User login
Effective treatment of recurrent bacterial vaginosis
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1
BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
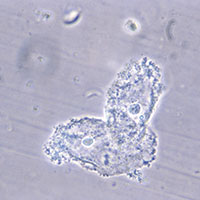
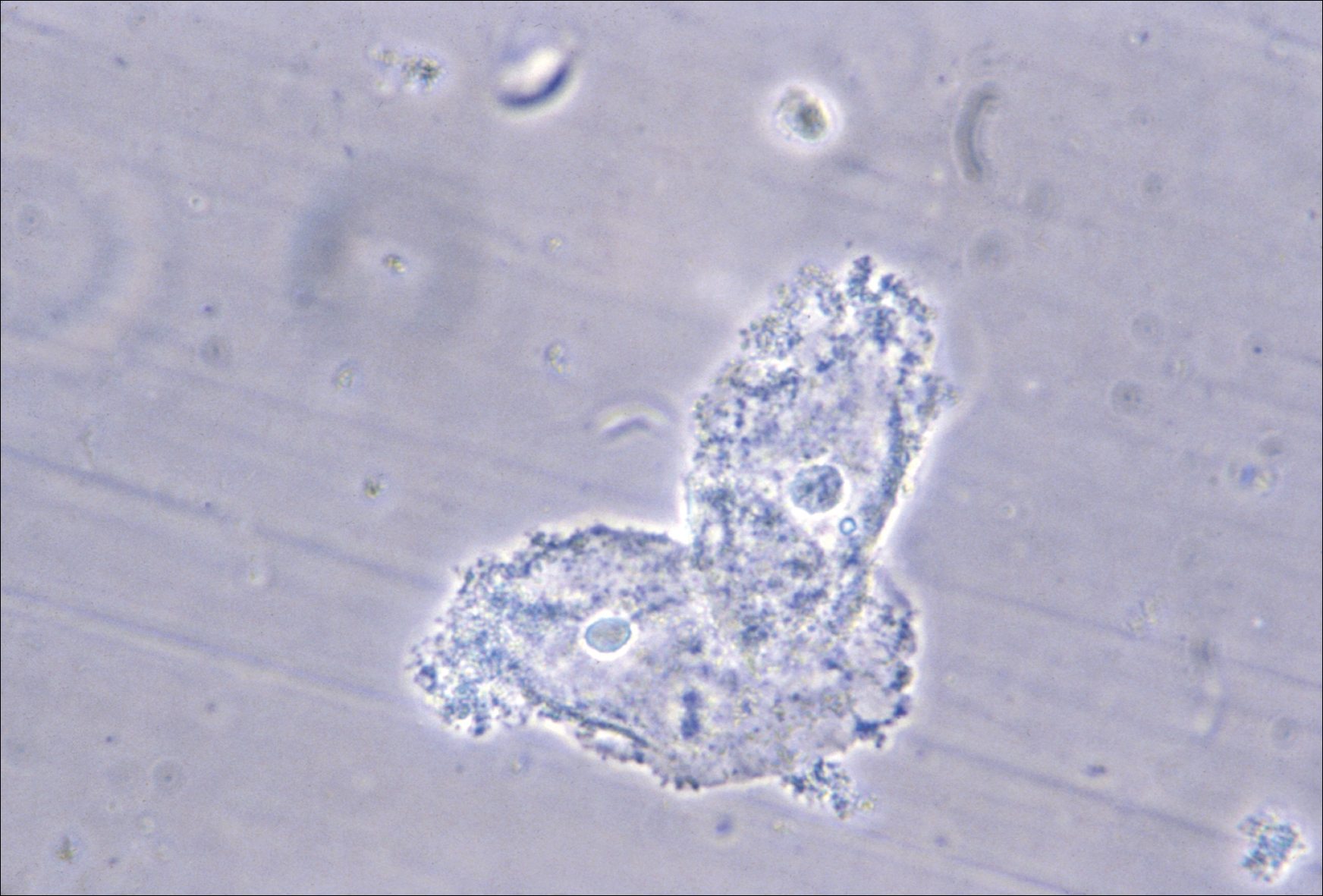
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
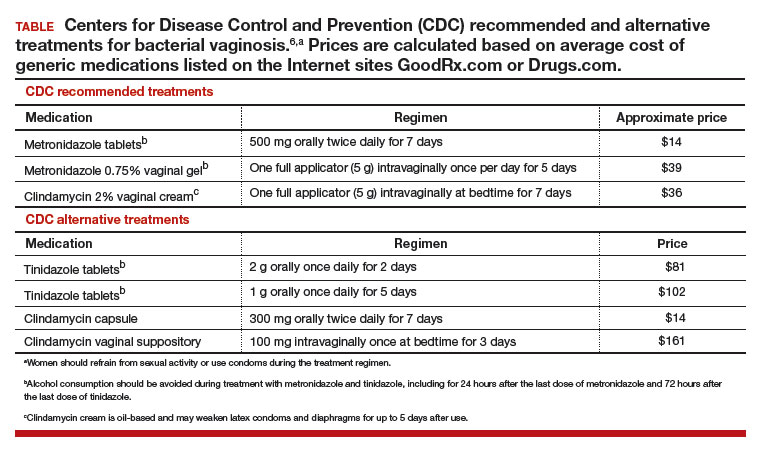
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.
Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1

BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.

Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1

BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.

Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
Appropriate diagnosis of tickborne infections
As summer is upon us, we enter the peak of tick season. Most reported cases of tickborne disease occur from April to October, and in this issue, Eickhoff and Blaylock offer a timely review of less common (non-Lyme disease) but significant tickborne infections.
In areas endemic for infection with Rickettsia rickettsii, the organism responsible for Rocky Mountain spotted fever (RMSF), physicians and many patients are keenly aware of the signs and symptoms of the disease and are quick to offer and accept empiric antibiotic (doxycycline) therapy. Empiric therapy at the first suspicion of RMSF is appropriate, as untreated infection carries a 20% death rate. Vigilance for early Lyme disease (caused by Borrelia burgdorferi) is also high in true endemic areas, likely because of public awareness and concern for various documented—and some touted but unproven—associated morbidities.
Other tickborne infections are likely underrecognized by physicians who are not experts in infectious disease, and thus are not treated early. There are many reasons for this, including the relative infrequency of severe disease, the nonspecific clinical signs of early infection, and the spreading of infections to geographic areas where they are traditionally not considered endemic.
Additional features likely contribute to delayed diagnosis. Surveys of patients with documented RMSF or Lyme disease show that a large proportion of infections occur in patients who have no history of camping or hiking. Most people are not even aware that they have been harboring a feeding tick, as many ticks are quite small and attachment is painless. Because some ticks survive more than a year, they may stay alive in clothes and closets throughout the winter months and occasionally cause nonseasonal infections.
Geography and entomology matter; the matching of a specific tick vector to a specific disease is tight. With the slow migration of some tick species along with their nonhuman animal hosts into new territories due to temperature changes and urbanization, some diseases are appearing in areas of the country where they had not been previously diagnosed. We must be aware of these changes, and the US Centers for Disease Control and Prevention (CDC) offers useful updated infection maps on their website.
The diagnosis of acute infection is often delayed because of late consideration of the possibility of the disease. In addition, some tests are serologic and require the passage of time before a diagnostic result is obtained. But an increasing and distinct problem is the overdiagnosis and long-term treatment of some patients whose infection is undocumented, perpetuating concern over the unproven entity of chronic infection, the most prevalent being the diagnosis and treatment of “chronic Lyme disease.” Close attention must be paid to the manner of diagnosis and the specific tests used to purportedly confirm the diagnosis of infection. This has been an ongoing challenge in managing patients with chronic fatigue and malaise, a vexing and significant clinical problem without a ready solution in patients who have undergone an extensive evaluation. It is obviously tempting for patients to grasp at any “diagnostic” answer. But chronic and repeated therapy for nonexistent infection is not benign. The CDC has published lists of tests for Lyme disease in particular that are considered to have inadequately established accuracy and clinical utility; these include lymphocyte transformation tests, quantitative CD57 lymphocyte assays, and urinary antigen “capture assays.”
Recognizing and treating acute tickborne infections is crucial, as in distinguishing them from their mimics, which include some systemic autoimmune diseases. But we should not allow the fear of undertreatment of early infection to morph into unwarranted overtreatment of nonexistent chronic infection, just as we should not prematurely assume that ongoing symptoms of fatigue and malaise after a presumed tickborne infection are from the psychologically crippling fear of ongoing morbidity. Periodic reappraisal of the patient and his or her symptoms is warranted.
