User login
What’s Eating You? Head Lice (Pediculus humanus capitis)
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
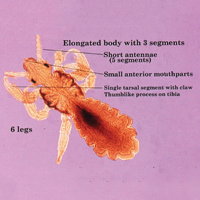
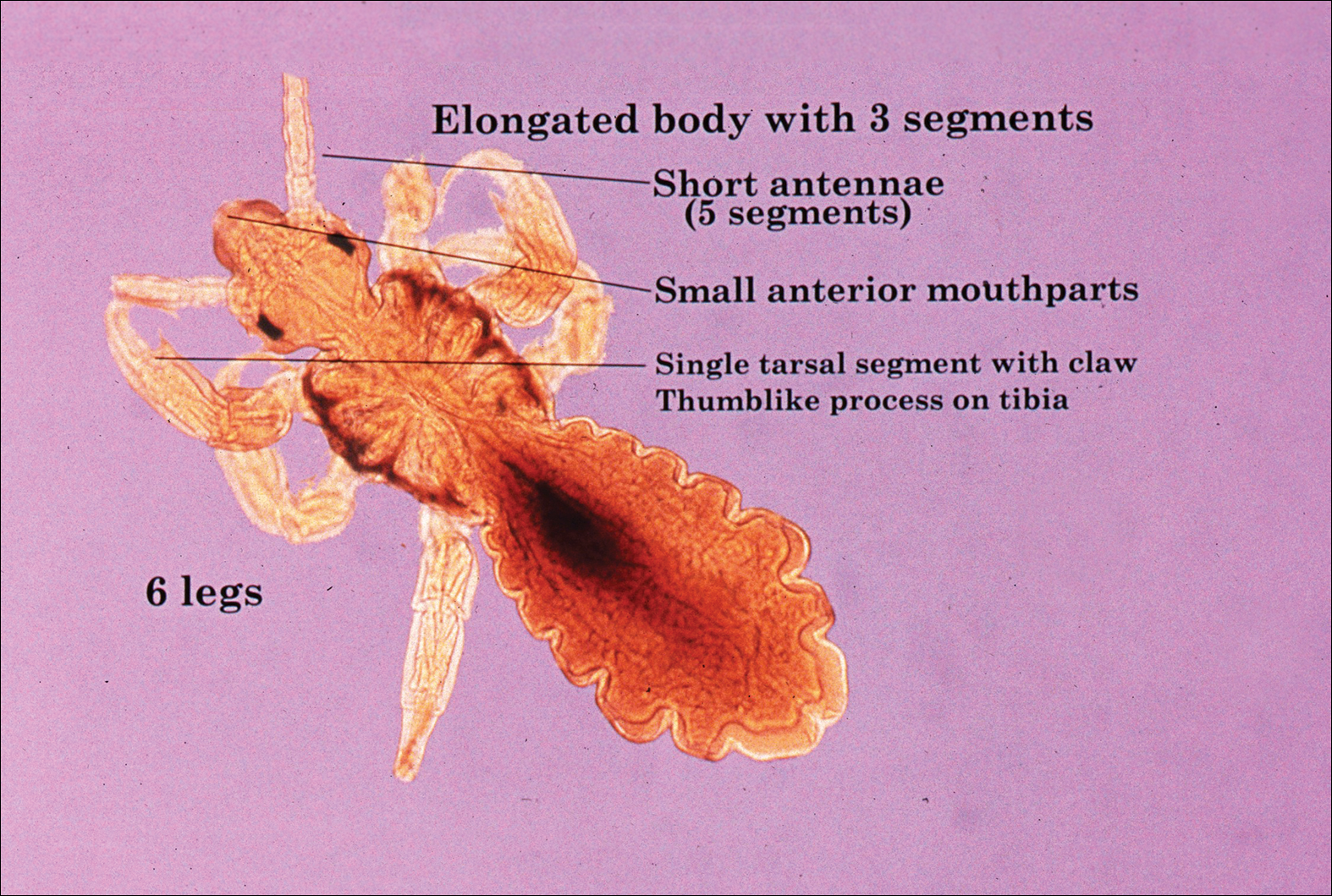
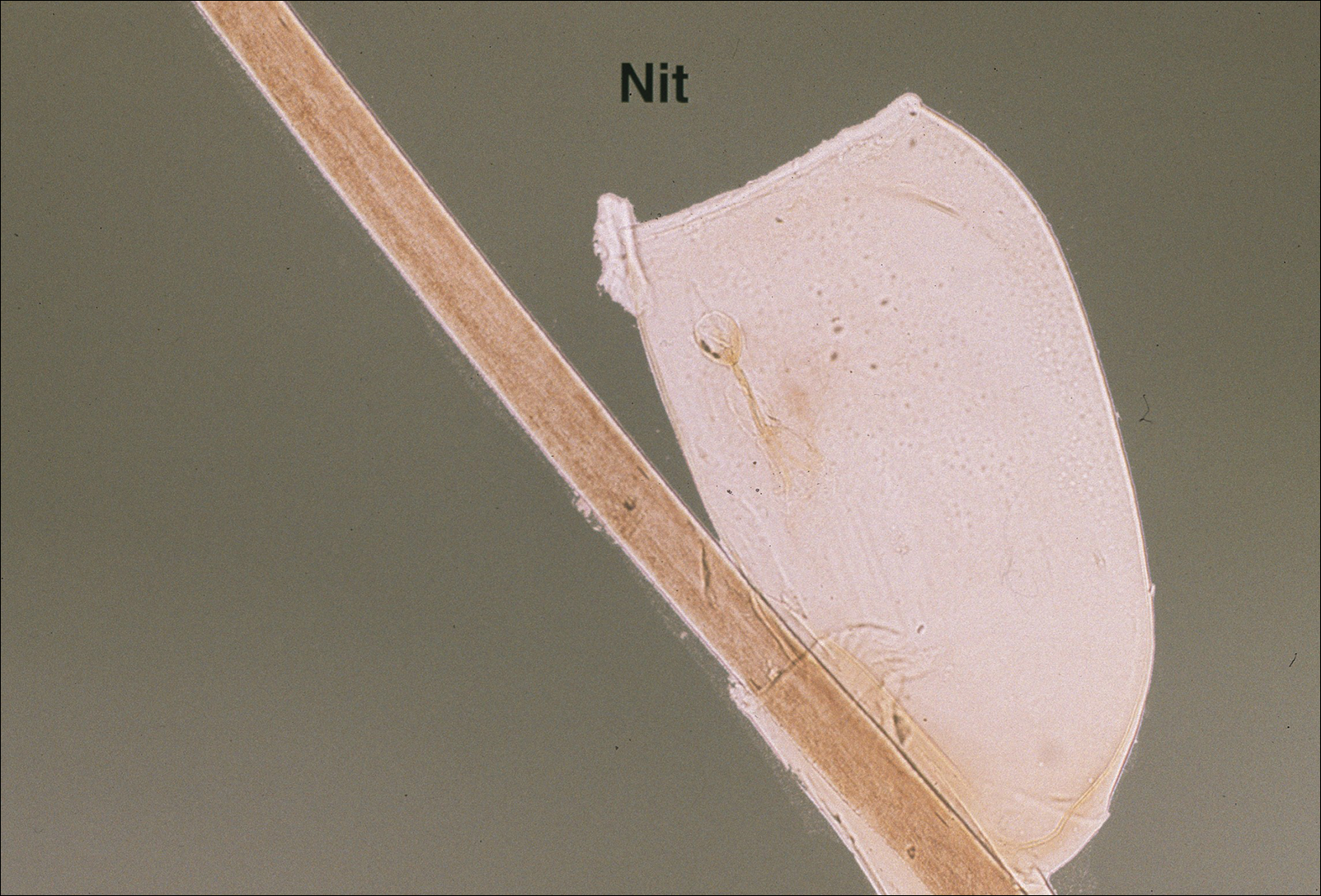
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4
Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
Practice Points
- Transmission of head lice occurs most frequently from direct head-to-head contact; however, head lice can survive up to 4 days on fomites.
