User login
Hypertension and Diabetes: Addressing Common Comorbidities
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in patients with diabetes.1 ASCVD is defined by the American College of Cardiology and the American Heart Association (ACC/AHA) as acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin.2 Risk factors for ASCVD include hypertension, dyslipidemia, smoking, family history of premature coronary disease, chronic kidney disease, and albuminuria.3
Hypertension, a modifiable risk factor, is prevalent in patients with diabetes. Multiple studies have shown that antihypertensive therapy in these patients reduces ASCVD events; therefore, blood pressure control is necessary.1,3 The American Diabetes Association’s (ADA) 2018 Standards of Medical Care in Diabetes offers guidance on the assessment and treatment of hypertension in patients with diabetes—including the organization’s position statement on hypertensive treatment with comorbid diabetes.1,3 These guidelines are relevant and useful to both primary care and specialty providers who manage these complex patients.
Screening and Diagnosis
Every clinical care visit for patients with diabetes should include a blood pressure measurement. (Evaluation for orthostatic hypotension should also be performed at the initial visit, to help guide future treatment.1) For accuracy, blood pressure should be assessed
- By a trained individual using the appropriate size cuff
- In both arms on the initial visit
- With the patient seated, with feet on the floor and arm at heart level
- After five minutes of rest
- With two to three readings taken one to two minutes apart and results averaged.1
If blood pressure is found to be elevated and the patient has no known history of hypertension, the elevated blood pressure should be reassessed on another visit within one month to confirm the diagnosis.1 Patients should also monitor blood pressure at home to distinguish between white coat and masked hypertension.1 Home blood pressures should be measured with arm cuffs that are the appropriate size. The bladder of the cuff should encircle 80% of the arm, should not cover clothing, and should be placed on the upper arm at the midpoint of the sternum.1
The ACC/AHA’s 2017 guidelines define stage 1 hypertension as 130-139/80-89 mm Hg and stage 2 hypertension as ≥ 140/90 mm Hg.4 The ADA defines hypertension as a sustained blood pressure ≥ 140/90 mm Hg, noting that the definition is “based on unambiguous data that levels above this threshold are strongly associated with ASCVD, death, disability, and microvascular complications.”1
BLOOD PRESSURE TARGETS
Evidence has shown that treatment of blood pressure to a goal of ≤ 140/90 mm Hg reduces cardiovascular events as well as microvascular complications.1 For patients with diabetes, the ADA recommends treatment to a systolic blood pressure goal of < 140 mm Hg and a diastolic blood pressure goal of < 90 mm Hg, while the ACC/AHA guidelines recommend a goal of < 130/80 mm Hg.1,4
The ADA does note that lower blood pressure targets (eg, < 130/80 mm Hg) can be appropriate for individuals at high risk for cardiovascular disease if no treatment burdens (eg, adverse effects, costs) are imposed.1 This is important, since patients with diabetes often have multiple risk factors for ASCVD and will be considered high risk. Studies suggest lower blood pressure targets may decrease the risk for stroke and albuminuria but offer little to no effect on other ASCVD events, occurrence of heart failure, or other conditions associated with diabetes (eg, peripheral neuropathy).1
Continue to: LIFESTYLE MANAGEMENT
LIFESTYLE MANAGEMENT
Patients with diabetes and elevated blood pressure (> 120/80 mm Hg, per the 2017 ACC/AHA guidelines) are at high risk for hypertension and its complications.1,4 Lifestyle management—which includes weight loss, a healthy diet, increase in physical activity, and moderation in alcohol intake—is an important component of preventing or delaying a hypertension diagnosis.1,4
Both the ADA and the ACC/AHA recommend that patients with diabetes follow the Dietary Approaches to Stop Hypertension (DASH) diet.1,4 Guidelines include restricting sodium intake to < 2,300 mg/d, consuming 8-10 servings/d of fruits and vegetables and 2-3 servings/d of low-fat dairy products, limiting alcohol consumption to two servings/d for men and one serving/d for women, and increasing physical activity to include at least 30-45 min/d of aerobic exercise.1,4
PHARMACOLOGIC TREATMENT
Initial treatment for patients with hypertension and diabetes depends on the severity of the hypertension and should include drug classes that have demonstrated success in reducing ASCVD events: ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, and dihydropyridine calcium channel blockers. The ADA offers additional guidance:
Blood pressure ≥ 140/90 mm Hg should be treated with lifestyle modifications and simultaneous initiation of a single drug, with timely titration of pharmacologic therapy to achieve blood pressure goals.
Continue to: Blood pressure ≥ 160/100 mm Hg
Blood pressure ≥ 160/100 mm Hg should be treated with lifestyle therapy and prompt initiation and timely titration of two drugs or a single-pill combination of drugs.
Multidrug therapy is generally required to achieve blood pressure targets—but ACE inhibitors and ARBs should not be used in combination due to the increased risk for adverse effects.
Firstline therapy is an ACE inhibitor or an ARB, at the maximum tolerated dose, in patients with diabetes and a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Monitoring of estimated glomerular filtration rate and serum potassium levels is needed in patients treated with an ACE inhibitor, ARB, or diuretic.1
RESISTANT HYPERTENSION
Patients with diabetes who have a blood pressure ≥ 140/90 mm Hg despite treatment that includes lifestyle management, two antihypertensives, and a diuretic, or who achieve blood pressure control with four or more medications, are considered to have resistant hypertension.1,5 Factors such as pseudoresistance (lack of medication adherence or poor measurement technique), masked hypertension, and white coat hypertension should be ruled out in making the diagnosis of resistant hypertension. Once these have been excluded, patients should be referred for a workup of their resistant hypertension to evaluate causes of secondary hypertension. These can include endocrine issues, renal arterial disease, edema in advanced kidney disease, hormones, and drugs such as NSAIDs and decongestants.1
Continue to: PATIENT-CENTERED CARE
PATIENT-CENTERED CARE
When evaluating and treating a patient with diabetes, it is important to consider
- What is the patient’s overall risk for atherosclerotic cardiovascular disease?
- Does he/she have an increased risk for stroke? If so, lower blood pressure targets may be appropriate.
- Is more than one antihypertensive agent (ACE inhibitor, ARB, or diuretic) being used? If so, close monitoring of estimated glomerular filtration rate and potassium (as well as other indications of adverse effects) is important.
The treatment regimen should be a shared decision-making process between the clinician and patient and should be individualized to each patient and his/her existing comorbidities.
CONCLUSION
Clinical trials and meta-analyses support target blood pressure management to < 140/90 mm Hg in most adults with diabetes, while lower targets (< 130/80 mm Hg) may be beneficial for patients with diabetes and a high risk for cardiovascular disease.1,5 Lifestyle management should be initiated and continued in patients with a blood pressure > 120/80 mm Hg and in those diagnosed with hypertension.1 Medications that reduce cardiovascular events should be used in management, with ACE inhibitors or ARBs being firstline treatment in patients with albuminuria.1
For more information on hypertensive treatment in special populations (eg, pregnant women and older adults), see the ADA’s full position statement.1
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
1. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014; 129(25 suppl 2):S1-S45.
3. American Diabetes Association. Position Statement 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
5. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916.
What’s New in Diabetes Management: Psychosocial Care
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
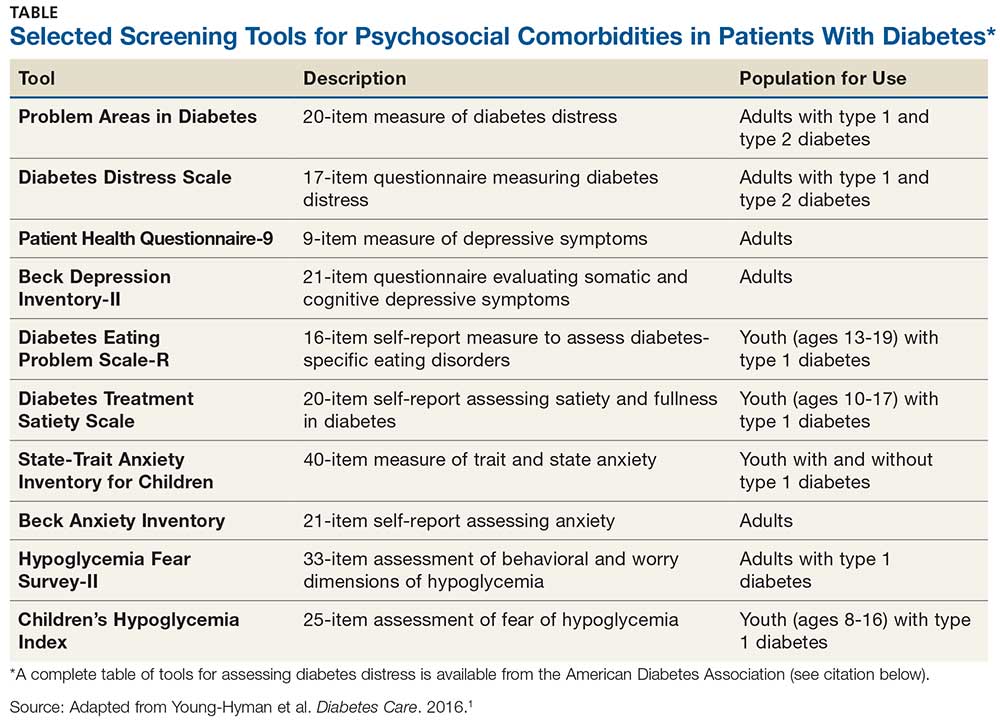
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.
PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.

PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
The wide array of comorbidities and treatment variables can make diabetes a difficult disease to manage—and to live with. Providers must be equipped to address the complexities and complications that affect patients with diabetes. In December 2016, the American Diabetes Association published a position statement recognizing the psychosocial factors (environmental, social, behavioral, and emotional) that affect medical outcomes and psychological well-being in persons with diabetes. These include self-management, diabetes distress, psychological comorbidities, and life-course considerations.1
SELF-MANAGEMENT
A patient’s perception of his or her ability to self-manage diabetes is an important psychosocial factor in treatment and management outcomes. Training patients with diabetes in self-care skills and the use of technologies—at the time of diagnosis, annually, and/or when complications or transitions in care occur—can empower patients to assume an active role in their daily management. These interventions can be tailored to address specific, individualized problems that contribute to suboptimal glycemic outcomes, such as issues in numeracy or coping, food insecurity, or lack of support. Employing a nonjudgmental approach that normalizes periodic lapses in self-management may help encourage patients and minimize their resistance to self-management.
DIABETES DISTRESS
The frustration, worry, anger, guilt, and burnout imposed by diabetes and its management (via glucose monitoring, medication dosing, and insulin titration) is known as diabetes distress. With a reported prevalence of 18% to 45%, this disease burden is quite common.2 Because high levels of diabetes distress are associated with low self-efficacy, poor glycemic outcomes, and suboptimal exercise/dietary habits, referral for counseling should be considered if a patient expresses feelings of distress.
Use of validated screening tools, such as Problem Areas in Diabetes (PAID)3,4 or the Diabetes Distress Scale (DDS)5, can aid in routine monitoring for diabetes distress. (See the Table for more information.) If distress is identified in specific self-care areas, further patient education on self-management is appropriate.

PSYCHOLOGICAL COMORBIDITIES
Depression, anxiety, disordered eating, and serious mental illness (eg, schizophrenia) are known psychological comorbidities of diabetes. Screening for symptoms using patient-appropriate, standardized/validated tools should occur at initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance.
Depression
Patients with diabetes should be screened for depression when medical status worsens or when complications occur; it is recommended to include caregivers and family members in this assessment. Patients who screen positive for depression should be referred to mental health providers who have experience with cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches, and who can provide collaborative care alongside the diabetes treatment team. Once diagnosed with depression, patients should be screened annually.
Anxiety
Expression of fear, dread, or irrational thoughts, avoidant and/or repetitious behaviors, and social withdrawal are signs of anxiety that should prompt screening. Consider screening for anxiety in patients who express worry about diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interferes with self-management behaviors.
Continue to: Patients with hypoglycemia unawareness...
Patients with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, can increase self-monitoring of glucose with a glucometer or continuous glucose monitor. Blood Glucose Awareness Training (or other similar evidence-based intervention) can be used to help reestablish awareness of hypoglycemia and reduce fear of hypoglycemia.6-8 Providers can deliver hypoglycemia awareness education in the clinic.
Disordered Eating
When hyperglycemia and weight loss are unexplained by self-reported medication dosing, diet, and exercise, consider screening for disordered or disrupted eating (see Table for screening tools). In addition, reviewing the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake.
Cognitive Impairment
Since research has shown significantly increased rates of diabetes among persons with serious mental illness (eg, schizophrenia), annual screening for prediabetes and diabetes is recommended for those taking atypical antipsychotic medications. Furthermore, some of the effects of serious mental illness—such as disordered thinking and impaired judgment—make it difficult for a patient to engage in risk-reducing behaviors or (if diagnosed) to manage diabetes. Therefore, monitoring of diabetes self-care activities should be incorporated into treatment goals for persons with these comorbid conditions.
Continue to: CONCLUSION
CONCLUSION
As providers, we should be familiar with the evidence-based, validated tools available to identify the psychosocial comorbidities of diabetes. Screening and assessing patients for psychosocial/behavioral challenges should be performed at an initial visit, at periodic intervals, and whenever there is a change in disease, treatment, or life circumstances.
Health care alliances with behavioral/mental health providers who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes are key. Patient-centered care is essential to promote optimal medical outcomes and psychological well-being. As members of the health care team, we must be respectful and responsive to patient preferences, needs, and values; clinical decisions should be guided by patient values. If A1C is not at goal despite maximized medication therapy and lifestyle modification, consider identifying and addressing any psychosocial factors that may be involved.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
1. Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140.
2. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472-2478.
3. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760.
4. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72.
5. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631.
6. Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood Glucose Awareness Training (BGAT-2). Diabetes Care. 2001;24(4):637-642.
7. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264.
8. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806.
The Gut Microbiome in Type 2 Diabetes
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
Insulin Pump Therapy: Who, Why, and How
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
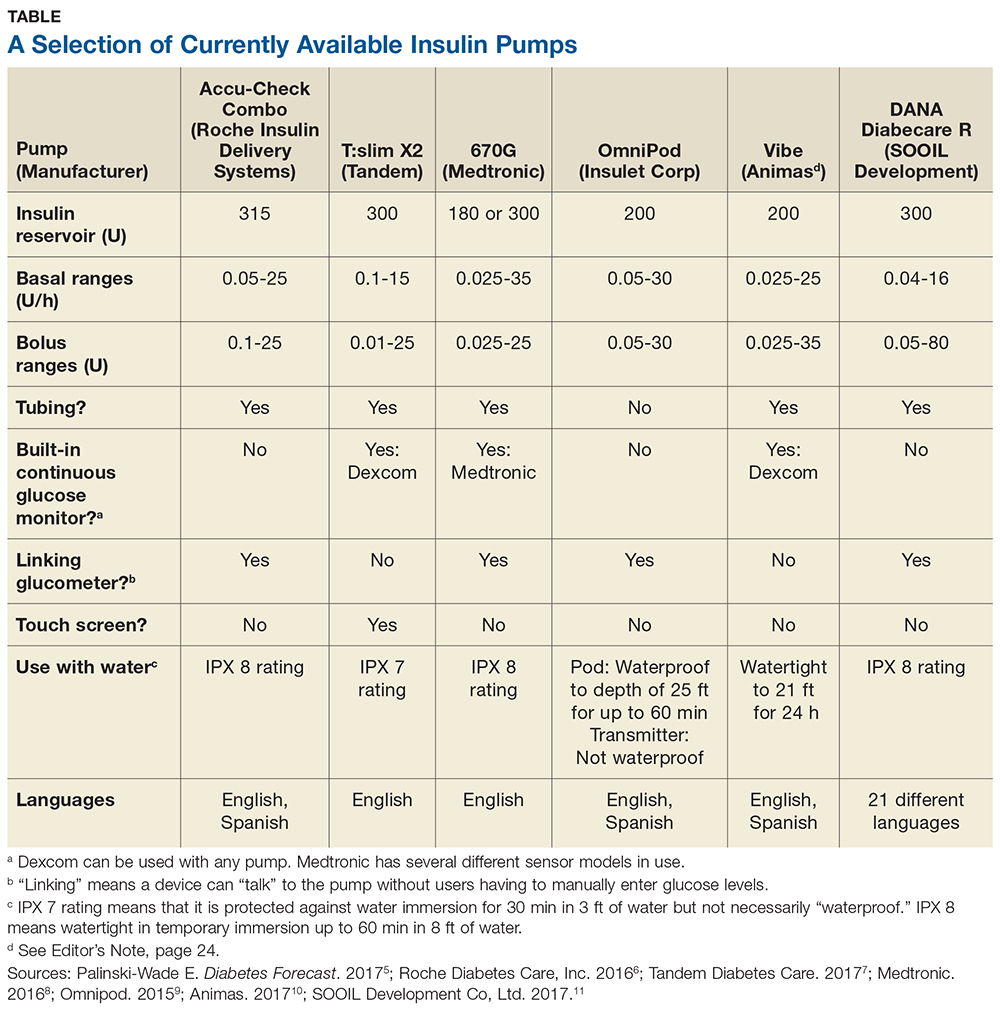
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12

Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12

Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
Thyroid Storm: Early Management and Prevention
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).
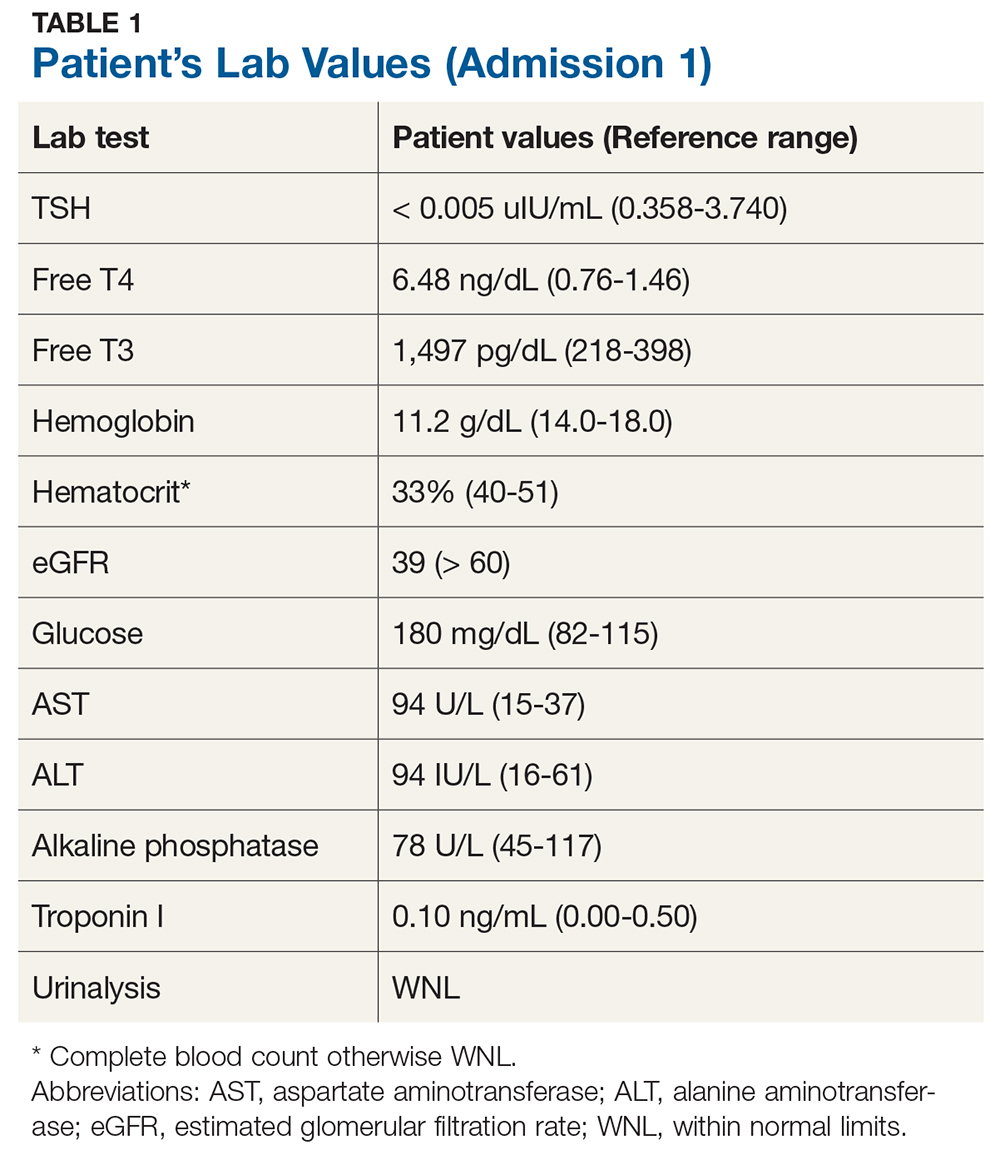
Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.
Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.
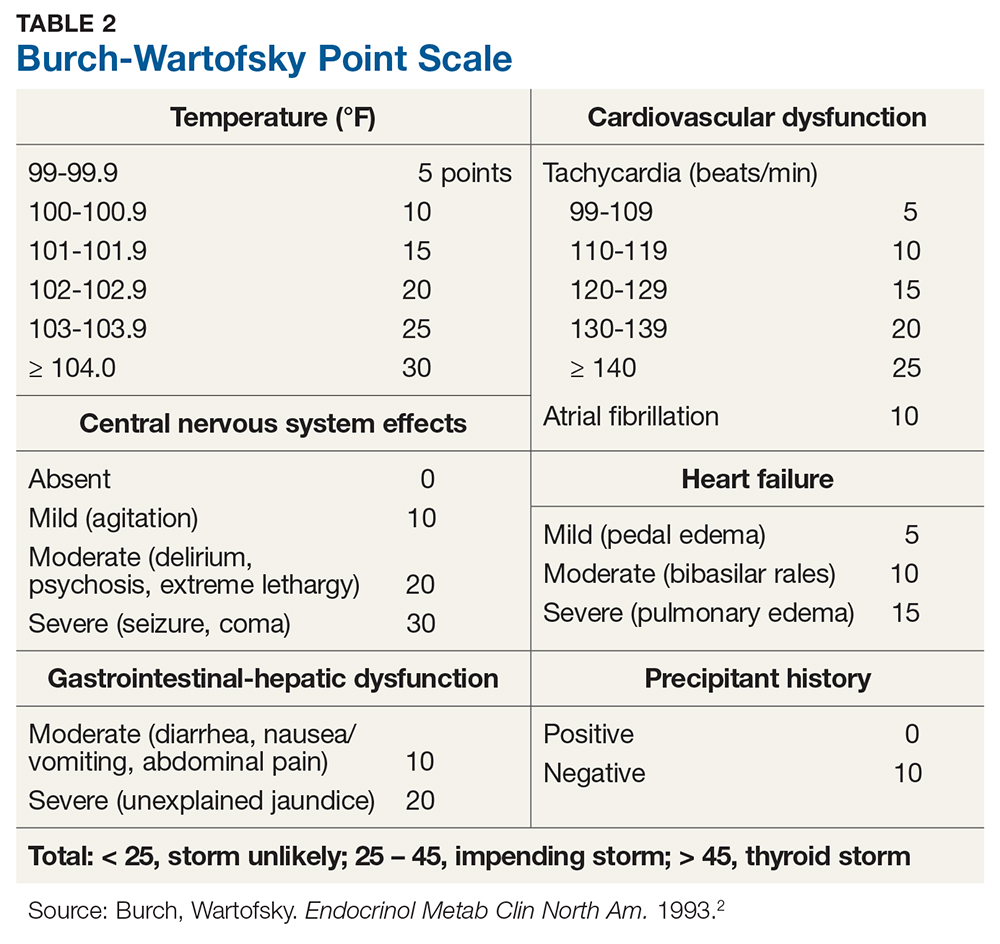
Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).

Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.

Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.

Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
A 73-year-old man is transported to the emergency department (ED) by ambulance for nausea, vomiting, diarrhea, and weakness of three days’ duration. Earlier today, he presented to his primary care provider with these symptoms and was found to be hypotensive; he was advised to go to the ED but instead went home against medical advice.
The patient’s medical history is significant for type 2 diabetes, stage 3b chronic kidney disease, dyslipidemia, hypertension, coronary artery disease, and benign prostatic hyperplasia. He has undergone stent placement and triple coronary artery bypass graft surgery. His medication list includes insulin glargine, glimepiride, liraglutide, atorvastatin, benazepril, carvedilol, amlodipine, clopidogrel, and tamsulosin.
Upon admission, the patient has a pulse of 98 beats/min; temperature, 98.2°F; respiratory rate, 18 breaths/min-1; and PO2, 98 mm Hg. An ECG, chest radiograph, and CT (without contrast) of the head, chest, and abdomen are all within normal limits. Lab evaluation is significant for severe thyrotoxicosis (see Table 1).

Endocrinology consult is requested. Further testing yields the following findings
- Thyroid-stimulating immunoglobulin: 309% (reference range, < 30%)
- Nuclear medicine thyroid scan with uptake: 6-hour uptake of 70.3% (10%-25%) and 24-hour uptake, 81.8% (15%-35%)
- Homogeneous radiotracer uptake within the thyroid gland: no evidence of hot or cold nodules
- Thyroid ultrasound: bilateral enlarged heterogeneous gland and multiple subcentimeter nodules (largest measuring 6 × 7 mm)
These results confirm a diagnosis of Graves’ disease. Treatment options, including antithyroid medications, radioactive iodine ablation (RAI), and surgery, are discussed. The patient is treated with RAI therapy (10 mCi) and discharged from the hospital.

Six days later, however, he returns to the ED with severe intermittent dizziness and lightheadedness of two hours’ duration, new-onset atrial fibrillation (A-fib), and mild shortness of breath. His vital signs include a pulse of 116 beats/min; temperature, 98.1°F; respiratory rate, 18 breaths/min-1, blood pressure, 154/88 mm Hg; and PO2, 100 mm Hg.
His lab values include
- TSH < 0.005 uIU/mL
- Free T4, 8.01 ng/dL
- Free T3, 3,701 pg/dL
- eGFR, 60 mL/min/1.73 m2
Cardiology consult is requested. A pacemaker is placed for bradycardia-tachycardia syndrome, and the patient is put on rivaroxaban for stroke prevention.
The endocrinologist suspects post-RAI thyroiditis or ineffective RAI treatment. The patient is started on methimazole (10 mg bid), and his carvedilol is replaced with metoprolol (50 mg bid).
Two weeks postdischarge, the patient returns to the office. Although he says he’s doing better, he seems uneasy and agitated and has a pulse of 120 beats/min. His methimazole and metoprolol are increased (to 10 mg tid and 50 mg tid, respectively).
Another two weeks later, lab results still show elevated thyroid levels—now with increased enzyme levels on liver function testing. The patient reports worsening dizziness and shortness of breath. He is sent back to hospital and admitted for inpatient management, with urgent surgical consult for thyroidectomy. Total thyroidectomy is successfully performed, and the final pathology report shows a benign goiter.
DISCUSSION
Thyroid storm is an extreme form of thyrotoxicosis with an associated mortality rate of 8% to 25%.1 When thyroid hormone levels are elevated, adrenaline receptors are upregulated—but, while it is possible for persistent thyrotoxicosis to progress to thyroid storm on its own, a surge of adrenaline is usually needed. Most cases are triggered by acute stressors (ie, myocardial infarction, surgery, anesthesia, labor and delivery) in the context of underlying thyrotoxicosis.1
Diagnosis of thyroid storm is made clinically in patients who are thyrotoxic and present with systemic decompensation (ie, altered mental status, cardiovascular dysfunction, hyperpyrexia). Although no universally accepted criteria currently exist, the Burch-Wartofsky Point Scale (BWPS; see Table 2) can be used to assess disease severity and guide the extent of treatment and monitoring.2 However, this measure should not replace clinical judgment—the distinction between compensated thyrotoxicosis and decompensating thyrotoxicosis (thyroid storm) should be made by sound but prompt clinical assessment.