As summer is upon us, we enter the peak of tick season. Most reported cases of tickborne disease occur from April to October, and in this issue, Eickhoff and Blaylock offer a timely review of less common (non-Lyme disease) but significant tickborne infections.
In areas endemic for infection with Rickettsia rickettsii, the organism responsible for Rocky Mountain spotted fever (RMSF), physicians and many patients are keenly aware of the signs and symptoms of the disease and are quick to offer and accept empiric antibiotic (doxycycline) therapy. Empiric therapy at the first suspicion of RMSF is appropriate, as untreated infection carries a 20% death rate. Vigilance for early Lyme disease (caused by Borrelia burgdorferi) is also high in true endemic areas, likely because of public awareness and concern for various documented—and some touted but unproven—associated morbidities.
Other tickborne infections are likely underrecognized by physicians who are not experts in infectious disease, and thus are not treated early. There are many reasons for this, including the relative infrequency of severe disease, the nonspecific clinical signs of early infection, and the spreading of infections to geographic areas where they are traditionally not considered endemic.
Additional features likely contribute to delayed diagnosis. Surveys of patients with documented RMSF or Lyme disease show that a large proportion of infections occur in patients who have no history of camping or hiking. Most people are not even aware that they have been harboring a feeding tick, as many ticks are quite small and attachment is painless. Because some ticks survive more than a year, they may stay alive in clothes and closets throughout the winter months and occasionally cause nonseasonal infections.
Geography and entomology matter; the matching of a specific tick vector to a specific disease is tight. With the slow migration of some tick species along with their nonhuman animal hosts into new territories due to temperature changes and urbanization, some diseases are appearing in areas of the country where they had not been previously diagnosed. We must be aware of these changes, and the US Centers for Disease Control and Prevention (CDC) offers useful updated infection maps on their website.
The diagnosis of acute infection is often delayed because of late consideration of the possibility of the disease. In addition, some tests are serologic and require the passage of time before a diagnostic result is obtained. But an increasing and distinct problem is the overdiagnosis and long-term treatment of some patients whose infection is undocumented, perpetuating concern over the unproven entity of chronic infection, the most prevalent being the diagnosis and treatment of “chronic Lyme disease.” Close attention must be paid to the manner of diagnosis and the specific tests used to purportedly confirm the diagnosis of infection. This has been an ongoing challenge in managing patients with chronic fatigue and malaise, a vexing and significant clinical problem without a ready solution in patients who have undergone an extensive evaluation. It is obviously tempting for patients to grasp at any “diagnostic” answer. But chronic and repeated therapy for nonexistent infection is not benign. The CDC has published lists of tests for Lyme disease in particular that are considered to have inadequately established accuracy and clinical utility; these include lymphocyte transformation tests, quantitative CD57 lymphocyte assays, and urinary antigen “capture assays.”
Recognizing and treating acute tickborne infections is crucial, as in distinguishing them from their mimics, which include some systemic autoimmune diseases. But we should not allow the fear of undertreatment of early infection to morph into unwarranted overtreatment of nonexistent chronic infection, just as we should not prematurely assume that ongoing symptoms of fatigue and malaise after a presumed tickborne infection are from the psychologically crippling fear of ongoing morbidity. Periodic reappraisal of the patient and his or her symptoms is warranted.
As summer is upon us, we enter the peak of tick season. Most reported cases of tickborne disease occur from April to October, and in this issue, Eickhoff and Blaylock offer a timely review of less common (non-Lyme disease) but significant tickborne infections.
In areas endemic for infection with Rickettsia rickettsii, the organism responsible for Rocky Mountain spotted fever (RMSF), physicians and many patients are keenly aware of the signs and symptoms of the disease and are quick to offer and accept empiric antibiotic (doxycycline) therapy. Empiric therapy at the first suspicion of RMSF is appropriate, as untreated infection carries a 20% death rate. Vigilance for early Lyme disease (caused by Borrelia burgdorferi) is also high in true endemic areas, likely because of public awareness and concern for various documented—and some touted but unproven—associated morbidities.
Other tickborne infections are likely underrecognized by physicians who are not experts in infectious disease, and thus are not treated early. There are many reasons for this, including the relative infrequency of severe disease, the nonspecific clinical signs of early infection, and the spreading of infections to geographic areas where they are traditionally not considered endemic.
