User login
Managing gynecologic cancers during the COVID-19 pandemic
To manage patients with gynecologic cancers, oncologists in the United States and Europe are recommending reducing outpatient visits, delaying surgeries, prolonging chemotherapy regimens, and generally trying to keep cancer patients away from those who have tested positive for COVID-19.
“We recognize that, in this special situation, we must continue to provide our gynecologic oncology patients with the highest quality of medical services,” Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center in Houston and associates wrote in an editorial published in the International Journal of Gynecological Cancer.
At the same time, the authors added, the safety of patients, their families, and medical staff needs to be assured.
Dr. Ramirez and colleagues’ editorial includes recommendations on how to optimize the care of patients with gynecologic cancers while prioritizing safety and minimizing the burden to the healthcare system. The group’s recommendations outline when surgery, radiotherapy, and other treatments might be safely postponed and when they need to proceed out of urgency.
Some authors of the editorial also described their experiences with COVID-19 during a webinar on managing patients with advanced ovarian cancer, which was hosted by the European Society of Gynaecological Oncology (ESGO).
A lack of resources
In Spain, health resources “are collapsed” by the pandemic, editorial author Luis Chiva, MD, said during the webinar.
At his institution, the Clínica Universidad de Navarra in Madrid, 98% of the 1,500 intensive care beds were occupied by COVID-19 patients at the end of March. So the hope was to be able to refer their patients to other communities where there may still be some capacity.
Another problem in Spain is the high percentage of health workers infected with SARS-CoV-2, the virus behind COVID-19. More than 15,000 health workers were recently reported to be sick or self-isolating, which is around 14% of the health care workforce in the country.
Dr. Chiva noted that this puts those treating gynecologic cancers in a difficult position. On the one hand, surgery to remove a high-risk ovarian mass should not be delayed, but the majority of hospitals in Spain simply cannot perform this type of surgery during the pandemic.
“Unfortunately, due to this specific situation, almost, I would say in 80%-90% of hospitals, we are only able to carry out emergency surgical procedures,” Dr. Chiva said. That’s general emergency procedures such as appendectomies, removing blockages, and dealing with hemorrhages, not gynecologic surgeries. “It’s almost impossible to schedule the typical oncological cases,” he said.
Even with the Hospital IFEMA now set up at the Feria de Madrid, which is usually used to host large-scale events, there are “minimal options for performing standard oncological surgery,” Dr. Chiva said. He estimated that just 5% of hospitals in Spain are able to perform oncologic surgeries as normal, with maybe 15% able to offer surgery without the backup of postsurgical intensive care.
‘Ring-fencing’
“This is really an unusual time for us,” commented Jonathan Ledermann, MD, vice president of ESGO and a professor of medical oncology at University College London, who moderated the webinar.
“This is affecting the way in which we diagnose our patients and have access to care,” he said. “It compromises the way in which we treat patients. We have to adjust our treatment pathways. We have to look at the risks of coronavirus infection in cancer patients and how we manage patients in a socially distancing environment. We also need to think about managing gynecological oncology departments in the face of disease amongst staff, the risks of transmission, and the reduced clinical service.”
Dr. Ledermann noted that “ring-fencing” a few hospitals to deal only with patients free of COVID-19 might be a way forward. This approach has been used in Northern Italy and was recently started in London.
“We try to divide and have separate access between COVID-positive and -negative patients,” said Anna Fagotti, MD, an assistant professor at Fondazione Policlinico Universitario Agostino Gemelli IRCCS in Rome and another coauthor of the editorial.
“We are trying to divide the work flow of patients and try to ensure treatment to cancer patients as much as we can,” she explained. “This means that it’s a very difficult situation, and, every time, you have to deal with the number of places available as some places have been taken by other patients from the emergency room. We are still trying to have a number of beds and intensive care unit beds available for our patients.”
Setting up dedicated hospitals is a good idea, but it has to be done before the “tsunami” of cases hits and there are no more intensive care beds or ventilators, according to Antonio González-Martín, MD, of Clínica Universidad de Navarra in Madrid, another coauthor of the editorial.
Limiting hospital visits
Strategies to limit the number of times patients need to come into hospital for appointments and treatment is key to getting through the pandemic, Sandro Pignata, MD, of Instituto Nazionale Tumori IRCCS Fondazione G. Pascale in Naples, Italy, said during the webinar.
