User login
Managing Diabetes in Women of Childbearing Age
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
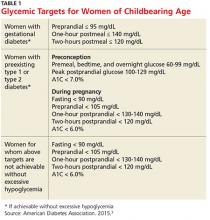
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
Are Those Glucometer Results Accurate?
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
Investigating Unstable Thyroid Function
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
Be Sure to Look for Secondary Diabetes
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
Weighing the Options for Obesity Meds
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
Managing Thyroid Disease in Pregnancy
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.
Pituitary Incidentaloma
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Noninsulinoma Pancreatogenous Hypoglycemia Syndrome Following Gastric Bypass Surgery
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
A 28-year-old white woman, KR, presents to primary care with episodic diaphoresis and weakness that occur one to two hours after meals. There is no history of syncope or seizures. The hypoglycemic symptoms abate with intake of oral glucose and do not occur when the patient fasts.
KR underwent Roux-en-Y gastric bypass surgery 12 months ago. At the time, her body weight was 250 lbs and her height, 62 in (BMI, 46). She has lost 60 lbs since surgery (current BMI, 35). KR has no comorbid medical conditions. She denies use of insulin injection or oral hypoglycemic medication, as well as alcohol consumption. There is no history of diarrhea or abdominal pain. Her only medication is a daily multivitamin.
Physical exam reveals a blood pressure of 126/80 mm Hg; pulse, 82 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 98%. Heart rate is regular with no murmur. Lungs are clear to auscultation. Abdominal and neurologic exams are unremarkable; musculoskeletal strength and orthostatic vital signs are normal.
The patient is instructed to test her blood sugar with a glucometer and return to the clinic in two weeks. Fingerstick monitoring reveals that her serum glucose level drops into the 40 to 50 mg/dL range approximately one to two hours after meals containing > 45 g of carbohydrate. Her fasting serum glucose readings are in the 80 to 95 mg/dL range.
The patient is presumptively diagnosed with dumping syndrome and receives nutritional counseling; she is instructed to reduce intake of simple carbohydrates and increase the protein content of meals. Despite these dietary modifications, the episodes of hypoglycemia persist.
The patient is then referred to endocrinology. Fasting labwork reveals a serum glucose level of 85 mg/dL; normal adrenocorticotropic hormone (ACTH) and cortisol levels; C-peptide level, 2.46 ng/mL (reference range, 0.80–4.00 ng/mL); and insulin level, 6.4 mIU/mL (reference range 2.6–24.9 mIU/mL). A 75-g two-hour oral glucose tolerance test (OGTT) reveals peak serum glucose of 180 mg/dL at 30 minutes followed by a nadir serum glucose of 48 mg/dL at 110 minutes, accompanied by hypoglycemic symptoms. The insulin and C-peptide levels are elevated during the entire two-hour test. The serum cortisol level is 22 mg/dL when the glucose level is 48 mg/dL. CT of the abdomen, previously ordered by the patient’s primary care provider, was unremarkable.
Since there is no laboratory evidence of fasting hypoglycemia and no pancreatic abnormalities are seen on imaging studies, the possibility of insulinoma is excluded from the differential diagnosis. Adrenal insufficiency is excluded based on the normal ACTH and cortisol levels. The possibility of noninsulinoma pancreatogenous hypoglycemia syndrome is considered.
The patient is prescribed verapamil ER 100 mg/d and notes significant reduction in the frequency of hypoglycemic episodes and symptoms. She is scheduled for follow-up in four weeks to assess for any changes in the frequency or severity of her hypoglycemic episodes.
BACKGROUND
Postprandial hypoglycemia is a rare but potentially serious complication of bariatric surgery procedures that divert nutrients into the small bowel.1,2 The Bariatric Outcomes Longitudinal Database revealed a 0.1% incidence of hypoglycemia in patients who underwent Roux-en-Y gastric bypass surgery.3
The most common cause of hypoglycemia following gastric bypass surgery is dumping syndrome, which involves rapid emptying of gastric contents with reactive hypoglycemia due to increased postprandial insulin release. In dumping syndrome, hypoglycemic symptoms—flushing, diaphoresis, weakness, and dizziness—typically occur within two to three hours after meals; patients do not experience the more severe symptoms of neuroglycopenia (eg, cognitive impairment, seizures, and loss of consciousness).4 The symptoms of dumping syndrome typically improve with reduced intake of simple carbohydrates and increased protein consumption.1
Other causes of postprandial hypoglycemia include insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Although both diagnoses are rare, they should be considered if no improvement in hypoglycemic symptoms occurs after dietary modification.1
Insulinoma is the most common cause of persistent hyperinsulinemic hypoglycemia. It is defined by Whipple’s triad: symptomatic hypoglycemia during fasting, a serum glucose level > 50 mg/dL at the time of symptom onset, and relief of symptoms after administration of glucose.5
NIPHS is less common than insulinoma. It is characterized by postprandial hypoglycemia due to increased insulin secretion resulting from pancreatic b-cell hyperplasia. Hypoglycemia does not typically occur during a 72-hour fast. In addition, pancreatic imaging studies yield normal results in cases of NIPHS. The selective arterial calcium stimulation test is positive in NIPHS.5 NIPHS is definitively diagnosed by histopathologic examination of the pancreas, which reveals nesidioblastosis.6
Nesidioblastosis involves pathologic b-cell overgrowth in the pancreas that results in excess insulin secretion.4 Nesidioblastosis is characterized by pancreatic b-cell hypertrophy, islet hyperplasia, and increased b-cell mass.2
