User login
Ob.gyns. reveal heavier suicide ideation burden than most specialists
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.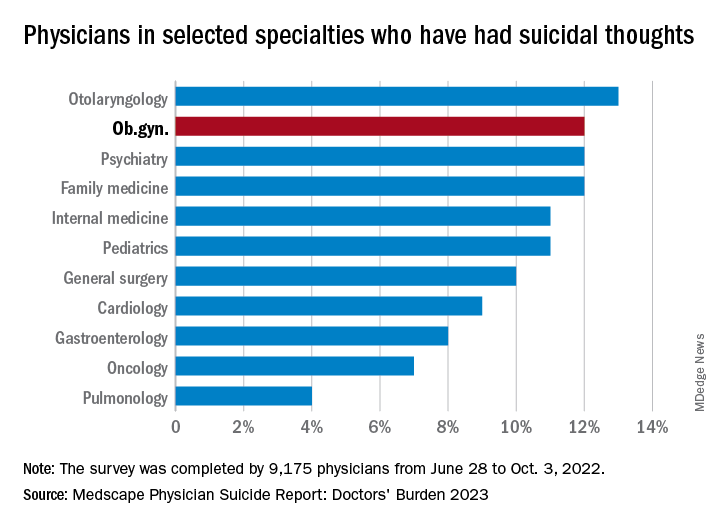
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.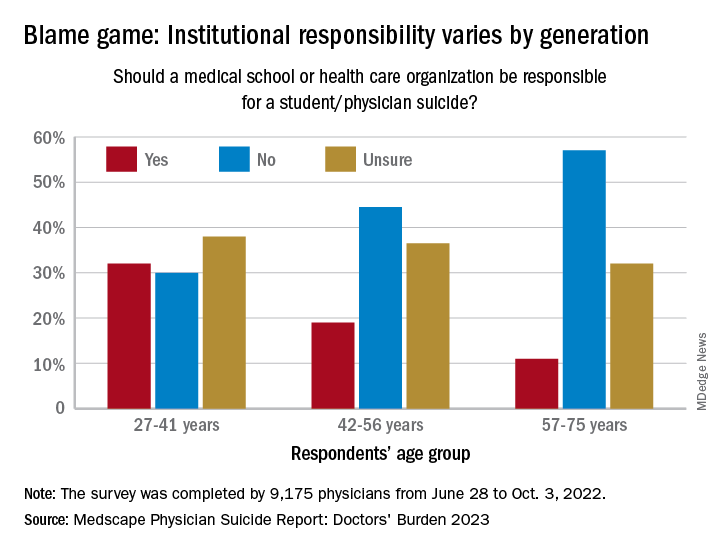
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Ob.gyn. loses PhD after committee finds he made up research
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
Telehealth doctor indicted on health care fraud, opioid distribution charges
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
New documentary highlights human toll of high insulin cost
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We have seen the future of healthy muffins, and its name is Roselle
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
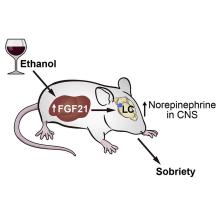
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Inclusive reminder: LGBTQ community may donate stem cells
In fact, gay men have been able to donate stem cells in the United States since 2015. That’s when National Marrow Donor Program’s Be the Match registry lifted restrictions on men who have sex with men (MSM).
Physicians say advocacy is still necessary, because LGBTQ people may assume they can’t donate or be wary of clinicians. “The LGBTQIA+ population in general has experienced a lot of issues with the medical-industrial complex in terms of discrimination and inappropriate care,” said UT Southwestern Medical Center pathologist Brian Adkins, MD, who manages the blood bank at Children’s Health in Dallas, in an interview. “There’s a weariness there that may produce some hesitancy to interact with the donation process.”
An estimated 6.8 million people give blood in the United States each year, and an estimated 9 million people are registered as potential stem cell donors. A total of 22,013 hematopoietic cell transplantation procedures were performed in 2020, according to the U.S. Health Resources and Services Administration.
Expanding the number of LGBTQ donors, especially those born as biological males, could pay major dividends. As Dr. Adkins noted, the ideal stem cell donor is young – Be the Match says doctors generally prefer donors aged 18-35 – and male. According to a 2021 Gallup Poll, 21% of those born from 1997 to 2003 (Generation Z) say they’re LGBTQ, as do 11% of those born from 1981 to 1996 (Millennials).
In North America, the most extensive outreach to the LGBTQ community about stem cell donation has been launched in Canada. There, an organization called Stem Cell Club focuses on encouraging college students and other young people to register as potential stem cell donors.
Stem Cell Club has several campaigns aimed at ethnic minority groups, and its Saving Lives With Pride project focuses on MSM. The project’s web page includes testimonials from a woman whose life was saved by an unrelated gay male donor and from a gay male nurse who recovered from blood cancer thanks to a stem cell donation. The site also includes videos about stem cell donation featuring LGBTQ young people and Canadian hematologists.
“Our specialized collection center will treat donors with the highest levels of respect and courtesy, indeed as heroes of their unselfish gift that can truly save a life,” says Ottawa Hospital transplant hematologist David Allan, MD, in one of the videos.
