User login
Gods and Monsters
For the first time in history, four generations of physicians work side by side in the U.S. health care system. An expanding population, longer life expectancies, and later retirement ages all contribute to this phenomenon. Each of these generations has made significant contributions to modern surgery and how we practice it. For better and for worse.
Traditionalists, or the Greatest Generation, were true surgical pioneers. DeBakey, Cooley, Fogarty, their names now adorn everything from instruments to medical centers. They truly founded the modern system of surgery. Born between 1900 and 1945, Traditionalists were forged in the crucibles of the Great War and the Great Depression. Their core values were hard work, discipline, and sacrifice. A large number were combat veterans who valued conformity and adherence to the rules. Traditionalists set up our current hierarchical departments of surgery. Mirroring their values, they employed a military chain of command approach. Many traditionalists rose to positions of absolute power, and some were corrupted by this power. Gods became monsters. Abuse, both verbal and physical, came to be commonplace and accepted in the surgical work environment.
Born between 1946 and 1964, Baby Boomers were raised in the aftermath of a war none of them saw. More optimistic and idealistic than the Traditionalists, the Boomers valued success. Their goals became more individualistic. Chasing money, titles, and recognition, Boomers wanted to build a stellar career. Fifty-hour work weeks became 70, 80, or 90. Ambition led to wealth, dramatic successes, and remarkable careers. Their choices also led to divorce, drug abuse, and suicide. While burnout has become a modern concern, its roots are clearly tied to this era. Now serving as our deans and department chairs, the Boomers also made several notable contributions. Specific to our field, Boomers oversaw the development of vascular surgery as an independent specialty and the expansion of fellowship training programs. Coming of age in the 1960s, Boomers also led the integration of our field with the acceptance of both minorities and women.
When I first heard the term “Generation X” I thought “Dumb name, won’t last.” Not my best prediction. Born between 1965 and 1980, Generation X grew up during the home computer revolution. Quick to adopt new technologies, Gen Xers were far more adaptive to change than previous generations. Labeled as having short attention spans, most Gen Xers were task/goal oriented. While these attributes helped drive the endovascular revolution, they also may be the reason we have approximately 983 FDA-approved devices to treat SFA disease. Generation X entered surgical training eager to please the more senior Traditionalists and Boomers. This wouldn’t last. Children of divorce and latch-key kids, Generation Xers are eclectic, resourceful, and self-reliant. Most of all they value freedom. Watching their predecessors work themselves and others to near death, Generation X revolted. Uncapped duty hours, limitless call, and pyramidal residencies were all institutions in the 1980s, and they all fell. Generation X were portrayed as nihilistic slackers, but their true motivation was often distrust of institutions. Watching the Boomers descend into burnout, Xers tried to achieve a more reasonable work-life balance. Though they successfully fought for lessening the abuses of surgical training, few Gen Xers actually reaped the benefits. I vividly recall watching slack-jawed as an intern scrubbed out of a case to go home because he was post call. A Martian landing in the OR and offering to assist with the anastomosis would have brought no less amazement.
With their careers spanning the endovascular revolution, Generation X has seen perhaps the greatest era of transformation in our profession. Our competition is no longer general or cardiac surgery, but rather interventional radiology and interventional cardiology. Gen X is also the first generation to earn less than its predecessors. Throw in their obscene tuition payments and one can see how Gen Xers fell well short of the financial heights of the Traditionalists and Boomers. The Gen Xers are the masters of the work hard/play hard ethos. You will see them at VEITH entertaining their European colleagues at 3 a.m. and then running the 6 a.m. breakfast sessions. While the Boomers often seemed old by 40, Xers appear desperate to salvage their lost youth.
Born between 1981 and 2006, Millennials are already the most populous generation. Their chief attributes are confidence, sociability, and a realistic outlook. Knowing they can’t please everyone, they rarely try. They want work to be meaningful in and of itself. They also value teamwork over individual approaches. Millennials are civic minded and have a strong sense of volunteerism. Their parents often tried to shelter them from the evils of the world, and they were the first generation of children with schedules. Because of their upbringing, Millennials are far more likely to seek guidance than the independent-minded Gen Xers. Raised to believe their voice mattered, they are now often reviled for it. It is with some degree of awe that I watch our Millennial students brazenly march into the dean’s and chancellor’s office to discuss their “careers.” As a medical student I first saw my dean at graduation, and I certainly didn’t even know what a chancellor was. Generation X is often baffled by the self-interest Millennials exude. But we shouldn’t be, we have seen it before. Raised by Baby Boomers (The Me Generation), Millennials inherited their self-driven outlook. This is also the reason Boomers and Millennials struggle to work together. They are too alike. Boomers see Millennials as “snowflakes” who are scared of work and selfie obsessed. Millennials bristle at the authoritarian nature of Boomers.
For vascular surgery to advance as a field, we need to recruit, train, and mentor this new generation. If only there was some guide: “The Proper Care and Feeding of Millennials." As senior attendings, program directors, and section chiefs, Generation X must now serve as a bridge between two larger forces, the Boomers and their offspring, the Millennials. Of course, whatever generation you are from is the best, but we must confront our biases. It is easy to seek out the same personalities to be your trainees and partners. Don’t. This pool will shrink every year. Millennials are more self-aware of their capabilities and therefore of their limitations. We may become flustered by their need for hand-holding, but what if it is appropriate? Was all of the autonomy you were granted during training truly good for the patients? Graduated responsibility and roles that push their limits help Millennials grow. I know they don’t value punctuality or dress codes, but they are better team players and openly motivated by learning. I formed our integrated vascular residency with two positions per year specifically to foster the team building Millennials crave. Yes, this is the generation that got 8th-place trophies so you must constantly award progress. Fortunately, now that surgery is unencumbered by such things as massive salaries and status, Millennials enter our workforce with purer intentions.
