend to be low among general gastroenterology fellows; in a survey, only 9% and 4.5% of trainees in standard gastrointestinal fellowships had anticipated volumes of more than 200 ERCP and EUS procedures, respectively.6 The unique skills required to safely and effectively perform ERCP and EUS, along with the growing portfolio of therapeutic procedures such as endoscopic mucosal resection (EMR), endoluminal stent placement, deep enteroscopy, advanced closure techniques, bariatric endoscopy, therapeutic EUS, and submucosal endoscopy (including endoscopic submucosal dissection and peroral endoscopic myotomy), has led to the development of dedicated postgraduate advanced endoscopy training programs.7-9
Status of advanced endoscopy training in the United States
Advanced endoscopy fellowships are typically year-long training programs completed at tertiary care centers. Over the last 2 decades, there has been a dramatic increase in the number of advanced endoscopy training positions.9 In 2012, the American Society for Gastrointestinal Endoscopy established a match program to standardize the application process (www.asgematch.com).10 Since its inception, there have been approximately 100 applicants per year and 60 participating programs. In the 2018 match, there were 90 advanced endoscopy applicants for 69 positions. Each year, about 20% of graduating gastroenterology fellows apply for advanced endoscopy fellowship, and applicant match rates are approximately 60%.
The goal of advanced endoscopy fellowship is to teach trainees to safely and effectively perform high-risk endoscopic procedures.1,11,12 Without ACGME oversight, no defined curricular requirements exist, and programs can be quite variable. Stronger programs offer close mentorship, conferences, comprehensive didactics, research support, and regular feedback. All programs participating in this year’s match offered training in both ERCP and EUS with most offering training in EMR, ablation, and deep enteroscopy.10 Many programs also offered training in endoluminal stenting and advanced closure techniques, such as suturing. More than half offered training in endoscopic submucosal dissection, peroral endoscopic myotomy, and bariatric endoscopy, but trainee hands-on time is usually limited, and competence is not guaranteed. A recent, large, multicenter, prospective study found that the median number of ERCPs and EUSs performed by trainees during advancing endoscopy training was 350 (range 125-500) and 300 (range 155-650), respectively.2 Median number of ERCPs performed in patients with native papilla was 51 (range 32-79). Most ERCPs were performed for biliary indications, and most EUSs were performed for pancreaticobiliary indications. The study found that most advanced endoscopy trainees have limited exposure to interventional EUS procedures, ERCPs for pancreatic indications, and ERCPs requiring advanced cannulation techniques.
Competency assessment
Advanced endoscopy fellowship programs must ensure trainees have achieved technical and cognitive competence and are safe for independent practice. Methods to assess trainee competence in advanced procedures have changed significantly over the last several years.1 Historically, endoscopic training was based on an apprenticeship model. Procedural volume and subjective assessments from trainers were used as surrogates for competence. Most current societal guidelines now recommend competency thresholds – a minimum number of supervised procedures that a trainee should complete before competency can be assessed – instead of absolute procedure volume requirements.4,13,14 The ASGE recommends that at least 200 supervised independent ERCPs, including 80 independent sphincterotomies and 60 biliary stent placements, should be performed before assessing competence.4 Similarly, 225 supervised independent EUS cases are recommended before assessing competence. Importantly, these guidelines are not validated and do not account for the inherent variability in which different trainees acquire endoscopic skills.15-18
Because of the limitations of volume-based assessments of competence, a greater emphasis has been placed on developing comprehensive, standardized competency assessments. With the ACGME’s adoption of the Next Accreditation System (NAS), a greater emphasis has been placed on competency-based medical education throughout the United States. The goal of the Next Accreditation System is to ensure that specific milestones are achieved by trainees and that trainee progress is clearly reported. Similarly, within advanced endoscopic training, it is now accepted that a minimum procedural volume is a necessary, but insufficient, marker of competence.1 Therefore, recent work has focused on defining milestones, developing assessment tools with strong validity, establishing trainee learning curves, and providing trainees with continuous feedback that allows for targeted improvement. Although the data are limited, a few studies have assessed learning curves among trainees. A prospective study of 15 trainees from the Netherlands found that trainees acquire competence in ERCP skills at variable rates; specifically, trainees achieved competence in native papilla cannulation later than other ERCP skills.18 Similarly, a recent prospective multicenter study of advanced endoscopy trainees using a standardized assessment tool and cumulative sum analysis found significant variability in the learning curves for cognitive and technical aspects of ERCP.15
The EUS and ERCP Skills Assessment Tool (TEESAT) is a competence assessment tool for EUS and ERCP with strong validity evidence.2,15,19-21 The tool assesses several individual technical and cognitive skills, in addition to a global assessment of competence, and should be used in a continuous fashion throughout fellowship training. A prospective, multicenter study using the TEESAT showed substantial variability in EUS and ERCP learning curves among trainees and demonstrated the feasibility of creating a national, centralized database that allows for continuous monitoring and reporting of individualized learning curves for EUS and ERCP among advanced endoscopy trainees.2 Such a database is an important step in evolving with the ACGME/NAS reporting requirement and would allow for fellowship program directors and trainers to identify specific trainee deficiencies in order to deliver targeted remediation.