- Patients present with scalp pruritus and bite reactions (papules or wheals), but pediculosis can be asymptomatic, particularly with the first exposure before the immune system has developed sensitivity to the louse saliva.
- Topical pyrethroids are available over-the-counter and are considered first-line therapy; however, resistance to pyrethroids has become an important problem in the United States and worldwide.
- Newer topical treatments such as benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, and ivermectin lotion 0.5% can be prescribed as alternative therapies, particularly if resistance to pyrethroids is a concern.
What’s Eating You? Scabies in the Developing World
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
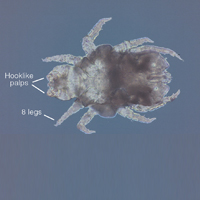
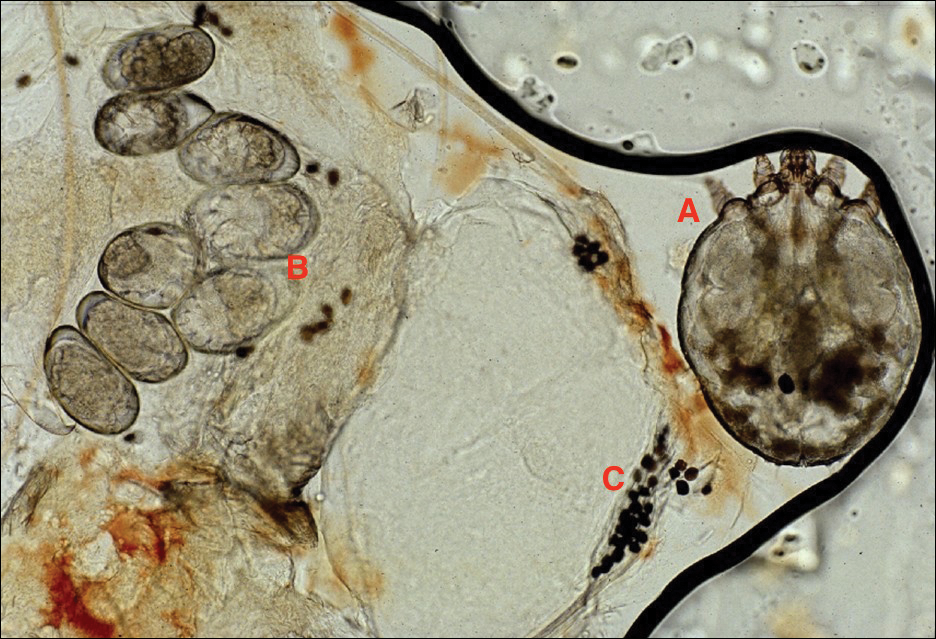
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
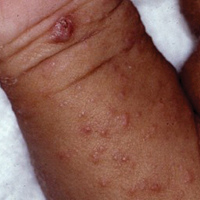
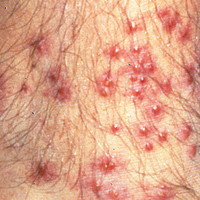
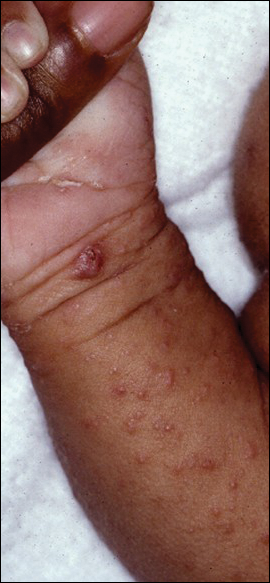
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.
What’s Eating You? Minute Brown Scavenger Beetle
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Practice Points
- Examining the specimens brought by a patient with delusional infestation is important for the therapeutic relationship.
- Clinicians must be able to recognize nonpathogenic insects that may incidentally be present in the specimen such as the minute brown scavenger beetle.
What's Eating You? Sticktight Flea Revisited
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).
Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Practice Points
- Although the primary host of the sticktight flea is poultry, it has been found in many species of birds and mammals, including humans.
- Flea bites cause irritation and itching for hosts, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.
- Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin.
What’s Eating You? Chiggers
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).
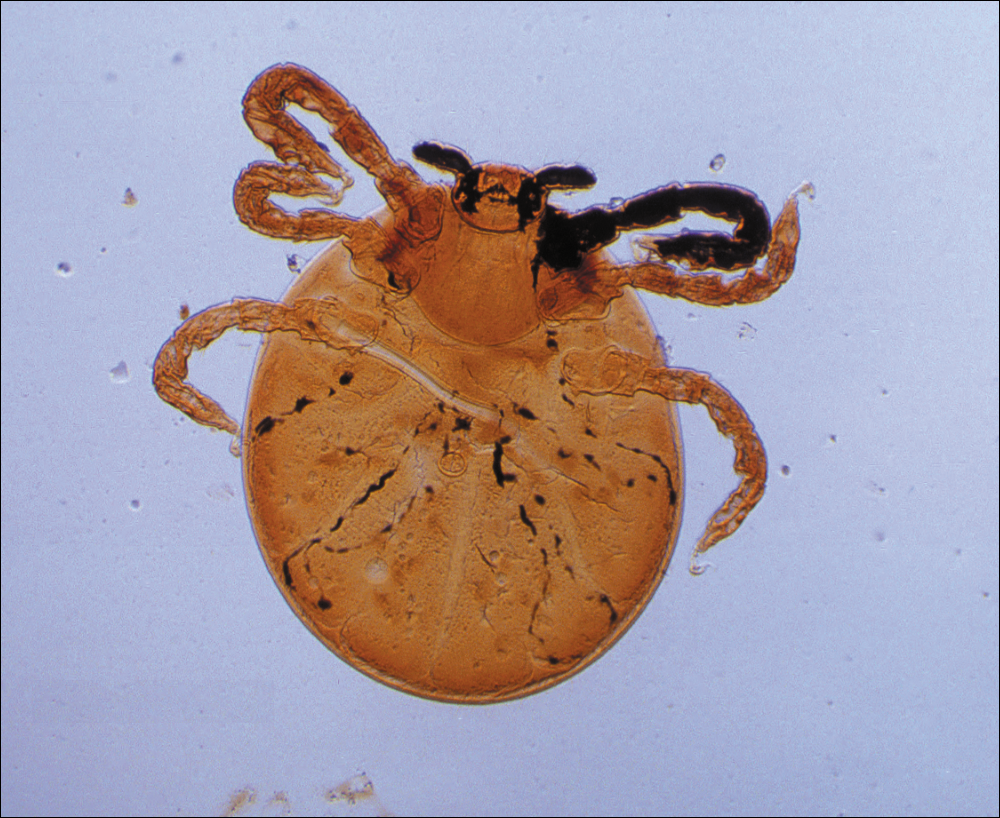
The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.