We may want to make Millennials match our values, traits, and behaviors, but each generation has departed radically from the ethos of their predecessors. Let’s see what the kids can do.
Dr. Sheahan is a professor of surgery and Program Director, Vascular Surgery Residency and Fellowship Programs, Louisiana State University Health Sciences Center, School of Medicine, New Orleans. He is also the Deputy Medical Editor of Vascular Specialist.
For the first time in history, four generations of physicians work side by side in the U.S. health care system. An expanding population, longer life expectancies, and later retirement ages all contribute to this phenomenon. Each of these generations has made significant contributions to modern surgery and how we practice it. For better and for worse.
Traditionalists, or the Greatest Generation, were true surgical pioneers. DeBakey, Cooley, Fogarty, their names now adorn everything from instruments to medical centers. They truly founded the modern system of surgery. Born between 1900 and 1945, Traditionalists were forged in the crucibles of the Great War and the Great Depression. Their core values were hard work, discipline, and sacrifice. A large number were combat veterans who valued conformity and adherence to the rules. Traditionalists set up our current hierarchical departments of surgery. Mirroring their values, they employed a military chain of command approach. Many traditionalists rose to positions of absolute power, and some were corrupted by this power. Gods became monsters. Abuse, both verbal and physical, came to be commonplace and accepted in the surgical work environment.
Born between 1946 and 1964, Baby Boomers were raised in the aftermath of a war none of them saw. More optimistic and idealistic than the Traditionalists, the Boomers valued success. Their goals became more individualistic. Chasing money, titles, and recognition, Boomers wanted to build a stellar career. Fifty-hour work weeks became 70, 80, or 90. Ambition led to wealth, dramatic successes, and remarkable careers. Their choices also led to divorce, drug abuse, and suicide. While burnout has become a modern concern, its roots are clearly tied to this era. Now serving as our deans and department chairs, the Boomers also made several notable contributions. Specific to our field, Boomers oversaw the development of vascular surgery as an independent specialty and the expansion of fellowship training programs. Coming of age in the 1960s, Boomers also led the integration of our field with the acceptance of both minorities and women.
When I first heard the term “Generation X” I thought “Dumb name, won’t last.” Not my best prediction. Born between 1965 and 1980, Generation X grew up during the home computer revolution. Quick to adopt new technologies, Gen Xers were far more adaptive to change than previous generations. Labeled as having short attention spans, most Gen Xers were task/goal oriented. While these attributes helped drive the endovascular revolution, they also may be the reason we have approximately 983 FDA-approved devices to treat SFA disease. Generation X entered surgical training eager to please the more senior Traditionalists and Boomers. This wouldn’t last. Children of divorce and latch-key kids, Generation Xers are eclectic, resourceful, and self-reliant. Most of all they value freedom. Watching their predecessors work themselves and others to near death, Generation X revolted. Uncapped duty hours, limitless call, and pyramidal residencies were all institutions in the 1980s, and they all fell. Generation X were portrayed as nihilistic slackers, but their true motivation was often distrust of institutions. Watching the Boomers descend into burnout, Xers tried to achieve a more reasonable work-life balance. Though they successfully fought for lessening the abuses of surgical training, few Gen Xers actually reaped the benefits. I vividly recall watching slack-jawed as an intern scrubbed out of a case to go home because he was post call. A Martian landing in the OR and offering to assist with the anastomosis would have brought no less amazement.
With their careers spanning the endovascular revolution, Generation X has seen perhaps the greatest era of transformation in our profession. Our competition is no longer general or cardiac surgery, but rather interventional radiology and interventional cardiology. Gen X is also the first generation to earn less than its predecessors. Throw in their obscene tuition payments and one can see how Gen Xers fell well short of the financial heights of the Traditionalists and Boomers. The Gen Xers are the masters of the work hard/play hard ethos. You will see them at VEITH entertaining their European colleagues at 3 a.m. and then running the 6 a.m. breakfast sessions. While the Boomers often seemed old by 40, Xers appear desperate to salvage their lost youth.
Born between 1981 and 2006, Millennials are already the most populous generation. Their chief attributes are confidence, sociability, and a realistic outlook. Knowing they can’t please everyone, they rarely try. They want work to be meaningful in and of itself. They also value teamwork over individual approaches. Millennials are civic minded and have a strong sense of volunteerism. Their parents often tried to shelter them from the evils of the world, and they were the first generation of children with schedules. Because of their upbringing, Millennials are far more likely to seek guidance than the independent-minded Gen Xers. Raised to believe their voice mattered, they are now often reviled for it. It is with some degree of awe that I watch our Millennial students brazenly march into the dean’s and chancellor’s office to discuss their “careers.” As a medical student I first saw my dean at graduation, and I certainly didn’t even know what a chancellor was. Generation X is often baffled by the self-interest Millennials exude. But we shouldn’t be, we have seen it before. Raised by Baby Boomers (The Me Generation), Millennials inherited their self-driven outlook. This is also the reason Boomers and Millennials struggle to work together. They are too alike. Boomers see Millennials as “snowflakes” who are scared of work and selfie obsessed. Millennials bristle at the authoritarian nature of Boomers.
