User login
Palliative care update highlights role of nonspecialists
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
ACR readies first-ever guidelines on managing reproductive health in rheumatology
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ACR ANNUAL MEETING
Moderate hypofractionation preferred in new guideline for localized PC
Moderate hypofractionation is preferred over conventional fractionation in treatment of patients with localized prostate cancer who are candidates for external beam radiotherapy (EBRT), according to new a clinical practice guideline.
A meta-analysis of randomized clinical trials showed that moderate fractionation delivered the same efficacy as did conventional fractionation with a mild increase in gastrointestinal toxicity, reported lead author Scott C. Morgan, MD of OSF Medical Group in Bloomington, Illinois, and his colleagues. The drawback of toxicity is outweighed by distinct advantages in resource utilization and patient convenience, which make moderate hypofractionation the winning choice.
For many types of cancer, a shift toward fewer fractions of higher radiation is ongoing, driven largely by technological advances in radiation planning and delivery.
“Technical advances have permitted more precise and conformal delivery of escalated doses of radiation to the prostate, thereby improving the therapeutic ratio,” the authors wrote in the Journal of Clinical Oncology.
Fractionation is typically limited by adjacent tissue sensitivity, but prostate tumors are more sensitive to radiation than the rectum, allowing for higher doses of radiation without damaging healthy tissue. While conventional fractionation doses are between 180 and 200 cGy, moderate hypofractionation delivers doses of 240-340 cGy. Ultrahypofractionation is defined by doses equal to or greater than 500 cGy (the upper limit of the linear-quadratic model of cell survival).
The present guideline was developed through a 2-year, collaborative effort between the American Society of Radiation Oncology, the Society of Clinical Oncology, and the American Urological Association. Task force members included urologic surgeons and oncologists, medical physicists, and radiation oncologists from academic and nonacademic settings. A patient representative and radiation oncology resident also were involved. After completing a systematic literature review, the team developed recommendations with varying degrees of strength. Supporting evidence quality and level of consensus also were described.
Of note, the guideline calls for moderate hypofractionation for patients with localized prostate cancer regardless of urinary function, anatomy, comorbidity, or age, with or without radiation to the seminal vesicles. Along with this recommendation, clinicians should discuss with patients the small increased risk of acute gastrointestinal toxicity, compared with conventional fractionation and the limited follow-up time in most relevant clinical trials (often less than 5 years).
The guideline conveyed more skepticism regarding ultrahypofractionation because of a lack of supporting evidence and comparative trials. As such, the authors conditionally recommended ultrahypofractionation for low-risk and intermediate patients, the latter of whom should be encouraged to enter clinical trials.
“The conditional recommendations regarding ultrahypofractionation underscore the importance of shared decision making between clinicians and patients in this setting,” the authors wrote. “The decision to use ultrahypofractionated EBRT at this time should follow a detailed discussion of the existing uncertainties in the risk-benefit balance associated with this treatment approach and should be informed at all stages by the patient’s values and preferences.”
The authors reported financial affiliations with Amgen, GlaxoSmithKline, Bristol-Myers Squibb, and others.
SOURCE: Morgan et al. J Clin Oncol. 2018 Oct 11. doi: 10.1200/JCO.18.01097.
Moderate hypofractionation is preferred over conventional fractionation in treatment of patients with localized prostate cancer who are candidates for external beam radiotherapy (EBRT), according to new a clinical practice guideline.
A meta-analysis of randomized clinical trials showed that moderate fractionation delivered the same efficacy as did conventional fractionation with a mild increase in gastrointestinal toxicity, reported lead author Scott C. Morgan, MD of OSF Medical Group in Bloomington, Illinois, and his colleagues. The drawback of toxicity is outweighed by distinct advantages in resource utilization and patient convenience, which make moderate hypofractionation the winning choice.
For many types of cancer, a shift toward fewer fractions of higher radiation is ongoing, driven largely by technological advances in radiation planning and delivery.
“Technical advances have permitted more precise and conformal delivery of escalated doses of radiation to the prostate, thereby improving the therapeutic ratio,” the authors wrote in the Journal of Clinical Oncology.
Fractionation is typically limited by adjacent tissue sensitivity, but prostate tumors are more sensitive to radiation than the rectum, allowing for higher doses of radiation without damaging healthy tissue. While conventional fractionation doses are between 180 and 200 cGy, moderate hypofractionation delivers doses of 240-340 cGy. Ultrahypofractionation is defined by doses equal to or greater than 500 cGy (the upper limit of the linear-quadratic model of cell survival).
The present guideline was developed through a 2-year, collaborative effort between the American Society of Radiation Oncology, the Society of Clinical Oncology, and the American Urological Association. Task force members included urologic surgeons and oncologists, medical physicists, and radiation oncologists from academic and nonacademic settings. A patient representative and radiation oncology resident also were involved. After completing a systematic literature review, the team developed recommendations with varying degrees of strength. Supporting evidence quality and level of consensus also were described.
Of note, the guideline calls for moderate hypofractionation for patients with localized prostate cancer regardless of urinary function, anatomy, comorbidity, or age, with or without radiation to the seminal vesicles. Along with this recommendation, clinicians should discuss with patients the small increased risk of acute gastrointestinal toxicity, compared with conventional fractionation and the limited follow-up time in most relevant clinical trials (often less than 5 years).