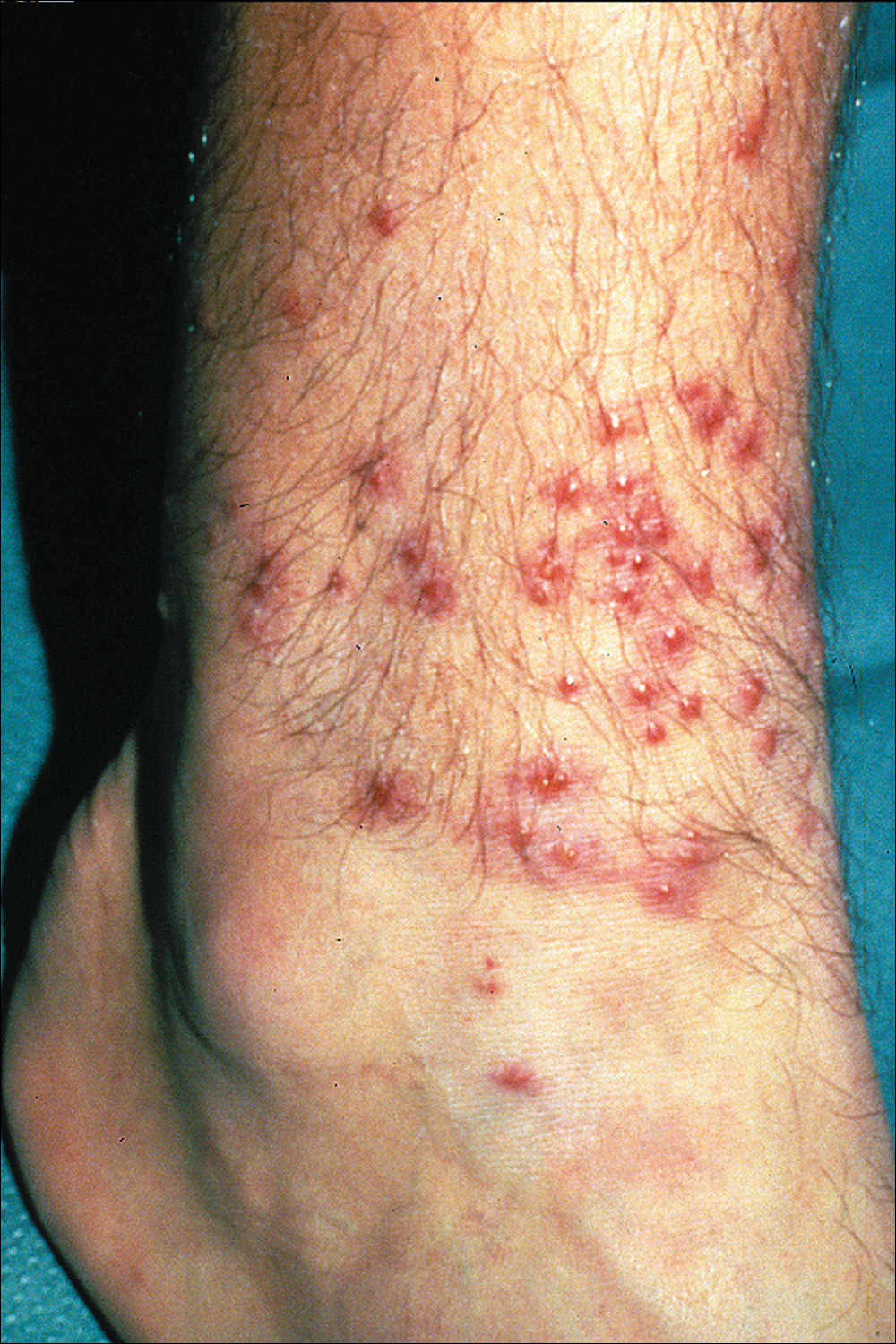
Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Practice Points
- The classic clinical presentation of chigger bites includes severe pruritus, cutaneous swelling, and erythematous papules and papulovesicles appearing in groups, most commonly affecting the legs and waistline.
- Because itching generally subsides within 72 hours of the chigger bite and cutaneous lesions typically heal within 1 to 2 weeks, treatment is focused on symptomatic relief.
- Symptomatic relief may be achieved by means of topical antipruritics or oral antihistamines as well as potent topical corticosteroids or an intralesional triamcinolone acetonide injection in severe cases.
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
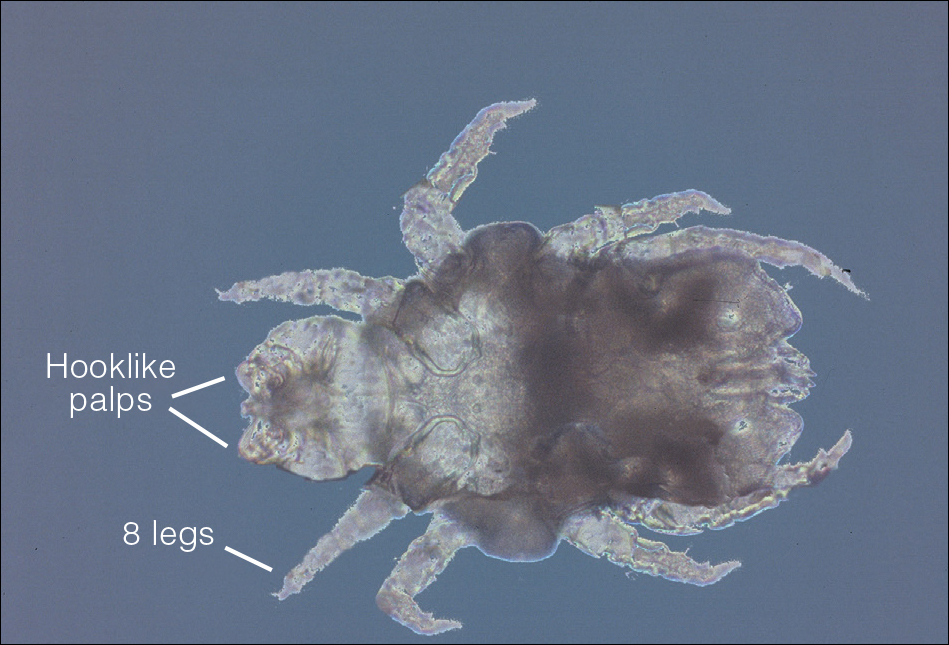
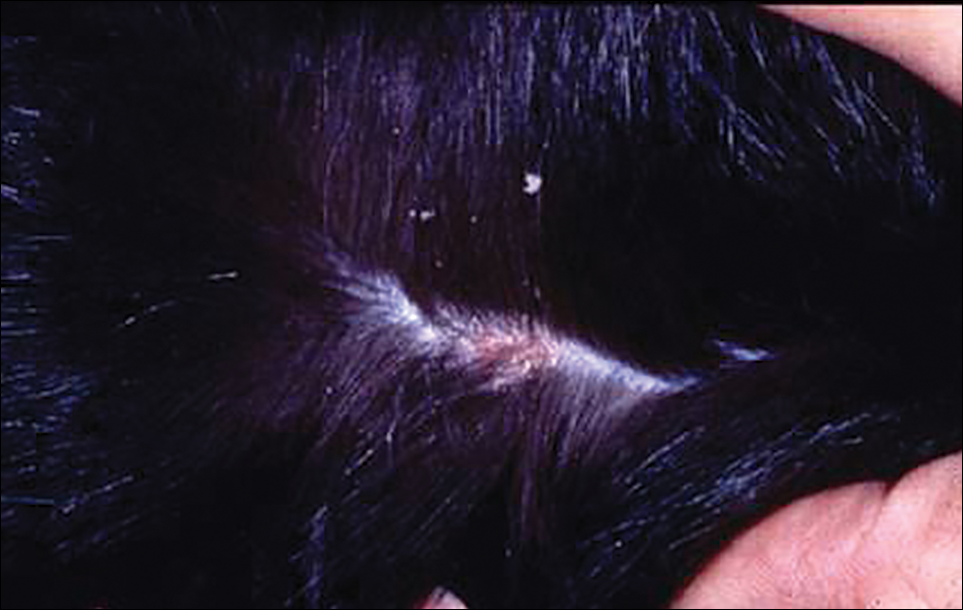
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
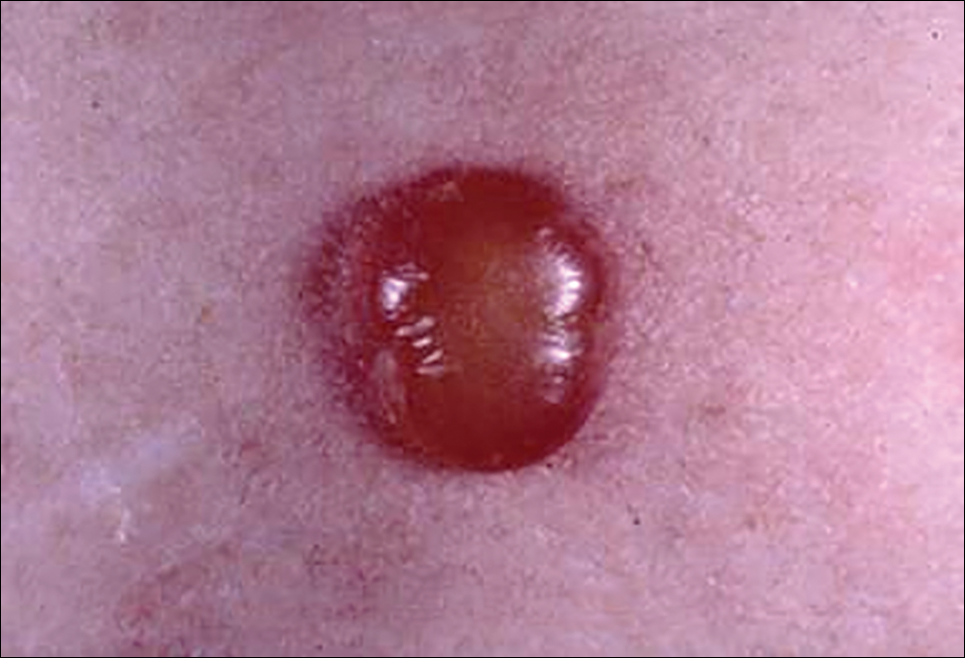
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.