Stem Cell Club was founded by transplant hematologist Warren Fingrut, MD, a research fellow at Memorial Sloan Kettering Cancer Center. In an interview, he said the organization’s LGBTQ project has promoted stem cell donation at several annual gay pride events and will continue the outreach this coming summer. In 2018 and 2019, advocates recruited 354 potential stem-cell donors (40% male, 42 non-White) at five pride events, Dr. Fingrut and colleagues reported last year in the journal Bone Marrow Transplantation.
For a new study, researchers interviewed 37 gay and bisexual men from five Canadian provinces about stem cell donation. Dr. Fingrut and colleagues reported the findings in February in an abstract at the Transplantation & Cellular Therapy Meetings.
Most participants didn’t know they “are eligible to donate stem cells, with many confusing stem cell versus blood donor eligibility criteria,” the researchers reported. According to Dr. Fingrut, some of the men “felt they were treated as second-class citizens, and that translated into frustration and decreased motivation to donate. There were concerns that they would be treated as though they shouldn’t be there.”
Canada has allowed gay men to donate stem cells for at least 10 years, Dr. Fingrut said. In 2022, Canadian officials said blood banks would no longer require MSM donors to have been abstinent from sex for 3 months, the BBC reported. However, donors will be asked about high-risk sexual behaviors.
The United States, where HIV spread through the blood supply during the early years of the AIDS pandemic and killed thousands of hemophiliacs, has much been slower to change its policies. For decades, starting in the 1980s, both blood banks and stem cell donation programs chose to lower the risk by turning away MSM donors.
Policies only began to change in recent years. Be the Match’s registry led the way by welcoming MSM in 2015. Stem cell donations go through more extensive testing than blood donations, Dr. Adkins said, so it’s more likely that HIV will be screened out. Also, he said, officials probably realized “it was necessary to widen the donor pool in order to best serve the patients” because it’s so hard to find matched stem-cell donors.
Be the Match has also stepped up its outreach to the LGBTQ community. “During Pride Month in 2022, Be The Match sponsored booths at events in 12 major markets from coast to coast,” said Jamie Margolis, senior vice president of Donor Services. “These efforts enabled us to increase awareness among more than 500,000 festival attendees and added more than 2,000 new members to the Be The Match Registry. We also produced a social media awareness campaign featuring one of our own employees, who is a cofounder of the Pride Employee Resource Group at Be The Match and a recent blood stem cell donor.”
In 2020 as blood banks became desperate for donations during the early days of the COVID-19 pandemic, the FDA changed its policy and required MSM to be abstinent for 3 months instead of 1 year before giving blood. (Prior to December 2014, any man who’d had sex with a man, even once, was indefinitely banned from giving blood.)
The 3-month policy instituted in 2020 drew fire from critics such as the American Medical Association, which noted the regulation treated men differently if they had unprotected sex with a single man versus with multiple women.
Now, the FDA is proposing that it once again change the policy about blood donations: It is recommending that there be no special polices regarding MSM. “All prospective donors who report having a new sexual partner or more than one sexual partner and had anal sex in the past 3 months would be deferred from donation.”
Under the proposal, anyone who’s ever had HIV will not be able to donate. (They can’t donate stem cells either.) And the FDA proposes restrictions on those who take pre-exposure prophylaxis or postexposure prophylaxis for HIV.
Margolis, of Be the Match, noted that some members of the LGBTQ community may not be able to donate to Be The Match BioTherapies, which works with cell and gene therapy developers worldwide to provide cellular starting material. “These therapies may have different requirements than those for blood stem-cell transplants. Men who have had sex with men in the past 5 years or women who have had sex with a man who has had sex with a man in the past 5 years may not be able to donate to Be The Match BioTherapies. While we understand this could be upsetting or frustrating for someone who desires to be a part of these therapies, we are committed to following medical guidelines and regulations, while also advocating for our donors and the LBGTQIA+ community as a whole.”
MSM aren’t the only target of outreach by proponents of stem cell donation. In 2019, UT Southwestern’s Dr. Adkins and colleagues wrote a commentary in Bone Marrow Transplantation that called for bone marrow donation centers to do more to be welcoming to transgender donors. “The largest age group identifying as transgender is 18-24 years of life, which overlaps considerably with the population of hematopoietic stem cell donors, which tend to be younger individuals,” the researchers wrote.
The transgender community was “simply overlooked,” Dr. Adkins said. Since then, as he pointed out, things have changed. Now, Be the Match’s website notes that “members of the LGBTQIA+ community CAN join the registry and donate.” The organization says that “for medical reasons, everyone is asked to provide their sex assigned at birth when they register. Should you be called as a match, pronouns and gender identity are respected throughout the process.”
In addition, the site says people on prescription hormone therapy are not excluded from joining the registry. Patients who have undergone surgery within the last 12 months, including sex-reassignment procedures, “will be asked about the current status of their recovery and whether they are still seeing a physician for follow-up in regards to the surgery.”
What’s next? Dr. Fingrut said he expects the lifting of strict rules about MSM and blood donation will boost stem cell donation in the community.
There seems to be plenty of room for more outreach. Cole Williams, founder of Pride & Plasma, which advocates for allowing gay men to give blood, suggested in an interview that advocates who want to increase stem cell donation in the LGBTQ community reach out to its community centers, health organizations, providers, and clinics.
So far, though, “I haven’t seen a big call for registration of any individuals unless they have a personal relation to bone marrow donation,” he said.
Dr. Fingrut and Dr. Adkins report no disclosures.