The impact of individualized feedback on trainee learning curves and EUS and ERCP quality indicators was addressed in a recently published prospective multicenter cohort study.22 In phase 1 of the study, 24 advanced endoscopy trainees from 20 programs were assessed using the TEESAT and given quarterly feedback. By the end of training, 92% and 74% of fellows had achieved overall technical competence in EUS and ERCP, respectively. In phase 2, trainees were assessed in their first year of independent practice to determine whether participation in competency-based fellowship programs results in high-quality care in independent practice. The study found that most trainees met performance thresholds for quality indicators in EUS (94% diagnostic rate of adequate samples and 84% diagnostic yield of malignancy in pancreatic masses) and ERCP (95% overall cannulation rate). While competence could not be confirmed for all trainees after fellowship completion, most met quality indicator thresholds for EUS and ERCP during the first year of independent practice. These data provide construct validity evidence for TEESAT and the data collection and reporting system that provides periodic feedback using learning curves and ultimately affirm the effectiveness of current training programs.
Establishing minimal standards for training programs
Although the ASGE offers rudimentary metrics to characterize fellowships through the match program, a more comprehensive evaluation of advanced endoscopy training programs would be of value to potential trainees. It is in this context that we offered the minimum ERCP (~250 cases for Grade 1 ERCP and ~300 cases for Grade 2 ERCP) and EUS (~225 cases) volumes that should serve as a basis for a more rigorous assessment of advanced endoscopy training programs. We also recently proposed structure, process, and outcomes measures that should be defined along with associated benchmarks (Table 1). These quality metrics could then be utilized to guide trainees in the selection of a program.

Conclusion
Advanced endoscopy training is a critical first step to ensuring endoscopists have the procedural and cognitive skills necessary to safely and effectively perform these high-risk procedures. As the portfolio of new procedures grows longer and more complex, it will become even more important for training programs to establish a standardized curriculum, adopt universal competency assessment tools, and provide continuous and targeted feedback to their trainees.
References
1. Wani S et al. Gastrointest Endosc. 2018;87:1371-82.
2. Wani S et al. Clin Gastroenterol Hepatol. 2017;15:1758-67 e11.
3. Patel SG et al. Am J Gastroenterol. 2015;110:956-62.
4. Committee ASoP et al. Gastrointest Endosc. 2017;85:273-81.
5. Cote GA et al. Gastrointest Endosc. 2011;74:65-73 e12.
6. Cotton PB et al. Gastrointest Endosc 2017;86:866-9.
7. Moffatt DC et al. Gastrointest Endosc. 2014;79:615-22.
8. Training and Education Committee of the American Gastroenterological Association. Gastroenterology 1988;94:1083-6.
9. Elta GH et al. Gastroenterology 2015;148:488-90.
10. www.asgematch.com. (Accessed June 21, 2018)
11. Jowell PS et al. Ann Intern Med 1996;125:983-9.
12. Eisen GM et al. Gastrointest Endosc 2002;55:780-3.
13. Polkowski M et al. Endoscopy 2012;44:190-206.
14. Committee AT et al. Gastrointest Endosc 2016;83:279-89.
15. Wani S et al. Gastrointest Endosc 2016;83:711-9 e11.
16. Northup PG et al. Gastroenterology 2013;144:677-80.
17. Eisen GM et al. Gastrointest Endosc 2001;53:846-8.
18. Ekkelenkamp VE et al. Endoscopy 2014;46:949-55.
19. Wani S et al. Clin Gastroenterol Hepatol 2015;13:1318-25 e2.
20. Wani S et al. Gastrointest Endosc 2013;77:558-65.
Dr. Duloy is a therapeutic gastroenterology fellow; Dr. Wani is an associate professor of medicine, University of Colorado Anschutz Medical Campus, Aurora, Colo.