“It will be imperative to explore options that reduce the number of procedures or surgical interventions that may be associated with prolonged operative time, risk of major blood loss, necessitating blood products, risk of infection to the medical personnel, or admission to intensive care units,” Dr. Ramirez and colleagues wrote in their editorial.
“In considering management of disease, we must recognize that, in many centers, access to routine visits and surgery may be either completely restricted or significantly reduced. We must, therefore, consider options that may still offer our patients a treatment plan that addresses their disease while at the same time limiting risk of exposure,” the authors wrote.
The authors declared no competing interests or specific funding in relation to their work, and the webinar participants had no conflicts of interest.
SOURCE: Ramirez PT et al. Int J Gynecol Cancer. 2020 Mar 27. doi: 10.1136/ijgc-2020-001419.
To manage patients with gynecologic cancers, oncologists in the United States and Europe are recommending reducing outpatient visits, delaying surgeries, prolonging chemotherapy regimens, and generally trying to keep cancer patients away from those who have tested positive for COVID-19.
“We recognize that, in this special situation, we must continue to provide our gynecologic oncology patients with the highest quality of medical services,” Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center in Houston and associates wrote in an editorial published in the International Journal of Gynecological Cancer.
At the same time, the authors added, the safety of patients, their families, and medical staff needs to be assured.
Dr. Ramirez and colleagues’ editorial includes recommendations on how to optimize the care of patients with gynecologic cancers while prioritizing safety and minimizing the burden to the healthcare system. The group’s recommendations outline when surgery, radiotherapy, and other treatments might be safely postponed and when they need to proceed out of urgency.
Some authors of the editorial also described their experiences with COVID-19 during a webinar on managing patients with advanced ovarian cancer, which was hosted by the European Society of Gynaecological Oncology (ESGO).
A lack of resources
In Spain, health resources “are collapsed” by the pandemic, editorial author Luis Chiva, MD, said during the webinar.
At his institution, the Clínica Universidad de Navarra in Madrid, 98% of the 1,500 intensive care beds were occupied by COVID-19 patients at the end of March. So the hope was to be able to refer their patients to other communities where there may still be some capacity.
Another problem in Spain is the high percentage of health workers infected with SARS-CoV-2, the virus behind COVID-19. More than 15,000 health workers were recently reported to be sick or self-isolating, which is around 14% of the health care workforce in the country.
Dr. Chiva noted that this puts those treating gynecologic cancers in a difficult position. On the one hand, surgery to remove a high-risk ovarian mass should not be delayed, but the majority of hospitals in Spain simply cannot perform this type of surgery during the pandemic.
“Unfortunately, due to this specific situation, almost, I would say in 80%-90% of hospitals, we are only able to carry out emergency surgical procedures,” Dr. Chiva said. That’s general emergency procedures such as appendectomies, removing blockages, and dealing with hemorrhages, not gynecologic surgeries. “It’s almost impossible to schedule the typical oncological cases,” he said.
Even with the Hospital IFEMA now set up at the Feria de Madrid, which is usually used to host large-scale events, there are “minimal options for performing standard oncological surgery,” Dr. Chiva said. He estimated that just 5% of hospitals in Spain are able to perform oncologic surgeries as normal, with maybe 15% able to offer surgery without the backup of postsurgical intensive care.
‘Ring-fencing’
“This is really an unusual time for us,” commented Jonathan Ledermann, MD, vice president of ESGO and a professor of medical oncology at University College London, who moderated the webinar.
“This is affecting the way in which we diagnose our patients and have access to care,” he said. “It compromises the way in which we treat patients. We have to adjust our treatment pathways. We have to look at the risks of coronavirus infection in cancer patients and how we manage patients in a socially distancing environment. We also need to think about managing gynecological oncology departments in the face of disease amongst staff, the risks of transmission, and the reduced clinical service.”
Dr. Ledermann noted that “ring-fencing” a few hospitals to deal only with patients free of COVID-19 might be a way forward. This approach has been used in Northern Italy and was recently started in London.
“We try to divide and have separate access between COVID-positive and -negative patients,” said Anna Fagotti, MD, an assistant professor at Fondazione Policlinico Universitario Agostino Gemelli IRCCS in Rome and another coauthor of the editorial.
“We are trying to divide the work flow of patients and try to ensure treatment to cancer patients as much as we can,” she explained. “This means that it’s a very difficult situation, and, every time, you have to deal with the number of places available as some places have been taken by other patients from the emergency room. We are still trying to have a number of beds and intensive care unit beds available for our patients.”