Once thyroid storm is suspected, aggressive treatment should be implemented to improve the systemic thyrotoxic state. Propylthiouracil (PTU) is preferred over methimazole, as it blocks T4 to T3 conversion in addition to blocking new hormone synthesis. Propranolol is the best choice of ß-blocker because it also blocks T4 to T3 conversion and controls cardiac rhythm.
Iodine can rapidly block new hormone synthesis and release; it is often used to reduce thyroid hormone levels prior to emergency thyroid surgery. However, it should be given at least one hour after a dose of PTU. Hydrocortisone is given prophylactically for relative adrenal insufficiency (due to rapid cortisol clearance during thyrotoxic state); it may block T4 to T3 conversion as well. Volume resuscitation, respiratory care, temperature control (eg, antipyretics, cooling blankets), and nutritional support should also be incorporated, ideally in the intensive care unit (ICU). During or after thyroid storm management, treatment of the precipitating event/illness and of hyperthyroidism should be initiated to prevent recurrence.1
The patient’s initial BWPS was 30 (gastrointestinal [GI] score 10 + central nervous system [CNS] score 10 + without precipitating factor 10), which put him in the “impending storm” category. At his second ED visit, his BWPS was 40 (cardiovascular score 10 + A-fib 10 + GI score 10 + CNS score 10 + precipitating factor [RAI ablation] score 0)—still in the “impending storm” category but certainly indicating a worsened state.
RAI for hyperthyroidism can transiently increase thyroid hormone levels due to inflammation of the gland. To prevent exacerbation of the thyrotoxic state, pretreatment with methimazole should be considered in patients with risk factors (eg, older age, cardiovascular complications, cerebrovascular disease, pulmonary disease, renal failure, infection, trauma, and poorly controlled diabetes). Patients should also be placed on ß-blockers prior to treatment, in anticipation of a transient rise in thyroid hormone levels.
Due to this patient’s age, severity of thyrotoxicosis, and multiple risk factors, strong consideration should have been given to pretreating him with antithyroid medication and a ß-blocker before definitive treatment was given. This would have potentially averted his subsequent hospital visits and urgent need for thyroidectomy.
CONCLUSION
Thyroid storm is an uncommon but serious medical condition with a high mortality rate. Prompt recognition and an aggressive multimodal treatment approach, ideally in the ICU, are paramount to stabilize patients and seek definitive treatment.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; 22(2):263-277.
Which Diet for Type 2 Diabetes?
Prescribed diets can be trying for both patients and providers; patients often struggle to adhere to them, and providers must determine which plan is suitable for which patient. The optimal diet for patients with diabetes—and whether it is sustainable—remains controversial.
A plant-based diet high in polyunsaturated and monounsaturated fats, with limited saturated fat and avoidance of trans-fatty acids, is supported by the American Association of Clinical Endocrinologists. Caloric restriction is recommended when weight loss is appropriate.1 The American Diabetes Association (ADA) recommends a Mediterranean-style diet rich in monounsaturated fats with carbohydrates from whole grains, vegetables, fruits, legumes, and dairy products, and an emphasis on foods higher in fiber and lower in glycemic load.2
Additionally, the ADA, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics advise that all individuals with diabetes receive individualized Medical Nutrition Therapy (MNT), preferably with a registered dietitian nutritionist (RDN) knowledgeable and skilled in providing diabetes-specific nutrition education. MNT delivered by an RDN has been shown to reduce A1C levels by up to 2% in people with type 2 diabetes (T2DM).3
This flexibility in recommendations creates uncertainty about the correct dietary choice. Several diet plans are endorsed for the management of diabetes, including Mediterranean, low carbohydrate, Paleolithic, vegan, high fiber, and glycemic index (GI). Which should your patients adhere to? Several randomized controlled trials (RCTs), meta-analyses, and literature reviews have examined and compared the benefits of these eating habits for management of diabetes.
MEDITERRANEAN
The Mediterranean diet incorporates plant foods such as greens, tomatoes, onions, garlic, herbs, whole grains, legumes, nuts, and olive oil as the primary source of fat. A crossover trial of adults with T2DM demonstrated a statistically significant A1C reduction (from 7.1% to 6.8%) after 12 weeks on the Mediterranean diet.4
In a systematic review of 20 RCTs, Ajala et al analyzed data for nearly 3,500 patients with T2DM who adhered to either a low-carbohydrate, vegetarian, vegan, low-GI, high-fiber, Mediterranean, or high-protein diet for at least six months. The researchers found that Mediterranean, low-carbohydrate, low-GI, and high-protein diets all led to A1C reductions—but the largest reduction was observed with patients on the Mediterranean diet. Low-carbohydrate and Mediterranean diets resulted in the most weight loss.5
LOW-CARBOHYDRATE
Low-carbohydrate diets have decreased in popularity due to concerns about their effects on renal function, possible lack of nutrients, and speculation that their macronutrient composition may have effects on weight beyond those explained by caloric deficit. A meta-analysis of 13 studies of adults with T2DM following a low-carbohydrate diet (≤ 45% of calories from carbohydrates) demonstrated beneficial effects on fasting glucose, A1C, and triglyceride levels. Nine of the studies evaluated glycemic control and found A1C reduction with lower carbohydrate diets; the greatest reductions in A1C and triglycerides were correlated with the lowest carbohydrate intakes. No significant effects were seen for total, HDL, or LDL cholesterol.6
In the literature review by Ajala et al, low-carbohydrate, low-GI, and Mediterranean diets all improved lipid profiles. HDL cholesterol increased the most with a low-carbohydrate diet.5
A two-week study of 10 adults with T2DM found that just one week on a low-carbohydrate diet decreased the average 24-h plasma glucose from 135 mg/dL to 113 mg/dL. Over the two-week study period, triglycerides decreased by 35%, cholesterol by 10%, and A1C by 0.5%. Patients were allowed to consume as much protein and fat as desired. Food sources included beef and ground turkey patties, chicken breasts, turkey, ham, steamed vegetables, butter, diet gelatin, and a limited amount of cheese. Mean calorie intake decreased from 3,111 to 2,164 calories/d. Carbohydrate intake decreased from 300 to 20 g/d. Weight loss was entirely explained by the mean energy deficit.6 Patients experienced no difference in hunger, satisfaction, or energy level with a low-carb diet compared to their usual diet.7
A literature review of six studies examined the effects of low-carb diets (between 20-95 g/d) on body weight and A1C in patients with T2DM. Three of the studies restricted carbohydrate intake to less than 50 g/d. All reported reductions in body weight and A1C. In two studies, the majority of the weight loss was explained by a decrease in body fat, not loss of water weight. No deleterious effects on cardiovascular disease risk, renal function, or nutritional intake were seen. The researchers concluded that low-carb diets are safe and effective over the short term for people with T2DM.8
PALEOLITHIC
The Paleolithic diet (also referred to as the caveman diet, Stone Age diet, and hunter-gatherer diet) involves eating foods believed to have been available to humans before agriculture—this period began about 2.5 million years ago and ended about 100,000 years ago. Food sources include wild animal meat (lean meat and fish) and uncultivated plant foods (vegetables, fruits, roots, eggs, and nuts). It excludes grains, legumes, dairy products, salt, refined sugar, and processed oils.
In a randomized crossover study of 13 participants with T2DM, the Paleolithic diet improved glucose control and several cardiovascular disease markers, compared to a standard diabetes diet. The Paleolithic diet resulted in significantly lower A1C, triglycerides, diastolic blood pressure, body weight, BMI, and waist circumference, as well as increased HDL. Despite receiving no instruction to restrict calories, patients on the Paleolithic diet consumed fewer calories and carbohydrates, and more protein and fat, than those on the standard diabetes diet. The caloric deficit accounted almost exactly for the observed difference in weight loss between the two groups.9
GLYCEMIC INDEX
The GI measures the blood glucose level increase in the two hours after eating a particular food, with 100 representing the effect of glucose consumption. Low-GI food sources include beans, peas, lentils, pasta, pumpernickel bread, bulgur, parboiled rice, barley, and oats, while high-GI foods include potatoes, wheat flour, white bread, most breakfast cereals, and rice.
A meta-analysis compared the effects of high- and low-GI diets on glycemic control in 356 patients with diabetes. Ten of 14 studies documented improvements in A1C and postprandial plasma glucose with lower GI diets. Low-GI diets reduced A1C by 0.43% after an average duration of 10 weeks. The average GI was 83 for high-GI diets and 65 for low-GI diets. The researchers concluded that selecting low-GI foods has a small but clinically relevant effect on medium-term glycemic control, similar to that offered by medications that target postprandial blood glucose excursions.10
Low-GI diets resulted in lower A1C and higher HDL but no significant change in weight, according to Ajala et al.5
HIGH-FIBER
A survey of 15 studies examined the relationship between fiber intake and glycemic control. Interventions ranged from an additional 4 to 40 g of fiber per day, with a mean increase of 18.3 g/d. Additional fiber lowered A1C by 0.26% in 3 to 12 weeks, compared to placebo. The overall mean fasting blood glucose reduction was 15.32 mg/dL. No study lasted more than 12 weeks, but it is inferred that a longer study could result in a greater A1C reduction. Current dietary guidelines for patients with diabetes exceed the amount of fiber included in most of these studies.11
VEGAN
Ajala et al observed that patients on a vegan diet had lower total cholesterol, LDL, and A1C levels, compared to those on a low-fat diet. At 18 months, the vegetarian diet demonstrated improvement in glucose control and lipids, but not weight loss.5
In one RCT, a low-fat vegan diet was shown to improve glycemic control and lipid levels more than a conventional diabetes diet did. A1C decreased by 1.23% over 22 weeks, compared to 0.38% in the conventional diet group. Body weight decreased by 6.5 kg and LDL cholesterol decreased by 21.2% with the vegan diet, compared with a weight loss of 3.1 kg and a 10.7% LDL reduction in the conventional diet group.12
Patients on the vegan diet derived energy primarily from carbohydrates (75%), protein (15%), and fat (10%) by eating fruits, vegetables, grains, and legumes. Portion size and caloric and carbohydrate intake were not restricted. The conventional diet involved a caloric intake mainly from a combination of carbohydrates and monounsaturated fats (60% to 70%), protein (15% to 20%), and saturated fat (< 7%). The diet was individualized based on caloric needs and participants’ lipid levels. All participants were given calorie intake deficits of 500 to 1000 kcal/d.13 Participants rated both diets as satisfactory, with no significant differences between groups. The researchers concluded that a low-fat vegan diet has acceptability similar to that of a more conventional diabetes diet.12
CONCLUSION
Diabetes management strategies may incorporate a variety of dietary plans. While study populations are small and study durations relatively short, the aforementioned diets show improvement in biochemical markers such as fasting glucose, A1C, and lipid levels. The Mediterranean diet is believed to be sustainable over the long term, given the duration of time that people in the region have survived on it. Low-carbohydrate diets, including the Atkins and Paleolithic diets, are very effective at lowering A1C and triglycerides. Vegetarian/vegan diets may be more acceptable to patients than previously thought.
The long-term impact of any eating pattern will likely relate to adherence; adherence is more likely when patients find a diet to be acceptable, palatable, and easy to prepare. Diet selection should incorporate patient preferences and lifestyle choices, and when possible, should involve an RDN with expertise in diabetes.
1. American Association of Clinical Endocrinologists; American College of Endocrinology. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm. Endocr Pract. 2016;22(1):84-113.
2. American Diabetes Association. Standards of medical care in diabetes—2016. Clin Diabetes. 2016;34(1):3-21.
3. Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition. J Acad Nutr Diet. 2015:115(8):1323-1334.
4. Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21(9):740-747.
5. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
6. Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403-411.
7. Kirk JK, Graves DE, Craven TE, et al. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91-100.
8. Dyson PA. A review of low and reduced carbohydrate diets and weight loss in type 2 diabetes. J Hum Nutr Diet. 2008;21(6):530-538.
9. Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
10. Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261-2267.
11. Post RE, Mainous AG III, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25(1):16-23.
12. Barnard ND, Gloede L, Cohen J, et al. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009;109(2):263-272.
13. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-week clinical trial. Am J Clin Nutr. 2009;89(5):1588S-1596S.
Prescribed diets can be trying for both patients and providers; patients often struggle to adhere to them, and providers must determine which plan is suitable for which patient. The optimal diet for patients with diabetes—and whether it is sustainable—remains controversial.
A plant-based diet high in polyunsaturated and monounsaturated fats, with limited saturated fat and avoidance of trans-fatty acids, is supported by the American Association of Clinical Endocrinologists. Caloric restriction is recommended when weight loss is appropriate.1 The American Diabetes Association (ADA) recommends a Mediterranean-style diet rich in monounsaturated fats with carbohydrates from whole grains, vegetables, fruits, legumes, and dairy products, and an emphasis on foods higher in fiber and lower in glycemic load.2
Additionally, the ADA, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics advise that all individuals with diabetes receive individualized Medical Nutrition Therapy (MNT), preferably with a registered dietitian nutritionist (RDN) knowledgeable and skilled in providing diabetes-specific nutrition education. MNT delivered by an RDN has been shown to reduce A1C levels by up to 2% in people with type 2 diabetes (T2DM).3
This flexibility in recommendations creates uncertainty about the correct dietary choice. Several diet plans are endorsed for the management of diabetes, including Mediterranean, low carbohydrate, Paleolithic, vegan, high fiber, and glycemic index (GI). Which should your patients adhere to? Several randomized controlled trials (RCTs), meta-analyses, and literature reviews have examined and compared the benefits of these eating habits for management of diabetes.
MEDITERRANEAN
The Mediterranean diet incorporates plant foods such as greens, tomatoes, onions, garlic, herbs, whole grains, legumes, nuts, and olive oil as the primary source of fat. A crossover trial of adults with T2DM demonstrated a statistically significant A1C reduction (from 7.1% to 6.8%) after 12 weeks on the Mediterranean diet.4
In a systematic review of 20 RCTs, Ajala et al analyzed data for nearly 3,500 patients with T2DM who adhered to either a low-carbohydrate, vegetarian, vegan, low-GI, high-fiber, Mediterranean, or high-protein diet for at least six months. The researchers found that Mediterranean, low-carbohydrate, low-GI, and high-protein diets all led to A1C reductions—but the largest reduction was observed with patients on the Mediterranean diet. Low-carbohydrate and Mediterranean diets resulted in the most weight loss.5
LOW-CARBOHYDRATE
Low-carbohydrate diets have decreased in popularity due to concerns about their effects on renal function, possible lack of nutrients, and speculation that their macronutrient composition may have effects on weight beyond those explained by caloric deficit. A meta-analysis of 13 studies of adults with T2DM following a low-carbohydrate diet (≤ 45% of calories from carbohydrates) demonstrated beneficial effects on fasting glucose, A1C, and triglyceride levels. Nine of the studies evaluated glycemic control and found A1C reduction with lower carbohydrate diets; the greatest reductions in A1C and triglycerides were correlated with the lowest carbohydrate intakes. No significant effects were seen for total, HDL, or LDL cholesterol.6
In the literature review by Ajala et al, low-carbohydrate, low-GI, and Mediterranean diets all improved lipid profiles. HDL cholesterol increased the most with a low-carbohydrate diet.5
A two-week study of 10 adults with T2DM found that just one week on a low-carbohydrate diet decreased the average 24-h plasma glucose from 135 mg/dL to 113 mg/dL. Over the two-week study period, triglycerides decreased by 35%, cholesterol by 10%, and A1C by 0.5%. Patients were allowed to consume as much protein and fat as desired. Food sources included beef and ground turkey patties, chicken breasts, turkey, ham, steamed vegetables, butter, diet gelatin, and a limited amount of cheese. Mean calorie intake decreased from 3,111 to 2,164 calories/d. Carbohydrate intake decreased from 300 to 20 g/d. Weight loss was entirely explained by the mean energy deficit.6 Patients experienced no difference in hunger, satisfaction, or energy level with a low-carb diet compared to their usual diet.7
A literature review of six studies examined the effects of low-carb diets (between 20-95 g/d) on body weight and A1C in patients with T2DM. Three of the studies restricted carbohydrate intake to less than 50 g/d. All reported reductions in body weight and A1C. In two studies, the majority of the weight loss was explained by a decrease in body fat, not loss of water weight. No deleterious effects on cardiovascular disease risk, renal function, or nutritional intake were seen. The researchers concluded that low-carb diets are safe and effective over the short term for people with T2DM.8
PALEOLITHIC
The Paleolithic diet (also referred to as the caveman diet, Stone Age diet, and hunter-gatherer diet) involves eating foods believed to have been available to humans before agriculture—this period began about 2.5 million years ago and ended about 100,000 years ago. Food sources include wild animal meat (lean meat and fish) and uncultivated plant foods (vegetables, fruits, roots, eggs, and nuts). It excludes grains, legumes, dairy products, salt, refined sugar, and processed oils.
In a randomized crossover study of 13 participants with T2DM, the Paleolithic diet improved glucose control and several cardiovascular disease markers, compared to a standard diabetes diet. The Paleolithic diet resulted in significantly lower A1C, triglycerides, diastolic blood pressure, body weight, BMI, and waist circumference, as well as increased HDL. Despite receiving no instruction to restrict calories, patients on the Paleolithic diet consumed fewer calories and carbohydrates, and more protein and fat, than those on the standard diabetes diet. The caloric deficit accounted almost exactly for the observed difference in weight loss between the two groups.9
GLYCEMIC INDEX
The GI measures the blood glucose level increase in the two hours after eating a particular food, with 100 representing the effect of glucose consumption. Low-GI food sources include beans, peas, lentils, pasta, pumpernickel bread, bulgur, parboiled rice, barley, and oats, while high-GI foods include potatoes, wheat flour, white bread, most breakfast cereals, and rice.
A meta-analysis compared the effects of high- and low-GI diets on glycemic control in 356 patients with diabetes. Ten of 14 studies documented improvements in A1C and postprandial plasma glucose with lower GI diets. Low-GI diets reduced A1C by 0.43% after an average duration of 10 weeks. The average GI was 83 for high-GI diets and 65 for low-GI diets. The researchers concluded that selecting low-GI foods has a small but clinically relevant effect on medium-term glycemic control, similar to that offered by medications that target postprandial blood glucose excursions.10
Low-GI diets resulted in lower A1C and higher HDL but no significant change in weight, according to Ajala et al.5
HIGH-FIBER
A survey of 15 studies examined the relationship between fiber intake and glycemic control. Interventions ranged from an additional 4 to 40 g of fiber per day, with a mean increase of 18.3 g/d. Additional fiber lowered A1C by 0.26% in 3 to 12 weeks, compared to placebo. The overall mean fasting blood glucose reduction was 15.32 mg/dL. No study lasted more than 12 weeks, but it is inferred that a longer study could result in a greater A1C reduction. Current dietary guidelines for patients with diabetes exceed the amount of fiber included in most of these studies.11
VEGAN
Ajala et al observed that patients on a vegan diet had lower total cholesterol, LDL, and A1C levels, compared to those on a low-fat diet. At 18 months, the vegetarian diet demonstrated improvement in glucose control and lipids, but not weight loss.5
In one RCT, a low-fat vegan diet was shown to improve glycemic control and lipid levels more than a conventional diabetes diet did. A1C decreased by 1.23% over 22 weeks, compared to 0.38% in the conventional diet group. Body weight decreased by 6.5 kg and LDL cholesterol decreased by 21.2% with the vegan diet, compared with a weight loss of 3.1 kg and a 10.7% LDL reduction in the conventional diet group.12
Patients on the vegan diet derived energy primarily from carbohydrates (75%), protein (15%), and fat (10%) by eating fruits, vegetables, grains, and legumes. Portion size and caloric and carbohydrate intake were not restricted. The conventional diet involved a caloric intake mainly from a combination of carbohydrates and monounsaturated fats (60% to 70%), protein (15% to 20%), and saturated fat (< 7%). The diet was individualized based on caloric needs and participants’ lipid levels. All participants were given calorie intake deficits of 500 to 1000 kcal/d.13 Participants rated both diets as satisfactory, with no significant differences between groups. The researchers concluded that a low-fat vegan diet has acceptability similar to that of a more conventional diabetes diet.12
CONCLUSION
Diabetes management strategies may incorporate a variety of dietary plans. While study populations are small and study durations relatively short, the aforementioned diets show improvement in biochemical markers such as fasting glucose, A1C, and lipid levels. The Mediterranean diet is believed to be sustainable over the long term, given the duration of time that people in the region have survived on it. Low-carbohydrate diets, including the Atkins and Paleolithic diets, are very effective at lowering A1C and triglycerides. Vegetarian/vegan diets may be more acceptable to patients than previously thought.
The long-term impact of any eating pattern will likely relate to adherence; adherence is more likely when patients find a diet to be acceptable, palatable, and easy to prepare. Diet selection should incorporate patient preferences and lifestyle choices, and when possible, should involve an RDN with expertise in diabetes.
Prescribed diets can be trying for both patients and providers; patients often struggle to adhere to them, and providers must determine which plan is suitable for which patient. The optimal diet for patients with diabetes—and whether it is sustainable—remains controversial.