Nesidioblastosis is the leading cause of hyperinsulinemia in newborns and infants (annual incidence, 1 in 50,000 births) but is quite rare in adults, occurring in 0.5% to 7.0% of all those with hyperinsulinism.7,8 Islet cell hypertrophy—characteristic of nesidioblastosis—is seen in both adults and children, whereas genetic mutations are present only in infants.7
Although rare in adults, nesidioblastosis is more common in the setting of gastric bypass than in the general population.7 As of 2011, there have been 40 cases of nesidioblastosis in adults who received gastric bypass.2 With the rapid increase in the number of these surgeries performed each year, nesidioblastosis should be considered in the differential diagnosis for patients who experience hypoglycemia following the procedure.2,7
Continue for hormonal mechanisms >>
HORMONAL MECHANISMS
There are multiple theories regarding the etiology of b-cell hyperplasia following bariatric surgery. The specific causes for NIPHS after gastric bypass remain under investigation.2
The most common theory is that b-cell hyperplasia may occur as a result of the surgical procedure itself and not due to obesity. The rapid delivery of food to the distal ileum after gastric bypass surgery may result in elevated production of incretin hormones (eg, GLP-1 and GIP), which increase b-cell proliferation, insulin secretion, and insulin sensitivity.7
Roux-en-Y gastric bypass also impairs ghrelin secretion. Ghrelin normally acts to suppress insulin secretion and directly opposes the action of insulin. Reduced levels of ghrelin may increase the likelihood of hypoglycemia. Other hormones that may contribute to the metabolic effects of bariatric surgery include peptide YY, oxyntomodulin, and others as yet unidentified.5,6
CLINICAL MANIFESTATIONS
NIPHS is characterized by moderate to severe postprandial hypoglycemia. Symptoms include confusion, diaphoresis, tremulousness, anxiety, weakness, blurred vision, and disorientation, as well as more severe neuroglycopenic symptoms, such as cognitive impairment, seizures, and loss of consciousness.5
These symptoms do not typically manifest until several months after gastric bypass surgery. (By contrast, symptoms experienced with dumping syndrome typically manifest shortly after the procedure.) Of note, hypoglycemic symptoms of NIPHS do not typically improve after dietary modifications aimed at reducing carbohydrate intake.2
DIAGNOSIS
Diagnosis of NIPHS is based on hypoglycemic/neuroglycopenic signs and symptoms without fasting hypoglycemia; endogenous hyperinsulinemia in the presence of hypoglycemia; negative localization studies for insulinoma (using triple-phase spiral CT); and positive selective arterial calcium stimulation test.4,6
If fasting hypoglycemia is reported or suspected, the patient should be evaluated for insulinoma using a 72-hour fast. During it, glucose, insulin, C-peptide, and pro-insulin levels should be tested every six hours; results will be normal in patients with NIPHS.5
The use of OGTT is controversial, as patients can experience variable degrees of postprandial hyperinsulinism and symptomatic hypoglycemia during the test. There are no guidelines on whether to perform OGTT in the work-up for NIPHS. In research protocols, it is common to perform a five-hour OGTT; subjects consume a mixed meal containing 50 g of carbohydrates, then their glucose, insulin, and C-peptide levels are tested every 30 to 60 minutes (or sooner if hypoglycemic symptoms occur).
Elevated insulin and C-peptide levels in the setting of hypoglycemia are characteristic findings in patients with NIPHS.5,9 In the setting of hypoglycemia, a cortisol level > 20 mg/dL is considered an appropriate adrenal response and excludes adrenal insufficiency. Triple-phase CT of the abdomen should be performed to rule out insulinoma if strongly suspected and if work-up for NIPHS is negative.5
The selective arterial calcium stimulation test is employed to confirm the diagnosis of NIPHS and to guide the extent of pancreatic resection, in an effort to minimize postoperative complications of insulin-dependent diabetes and exocrine insufficiency. In this procedure, the splenic, gastroduodenal, superior mesenteric, and hepatic arteries that supply the pancreas are selectively injected with calcium gluconate. After injection of calcium, the insulin level is measured within each artery.4,5,7 The selective arterial calcium stimulation test can also be used to localize an insulinoma. NIPHS is distinguished from insulinoma by a diffuse increase in insulin secreted from multiple segments of the arteries that supply the pancreas, following calcium stimulation.4,5,7
Continue for treatment >>
TREATMENT
There is no consensus on treatment of NIPHS in postbariatric surgery patients, and no “gold standard” exists. Pharmacologic treatment is recommended prior to surgical intervention in patients who present with symptomatic hypoglycemia without loss of consciousness or seizures.1
Pharmacologic treatments include calcium channel blockers (eg, verapamil or nifedipine), the b-cell inhibitor diazoxide, the secretory inhibitor octreotide, and a-glucosidase inhibitors.1 In one hospital group, patients were initially treated with verapamil ER 100 mg/d.5 If patients did not respond to this therapy or developed adverse effects, diazoxide was added (starting dose, 25 mg tid, titrated to 75 mg tid).5 If this combination did not produce results, octreotide (dose ranging from 25 mg/d to 50 mg tid, subcutaneously) was added. Acarbose can also be added, with the typical starting dose of 50 mg tid.1
Distal or subtotal pancreatectomy to debulk the hypertrophic islets is the most common surgical method used in patients with severe hypoglycemia that is refractory to medical management.2,5 The extent of pancreatic resection is guided by calcium angiography and typically ranges from 80% to 95%.7 Smaller pancreatic resection is associated with higher risk for persistent postoperative hypoglycemia.5 Complications associated with pancreatectomy include insulin-dependent diabetes and exocrine insufficiency.5