For vascular surgery to advance as a field, we need to recruit, train, and mentor this new generation. If only there was some guide: “The Proper Care and Feeding of Millennials." As senior attendings, program directors, and section chiefs, Generation X must now serve as a bridge between two larger forces, the Boomers and their offspring, the Millennials. Of course, whatever generation you are from is the best, but we must confront our biases. It is easy to seek out the same personalities to be your trainees and partners. Don’t. This pool will shrink every year. Millennials are more self-aware of their capabilities and therefore of their limitations. We may become flustered by their need for hand-holding, but what if it is appropriate? Was all of the autonomy you were granted during training truly good for the patients? Graduated responsibility and roles that push their limits help Millennials grow. I know they don’t value punctuality or dress codes, but they are better team players and openly motivated by learning. I formed our integrated vascular residency with two positions per year specifically to foster the team building Millennials crave. Yes, this is the generation that got 8th-place trophies so you must constantly award progress. Fortunately, now that surgery is unencumbered by such things as massive salaries and status, Millennials enter our workforce with purer intentions.
We may want to make Millennials match our values, traits, and behaviors, but each generation has departed radically from the ethos of their predecessors. Let’s see what the kids can do.
Dr. Sheahan is a professor of surgery and Program Director, Vascular Surgery Residency and Fellowship Programs, Louisiana State University Health Sciences Center, School of Medicine, New Orleans. He is also the Deputy Medical Editor of Vascular Specialist.
For the first time in history, four generations of physicians work side by side in the U.S. health care system. An expanding population, longer life expectancies, and later retirement ages all contribute to this phenomenon. Each of these generations has made significant contributions to modern surgery and how we practice it. For better and for worse.
Traditionalists, or the Greatest Generation, were true surgical pioneers. DeBakey, Cooley, Fogarty, their names now adorn everything from instruments to medical centers. They truly founded the modern system of surgery. Born between 1900 and 1945, Traditionalists were forged in the crucibles of the Great War and the Great Depression. Their core values were hard work, discipline, and sacrifice. A large number were combat veterans who valued conformity and adherence to the rules. Traditionalists set up our current hierarchical departments of surgery. Mirroring their values, they employed a military chain of command approach. Many traditionalists rose to positions of absolute power, and some were corrupted by this power. Gods became monsters. Abuse, both verbal and physical, came to be commonplace and accepted in the surgical work environment.
Born between 1946 and 1964, Baby Boomers were raised in the aftermath of a war none of them saw. More optimistic and idealistic than the Traditionalists, the Boomers valued success. Their goals became more individualistic. Chasing money, titles, and recognition, Boomers wanted to build a stellar career. Fifty-hour work weeks became 70, 80, or 90. Ambition led to wealth, dramatic successes, and remarkable careers. Their choices also led to divorce, drug abuse, and suicide. While burnout has become a modern concern, its roots are clearly tied to this era. Now serving as our deans and department chairs, the Boomers also made several notable contributions. Specific to our field, Boomers oversaw the development of vascular surgery as an independent specialty and the expansion of fellowship training programs. Coming of age in the 1960s, Boomers also led the integration of our field with the acceptance of both minorities and women.
When I first heard the term “Generation X” I thought “Dumb name, won’t last.” Not my best prediction. Born between 1965 and 1980, Generation X grew up during the home computer revolution. Quick to adopt new technologies, Gen Xers were far more adaptive to change than previous generations. Labeled as having short attention spans, most Gen Xers were task/goal oriented. While these attributes helped drive the endovascular revolution, they also may be the reason we have approximately 983 FDA-approved devices to treat SFA disease. Generation X entered surgical training eager to please the more senior Traditionalists and Boomers. This wouldn’t last. Children of divorce and latch-key kids, Generation Xers are eclectic, resourceful, and self-reliant. Most of all they value freedom. Watching their predecessors work themselves and others to near death, Generation X revolted. Uncapped duty hours, limitless call, and pyramidal residencies were all institutions in the 1980s, and they all fell. Generation X were portrayed as nihilistic slackers, but their true motivation was often distrust of institutions. Watching the Boomers descend into burnout, Xers tried to achieve a more reasonable work-life balance. Though they successfully fought for lessening the abuses of surgical training, few Gen Xers actually reaped the benefits. I vividly recall watching slack-jawed as an intern scrubbed out of a case to go home because he was post call. A Martian landing in the OR and offering to assist with the anastomosis would have brought no less amazement.
With their careers spanning the endovascular revolution, Generation X has seen perhaps the greatest era of transformation in our profession. Our competition is no longer general or cardiac surgery, but rather interventional radiology and interventional cardiology. Gen X is also the first generation to earn less than its predecessors. Throw in their obscene tuition payments and one can see how Gen Xers fell well short of the financial heights of the Traditionalists and Boomers. The Gen Xers are the masters of the work hard/play hard ethos. You will see them at VEITH entertaining their European colleagues at 3 a.m. and then running the 6 a.m. breakfast sessions. While the Boomers often seemed old by 40, Xers appear desperate to salvage their lost youth.
Born between 1981 and 2006, Millennials are already the most populous generation. Their chief attributes are confidence, sociability, and a realistic outlook. Knowing they can’t please everyone, they rarely try. They want work to be meaningful in and of itself. They also value teamwork over individual approaches. Millennials are civic minded and have a strong sense of volunteerism. Their parents often tried to shelter them from the evils of the world, and they were the first generation of children with schedules. Because of their upbringing, Millennials are far more likely to seek guidance than the independent-minded Gen Xers. Raised to believe their voice mattered, they are now often reviled for it. It is with some degree of awe that I watch our Millennial students brazenly march into the dean’s and chancellor’s office to discuss their “careers.” As a medical student I first saw my dean at graduation, and I certainly didn’t even know what a chancellor was. Generation X is often baffled by the self-interest Millennials exude. But we shouldn’t be, we have seen it before. Raised by Baby Boomers (The Me Generation), Millennials inherited their self-driven outlook. This is also the reason Boomers and Millennials struggle to work together. They are too alike. Boomers see Millennials as “snowflakes” who are scared of work and selfie obsessed. Millennials bristle at the authoritarian nature of Boomers.