Additional features likely contribute to delayed diagnosis. Surveys of patients with documented RMSF or Lyme disease show that a large proportion of infections occur in patients who have no history of camping or hiking. Most people are not even aware that they have been harboring a feeding tick, as many ticks are quite small and attachment is painless. Because some ticks survive more than a year, they may stay alive in clothes and closets throughout the winter months and occasionally cause nonseasonal infections.
Geography and entomology matter; the matching of a specific tick vector to a specific disease is tight. With the slow migration of some tick species along with their nonhuman animal hosts into new territories due to temperature changes and urbanization, some diseases are appearing in areas of the country where they had not been previously diagnosed. We must be aware of these changes, and the US Centers for Disease Control and Prevention (CDC) offers useful updated infection maps on their website.
The diagnosis of acute infection is often delayed because of late consideration of the possibility of the disease. In addition, some tests are serologic and require the passage of time before a diagnostic result is obtained. But an increasing and distinct problem is the overdiagnosis and long-term treatment of some patients whose infection is undocumented, perpetuating concern over the unproven entity of chronic infection, the most prevalent being the diagnosis and treatment of “chronic Lyme disease.” Close attention must be paid to the manner of diagnosis and the specific tests used to purportedly confirm the diagnosis of infection. This has been an ongoing challenge in managing patients with chronic fatigue and malaise, a vexing and significant clinical problem without a ready solution in patients who have undergone an extensive evaluation. It is obviously tempting for patients to grasp at any “diagnostic” answer. But chronic and repeated therapy for nonexistent infection is not benign. The CDC has published lists of tests for Lyme disease in particular that are considered to have inadequately established accuracy and clinical utility; these include lymphocyte transformation tests, quantitative CD57 lymphocyte assays, and urinary antigen “capture assays.”
Recognizing and treating acute tickborne infections is crucial, as in distinguishing them from their mimics, which include some systemic autoimmune diseases. But we should not allow the fear of undertreatment of early infection to morph into unwarranted overtreatment of nonexistent chronic infection, just as we should not prematurely assume that ongoing symptoms of fatigue and malaise after a presumed tickborne infection are from the psychologically crippling fear of ongoing morbidity. Periodic reappraisal of the patient and his or her symptoms is warranted.
Glutamate’s exciting roles in body, brain, and mind: A fertile future pharmacotherapy target
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
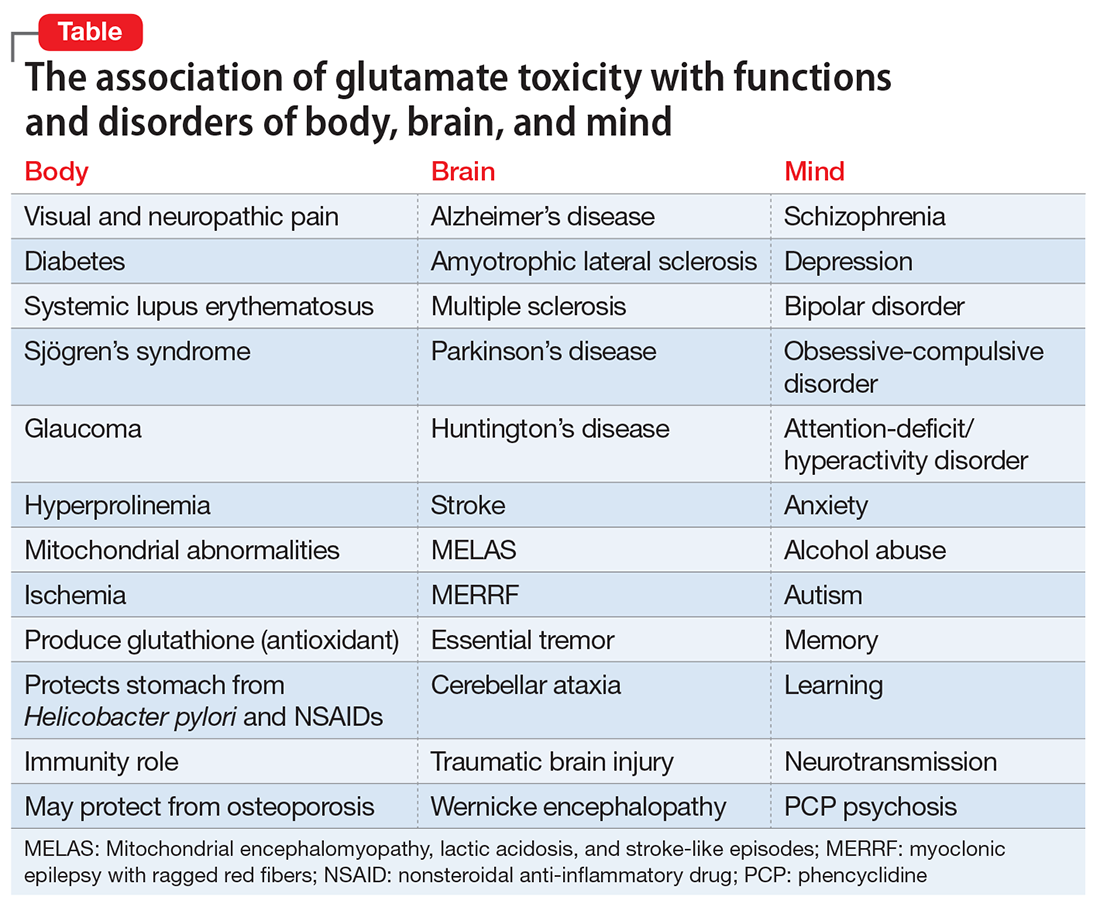
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.
GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
Improving cancer care through modern portfolio theory
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
Endo hubris
I began to write this opinion piece on the red-eye flying back from the outstanding Vascular Annual Meeting, recently held in San Diego. I attended breakfast sessions at 6 a.m., as well as presentations and meetings going on well into the evening. I now have bloodshot eyes, tachycardia, and a heightened perception of things.
It is possible that I am just sleep deprived or maybe it’s a result of inhaling the ubiquitous “medical” marijuana vapors that constantly wafted outside the convention center. Whatever the reason, I have returned in a feverish state of excitement that accompanies a sudden significant insight ... that some of us are under the influence, not of some hallucinogenic, but rather an endovascular- induced euphoria whose spell has made us forget that we are still surgeons.
The exhibition hall had the few customary surgical equipment companies with their surgical loupes, vascular clamps, and needle holders. But it was the expansive displays of every type of endovascular tool, guidewires, catheters, x-ray equipment, endografts, and stents, that attracted the crowds. Not a single vascular ultrasound company showed off their duplex scanners despite the fact that vascular surgeons advanced the use of these instruments and made them as omnipresent as they now are in clinical practice. These companies now find it more profitable to show off their wares at cardiology and radiology meetings.
In the lecture hall, audiences packed in to hear the latest method to treat aortic aneurysms with every variety of endovascular device, but there was not a single presentation on open surgical alternatives. Further, an excellent vascular surgery resident was asked how he would handle a pararenal aneurysm in community practice. He admitted that, unless he could be assisted by an experienced surgeon, he would be uncomfortable performing an open surgical repair. Thus, he would be forced to perform an endovascular treatment even if it would not be the best alternative for the patient. But in the future, will there actually be accomplished surgeons who can assist the newly minted vascular surgeon with open procedures? At a breakfast session dedicated to the aging vascular surgeon, we learned that many will soon be retiring since 46% of the Society are now over the age of 50, and 30% are more than 60 years of age.
Where am I going with this? It is apparent my aphorism that a vascular surgeon can “Operate, Medicate, and Dilate” may no longer hold. Perhaps it should now be “Stent, Ablate, Dilate, and Dilate Again.” Future generations of vascular “surgeons” may be no more than cardiologists who don’t treat the heart, able to perform endovascular revascularizations but unable to perform open operations.
Training paradigms may be affected by middle-aged attending surgeons attempting to prove that they are current by adopting endo-treatments rather than open surgery. Some academic surgeons may see the need to experiment with new endo-techniques to advance their careers. Some may see profit patenting new devices. Some may just be enthralled by what Bruce Brenner refers to as “Endo hubris.” Irrespective, dwindling open procedures will inevitably result in inexperienced open surgeons who will feel comfortable only in an endovascular suite.
Payment inequities that undervalue open procedures and overvalue endovascular ones will promote a preference for endovascular alternatives, many of which are less physically demanding than open surgical procedures and bypasses. Thus, the preference for endovascular procedures will be perpetuated.
Concurrently, our cardiology colleagues will be honing their endovascular skills not only in the coronary arteries but also on the periphery. Soon they will claim superiority. Hospital administrators, already under the spell of Cardiology, will notice the declining profit generated by poorly performed open surgery and resultant prolonged hospitalizations. They will then continue to relegate vascular surgeons to even lesser roles than they now have in so-called Heart and Vascular Centers.
