User login
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
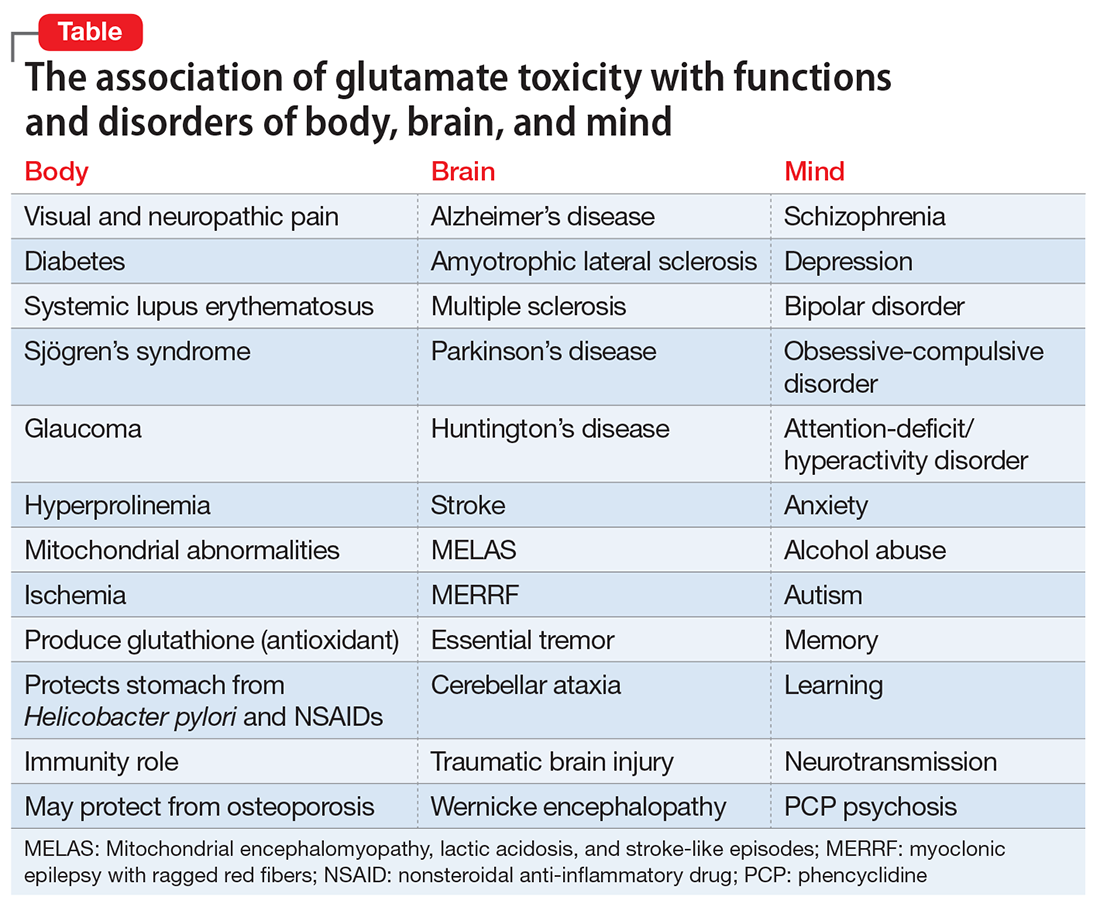
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.
GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