A plant-based diet high in polyunsaturated and monounsaturated fats, with limited saturated fat and avoidance of trans-fatty acids, is supported by the American Association of Clinical Endocrinologists. Caloric restriction is recommended when weight loss is appropriate.1 The American Diabetes Association (ADA) recommends a Mediterranean-style diet rich in monounsaturated fats with carbohydrates from whole grains, vegetables, fruits, legumes, and dairy products, and an emphasis on foods higher in fiber and lower in glycemic load.2
Additionally, the ADA, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics advise that all individuals with diabetes receive individualized Medical Nutrition Therapy (MNT), preferably with a registered dietitian nutritionist (RDN) knowledgeable and skilled in providing diabetes-specific nutrition education. MNT delivered by an RDN has been shown to reduce A1C levels by up to 2% in people with type 2 diabetes (T2DM).3
This flexibility in recommendations creates uncertainty about the correct dietary choice. Several diet plans are endorsed for the management of diabetes, including Mediterranean, low carbohydrate, Paleolithic, vegan, high fiber, and glycemic index (GI). Which should your patients adhere to? Several randomized controlled trials (RCTs), meta-analyses, and literature reviews have examined and compared the benefits of these eating habits for management of diabetes.
MEDITERRANEAN
The Mediterranean diet incorporates plant foods such as greens, tomatoes, onions, garlic, herbs, whole grains, legumes, nuts, and olive oil as the primary source of fat. A crossover trial of adults with T2DM demonstrated a statistically significant A1C reduction (from 7.1% to 6.8%) after 12 weeks on the Mediterranean diet.4
In a systematic review of 20 RCTs, Ajala et al analyzed data for nearly 3,500 patients with T2DM who adhered to either a low-carbohydrate, vegetarian, vegan, low-GI, high-fiber, Mediterranean, or high-protein diet for at least six months. The researchers found that Mediterranean, low-carbohydrate, low-GI, and high-protein diets all led to A1C reductions—but the largest reduction was observed with patients on the Mediterranean diet. Low-carbohydrate and Mediterranean diets resulted in the most weight loss.5
LOW-CARBOHYDRATE
Low-carbohydrate diets have decreased in popularity due to concerns about their effects on renal function, possible lack of nutrients, and speculation that their macronutrient composition may have effects on weight beyond those explained by caloric deficit. A meta-analysis of 13 studies of adults with T2DM following a low-carbohydrate diet (≤ 45% of calories from carbohydrates) demonstrated beneficial effects on fasting glucose, A1C, and triglyceride levels. Nine of the studies evaluated glycemic control and found A1C reduction with lower carbohydrate diets; the greatest reductions in A1C and triglycerides were correlated with the lowest carbohydrate intakes. No significant effects were seen for total, HDL, or LDL cholesterol.6
In the literature review by Ajala et al, low-carbohydrate, low-GI, and Mediterranean diets all improved lipid profiles. HDL cholesterol increased the most with a low-carbohydrate diet.5
A two-week study of 10 adults with T2DM found that just one week on a low-carbohydrate diet decreased the average 24-h plasma glucose from 135 mg/dL to 113 mg/dL. Over the two-week study period, triglycerides decreased by 35%, cholesterol by 10%, and A1C by 0.5%. Patients were allowed to consume as much protein and fat as desired. Food sources included beef and ground turkey patties, chicken breasts, turkey, ham, steamed vegetables, butter, diet gelatin, and a limited amount of cheese. Mean calorie intake decreased from 3,111 to 2,164 calories/d. Carbohydrate intake decreased from 300 to 20 g/d. Weight loss was entirely explained by the mean energy deficit.6 Patients experienced no difference in hunger, satisfaction, or energy level with a low-carb diet compared to their usual diet.7
A literature review of six studies examined the effects of low-carb diets (between 20-95 g/d) on body weight and A1C in patients with T2DM. Three of the studies restricted carbohydrate intake to less than 50 g/d. All reported reductions in body weight and A1C. In two studies, the majority of the weight loss was explained by a decrease in body fat, not loss of water weight. No deleterious effects on cardiovascular disease risk, renal function, or nutritional intake were seen. The researchers concluded that low-carb diets are safe and effective over the short term for people with T2DM.8
PALEOLITHIC
The Paleolithic diet (also referred to as the caveman diet, Stone Age diet, and hunter-gatherer diet) involves eating foods believed to have been available to humans before agriculture—this period began about 2.5 million years ago and ended about 100,000 years ago. Food sources include wild animal meat (lean meat and fish) and uncultivated plant foods (vegetables, fruits, roots, eggs, and nuts). It excludes grains, legumes, dairy products, salt, refined sugar, and processed oils.
In a randomized crossover study of 13 participants with T2DM, the Paleolithic diet improved glucose control and several cardiovascular disease markers, compared to a standard diabetes diet. The Paleolithic diet resulted in significantly lower A1C, triglycerides, diastolic blood pressure, body weight, BMI, and waist circumference, as well as increased HDL. Despite receiving no instruction to restrict calories, patients on the Paleolithic diet consumed fewer calories and carbohydrates, and more protein and fat, than those on the standard diabetes diet. The caloric deficit accounted almost exactly for the observed difference in weight loss between the two groups.9
GLYCEMIC INDEX
The GI measures the blood glucose level increase in the two hours after eating a particular food, with 100 representing the effect of glucose consumption. Low-GI food sources include beans, peas, lentils, pasta, pumpernickel bread, bulgur, parboiled rice, barley, and oats, while high-GI foods include potatoes, wheat flour, white bread, most breakfast cereals, and rice.
A meta-analysis compared the effects of high- and low-GI diets on glycemic control in 356 patients with diabetes. Ten of 14 studies documented improvements in A1C and postprandial plasma glucose with lower GI diets. Low-GI diets reduced A1C by 0.43% after an average duration of 10 weeks. The average GI was 83 for high-GI diets and 65 for low-GI diets. The researchers concluded that selecting low-GI foods has a small but clinically relevant effect on medium-term glycemic control, similar to that offered by medications that target postprandial blood glucose excursions.10
Low-GI diets resulted in lower A1C and higher HDL but no significant change in weight, according to Ajala et al.5
HIGH-FIBER
A survey of 15 studies examined the relationship between fiber intake and glycemic control. Interventions ranged from an additional 4 to 40 g of fiber per day, with a mean increase of 18.3 g/d. Additional fiber lowered A1C by 0.26% in 3 to 12 weeks, compared to placebo. The overall mean fasting blood glucose reduction was 15.32 mg/dL. No study lasted more than 12 weeks, but it is inferred that a longer study could result in a greater A1C reduction. Current dietary guidelines for patients with diabetes exceed the amount of fiber included in most of these studies.11
VEGAN
Ajala et al observed that patients on a vegan diet had lower total cholesterol, LDL, and A1C levels, compared to those on a low-fat diet. At 18 months, the vegetarian diet demonstrated improvement in glucose control and lipids, but not weight loss.5
In one RCT, a low-fat vegan diet was shown to improve glycemic control and lipid levels more than a conventional diabetes diet did. A1C decreased by 1.23% over 22 weeks, compared to 0.38% in the conventional diet group. Body weight decreased by 6.5 kg and LDL cholesterol decreased by 21.2% with the vegan diet, compared with a weight loss of 3.1 kg and a 10.7% LDL reduction in the conventional diet group.12
Patients on the vegan diet derived energy primarily from carbohydrates (75%), protein (15%), and fat (10%) by eating fruits, vegetables, grains, and legumes. Portion size and caloric and carbohydrate intake were not restricted. The conventional diet involved a caloric intake mainly from a combination of carbohydrates and monounsaturated fats (60% to 70%), protein (15% to 20%), and saturated fat (< 7%). The diet was individualized based on caloric needs and participants’ lipid levels. All participants were given calorie intake deficits of 500 to 1000 kcal/d.13 Participants rated both diets as satisfactory, with no significant differences between groups. The researchers concluded that a low-fat vegan diet has acceptability similar to that of a more conventional diabetes diet.12
CONCLUSION
Diabetes management strategies may incorporate a variety of dietary plans. While study populations are small and study durations relatively short, the aforementioned diets show improvement in biochemical markers such as fasting glucose, A1C, and lipid levels. The Mediterranean diet is believed to be sustainable over the long term, given the duration of time that people in the region have survived on it. Low-carbohydrate diets, including the Atkins and Paleolithic diets, are very effective at lowering A1C and triglycerides. Vegetarian/vegan diets may be more acceptable to patients than previously thought.
The long-term impact of any eating pattern will likely relate to adherence; adherence is more likely when patients find a diet to be acceptable, palatable, and easy to prepare. Diet selection should incorporate patient preferences and lifestyle choices, and when possible, should involve an RDN with expertise in diabetes.
1. American Association of Clinical Endocrinologists; American College of Endocrinology. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm. Endocr Pract. 2016;22(1):84-113.
2. American Diabetes Association. Standards of medical care in diabetes—2016. Clin Diabetes. 2016;34(1):3-21.
3. Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition. J Acad Nutr Diet. 2015:115(8):1323-1334.
4. Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21(9):740-747.
5. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
6. Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403-411.
7. Kirk JK, Graves DE, Craven TE, et al. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91-100.
8. Dyson PA. A review of low and reduced carbohydrate diets and weight loss in type 2 diabetes. J Hum Nutr Diet. 2008;21(6):530-538.
9. Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
10. Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261-2267.
11. Post RE, Mainous AG III, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25(1):16-23.
12. Barnard ND, Gloede L, Cohen J, et al. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009;109(2):263-272.
13. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-week clinical trial. Am J Clin Nutr. 2009;89(5):1588S-1596S.
1. American Association of Clinical Endocrinologists; American College of Endocrinology. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm. Endocr Pract. 2016;22(1):84-113.
2. American Diabetes Association. Standards of medical care in diabetes—2016. Clin Diabetes. 2016;34(1):3-21.
3. Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition. J Acad Nutr Diet. 2015:115(8):1323-1334.
4. Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21(9):740-747.
5. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
6. Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403-411.
7. Kirk JK, Graves DE, Craven TE, et al. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91-100.
8. Dyson PA. A review of low and reduced carbohydrate diets and weight loss in type 2 diabetes. J Hum Nutr Diet. 2008;21(6):530-538.
9. Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
10. Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261-2267.
11. Post RE, Mainous AG III, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25(1):16-23.
12. Barnard ND, Gloede L, Cohen J, et al. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009;109(2):263-272.
13. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-week clinical trial. Am J Clin Nutr. 2009;89(5):1588S-1596S.
Hypertriglyceridemia: Identifying Secondary Causes
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
Screening for cardiovascular (CV) risk often includes a routine serum fasting lipid profile. However, with the focus on LDL cholesterol, triglyceride measurement is frequently overlooked. Yet this element of the lipid profile is particularly important, given its strong association with not only atherosclerotic coronary heart disease but also pancreatitis.
Hypertriglyceridemia is defined as a serum triglyceride level that exceeds 150 mg/dL. In the US, an estimated 25% of patients have hypertriglyceridemia.1 Of these, 33.1% have “borderline high” triglyceride levels (150 to 199 mg/dL), 17.8% have “high” levels (200 to 499 mg/dL), and 1.7% have “very high” levels (> 500 mg/dL).1,2
Most of the time, hypertriglyceridemia is caused (or at least exacerbated) by underlying etiology. The best way to identify and manage these secondary causes is through a systematic approach.
CONSIDER THE EVIDENCE
For mild to moderately elevated (borderline high) triglyceride levels, our reflex reaction may be to recommend a triglyceride-lowering medication, such as fenofibrate. But this may not be the best answer. Although there is increasing evidence of an independent association between elevated triglyceride levels and CV risk, it remains unclear whether targeting them specifically can reduce that risk.3
In well-designed, peer-reviewed clinical trials, statins have been shown to reduce CV risk in patients with known cardiovascular disease (CVD) and those at high risk for CVD, as well as in primary prevention. However, these trials also suggest that significant residual CV risk remains after statin therapy.4
Several trials have attempted to prove residual risk reduction following combination therapy including statins—with inconclusive results:
ACCORD: Fenofibrate showed no overall macrovascular benefit when added to a statin in patients with type 2 diabetes and a triglyceride level < 204 mg/dL.3,5
AIM-HIGH: There was a 25% reduction in triglyceride levels when niacin was added to a regimen of a statin +/- ezetimibe, with an aggressive LDL treatment target (40 to 80 mg/dL). But the study was stopped early due to the lack of expected reduction in CVD events.4,6
JELIS: A reduction in major CV events was seen with 1,800 mg/d of eicosapentaenoic acid (EPA) supplementation plus a low-dose statin, compared to statin monotherapy. However, there was minimal change in triglyceride levels, leading the researchers to hypothesize that multiple mechanisms—such as decreasing oxidative stress, platelet aggregation, plaque formation and stabilization—contributed to the outcome.4,7
Informed by the JELIS results, the much-anticipated REDUCE-IT trial is currently in progress to address the lingering question of whether combination therapy can reduce residual CV risk. In this trial, EPA omega-3 fatty acid is being added to the regimen of statin-treated patients with persistently elevated triglycerides. Results are expected in 2017 to 2018.8
Remember that a triglyceride level of 150 mg/dL is a parameter—it does not represent a therapeutic target. There is insufficient evidence that treating to this level improves CV risk beyond LDL target recommendations.7
The National Lipid Association Expert Panel’s consensus view is that non-HDL is a better primary target than triglycerides alone or LDL. Using non-HDL as a target for intervention also simplifies the management of patients with high triglycerides (200 to 499 mg/dL). The non-HDL goal is considered to be 30 mg/dL greater than the LDL target. For patients with diabetes and those with CVD, the individualized non-HDL targets are 130 mg/dL and 100 mg/dL, respectively.9
REVIEW THE MEDICATION LIST
Several commonly used medications, including ß-blockers and thiazide diuretics, can increase triglyceride levels.10 Other medications with exacerbating effects on triglycerides include corticosteroids, retrovirals, immunosuppressants, retinoids, and some antipsychotics.10 Bile acid sequestrants (eg, colesevelam) should be avoided in patients with elevated triglycerides (> 200 mg/dL).7
In women, oral estrogen (ie, menopausal hormone replacement and oral birth control) can greatly exacerbate triglyceride levels, making transdermal delivery a better option. Tamoxifen, the hormonal medication used for breast cancer prophylaxis, can also increase triglyceride levels.11
LOOK FOR UNDERLYING CONDITIONS
Among those to consider: Hypothyroidism is common and easily ruled out by a simple blood test. Nephrotic syndrome should be ruled out, particularly in patients with concomitant renal dysfunction and peripheral edema, by checking a random urine protein-to-creatinine ratio or 24-hour urine for protein. Other factors that should be explored because of their potential effect on lipid metabolism include obesity and excessive intake of sugary beverages (ie, soda, fruit juice) and alcohol.11
High triglyceride levels occurring with low HDL are characteristic of insulin resistance and concerning for metabolic syndrome and/or polycystic ovarian syndrome.3,12 Often, patients will have underlying prediabetes (fasting glucose ≥ 100 mg/dL or random glucose ≥ 140 mg/dL with an A1C > 5.7%13) or covert type 2 diabetes. Another underdiagnosed but very common condition, obstructive sleep apnea, can greatly affect insulin sensitivity and has been associated with lipid abnormalities and metabolic syndrome.14
EXAMINE YOUR PATIENT
The physical exam is an essential component of assessment for patients with high triglycerides. As discussed, elevated triglycerides and low HDL are hallmarks for insulin resistance. As triglyceride levels are affected by obesity and body fat distribution, measuring BMI and assessing for visceral adiposity are an important part of the physical exam.4
The physical exam may also yield dermatologic clues, such as skin tags or acanthosis nigricans, a dark, velvety lesion usually found on the posterior and lateral neck creases, axillae, groin, and elbows.13 In rare cases—usually those with genetic involvement from a familial lipid metabolism disorder—patients may exhibit xanthomas. These cutaneous, lipid-rich lesions can appear as flat, yellowish plaques on various parts of the body, such as the eyelids (xanthelasma) or tendons of the hands, feet, and heels. Widespread, eruptive xanthomas, which manifest as pruritic pink papules with creamy centers, are associated with severe emergent triglyceride elevation and pancreatitis.10
CONSIDER NONPHARMACOLOGIC MANAGEMENT
In mild to moderate hypertriglyceridemia, intensive lifestyle changes are considered firstline therapy. Weight loss is recommended in obese patients; a 5% to 10% reduction in body weight can lower triglycerides by 20%.15
A quick 24-hour diet recall, including beverages, is helpful for identifying key issues. The goal should be to reduce carbohydrates—in particular, simple, high glycemic index, processed foods—as well as total and saturated fats. A substantial problem in our population is the consumption of high-fructose beverages and fruit juices. Referral to a dietitian can be very helpful, not only for initial meal planning but also for continuing counseling on successful long-term weight loss and maintenance.
Exercise is also very helpful for improving lipid parameters. A daily minimum of 30 to 60 minutes of intermittent aerobic exercise or mild resistance exercise has been shown to reduce triglyceride levels.10
PRESCRIBE APPROPRIATELY
The most important indication for treatment of hypertriglyceridemia is reduction of CVD risk. However, in patients with very high triglyceride levels (> 500 mg/dL), the goal is to decrease risk for life-threatening pancreatitis.15 Lipid-lowering medications and dietary restrictions should be promptly employed.
There are medications, as discussed earlier, that specifically lower triglycerides. Fibrates offer the most robust decrease, with a 20% to 50% reduction in triglyceride levels. Fenofibrate is considered a safer option when used in combination with a statin, due to the risk for significant muscle toxicity with gemfibrozil. There is some evidence that adding a fibrate may actually increase risk for pancreatitis; since this risk is otherwise low in patients with mild to moderate triglyceride elevation, the addition of a fibrate to their regimen should be avoided.3
Statins are the drug of choice when CV risk reduction is the goal (for patients with hypertriglyceridemia < 500 mg/dL). In addition to lowering LDL, statins can reduce triglycerides by 7% to 30%, depending on the dose.15
Other triglyceride-lowering medications include omega-3 fatty acids and niacin preparations. Prescription-strength omega-3 fatty acids have been found to lower serum triglyceride levels by 50% or more; the newest preparation, icosapent ethyl, demonstrated up to 45% reduction without significant effect on LDL levels.3 (Other preparations have been shown to substantially increase LDL in many cases.) Niacin (1,500 to 2,000 mg/d) can decrease triglycerides by 15% to 25%. However, it is no longer recommended for CV risk reduction; recent data indicate it may increase stroke risk when used in combination with statins.3,10 In April 2016, the FDA revoked its approval of the co-administration of niacin and fenofibrate with statin therapy, due to a lack of CV benefit.16
Other secondline options to consider for patients with insulin resistance or diabetes are metformin and pioglitazone. These medications have been shown to improve insulin sensitivity and decrease LDL and triglycerides in patients with prediabetes. Pioglitazone has proven beneficial in the treatment of steatohepatitis.17 Insulin is an excellent rapid triglyceride-lowering agent for patients with diabetes. It is important to reinforce that reduction of glucose is a key component in reduction of triglyceride levels.3
CONCLUSION
Hypertriglyceridemia is a complex condition that requires individualized and comprehensive management strategies. Clinicians must be able to identify and address secondary causes. Treatment options should be tailored to decrease CV and pancreatitis risk, and medication recommendations should be evidenced based and carefully selected to mitigate potential adverse effects. Patients should receive education and lifestyle management support to help motivate and equip them to employ strategies to improve their health.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
1. CDC. Trends in elevated triglyceride in adults: United States, 2001-2012. www.cdc.gov/nchs/data/databriefs/db198.pdf. Accessed December 27, 2016.
2. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-426.
3. Rosenson RS. Approach to the patient with hypertriglyceridemia. www.uptodate.com/contents/approach-to-the-patient-with-hypertriglyceridemia. Accessed December 28, 2016.
4. Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6): 544-552.
5. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-1574.
6. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol. Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH). Am Heart J. 2011;161(3):471-477.
7. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
8. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357-366.
9. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol. 2015;9(2):129-169.
10. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
12. Amini L, Sadeghi MR, Oskuie F, Maleki H. Lipid profile in women with polycystic ovary syndrome. Crescent J Med Biol Sci. 2014;1(4):147-150.
13. Mantzoros C. Insulin resistance: definition and clinical spectrum. www.uptodate.com/contents/insulin-resistance-definition-and-clinical-spectrum. Accessed December 28, 2016.
14. Lin M, Lin H, Lee P, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19(3):809-817.
15. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
16. FDA. Withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://s3.amazonaws.com/public-inspection.federalregister.gov/2016-08887.pdf. Accessed December 28, 2016.
17. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012: 716404.
Thyroid Cancer: Incidence on the Rise
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2
RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.
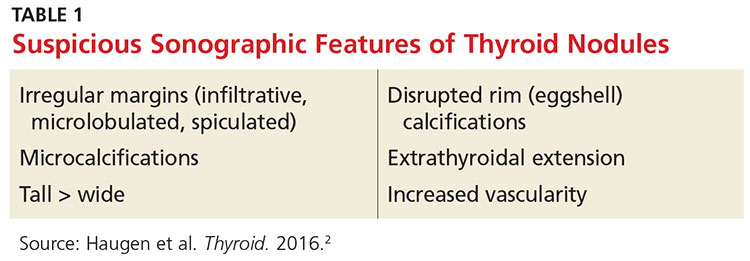
Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3
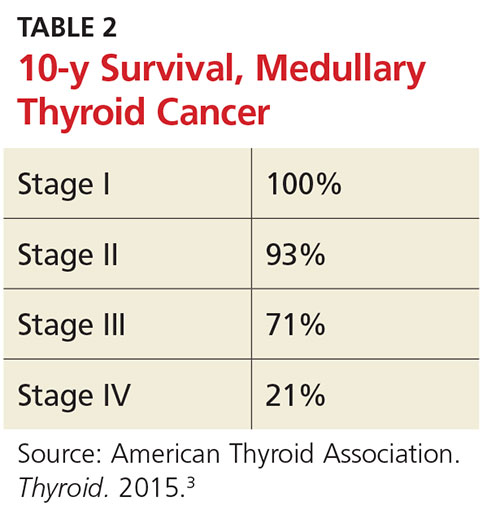
Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2

RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.

Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3

Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
Detection of thyroid cancer is widespread, increasing by about 4.5% annually. In the past year, approximately 64,300 new cases were identified. An estimated one in 100 people will be diagnosed with thyroid cancer during their lifetime, making it the eighth most common cancer in the United States.1
Incidental thyroid nodules found on carotid ultrasounds and other neck imaging may account for much of the increase; evaluation of these “incidentalomas” may account for the doubling incidence of thyroid cancer cases. (For more on thyroid nodules, see “To Cut or Not to Cut?” Clinician Reviews. 2016;26[8]:34-36.) If this pace continues, thyroid cancer may become the third most common cancer among women in the US by 2019.2

RISK FACTORS
Generally, women are diagnosed with thyroid cancer more frequently than men.3 Other risk factors include
- Age (40 to 60 in women; 60 to 80 in men; median age at diagnosis, 51)
- Inherited conditions, such as multiple endocrine neoplasia (MEN) or familial medullary and nonmedullary thyroid carcinoma
- Other cancers, including breast cancer and familial adenomatous polyposis
- Iodine deficiency
- Radiation exposure, particularly head and neck radiation in childhood. This can be through treatment of acne, tinea capitis, enlarged tonsils, or adenoids (usually prior to 1960); treatment of lymphoma, Wilms tumor, or neuroblastoma; or proximity to Chernobyl in 1986.1,2
BIOPSY RECOMMENDATIONS
While thyroid nodules are fairly common, only 7% to 15% of nodules are found to be malignant.2 However, all patients presenting with a palpable thyroid nodule should undergo thyroid ultrasound for further evaluation.
According to American Thyroid Association guidelines, all nodules 2 cm or larger should be evaluated with fine needle aspiration (FNA) due to a concern for metastatic thyroid cancer in larger nodules.2 Some clinicians prefer to aspirate nodules 1 cm or larger. Nodules that are smaller than 2 cm with sonographic features suspicious for thyroid cancer (see Table 1) should be biopsied.

Nodules that are spongiform in appearance or are completely cystic with no solid components may be monitored without FNA.2
The FNA is typically performed by an endocrinologist under ultrasound guidance. No anesthetic is required, but a topical ethyl chloride spray can assist with patient comfort. Three to four passes are made into the nodule with a 27-gauge needle; most patients describe pressure or a pinching sensation, rather than pain, during the procedure. After the procedure, ice applied to the FNA area may help with patient comfort.
TYPES OF THYROID CANCER
Four possible types of thyroid cancer are identified on pathology after FNA: papillary, follicular, medullary, and anaplastic. Differentiated thyroid cancers, which encompass papillary and follicular cancers, are the most commonly diagnosed. Approximately 90% of thyroid cancers fall into this category.2
In most cases of differentiated thyroid cancer, patients can be treated with thyroidectomy alone if the cancer remains confined to the thyroid.2 Just over two-thirds of differentiated thyroid cancer cases are localized in the thyroid. The five-year survival rate for these patients is nearly 100%.1
About 27% of differentiated thyroid cancer is also found in neck lymph nodes; these patients may be treated with thyroidectomy and radioactive iodine.2 The five-year survival rate in these cases is nearly 98%.1 Chemotherapy is generally not needed for differentiated thyroid cancers.
Medullary thyroid cancer (MTC) is diagnosed in up to 4% of thyroid cancer patients. Characterized by high levels of calcitonin, MTC can be genetically mediated or sporadic. MTC is associated with a variety of RET oncogene mutations; genetic testing of family members is recommended, as well as prophylactic thyroidectomy when high-risk RET oncogenes are detected.3
The 10-year survival prognosis for MTC patients varies according to stage at diagnosis (see Table 2). Up to 70% of patients with a palpable MTC nodule present with metastasis consistent with stage III or IV disease.3