The guideline conveyed more skepticism regarding ultrahypofractionation because of a lack of supporting evidence and comparative trials. As such, the authors conditionally recommended ultrahypofractionation for low-risk and intermediate patients, the latter of whom should be encouraged to enter clinical trials.
“The conditional recommendations regarding ultrahypofractionation underscore the importance of shared decision making between clinicians and patients in this setting,” the authors wrote. “The decision to use ultrahypofractionated EBRT at this time should follow a detailed discussion of the existing uncertainties in the risk-benefit balance associated with this treatment approach and should be informed at all stages by the patient’s values and preferences.”
The authors reported financial affiliations with Amgen, GlaxoSmithKline, Bristol-Myers Squibb, and others.
SOURCE: Morgan et al. J Clin Oncol. 2018 Oct 11. doi: 10.1200/JCO.18.01097.
Moderate hypofractionation is preferred over conventional fractionation in treatment of patients with localized prostate cancer who are candidates for external beam radiotherapy (EBRT), according to new a clinical practice guideline.
A meta-analysis of randomized clinical trials showed that moderate fractionation delivered the same efficacy as did conventional fractionation with a mild increase in gastrointestinal toxicity, reported lead author Scott C. Morgan, MD of OSF Medical Group in Bloomington, Illinois, and his colleagues. The drawback of toxicity is outweighed by distinct advantages in resource utilization and patient convenience, which make moderate hypofractionation the winning choice.
For many types of cancer, a shift toward fewer fractions of higher radiation is ongoing, driven largely by technological advances in radiation planning and delivery.
“Technical advances have permitted more precise and conformal delivery of escalated doses of radiation to the prostate, thereby improving the therapeutic ratio,” the authors wrote in the Journal of Clinical Oncology.
Fractionation is typically limited by adjacent tissue sensitivity, but prostate tumors are more sensitive to radiation than the rectum, allowing for higher doses of radiation without damaging healthy tissue. While conventional fractionation doses are between 180 and 200 cGy, moderate hypofractionation delivers doses of 240-340 cGy. Ultrahypofractionation is defined by doses equal to or greater than 500 cGy (the upper limit of the linear-quadratic model of cell survival).
The present guideline was developed through a 2-year, collaborative effort between the American Society of Radiation Oncology, the Society of Clinical Oncology, and the American Urological Association. Task force members included urologic surgeons and oncologists, medical physicists, and radiation oncologists from academic and nonacademic settings. A patient representative and radiation oncology resident also were involved. After completing a systematic literature review, the team developed recommendations with varying degrees of strength. Supporting evidence quality and level of consensus also were described.
Of note, the guideline calls for moderate hypofractionation for patients with localized prostate cancer regardless of urinary function, anatomy, comorbidity, or age, with or without radiation to the seminal vesicles. Along with this recommendation, clinicians should discuss with patients the small increased risk of acute gastrointestinal toxicity, compared with conventional fractionation and the limited follow-up time in most relevant clinical trials (often less than 5 years).
The guideline conveyed more skepticism regarding ultrahypofractionation because of a lack of supporting evidence and comparative trials. As such, the authors conditionally recommended ultrahypofractionation for low-risk and intermediate patients, the latter of whom should be encouraged to enter clinical trials.
“The conditional recommendations regarding ultrahypofractionation underscore the importance of shared decision making between clinicians and patients in this setting,” the authors wrote. “The decision to use ultrahypofractionated EBRT at this time should follow a detailed discussion of the existing uncertainties in the risk-benefit balance associated with this treatment approach and should be informed at all stages by the patient’s values and preferences.”
The authors reported financial affiliations with Amgen, GlaxoSmithKline, Bristol-Myers Squibb, and others.
SOURCE: Morgan et al. J Clin Oncol. 2018 Oct 11. doi: 10.1200/JCO.18.01097.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Moderate hypofractionation is preferred over conventional fractionation in treatment of patients with localized prostate cancer who are candidates for external beam radiotherapy (EBRT).
Major finding: The guideline panel reached a 94% consensus for the recommendation of moderate hypofractionation over conventional fractionation regardless of urinary function, anatomy, comorbidity, or age.
Study details: An evidence-based clinical practice guideline developed by the American Society of Radiation Oncology (ASTRO), the American Society of Clinical Oncology (ASCO), and the American Urological Association (AUA).
Disclosures: The authors reported financial affiliations with Amgen, GlaxoSmithKline, Bristol-Myers Squibb, and others.
Source: Morgan et al. J Clin Oncol. 2018 Oct 11. doi: 10.1200/JCO.18.01097.
Guidelines outline patient-centered approach to type 2 diabetes
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
REPORTING FROM EASD 2018
Endocrine Society updates guidelines for congenital adrenal hyperplasia
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
New secondary fracture–prevention recommendations carry simple messages
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
Ensuring that older adults who have experienced a hip or vertebral fracture understand they likely have osteoporosis, and offering prompt drug treatment for the condition, are among five fundamental
The recommendations were announced at the annual meeting of the American Society for Bone and Mineral Research (ASBMR) in Montreal. The 40-member group that developed the recommendations, called the ASBMR Secondary Fracture Prevention Initiative Coalition, includes the American Association of Clinical Endocrinologists/American College of Endocrinology, the Endocrine Society, the American College of Rheumatology, the American College of Physicians, the American Geriatrics Society, the American Academy of Physical Medicine and Rehabilitation, and the American Academy of Orthopaedic Surgeons.