In fact, gay men have been able to donate stem cells in the United States since 2015. That’s when National Marrow Donor Program’s Be the Match registry lifted restrictions on men who have sex with men (MSM).
Physicians say advocacy is still necessary, because LGBTQ people may assume they can’t donate or be wary of clinicians. “The LGBTQIA+ population in general has experienced a lot of issues with the medical-industrial complex in terms of discrimination and inappropriate care,” said UT Southwestern Medical Center pathologist Brian Adkins, MD, who manages the blood bank at Children’s Health in Dallas, in an interview. “There’s a weariness there that may produce some hesitancy to interact with the donation process.”
An estimated 6.8 million people give blood in the United States each year, and an estimated 9 million people are registered as potential stem cell donors. A total of 22,013 hematopoietic cell transplantation procedures were performed in 2020, according to the U.S. Health Resources and Services Administration.
Expanding the number of LGBTQ donors, especially those born as biological males, could pay major dividends. As Dr. Adkins noted, the ideal stem cell donor is young – Be the Match says doctors generally prefer donors aged 18-35 – and male. According to a 2021 Gallup Poll, 21% of those born from 1997 to 2003 (Generation Z) say they’re LGBTQ, as do 11% of those born from 1981 to 1996 (Millennials).
In North America, the most extensive outreach to the LGBTQ community about stem cell donation has been launched in Canada. There, an organization called Stem Cell Club focuses on encouraging college students and other young people to register as potential stem cell donors.
Stem Cell Club has several campaigns aimed at ethnic minority groups, and its Saving Lives With Pride project focuses on MSM. The project’s web page includes testimonials from a woman whose life was saved by an unrelated gay male donor and from a gay male nurse who recovered from blood cancer thanks to a stem cell donation. The site also includes videos about stem cell donation featuring LGBTQ young people and Canadian hematologists.
“Our specialized collection center will treat donors with the highest levels of respect and courtesy, indeed as heroes of their unselfish gift that can truly save a life,” says Ottawa Hospital transplant hematologist David Allan, MD, in one of the videos.
Stem Cell Club was founded by transplant hematologist Warren Fingrut, MD, a research fellow at Memorial Sloan Kettering Cancer Center. In an interview, he said the organization’s LGBTQ project has promoted stem cell donation at several annual gay pride events and will continue the outreach this coming summer. In 2018 and 2019, advocates recruited 354 potential stem-cell donors (40% male, 42 non-White) at five pride events, Dr. Fingrut and colleagues reported last year in the journal Bone Marrow Transplantation.
For a new study, researchers interviewed 37 gay and bisexual men from five Canadian provinces about stem cell donation. Dr. Fingrut and colleagues reported the findings in February in an abstract at the Transplantation & Cellular Therapy Meetings.
Most participants didn’t know they “are eligible to donate stem cells, with many confusing stem cell versus blood donor eligibility criteria,” the researchers reported. According to Dr. Fingrut, some of the men “felt they were treated as second-class citizens, and that translated into frustration and decreased motivation to donate. There were concerns that they would be treated as though they shouldn’t be there.”
Canada has allowed gay men to donate stem cells for at least 10 years, Dr. Fingrut said. In 2022, Canadian officials said blood banks would no longer require MSM donors to have been abstinent from sex for 3 months, the BBC reported. However, donors will be asked about high-risk sexual behaviors.
The United States, where HIV spread through the blood supply during the early years of the AIDS pandemic and killed thousands of hemophiliacs, has much been slower to change its policies. For decades, starting in the 1980s, both blood banks and stem cell donation programs chose to lower the risk by turning away MSM donors.
Policies only began to change in recent years. Be the Match’s registry led the way by welcoming MSM in 2015. Stem cell donations go through more extensive testing than blood donations, Dr. Adkins said, so it’s more likely that HIV will be screened out. Also, he said, officials probably realized “it was necessary to widen the donor pool in order to best serve the patients” because it’s so hard to find matched stem-cell donors.
Be the Match has also stepped up its outreach to the LGBTQ community. “During Pride Month in 2022, Be The Match sponsored booths at events in 12 major markets from coast to coast,” said Jamie Margolis, senior vice president of Donor Services. “These efforts enabled us to increase awareness among more than 500,000 festival attendees and added more than 2,000 new members to the Be The Match Registry. We also produced a social media awareness campaign featuring one of our own employees, who is a cofounder of the Pride Employee Resource Group at Be The Match and a recent blood stem cell donor.”
In 2020 as blood banks became desperate for donations during the early days of the COVID-19 pandemic, the FDA changed its policy and required MSM to be abstinent for 3 months instead of 1 year before giving blood. (Prior to December 2014, any man who’d had sex with a man, even once, was indefinitely banned from giving blood.)
The 3-month policy instituted in 2020 drew fire from critics such as the American Medical Association, which noted the regulation treated men differently if they had unprotected sex with a single man versus with multiple women.
Now, the FDA is proposing that it once again change the policy about blood donations: It is recommending that there be no special polices regarding MSM. “All prospective donors who report having a new sexual partner or more than one sexual partner and had anal sex in the past 3 months would be deferred from donation.”
Under the proposal, anyone who’s ever had HIV will not be able to donate. (They can’t donate stem cells either.) And the FDA proposes restrictions on those who take pre-exposure prophylaxis or postexposure prophylaxis for HIV.