For vascular surgery to advance as a field, we need to recruit, train, and mentor this new generation. If only there was some guide: “The Proper Care and Feeding of Millennials." As senior attendings, program directors, and section chiefs, Generation X must now serve as a bridge between two larger forces, the Boomers and their offspring, the Millennials. Of course, whatever generation you are from is the best, but we must confront our biases. It is easy to seek out the same personalities to be your trainees and partners. Don’t. This pool will shrink every year. Millennials are more self-aware of their capabilities and therefore of their limitations. We may become flustered by their need for hand-holding, but what if it is appropriate? Was all of the autonomy you were granted during training truly good for the patients? Graduated responsibility and roles that push their limits help Millennials grow. I know they don’t value punctuality or dress codes, but they are better team players and openly motivated by learning. I formed our integrated vascular residency with two positions per year specifically to foster the team building Millennials crave. Yes, this is the generation that got 8th-place trophies so you must constantly award progress. Fortunately, now that surgery is unencumbered by such things as massive salaries and status, Millennials enter our workforce with purer intentions.
We may want to make Millennials match our values, traits, and behaviors, but each generation has departed radically from the ethos of their predecessors. Let’s see what the kids can do.
Dr. Sheahan is a professor of surgery and Program Director, Vascular Surgery Residency and Fellowship Programs, Louisiana State University Health Sciences Center, School of Medicine, New Orleans. He is also the Deputy Medical Editor of Vascular Specialist.
From the Editors: Hanging up the scalpel
The decision to stop practicing surgery is a monumental one when you have been a surgeon for almost 40 years, have loved operating, and have defined yourself by the word “surgeon.”
The decision to cease operating should at best be a personal one that the surgeon makes, rather than one imposed by others. The “others” could be an institutional policy mandating retirement at a given age, the results of a series of psychomotor examinations, or even a kind department chair’s suggestion that you should stop operating because your complications have increased and it is in your patients’ best interests. As we approach “a certain age,” I suspect that most surgeons would prefer to decide their own fate and, especially, to avoid the last of the three above options.
Literature is emerging about the aging physician and how best the decisions should be made about ceasing practice. A recent such article published online by some dear and respected colleagues (JAMA Surg. 2017 July 19;doi:10.1001/jamasurg.2017.2342) proposes that institutions and professional organizations develop policies to address the aging physician that leave “flexibility to customize the approach” lest regulators and legislators impose “more draconian measures.” Their suggestions include mandatory cognitive evaluation, voluntary annual physical examinations, and confidential peer evaluations of wellness and competence as physicians reach a certain (unspecified) age.
I most certainly concur with the authors’ well-reasoned arguments. As they relate, only a handful of institutions to date have developed policies that require assessments of physician wellness and competence at a given age. Most institutions still rely on physicians’ voluntary submission to physical examinations, cognitive testing, or peer referral of a colleague if declining function is observed. Yet we all know that individuals tend to overlook signs of declining physical and cognitive function both in themselves and in colleagues. Moreover, we all know that even the most carefully designed and implemented tests have shortcomings and may fail to identify the exact nature of an individual’s malady or fail to identify a remediable issue early. And just as individuals’ physical and cognitive abilities decline at different chronological ages, problems with burnout, mental illness, and substance abuse have no reliable age threshold and may be difficult to diagnose accurately.
Whatever the age of the individual, it is critical that a decline in function of a practitioner be addressed promptly and effectively, for the benefit of the affected individual, his or her patients, and the institution. It is therefore most appropriate for every institution to develop a firm policy to deal with concerns of competency of all staff members, regardless of age.
It is also appropriate for peers to pay attention to a colleague’s stumbles and have the courage to first initiate a dialogue directly with that person, referring the issue to an individual in authority if the direct approach fails. A culture that promotes responsible self-policing protects patients and the reputations of both the affected individual and the institution.
Most of us with “seniority” will recall situations during our training when surgeons with diminished physical or cognitive capacity continued operating well beyond their prime. In those days, it was not unusual for a chief resident to be told, “Your job is to scrub with Dr. X and keep him out of trouble.” As inappropriate as that was, we complied, all the while vowing that we would never let ourselves be in the same position when we aged.
It therefore became my habit as I aged to “listen to my body” and pay attention to evidence that my skills might be declining and perhaps it was time to hang up the scalpel. As an almost lifelong runner, I marked my athletic decline by noting an increase in minutes per mile from 7 to 14 over 40 years and wondered whether my cognitive decline might be comparable, if not so obvious. I had to admit to a bit of lost hand dexterity, less sharpness of eyesight, and slowed memory for the names of people and even of surgical instruments. Although I believed that my diagnostic acumen and decisions were unaffected, I weathered a sleepless night on call less well, requiring two or more full nights of eight hours’ sleep to recover my energy completely.
Part of the reluctance to cease surgical practice that I share with many colleagues my age is the fear of becoming irrelevant and unproductive. It was therefore critical to prepare for retirement from practice by identifying activities that I considered both meaningful and also challenging: writing and editing, teaching students and residents in surgical skills labs, teaching residents “open” surgical techniques on cadavers, advising younger colleagues when they have a challenging case in my area of expertise, and filling a myriad of needs in our department that match my skill set but that my younger counterparts are too busy to attend to.