Additional fundamental recommendations from the Coalition advised ensuring that patients’ primary health care providers are aware of the fracture, regularly assessing the risk of falls, and routinely reevaluating patients who are being treated for osteoporosis. These suggestions were developed in response to growing evidence of a rising trend in osteoporosis patients not being prescribed appropriate medications or not taking them, the ASBMR said.
“The very simple message is if you’ve got somebody who has had a hip fracture or a vertebral fracture, that needs secondary prevention just like somebody who’s had an MI needs to be on a statin and a beta blocker,” said coalition cochair Sundeep Khosla, MD, a past president of the ASBMR and director of the Center for Clinical and Translational Science at the Mayo Clinic, Rochester, Minn. “You can’t ignore the fracture because it’s not immediately life-threatening. Down the road they’re going to have another hip fracture if nothing is done.”
Only 23% of elderly patients who have a hip fracture receive osteoporosis medication to reduce future fracture risk, according to the ASBMR. A 30-year downward trend in the number of hip fractures in the United States has recently plateaued, raising concerns this may have been caused by doctors and patients not following diagnostic and treatment guidelines, the organization noted.
The reasons for the plateau are uncertain, Dr. Khosla said, but could include a reluctance by patients to take bisphosphonates following some reports of relatively rare side effects, such as atypical femoral fractures and osteonecrosis of the jaw. In addition, he said, reimbursement for dual-energy x-ray absorptiometry (DEXA) scans to measure bone mineral density has gone down, which has led to fewer osteoporosis diagnoses. But fracture prevention is important, he said. Of the 300,000 hip fractures in the United States each year, one of every two patients never regains their previous functioning. In addition, one of every four hip fracture patients ends up in a nursing home or dies within a year, according to the ASBMR.
The recommendations and more data about osteoporosis treatment are available on the coalition’s website, www.secondaryfractures.org. An action plan for clinicians should be added to the site sometime this fall, Dr. Khosla said.
There are five fundamental recommendations:
First, communicate three simple messages to patients and their family/caregivers throughout the fracture care and healing process. These include: Their broken bone likely means they have osteoporosis and are at high risk for breaking more bones; breaking bones means they may have to use a walker, cane, or wheelchair or move from their home to a residential facility and will be at higher risk for premature death; and there are actions they can take to reduce their risk.
This is key, said coalition cochair Douglas P. Kiel, MD, a past president of ASBMR, the director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston, and a professor of medicine at Harvard Medical School, also in Boston.
“If you talk to people who have had a broken bone, they view this as an accident and not that they have anything wrong with them,” he said. “The communication should be that if you broke something, it is not a random, chance event. You have osteoporosis, and if you don’t do anything about it, you’re going to be at great risk of a life-threatening, independence-threatening fracture in the future.”
Second, ensure the patient’s primary health care provider is made aware of the occurrence of the fracture. Take action to be sure the communication is made.
Third, regularly assess the risk of falling in women and men age 65 years or older who have had a hip or vertebral fracture. At minimum, take a history of falls within the last year, minimize the use of medications associated with increased risk for falls, evaluate patients for conditions associated with an increased risk for falls, and strongly consider referring patients to physical and/or occupational therapy or a physiatrist for evaluation and interventions to improve impairments in mobility, gait, and balance to reduce the risk for falls.
Fourth, reduce the risk of additional fractures by offering pharmacologic therapy for osteoporosis to women and men age 65 years or older who have had a hip or vertebral fracture. This can begin in the hospital and be included in discharge orders. Do not delay initiation of therapy for bone mineral density (BMD) testing. Consider patients’ oral health before starting therapy with bisphosphonates or denosumab (Prolia).
Most hip fracture patients leave the hospital without osteoporosis medications, Dr. Kiel said. It could be that hospital-based physicians are concerned patients are still unsteady such that they may not want to start patients on a new medication when they’re discharging them. Physicians in rehabilitation units may not prescribe these medications because they feel they have the patients for a short time, so by the time the patient returns to their primary care provider, the patient may have the same mistaken impression the fracture was an accident.
“We’re advocating not to delay treatment for any of these care transitions or because you think they need a BMD test,” Dr. Kiel said. “Just get them treated like they do with heart attacks.”
Finally, follow and reevaluate women and men age 65 years or older who have had a hip or vertebral fracture and are being treated for osteoporosis because it is a life-long chronic condition. This can help reinforce key messages about osteoporosis and associated fractures, identify any barriers to treatment adherence, assess the risk of falls, evaluate the effectiveness of a treatment plan, monitor for adverse effects, and determine whether any changes in treatment should be made, including whether any osteoporosis pharmacotherapy should be changed or discontinued.
Ideally, patients should be managed in the context of a multidisciplinary clinical system that includes case management, such as a fracture liaison service, according to the recommendations.
Besides the fundamental five recommendations, the documents lists another seven that deal with referring patients, prescribing vitamin D, counseling on lifestyle and diet, discussing pharmacotherapy benefits and risks, weighing first-line therapy options, and determining the duration of pharmacotherapy.