Margolis, of Be the Match, noted that some members of the LGBTQ community may not be able to donate to Be The Match BioTherapies, which works with cell and gene therapy developers worldwide to provide cellular starting material. “These therapies may have different requirements than those for blood stem-cell transplants. Men who have had sex with men in the past 5 years or women who have had sex with a man who has had sex with a man in the past 5 years may not be able to donate to Be The Match BioTherapies. While we understand this could be upsetting or frustrating for someone who desires to be a part of these therapies, we are committed to following medical guidelines and regulations, while also advocating for our donors and the LBGTQIA+ community as a whole.”
MSM aren’t the only target of outreach by proponents of stem cell donation. In 2019, UT Southwestern’s Dr. Adkins and colleagues wrote a commentary in Bone Marrow Transplantation that called for bone marrow donation centers to do more to be welcoming to transgender donors. “The largest age group identifying as transgender is 18-24 years of life, which overlaps considerably with the population of hematopoietic stem cell donors, which tend to be younger individuals,” the researchers wrote.
The transgender community was “simply overlooked,” Dr. Adkins said. Since then, as he pointed out, things have changed. Now, Be the Match’s website notes that “members of the LGBTQIA+ community CAN join the registry and donate.” The organization says that “for medical reasons, everyone is asked to provide their sex assigned at birth when they register. Should you be called as a match, pronouns and gender identity are respected throughout the process.”
In addition, the site says people on prescription hormone therapy are not excluded from joining the registry. Patients who have undergone surgery within the last 12 months, including sex-reassignment procedures, “will be asked about the current status of their recovery and whether they are still seeing a physician for follow-up in regards to the surgery.”
What’s next? Dr. Fingrut said he expects the lifting of strict rules about MSM and blood donation will boost stem cell donation in the community.
There seems to be plenty of room for more outreach. Cole Williams, founder of Pride & Plasma, which advocates for allowing gay men to give blood, suggested in an interview that advocates who want to increase stem cell donation in the LGBTQ community reach out to its community centers, health organizations, providers, and clinics.
So far, though, “I haven’t seen a big call for registration of any individuals unless they have a personal relation to bone marrow donation,” he said.
Dr. Fingrut and Dr. Adkins report no disclosures.
In fact, gay men have been able to donate stem cells in the United States since 2015. That’s when National Marrow Donor Program’s Be the Match registry lifted restrictions on men who have sex with men (MSM).
Physicians say advocacy is still necessary, because LGBTQ people may assume they can’t donate or be wary of clinicians. “The LGBTQIA+ population in general has experienced a lot of issues with the medical-industrial complex in terms of discrimination and inappropriate care,” said UT Southwestern Medical Center pathologist Brian Adkins, MD, who manages the blood bank at Children’s Health in Dallas, in an interview. “There’s a weariness there that may produce some hesitancy to interact with the donation process.”
An estimated 6.8 million people give blood in the United States each year, and an estimated 9 million people are registered as potential stem cell donors. A total of 22,013 hematopoietic cell transplantation procedures were performed in 2020, according to the U.S. Health Resources and Services Administration.
Expanding the number of LGBTQ donors, especially those born as biological males, could pay major dividends. As Dr. Adkins noted, the ideal stem cell donor is young – Be the Match says doctors generally prefer donors aged 18-35 – and male. According to a 2021 Gallup Poll, 21% of those born from 1997 to 2003 (Generation Z) say they’re LGBTQ, as do 11% of those born from 1981 to 1996 (Millennials).
In North America, the most extensive outreach to the LGBTQ community about stem cell donation has been launched in Canada. There, an organization called Stem Cell Club focuses on encouraging college students and other young people to register as potential stem cell donors.
Stem Cell Club has several campaigns aimed at ethnic minority groups, and its Saving Lives With Pride project focuses on MSM. The project’s web page includes testimonials from a woman whose life was saved by an unrelated gay male donor and from a gay male nurse who recovered from blood cancer thanks to a stem cell donation. The site also includes videos about stem cell donation featuring LGBTQ young people and Canadian hematologists.
“Our specialized collection center will treat donors with the highest levels of respect and courtesy, indeed as heroes of their unselfish gift that can truly save a life,” says Ottawa Hospital transplant hematologist David Allan, MD, in one of the videos.
Stem Cell Club was founded by transplant hematologist Warren Fingrut, MD, a research fellow at Memorial Sloan Kettering Cancer Center. In an interview, he said the organization’s LGBTQ project has promoted stem cell donation at several annual gay pride events and will continue the outreach this coming summer. In 2018 and 2019, advocates recruited 354 potential stem-cell donors (40% male, 42 non-White) at five pride events, Dr. Fingrut and colleagues reported last year in the journal Bone Marrow Transplantation.
For a new study, researchers interviewed 37 gay and bisexual men from five Canadian provinces about stem cell donation. Dr. Fingrut and colleagues reported the findings in February in an abstract at the Transplantation & Cellular Therapy Meetings.
Most participants didn’t know they “are eligible to donate stem cells, with many confusing stem cell versus blood donor eligibility criteria,” the researchers reported. According to Dr. Fingrut, some of the men “felt they were treated as second-class citizens, and that translated into frustration and decreased motivation to donate. There were concerns that they would be treated as though they shouldn’t be there.”