Medullary thyroid cancer is treated with total thyroidectomy and cervical lymph node dissection. Radioactive iodine has not been proven effective for MTC patients, unless there is also papillary or follicular thyroid cancer present.3
Anaplastic thyroid cancer has the highest mortality rate of all types of thyroid cancer. Fortunately, it is relatively rare, occurring in only 1.7% of thyroid cancer patients. The one-year survival rate is 20%, with a median postdiagnosis survival prognosis of approximately five months. Anaplastic thyroid cancer is treated with total thyroidectomy and radical neck dissection when it is considered resectable. Metastatic lesions in the brain or spine are often indicators of unresectable disease. In some cases, external beam radiation therapy is used as palliative treatment.4
PEDIATRIC INCIDENCE
Thyroid cancer in children is rare, making up only 1.8% of all pediatric cancers diagnosed in the US annually. Patients are most often between ages 15 and 19, but it is possible for thyroid cancer to manifest in younger patients. Thyroid nodules are more likely to be malignant in children, with a greater incidence of metastatic disease at diagnosis. Prognosis is generally better in children than in adults, however, even with extensive disease.5
Children with prior history of other types of cancer treated with radiation, such as Hodgkin lymphoma or leukemia, are at increased risk for thyroid cancer and should be monitored.5 Children with a family history of MEN or MTC and evidence of RET oncogenes should be monitored starting as early as age 3 with thyroid exam, ultrasound, and measurement of calcitonin levels.3 Prophylactic thyroidectomy is an option in the first few months of life, depending on the presence of specific RET oncogenes.3
CHEMOTHERAPY
Chemotherapy may be helpful for metastatic medullary or anaplastic thyroid cancer, particularly in patients with unresectable disease. Though not usually curative, it may increase progression-free survival time. New chemotherapy agents approved for use in metastatic MTC include cabozantinib and vandetanib.3 Carboplatin, docetaxel, doxorubicin, and paclitaxel are used in treatment of anaplastic thyroid cancer.4
LONG-TERM PATIENT MANAGEMENT
After thyroidectomy and radioactive iodine treatment, follicular cell cancers (eg, papillary, follicular, anaplastic) are managed by following patients’ thyroid-stimulating hormone (TSH), thyroglobulin, and antithyroglobulin antibody levels. A cervical ultrasound is performed to detect possible disease in lymph nodes.2
Levothyroxine is dosed to suppress TSH below the recommended levels for hypothyroid patients in order to prevent disease recurrence. Low-risk patients may have TSH suppression below 1 to 2 mU/L, while high-risk patients may be managed with TSH levels below 0.1 mU/L.2
Lab levels should be checked annually and a cervical ultrasound performed at six to 12 months, then periodically thereafter depending on patient risk status.2 Patients with long-term TSH suppression must be monitored for atrial fibrillation and osteoporosis.
Patients who have been treated for medullary thyroid cancer require a different long-term management strategy. Patients should have ultrasound and measurement of TSH as well as calcitonin and carcinoembryonic antigen levels every six to 12 months.3 TSH suppression is not required; TSH may be maintained at typical euthyroid levels.
A FINAL THOUGHT
For clinicians, it’s easy to attempt to minimize thyroid cancer, since the disease is curable for most patients without the burden of chemotherapy and external radiation. However, for a patient, this is still a cancer diagnosis, with the accompanying surgery and required lifelong monitoring. It can be very disruptive to the lives of both patients and their families.
Support groups are available to help patients navigate their new reality. The Thyroid Cancer Survivors’ Association (www.thyca.org) has resources that may be beneficial to patients (and caregivers) as they learn how to live as a thyroid cancer survivor.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 16, 2016.
2. Haugen BR, Alexander EK, Bible KC, et al; American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133.
3. Wells SA Jr, Asa SL, Dralle H, et al; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
4. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for the management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104-1139.
5. Francis GL, Waguespack SG, Bauer AJ, et al; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716-759.
To Cut or Not to Cut? Evaluating Surgical Criteria for Benign & Nondiagnostic Thyroid Nodules
A new-onset thyroid nodule, found on exam or incidentally on imaging, is a common presentation at primary care and specialist clinics. Palpable nodules are present in 4% to 7% of the population.1 However, more sensitive evaluation with thyroid ultrasound (US) suggests an incidence as high as 70%.2
According to the American Cancer Society, in 2015, there were approximately 62,450 new cases of thyroid cancer in the United States (with 2.5 times as many occurring in women as in men).3 In fact, thyroid cancer is the most rapidly increasing cancer in the United States—attributable in part to the increased use of thyroid US and incidental detection.3
The high prevalence of thyroid nodules makes appropriate evaluation and treatment crucial. This article, through a case study, explores the evaluation of a thyroid nodule and the recommendation for and against thyroidectomy.
Felicia, 49, presents to the endocrine clinic as a new patient with questions about multinodular goiter (MNG). She has been advised by ENT to have a left-sided dominant nodule surgically removed while under anesthesia during her upcoming chronic sinusitis surgery. Felicia would like to avoid thyroid surgery, if possible. Her most recent thyroid US, performed three months ago, showed a right lobe with multiple colloid nodules with inspissated colloid, the largest of which is 1.5 cm, and a 4-cm complex, solid, cystic nodule with inspissated colloid in the cystic spaces replacing the entire left thyroid lobe.
HistoryThe first step is establishing a history of the nodule(s) in question. Key questions are listed in Table 1. The onset and progression of a thyroid nodule must be determined; ideally, the provider should review any previous studies related to the thyroid gland. This will help determine if the nodule is new, if it has been evaluated in the past, and if it has changed significantly.
A thorough history can identify risk factors for malignancy, which include a personal history of cancer or radiation exposure, as well as a family history of thyroid cancer or malignant endocrine syndromes.
Felicia denies any family or personal medical history concerning for malignancy. She notes that she has two sisters with MNG. She denies any neck pain, compressive/obstructive symptoms, and hypo- or hyperthyroid symptoms.
She reports that she was found to have a goiter on exam and was subsequently diagnosed with MNG in 2008. Thyroid US showed a 2.3-cm complex, largely solid mass in the right mid-pole and a 3.3-cm largely cystic lesion in the left mid-pole. She was referred for right-sided fine-needle aspiration (FNA); results were consistent with benign colloid nodule. The left-sided nodule was not biopsied at that time, due to a largely cystic component.
Felicia underwent a follow-up US in 2011; it showed a 1.6-cm right mid-pole nodule with multiple nonspecific echogenic areas; a 1-cm benign-appearing nodule; and a 3.7-cm highly vascular heterogeneous mass with some colloid components with indeterminate component in the left lower and mid-pole. She reports that she did not follow up in 2011. Her next evaluation was the current thyroid US. She has never had FNA of the left-sided dominant nodule.
Continue for symptomatic vs asymptomatic thyroid nodules >>
Symptomatic vs Asymptomatic Thyroid NodulesEvaluation of a symptomatic thyroid nodule can help to determine the need for surgery, as well as assess the level of interference with a patient’s activities of daily living and the potential for functional abnormalities. However, both local neck and constitutional symptoms may be nonspecific and unrelated to the thyroid gland’s structure or function. Therefore, the provider should exercise caution in making recommendations based on reported symptoms alone.
Symptoms indicative of the need for surgical intervention include neck pain, increased neck pressure, foreign body sensation, dysphonia, dyspnea, and dysphagia. However, it is essential to determine if these symptoms are likely due to a thyroid nodule or if they can be attributed to a secondary cause (eg, postnasal drip, vocal cord dysfunction, gastroesophageal reflux disease, or esophageal stricture).
If the findings are inconsistent with the clinical picture, secondary evaluation is prudent to avoid an unnecessary procedure.
Physical ExamPalpation of a thyroid nodule is an unreliable indicator of risk for malignancy. Palpation alone does not allow for detection of all nodules, particularly smaller ones, and specific characteristics are not discernible. Imaging studies are required to accurately evaluate a thyroid nodule and determine the most appropriate course of action.
Palpation can be used to evaluate for a larger and/or fixed nodule, thyroid gland/nodule tenderness, and cervical lymphadenopathy. Physical exam can also assess for signs of hypo- or hyperthyroidism, including abnormal pulse rate or blood pressure, tremor, hypo- or hyperreflexia, and integumentary abnormalities (eg, hair loss, abnormal skin temperature, and nail changes).
Continue for serologic evaluation >>
Serologic Evaluation
If a thyroid nodule is suspected on exam or found on imaging, assessment of thyroid function, via thyroid-stimulating hormone (TSH) measurement, is the recommended first step. If TSH is elevated, further evaluation for hypothyroidism is recommended, with testing for free thyroxine (T4) and antithyroid peroxidase (TPO) antibodies.4 If TSH is suppressed, further evaluation with free T4 and assessment for underlying causes of hyperthyroidism are indicated, including work-up for toxic nodular goiter.
Routine monitoring of serum calcitonin level is not recommended. However, if there is suspicion for medullary thyroid cancer—based on either US findings or family history—serologic screening for abnormal calcitonin level may be indicated.4,5
Felicia’s lab results include a TSH of 1.30 µIU/mL (reference range, 0.30-3.00 µIU/mL). Based on this finding, what (if any) further serologic testing is recommended? None: With normal TSH and no concerning family or personal history, additional laboratory evaluation is not indicated.
Imaging a Thyroid Nodule
Thyroid US is the most sensitive imaging study for evaluating thyroid nodule characteristics. Thyroid uptake and scan is not indicated unless TSH is suppressed and evaluation for toxic nodular goiter is needed. Additional imaging studies, such as CT or MRI, are not recommended for thyroid nodule evaluation.
Based on the thyroid US, what characteristics of Felicia’s nodule are suggestive of a benign nodule? Of a malignant nodule? (See Table 2.)
FNA of the left-sided dominant nodule is indicated, based on the US findings of a partially solid component and size > 1 cm. Unfortunately, FNA is nondiagnostic, because it yielded cystic fluid only with scant follicular cells for evaluation.
Continue to now what? >>
Now What?
While FNA most definitively distinguishes between benign and malignant nodules, the test is limited. An indeterminate, or nondiagnostic, finding occurs in 10% to 15% of cases and is more likely in nodules with a large cystic component.1
Even a benign finding on FNA of a larger nodule should be viewed with caution, since aspiration is unlikely to pinpoint small insidious malignant cells nestled among a larger collection of benign tissue.3 In many situations, a patient receives FNA results and asks, “What should we do now?”
Nondiagnostic nodules
When FNA is indeterminate, the next step depends on the characteristics of the nodule. For a solid nodule, repeat FNA is recommended.4,5 For nodules with repeatedly nondiagnostic FNAs, the American Academy of Clinical Endocrinologists and the American Thyroid Association recommend that a solid nodule be considered for surgical removal unless the nodule has “clearly favorable clinical and US features.”4,5
Surgical excision should be considered for cysts that recur, those that are larger (> 4 cm), and those that are repeatedly nondiagnostic on FNA. Personal and family history should be taken into account when nodules that are nondiagnostic on FNA demonstrate suspicious characteristics on US.6
An analysis by Renshaw determined that risk for malignancy in a nodule with a single nondiagnostic FNA was about 20%. For nodules that underwent repeat FNA, the risk was 0% for those that were again nondiagnostic. This significant difference led the author to conclude that “patients with two sequential nondiagnostic thyroid aspirates have a very low risk of malignancy.”7
Consider the time commitment, financial burden, and emotional cost for the patient of repeated evaluation with thyroid US and possibly FNA. In recurrent cases, the risks associated with surgery begin to be outweighed by the cost and burden of prolonged observation.
Benign nodules
With a biopsy-proven benign nodule, observation is recommended unless certain criteria are present: local neck compressive/obstructive symptoms that can be confidently attributed to a thyroid nodule; patient preference (eg, due to anxiety or aesthetics); or higher index of suspicion (eg, history of previous radiation exposure, progressive nodule growth, or suspicious characteristics on US).4,5
If surgical removal of a benign thyroid nodule is recommended, it is imperative to discuss the risks with patients. In addition to traditional surgery risks, thyroidectomy is associated with transient or permanent postoperative hypoparathyroidism, as well as vocal hoarseness or changes in vocal quality due to the proximity of the recurrent laryngeal nerve. Additionally, patients should be advised of the potential for surgical hypothyroidism with hemithyroidectomy and certain irreversible hypothyroidism with total thyroidectomy.
After a discussion of the risks and cost of observation versus surgery, an informed decision between provider and patient can ultimately be reached.
Would thyroidectomy be recommended for Felicia? After a thorough discussion, it is decided that surgery is not indicated at this time. Relevant factors include the benign thyroid US characteristics, lack of clinical neck compressive symptoms, and patient preference.
According to the American Thyroid Association guidelines, Felicia’s risk for malignancy for the nodule in question is < 3%, since it is a partially cystic nodule without any suspicious sonographic features. By foregoing surgery, Felicia will need repeated imaging studies and possibly repeat serologic studies and FNA in the future.
References
1. Stang MT, Carty SE. Recent developments in predicting thyroid malignancy. Curr Opin Oncol. 2008;21(1):11-17.
2. Hambleton C, Kandil E. Appropriate and accurate diagnosis of thyroid nodules: a review of thyroid fine-needle aspiration. Int J Clin Exp Med. 2013;6(6):413-422.
3. American Cancer Society. Thyroid cancer (2014). www.cancer.org/acs/groups/cid/documents/webcontent/003144-pdf.pdf. Accessed June 29, 2016.
4. Gharib H, Papini E, Garber J, et al; AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 Update. Endocrine Pract. 2016;22(suppl 1):1-60.
5. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26(1):1-133.
6. Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008; 13(2):105-112.
7. Renshaw A. Significance of repeatedly nondiagnostic thyroid fine-needle aspirations. Am J Clin Pathol. 2011;135(5):750-752.
A new-onset thyroid nodule, found on exam or incidentally on imaging, is a common presentation at primary care and specialist clinics. Palpable nodules are present in 4% to 7% of the population.1 However, more sensitive evaluation with thyroid ultrasound (US) suggests an incidence as high as 70%.2
According to the American Cancer Society, in 2015, there were approximately 62,450 new cases of thyroid cancer in the United States (with 2.5 times as many occurring in women as in men).3 In fact, thyroid cancer is the most rapidly increasing cancer in the United States—attributable in part to the increased use of thyroid US and incidental detection.3
The high prevalence of thyroid nodules makes appropriate evaluation and treatment crucial. This article, through a case study, explores the evaluation of a thyroid nodule and the recommendation for and against thyroidectomy.
Felicia, 49, presents to the endocrine clinic as a new patient with questions about multinodular goiter (MNG). She has been advised by ENT to have a left-sided dominant nodule surgically removed while under anesthesia during her upcoming chronic sinusitis surgery. Felicia would like to avoid thyroid surgery, if possible. Her most recent thyroid US, performed three months ago, showed a right lobe with multiple colloid nodules with inspissated colloid, the largest of which is 1.5 cm, and a 4-cm complex, solid, cystic nodule with inspissated colloid in the cystic spaces replacing the entire left thyroid lobe.
HistoryThe first step is establishing a history of the nodule(s) in question. Key questions are listed in Table 1. The onset and progression of a thyroid nodule must be determined; ideally, the provider should review any previous studies related to the thyroid gland. This will help determine if the nodule is new, if it has been evaluated in the past, and if it has changed significantly.
A thorough history can identify risk factors for malignancy, which include a personal history of cancer or radiation exposure, as well as a family history of thyroid cancer or malignant endocrine syndromes.
Felicia denies any family or personal medical history concerning for malignancy. She notes that she has two sisters with MNG. She denies any neck pain, compressive/obstructive symptoms, and hypo- or hyperthyroid symptoms.
She reports that she was found to have a goiter on exam and was subsequently diagnosed with MNG in 2008. Thyroid US showed a 2.3-cm complex, largely solid mass in the right mid-pole and a 3.3-cm largely cystic lesion in the left mid-pole. She was referred for right-sided fine-needle aspiration (FNA); results were consistent with benign colloid nodule. The left-sided nodule was not biopsied at that time, due to a largely cystic component.
Felicia underwent a follow-up US in 2011; it showed a 1.6-cm right mid-pole nodule with multiple nonspecific echogenic areas; a 1-cm benign-appearing nodule; and a 3.7-cm highly vascular heterogeneous mass with some colloid components with indeterminate component in the left lower and mid-pole. She reports that she did not follow up in 2011. Her next evaluation was the current thyroid US. She has never had FNA of the left-sided dominant nodule.
Continue for symptomatic vs asymptomatic thyroid nodules >>
Symptomatic vs Asymptomatic Thyroid NodulesEvaluation of a symptomatic thyroid nodule can help to determine the need for surgery, as well as assess the level of interference with a patient’s activities of daily living and the potential for functional abnormalities. However, both local neck and constitutional symptoms may be nonspecific and unrelated to the thyroid gland’s structure or function. Therefore, the provider should exercise caution in making recommendations based on reported symptoms alone.
Symptoms indicative of the need for surgical intervention include neck pain, increased neck pressure, foreign body sensation, dysphonia, dyspnea, and dysphagia. However, it is essential to determine if these symptoms are likely due to a thyroid nodule or if they can be attributed to a secondary cause (eg, postnasal drip, vocal cord dysfunction, gastroesophageal reflux disease, or esophageal stricture).
If the findings are inconsistent with the clinical picture, secondary evaluation is prudent to avoid an unnecessary procedure.
Physical ExamPalpation of a thyroid nodule is an unreliable indicator of risk for malignancy. Palpation alone does not allow for detection of all nodules, particularly smaller ones, and specific characteristics are not discernible. Imaging studies are required to accurately evaluate a thyroid nodule and determine the most appropriate course of action.
Palpation can be used to evaluate for a larger and/or fixed nodule, thyroid gland/nodule tenderness, and cervical lymphadenopathy. Physical exam can also assess for signs of hypo- or hyperthyroidism, including abnormal pulse rate or blood pressure, tremor, hypo- or hyperreflexia, and integumentary abnormalities (eg, hair loss, abnormal skin temperature, and nail changes).
Continue for serologic evaluation >>
Serologic Evaluation
If a thyroid nodule is suspected on exam or found on imaging, assessment of thyroid function, via thyroid-stimulating hormone (TSH) measurement, is the recommended first step. If TSH is elevated, further evaluation for hypothyroidism is recommended, with testing for free thyroxine (T4) and antithyroid peroxidase (TPO) antibodies.4 If TSH is suppressed, further evaluation with free T4 and assessment for underlying causes of hyperthyroidism are indicated, including work-up for toxic nodular goiter.
Routine monitoring of serum calcitonin level is not recommended. However, if there is suspicion for medullary thyroid cancer—based on either US findings or family history—serologic screening for abnormal calcitonin level may be indicated.4,5
Felicia’s lab results include a TSH of 1.30 µIU/mL (reference range, 0.30-3.00 µIU/mL). Based on this finding, what (if any) further serologic testing is recommended? None: With normal TSH and no concerning family or personal history, additional laboratory evaluation is not indicated.
Imaging a Thyroid Nodule
Thyroid US is the most sensitive imaging study for evaluating thyroid nodule characteristics. Thyroid uptake and scan is not indicated unless TSH is suppressed and evaluation for toxic nodular goiter is needed. Additional imaging studies, such as CT or MRI, are not recommended for thyroid nodule evaluation.
Based on the thyroid US, what characteristics of Felicia’s nodule are suggestive of a benign nodule? Of a malignant nodule? (See Table 2.)
FNA of the left-sided dominant nodule is indicated, based on the US findings of a partially solid component and size > 1 cm. Unfortunately, FNA is nondiagnostic, because it yielded cystic fluid only with scant follicular cells for evaluation.
Continue to now what? >>
Now What?
While FNA most definitively distinguishes between benign and malignant nodules, the test is limited. An indeterminate, or nondiagnostic, finding occurs in 10% to 15% of cases and is more likely in nodules with a large cystic component.1
Even a benign finding on FNA of a larger nodule should be viewed with caution, since aspiration is unlikely to pinpoint small insidious malignant cells nestled among a larger collection of benign tissue.3 In many situations, a patient receives FNA results and asks, “What should we do now?”
Nondiagnostic nodules
When FNA is indeterminate, the next step depends on the characteristics of the nodule. For a solid nodule, repeat FNA is recommended.4,5 For nodules with repeatedly nondiagnostic FNAs, the American Academy of Clinical Endocrinologists and the American Thyroid Association recommend that a solid nodule be considered for surgical removal unless the nodule has “clearly favorable clinical and US features.”4,5
Surgical excision should be considered for cysts that recur, those that are larger (> 4 cm), and those that are repeatedly nondiagnostic on FNA. Personal and family history should be taken into account when nodules that are nondiagnostic on FNA demonstrate suspicious characteristics on US.6
An analysis by Renshaw determined that risk for malignancy in a nodule with a single nondiagnostic FNA was about 20%. For nodules that underwent repeat FNA, the risk was 0% for those that were again nondiagnostic. This significant difference led the author to conclude that “patients with two sequential nondiagnostic thyroid aspirates have a very low risk of malignancy.”7
Consider the time commitment, financial burden, and emotional cost for the patient of repeated evaluation with thyroid US and possibly FNA. In recurrent cases, the risks associated with surgery begin to be outweighed by the cost and burden of prolonged observation.
Benign nodules
With a biopsy-proven benign nodule, observation is recommended unless certain criteria are present: local neck compressive/obstructive symptoms that can be confidently attributed to a thyroid nodule; patient preference (eg, due to anxiety or aesthetics); or higher index of suspicion (eg, history of previous radiation exposure, progressive nodule growth, or suspicious characteristics on US).4,5
If surgical removal of a benign thyroid nodule is recommended, it is imperative to discuss the risks with patients. In addition to traditional surgery risks, thyroidectomy is associated with transient or permanent postoperative hypoparathyroidism, as well as vocal hoarseness or changes in vocal quality due to the proximity of the recurrent laryngeal nerve. Additionally, patients should be advised of the potential for surgical hypothyroidism with hemithyroidectomy and certain irreversible hypothyroidism with total thyroidectomy.
After a discussion of the risks and cost of observation versus surgery, an informed decision between provider and patient can ultimately be reached.
Would thyroidectomy be recommended for Felicia? After a thorough discussion, it is decided that surgery is not indicated at this time. Relevant factors include the benign thyroid US characteristics, lack of clinical neck compressive symptoms, and patient preference.
According to the American Thyroid Association guidelines, Felicia’s risk for malignancy for the nodule in question is < 3%, since it is a partially cystic nodule without any suspicious sonographic features. By foregoing surgery, Felicia will need repeated imaging studies and possibly repeat serologic studies and FNA in the future.
References
1. Stang MT, Carty SE. Recent developments in predicting thyroid malignancy. Curr Opin Oncol. 2008;21(1):11-17.
2. Hambleton C, Kandil E. Appropriate and accurate diagnosis of thyroid nodules: a review of thyroid fine-needle aspiration. Int J Clin Exp Med. 2013;6(6):413-422.
3. American Cancer Society. Thyroid cancer (2014). www.cancer.org/acs/groups/cid/documents/webcontent/003144-pdf.pdf. Accessed June 29, 2016.