EXPERT ANALYSIS FROM ASBMR 2018
Guideline: Early screening warranted if family history of nonhereditary colorectal cancer
New consensus guidelines strongly recommend screening colonoscopy for individuals who have at least one first-degree relative with nonhereditary colorectal cancer or advanced adenoma.
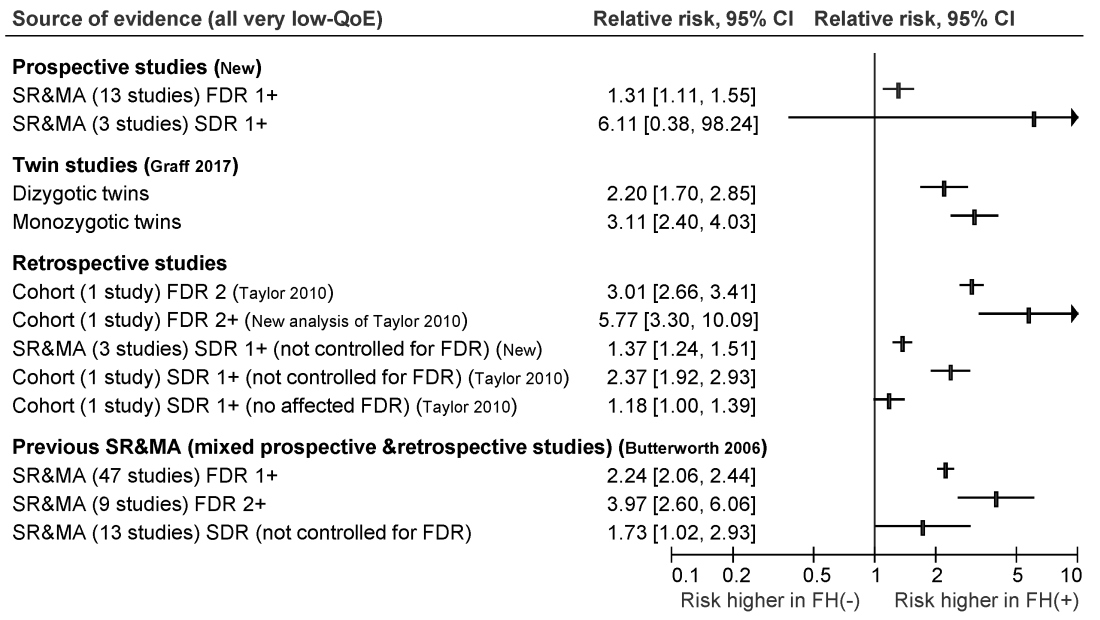
Published in the November issue of Gastroenterology, the guideline cites moderate-quality evidence for this recommendation and reserves fecal immunochemical testing for individuals who refuse colonoscopy, are at increased risk for complications, or face barriers accessing the procedure.
Most colorectal cancer screening guidelines have focused on average-risk individuals or those at highest risk because of heritable germline mutations. However, hereditary syndromes comprise only about 5% of colorectal cancers, noted Desmond Leddin, MB, MSc, FRCPC, FRCPI, of the University of Limerick, Ireland, and David A. Lieberman, MD, AGAF, FACG, of Oregon Health and Science University, Portland, with their associates from the Canadian Association of Gastroenterology Banff Consensus.
To develop the guideline, they searched the literature for studies of family history and colorectal cancer risk apart from hereditary Lynch syndrome, familial adenomatous polyposis, attenuated familial adenomatous polyposis, MUTYH-associated polyposis, Peutz-Jeghers syndrome, juvenile polyposis syndrome, Cowden syndrome, serrated (hyperplastic) polyposis syndrome, hereditary pancreatic cancer, and hereditary gastric cancer.
The ensuant guideline cites two new systematic reviews and meta-analyses of 16 prospective studies, as well as one twin study, four retrospective cohort studies, one new systematic review of retrospective studies, and three prior systematic reviews and meta-analyses. The authors note that this is the first guideline to use the GRADE (Grading of Recommendation Assessment, Development and Evaluation) approach to make screening recommendations for individuals who have a family history of nonhereditary colorectal cancer or advanced adenoma.
For those with one first-degree relative with colorectal cancer, the guideline recommends screening colonoscopy or fecal immunochemical testing beginning at age 40-50 years, or 10 years before the age of diagnosis of the first-degree relative, whichever is earlier. The authors recommend spacing subsequent screening colonoscopies by 5-10 years and spacing fecal immunochemical testing by 1-2 years. They offer the same recommendation for individuals with one or more first-degree relatives with confirmed advanced adenoma.
For individuals whose family history includes at least two first-degree relatives with colorectal cancer, the guideline recommends an initial screening colonoscopy at age 40, or 10 years earlier than the age of earliest-diagnosed first-degree relative, whichever is earlier. Screenings should occur every 5 years.
For persons with at least one second-degree relative with colorectal cancer, the guideline authors strongly recommend screening starting at age 50 with tests and intervals based on guidelines for average-risk individuals. Their recommendation is the same for individuals with at least one first-degree relative with nonadvanced adenoma or a polyp of unknown histology.
Given the low-quality evidence supporting most of these recommendations, the guideline calls for well designed observational studies to better quantify the risk of colorectal cancer among individuals with a family history of nonheritable disease. Studies should especially focus on the optimal age of first screening and appropriate screening intervals, the guideline authors wrote. Also, they call for randomized controlled trials to assess whether colonoscopy, fecal immunochemical testing, or fecal occult blood screening might significantly reduce long-term risk for colorectal cancer and improve survival in this population.