As our open skills deteriorate, patients will be forced to travel to the few centers where some exhausted elderly surgeons will perform the occasional open aortic procedure, distal bypass, or, heaven forbid, some ancient procedure referred to as a carotid endarterectomy.
Of course, some may argue that it is time that endovascular procedures replace “barbaric” or disfiguring open procedures. In fact, this may already be the case for the treatment of thoracic aortic pathology. However, I propose that the time for abandoning surgery has not arrived. There are now sufficient data to show that many endo-procedures are no panacea nor are they without significant limitations. Furthermore, if we abandon open surgery we essentially abandon our raison d’etre. What will then distinguish us from interventional radiologists and cardiologists?
I propose it is time for us to embrace our surgical prowess rather than to treat open procedures like Cinderella’s ugly sisters. I would encourage program directors not to stretch indications for use to justify endovascular treatments but rather adopt standard open procedures. If the volume of indicated open procedures is insufficient, then arrange for the trainee to visit another institution.
We must educate referring physicians and hospital administrators about the benefits of well performed open surgery, and we must make sure that such procedures are, in fact, successfully and expeditiously executed. Non-surgeons also need to be informed about some of the drawbacks of endovascular procedures so that they gain a realistic understanding of the pros and cons.
This does not mean being disparaging, but they should be educated about the occurrence and significance of endoleaks, stent fractures, early recurrence, and other complications. Thus informed, they will better understand the rationale for choosing a vascular surgeon to take care of their patients. They will realize that we remain the only specialists devoted to the understanding and treatment of vascular pathology and consequently able to perform the most appropriate and best treatment for that patient.
I am now fully awake. I am convinced that my evaluation of the events at the meeting were not some sleep-deprived or hallucinogenic aberration. I sincerely believe that it is time for drastic adjustments in our thinking about endovascular and open procedures. If not, vascular surgeons and our specialty may become irrelevant and the repercussions for our survival and that of our patients may be devastating. ■
Russell Samson, MD, a physician in the practice of Sarasota Vascular Specialists, Sarasota, Fl., and a clinical professor of surgery, Florida State University, Tallahassee. He is the medical editor of Vascular Specialist.
I began to write this opinion piece on the red-eye flying back from the outstanding Vascular Annual Meeting, recently held in San Diego. I attended breakfast sessions at 6 a.m., as well as presentations and meetings going on well into the evening. I now have bloodshot eyes, tachycardia, and a heightened perception of things.
It is possible that I am just sleep deprived or maybe it’s a result of inhaling the ubiquitous “medical” marijuana vapors that constantly wafted outside the convention center. Whatever the reason, I have returned in a feverish state of excitement that accompanies a sudden significant insight ... that some of us are under the influence, not of some hallucinogenic, but rather an endovascular- induced euphoria whose spell has made us forget that we are still surgeons.
The exhibition hall had the few customary surgical equipment companies with their surgical loupes, vascular clamps, and needle holders. But it was the expansive displays of every type of endovascular tool, guidewires, catheters, x-ray equipment, endografts, and stents, that attracted the crowds. Not a single vascular ultrasound company showed off their duplex scanners despite the fact that vascular surgeons advanced the use of these instruments and made them as omnipresent as they now are in clinical practice. These companies now find it more profitable to show off their wares at cardiology and radiology meetings.
In the lecture hall, audiences packed in to hear the latest method to treat aortic aneurysms with every variety of endovascular device, but there was not a single presentation on open surgical alternatives. Further, an excellent vascular surgery resident was asked how he would handle a pararenal aneurysm in community practice. He admitted that, unless he could be assisted by an experienced surgeon, he would be uncomfortable performing an open surgical repair. Thus, he would be forced to perform an endovascular treatment even if it would not be the best alternative for the patient. But in the future, will there actually be accomplished surgeons who can assist the newly minted vascular surgeon with open procedures? At a breakfast session dedicated to the aging vascular surgeon, we learned that many will soon be retiring since 46% of the Society are now over the age of 50, and 30% are more than 60 years of age.
Where am I going with this? It is apparent my aphorism that a vascular surgeon can “Operate, Medicate, and Dilate” may no longer hold. Perhaps it should now be “Stent, Ablate, Dilate, and Dilate Again.” Future generations of vascular “surgeons” may be no more than cardiologists who don’t treat the heart, able to perform endovascular revascularizations but unable to perform open operations.