It is not uncommon for patients to experience recurrent symptoms after subtotal pancreatectomy, but the symptoms are typically easier to manage pharmacologically than they were pre-operatively. Occasionally, a second surgery with 95% to complete pancreatectomy is employed if recurrent hypoglycemia develops that is refractory to medical management.5
Reversal of Roux-en-Y bypass surgery has been described as an attempted treatment method in several case reports of patients with NIPHS. In at least one patient, hyperinsulinemic hypoglycemia persisted after Roux-en-Y gastric bypass reversal.2 Adjustable gastric band placement was recently reported to reverse hypoglycemic symptoms and maintain weight loss, due to restricted gastric emptying.2 Conversion of Roux-en-Y gastric banding to gastric sleeve may also be employed to restore normal gastrointestinal continuity and resolve hypoglycemia, though limited data is available regarding the efficacy of this procedure.2
Close monitoring is necessary in patients treated with pharmacologic therapy to ensure that symptoms are well controlled and that surgery is not necessary.1
SUMMARY AND CONCLUSION
Symptomatic hypoglycemia is a potential complication associated with gastric bypass surgery and is most commonly caused by dumping syndrome. It is important to consider other causes of postprandial hypoglycemia, such as insulinoma and NIPHS, in patients who continue to experience hypoglycemia despite making dietary modifications.1,4
NIPHS is a rare and poorly understood complication of gastric bypass surgery involving pathologic b-cell overgrowth, leading to hyperinsulinemia and potentially severe hypoglycemia.6 Some patients may present with complete relief of symptoms with pharmacologic treatment, while others will need surgical treatment with subtotal pancreatectomy.1
The findings of increased levels of GLP-1 hormone in patients who have received gastric bypass surgery and the fact that only a very small subset of gastric bypass patients develop NIPHS with histologic features of nesidioblastosis are subjects for further research. Further understanding of the hormonal factors involved in the pathogenesis of NIPHS and adult-onset nesidioblastosis following gastric bypass surgery could lead to novel drug development to treat diabetes.6
REFERENCES
1. Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618-1621.
2. Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879-1888.
3. Sarwar H, Chapman III WH, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120-1124.
4. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254.
5. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc. 2010;24:2547-2555.
6. Cummings D. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med. 2005;353(3):300-302.
7. Clancy TE, Moore FD, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116-1119.
8. Kaczirek K, Niederle B. Nesidioblastosis: an old term and a new understanding. World J Surg. 2004;28:1227-1230.
9. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6): 2008-2017.
Targeting the Kidneys to Improve Glycemic Control
A 37-year-old woman with a history of papillary carcinoma (status post total thyroidectomy 12 years ago, with negative recurrence) presents for a check-up. She also has polycystic ovarian syndrome (PCOS) with obesity and is taking metformin XR (one 500-mg tablet bid). Her visit is uneventful, and she leaves the office with an order for labwork.
Results indicate normal thyroid function and negative thyroglobulin. However, her serum glucose level is 350 mg/dL, so the patient is called and informed of the result. She denies polyphagia, polydipsia, and polyuria. Repeat blood work confirms overt hyperglycemia (320 mg/dL) with an A1C of 13%, undetectable C-peptide, and negative glutamic acid decarboxylase 65 (GAD65) and islet cell antibodies.
She is advised to increase her metformin dose (to two 500-mg tablets bid) and is started on insulin detemir (20 U every evening), with instructions to increase the latter by three units every two to three days until a target fasting glucose level of 100 to 140 mg/dL is achieved. She is also advised to follow a low-carbohydrate diet and increase her exercise.
The patient returns in two weeks for follow-up. She remains asymptomatic and has now increased her insulin detemir to 34 U bid (she started splitting the dosage after it reached 50 U/d). However, her glucose is still in the low 200s in the morning and the high 200s during the day (after lunch and dinner).
Her overt hyperglycemia is most likely a result of her longstanding insulin resistance, essential lack of b-cell function, and PCOS-associated obesity. Once diabetes from autoimmunity is ruled out by laboratory findings (negative antibodies) and clinical assessment (classic metabolic syndrome features), we focus on her glycemic control.
Even with nearly 70 U/d of insulin, the patient’s glycemic improvement is disappointing, suggesting significant insulin resistance and glucose toxicity. Living in an era with numerous classes of antidiabetic medications, we have lengthy discussions on treatment options. Canagliflozin, recently (at the time) approved, is included. The patient is interested in this new medication, and it is a reasonable choice to get her out of the glucotoxic phase.
After a discussion of benefits and potential adverse effects, she is placed on canagliflozin 100 mg/d. Her glucose log in one week shows fasting glucose values in the range of 140 to 160 mg/dL and postprandial glucose values in the 180s. As a result, she lowers her insulin to 25 U bid. Her renal panel shows a potassium level of 4.3 mEq/L (reference range, 3.5 to 5.3) and a glomerular filtration rate (GFR) of 103 mL/min/1.73 m2. She is advised to further increase her canagliflozin to 300 mg and slowly titrate her insulin down as needed, with a target fasting glucose level of 80 to 110 mg/dL and a postprandial target of 100 to 140 mg/dL.
What are SGLT2 inhibitors, and how do they work?
What are SGLT2 inhibitors, and how do they work?
Sodium-GLucose co-Transporter 2 (SGLT2) inhibitors are a new class of antihyperglycemic agent. The first, canagliflozin, was approved by the FDA in March 2013, followed by dapagliflozin (January 2014) and empagliflozin (August 2014).