Merck provided unrestricted funding for the work. Dr. Leddin reported having no conflicts of interest. Dr. Lieberman and several coauthors disclosed financial relationships with companies other than Merck. One coauthor disclosed advisory and consulting relationships with Merck.
SOURCE: Leddin D et al. Gastroenterology. 2018 Aug 16. doi: 10.1053/j.gastro.2018.08.017.
New consensus guidelines strongly recommend screening colonoscopy for individuals who have at least one first-degree relative with nonhereditary colorectal cancer or advanced adenoma.

Published in the November issue of Gastroenterology, the guideline cites moderate-quality evidence for this recommendation and reserves fecal immunochemical testing for individuals who refuse colonoscopy, are at increased risk for complications, or face barriers accessing the procedure.
Most colorectal cancer screening guidelines have focused on average-risk individuals or those at highest risk because of heritable germline mutations. However, hereditary syndromes comprise only about 5% of colorectal cancers, noted Desmond Leddin, MB, MSc, FRCPC, FRCPI, of the University of Limerick, Ireland, and David A. Lieberman, MD, AGAF, FACG, of Oregon Health and Science University, Portland, with their associates from the Canadian Association of Gastroenterology Banff Consensus.
To develop the guideline, they searched the literature for studies of family history and colorectal cancer risk apart from hereditary Lynch syndrome, familial adenomatous polyposis, attenuated familial adenomatous polyposis, MUTYH-associated polyposis, Peutz-Jeghers syndrome, juvenile polyposis syndrome, Cowden syndrome, serrated (hyperplastic) polyposis syndrome, hereditary pancreatic cancer, and hereditary gastric cancer.
The ensuant guideline cites two new systematic reviews and meta-analyses of 16 prospective studies, as well as one twin study, four retrospective cohort studies, one new systematic review of retrospective studies, and three prior systematic reviews and meta-analyses. The authors note that this is the first guideline to use the GRADE (Grading of Recommendation Assessment, Development and Evaluation) approach to make screening recommendations for individuals who have a family history of nonhereditary colorectal cancer or advanced adenoma.
For those with one first-degree relative with colorectal cancer, the guideline recommends screening colonoscopy or fecal immunochemical testing beginning at age 40-50 years, or 10 years before the age of diagnosis of the first-degree relative, whichever is earlier. The authors recommend spacing subsequent screening colonoscopies by 5-10 years and spacing fecal immunochemical testing by 1-2 years. They offer the same recommendation for individuals with one or more first-degree relatives with confirmed advanced adenoma.
For individuals whose family history includes at least two first-degree relatives with colorectal cancer, the guideline recommends an initial screening colonoscopy at age 40, or 10 years earlier than the age of earliest-diagnosed first-degree relative, whichever is earlier. Screenings should occur every 5 years.
For persons with at least one second-degree relative with colorectal cancer, the guideline authors strongly recommend screening starting at age 50 with tests and intervals based on guidelines for average-risk individuals. Their recommendation is the same for individuals with at least one first-degree relative with nonadvanced adenoma or a polyp of unknown histology.
Given the low-quality evidence supporting most of these recommendations, the guideline calls for well designed observational studies to better quantify the risk of colorectal cancer among individuals with a family history of nonheritable disease. Studies should especially focus on the optimal age of first screening and appropriate screening intervals, the guideline authors wrote. Also, they call for randomized controlled trials to assess whether colonoscopy, fecal immunochemical testing, or fecal occult blood screening might significantly reduce long-term risk for colorectal cancer and improve survival in this population.
Merck provided unrestricted funding for the work. Dr. Leddin reported having no conflicts of interest. Dr. Lieberman and several coauthors disclosed financial relationships with companies other than Merck. One coauthor disclosed advisory and consulting relationships with Merck.
SOURCE: Leddin D et al. Gastroenterology. 2018 Aug 16. doi: 10.1053/j.gastro.2018.08.017.
New consensus guidelines strongly recommend screening colonoscopy for individuals who have at least one first-degree relative with nonhereditary colorectal cancer or advanced adenoma.

Published in the November issue of Gastroenterology, the guideline cites moderate-quality evidence for this recommendation and reserves fecal immunochemical testing for individuals who refuse colonoscopy, are at increased risk for complications, or face barriers accessing the procedure.
Most colorectal cancer screening guidelines have focused on average-risk individuals or those at highest risk because of heritable germline mutations. However, hereditary syndromes comprise only about 5% of colorectal cancers, noted Desmond Leddin, MB, MSc, FRCPC, FRCPI, of the University of Limerick, Ireland, and David A. Lieberman, MD, AGAF, FACG, of Oregon Health and Science University, Portland, with their associates from the Canadian Association of Gastroenterology Banff Consensus.
To develop the guideline, they searched the literature for studies of family history and colorectal cancer risk apart from hereditary Lynch syndrome, familial adenomatous polyposis, attenuated familial adenomatous polyposis, MUTYH-associated polyposis, Peutz-Jeghers syndrome, juvenile polyposis syndrome, Cowden syndrome, serrated (hyperplastic) polyposis syndrome, hereditary pancreatic cancer, and hereditary gastric cancer.