I now also have the freedom to pursue activities for which I had little time during the years of intense practice, including service on nonprofit boards and other community activities. There may even come a day when my definition of self has fully accepted the word “retired,” even though I hope that day is many years in the future.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The decision to stop practicing surgery is a monumental one when you have been a surgeon for almost 40 years, have loved operating, and have defined yourself by the word “surgeon.”
The decision to cease operating should at best be a personal one that the surgeon makes, rather than one imposed by others. The “others” could be an institutional policy mandating retirement at a given age, the results of a series of psychomotor examinations, or even a kind department chair’s suggestion that you should stop operating because your complications have increased and it is in your patients’ best interests. As we approach “a certain age,” I suspect that most surgeons would prefer to decide their own fate and, especially, to avoid the last of the three above options.
Literature is emerging about the aging physician and how best the decisions should be made about ceasing practice. A recent such article published online by some dear and respected colleagues (JAMA Surg. 2017 July 19;doi:10.1001/jamasurg.2017.2342) proposes that institutions and professional organizations develop policies to address the aging physician that leave “flexibility to customize the approach” lest regulators and legislators impose “more draconian measures.” Their suggestions include mandatory cognitive evaluation, voluntary annual physical examinations, and confidential peer evaluations of wellness and competence as physicians reach a certain (unspecified) age.
I most certainly concur with the authors’ well-reasoned arguments. As they relate, only a handful of institutions to date have developed policies that require assessments of physician wellness and competence at a given age. Most institutions still rely on physicians’ voluntary submission to physical examinations, cognitive testing, or peer referral of a colleague if declining function is observed. Yet we all know that individuals tend to overlook signs of declining physical and cognitive function both in themselves and in colleagues. Moreover, we all know that even the most carefully designed and implemented tests have shortcomings and may fail to identify the exact nature of an individual’s malady or fail to identify a remediable issue early. And just as individuals’ physical and cognitive abilities decline at different chronological ages, problems with burnout, mental illness, and substance abuse have no reliable age threshold and may be difficult to diagnose accurately.
Whatever the age of the individual, it is critical that a decline in function of a practitioner be addressed promptly and effectively, for the benefit of the affected individual, his or her patients, and the institution. It is therefore most appropriate for every institution to develop a firm policy to deal with concerns of competency of all staff members, regardless of age.
It is also appropriate for peers to pay attention to a colleague’s stumbles and have the courage to first initiate a dialogue directly with that person, referring the issue to an individual in authority if the direct approach fails. A culture that promotes responsible self-policing protects patients and the reputations of both the affected individual and the institution.
Most of us with “seniority” will recall situations during our training when surgeons with diminished physical or cognitive capacity continued operating well beyond their prime. In those days, it was not unusual for a chief resident to be told, “Your job is to scrub with Dr. X and keep him out of trouble.” As inappropriate as that was, we complied, all the while vowing that we would never let ourselves be in the same position when we aged.
It therefore became my habit as I aged to “listen to my body” and pay attention to evidence that my skills might be declining and perhaps it was time to hang up the scalpel. As an almost lifelong runner, I marked my athletic decline by noting an increase in minutes per mile from 7 to 14 over 40 years and wondered whether my cognitive decline might be comparable, if not so obvious. I had to admit to a bit of lost hand dexterity, less sharpness of eyesight, and slowed memory for the names of people and even of surgical instruments. Although I believed that my diagnostic acumen and decisions were unaffected, I weathered a sleepless night on call less well, requiring two or more full nights of eight hours’ sleep to recover my energy completely.
Part of the reluctance to cease surgical practice that I share with many colleagues my age is the fear of becoming irrelevant and unproductive. It was therefore critical to prepare for retirement from practice by identifying activities that I considered both meaningful and also challenging: writing and editing, teaching students and residents in surgical skills labs, teaching residents “open” surgical techniques on cadavers, advising younger colleagues when they have a challenging case in my area of expertise, and filling a myriad of needs in our department that match my skill set but that my younger counterparts are too busy to attend to.
I now also have the freedom to pursue activities for which I had little time during the years of intense practice, including service on nonprofit boards and other community activities. There may even come a day when my definition of self has fully accepted the word “retired,” even though I hope that day is many years in the future.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The decision to stop practicing surgery is a monumental one when you have been a surgeon for almost 40 years, have loved operating, and have defined yourself by the word “surgeon.”
The decision to cease operating should at best be a personal one that the surgeon makes, rather than one imposed by others. The “others” could be an institutional policy mandating retirement at a given age, the results of a series of psychomotor examinations, or even a kind department chair’s suggestion that you should stop operating because your complications have increased and it is in your patients’ best interests. As we approach “a certain age,” I suspect that most surgeons would prefer to decide their own fate and, especially, to avoid the last of the three above options.
Literature is emerging about the aging physician and how best the decisions should be made about ceasing practice. A recent such article published online by some dear and respected colleagues (JAMA Surg. 2017 July 19;doi:10.1001/jamasurg.2017.2342) proposes that institutions and professional organizations develop policies to address the aging physician that leave “flexibility to customize the approach” lest regulators and legislators impose “more draconian measures.” Their suggestions include mandatory cognitive evaluation, voluntary annual physical examinations, and confidential peer evaluations of wellness and competence as physicians reach a certain (unspecified) age.
I most certainly concur with the authors’ well-reasoned arguments. As they relate, only a handful of institutions to date have developed policies that require assessments of physician wellness and competence at a given age. Most institutions still rely on physicians’ voluntary submission to physical examinations, cognitive testing, or peer referral of a colleague if declining function is observed. Yet we all know that individuals tend to overlook signs of declining physical and cognitive function both in themselves and in colleagues. Moreover, we all know that even the most carefully designed and implemented tests have shortcomings and may fail to identify the exact nature of an individual’s malady or fail to identify a remediable issue early. And just as individuals’ physical and cognitive abilities decline at different chronological ages, problems with burnout, mental illness, and substance abuse have no reliable age threshold and may be difficult to diagnose accurately.