As glucose is filtered through the nephrons of the kidney, about 90% is reabsorbed via SGLT2 in the proximal tubule (SGLT1 is responsible for the remaining 10%) so that glucose calories are not eliminated through urine.1 In a healthy person, the renal glucose threshold is about 180 mg/dL.1 When blood glucose exceeds this level, glucose is excreted into the urine. However, in diabetic patients, this threshold is higher due to the up-regulation of SGLT2s (and other glucose transporters), which worsens hyperglycemia.1 SGLT2 inhibitors will reset the threshold, which in turn will increase glucosuria and thereby lower serum glucose.1
SGLT2 inhibitors lower A1C by about 0.7% to 0.8%.2 Independent of other mechanisms such as the degree of b-cell function or insulin resistance, these agents can be used regardless of the duration of diabetes3 if the GFR is intact (≥ 45 mL/min/1.73 m2 for canagliflozin and empagliflozin, ≥ 60 mL/min/ 1.73 m2 for dapagliflozin).4,5
What are the risks and benefits associated with these agents?
What are the risks and benefits associated with these agents?
Modest weight loss is seen with the use of SGLT2 inhibitors. Initial weight loss is believed to be related to volume loss, but more sustained weight loss is thought to be from loss of fat mass.6 This is not surprising, as excreting glucose means excreting calories through urine.
Risk for hypoglycemia is extremely low, which makes this therapeutic class an attractive option. However, caution should be exercised when SGLT2 inhibitors are combined with other agents known to cause hypoglycemia (sulfonylureas and insulin).6
The most common adverse effect is genital mycotic infection. Women with a history of recurrent genital mycotic infection and uncircumcised men are at the greatest risk.6
Due to increased glycosuria, which results in an osmotic diuresis, modest blood pressure improvement has been seen (3 to 4 mm Hg systolic and 1 to 2 mm Hg diastolic7,8) in patients taking SGLT2 inhibitors, which is an additional benefit for hypertensive diabetic patients.6 On the other hand, use of SGLT2 inhibitors can also cause dehydration and volume depletion and can raise serum creatinine in patients who are already taking diuretics (particularly loop diuretics).6 Drug tolerance and adherence can be improved by advising patients to expect transient increased urination (approximately 135 to 350 mL/d increase from baseline5,9) and emphasizing the importance of good hydration and maintaining good genital hygiene.
A slight increase in LDL cholesterol was seen in clinical trials of the SGLT2 inhibitors, although this phenomenon is poorly understood. However, HDL cholesterol increased as well, maintaining the LDL:HDL ratio.6 No long-term cardiovascular outcome data are available at this time; as with any new antidiabetic medication, postmarketing studies, as required by the FDA, are currently ongoing.6
What are the options in this therapeutic category, and how are they distinct?
What are the options in this therapeutic category, and how are they distinct?
As mentioned previously, there are currently three SGLT2 inhibitors on the market: canagliflozin, dapagliflozin, and empagliflozin. There are subtle clinical differences among these three agents, which might direct the clinician’s choice.
First, canagliflozin is available in dosages of 100 and 300 mg. The starting dosage is 100 mg, which can be titrated to 300 mg in patients with a GFR ≥ 60 mL/min/1.73 m2 who require a greater glucose-lowering effect. Those with a GFR < 60 mL/min/1.73 m2 but ≥ 45 mL/min/1.73 m2 are limited to the 100-mg dosage. Dapagliflozin is available in 5-mg and 10-mg dosages, the former being the starting dosage. But dapagliflozin is not recommended in patients whose GFR is < 60 mL/min/1.73 m2.4
Empagliflozin is available in dosages of 10 and 25 mg. The starting dosage of 10 mg can be increased to 25 mg if the patient has not achieved his/her target glucose level. Either can be used in patients with a GFR ≥ 45 mL/min/1.73 m2.5
Second, hyperkalemia was seen in patients taking canagliflozin but not in those taking dapagliflozin or empagliflozin. Therefore, serum potassium should be monitored and caution used, especially when patients are being treated with potassium-sparing diuretics and/or ACE inhibitors or angiotensin II receptor blockers.6
Third, dapagliflozin carries a warning for bladder cancer, as higher rates of newly diagnosed bladder cancer were seen with this drug compared with placebo or comparator drugs (0.17% vs 0.03%, respectively).4 However, this finding may have resulted from a randomization imbalance of patients in the study, and further research is needed to clarify this risk.6 It is not recommended that dapagliflozin be used in patients with active or a history of bladder cancer at this time.
With these agents, there is a paradoxical rise in glucagon that increases endogenous glucose production from the liver.10 The mechanism is poorly understood, but it might be due to the body’s compensatory (survival) mechanism to “make up” the loss of glucose through urine by increasing hepatic gluconeogenesis.
Using an incretin agent, such as dipeptidyl peptidase 4 (DPP-4) inhibitors or glucagon-like peptide 1 (GLP-1) receptor agonists, in conjunction with an SGLT2 inhibitor, has been suggested as a way to potentiate the glucose-lowering effect, as it may attenuate the paradoxical rise in glucagon.10 Since the incretin class is weight neutral (DPP-4 inhibitors) or associated with weight loss (GLP-1 agonists), using incretins with SGLT2 inhibitors might produce more significant weight loss, which has numerous additional benefits for diabetic patients.
SGLT2 inhibitors are currently approved as an adjunct to diet and exercise for patients with type 2 diabetes. They are not approved for those with type 1 diabetes, although the mechanism of action of these drugs (which is independent of the b-cell function) might make them effective in this population. Active pilot studies of this patient population are in progress.11
Conclusion
In summary, SGLT2 inhibitors are an exciting new class of antidiabetic medication that offers a unique mechanism to lower serum glucose. It is the only medication that will actually remove glucose from the body; by contrast, all other existing antidiabetic medications move glucose within the body (to liver, fat, muscle, etc).