Setting up dedicated hospitals is a good idea, but it has to be done before the “tsunami” of cases hits and there are no more intensive care beds or ventilators, according to Antonio González-Martín, MD, of Clínica Universidad de Navarra in Madrid, another coauthor of the editorial.
Limiting hospital visits
Strategies to limit the number of times patients need to come into hospital for appointments and treatment is key to getting through the pandemic, Sandro Pignata, MD, of Instituto Nazionale Tumori IRCCS Fondazione G. Pascale in Naples, Italy, said during the webinar.
“It will be imperative to explore options that reduce the number of procedures or surgical interventions that may be associated with prolonged operative time, risk of major blood loss, necessitating blood products, risk of infection to the medical personnel, or admission to intensive care units,” Dr. Ramirez and colleagues wrote in their editorial.
“In considering management of disease, we must recognize that, in many centers, access to routine visits and surgery may be either completely restricted or significantly reduced. We must, therefore, consider options that may still offer our patients a treatment plan that addresses their disease while at the same time limiting risk of exposure,” the authors wrote.
The authors declared no competing interests or specific funding in relation to their work, and the webinar participants had no conflicts of interest.
SOURCE: Ramirez PT et al. Int J Gynecol Cancer. 2020 Mar 27. doi: 10.1136/ijgc-2020-001419.
To manage patients with gynecologic cancers, oncologists in the United States and Europe are recommending reducing outpatient visits, delaying surgeries, prolonging chemotherapy regimens, and generally trying to keep cancer patients away from those who have tested positive for COVID-19.
“We recognize that, in this special situation, we must continue to provide our gynecologic oncology patients with the highest quality of medical services,” Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center in Houston and associates wrote in an editorial published in the International Journal of Gynecological Cancer.
At the same time, the authors added, the safety of patients, their families, and medical staff needs to be assured.
Dr. Ramirez and colleagues’ editorial includes recommendations on how to optimize the care of patients with gynecologic cancers while prioritizing safety and minimizing the burden to the healthcare system. The group’s recommendations outline when surgery, radiotherapy, and other treatments might be safely postponed and when they need to proceed out of urgency.
Some authors of the editorial also described their experiences with COVID-19 during a webinar on managing patients with advanced ovarian cancer, which was hosted by the European Society of Gynaecological Oncology (ESGO).
A lack of resources
In Spain, health resources “are collapsed” by the pandemic, editorial author Luis Chiva, MD, said during the webinar.
At his institution, the Clínica Universidad de Navarra in Madrid, 98% of the 1,500 intensive care beds were occupied by COVID-19 patients at the end of March. So the hope was to be able to refer their patients to other communities where there may still be some capacity.
Another problem in Spain is the high percentage of health workers infected with SARS-CoV-2, the virus behind COVID-19. More than 15,000 health workers were recently reported to be sick or self-isolating, which is around 14% of the health care workforce in the country.
Dr. Chiva noted that this puts those treating gynecologic cancers in a difficult position. On the one hand, surgery to remove a high-risk ovarian mass should not be delayed, but the majority of hospitals in Spain simply cannot perform this type of surgery during the pandemic.
“Unfortunately, due to this specific situation, almost, I would say in 80%-90% of hospitals, we are only able to carry out emergency surgical procedures,” Dr. Chiva said. That’s general emergency procedures such as appendectomies, removing blockages, and dealing with hemorrhages, not gynecologic surgeries. “It’s almost impossible to schedule the typical oncological cases,” he said.
Even with the Hospital IFEMA now set up at the Feria de Madrid, which is usually used to host large-scale events, there are “minimal options for performing standard oncological surgery,” Dr. Chiva said. He estimated that just 5% of hospitals in Spain are able to perform oncologic surgeries as normal, with maybe 15% able to offer surgery without the backup of postsurgical intensive care.
‘Ring-fencing’
“This is really an unusual time for us,” commented Jonathan Ledermann, MD, vice president of ESGO and a professor of medical oncology at University College London, who moderated the webinar.
“This is affecting the way in which we diagnose our patients and have access to care,” he said. “It compromises the way in which we treat patients. We have to adjust our treatment pathways. We have to look at the risks of coronavirus infection in cancer patients and how we manage patients in a socially distancing environment. We also need to think about managing gynecological oncology departments in the face of disease amongst staff, the risks of transmission, and the reduced clinical service.”