Whatever the age of the individual, it is critical that a decline in function of a practitioner be addressed promptly and effectively, for the benefit of the affected individual, his or her patients, and the institution. It is therefore most appropriate for every institution to develop a firm policy to deal with concerns of competency of all staff members, regardless of age.
It is also appropriate for peers to pay attention to a colleague’s stumbles and have the courage to first initiate a dialogue directly with that person, referring the issue to an individual in authority if the direct approach fails. A culture that promotes responsible self-policing protects patients and the reputations of both the affected individual and the institution.
Most of us with “seniority” will recall situations during our training when surgeons with diminished physical or cognitive capacity continued operating well beyond their prime. In those days, it was not unusual for a chief resident to be told, “Your job is to scrub with Dr. X and keep him out of trouble.” As inappropriate as that was, we complied, all the while vowing that we would never let ourselves be in the same position when we aged.
It therefore became my habit as I aged to “listen to my body” and pay attention to evidence that my skills might be declining and perhaps it was time to hang up the scalpel. As an almost lifelong runner, I marked my athletic decline by noting an increase in minutes per mile from 7 to 14 over 40 years and wondered whether my cognitive decline might be comparable, if not so obvious. I had to admit to a bit of lost hand dexterity, less sharpness of eyesight, and slowed memory for the names of people and even of surgical instruments. Although I believed that my diagnostic acumen and decisions were unaffected, I weathered a sleepless night on call less well, requiring two or more full nights of eight hours’ sleep to recover my energy completely.
Part of the reluctance to cease surgical practice that I share with many colleagues my age is the fear of becoming irrelevant and unproductive. It was therefore critical to prepare for retirement from practice by identifying activities that I considered both meaningful and also challenging: writing and editing, teaching students and residents in surgical skills labs, teaching residents “open” surgical techniques on cadavers, advising younger colleagues when they have a challenging case in my area of expertise, and filling a myriad of needs in our department that match my skill set but that my younger counterparts are too busy to attend to.
I now also have the freedom to pursue activities for which I had little time during the years of intense practice, including service on nonprofit boards and other community activities. There may even come a day when my definition of self has fully accepted the word “retired,” even though I hope that day is many years in the future.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
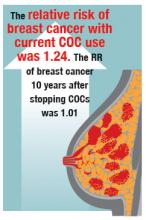
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
Watson, the game is a foot…or a palm
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
Are combination estrogen-progestin oral contraceptives associated with an increased risk of cancer?
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
Advancing clinical neuroscience literacy among psychiatric practitioners
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
Unskilled and unaware
In 1999, two psychologists, David Dunning and his student Justin Kruger, published a paper that demonstrated people who are really bad at something tend to believe that they are really good (J Pers Soc Psychol. 1999;77:1121-34). They also posited that most competent people underestimate their abilities while the rest of us overestimate them, and the worse we are, the more we overestimate our capabilities. In essence, they postulate that one needs a degree of skill in performing an activity in order to assess one's aptitude. In other words, it’s impossible to tell if you are bad at something if you’re too bad to know that you’re bad.
No, I’m not writing another diatribe about cardiologists (although this surely applies to some!). Rather this is a semi-apology to the vascular fellow who I featured in my last editorial, wherein I bemoaned that the endo-revolution resulted in some younger surgeons lacking open skills. That young man is an example of a highly competent trainee who probably underestimates his abilities to perform complex open procedures. In fact, an honest self-evaluation of my own clinical experience has made me realize that there is a corollary to newly minted vascular surgeons having limited open experience … rather, that some older surgeons, well versed in open surgery, may be inexperienced in some complex endo-procedures. The implications for the practice of vascular surgery are significant and warrant discussion. Perhaps my personal experience in learning endovascular methods will be revealing.
I performed my vascular fellowship at Montefiore with Frank Veith, MD, in 1980. At the time, Dr. Veith was a principal investigator in a multicenter, randomized trial to evaluate whether PTFE could be an acceptable substitute for saphenous vein in infra-inguinal bypass. As his fellow, I gained an enormous experience in these procedures. I stayed on in academic vascular surgery for another 6years honing my techniques in other forms of open surgery. In 1986 I moved to Sarasota, Fla., to start a private practice. Here, vascular surgery was performed by general surgeons who, although competent in the vascular procedures of that time, treated most infrapopliteal disease with an amputation. My calling card was my ability to do a distal bypass. What an anachronism! Of course, I still do a fair number of tibial bypasses, but femoropopliteal bypass is almost ready for the museum. The reason is that the tidal force of the endo-tsunami had just begun to wash up on the sunny beaches of Sarasota.