What’s Eating You? Lone Star Tick (Amblyomma americanum)
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
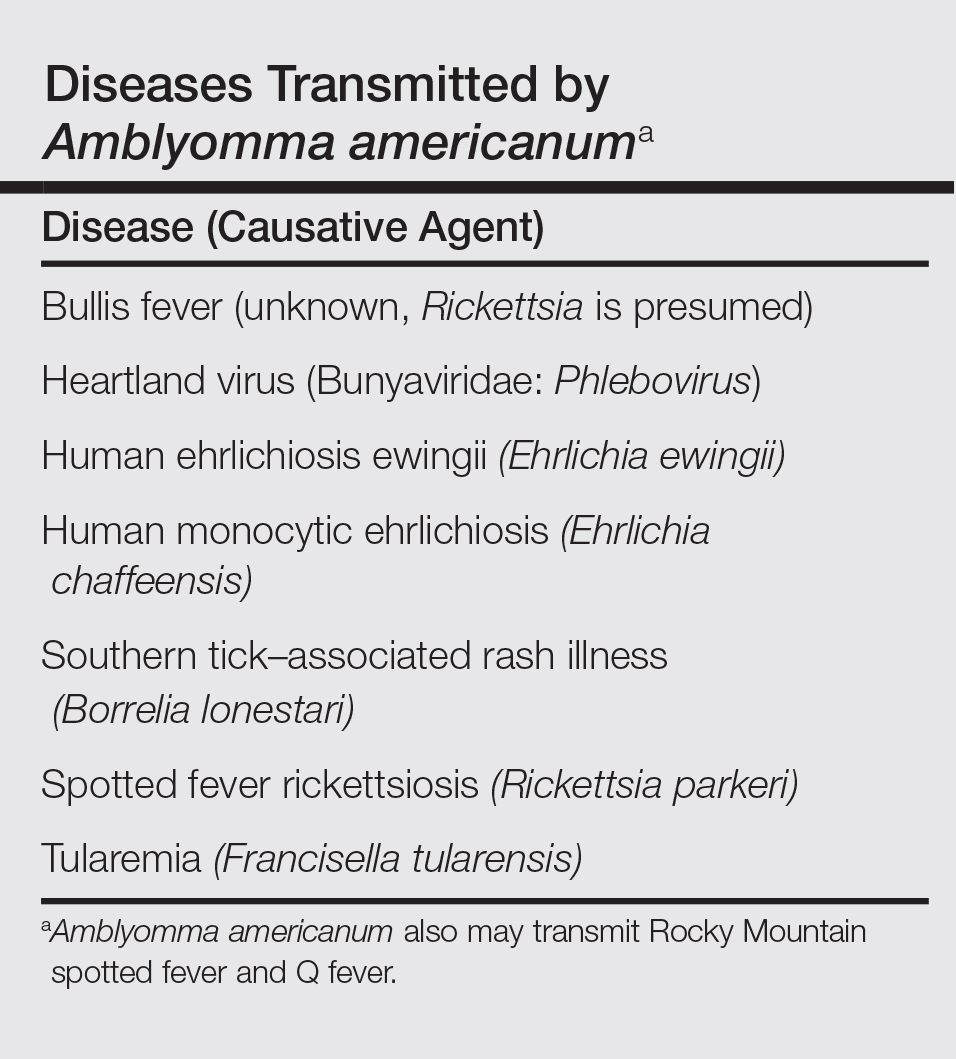
Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.
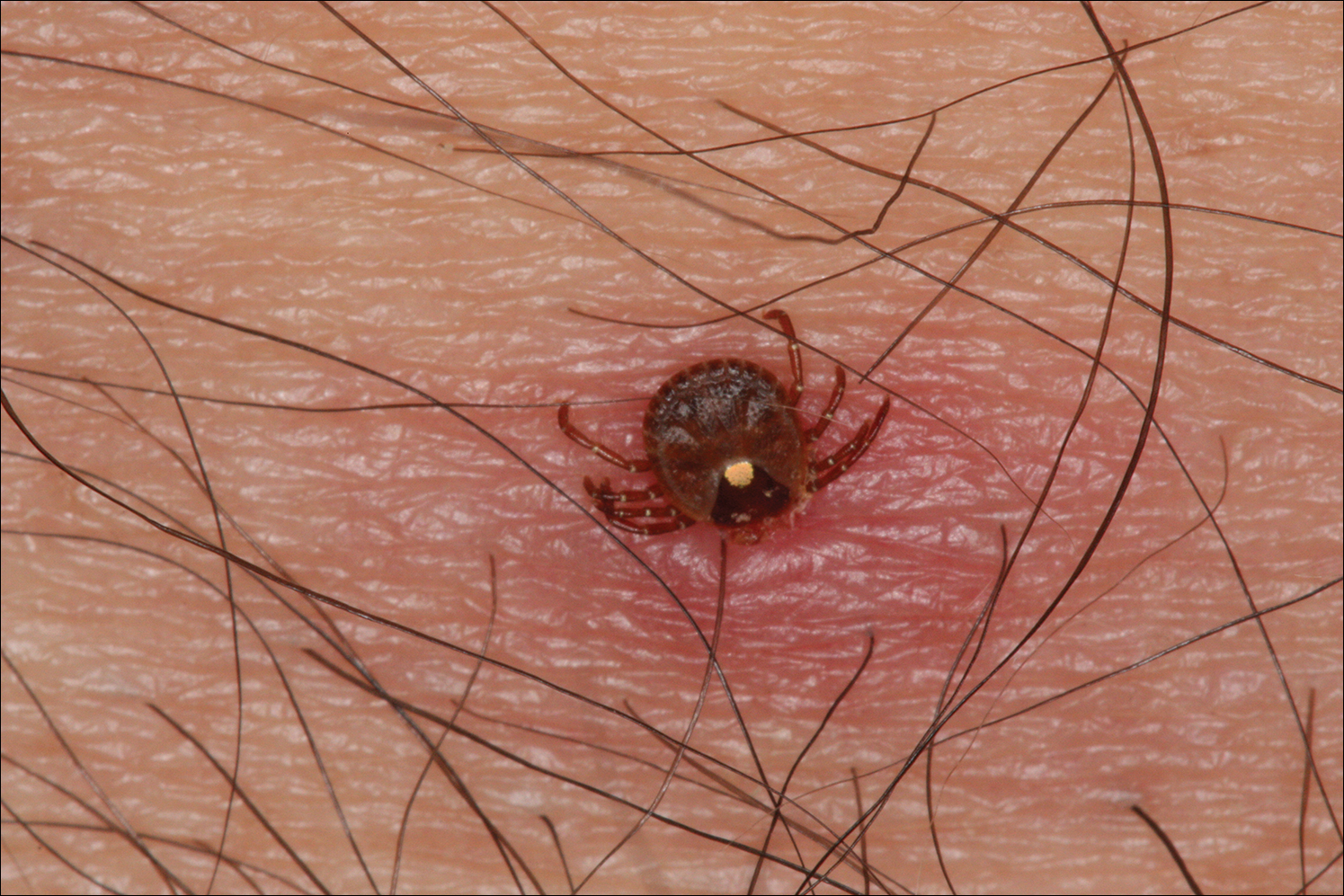
Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.