The ensuant guideline cites two new systematic reviews and meta-analyses of 16 prospective studies, as well as one twin study, four retrospective cohort studies, one new systematic review of retrospective studies, and three prior systematic reviews and meta-analyses. The authors note that this is the first guideline to use the GRADE (Grading of Recommendation Assessment, Development and Evaluation) approach to make screening recommendations for individuals who have a family history of nonhereditary colorectal cancer or advanced adenoma.
For those with one first-degree relative with colorectal cancer, the guideline recommends screening colonoscopy or fecal immunochemical testing beginning at age 40-50 years, or 10 years before the age of diagnosis of the first-degree relative, whichever is earlier. The authors recommend spacing subsequent screening colonoscopies by 5-10 years and spacing fecal immunochemical testing by 1-2 years. They offer the same recommendation for individuals with one or more first-degree relatives with confirmed advanced adenoma.
For individuals whose family history includes at least two first-degree relatives with colorectal cancer, the guideline recommends an initial screening colonoscopy at age 40, or 10 years earlier than the age of earliest-diagnosed first-degree relative, whichever is earlier. Screenings should occur every 5 years.
For persons with at least one second-degree relative with colorectal cancer, the guideline authors strongly recommend screening starting at age 50 with tests and intervals based on guidelines for average-risk individuals. Their recommendation is the same for individuals with at least one first-degree relative with nonadvanced adenoma or a polyp of unknown histology.
Given the low-quality evidence supporting most of these recommendations, the guideline calls for well designed observational studies to better quantify the risk of colorectal cancer among individuals with a family history of nonheritable disease. Studies should especially focus on the optimal age of first screening and appropriate screening intervals, the guideline authors wrote. Also, they call for randomized controlled trials to assess whether colonoscopy, fecal immunochemical testing, or fecal occult blood screening might significantly reduce long-term risk for colorectal cancer and improve survival in this population.
Merck provided unrestricted funding for the work. Dr. Leddin reported having no conflicts of interest. Dr. Lieberman and several coauthors disclosed financial relationships with companies other than Merck. One coauthor disclosed advisory and consulting relationships with Merck.
SOURCE: Leddin D et al. Gastroenterology. 2018 Aug 16. doi: 10.1053/j.gastro.2018.08.017.
FROM GASTROENTEROLOGY
New guidelines frame treatment options for Castleman disease
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
FROM BLOOD
Maximize well-woman visits for preventive services, counseling
A well-woman visit with an ob.gyn. should include preventive services and counseling, according to an updated committee opinion from the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice.
“A well-woman visit provides an excellent opportunity to counsel patients about maintaining a healthy lifestyle and minimizing health risks,” according to the opinion, published in Obstetrics & Gynecology. The updated opinion coincides with the release of the new Well-Woman Chart from the Women’s Preventive Services Initiative.
Previous research suggests that many women prefer an ob.gyn. or other women’s health care specialist not only for reproductive health care but also for services such as cervical cancer screening, contraception, and treatment for sexually transmitted infections, the committee members wrote. Although surveys of ob.gyns. show that most provide some level of overall health and primary care, the screening and other clinical preventive services were not consistent.
The committee opinion consequently recommends that the “periodic well-woman care visit should include screening, evaluation and counseling, and immunizations based on age and risk factors.” However, the committee acknowledged that the interval for specific services varies among patients, as does the scope of services provided in different settings.
“Taking a comprehensive history (specifically obtaining detailed information on symptoms and past medical and gynecologic history) will inform if certain components of the physical examination, including breast or pelvic examination, are indicated at that visit and will inform shared decision making for these examinations,” committee members wrote. Topics that should be addressed during lifespan include sexual health (which may include contraception, prepregnancy counseling, sexually transmitted infections, and infertility), vulvovaginal symptoms, and bone health.
Not all components of a physical may be required at a well-woman visit, but ob.gyns. can play a key role by encouraging and facilitating healthy behaviors, counseling on preventive health strategies, and engaging women in shared decision-making. Screening for smoking, poor diet, and lack of physical activity are important. Ob.gyns. also can be part of the team-based care for women that may include physician assistants, nurse practitioners, and other medical professionals.
The most notable change from the previous opinion is that it coincides with the Women’s Preventive Services Initiative’s release of a Well-Woman Chart, which is designed to help ob.gyns. navigate the implementation of ACOG’s well-woman guidance, Christopher Zahn, MD, vice president of practice activities for ACOG, said in an interview.
“In tandem, these documents support ob.gyns. and other women’s health care providers’ efforts to make well-woman visits more personalized care that prioritizes shared decision-making over a woman’s lifetime,” he said. The opinion statement also includes the Women’s Preventive Services Initiative as a source of information for recommendations on well-woman care, and includes new guidance on the elements of a physical exam, including the pelvic exam.
“Ob.gyns. care for women over their lifetime, and increasingly this includes a lot of preventive care. The committee opinion details ACOG’s overall approach to well-women care and the role of the ob.gyn. as a provider of preventive services,” said Dr. Zahn. “The accompanying well-woman chart, targeted to providers, summarizes needed preventive services ensuring that time can be spent effectively and productively during each well-woman visit. By centering shared decision making and care tailored to each woman’s health care needs at every life stage, the well-woman visit is a fundamental part of the patient-provider relationship.”