4. Gharib H, Papini E, Garber J, et al; AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 Update. Endocrine Pract. 2016;22(suppl 1):1-60.
5. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26(1):1-133.
6. Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008; 13(2):105-112.
7. Renshaw A. Significance of repeatedly nondiagnostic thyroid fine-needle aspirations. Am J Clin Pathol. 2011;135(5):750-752.
A new-onset thyroid nodule, found on exam or incidentally on imaging, is a common presentation at primary care and specialist clinics. Palpable nodules are present in 4% to 7% of the population.1 However, more sensitive evaluation with thyroid ultrasound (US) suggests an incidence as high as 70%.2
According to the American Cancer Society, in 2015, there were approximately 62,450 new cases of thyroid cancer in the United States (with 2.5 times as many occurring in women as in men).3 In fact, thyroid cancer is the most rapidly increasing cancer in the United States—attributable in part to the increased use of thyroid US and incidental detection.3
The high prevalence of thyroid nodules makes appropriate evaluation and treatment crucial. This article, through a case study, explores the evaluation of a thyroid nodule and the recommendation for and against thyroidectomy.
Felicia, 49, presents to the endocrine clinic as a new patient with questions about multinodular goiter (MNG). She has been advised by ENT to have a left-sided dominant nodule surgically removed while under anesthesia during her upcoming chronic sinusitis surgery. Felicia would like to avoid thyroid surgery, if possible. Her most recent thyroid US, performed three months ago, showed a right lobe with multiple colloid nodules with inspissated colloid, the largest of which is 1.5 cm, and a 4-cm complex, solid, cystic nodule with inspissated colloid in the cystic spaces replacing the entire left thyroid lobe.
HistoryThe first step is establishing a history of the nodule(s) in question. Key questions are listed in Table 1. The onset and progression of a thyroid nodule must be determined; ideally, the provider should review any previous studies related to the thyroid gland. This will help determine if the nodule is new, if it has been evaluated in the past, and if it has changed significantly.
A thorough history can identify risk factors for malignancy, which include a personal history of cancer or radiation exposure, as well as a family history of thyroid cancer or malignant endocrine syndromes.
Felicia denies any family or personal medical history concerning for malignancy. She notes that she has two sisters with MNG. She denies any neck pain, compressive/obstructive symptoms, and hypo- or hyperthyroid symptoms.
She reports that she was found to have a goiter on exam and was subsequently diagnosed with MNG in 2008. Thyroid US showed a 2.3-cm complex, largely solid mass in the right mid-pole and a 3.3-cm largely cystic lesion in the left mid-pole. She was referred for right-sided fine-needle aspiration (FNA); results were consistent with benign colloid nodule. The left-sided nodule was not biopsied at that time, due to a largely cystic component.
Felicia underwent a follow-up US in 2011; it showed a 1.6-cm right mid-pole nodule with multiple nonspecific echogenic areas; a 1-cm benign-appearing nodule; and a 3.7-cm highly vascular heterogeneous mass with some colloid components with indeterminate component in the left lower and mid-pole. She reports that she did not follow up in 2011. Her next evaluation was the current thyroid US. She has never had FNA of the left-sided dominant nodule.
Continue for symptomatic vs asymptomatic thyroid nodules >>
Symptomatic vs Asymptomatic Thyroid NodulesEvaluation of a symptomatic thyroid nodule can help to determine the need for surgery, as well as assess the level of interference with a patient’s activities of daily living and the potential for functional abnormalities. However, both local neck and constitutional symptoms may be nonspecific and unrelated to the thyroid gland’s structure or function. Therefore, the provider should exercise caution in making recommendations based on reported symptoms alone.
Symptoms indicative of the need for surgical intervention include neck pain, increased neck pressure, foreign body sensation, dysphonia, dyspnea, and dysphagia. However, it is essential to determine if these symptoms are likely due to a thyroid nodule or if they can be attributed to a secondary cause (eg, postnasal drip, vocal cord dysfunction, gastroesophageal reflux disease, or esophageal stricture).
If the findings are inconsistent with the clinical picture, secondary evaluation is prudent to avoid an unnecessary procedure.
Physical ExamPalpation of a thyroid nodule is an unreliable indicator of risk for malignancy. Palpation alone does not allow for detection of all nodules, particularly smaller ones, and specific characteristics are not discernible. Imaging studies are required to accurately evaluate a thyroid nodule and determine the most appropriate course of action.
Palpation can be used to evaluate for a larger and/or fixed nodule, thyroid gland/nodule tenderness, and cervical lymphadenopathy. Physical exam can also assess for signs of hypo- or hyperthyroidism, including abnormal pulse rate or blood pressure, tremor, hypo- or hyperreflexia, and integumentary abnormalities (eg, hair loss, abnormal skin temperature, and nail changes).
Continue for serologic evaluation >>
Serologic Evaluation
If a thyroid nodule is suspected on exam or found on imaging, assessment of thyroid function, via thyroid-stimulating hormone (TSH) measurement, is the recommended first step. If TSH is elevated, further evaluation for hypothyroidism is recommended, with testing for free thyroxine (T4) and antithyroid peroxidase (TPO) antibodies.4 If TSH is suppressed, further evaluation with free T4 and assessment for underlying causes of hyperthyroidism are indicated, including work-up for toxic nodular goiter.
Routine monitoring of serum calcitonin level is not recommended. However, if there is suspicion for medullary thyroid cancer—based on either US findings or family history—serologic screening for abnormal calcitonin level may be indicated.4,5
Felicia’s lab results include a TSH of 1.30 µIU/mL (reference range, 0.30-3.00 µIU/mL). Based on this finding, what (if any) further serologic testing is recommended? None: With normal TSH and no concerning family or personal history, additional laboratory evaluation is not indicated.
Imaging a Thyroid Nodule
Thyroid US is the most sensitive imaging study for evaluating thyroid nodule characteristics. Thyroid uptake and scan is not indicated unless TSH is suppressed and evaluation for toxic nodular goiter is needed. Additional imaging studies, such as CT or MRI, are not recommended for thyroid nodule evaluation.
Based on the thyroid US, what characteristics of Felicia’s nodule are suggestive of a benign nodule? Of a malignant nodule? (See Table 2.)
FNA of the left-sided dominant nodule is indicated, based on the US findings of a partially solid component and size > 1 cm. Unfortunately, FNA is nondiagnostic, because it yielded cystic fluid only with scant follicular cells for evaluation.
Continue to now what? >>
Now What?
While FNA most definitively distinguishes between benign and malignant nodules, the test is limited. An indeterminate, or nondiagnostic, finding occurs in 10% to 15% of cases and is more likely in nodules with a large cystic component.1
Even a benign finding on FNA of a larger nodule should be viewed with caution, since aspiration is unlikely to pinpoint small insidious malignant cells nestled among a larger collection of benign tissue.3 In many situations, a patient receives FNA results and asks, “What should we do now?”
Nondiagnostic nodules
When FNA is indeterminate, the next step depends on the characteristics of the nodule. For a solid nodule, repeat FNA is recommended.4,5 For nodules with repeatedly nondiagnostic FNAs, the American Academy of Clinical Endocrinologists and the American Thyroid Association recommend that a solid nodule be considered for surgical removal unless the nodule has “clearly favorable clinical and US features.”4,5
Surgical excision should be considered for cysts that recur, those that are larger (> 4 cm), and those that are repeatedly nondiagnostic on FNA. Personal and family history should be taken into account when nodules that are nondiagnostic on FNA demonstrate suspicious characteristics on US.6
An analysis by Renshaw determined that risk for malignancy in a nodule with a single nondiagnostic FNA was about 20%. For nodules that underwent repeat FNA, the risk was 0% for those that were again nondiagnostic. This significant difference led the author to conclude that “patients with two sequential nondiagnostic thyroid aspirates have a very low risk of malignancy.”7
Consider the time commitment, financial burden, and emotional cost for the patient of repeated evaluation with thyroid US and possibly FNA. In recurrent cases, the risks associated with surgery begin to be outweighed by the cost and burden of prolonged observation.
Benign nodules
With a biopsy-proven benign nodule, observation is recommended unless certain criteria are present: local neck compressive/obstructive symptoms that can be confidently attributed to a thyroid nodule; patient preference (eg, due to anxiety or aesthetics); or higher index of suspicion (eg, history of previous radiation exposure, progressive nodule growth, or suspicious characteristics on US).4,5
If surgical removal of a benign thyroid nodule is recommended, it is imperative to discuss the risks with patients. In addition to traditional surgery risks, thyroidectomy is associated with transient or permanent postoperative hypoparathyroidism, as well as vocal hoarseness or changes in vocal quality due to the proximity of the recurrent laryngeal nerve. Additionally, patients should be advised of the potential for surgical hypothyroidism with hemithyroidectomy and certain irreversible hypothyroidism with total thyroidectomy.
After a discussion of the risks and cost of observation versus surgery, an informed decision between provider and patient can ultimately be reached.
Would thyroidectomy be recommended for Felicia? After a thorough discussion, it is decided that surgery is not indicated at this time. Relevant factors include the benign thyroid US characteristics, lack of clinical neck compressive symptoms, and patient preference.
According to the American Thyroid Association guidelines, Felicia’s risk for malignancy for the nodule in question is < 3%, since it is a partially cystic nodule without any suspicious sonographic features. By foregoing surgery, Felicia will need repeated imaging studies and possibly repeat serologic studies and FNA in the future.
References
1. Stang MT, Carty SE. Recent developments in predicting thyroid malignancy. Curr Opin Oncol. 2008;21(1):11-17.
2. Hambleton C, Kandil E. Appropriate and accurate diagnosis of thyroid nodules: a review of thyroid fine-needle aspiration. Int J Clin Exp Med. 2013;6(6):413-422.
3. American Cancer Society. Thyroid cancer (2014). www.cancer.org/acs/groups/cid/documents/webcontent/003144-pdf.pdf. Accessed June 29, 2016.
4. Gharib H, Papini E, Garber J, et al; AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 Update. Endocrine Pract. 2016;22(suppl 1):1-60.
5. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26(1):1-133.
6. Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008; 13(2):105-112.
7. Renshaw A. Significance of repeatedly nondiagnostic thyroid fine-needle aspirations. Am J Clin Pathol. 2011;135(5):750-752.
Lipoprotein(a) and Cardiovascular Disease
Cardiovascular disease (CVD) is the leading cause of death in the United States. CVD-related diseases affect 83.6 million people in the US and are responsible for almost 800,000 deaths annually.1 The myriad underlying causes for these disorders range from inadequate lifestyle management to genetic abnormalities. One genetically determined abnormality is lipoprotein(a), or Lp(a).2-4
It is estimated that 25% of the US population has elevated Lp(a) levels (> 30 mg/dL) that are clinically significant.5 Lp(a) is recognized as an independent risk factor for CVD, stroke, retinal artery occlusions, and restenosis of vein grafts.2-5
Regardless of practice type, clinicians at some point in their career will see a “vasculopath.” Many of these patients have undiagnosed familial hypercholesterolemia, which affects 1 in 200 to 300 patients in the US and manifests with LDL cholesterol (LDL-C) levels ≥ 190 mg/dL.6-8 Other patients may have CVD with relatively “normal” traditional lipids, more aggressive premature disease, and/or progressive disease despite “usual therapy.”
As clinical lipid specialists working both in cardiology and endocrinology, the authors find the lack of evaluation for additional abnormalities in high-risk patients to be quite disturbing. The patient most commonly seen with the Lp(a) abnormality is one with CVD onset approximately one decade earlier than expected, along with a family history of premature CVD or closure of recently placed stents. Unfortunately, this may result in disease in the second or third decade for men and third or fourth decade for women.
Of course, CVD can leave patients with less productive lives and increase the burden to the health care system and to society. A positive outcome of identification of this apolipoprotein abnormality is that it may prompt evaluation of other family members prior to the inception of vascular disease. When it is identified in the asymptomatic, disease-free patient, aggressive risk reduction—in the form of lifestyle management and medication—may delay or prevent disease onset.
Continue for identification of the problem >>
Identification of the problem
Office visits seldom include a thorough and complete patient history. A “good” family history should include first-degree relatives. Time-constrained practitioners may take a rudimentary family history of immediate relatives when a pedigree of the patient would be more appropriate.
Pedigree assessment offers a more specific picture of disease in families and identifies prevalence and incidence. Busy clinicians could have patients use an online resource to generate their own family pedigree. Or, as in most practices, a medical assistant or other appropriate office staff could initiate the process in the chart.
Patients with premature or advanced disease and significant family history need further investigation. A suspect history would include multiple family members with disease earlier in life than expected and perhaps early cardiovascular death. The personal history of the patient may include multiple cardiovascular incidents despite therapeutic intervention; despite taking lipid-lowering and/or antiplatelet therapy, the patient will present with progressive disease. Often, disease manifests in multiple areas of the vasculature or as restenosis of previous interventions.
Genetics
Lp(a) results from a genetic variation of the apolipoprotein(a) (LPA) locus on chromosome 6q27. Lp(a) is comprised of an apolipoprotein(b) (apoB)–containing LDL molecule that is bonded to LPA. LPA is structurally similar to plasminogen, the precursor for plasmin that degrades fibrin in blood clots. Due to this similarity, LPA can competitively inhibit plasmin activity and thereby increase risk for thrombosis.4,9
Continue for physical examination >>
Physical Examination
Patients with very elevated LDL-C levels in whom Lp(a) is also high may present with other outward stigmata of dyslipidemia. Visualization of the eye may reveal evidence of severe dyslipidemia with arcus cornea. This arcus can present as unilateral, bilateral, inferior, superior, or mixed and is representative of the buildup of cholesterol that cannot be removed from the body by normal means. Further examination may reveal tendon xanthomas, which are also representative of a genetic cholesterol disorder—in most cases, familial hypercholesterolemia.7
Laboratory Workup
In patients who are known or suspected to be at high risk for CVD, the laboratory workup should include a fasting lipid panel, with Lp(a) and apoB; a comprehensive metabolic profile to establish renal and liver function (as therapeutic interventions utilize these organs for metabolism); and a fasting glucose measurement to rule out occult diabetes, which enhances risk factors. Thyroid function is also assessed, secondary to its deleterious effects on lipid metabolism.
Lp(a) results must be interpreted in the context of ethnicity; significance will vary. For example, both the African-American and Asian populations have been found to have high levels of Lp(a), but these are generally felt to be less atherogenic in African Americans. No major differences have been identified for other populations. It is, however, important to note that those patients with nephropathies and elevated Lp(a) carry a higher risk for coronary artery disease.
Lp(a) levels will remain relatively steady throughout life, negating the need for routine monitoring once a patient’s levels have been established. The exception is postmenopausal women, in whom Lp(a) levels may increase due to changes in estrogen. It is prudent to assess Lp(a) in women both pre- and postmenopause, based on data from the Women’s Health Study.10
Continue for diagnosis and treatment >>
Diagnosis and Treatment
Elevated Lp(a), which is found in 25% to 35% of the population, is diagnosed at a level > 30 mg/dL, regardless of sex.4,9 In conjunction with known disease, elevated Lp(a) is sufficient to warrant consideration of very aggressive treatment. In these circumstances, the provider may consider a target LDL-C level ≤ 70 mg/dL.6,7,11 In primary prevention, clinicians should consider lowering this threshold. Levels that may have been considered appropriate in a low- or moderate-risk patient (≤ 160 mg/dL and ≤ 130 mg/dL, respectively) may be reduced to ≤ 130 mg/dL and ≤ 100 mg/dL, respectively.6,11
There is no peer-reviewed evidence with regard to lifestyle management (exercise and diet) for reduction of Lp(a). However, it is reasonable to recommend that high-risk patients adopt healthier regimens.
Management of elevated Lp(a) includes consideration of pharmacologic intervention. Since Lp(a) is prothrombotic, all patients without contraindications should at least be taking low-dose (81-mg) aspirin. Those with evidence of thrombotic events may need lifetime antiplatelet therapy.12 Statin therapy has mixed and minimal effects on Lp(a), although it remains the mainstay of treatment due to its effects on LDL-C and other lipoproteins.13 Although long-term data are lacking, there is some anecdotal evidence of improvement with fibrate therapy. However, it is not recommended for treatment of elevated Lp(a).14
Nicotinic acid has had the longest and most robust history for reduction of Lp(a).9,12 However, recent studies examining combination therapy with statins and nicotinic acid have yielded discouraging results—and in some cases have suggested negative outcomes with this combination.15,16 High doses (4-5 g for immediate release and 2-3 g for sustained release) of nicotinic acid are necessary to produce beneficial results on Lp(a) or other lipid abnormalities (eg, elevated triglycerides, low HDL cholesterol).17 Use of OTC nicotinic acid is not recommended, since these products are considered dietary supplements and regulated as such, raising the potential for untoward adverse effects and/or the possibility that little to no active ingredient is present.18-20
Results from the Women’s Health Study and the Heart and Estrogen/progestin Replacement Study suggested that estrogen might be an effective therapy. In one analysis, women with elevated Lp(a) derived greater potential cardioprotective effects from hormone replacement therapy (HRT) than those with lower Lp(a), and the researchers noted a “significant interaction” between baseline Lp(a), HRT treatment, and CVD risk. However, use of HRT is not approved for treatment of vascular risk today, due to the potential for adverse effects.10,21
A novel therapy, in the form of PCSK9 inhibition, has been shown to reduce LDL-C significantly; reduction in Lp(a) was also observed. The FDA recently approved two PCSK9 inhibitors (alirocumab and evolocumab) for use, although the primary indication is for further reduction in LDL-C on top of the maximally tolerated dose of statin therapy, not for reduction of Lp(a).22,23
Apheresis has been shown to have positive effects in reducing ongoing vascular events in select patient populations. It is approved by the FDA for treatment of refractory LDL-C, mostly in patients with familial hypercholesterolemia, but it is not indicated for treatment of elevated Lp(a). However, since Lp(a) tracks with LDL-C, it is also removed during the process; about a 50% reduction in Lp(a) levels has been noted, although levels rebound posttreatment. To date, reimbursement issues remain in the absence of an FDA indication and due to the paucity of treatment centers in the US.24,25
Follow-up. The therapies mentioned require routine evaluation to assess tolerability and safety, as recommended in the prescribing information. Patients with known CVD should undergo an appropriate cardiac workup annually to evaluate for occult progression of disease. Patients require further evaluation of related cardiovascular risk factors and adherence with medication regimens. For primary prevention patients, annual follow-up is also recommended to assess for any changes in health status, lifestyle, or medication adherence.
Continue for conclusion >>
Conclusion
The average health care provider frequently performs the standard evaluation of a patient at risk for, or with, CVD. However, a subset of this population may be at increased cardiovascular risk due to Lp(a), a common genetic risk factor that can be responsible for premature or progressive CVD. Because of the aggressive nature of this disorder and the young age at which it influences the development of vascular disease, health care providers must be more vigilant about looking beyond the obvious in patients with familial hypercholesterolemia or family history of premature CVD.
Patients with progressive disease must be more thoroughly evaluated; there are already more than 63 million persons with elevated Lp(a) in the US—and many more undiagnosed—who may benefit from aggressive care. Underdiagnosis has been associated with decreased quality and productivity in the work environment, decreased quality of life, increased use of health dollars, and possibly early loss of life.
While the test for Lp(a) is readily available, the cost may not be covered by insurance and therefore may be passed on to the patient. It would behoove health care professionals to lobby for coverage as a means to reduce the prevalence of CVD, the number one cause of mortality in the US.
References
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive Summary: heart disease and stroke statistics—2014 Update: a report from the American Heart Association. Circulation. 2014;129:399-410.
2. Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data [published corrections appear in Arch Intern Med. 2008;168(10):1089 and Arch Intern Med. 2008;168(10):1096]. Arch Intern Med. 2008;168(6):598-608.
3. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
4. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
5. Scanu AM. Lipoprotein(a). A genetic risk factor for premature coronary heart disease. JAMA. 1992;267(24):3326-3329.
6. Goldberg AC, Hopkins PN, Toth PP; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S1-S8.
7. Ito M, McGowan MP, Moriarty PM; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S38-S45.
8. Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2014 Feb 28. [Epub ahead of print]
9. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein (a) as a cardiovascular risk factor: current status. Eur Heart J. 2010; 31(23):2844-2853.
10. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296 (11):1363-1370.
11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
12. Jacobson TA. Lipoprotein (a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88(11):1294-1311.
13. Hunninghake DB, Stein EA, Mellies MJ. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. J Clin Pharmacol. 1993;33 (6):574-580.
14. Jones PH, Pownall HJ, Patsch W, et al. Effect of gemfibrozil on levels of lipoprotein[a] in type 2 hyperlipoproteinemic subjects. J Lipid Res. 1996;37(6):1298-1308.
15. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011; 365(24):2255-2267.
16. Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371 (3):203-212.
17. Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82 (12A): 29U-34U.
18. Piepho RW. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am J Cardiol. 2000;86(12A):35L-40L.
19. Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007; 99(6A):22C-31C.
20. McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271(9):672-677.
21. Shlipak MG, Simon JA, Vittinghoff E, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA. 2000;283 (14):1845-1852.
22. Marbach JA, McKeon JL, Ross JL, Duffy D. Novel treatments for familial hypercholesterolemia: pharmacogenetics at work. Pharmacotherapy. 2014;34(9):961-972.
23. Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12): 1108-1118.
24. Sachais BS, Katz J, Ross J, Rader DJ. Long-term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20:252-255.
25. Waldmann E, Parhofer K. Lipoprotein apheresis to treat elevated lipoprotein(a). J Lipid Res. 2016 Feb 17. [Epub ahead of print]
Cardiovascular disease (CVD) is the leading cause of death in the United States. CVD-related diseases affect 83.6 million people in the US and are responsible for almost 800,000 deaths annually.1 The myriad underlying causes for these disorders range from inadequate lifestyle management to genetic abnormalities. One genetically determined abnormality is lipoprotein(a), or Lp(a).2-4
It is estimated that 25% of the US population has elevated Lp(a) levels (> 30 mg/dL) that are clinically significant.5 Lp(a) is recognized as an independent risk factor for CVD, stroke, retinal artery occlusions, and restenosis of vein grafts.2-5
Regardless of practice type, clinicians at some point in their career will see a “vasculopath.” Many of these patients have undiagnosed familial hypercholesterolemia, which affects 1 in 200 to 300 patients in the US and manifests with LDL cholesterol (LDL-C) levels ≥ 190 mg/dL.6-8 Other patients may have CVD with relatively “normal” traditional lipids, more aggressive premature disease, and/or progressive disease despite “usual therapy.”