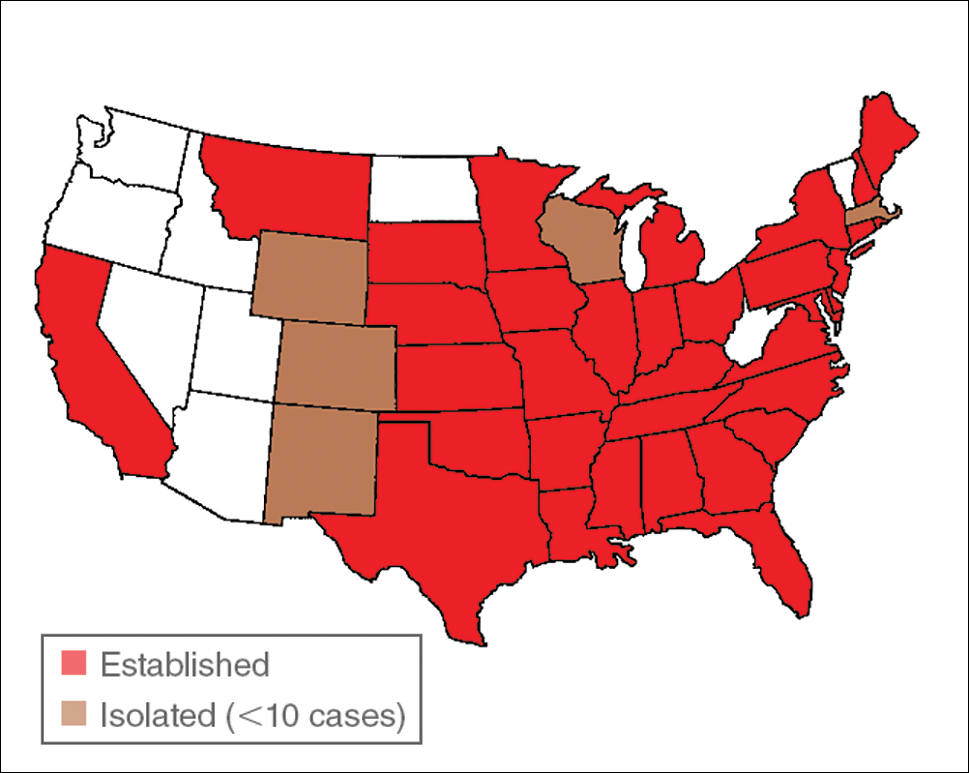
Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
Practice Points
- Amblyomma americanum (lone star tick) is widely distributed throughout the United States and is an important cause of several tick-borne illnesses.
- Prompt diagnosis and treatment of tick-borne disease improves patient outcomes.
- In some cases, tick bites may cause the human host to develop certain IgE antibodies that result in a delayed-onset anaphylaxis after ingestion of red meat.
Aquatic Antagonists: Cutaneous Sea Urchin Spine Injury
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.
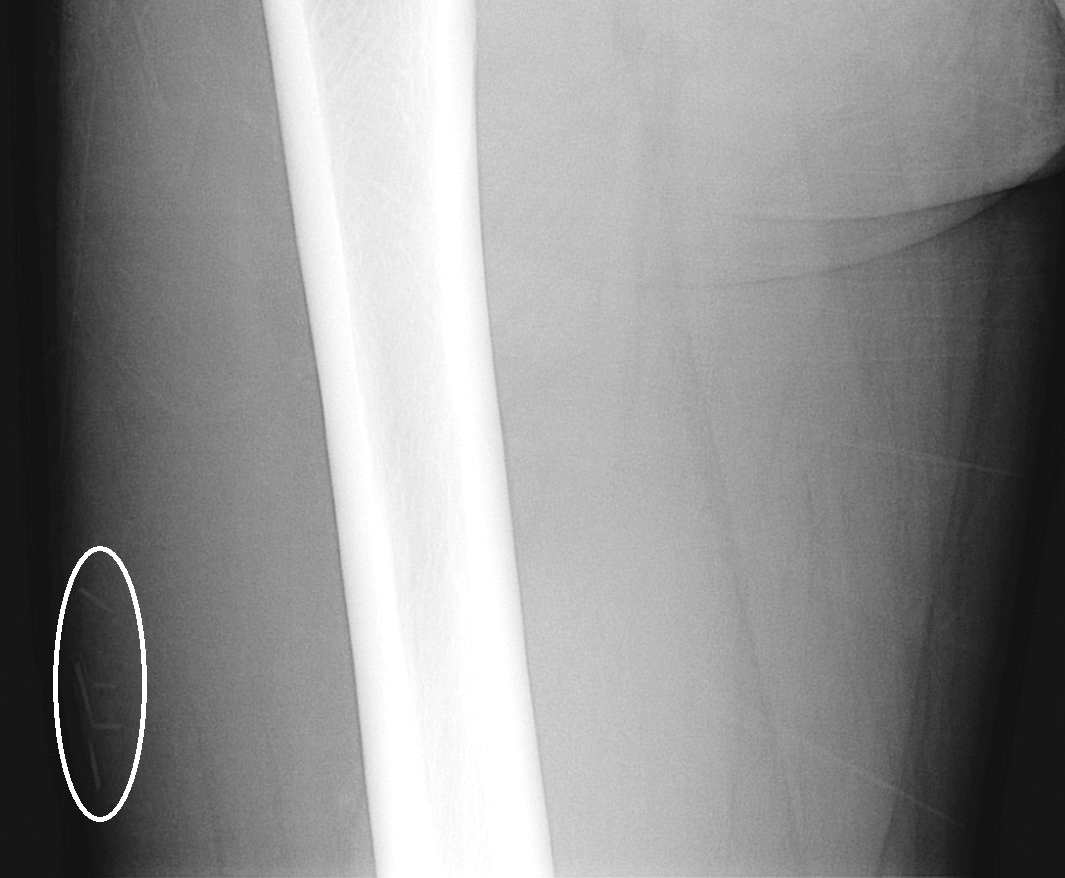
At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.