Dr. Ledermann noted that “ring-fencing” a few hospitals to deal only with patients free of COVID-19 might be a way forward. This approach has been used in Northern Italy and was recently started in London.
“We try to divide and have separate access between COVID-positive and -negative patients,” said Anna Fagotti, MD, an assistant professor at Fondazione Policlinico Universitario Agostino Gemelli IRCCS in Rome and another coauthor of the editorial.
“We are trying to divide the work flow of patients and try to ensure treatment to cancer patients as much as we can,” she explained. “This means that it’s a very difficult situation, and, every time, you have to deal with the number of places available as some places have been taken by other patients from the emergency room. We are still trying to have a number of beds and intensive care unit beds available for our patients.”
Setting up dedicated hospitals is a good idea, but it has to be done before the “tsunami” of cases hits and there are no more intensive care beds or ventilators, according to Antonio González-Martín, MD, of Clínica Universidad de Navarra in Madrid, another coauthor of the editorial.
Limiting hospital visits
Strategies to limit the number of times patients need to come into hospital for appointments and treatment is key to getting through the pandemic, Sandro Pignata, MD, of Instituto Nazionale Tumori IRCCS Fondazione G. Pascale in Naples, Italy, said during the webinar.
“It will be imperative to explore options that reduce the number of procedures or surgical interventions that may be associated with prolonged operative time, risk of major blood loss, necessitating blood products, risk of infection to the medical personnel, or admission to intensive care units,” Dr. Ramirez and colleagues wrote in their editorial.
“In considering management of disease, we must recognize that, in many centers, access to routine visits and surgery may be either completely restricted or significantly reduced. We must, therefore, consider options that may still offer our patients a treatment plan that addresses their disease while at the same time limiting risk of exposure,” the authors wrote.
The authors declared no competing interests or specific funding in relation to their work, and the webinar participants had no conflicts of interest.
SOURCE: Ramirez PT et al. Int J Gynecol Cancer. 2020 Mar 27. doi: 10.1136/ijgc-2020-001419.
FROM THE INTERNATIONAL JOURNAL OF GYNECOLOGICAL CANCER
Treating rectal cancer in the COVID-19 era: Expert guidance
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
AGA CPU: Screening and surveillance for hepatocellular carcinoma in patients with NAFLD
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
FROM GASTROENTEROLOGY
Colorectal cancer: Proposed treatment guidelines for the COVID-19 era
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer
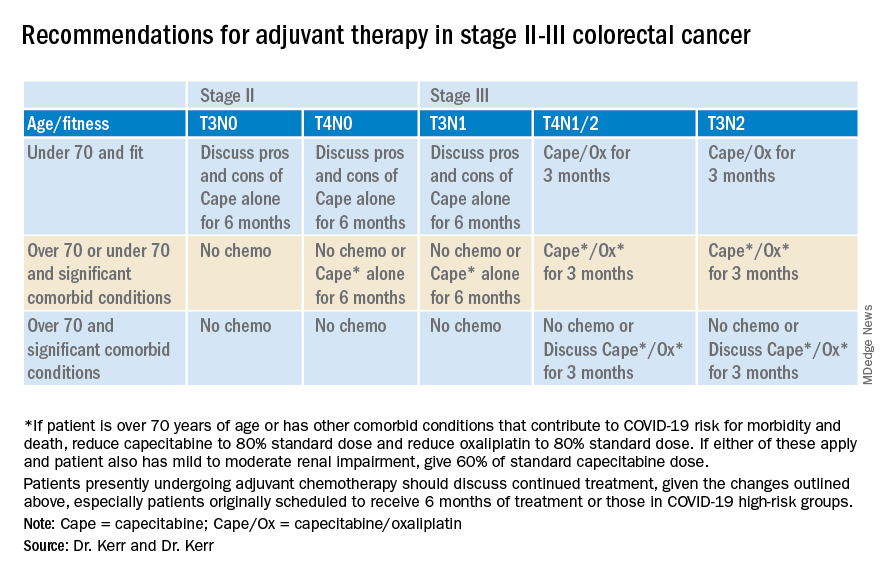
Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
AASLD: Liver transplants should proceed despite COVID-19
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
Guidelines on delaying cancer surgery during COVID-19
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Perspective from the heartland: Cancer care and research during a public health crisis
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
AGA and colleague societies issue clinical insights for COVID-19
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.