There is no curative medication for diabetes. But with an increasing diabetic population and an emphasis on individualizing antihyperglycemic regimens, we always welcome medications with novel mechanisms of action. Due to SLGT2 inhibitors’ recent approval, however, short-term and long-term adverse effects are unknown, and ongoing postmarketing surveillance should be closely followed.
References
1. Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008;14:782-790.
2. Berhan A, Barker A. Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized double-blind controlled trials. BMC Endocr Disord. 2013;13(1):58.
3. Wilding JP, Norwood P, T’joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Diabetes Care. 2009;32:1656-1662.
4. Taylor JR. Dapagliflozin offers differences from other SGLT2 inhibitors. Endocrine Today. May 2014.
5. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2014.
6. Bakris G, Fonseca VA, Peters AL, Wysham CH. Clinical perspectives on the role of the kidney in the pathophysiology of T2DM: emerging options for treatment [video series]. 2013. www.thedoctorschannel.com/view/the-kid ney-in-t2dm-cme-part-1/. Accessed September 12, 2014.
7. Vercruysse F. Efficacy and safety of canagliflozin in subjects with type 2 diabetes mellitus inadequately controlled with metformin plus sulphonylurea over 52 weeks [abstract 934]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
8. Hach T. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials [abstract 943]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
9. List JF, Woo V, Morales E, et al. Sodium-glucose co-transport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care. 2009;32(4):650-657.
10. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(5):2287.
11. Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37(5):1480-1483.
A 37-year-old woman with a history of papillary carcinoma (status post total thyroidectomy 12 years ago, with negative recurrence) presents for a check-up. She also has polycystic ovarian syndrome (PCOS) with obesity and is taking metformin XR (one 500-mg tablet bid). Her visit is uneventful, and she leaves the office with an order for labwork.
Results indicate normal thyroid function and negative thyroglobulin. However, her serum glucose level is 350 mg/dL, so the patient is called and informed of the result. She denies polyphagia, polydipsia, and polyuria. Repeat blood work confirms overt hyperglycemia (320 mg/dL) with an A1C of 13%, undetectable C-peptide, and negative glutamic acid decarboxylase 65 (GAD65) and islet cell antibodies.
She is advised to increase her metformin dose (to two 500-mg tablets bid) and is started on insulin detemir (20 U every evening), with instructions to increase the latter by three units every two to three days until a target fasting glucose level of 100 to 140 mg/dL is achieved. She is also advised to follow a low-carbohydrate diet and increase her exercise.
The patient returns in two weeks for follow-up. She remains asymptomatic and has now increased her insulin detemir to 34 U bid (she started splitting the dosage after it reached 50 U/d). However, her glucose is still in the low 200s in the morning and the high 200s during the day (after lunch and dinner).
Her overt hyperglycemia is most likely a result of her longstanding insulin resistance, essential lack of b-cell function, and PCOS-associated obesity. Once diabetes from autoimmunity is ruled out by laboratory findings (negative antibodies) and clinical assessment (classic metabolic syndrome features), we focus on her glycemic control.
Even with nearly 70 U/d of insulin, the patient’s glycemic improvement is disappointing, suggesting significant insulin resistance and glucose toxicity. Living in an era with numerous classes of antidiabetic medications, we have lengthy discussions on treatment options. Canagliflozin, recently (at the time) approved, is included. The patient is interested in this new medication, and it is a reasonable choice to get her out of the glucotoxic phase.
After a discussion of benefits and potential adverse effects, she is placed on canagliflozin 100 mg/d. Her glucose log in one week shows fasting glucose values in the range of 140 to 160 mg/dL and postprandial glucose values in the 180s. As a result, she lowers her insulin to 25 U bid. Her renal panel shows a potassium level of 4.3 mEq/L (reference range, 3.5 to 5.3) and a glomerular filtration rate (GFR) of 103 mL/min/1.73 m2. She is advised to further increase her canagliflozin to 300 mg and slowly titrate her insulin down as needed, with a target fasting glucose level of 80 to 110 mg/dL and a postprandial target of 100 to 140 mg/dL.
What are SGLT2 inhibitors, and how do they work?
What are SGLT2 inhibitors, and how do they work?
Sodium-GLucose co-Transporter 2 (SGLT2) inhibitors are a new class of antihyperglycemic agent. The first, canagliflozin, was approved by the FDA in March 2013, followed by dapagliflozin (January 2014) and empagliflozin (August 2014).
As glucose is filtered through the nephrons of the kidney, about 90% is reabsorbed via SGLT2 in the proximal tubule (SGLT1 is responsible for the remaining 10%) so that glucose calories are not eliminated through urine.1 In a healthy person, the renal glucose threshold is about 180 mg/dL.1 When blood glucose exceeds this level, glucose is excreted into the urine. However, in diabetic patients, this threshold is higher due to the up-regulation of SGLT2s (and other glucose transporters), which worsens hyperglycemia.1 SGLT2 inhibitors will reset the threshold, which in turn will increase glucosuria and thereby lower serum glucose.1
SGLT2 inhibitors lower A1C by about 0.7% to 0.8%.2 Independent of other mechanisms such as the degree of b-cell function or insulin resistance, these agents can be used regardless of the duration of diabetes3 if the GFR is intact (≥ 45 mL/min/1.73 m2 for canagliflozin and empagliflozin, ≥ 60 mL/min/ 1.73 m2 for dapagliflozin).4,5
What are the risks and benefits associated with these agents?