As clinical lipid specialists working both in cardiology and endocrinology, the authors find the lack of evaluation for additional abnormalities in high-risk patients to be quite disturbing. The patient most commonly seen with the Lp(a) abnormality is one with CVD onset approximately one decade earlier than expected, along with a family history of premature CVD or closure of recently placed stents. Unfortunately, this may result in disease in the second or third decade for men and third or fourth decade for women.
Of course, CVD can leave patients with less productive lives and increase the burden to the health care system and to society. A positive outcome of identification of this apolipoprotein abnormality is that it may prompt evaluation of other family members prior to the inception of vascular disease. When it is identified in the asymptomatic, disease-free patient, aggressive risk reduction—in the form of lifestyle management and medication—may delay or prevent disease onset.
Continue for identification of the problem >>
Identification of the problem
Office visits seldom include a thorough and complete patient history. A “good” family history should include first-degree relatives. Time-constrained practitioners may take a rudimentary family history of immediate relatives when a pedigree of the patient would be more appropriate.
Pedigree assessment offers a more specific picture of disease in families and identifies prevalence and incidence. Busy clinicians could have patients use an online resource to generate their own family pedigree. Or, as in most practices, a medical assistant or other appropriate office staff could initiate the process in the chart.
Patients with premature or advanced disease and significant family history need further investigation. A suspect history would include multiple family members with disease earlier in life than expected and perhaps early cardiovascular death. The personal history of the patient may include multiple cardiovascular incidents despite therapeutic intervention; despite taking lipid-lowering and/or antiplatelet therapy, the patient will present with progressive disease. Often, disease manifests in multiple areas of the vasculature or as restenosis of previous interventions.
Genetics
Lp(a) results from a genetic variation of the apolipoprotein(a) (LPA) locus on chromosome 6q27. Lp(a) is comprised of an apolipoprotein(b) (apoB)–containing LDL molecule that is bonded to LPA. LPA is structurally similar to plasminogen, the precursor for plasmin that degrades fibrin in blood clots. Due to this similarity, LPA can competitively inhibit plasmin activity and thereby increase risk for thrombosis.4,9
Continue for physical examination >>
Physical Examination
Patients with very elevated LDL-C levels in whom Lp(a) is also high may present with other outward stigmata of dyslipidemia. Visualization of the eye may reveal evidence of severe dyslipidemia with arcus cornea. This arcus can present as unilateral, bilateral, inferior, superior, or mixed and is representative of the buildup of cholesterol that cannot be removed from the body by normal means. Further examination may reveal tendon xanthomas, which are also representative of a genetic cholesterol disorder—in most cases, familial hypercholesterolemia.7
Laboratory Workup
In patients who are known or suspected to be at high risk for CVD, the laboratory workup should include a fasting lipid panel, with Lp(a) and apoB; a comprehensive metabolic profile to establish renal and liver function (as therapeutic interventions utilize these organs for metabolism); and a fasting glucose measurement to rule out occult diabetes, which enhances risk factors. Thyroid function is also assessed, secondary to its deleterious effects on lipid metabolism.
Lp(a) results must be interpreted in the context of ethnicity; significance will vary. For example, both the African-American and Asian populations have been found to have high levels of Lp(a), but these are generally felt to be less atherogenic in African Americans. No major differences have been identified for other populations. It is, however, important to note that those patients with nephropathies and elevated Lp(a) carry a higher risk for coronary artery disease.
Lp(a) levels will remain relatively steady throughout life, negating the need for routine monitoring once a patient’s levels have been established. The exception is postmenopausal women, in whom Lp(a) levels may increase due to changes in estrogen. It is prudent to assess Lp(a) in women both pre- and postmenopause, based on data from the Women’s Health Study.10
Continue for diagnosis and treatment >>
Diagnosis and Treatment
Elevated Lp(a), which is found in 25% to 35% of the population, is diagnosed at a level > 30 mg/dL, regardless of sex.4,9 In conjunction with known disease, elevated Lp(a) is sufficient to warrant consideration of very aggressive treatment. In these circumstances, the provider may consider a target LDL-C level ≤ 70 mg/dL.6,7,11 In primary prevention, clinicians should consider lowering this threshold. Levels that may have been considered appropriate in a low- or moderate-risk patient (≤ 160 mg/dL and ≤ 130 mg/dL, respectively) may be reduced to ≤ 130 mg/dL and ≤ 100 mg/dL, respectively.6,11
There is no peer-reviewed evidence with regard to lifestyle management (exercise and diet) for reduction of Lp(a). However, it is reasonable to recommend that high-risk patients adopt healthier regimens.
Management of elevated Lp(a) includes consideration of pharmacologic intervention. Since Lp(a) is prothrombotic, all patients without contraindications should at least be taking low-dose (81-mg) aspirin. Those with evidence of thrombotic events may need lifetime antiplatelet therapy.12 Statin therapy has mixed and minimal effects on Lp(a), although it remains the mainstay of treatment due to its effects on LDL-C and other lipoproteins.13 Although long-term data are lacking, there is some anecdotal evidence of improvement with fibrate therapy. However, it is not recommended for treatment of elevated Lp(a).14
Nicotinic acid has had the longest and most robust history for reduction of Lp(a).9,12 However, recent studies examining combination therapy with statins and nicotinic acid have yielded discouraging results—and in some cases have suggested negative outcomes with this combination.15,16 High doses (4-5 g for immediate release and 2-3 g for sustained release) of nicotinic acid are necessary to produce beneficial results on Lp(a) or other lipid abnormalities (eg, elevated triglycerides, low HDL cholesterol).17 Use of OTC nicotinic acid is not recommended, since these products are considered dietary supplements and regulated as such, raising the potential for untoward adverse effects and/or the possibility that little to no active ingredient is present.18-20
Results from the Women’s Health Study and the Heart and Estrogen/progestin Replacement Study suggested that estrogen might be an effective therapy. In one analysis, women with elevated Lp(a) derived greater potential cardioprotective effects from hormone replacement therapy (HRT) than those with lower Lp(a), and the researchers noted a “significant interaction” between baseline Lp(a), HRT treatment, and CVD risk. However, use of HRT is not approved for treatment of vascular risk today, due to the potential for adverse effects.10,21
A novel therapy, in the form of PCSK9 inhibition, has been shown to reduce LDL-C significantly; reduction in Lp(a) was also observed. The FDA recently approved two PCSK9 inhibitors (alirocumab and evolocumab) for use, although the primary indication is for further reduction in LDL-C on top of the maximally tolerated dose of statin therapy, not for reduction of Lp(a).22,23
Apheresis has been shown to have positive effects in reducing ongoing vascular events in select patient populations. It is approved by the FDA for treatment of refractory LDL-C, mostly in patients with familial hypercholesterolemia, but it is not indicated for treatment of elevated Lp(a). However, since Lp(a) tracks with LDL-C, it is also removed during the process; about a 50% reduction in Lp(a) levels has been noted, although levels rebound posttreatment. To date, reimbursement issues remain in the absence of an FDA indication and due to the paucity of treatment centers in the US.24,25
Follow-up. The therapies mentioned require routine evaluation to assess tolerability and safety, as recommended in the prescribing information. Patients with known CVD should undergo an appropriate cardiac workup annually to evaluate for occult progression of disease. Patients require further evaluation of related cardiovascular risk factors and adherence with medication regimens. For primary prevention patients, annual follow-up is also recommended to assess for any changes in health status, lifestyle, or medication adherence.
Continue for conclusion >>
Conclusion
The average health care provider frequently performs the standard evaluation of a patient at risk for, or with, CVD. However, a subset of this population may be at increased cardiovascular risk due to Lp(a), a common genetic risk factor that can be responsible for premature or progressive CVD. Because of the aggressive nature of this disorder and the young age at which it influences the development of vascular disease, health care providers must be more vigilant about looking beyond the obvious in patients with familial hypercholesterolemia or family history of premature CVD.
Patients with progressive disease must be more thoroughly evaluated; there are already more than 63 million persons with elevated Lp(a) in the US—and many more undiagnosed—who may benefit from aggressive care. Underdiagnosis has been associated with decreased quality and productivity in the work environment, decreased quality of life, increased use of health dollars, and possibly early loss of life.
While the test for Lp(a) is readily available, the cost may not be covered by insurance and therefore may be passed on to the patient. It would behoove health care professionals to lobby for coverage as a means to reduce the prevalence of CVD, the number one cause of mortality in the US.
References
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive Summary: heart disease and stroke statistics—2014 Update: a report from the American Heart Association. Circulation. 2014;129:399-410.
2. Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data [published corrections appear in Arch Intern Med. 2008;168(10):1089 and Arch Intern Med. 2008;168(10):1096]. Arch Intern Med. 2008;168(6):598-608.
3. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
4. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
5. Scanu AM. Lipoprotein(a). A genetic risk factor for premature coronary heart disease. JAMA. 1992;267(24):3326-3329.
6. Goldberg AC, Hopkins PN, Toth PP; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S1-S8.
7. Ito M, McGowan MP, Moriarty PM; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S38-S45.
8. Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2014 Feb 28. [Epub ahead of print]
9. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein (a) as a cardiovascular risk factor: current status. Eur Heart J. 2010; 31(23):2844-2853.
10. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296 (11):1363-1370.
11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
12. Jacobson TA. Lipoprotein (a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88(11):1294-1311.
13. Hunninghake DB, Stein EA, Mellies MJ. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. J Clin Pharmacol. 1993;33 (6):574-580.
14. Jones PH, Pownall HJ, Patsch W, et al. Effect of gemfibrozil on levels of lipoprotein[a] in type 2 hyperlipoproteinemic subjects. J Lipid Res. 1996;37(6):1298-1308.
15. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011; 365(24):2255-2267.
16. Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371 (3):203-212.
17. Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82 (12A): 29U-34U.
18. Piepho RW. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am J Cardiol. 2000;86(12A):35L-40L.
19. Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007; 99(6A):22C-31C.
20. McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271(9):672-677.
21. Shlipak MG, Simon JA, Vittinghoff E, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA. 2000;283 (14):1845-1852.
22. Marbach JA, McKeon JL, Ross JL, Duffy D. Novel treatments for familial hypercholesterolemia: pharmacogenetics at work. Pharmacotherapy. 2014;34(9):961-972.
23. Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12): 1108-1118.
24. Sachais BS, Katz J, Ross J, Rader DJ. Long-term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20:252-255.
25. Waldmann E, Parhofer K. Lipoprotein apheresis to treat elevated lipoprotein(a). J Lipid Res. 2016 Feb 17. [Epub ahead of print]
Cardiovascular disease (CVD) is the leading cause of death in the United States. CVD-related diseases affect 83.6 million people in the US and are responsible for almost 800,000 deaths annually.1 The myriad underlying causes for these disorders range from inadequate lifestyle management to genetic abnormalities. One genetically determined abnormality is lipoprotein(a), or Lp(a).2-4
It is estimated that 25% of the US population has elevated Lp(a) levels (> 30 mg/dL) that are clinically significant.5 Lp(a) is recognized as an independent risk factor for CVD, stroke, retinal artery occlusions, and restenosis of vein grafts.2-5
Regardless of practice type, clinicians at some point in their career will see a “vasculopath.” Many of these patients have undiagnosed familial hypercholesterolemia, which affects 1 in 200 to 300 patients in the US and manifests with LDL cholesterol (LDL-C) levels ≥ 190 mg/dL.6-8 Other patients may have CVD with relatively “normal” traditional lipids, more aggressive premature disease, and/or progressive disease despite “usual therapy.”
As clinical lipid specialists working both in cardiology and endocrinology, the authors find the lack of evaluation for additional abnormalities in high-risk patients to be quite disturbing. The patient most commonly seen with the Lp(a) abnormality is one with CVD onset approximately one decade earlier than expected, along with a family history of premature CVD or closure of recently placed stents. Unfortunately, this may result in disease in the second or third decade for men and third or fourth decade for women.
Of course, CVD can leave patients with less productive lives and increase the burden to the health care system and to society. A positive outcome of identification of this apolipoprotein abnormality is that it may prompt evaluation of other family members prior to the inception of vascular disease. When it is identified in the asymptomatic, disease-free patient, aggressive risk reduction—in the form of lifestyle management and medication—may delay or prevent disease onset.
Continue for identification of the problem >>
Identification of the problem
Office visits seldom include a thorough and complete patient history. A “good” family history should include first-degree relatives. Time-constrained practitioners may take a rudimentary family history of immediate relatives when a pedigree of the patient would be more appropriate.
Pedigree assessment offers a more specific picture of disease in families and identifies prevalence and incidence. Busy clinicians could have patients use an online resource to generate their own family pedigree. Or, as in most practices, a medical assistant or other appropriate office staff could initiate the process in the chart.
Patients with premature or advanced disease and significant family history need further investigation. A suspect history would include multiple family members with disease earlier in life than expected and perhaps early cardiovascular death. The personal history of the patient may include multiple cardiovascular incidents despite therapeutic intervention; despite taking lipid-lowering and/or antiplatelet therapy, the patient will present with progressive disease. Often, disease manifests in multiple areas of the vasculature or as restenosis of previous interventions.
Genetics
Lp(a) results from a genetic variation of the apolipoprotein(a) (LPA) locus on chromosome 6q27. Lp(a) is comprised of an apolipoprotein(b) (apoB)–containing LDL molecule that is bonded to LPA. LPA is structurally similar to plasminogen, the precursor for plasmin that degrades fibrin in blood clots. Due to this similarity, LPA can competitively inhibit plasmin activity and thereby increase risk for thrombosis.4,9
Continue for physical examination >>
Physical Examination
Patients with very elevated LDL-C levels in whom Lp(a) is also high may present with other outward stigmata of dyslipidemia. Visualization of the eye may reveal evidence of severe dyslipidemia with arcus cornea. This arcus can present as unilateral, bilateral, inferior, superior, or mixed and is representative of the buildup of cholesterol that cannot be removed from the body by normal means. Further examination may reveal tendon xanthomas, which are also representative of a genetic cholesterol disorder—in most cases, familial hypercholesterolemia.7
Laboratory Workup
In patients who are known or suspected to be at high risk for CVD, the laboratory workup should include a fasting lipid panel, with Lp(a) and apoB; a comprehensive metabolic profile to establish renal and liver function (as therapeutic interventions utilize these organs for metabolism); and a fasting glucose measurement to rule out occult diabetes, which enhances risk factors. Thyroid function is also assessed, secondary to its deleterious effects on lipid metabolism.
Lp(a) results must be interpreted in the context of ethnicity; significance will vary. For example, both the African-American and Asian populations have been found to have high levels of Lp(a), but these are generally felt to be less atherogenic in African Americans. No major differences have been identified for other populations. It is, however, important to note that those patients with nephropathies and elevated Lp(a) carry a higher risk for coronary artery disease.
Lp(a) levels will remain relatively steady throughout life, negating the need for routine monitoring once a patient’s levels have been established. The exception is postmenopausal women, in whom Lp(a) levels may increase due to changes in estrogen. It is prudent to assess Lp(a) in women both pre- and postmenopause, based on data from the Women’s Health Study.10
Continue for diagnosis and treatment >>
Diagnosis and Treatment
Elevated Lp(a), which is found in 25% to 35% of the population, is diagnosed at a level > 30 mg/dL, regardless of sex.4,9 In conjunction with known disease, elevated Lp(a) is sufficient to warrant consideration of very aggressive treatment. In these circumstances, the provider may consider a target LDL-C level ≤ 70 mg/dL.6,7,11 In primary prevention, clinicians should consider lowering this threshold. Levels that may have been considered appropriate in a low- or moderate-risk patient (≤ 160 mg/dL and ≤ 130 mg/dL, respectively) may be reduced to ≤ 130 mg/dL and ≤ 100 mg/dL, respectively.6,11
There is no peer-reviewed evidence with regard to lifestyle management (exercise and diet) for reduction of Lp(a). However, it is reasonable to recommend that high-risk patients adopt healthier regimens.
Management of elevated Lp(a) includes consideration of pharmacologic intervention. Since Lp(a) is prothrombotic, all patients without contraindications should at least be taking low-dose (81-mg) aspirin. Those with evidence of thrombotic events may need lifetime antiplatelet therapy.12 Statin therapy has mixed and minimal effects on Lp(a), although it remains the mainstay of treatment due to its effects on LDL-C and other lipoproteins.13 Although long-term data are lacking, there is some anecdotal evidence of improvement with fibrate therapy. However, it is not recommended for treatment of elevated Lp(a).14
Nicotinic acid has had the longest and most robust history for reduction of Lp(a).9,12 However, recent studies examining combination therapy with statins and nicotinic acid have yielded discouraging results—and in some cases have suggested negative outcomes with this combination.15,16 High doses (4-5 g for immediate release and 2-3 g for sustained release) of nicotinic acid are necessary to produce beneficial results on Lp(a) or other lipid abnormalities (eg, elevated triglycerides, low HDL cholesterol).17 Use of OTC nicotinic acid is not recommended, since these products are considered dietary supplements and regulated as such, raising the potential for untoward adverse effects and/or the possibility that little to no active ingredient is present.18-20
Results from the Women’s Health Study and the Heart and Estrogen/progestin Replacement Study suggested that estrogen might be an effective therapy. In one analysis, women with elevated Lp(a) derived greater potential cardioprotective effects from hormone replacement therapy (HRT) than those with lower Lp(a), and the researchers noted a “significant interaction” between baseline Lp(a), HRT treatment, and CVD risk. However, use of HRT is not approved for treatment of vascular risk today, due to the potential for adverse effects.10,21
A novel therapy, in the form of PCSK9 inhibition, has been shown to reduce LDL-C significantly; reduction in Lp(a) was also observed. The FDA recently approved two PCSK9 inhibitors (alirocumab and evolocumab) for use, although the primary indication is for further reduction in LDL-C on top of the maximally tolerated dose of statin therapy, not for reduction of Lp(a).22,23
Apheresis has been shown to have positive effects in reducing ongoing vascular events in select patient populations. It is approved by the FDA for treatment of refractory LDL-C, mostly in patients with familial hypercholesterolemia, but it is not indicated for treatment of elevated Lp(a). However, since Lp(a) tracks with LDL-C, it is also removed during the process; about a 50% reduction in Lp(a) levels has been noted, although levels rebound posttreatment. To date, reimbursement issues remain in the absence of an FDA indication and due to the paucity of treatment centers in the US.24,25
Follow-up. The therapies mentioned require routine evaluation to assess tolerability and safety, as recommended in the prescribing information. Patients with known CVD should undergo an appropriate cardiac workup annually to evaluate for occult progression of disease. Patients require further evaluation of related cardiovascular risk factors and adherence with medication regimens. For primary prevention patients, annual follow-up is also recommended to assess for any changes in health status, lifestyle, or medication adherence.
Continue for conclusion >>
Conclusion
The average health care provider frequently performs the standard evaluation of a patient at risk for, or with, CVD. However, a subset of this population may be at increased cardiovascular risk due to Lp(a), a common genetic risk factor that can be responsible for premature or progressive CVD. Because of the aggressive nature of this disorder and the young age at which it influences the development of vascular disease, health care providers must be more vigilant about looking beyond the obvious in patients with familial hypercholesterolemia or family history of premature CVD.
Patients with progressive disease must be more thoroughly evaluated; there are already more than 63 million persons with elevated Lp(a) in the US—and many more undiagnosed—who may benefit from aggressive care. Underdiagnosis has been associated with decreased quality and productivity in the work environment, decreased quality of life, increased use of health dollars, and possibly early loss of life.
While the test for Lp(a) is readily available, the cost may not be covered by insurance and therefore may be passed on to the patient. It would behoove health care professionals to lobby for coverage as a means to reduce the prevalence of CVD, the number one cause of mortality in the US.
References
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive Summary: heart disease and stroke statistics—2014 Update: a report from the American Heart Association. Circulation. 2014;129:399-410.
2. Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data [published corrections appear in Arch Intern Med. 2008;168(10):1089 and Arch Intern Med. 2008;168(10):1096]. Arch Intern Med. 2008;168(6):598-608.
3. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
4. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
5. Scanu AM. Lipoprotein(a). A genetic risk factor for premature coronary heart disease. JAMA. 1992;267(24):3326-3329.
6. Goldberg AC, Hopkins PN, Toth PP; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S1-S8.
7. Ito M, McGowan MP, Moriarty PM; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipid. 2011;5(3 suppl):S38-S45.
8. Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2014 Feb 28. [Epub ahead of print]
9. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein (a) as a cardiovascular risk factor: current status. Eur Heart J. 2010; 31(23):2844-2853.
10. Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296 (11):1363-1370.
11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
12. Jacobson TA. Lipoprotein (a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88(11):1294-1311.
13. Hunninghake DB, Stein EA, Mellies MJ. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. J Clin Pharmacol. 1993;33 (6):574-580.
14. Jones PH, Pownall HJ, Patsch W, et al. Effect of gemfibrozil on levels of lipoprotein[a] in type 2 hyperlipoproteinemic subjects. J Lipid Res. 1996;37(6):1298-1308.
15. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011; 365(24):2255-2267.
16. Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371 (3):203-212.
17. Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82 (12A): 29U-34U.
18. Piepho RW. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am J Cardiol. 2000;86(12A):35L-40L.
19. Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007; 99(6A):22C-31C.
20. McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271(9):672-677.
21. Shlipak MG, Simon JA, Vittinghoff E, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA. 2000;283 (14):1845-1852.
22. Marbach JA, McKeon JL, Ross JL, Duffy D. Novel treatments for familial hypercholesterolemia: pharmacogenetics at work. Pharmacotherapy. 2014;34(9):961-972.
23. Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12): 1108-1118.
24. Sachais BS, Katz J, Ross J, Rader DJ. Long-term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20:252-255.
25. Waldmann E, Parhofer K. Lipoprotein apheresis to treat elevated lipoprotein(a). J Lipid Res. 2016 Feb 17. [Epub ahead of print]