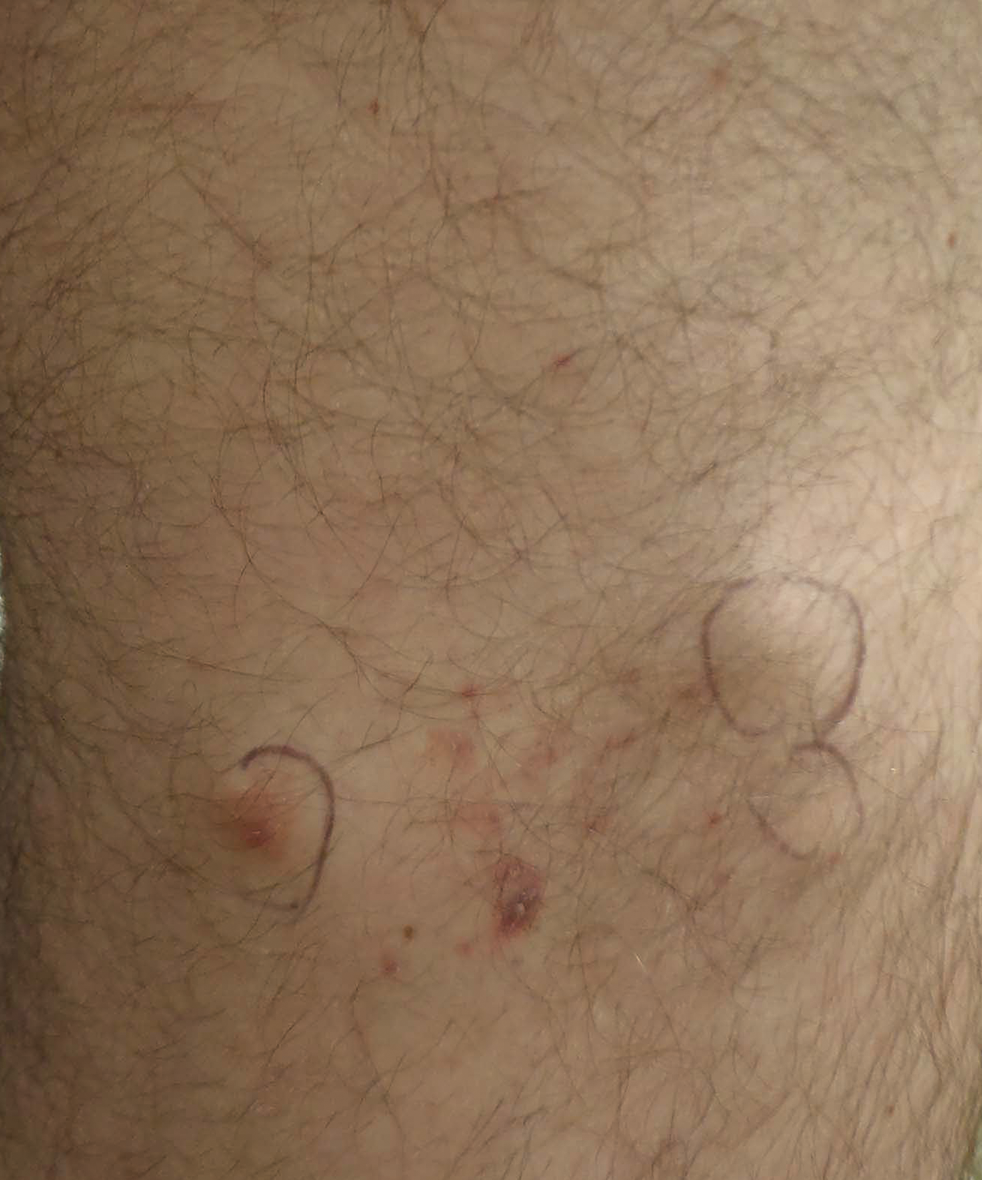
Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.
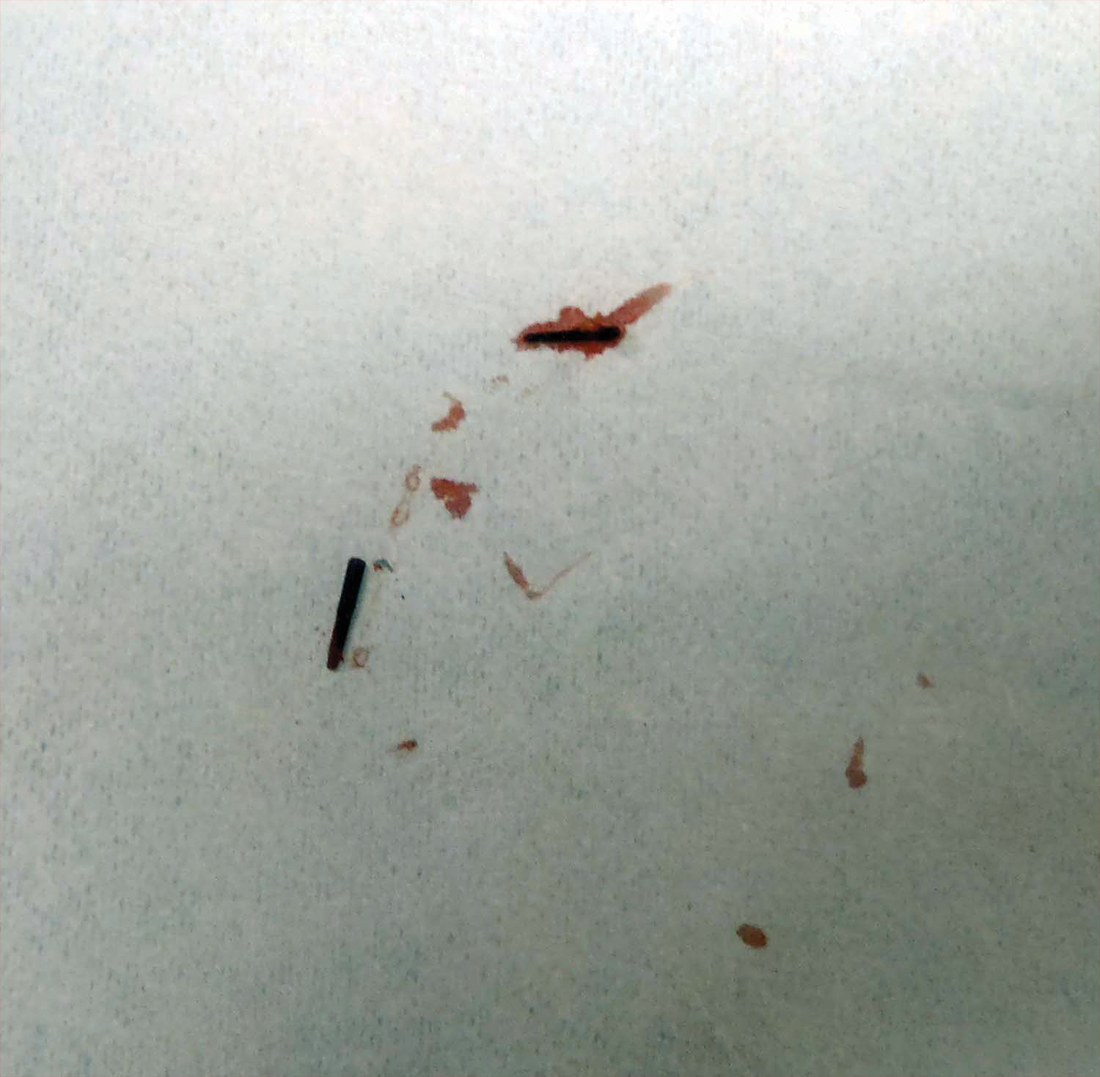
At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Practice Points
- Radiographic imaging may aid in the identification of sea urchin spines, especially if there are no visible or palpable skin nodules.
- Treatment of sea urchin spine injuries typically involves surgical removal of the spines with local anesthesia and cutaneous extraction.
- Prompt extraction of sea urchin spines can improve pain symptoms and decrease the likelihood of granuloma formation, infection, and extracutaneous complications.