What are the risks and benefits associated with these agents?
Modest weight loss is seen with the use of SGLT2 inhibitors. Initial weight loss is believed to be related to volume loss, but more sustained weight loss is thought to be from loss of fat mass.6 This is not surprising, as excreting glucose means excreting calories through urine.
Risk for hypoglycemia is extremely low, which makes this therapeutic class an attractive option. However, caution should be exercised when SGLT2 inhibitors are combined with other agents known to cause hypoglycemia (sulfonylureas and insulin).6
The most common adverse effect is genital mycotic infection. Women with a history of recurrent genital mycotic infection and uncircumcised men are at the greatest risk.6
Due to increased glycosuria, which results in an osmotic diuresis, modest blood pressure improvement has been seen (3 to 4 mm Hg systolic and 1 to 2 mm Hg diastolic7,8) in patients taking SGLT2 inhibitors, which is an additional benefit for hypertensive diabetic patients.6 On the other hand, use of SGLT2 inhibitors can also cause dehydration and volume depletion and can raise serum creatinine in patients who are already taking diuretics (particularly loop diuretics).6 Drug tolerance and adherence can be improved by advising patients to expect transient increased urination (approximately 135 to 350 mL/d increase from baseline5,9) and emphasizing the importance of good hydration and maintaining good genital hygiene.
A slight increase in LDL cholesterol was seen in clinical trials of the SGLT2 inhibitors, although this phenomenon is poorly understood. However, HDL cholesterol increased as well, maintaining the LDL:HDL ratio.6 No long-term cardiovascular outcome data are available at this time; as with any new antidiabetic medication, postmarketing studies, as required by the FDA, are currently ongoing.6
What are the options in this therapeutic category, and how are they distinct?
What are the options in this therapeutic category, and how are they distinct?
As mentioned previously, there are currently three SGLT2 inhibitors on the market: canagliflozin, dapagliflozin, and empagliflozin. There are subtle clinical differences among these three agents, which might direct the clinician’s choice.
First, canagliflozin is available in dosages of 100 and 300 mg. The starting dosage is 100 mg, which can be titrated to 300 mg in patients with a GFR ≥ 60 mL/min/1.73 m2 who require a greater glucose-lowering effect. Those with a GFR < 60 mL/min/1.73 m2 but ≥ 45 mL/min/1.73 m2 are limited to the 100-mg dosage. Dapagliflozin is available in 5-mg and 10-mg dosages, the former being the starting dosage. But dapagliflozin is not recommended in patients whose GFR is < 60 mL/min/1.73 m2.4
Empagliflozin is available in dosages of 10 and 25 mg. The starting dosage of 10 mg can be increased to 25 mg if the patient has not achieved his/her target glucose level. Either can be used in patients with a GFR ≥ 45 mL/min/1.73 m2.5
Second, hyperkalemia was seen in patients taking canagliflozin but not in those taking dapagliflozin or empagliflozin. Therefore, serum potassium should be monitored and caution used, especially when patients are being treated with potassium-sparing diuretics and/or ACE inhibitors or angiotensin II receptor blockers.6
Third, dapagliflozin carries a warning for bladder cancer, as higher rates of newly diagnosed bladder cancer were seen with this drug compared with placebo or comparator drugs (0.17% vs 0.03%, respectively).4 However, this finding may have resulted from a randomization imbalance of patients in the study, and further research is needed to clarify this risk.6 It is not recommended that dapagliflozin be used in patients with active or a history of bladder cancer at this time.
With these agents, there is a paradoxical rise in glucagon that increases endogenous glucose production from the liver.10 The mechanism is poorly understood, but it might be due to the body’s compensatory (survival) mechanism to “make up” the loss of glucose through urine by increasing hepatic gluconeogenesis.
Using an incretin agent, such as dipeptidyl peptidase 4 (DPP-4) inhibitors or glucagon-like peptide 1 (GLP-1) receptor agonists, in conjunction with an SGLT2 inhibitor, has been suggested as a way to potentiate the glucose-lowering effect, as it may attenuate the paradoxical rise in glucagon.10 Since the incretin class is weight neutral (DPP-4 inhibitors) or associated with weight loss (GLP-1 agonists), using incretins with SGLT2 inhibitors might produce more significant weight loss, which has numerous additional benefits for diabetic patients.
SGLT2 inhibitors are currently approved as an adjunct to diet and exercise for patients with type 2 diabetes. They are not approved for those with type 1 diabetes, although the mechanism of action of these drugs (which is independent of the b-cell function) might make them effective in this population. Active pilot studies of this patient population are in progress.11
Conclusion
In summary, SGLT2 inhibitors are an exciting new class of antidiabetic medication that offers a unique mechanism to lower serum glucose. It is the only medication that will actually remove glucose from the body; by contrast, all other existing antidiabetic medications move glucose within the body (to liver, fat, muscle, etc).
There is no curative medication for diabetes. But with an increasing diabetic population and an emphasis on individualizing antihyperglycemic regimens, we always welcome medications with novel mechanisms of action. Due to SLGT2 inhibitors’ recent approval, however, short-term and long-term adverse effects are unknown, and ongoing postmarketing surveillance should be closely followed.
References
1. Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008;14:782-790.
2. Berhan A, Barker A. Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized double-blind controlled trials. BMC Endocr Disord. 2013;13(1):58.
3. Wilding JP, Norwood P, T’joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Diabetes Care. 2009;32:1656-1662.
4. Taylor JR. Dapagliflozin offers differences from other SGLT2 inhibitors. Endocrine Today. May 2014.
5. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2014.