While at Montefiore I had witnessed the beginning of the endovascular wave. I realized that if I didn’t learn this new technology, I might well have become a surgical dinosaur. Accordingly, soon after arriving in Sarasota, I left town to spend a week with a pioneering radiologist who allowed me to observe his team’s early experience with aortic endografts. I left my practice a second time to visit with a very busy invasive cardiologist where I had hands-on experience with balloon angioplasty and early Palmaz stents. On my return, I cautiously started performing diagnostic arteriograms in the operating room using early C-arms. Eventually, my partner, David Showalter, MD, and I convinced the hospital to outfit a room as a semi “hybrid” suite, a fixed sliding X-ray table coupled with the most advanced C-arm of the time. Over the objections of local radiologists and cardiologists, we ultimately obtained privileges to perform our endo cases in their radiology suites and cath labs. This allowed us to expand our endovascular experience, first by improving our proficiency as diagnostic arteriographers, then by advancing our angioplasty and, ultimately, stent techniques. In the interim, however, endovascular technology had flourished with the introduction of TEVAR, FEVAR, chimneys and snorkels, rotor-rooters, lasers, drills, drug-eluting balloons and stents, radial and tibial access. Unfortunately, I must not have read Dale Carnegie’s book on how to win friends and influence people since by then I had alienated some of the general surgeons and all the radiologists and cardiologists. Accordingly, we had to train ourselves on these new devices and indications. Fortunately, training programs were by then producing endo-competent vascular surgeons and we were able to incorporate, and learn from, two of these younger surgeons, Michael Lepore, MD, and Deepak Nair, MD, who had joined our practice.
I suspect that many vascular surgeons who trained in the early eighties, and perhaps even nineties, were similarly self-taught. In fact, I suggest that some program directors, who now teach endovascular procedures, also had to learn on the job. This does not imply that we are all less skilled. Rather, that our generation of vascular surgeons come to the endo table with prejudices that favor open surgery and which may prevent us from fully embracing new technologies. Further, the host of new equipment alternatives makes it almost impossible to gain a global experience unless one has an extensive clinical practice or works within a large group or academic program. Thus, if we are not exposed to these devices and are not aware of their pluses and minuses, we might not be as good at them as we think we are.
An even more unfortunate repercussion of the endo-tsunami drowning open skills of young surgeons is that it may be having a similar effect on their more senior colleagues. Surgeons over the age of 50 are now in the majority and most have appropriately embraced endovascular procedures. However, in so doing their open volume falls and their expertise in this segment of their practice must diminish. I realize that there are many surgeons of my generation who are masters of all techniques, and I applaud their resilience. However, some may need to acknowledge that they may be just a little less proficient in the operating room. Accordingly, we need to be careful not to cast too many stones at our junior colleagues.
So, there are young vascular surgeons who may have lesser open skills, older surgeons who may have lesser endo skills, and some senior surgeons who may not be totally expert at either skill. I propose that it is now up to those of you, in the middle of your careers, to make sure that you keep up with changing paradigms, never lose your hard-earned skills, teach the new graduates all you can and, even more importantly, always remain aware of your inadequacies.
Russell Samson, MD, is a physician in the practice of Sarasota Vascular Specialists and clinical professor of surgery, Florida State University, Tallahassee. He is also the medical editor of Vascular Specialist.
In 1999, two psychologists, David Dunning and his student Justin Kruger, published a paper that demonstrated people who are really bad at something tend to believe that they are really good (J Pers Soc Psychol. 1999;77:1121-34). They also posited that most competent people underestimate their abilities while the rest of us overestimate them, and the worse we are, the more we overestimate our capabilities. In essence, they postulate that one needs a degree of skill in performing an activity in order to assess one's aptitude. In other words, it’s impossible to tell if you are bad at something if you’re too bad to know that you’re bad.
No, I’m not writing another diatribe about cardiologists (although this surely applies to some!). Rather this is a semi-apology to the vascular fellow who I featured in my last editorial, wherein I bemoaned that the endo-revolution resulted in some younger surgeons lacking open skills. That young man is an example of a highly competent trainee who probably underestimates his abilities to perform complex open procedures. In fact, an honest self-evaluation of my own clinical experience has made me realize that there is a corollary to newly minted vascular surgeons having limited open experience … rather, that some older surgeons, well versed in open surgery, may be inexperienced in some complex endo-procedures. The implications for the practice of vascular surgery are significant and warrant discussion. Perhaps my personal experience in learning endovascular methods will be revealing.
I performed my vascular fellowship at Montefiore with Frank Veith, MD, in 1980. At the time, Dr. Veith was a principal investigator in a multicenter, randomized trial to evaluate whether PTFE could be an acceptable substitute for saphenous vein in infra-inguinal bypass. As his fellow, I gained an enormous experience in these procedures. I stayed on in academic vascular surgery for another 6years honing my techniques in other forms of open surgery. In 1986 I moved to Sarasota, Fla., to start a private practice. Here, vascular surgery was performed by general surgeons who, although competent in the vascular procedures of that time, treated most infrapopliteal disease with an amputation. My calling card was my ability to do a distal bypass. What an anachronism! Of course, I still do a fair number of tibial bypasses, but femoropopliteal bypass is almost ready for the museum. The reason is that the tidal force of the endo-tsunami had just begun to wash up on the sunny beaches of Sarasota.
While at Montefiore I had witnessed the beginning of the endovascular wave. I realized that if I didn’t learn this new technology, I might well have become a surgical dinosaur. Accordingly, soon after arriving in Sarasota, I left town to spend a week with a pioneering radiologist who allowed me to observe his team’s early experience with aortic endografts. I left my practice a second time to visit with a very busy invasive cardiologist where I had hands-on experience with balloon angioplasty and early Palmaz stents. On my return, I cautiously started performing diagnostic arteriograms in the operating room using early C-arms. Eventually, my partner, David Showalter, MD, and I convinced the hospital to outfit a room as a semi “hybrid” suite, a fixed sliding X-ray table coupled with the most advanced C-arm of the time. Over the objections of local radiologists and cardiologists, we ultimately obtained privileges to perform our endo cases in their radiology suites and cath labs. This allowed us to expand our endovascular experience, first by improving our proficiency as diagnostic arteriographers, then by advancing our angioplasty and, ultimately, stent techniques. In the interim, however, endovascular technology had flourished with the introduction of TEVAR, FEVAR, chimneys and snorkels, rotor-rooters, lasers, drills, drug-eluting balloons and stents, radial and tibial access. Unfortunately, I must not have read Dale Carnegie’s book on how to win friends and influence people since by then I had alienated some of the general surgeons and all the radiologists and cardiologists. Accordingly, we had to train ourselves on these new devices and indications. Fortunately, training programs were by then producing endo-competent vascular surgeons and we were able to incorporate, and learn from, two of these younger surgeons, Michael Lepore, MD, and Deepak Nair, MD, who had joined our practice.