Training paradigms may be affected by middle-aged attending surgeons attempting to prove that they are current by adopting endo-treatments rather than open surgery. Some academic surgeons may see the need to experiment with new endo-techniques to advance their careers. Some may see profit patenting new devices. Some may just be enthralled by what Bruce Brenner refers to as “Endo hubris.” Irrespective, dwindling open procedures will inevitably result in inexperienced open surgeons who will feel comfortable only in an endovascular suite.
Payment inequities that undervalue open procedures and overvalue endovascular ones will promote a preference for endovascular alternatives, many of which are less physically demanding than open surgical procedures and bypasses. Thus, the preference for endovascular procedures will be perpetuated.
Concurrently, our cardiology colleagues will be honing their endovascular skills not only in the coronary arteries but also on the periphery. Soon they will claim superiority. Hospital administrators, already under the spell of Cardiology, will notice the declining profit generated by poorly performed open surgery and resultant prolonged hospitalizations. They will then continue to relegate vascular surgeons to even lesser roles than they now have in so-called Heart and Vascular Centers.
As our open skills deteriorate, patients will be forced to travel to the few centers where some exhausted elderly surgeons will perform the occasional open aortic procedure, distal bypass, or, heaven forbid, some ancient procedure referred to as a carotid endarterectomy.
Of course, some may argue that it is time that endovascular procedures replace “barbaric” or disfiguring open procedures. In fact, this may already be the case for the treatment of thoracic aortic pathology. However, I propose that the time for abandoning surgery has not arrived. There are now sufficient data to show that many endo-procedures are no panacea nor are they without significant limitations. Furthermore, if we abandon open surgery we essentially abandon our raison d’etre. What will then distinguish us from interventional radiologists and cardiologists?
I propose it is time for us to embrace our surgical prowess rather than to treat open procedures like Cinderella’s ugly sisters. I would encourage program directors not to stretch indications for use to justify endovascular treatments but rather adopt standard open procedures. If the volume of indicated open procedures is insufficient, then arrange for the trainee to visit another institution.
We must educate referring physicians and hospital administrators about the benefits of well performed open surgery, and we must make sure that such procedures are, in fact, successfully and expeditiously executed. Non-surgeons also need to be informed about some of the drawbacks of endovascular procedures so that they gain a realistic understanding of the pros and cons.
This does not mean being disparaging, but they should be educated about the occurrence and significance of endoleaks, stent fractures, early recurrence, and other complications. Thus informed, they will better understand the rationale for choosing a vascular surgeon to take care of their patients. They will realize that we remain the only specialists devoted to the understanding and treatment of vascular pathology and consequently able to perform the most appropriate and best treatment for that patient.
I am now fully awake. I am convinced that my evaluation of the events at the meeting were not some sleep-deprived or hallucinogenic aberration. I sincerely believe that it is time for drastic adjustments in our thinking about endovascular and open procedures. If not, vascular surgeons and our specialty may become irrelevant and the repercussions for our survival and that of our patients may be devastating. ■
Russell Samson, MD, a physician in the practice of Sarasota Vascular Specialists, Sarasota, Fl., and a clinical professor of surgery, Florida State University, Tallahassee. He is the medical editor of Vascular Specialist.
I began to write this opinion piece on the red-eye flying back from the outstanding Vascular Annual Meeting, recently held in San Diego. I attended breakfast sessions at 6 a.m., as well as presentations and meetings going on well into the evening. I now have bloodshot eyes, tachycardia, and a heightened perception of things.
It is possible that I am just sleep deprived or maybe it’s a result of inhaling the ubiquitous “medical” marijuana vapors that constantly wafted outside the convention center. Whatever the reason, I have returned in a feverish state of excitement that accompanies a sudden significant insight ... that some of us are under the influence, not of some hallucinogenic, but rather an endovascular- induced euphoria whose spell has made us forget that we are still surgeons.
The exhibition hall had the few customary surgical equipment companies with their surgical loupes, vascular clamps, and needle holders. But it was the expansive displays of every type of endovascular tool, guidewires, catheters, x-ray equipment, endografts, and stents, that attracted the crowds. Not a single vascular ultrasound company showed off their duplex scanners despite the fact that vascular surgeons advanced the use of these instruments and made them as omnipresent as they now are in clinical practice. These companies now find it more profitable to show off their wares at cardiology and radiology meetings.