6. Bakris G, Fonseca VA, Peters AL, Wysham CH. Clinical perspectives on the role of the kidney in the pathophysiology of T2DM: emerging options for treatment [video series]. 2013. www.thedoctorschannel.com/view/the-kid ney-in-t2dm-cme-part-1/. Accessed September 12, 2014.
7. Vercruysse F. Efficacy and safety of canagliflozin in subjects with type 2 diabetes mellitus inadequately controlled with metformin plus sulphonylurea over 52 weeks [abstract 934]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
8. Hach T. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials [abstract 943]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
9. List JF, Woo V, Morales E, et al. Sodium-glucose co-transport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care. 2009;32(4):650-657.
10. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(5):2287.
11. Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37(5):1480-1483.
A 37-year-old woman with a history of papillary carcinoma (status post total thyroidectomy 12 years ago, with negative recurrence) presents for a check-up. She also has polycystic ovarian syndrome (PCOS) with obesity and is taking metformin XR (one 500-mg tablet bid). Her visit is uneventful, and she leaves the office with an order for labwork.
Results indicate normal thyroid function and negative thyroglobulin. However, her serum glucose level is 350 mg/dL, so the patient is called and informed of the result. She denies polyphagia, polydipsia, and polyuria. Repeat blood work confirms overt hyperglycemia (320 mg/dL) with an A1C of 13%, undetectable C-peptide, and negative glutamic acid decarboxylase 65 (GAD65) and islet cell antibodies.
She is advised to increase her metformin dose (to two 500-mg tablets bid) and is started on insulin detemir (20 U every evening), with instructions to increase the latter by three units every two to three days until a target fasting glucose level of 100 to 140 mg/dL is achieved. She is also advised to follow a low-carbohydrate diet and increase her exercise.
The patient returns in two weeks for follow-up. She remains asymptomatic and has now increased her insulin detemir to 34 U bid (she started splitting the dosage after it reached 50 U/d). However, her glucose is still in the low 200s in the morning and the high 200s during the day (after lunch and dinner).
Her overt hyperglycemia is most likely a result of her longstanding insulin resistance, essential lack of b-cell function, and PCOS-associated obesity. Once diabetes from autoimmunity is ruled out by laboratory findings (negative antibodies) and clinical assessment (classic metabolic syndrome features), we focus on her glycemic control.
Even with nearly 70 U/d of insulin, the patient’s glycemic improvement is disappointing, suggesting significant insulin resistance and glucose toxicity. Living in an era with numerous classes of antidiabetic medications, we have lengthy discussions on treatment options. Canagliflozin, recently (at the time) approved, is included. The patient is interested in this new medication, and it is a reasonable choice to get her out of the glucotoxic phase.
After a discussion of benefits and potential adverse effects, she is placed on canagliflozin 100 mg/d. Her glucose log in one week shows fasting glucose values in the range of 140 to 160 mg/dL and postprandial glucose values in the 180s. As a result, she lowers her insulin to 25 U bid. Her renal panel shows a potassium level of 4.3 mEq/L (reference range, 3.5 to 5.3) and a glomerular filtration rate (GFR) of 103 mL/min/1.73 m2. She is advised to further increase her canagliflozin to 300 mg and slowly titrate her insulin down as needed, with a target fasting glucose level of 80 to 110 mg/dL and a postprandial target of 100 to 140 mg/dL.
What are SGLT2 inhibitors, and how do they work?
What are SGLT2 inhibitors, and how do they work?
Sodium-GLucose co-Transporter 2 (SGLT2) inhibitors are a new class of antihyperglycemic agent. The first, canagliflozin, was approved by the FDA in March 2013, followed by dapagliflozin (January 2014) and empagliflozin (August 2014).
As glucose is filtered through the nephrons of the kidney, about 90% is reabsorbed via SGLT2 in the proximal tubule (SGLT1 is responsible for the remaining 10%) so that glucose calories are not eliminated through urine.1 In a healthy person, the renal glucose threshold is about 180 mg/dL.1 When blood glucose exceeds this level, glucose is excreted into the urine. However, in diabetic patients, this threshold is higher due to the up-regulation of SGLT2s (and other glucose transporters), which worsens hyperglycemia.1 SGLT2 inhibitors will reset the threshold, which in turn will increase glucosuria and thereby lower serum glucose.1
SGLT2 inhibitors lower A1C by about 0.7% to 0.8%.2 Independent of other mechanisms such as the degree of b-cell function or insulin resistance, these agents can be used regardless of the duration of diabetes3 if the GFR is intact (≥ 45 mL/min/1.73 m2 for canagliflozin and empagliflozin, ≥ 60 mL/min/ 1.73 m2 for dapagliflozin).4,5
What are the risks and benefits associated with these agents?
What are the risks and benefits associated with these agents?
Modest weight loss is seen with the use of SGLT2 inhibitors. Initial weight loss is believed to be related to volume loss, but more sustained weight loss is thought to be from loss of fat mass.6 This is not surprising, as excreting glucose means excreting calories through urine.
Risk for hypoglycemia is extremely low, which makes this therapeutic class an attractive option. However, caution should be exercised when SGLT2 inhibitors are combined with other agents known to cause hypoglycemia (sulfonylureas and insulin).6
The most common adverse effect is genital mycotic infection. Women with a history of recurrent genital mycotic infection and uncircumcised men are at the greatest risk.6
Due to increased glycosuria, which results in an osmotic diuresis, modest blood pressure improvement has been seen (3 to 4 mm Hg systolic and 1 to 2 mm Hg diastolic7,8) in patients taking SGLT2 inhibitors, which is an additional benefit for hypertensive diabetic patients.6 On the other hand, use of SGLT2 inhibitors can also cause dehydration and volume depletion and can raise serum creatinine in patients who are already taking diuretics (particularly loop diuretics).6 Drug tolerance and adherence can be improved by advising patients to expect transient increased urination (approximately 135 to 350 mL/d increase from baseline5,9) and emphasizing the importance of good hydration and maintaining good genital hygiene.