Dr. Zahn noted that ongoing, high-quality research is essential to determine what strategies are most effective for women’s preventive care needs at every life stage. “Further research is also needed to identify screening strategies for women in certain higher risk groups and to reduce disparities in outcomes in certain populations of women. From cancer screening to new contraceptive methods, to managing symptoms of menopause, the more research we have to support our recommendations for these services, the more effectively we can care for women and help to keep them healthy for many, many years,” he emphasized.
The committee recommended additional resources for ob.gyns. and other health care providers, as well as for patients. The resources are available online at www.acog.org/More-Info/WellWoman.
The new opinion statement, which replaces the previous opinion issued in 2012, was developed by the ACOG Committee on Gynecologic Practice in collaboration with committee member Catherine Witkop, MD, MPH, of the Uniformed Health Sciences University in Bethesda, Md. The committee members had no relevant financial conflicts to disclose.
A well-woman visit with an ob.gyn. should include preventive services and counseling, according to an updated committee opinion from the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice.
“A well-woman visit provides an excellent opportunity to counsel patients about maintaining a healthy lifestyle and minimizing health risks,” according to the opinion, published in Obstetrics & Gynecology. The updated opinion coincides with the release of the new Well-Woman Chart from the Women’s Preventive Services Initiative.
Previous research suggests that many women prefer an ob.gyn. or other women’s health care specialist not only for reproductive health care but also for services such as cervical cancer screening, contraception, and treatment for sexually transmitted infections, the committee members wrote. Although surveys of ob.gyns. show that most provide some level of overall health and primary care, the screening and other clinical preventive services were not consistent.
The committee opinion consequently recommends that the “periodic well-woman care visit should include screening, evaluation and counseling, and immunizations based on age and risk factors.” However, the committee acknowledged that the interval for specific services varies among patients, as does the scope of services provided in different settings.
“Taking a comprehensive history (specifically obtaining detailed information on symptoms and past medical and gynecologic history) will inform if certain components of the physical examination, including breast or pelvic examination, are indicated at that visit and will inform shared decision making for these examinations,” committee members wrote. Topics that should be addressed during lifespan include sexual health (which may include contraception, prepregnancy counseling, sexually transmitted infections, and infertility), vulvovaginal symptoms, and bone health.
Not all components of a physical may be required at a well-woman visit, but ob.gyns. can play a key role by encouraging and facilitating healthy behaviors, counseling on preventive health strategies, and engaging women in shared decision-making. Screening for smoking, poor diet, and lack of physical activity are important. Ob.gyns. also can be part of the team-based care for women that may include physician assistants, nurse practitioners, and other medical professionals.
The most notable change from the previous opinion is that it coincides with the Women’s Preventive Services Initiative’s release of a Well-Woman Chart, which is designed to help ob.gyns. navigate the implementation of ACOG’s well-woman guidance, Christopher Zahn, MD, vice president of practice activities for ACOG, said in an interview.
“In tandem, these documents support ob.gyns. and other women’s health care providers’ efforts to make well-woman visits more personalized care that prioritizes shared decision-making over a woman’s lifetime,” he said. The opinion statement also includes the Women’s Preventive Services Initiative as a source of information for recommendations on well-woman care, and includes new guidance on the elements of a physical exam, including the pelvic exam.
“Ob.gyns. care for women over their lifetime, and increasingly this includes a lot of preventive care. The committee opinion details ACOG’s overall approach to well-women care and the role of the ob.gyn. as a provider of preventive services,” said Dr. Zahn. “The accompanying well-woman chart, targeted to providers, summarizes needed preventive services ensuring that time can be spent effectively and productively during each well-woman visit. By centering shared decision making and care tailored to each woman’s health care needs at every life stage, the well-woman visit is a fundamental part of the patient-provider relationship.”
Dr. Zahn noted that ongoing, high-quality research is essential to determine what strategies are most effective for women’s preventive care needs at every life stage. “Further research is also needed to identify screening strategies for women in certain higher risk groups and to reduce disparities in outcomes in certain populations of women. From cancer screening to new contraceptive methods, to managing symptoms of menopause, the more research we have to support our recommendations for these services, the more effectively we can care for women and help to keep them healthy for many, many years,” he emphasized.
The committee recommended additional resources for ob.gyns. and other health care providers, as well as for patients. The resources are available online at www.acog.org/More-Info/WellWoman.
The new opinion statement, which replaces the previous opinion issued in 2012, was developed by the ACOG Committee on Gynecologic Practice in collaboration with committee member Catherine Witkop, MD, MPH, of the Uniformed Health Sciences University in Bethesda, Md. The committee members had no relevant financial conflicts to disclose.
A well-woman visit with an ob.gyn. should include preventive services and counseling, according to an updated committee opinion from the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice.
“A well-woman visit provides an excellent opportunity to counsel patients about maintaining a healthy lifestyle and minimizing health risks,” according to the opinion, published in Obstetrics & Gynecology. The updated opinion coincides with the release of the new Well-Woman Chart from the Women’s Preventive Services Initiative.
Previous research suggests that many women prefer an ob.gyn. or other women’s health care specialist not only for reproductive health care but also for services such as cervical cancer screening, contraception, and treatment for sexually transmitted infections, the committee members wrote. Although surveys of ob.gyns. show that most provide some level of overall health and primary care, the screening and other clinical preventive services were not consistent.