In the lecture hall, audiences packed in to hear the latest method to treat aortic aneurysms with every variety of endovascular device, but there was not a single presentation on open surgical alternatives. Further, an excellent vascular surgery resident was asked how he would handle a pararenal aneurysm in community practice. He admitted that, unless he could be assisted by an experienced surgeon, he would be uncomfortable performing an open surgical repair. Thus, he would be forced to perform an endovascular treatment even if it would not be the best alternative for the patient. But in the future, will there actually be accomplished surgeons who can assist the newly minted vascular surgeon with open procedures? At a breakfast session dedicated to the aging vascular surgeon, we learned that many will soon be retiring since 46% of the Society are now over the age of 50, and 30% are more than 60 years of age.
Where am I going with this? It is apparent my aphorism that a vascular surgeon can “Operate, Medicate, and Dilate” may no longer hold. Perhaps it should now be “Stent, Ablate, Dilate, and Dilate Again.” Future generations of vascular “surgeons” may be no more than cardiologists who don’t treat the heart, able to perform endovascular revascularizations but unable to perform open operations.
Training paradigms may be affected by middle-aged attending surgeons attempting to prove that they are current by adopting endo-treatments rather than open surgery. Some academic surgeons may see the need to experiment with new endo-techniques to advance their careers. Some may see profit patenting new devices. Some may just be enthralled by what Bruce Brenner refers to as “Endo hubris.” Irrespective, dwindling open procedures will inevitably result in inexperienced open surgeons who will feel comfortable only in an endovascular suite.
Payment inequities that undervalue open procedures and overvalue endovascular ones will promote a preference for endovascular alternatives, many of which are less physically demanding than open surgical procedures and bypasses. Thus, the preference for endovascular procedures will be perpetuated.
Concurrently, our cardiology colleagues will be honing their endovascular skills not only in the coronary arteries but also on the periphery. Soon they will claim superiority. Hospital administrators, already under the spell of Cardiology, will notice the declining profit generated by poorly performed open surgery and resultant prolonged hospitalizations. They will then continue to relegate vascular surgeons to even lesser roles than they now have in so-called Heart and Vascular Centers.
As our open skills deteriorate, patients will be forced to travel to the few centers where some exhausted elderly surgeons will perform the occasional open aortic procedure, distal bypass, or, heaven forbid, some ancient procedure referred to as a carotid endarterectomy.
Of course, some may argue that it is time that endovascular procedures replace “barbaric” or disfiguring open procedures. In fact, this may already be the case for the treatment of thoracic aortic pathology. However, I propose that the time for abandoning surgery has not arrived. There are now sufficient data to show that many endo-procedures are no panacea nor are they without significant limitations. Furthermore, if we abandon open surgery we essentially abandon our raison d’etre. What will then distinguish us from interventional radiologists and cardiologists?
I propose it is time for us to embrace our surgical prowess rather than to treat open procedures like Cinderella’s ugly sisters. I would encourage program directors not to stretch indications for use to justify endovascular treatments but rather adopt standard open procedures. If the volume of indicated open procedures is insufficient, then arrange for the trainee to visit another institution.
We must educate referring physicians and hospital administrators about the benefits of well performed open surgery, and we must make sure that such procedures are, in fact, successfully and expeditiously executed. Non-surgeons also need to be informed about some of the drawbacks of endovascular procedures so that they gain a realistic understanding of the pros and cons.
This does not mean being disparaging, but they should be educated about the occurrence and significance of endoleaks, stent fractures, early recurrence, and other complications. Thus informed, they will better understand the rationale for choosing a vascular surgeon to take care of their patients. They will realize that we remain the only specialists devoted to the understanding and treatment of vascular pathology and consequently able to perform the most appropriate and best treatment for that patient.
I am now fully awake. I am convinced that my evaluation of the events at the meeting were not some sleep-deprived or hallucinogenic aberration. I sincerely believe that it is time for drastic adjustments in our thinking about endovascular and open procedures. If not, vascular surgeons and our specialty may become irrelevant and the repercussions for our survival and that of our patients may be devastating. ■
Russell Samson, MD, a physician in the practice of Sarasota Vascular Specialists, Sarasota, Fl., and a clinical professor of surgery, Florida State University, Tallahassee. He is the medical editor of Vascular Specialist.
Acute cholecystitis: Not always routine
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
Fighting the reflux reflex
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.