Canada has allowed gay men to donate stem cells for at least 10 years, Dr. Fingrut said. In 2022, Canadian officials said blood banks would no longer require MSM donors to have been abstinent from sex for 3 months, the BBC reported. However, donors will be asked about high-risk sexual behaviors.
The United States, where HIV spread through the blood supply during the early years of the AIDS pandemic and killed thousands of hemophiliacs, has much been slower to change its policies. For decades, starting in the 1980s, both blood banks and stem cell donation programs chose to lower the risk by turning away MSM donors.
Policies only began to change in recent years. Be the Match’s registry led the way by welcoming MSM in 2015. Stem cell donations go through more extensive testing than blood donations, Dr. Adkins said, so it’s more likely that HIV will be screened out. Also, he said, officials probably realized “it was necessary to widen the donor pool in order to best serve the patients” because it’s so hard to find matched stem-cell donors.
Be the Match has also stepped up its outreach to the LGBTQ community. “During Pride Month in 2022, Be The Match sponsored booths at events in 12 major markets from coast to coast,” said Jamie Margolis, senior vice president of Donor Services. “These efforts enabled us to increase awareness among more than 500,000 festival attendees and added more than 2,000 new members to the Be The Match Registry. We also produced a social media awareness campaign featuring one of our own employees, who is a cofounder of the Pride Employee Resource Group at Be The Match and a recent blood stem cell donor.”
In 2020 as blood banks became desperate for donations during the early days of the COVID-19 pandemic, the FDA changed its policy and required MSM to be abstinent for 3 months instead of 1 year before giving blood. (Prior to December 2014, any man who’d had sex with a man, even once, was indefinitely banned from giving blood.)
The 3-month policy instituted in 2020 drew fire from critics such as the American Medical Association, which noted the regulation treated men differently if they had unprotected sex with a single man versus with multiple women.
Now, the FDA is proposing that it once again change the policy about blood donations: It is recommending that there be no special polices regarding MSM. “All prospective donors who report having a new sexual partner or more than one sexual partner and had anal sex in the past 3 months would be deferred from donation.”
Under the proposal, anyone who’s ever had HIV will not be able to donate. (They can’t donate stem cells either.) And the FDA proposes restrictions on those who take pre-exposure prophylaxis or postexposure prophylaxis for HIV.
Margolis, of Be the Match, noted that some members of the LGBTQ community may not be able to donate to Be The Match BioTherapies, which works with cell and gene therapy developers worldwide to provide cellular starting material. “These therapies may have different requirements than those for blood stem-cell transplants. Men who have had sex with men in the past 5 years or women who have had sex with a man who has had sex with a man in the past 5 years may not be able to donate to Be The Match BioTherapies. While we understand this could be upsetting or frustrating for someone who desires to be a part of these therapies, we are committed to following medical guidelines and regulations, while also advocating for our donors and the LBGTQIA+ community as a whole.”
MSM aren’t the only target of outreach by proponents of stem cell donation. In 2019, UT Southwestern’s Dr. Adkins and colleagues wrote a commentary in Bone Marrow Transplantation that called for bone marrow donation centers to do more to be welcoming to transgender donors. “The largest age group identifying as transgender is 18-24 years of life, which overlaps considerably with the population of hematopoietic stem cell donors, which tend to be younger individuals,” the researchers wrote.
The transgender community was “simply overlooked,” Dr. Adkins said. Since then, as he pointed out, things have changed. Now, Be the Match’s website notes that “members of the LGBTQIA+ community CAN join the registry and donate.” The organization says that “for medical reasons, everyone is asked to provide their sex assigned at birth when they register. Should you be called as a match, pronouns and gender identity are respected throughout the process.”
In addition, the site says people on prescription hormone therapy are not excluded from joining the registry. Patients who have undergone surgery within the last 12 months, including sex-reassignment procedures, “will be asked about the current status of their recovery and whether they are still seeing a physician for follow-up in regards to the surgery.”
What’s next? Dr. Fingrut said he expects the lifting of strict rules about MSM and blood donation will boost stem cell donation in the community.
There seems to be plenty of room for more outreach. Cole Williams, founder of Pride & Plasma, which advocates for allowing gay men to give blood, suggested in an interview that advocates who want to increase stem cell donation in the LGBTQ community reach out to its community centers, health organizations, providers, and clinics.
So far, though, “I haven’t seen a big call for registration of any individuals unless they have a personal relation to bone marrow donation,” he said.
Dr. Fingrut and Dr. Adkins report no disclosures.
Specialty and age may contribute to suicidal thoughts among physicians
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
For young people on Medicare, a hysterectomy sometimes is more affordable than birth control
Sam Chavarría said her doctor was clear about the birth defects her medication could cause if she became pregnant but agreed to keep her on it as long as she had an IUD.
As she was waiting to get her contraceptive intrauterine device replaced at her local clinic, however, the billing nurse told her that her insurance wouldn’t cover the removal – or a new IUD. Chavarría didn’t understand why not.
“Then she said very delicately, ‘Well, people on this insurance typically tend to be older,’ ” Chavarría recalled.
Although Chavarría is 34, she is enrolled in Medicare, the government insurance program designed for those 65 and older. Chavarría, who lives in Houston, is disabled by fibromyalgia, rheumatoid arthritis, and mental health issues. Medicare automatically enrolls anyone who has received Social Security disability benefits for two years and this was her first time getting an IUD while in the government program.