A slight increase in LDL cholesterol was seen in clinical trials of the SGLT2 inhibitors, although this phenomenon is poorly understood. However, HDL cholesterol increased as well, maintaining the LDL:HDL ratio.6 No long-term cardiovascular outcome data are available at this time; as with any new antidiabetic medication, postmarketing studies, as required by the FDA, are currently ongoing.6
What are the options in this therapeutic category, and how are they distinct?
What are the options in this therapeutic category, and how are they distinct?
As mentioned previously, there are currently three SGLT2 inhibitors on the market: canagliflozin, dapagliflozin, and empagliflozin. There are subtle clinical differences among these three agents, which might direct the clinician’s choice.
First, canagliflozin is available in dosages of 100 and 300 mg. The starting dosage is 100 mg, which can be titrated to 300 mg in patients with a GFR ≥ 60 mL/min/1.73 m2 who require a greater glucose-lowering effect. Those with a GFR < 60 mL/min/1.73 m2 but ≥ 45 mL/min/1.73 m2 are limited to the 100-mg dosage. Dapagliflozin is available in 5-mg and 10-mg dosages, the former being the starting dosage. But dapagliflozin is not recommended in patients whose GFR is < 60 mL/min/1.73 m2.4
Empagliflozin is available in dosages of 10 and 25 mg. The starting dosage of 10 mg can be increased to 25 mg if the patient has not achieved his/her target glucose level. Either can be used in patients with a GFR ≥ 45 mL/min/1.73 m2.5
Second, hyperkalemia was seen in patients taking canagliflozin but not in those taking dapagliflozin or empagliflozin. Therefore, serum potassium should be monitored and caution used, especially when patients are being treated with potassium-sparing diuretics and/or ACE inhibitors or angiotensin II receptor blockers.6
Third, dapagliflozin carries a warning for bladder cancer, as higher rates of newly diagnosed bladder cancer were seen with this drug compared with placebo or comparator drugs (0.17% vs 0.03%, respectively).4 However, this finding may have resulted from a randomization imbalance of patients in the study, and further research is needed to clarify this risk.6 It is not recommended that dapagliflozin be used in patients with active or a history of bladder cancer at this time.
With these agents, there is a paradoxical rise in glucagon that increases endogenous glucose production from the liver.10 The mechanism is poorly understood, but it might be due to the body’s compensatory (survival) mechanism to “make up” the loss of glucose through urine by increasing hepatic gluconeogenesis.
Using an incretin agent, such as dipeptidyl peptidase 4 (DPP-4) inhibitors or glucagon-like peptide 1 (GLP-1) receptor agonists, in conjunction with an SGLT2 inhibitor, has been suggested as a way to potentiate the glucose-lowering effect, as it may attenuate the paradoxical rise in glucagon.10 Since the incretin class is weight neutral (DPP-4 inhibitors) or associated with weight loss (GLP-1 agonists), using incretins with SGLT2 inhibitors might produce more significant weight loss, which has numerous additional benefits for diabetic patients.
SGLT2 inhibitors are currently approved as an adjunct to diet and exercise for patients with type 2 diabetes. They are not approved for those with type 1 diabetes, although the mechanism of action of these drugs (which is independent of the b-cell function) might make them effective in this population. Active pilot studies of this patient population are in progress.11
Conclusion
In summary, SGLT2 inhibitors are an exciting new class of antidiabetic medication that offers a unique mechanism to lower serum glucose. It is the only medication that will actually remove glucose from the body; by contrast, all other existing antidiabetic medications move glucose within the body (to liver, fat, muscle, etc).
There is no curative medication for diabetes. But with an increasing diabetic population and an emphasis on individualizing antihyperglycemic regimens, we always welcome medications with novel mechanisms of action. Due to SLGT2 inhibitors’ recent approval, however, short-term and long-term adverse effects are unknown, and ongoing postmarketing surveillance should be closely followed.
References
1. Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008;14:782-790.
2. Berhan A, Barker A. Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized double-blind controlled trials. BMC Endocr Disord. 2013;13(1):58.
3. Wilding JP, Norwood P, T’joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Diabetes Care. 2009;32:1656-1662.
4. Taylor JR. Dapagliflozin offers differences from other SGLT2 inhibitors. Endocrine Today. May 2014.
5. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2014.
6. Bakris G, Fonseca VA, Peters AL, Wysham CH. Clinical perspectives on the role of the kidney in the pathophysiology of T2DM: emerging options for treatment [video series]. 2013. www.thedoctorschannel.com/view/the-kid ney-in-t2dm-cme-part-1/. Accessed September 12, 2014.
7. Vercruysse F. Efficacy and safety of canagliflozin in subjects with type 2 diabetes mellitus inadequately controlled with metformin plus sulphonylurea over 52 weeks [abstract 934]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
8. Hach T. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials [abstract 943]. Presented at the 49th European Association for the Study of Diabetes Annual Meeting: Barcelona; September 24, 2013.
9. List JF, Woo V, Morales E, et al. Sodium-glucose co-transport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care. 2009;32(4):650-657.
10. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(5):2287.
11. Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37(5):1480-1483.
Subacute Thyroiditis
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.