Introduction
Comprehensive training in endoscopic retrograde cholangioscopy (ERCP) and endoscopic ultrasound (EUS) is difficult to achieve within the curriculum of a standard 3-year Accreditation Council for Graduate Medical Education (ACGME)–accredited gastroenterology fellowship. ERCP and EUS are technically challenging, operator-dependent procedures that require specialized cognitive, technical, and integrative skills.1-4 A survey of physicians performing ERCP found that only 60% felt “very comfortable” performing the procedure after completion of a standard gastroenterology fellowship.5 Procedural volumes in ERCP and EUS tend to be low among general gastroenterology fellows; in a survey, only 9% and 4.5% of trainees in standard gastrointestinal fellowships had anticipated volumes of more than 200 ERCP and EUS procedures, respectively.6 The unique skills required to safely and effectively perform ERCP and EUS, along with the growing portfolio of therapeutic procedures such as endoscopic mucosal resection (EMR), endoluminal stent placement, deep enteroscopy, advanced closure techniques, bariatric endoscopy, therapeutic EUS, and submucosal endoscopy (including endoscopic submucosal dissection and peroral endoscopic myotomy), has led to the development of dedicated postgraduate advanced endoscopy training programs.7-9
Status of advanced endoscopy training in the United States
Advanced endoscopy fellowships are typically year-long training programs completed at tertiary care centers. Over the last 2 decades, there has been a dramatic increase in the number of advanced endoscopy training positions.9 In 2012, the American Society for Gastrointestinal Endoscopy established a match program to standardize the application process (www.asgematch.com).10 Since its inception, there have been approximately 100 applicants per year and 60 participating programs. In the 2018 match, there were 90 advanced endoscopy applicants for 69 positions. Each year, about 20% of graduating gastroenterology fellows apply for advanced endoscopy fellowship, and applicant match rates are approximately 60%.
The goal of advanced endoscopy fellowship is to teach trainees to safely and effectively perform high-risk endoscopic procedures.1,11,12 Without ACGME oversight, no defined curricular requirements exist, and programs can be quite variable. Stronger programs offer close mentorship, conferences, comprehensive didactics, research support, and regular feedback. All programs participating in this year’s match offered training in both ERCP and EUS with most offering training in EMR, ablation, and deep enteroscopy.10 Many programs also offered training in endoluminal stenting and advanced closure techniques, such as suturing. More than half offered training in endoscopic submucosal dissection, peroral endoscopic myotomy, and bariatric endoscopy, but trainee hands-on time is usually limited, and competence is not guaranteed. A recent, large, multicenter, prospective study found that the median number of ERCPs and EUSs performed by trainees during advancing endoscopy training was 350 (range 125-500) and 300 (range 155-650), respectively.2 Median number of ERCPs performed in patients with native papilla was 51 (range 32-79). Most ERCPs were performed for biliary indications, and most EUSs were performed for pancreaticobiliary indications. The study found that most advanced endoscopy trainees have limited exposure to interventional EUS procedures, ERCPs for pancreatic indications, and ERCPs requiring advanced cannulation techniques.
Competency assessment
Advanced endoscopy fellowship programs must ensure trainees have achieved technical and cognitive competence and are safe for independent practice. Methods to assess trainee competence in advanced procedures have changed significantly over the last several years.1 Historically, endoscopic training was based on an apprenticeship model. Procedural volume and subjective assessments from trainers were used as surrogates for competence. Most current societal guidelines now recommend competency thresholds – a minimum number of supervised procedures that a trainee should complete before competency can be assessed – instead of absolute procedure volume requirements.4,13,14 The ASGE recommends that at least 200 supervised independent ERCPs, including 80 independent sphincterotomies and 60 biliary stent placements, should be performed before assessing competence.4 Similarly, 225 supervised independent EUS cases are recommended before assessing competence. Importantly, these guidelines are not validated and do not account for the inherent variability in which different trainees acquire endoscopic skills.15-18
Because of the limitations of volume-based assessments of competence, a greater emphasis has been placed on developing comprehensive, standardized competency assessments. With the ACGME’s adoption of the Next Accreditation System (NAS), a greater emphasis has been placed on competency-based medical education throughout the United States. The goal of the Next Accreditation System is to ensure that specific milestones are achieved by trainees and that trainee progress is clearly reported. Similarly, within advanced endoscopic training, it is now accepted that a minimum procedural volume is a necessary, but insufficient, marker of competence.1 Therefore, recent work has focused on defining milestones, developing assessment tools with strong validity, establishing trainee learning curves, and providing trainees with continuous feedback that allows for targeted improvement. Although the data are limited, a few studies have assessed learning curves among trainees. A prospective study of 15 trainees from the Netherlands found that trainees acquire competence in ERCP skills at variable rates; specifically, trainees achieved competence in native papilla cannulation later than other ERCP skills.18 Similarly, a recent prospective multicenter study of advanced endoscopy trainees using a standardized assessment tool and cumulative sum analysis found significant variability in the learning curves for cognitive and technical aspects of ERCP.15
The EUS and ERCP Skills Assessment Tool (TEESAT) is a competence assessment tool for EUS and ERCP with strong validity evidence.2,15,19-21 The tool assesses several individual technical and cognitive skills, in addition to a global assessment of competence, and should be used in a continuous fashion throughout fellowship training. A prospective, multicenter study using the TEESAT showed substantial variability in EUS and ERCP learning curves among trainees and demonstrated the feasibility of creating a national, centralized database that allows for continuous monitoring and reporting of individualized learning curves for EUS and ERCP among advanced endoscopy trainees.2 Such a database is an important step in evolving with the ACGME/NAS reporting requirement and would allow for fellowship program directors and trainers to identify specific trainee deficiencies in order to deliver targeted remediation.