Without insurance, just removing her expired IUD would cost Chavarría $350 out of pocket; exchanging it for a new one would be $2,000. She left the clinic in tears.
Chavarría’s experience is not rare. Medicare was originally intended for people of retirement age. Over the years, the program has evolved to include new populations, such as those who have disabilities or are critically ill, said Jennifer Lea Huer, a public health expert at Yale University, New Haven, Conn. In 2020, 1.7 million people ages 18-44 were enrolled in Medicare.
An estimated 70% of childbearing-age women on Medicare are also eligible for Medicaid, a state and federal program for those with low incomes, which should fill the gap for contraception. It’s not clear how many transgender or nonbinary people – who also might need contraception – are on Medicare or are eligible for Medicaid.
Medicaid, like the plans offered via the federal Affordable Care Act, mandates coverage of birth control. But those who aren’t eligible for Medicaid are left in the lurch – Medicare’s origins mean it does not require access to birth control.
Traditional Medicare includes two parts: Part A covers hospital costs, while Part B covers physicians’ care and certain other services, such as ambulance rides. Neither ordinarily includes contraception.
People can get contraception through a Medicare Advantage plan or Part D of Medicare, which covers prescription drugs, but those come at a cost. And even people who pay for Part D often aren’t covered for some types of birth control, such as IUDs.
“So, if you are disabled, if you are locked outside of the labor market, if you do not have the means or any other way to financially support yourself, you were likely still on traditional Medicare, which is Part A and Part B,” Huer said. “In which case, your access to contraception is incredibly difficult.”
Contraception for those with traditional Medicare is given on a case-by-case basis, Huer said. It can be covered only if a doctor can make a credible case that the patient needs it for medical reasons – because their body cannot sustain a pregnancy – as opposed to merely wanting to avoid one.
“You have to have a champion physician who’s willing to partner with you and make those arguments,” Huer said.
That’s what Chavarría’s doctor tried to do. Before she left the clinic, staffers there told her they would try to make the case she needed the IUD for medical reasons. The IUD exchange was scheduled almost 10 weeks later, but during those weeks, she got pregnant. Her body couldn’t sustain a pregnancy, so she and her partner rushed to get an abortion just before Texas tightened its rules Sept. 1, 2021.
“If Medicare had just covered the IUD removal or exchange to begin with, none of this would have happened,” Chavarría said. “It would have saved me having to make a really tough decision that I never thought I’d have to make.”
Women with disabilities often face a stigma from health care practitioners, especially when it comes to birth control, said Willi Horner-Johnson, a public health researcher specializing in disabilities at Oregon Health & Science University, Portland. In her research, women with disabilities have described being treated like children or having to go to multiple doctors to find someone with whom they felt comfortable.
“We don’t want to acknowledge that disabled people have sex,” said Miriam Garber, a 36-year-old sex worker who lives in Rhode Island and is also on Medicare because of her disabilities. Garber got an IUD from Planned Parenthood because her insurance wouldn’t cover it.
Even those who pay for Part D to have their prescription drugs covered and have a “champion physician” face difficulties. Liz Moore, a nonbinary person in their 30s who lives in the Washington, D.C., area, could not get Medicare to pay for the Mirena IUD their doctor prescribed for their polycystic ovary syndrome. Moore is disabled with fibromyalgia and dysautonomia, a condition of the autonomic nervous system, which regulates breathing, heart rate, and more.
“After literally months of phone calls, it seemed like my Medicare Part D and original Medicare could not agree on who should pay for my IUD,” they wrote in a direct message. “Was it a prescription or durable medical equipment?”
When Moore finally learned it would cost $800 upfront, they said, they decided to get a hysterectomy – which Medicare would pay for – instead.
Chavarría’s doctor told her a tubal ligation also was more likely to be approved by Medicare than an IUD, because older people have that procedure more often. Like all surgeries, both come with risks of complications and recovery.
Even for those on both Medicare and Medicaid, getting contraception also isn’t always easy, as in Katie Elizabeth Walsh’s case.
Walsh, 34, who lives in northeastern Connecticut, is disabled by a traumatic brain injury, depression, and chronic fatigue syndrome. She got an IUD at an ob.gyn. clinic and was told there her insurance would cover it.
Then she got a bill for nearly $2,000.
Medicaid should cover contraceptive devices for dual-eligibility people, according to Centers for Medicare & Medicaid Services policy guidance, but when Walsh tried to get her bill covered, Medicare and Medicaid could not agree on which of them should pay.
“Every single time I have called one of the insurance offices, they are like, ‘Oh, no, you have to talk to the other one, and we don’t really talk to each other,’ ” Walsh said.
Walsh said the hassle to get her contraception covered feels like a kick in the stomach: “Like truly you do not have a place in this world, and your insurance is telling you that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sam Chavarría said her doctor was clear about the birth defects her medication could cause if she became pregnant but agreed to keep her on it as long as she had an IUD.
As she was waiting to get her contraceptive intrauterine device replaced at her local clinic, however, the billing nurse told her that her insurance wouldn’t cover the removal – or a new IUD. Chavarría didn’t understand why not.
“Then she said very delicately, ‘Well, people on this insurance typically tend to be older,’ ” Chavarría recalled.