What’s Eating You? Tick Bite Alopecia
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later
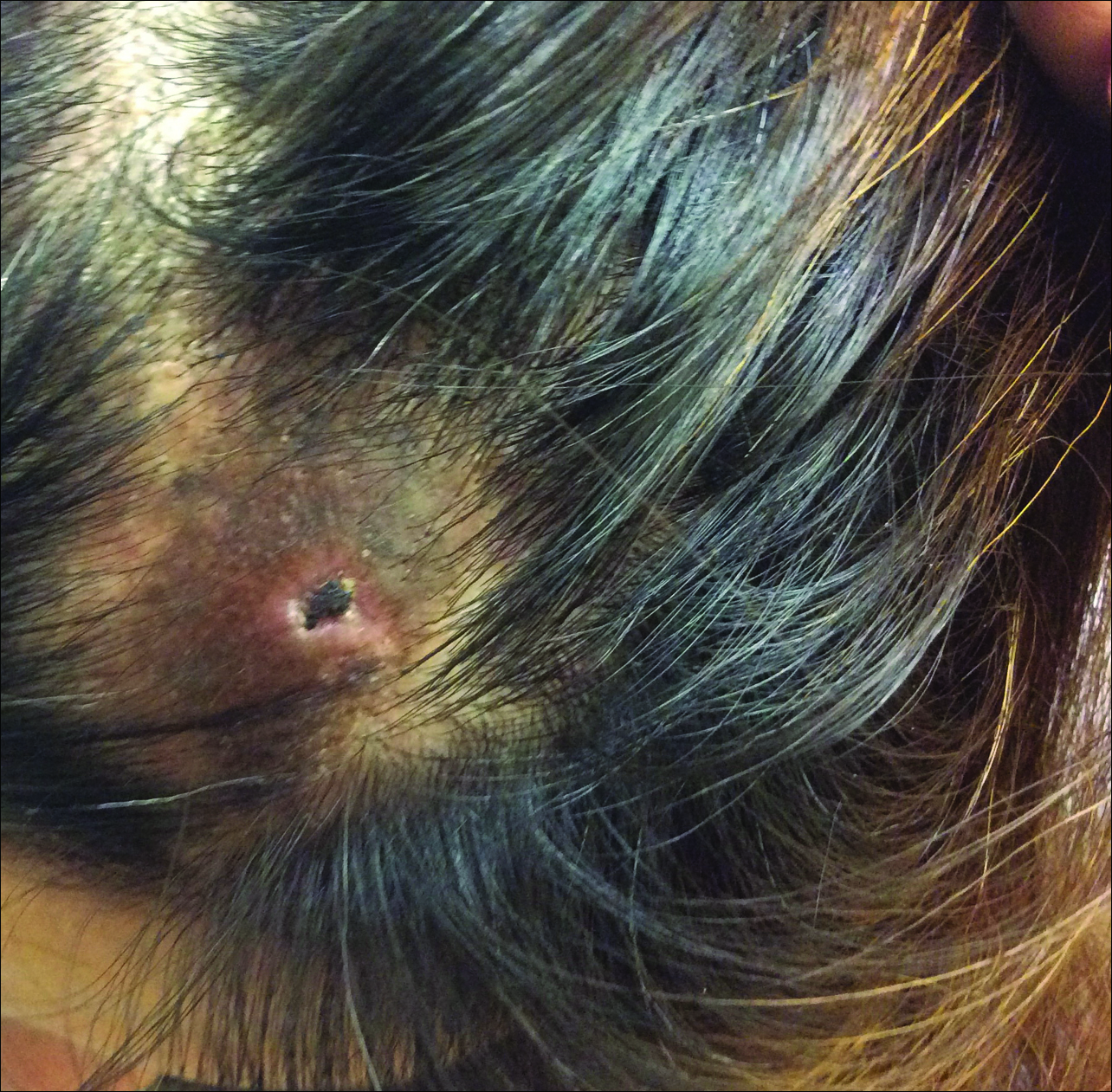
A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).
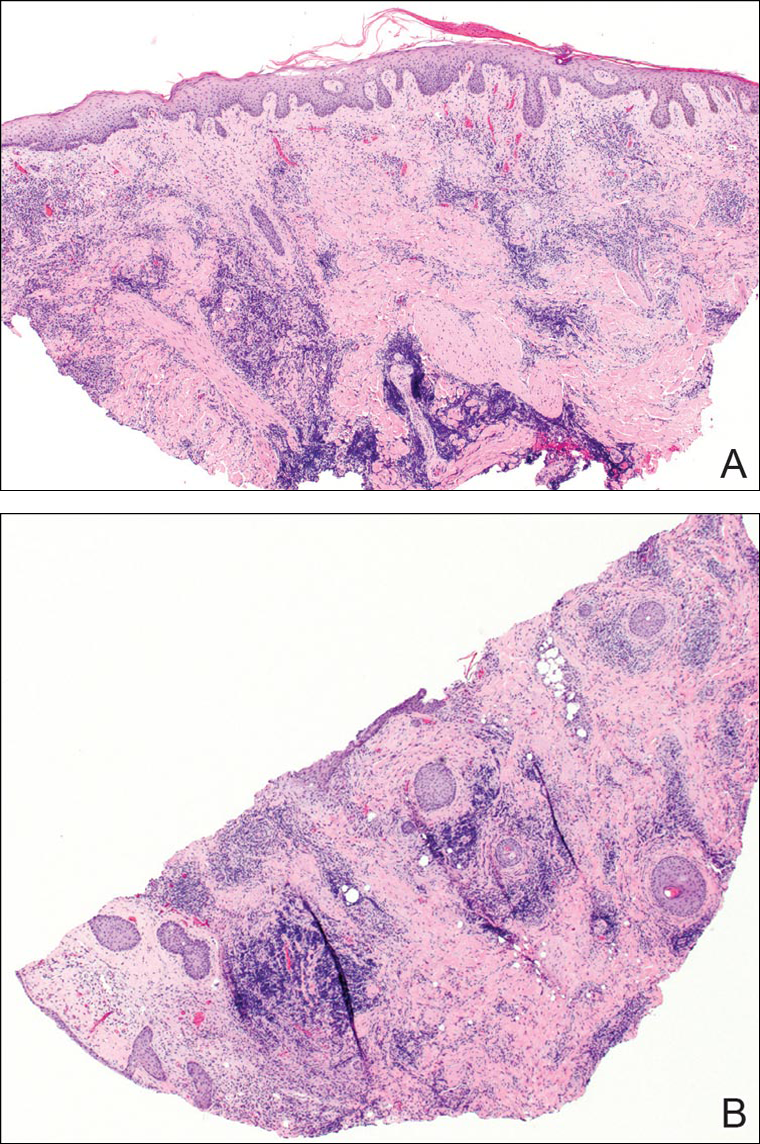
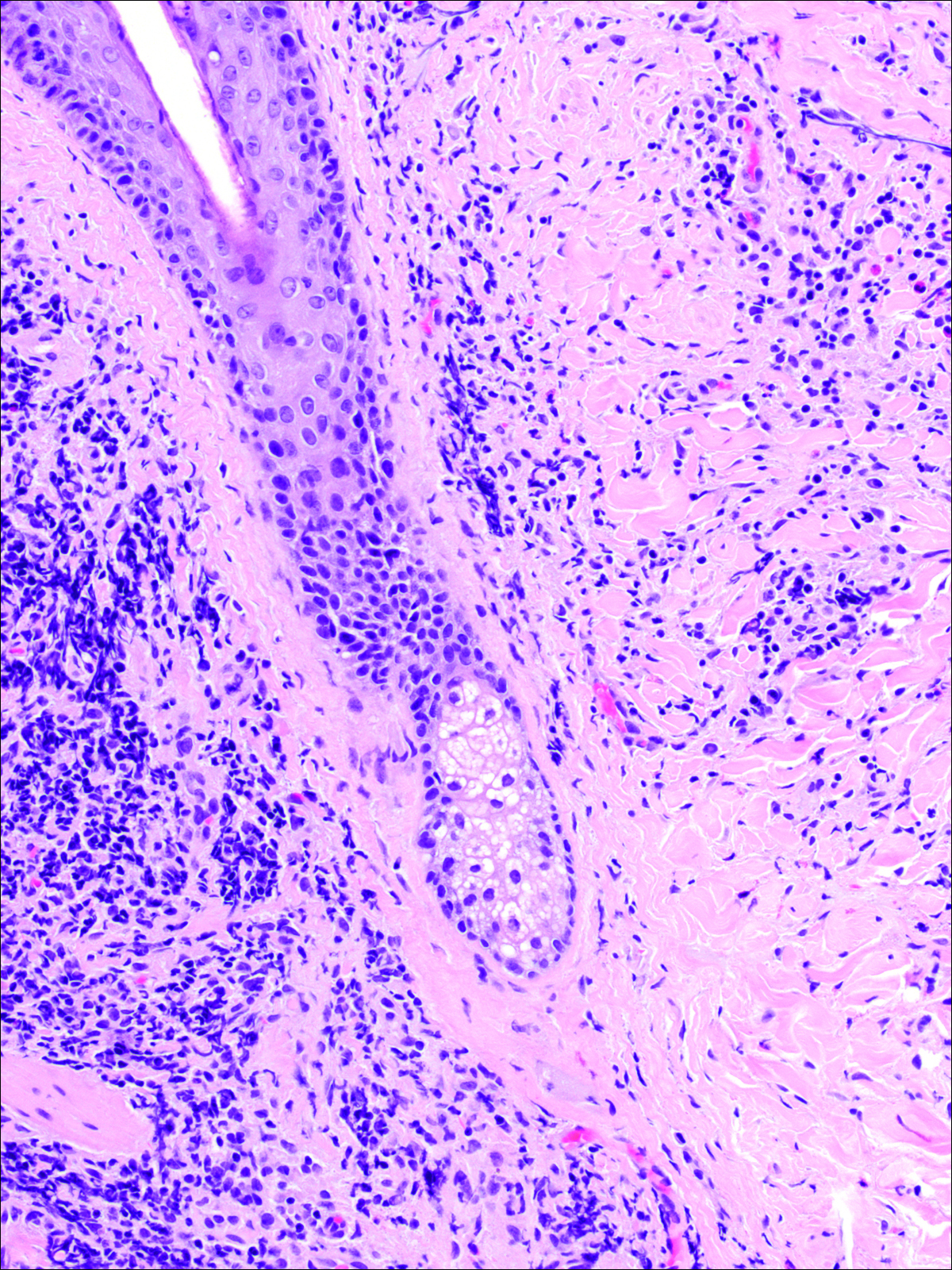
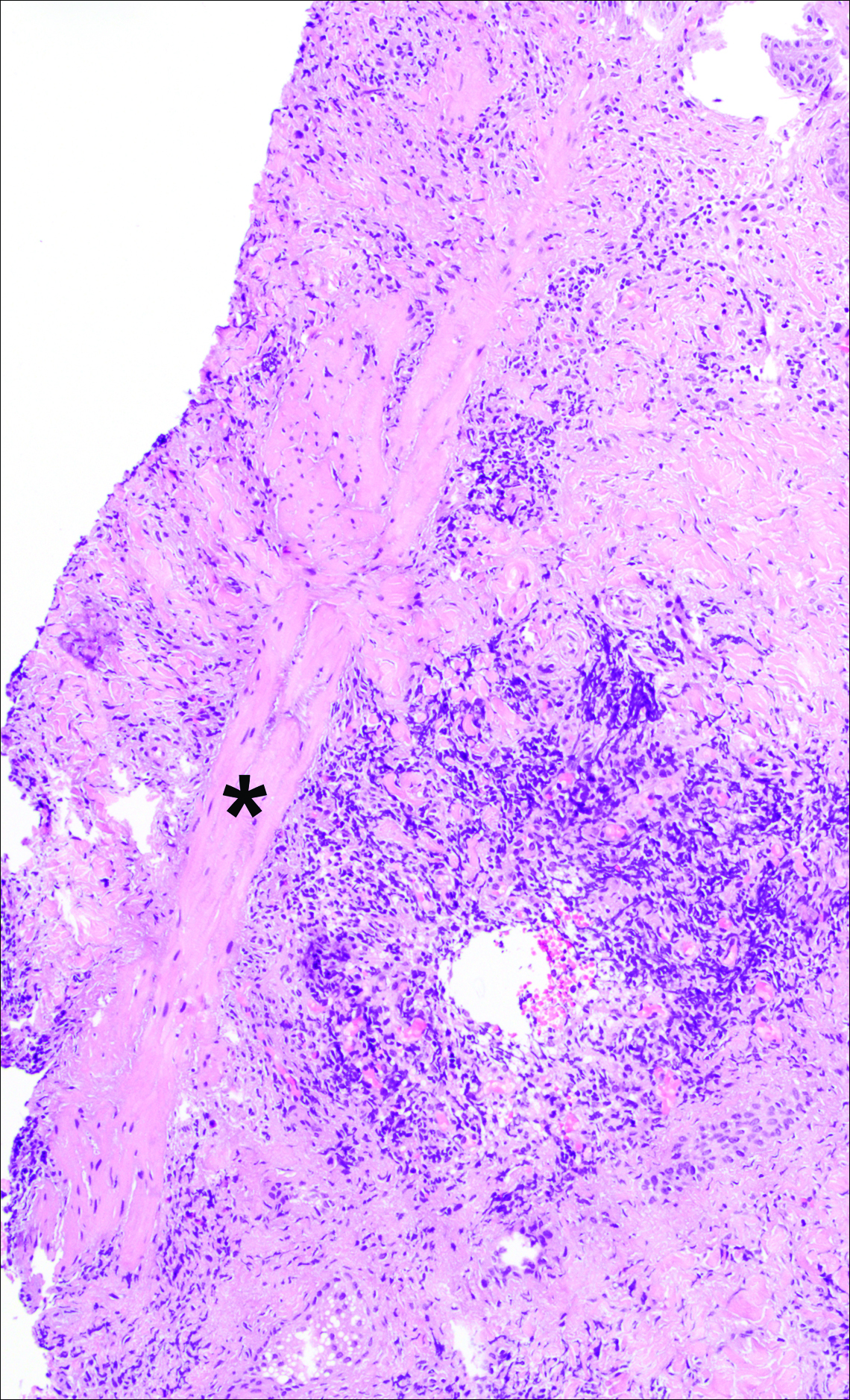
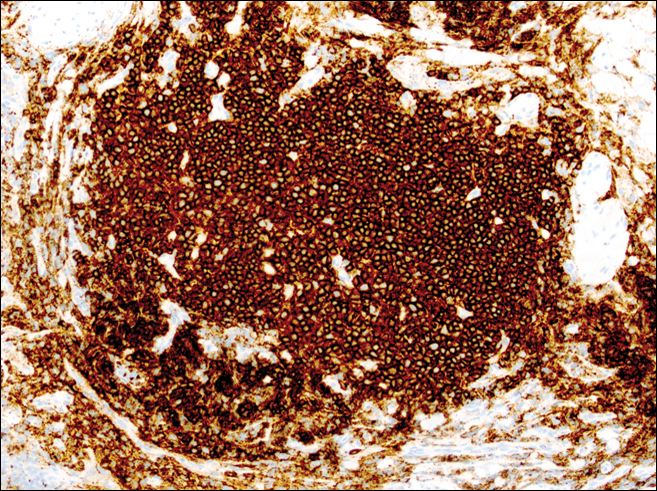
Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Practice Points
- Tick bite alopecia should be included in the differential diagnosis of both solitary and moth-eaten lesions of localized hair loss.
- In most cases, hair regrowth can be expected in a lesion of tick bite alopecia.
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.