The impact of individualized feedback on trainee learning curves and EUS and ERCP quality indicators was addressed in a recently published prospective multicenter cohort study.22 In phase 1 of the study, 24 advanced endoscopy trainees from 20 programs were assessed using the TEESAT and given quarterly feedback. By the end of training, 92% and 74% of fellows had achieved overall technical competence in EUS and ERCP, respectively. In phase 2, trainees were assessed in their first year of independent practice to determine whether participation in competency-based fellowship programs results in high-quality care in independent practice. The study found that most trainees met performance thresholds for quality indicators in EUS (94% diagnostic rate of adequate samples and 84% diagnostic yield of malignancy in pancreatic masses) and ERCP (95% overall cannulation rate). While competence could not be confirmed for all trainees after fellowship completion, most met quality indicator thresholds for EUS and ERCP during the first year of independent practice. These data provide construct validity evidence for TEESAT and the data collection and reporting system that provides periodic feedback using learning curves and ultimately affirm the effectiveness of current training programs.
Establishing minimal standards for training programs
Although the ASGE offers rudimentary metrics to characterize fellowships through the match program, a more comprehensive evaluation of advanced endoscopy training programs would be of value to potential trainees. It is in this context that we offered the minimum ERCP (~250 cases for Grade 1 ERCP and ~300 cases for Grade 2 ERCP) and EUS (~225 cases) volumes that should serve as a basis for a more rigorous assessment of advanced endoscopy training programs. We also recently proposed structure, process, and outcomes measures that should be defined along with associated benchmarks (Table 1). These quality metrics could then be utilized to guide trainees in the selection of a program.

Conclusion
Advanced endoscopy training is a critical first step to ensuring endoscopists have the procedural and cognitive skills necessary to safely and effectively perform these high-risk procedures. As the portfolio of new procedures grows longer and more complex, it will become even more important for training programs to establish a standardized curriculum, adopt universal competency assessment tools, and provide continuous and targeted feedback to their trainees.
References
1. Wani S et al. Gastrointest Endosc. 2018;87:1371-82.
2. Wani S et al. Clin Gastroenterol Hepatol. 2017;15:1758-67 e11.
3. Patel SG et al. Am J Gastroenterol. 2015;110:956-62.
4. Committee ASoP et al. Gastrointest Endosc. 2017;85:273-81.
5. Cote GA et al. Gastrointest Endosc. 2011;74:65-73 e12.
6. Cotton PB et al. Gastrointest Endosc 2017;86:866-9.
7. Moffatt DC et al. Gastrointest Endosc. 2014;79:615-22.
8. Training and Education Committee of the American Gastroenterological Association. Gastroenterology 1988;94:1083-6.
9. Elta GH et al. Gastroenterology 2015;148:488-90.
10. www.asgematch.com. (Accessed June 21, 2018)
11. Jowell PS et al. Ann Intern Med 1996;125:983-9.
12. Eisen GM et al. Gastrointest Endosc 2002;55:780-3.
13. Polkowski M et al. Endoscopy 2012;44:190-206.
14. Committee AT et al. Gastrointest Endosc 2016;83:279-89.
15. Wani S et al. Gastrointest Endosc 2016;83:711-9 e11.
16. Northup PG et al. Gastroenterology 2013;144:677-80.
17. Eisen GM et al. Gastrointest Endosc 2001;53:846-8.
18. Ekkelenkamp VE et al. Endoscopy 2014;46:949-55.
19. Wani S et al. Clin Gastroenterol Hepatol 2015;13:1318-25 e2.
20. Wani S et al. Gastrointest Endosc 2013;77:558-65.
Dr. Duloy is a therapeutic gastroenterology fellow; Dr. Wani is an associate professor of medicine, University of Colorado Anschutz Medical Campus, Aurora, Colo.