Although Chavarría is 34, she is enrolled in Medicare, the government insurance program designed for those 65 and older. Chavarría, who lives in Houston, is disabled by fibromyalgia, rheumatoid arthritis, and mental health issues. Medicare automatically enrolls anyone who has received Social Security disability benefits for two years and this was her first time getting an IUD while in the government program.
Without insurance, just removing her expired IUD would cost Chavarría $350 out of pocket; exchanging it for a new one would be $2,000. She left the clinic in tears.
Chavarría’s experience is not rare. Medicare was originally intended for people of retirement age. Over the years, the program has evolved to include new populations, such as those who have disabilities or are critically ill, said Jennifer Lea Huer, a public health expert at Yale University, New Haven, Conn. In 2020, 1.7 million people ages 18-44 were enrolled in Medicare.
An estimated 70% of childbearing-age women on Medicare are also eligible for Medicaid, a state and federal program for those with low incomes, which should fill the gap for contraception. It’s not clear how many transgender or nonbinary people – who also might need contraception – are on Medicare or are eligible for Medicaid.
Medicaid, like the plans offered via the federal Affordable Care Act, mandates coverage of birth control. But those who aren’t eligible for Medicaid are left in the lurch – Medicare’s origins mean it does not require access to birth control.
Traditional Medicare includes two parts: Part A covers hospital costs, while Part B covers physicians’ care and certain other services, such as ambulance rides. Neither ordinarily includes contraception.
People can get contraception through a Medicare Advantage plan or Part D of Medicare, which covers prescription drugs, but those come at a cost. And even people who pay for Part D often aren’t covered for some types of birth control, such as IUDs.
“So, if you are disabled, if you are locked outside of the labor market, if you do not have the means or any other way to financially support yourself, you were likely still on traditional Medicare, which is Part A and Part B,” Huer said. “In which case, your access to contraception is incredibly difficult.”
Contraception for those with traditional Medicare is given on a case-by-case basis, Huer said. It can be covered only if a doctor can make a credible case that the patient needs it for medical reasons – because their body cannot sustain a pregnancy – as opposed to merely wanting to avoid one.
“You have to have a champion physician who’s willing to partner with you and make those arguments,” Huer said.
That’s what Chavarría’s doctor tried to do. Before she left the clinic, staffers there told her they would try to make the case she needed the IUD for medical reasons. The IUD exchange was scheduled almost 10 weeks later, but during those weeks, she got pregnant. Her body couldn’t sustain a pregnancy, so she and her partner rushed to get an abortion just before Texas tightened its rules Sept. 1, 2021.
“If Medicare had just covered the IUD removal or exchange to begin with, none of this would have happened,” Chavarría said. “It would have saved me having to make a really tough decision that I never thought I’d have to make.”
Women with disabilities often face a stigma from health care practitioners, especially when it comes to birth control, said Willi Horner-Johnson, a public health researcher specializing in disabilities at Oregon Health & Science University, Portland. In her research, women with disabilities have described being treated like children or having to go to multiple doctors to find someone with whom they felt comfortable.
“We don’t want to acknowledge that disabled people have sex,” said Miriam Garber, a 36-year-old sex worker who lives in Rhode Island and is also on Medicare because of her disabilities. Garber got an IUD from Planned Parenthood because her insurance wouldn’t cover it.
Even those who pay for Part D to have their prescription drugs covered and have a “champion physician” face difficulties. Liz Moore, a nonbinary person in their 30s who lives in the Washington, D.C., area, could not get Medicare to pay for the Mirena IUD their doctor prescribed for their polycystic ovary syndrome. Moore is disabled with fibromyalgia and dysautonomia, a condition of the autonomic nervous system, which regulates breathing, heart rate, and more.
“After literally months of phone calls, it seemed like my Medicare Part D and original Medicare could not agree on who should pay for my IUD,” they wrote in a direct message. “Was it a prescription or durable medical equipment?”
When Moore finally learned it would cost $800 upfront, they said, they decided to get a hysterectomy – which Medicare would pay for – instead.
Chavarría’s doctor told her a tubal ligation also was more likely to be approved by Medicare than an IUD, because older people have that procedure more often. Like all surgeries, both come with risks of complications and recovery.
Even for those on both Medicare and Medicaid, getting contraception also isn’t always easy, as in Katie Elizabeth Walsh’s case.
Walsh, 34, who lives in northeastern Connecticut, is disabled by a traumatic brain injury, depression, and chronic fatigue syndrome. She got an IUD at an ob.gyn. clinic and was told there her insurance would cover it.
Then she got a bill for nearly $2,000.
Medicaid should cover contraceptive devices for dual-eligibility people, according to Centers for Medicare & Medicaid Services policy guidance, but when Walsh tried to get her bill covered, Medicare and Medicaid could not agree on which of them should pay.
“Every single time I have called one of the insurance offices, they are like, ‘Oh, no, you have to talk to the other one, and we don’t really talk to each other,’ ” Walsh said.
Walsh said the hassle to get her contraception covered feels like a kick in the stomach: “Like truly you do not have a place in this world, and your insurance is telling you that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sam Chavarría said her doctor was clear about the birth defects her medication could cause if she became pregnant but agreed to keep her on it as long as she had an IUD.
As she was waiting to get her contraceptive intrauterine device replaced at her local clinic, however, the billing nurse told her that her insurance wouldn’t cover the removal – or a new IUD. Chavarría didn’t understand why not.