The committee opinion consequently recommends that the “periodic well-woman care visit should include screening, evaluation and counseling, and immunizations based on age and risk factors.” However, the committee acknowledged that the interval for specific services varies among patients, as does the scope of services provided in different settings.
“Taking a comprehensive history (specifically obtaining detailed information on symptoms and past medical and gynecologic history) will inform if certain components of the physical examination, including breast or pelvic examination, are indicated at that visit and will inform shared decision making for these examinations,” committee members wrote. Topics that should be addressed during lifespan include sexual health (which may include contraception, prepregnancy counseling, sexually transmitted infections, and infertility), vulvovaginal symptoms, and bone health.
Not all components of a physical may be required at a well-woman visit, but ob.gyns. can play a key role by encouraging and facilitating healthy behaviors, counseling on preventive health strategies, and engaging women in shared decision-making. Screening for smoking, poor diet, and lack of physical activity are important. Ob.gyns. also can be part of the team-based care for women that may include physician assistants, nurse practitioners, and other medical professionals.
The most notable change from the previous opinion is that it coincides with the Women’s Preventive Services Initiative’s release of a Well-Woman Chart, which is designed to help ob.gyns. navigate the implementation of ACOG’s well-woman guidance, Christopher Zahn, MD, vice president of practice activities for ACOG, said in an interview.
“In tandem, these documents support ob.gyns. and other women’s health care providers’ efforts to make well-woman visits more personalized care that prioritizes shared decision-making over a woman’s lifetime,” he said. The opinion statement also includes the Women’s Preventive Services Initiative as a source of information for recommendations on well-woman care, and includes new guidance on the elements of a physical exam, including the pelvic exam.
“Ob.gyns. care for women over their lifetime, and increasingly this includes a lot of preventive care. The committee opinion details ACOG’s overall approach to well-women care and the role of the ob.gyn. as a provider of preventive services,” said Dr. Zahn. “The accompanying well-woman chart, targeted to providers, summarizes needed preventive services ensuring that time can be spent effectively and productively during each well-woman visit. By centering shared decision making and care tailored to each woman’s health care needs at every life stage, the well-woman visit is a fundamental part of the patient-provider relationship.”
Dr. Zahn noted that ongoing, high-quality research is essential to determine what strategies are most effective for women’s preventive care needs at every life stage. “Further research is also needed to identify screening strategies for women in certain higher risk groups and to reduce disparities in outcomes in certain populations of women. From cancer screening to new contraceptive methods, to managing symptoms of menopause, the more research we have to support our recommendations for these services, the more effectively we can care for women and help to keep them healthy for many, many years,” he emphasized.
The committee recommended additional resources for ob.gyns. and other health care providers, as well as for patients. The resources are available online at www.acog.org/More-Info/WellWoman.
The new opinion statement, which replaces the previous opinion issued in 2012, was developed by the ACOG Committee on Gynecologic Practice in collaboration with committee member Catherine Witkop, MD, MPH, of the Uniformed Health Sciences University in Bethesda, Md. The committee members had no relevant financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
New AAP policy statement addresses teen driver risks
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
The American Academy of Pediatric has issued a policy statement regarding teen drivers because, although becoming a driver is a rite of passage for many, there are unique risks associated with teen drivers – and important ways to address them.
“In 2015, among 15- to 20-year-old individuals, 1,886 young drivers died in MVCs [motor vehicle crashes], which is an increase of 9% from 2014,” the statement explains. “Another 195,000 young drivers were in MVCs, which is up 14% from 2014.”
Risk factors associated with crashes in teenage drivers include their inexperience, the presence of other teens in the car, high-speed and risky driving, distraction, lack of sleep, nighttime driving, and alcohol, marijuana, and medication use. Mobile phones and other electronic devices pose a major threat to driver safety because they contribute opportunities for three different kinds of distraction – visual, manual, and cognitive. Drug and alcohol use poses another threat; for example, tetrahydrocannabinol, found in marijuana, is associated with a 1.25 higher risk of crash, according Elizabeth M. Alderman, MD, and her associates on the Committee on Adolescence and the Council on Injury, Violence, and Poison Prevention who crafted the policy statement.
The policy statement also outlined several interventions to help mitigate these risks. Graduated driver licensing, which has been adopted in all 50 states, has helped reduce teen crashes by promoting skills development and reducing exposure to risky-driving situations. Parents can help model safer-driving practices and set expectations, as well as monitor and affect their teens’ driving behaviors during the supervised-driving phase. Driver education programs, on the other hand, counterintuitively have little effect on teen driving safety, which the authors suggested is because “the knowledge required to pass licensing exams is seldom related to an evidence-based understanding of the behaviors and skills associated with novice driver crash risk.” There also have been technological advances that can help improve driving safety among teenage drivers, such as automatic braking and lane-maintenance alerts.
“Policies, programs, and technologies exist to mitigate these risks but, in most cases, depend on active participation by the teenager and parents,” the authors concluded. “Pediatricians, communities, and governments need to take action to better educate teen drivers and their parents around these risks and strategies to reduce them.”
The full policy statement includes specific recommendations for anticipatory guidance, professional practice, community advocacy, and legislative advocacy. It also includes many resources and links to further information.
SOURCE: Alderman EM et al. Pediatrics. 2018 Oct;142(4):e20182163.
FROM PEDIATRICS