“Then she said very delicately, ‘Well, people on this insurance typically tend to be older,’ ” Chavarría recalled.
Although Chavarría is 34, she is enrolled in Medicare, the government insurance program designed for those 65 and older. Chavarría, who lives in Houston, is disabled by fibromyalgia, rheumatoid arthritis, and mental health issues. Medicare automatically enrolls anyone who has received Social Security disability benefits for two years and this was her first time getting an IUD while in the government program.
Without insurance, just removing her expired IUD would cost Chavarría $350 out of pocket; exchanging it for a new one would be $2,000. She left the clinic in tears.
Chavarría’s experience is not rare. Medicare was originally intended for people of retirement age. Over the years, the program has evolved to include new populations, such as those who have disabilities or are critically ill, said Jennifer Lea Huer, a public health expert at Yale University, New Haven, Conn. In 2020, 1.7 million people ages 18-44 were enrolled in Medicare.
An estimated 70% of childbearing-age women on Medicare are also eligible for Medicaid, a state and federal program for those with low incomes, which should fill the gap for contraception. It’s not clear how many transgender or nonbinary people – who also might need contraception – are on Medicare or are eligible for Medicaid.
Medicaid, like the plans offered via the federal Affordable Care Act, mandates coverage of birth control. But those who aren’t eligible for Medicaid are left in the lurch – Medicare’s origins mean it does not require access to birth control.
Traditional Medicare includes two parts: Part A covers hospital costs, while Part B covers physicians’ care and certain other services, such as ambulance rides. Neither ordinarily includes contraception.
People can get contraception through a Medicare Advantage plan or Part D of Medicare, which covers prescription drugs, but those come at a cost. And even people who pay for Part D often aren’t covered for some types of birth control, such as IUDs.
“So, if you are disabled, if you are locked outside of the labor market, if you do not have the means or any other way to financially support yourself, you were likely still on traditional Medicare, which is Part A and Part B,” Huer said. “In which case, your access to contraception is incredibly difficult.”
Contraception for those with traditional Medicare is given on a case-by-case basis, Huer said. It can be covered only if a doctor can make a credible case that the patient needs it for medical reasons – because their body cannot sustain a pregnancy – as opposed to merely wanting to avoid one.
“You have to have a champion physician who’s willing to partner with you and make those arguments,” Huer said.
That’s what Chavarría’s doctor tried to do. Before she left the clinic, staffers there told her they would try to make the case she needed the IUD for medical reasons. The IUD exchange was scheduled almost 10 weeks later, but during those weeks, she got pregnant. Her body couldn’t sustain a pregnancy, so she and her partner rushed to get an abortion just before Texas tightened its rules Sept. 1, 2021.
“If Medicare had just covered the IUD removal or exchange to begin with, none of this would have happened,” Chavarría said. “It would have saved me having to make a really tough decision that I never thought I’d have to make.”
Women with disabilities often face a stigma from health care practitioners, especially when it comes to birth control, said Willi Horner-Johnson, a public health researcher specializing in disabilities at Oregon Health & Science University, Portland. In her research, women with disabilities have described being treated like children or having to go to multiple doctors to find someone with whom they felt comfortable.
“We don’t want to acknowledge that disabled people have sex,” said Miriam Garber, a 36-year-old sex worker who lives in Rhode Island and is also on Medicare because of her disabilities. Garber got an IUD from Planned Parenthood because her insurance wouldn’t cover it.
Even those who pay for Part D to have their prescription drugs covered and have a “champion physician” face difficulties. Liz Moore, a nonbinary person in their 30s who lives in the Washington, D.C., area, could not get Medicare to pay for the Mirena IUD their doctor prescribed for their polycystic ovary syndrome. Moore is disabled with fibromyalgia and dysautonomia, a condition of the autonomic nervous system, which regulates breathing, heart rate, and more.
“After literally months of phone calls, it seemed like my Medicare Part D and original Medicare could not agree on who should pay for my IUD,” they wrote in a direct message. “Was it a prescription or durable medical equipment?”
When Moore finally learned it would cost $800 upfront, they said, they decided to get a hysterectomy – which Medicare would pay for – instead.
Chavarría’s doctor told her a tubal ligation also was more likely to be approved by Medicare than an IUD, because older people have that procedure more often. Like all surgeries, both come with risks of complications and recovery.
Even for those on both Medicare and Medicaid, getting contraception also isn’t always easy, as in Katie Elizabeth Walsh’s case.
Walsh, 34, who lives in northeastern Connecticut, is disabled by a traumatic brain injury, depression, and chronic fatigue syndrome. She got an IUD at an ob.gyn. clinic and was told there her insurance would cover it.
Then she got a bill for nearly $2,000.
Medicaid should cover contraceptive devices for dual-eligibility people, according to Centers for Medicare & Medicaid Services policy guidance, but when Walsh tried to get her bill covered, Medicare and Medicaid could not agree on which of them should pay.
“Every single time I have called one of the insurance offices, they are like, ‘Oh, no, you have to talk to the other one, and we don’t really talk to each other,’ ” Walsh said.
Walsh said the hassle to get her contraception covered feels like a kick in the stomach: “Like truly you do not have a place in this world, and your insurance is telling you that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Popular book by USC oncologist pulled because of plagiarism
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
Experts share real-world experience prescribing voclosporin, belimumab for lupus nephritis
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.