I suspect that many vascular surgeons who trained in the early eighties, and perhaps even nineties, were similarly self-taught. In fact, I suggest that some program directors, who now teach endovascular procedures, also had to learn on the job. This does not imply that we are all less skilled. Rather, that our generation of vascular surgeons come to the endo table with prejudices that favor open surgery and which may prevent us from fully embracing new technologies. Further, the host of new equipment alternatives makes it almost impossible to gain a global experience unless one has an extensive clinical practice or works within a large group or academic program. Thus, if we are not exposed to these devices and are not aware of their pluses and minuses, we might not be as good at them as we think we are.
An even more unfortunate repercussion of the endo-tsunami drowning open skills of young surgeons is that it may be having a similar effect on their more senior colleagues. Surgeons over the age of 50 are now in the majority and most have appropriately embraced endovascular procedures. However, in so doing their open volume falls and their expertise in this segment of their practice must diminish. I realize that there are many surgeons of my generation who are masters of all techniques, and I applaud their resilience. However, some may need to acknowledge that they may be just a little less proficient in the operating room. Accordingly, we need to be careful not to cast too many stones at our junior colleagues.
So, there are young vascular surgeons who may have lesser open skills, older surgeons who may have lesser endo skills, and some senior surgeons who may not be totally expert at either skill. I propose that it is now up to those of you, in the middle of your careers, to make sure that you keep up with changing paradigms, never lose your hard-earned skills, teach the new graduates all you can and, even more importantly, always remain aware of your inadequacies.
Russell Samson, MD, is a physician in the practice of Sarasota Vascular Specialists and clinical professor of surgery, Florida State University, Tallahassee. He is also the medical editor of Vascular Specialist.
In 1999, two psychologists, David Dunning and his student Justin Kruger, published a paper that demonstrated people who are really bad at something tend to believe that they are really good (J Pers Soc Psychol. 1999;77:1121-34). They also posited that most competent people underestimate their abilities while the rest of us overestimate them, and the worse we are, the more we overestimate our capabilities. In essence, they postulate that one needs a degree of skill in performing an activity in order to assess one's aptitude. In other words, it’s impossible to tell if you are bad at something if you’re too bad to know that you’re bad.
No, I’m not writing another diatribe about cardiologists (although this surely applies to some!). Rather this is a semi-apology to the vascular fellow who I featured in my last editorial, wherein I bemoaned that the endo-revolution resulted in some younger surgeons lacking open skills. That young man is an example of a highly competent trainee who probably underestimates his abilities to perform complex open procedures. In fact, an honest self-evaluation of my own clinical experience has made me realize that there is a corollary to newly minted vascular surgeons having limited open experience … rather, that some older surgeons, well versed in open surgery, may be inexperienced in some complex endo-procedures. The implications for the practice of vascular surgery are significant and warrant discussion. Perhaps my personal experience in learning endovascular methods will be revealing.
I performed my vascular fellowship at Montefiore with Frank Veith, MD, in 1980. At the time, Dr. Veith was a principal investigator in a multicenter, randomized trial to evaluate whether PTFE could be an acceptable substitute for saphenous vein in infra-inguinal bypass. As his fellow, I gained an enormous experience in these procedures. I stayed on in academic vascular surgery for another 6years honing my techniques in other forms of open surgery. In 1986 I moved to Sarasota, Fla., to start a private practice. Here, vascular surgery was performed by general surgeons who, although competent in the vascular procedures of that time, treated most infrapopliteal disease with an amputation. My calling card was my ability to do a distal bypass. What an anachronism! Of course, I still do a fair number of tibial bypasses, but femoropopliteal bypass is almost ready for the museum. The reason is that the tidal force of the endo-tsunami had just begun to wash up on the sunny beaches of Sarasota.
While at Montefiore I had witnessed the beginning of the endovascular wave. I realized that if I didn’t learn this new technology, I might well have become a surgical dinosaur. Accordingly, soon after arriving in Sarasota, I left town to spend a week with a pioneering radiologist who allowed me to observe his team’s early experience with aortic endografts. I left my practice a second time to visit with a very busy invasive cardiologist where I had hands-on experience with balloon angioplasty and early Palmaz stents. On my return, I cautiously started performing diagnostic arteriograms in the operating room using early C-arms. Eventually, my partner, David Showalter, MD, and I convinced the hospital to outfit a room as a semi “hybrid” suite, a fixed sliding X-ray table coupled with the most advanced C-arm of the time. Over the objections of local radiologists and cardiologists, we ultimately obtained privileges to perform our endo cases in their radiology suites and cath labs. This allowed us to expand our endovascular experience, first by improving our proficiency as diagnostic arteriographers, then by advancing our angioplasty and, ultimately, stent techniques. In the interim, however, endovascular technology had flourished with the introduction of TEVAR, FEVAR, chimneys and snorkels, rotor-rooters, lasers, drills, drug-eluting balloons and stents, radial and tibial access. Unfortunately, I must not have read Dale Carnegie’s book on how to win friends and influence people since by then I had alienated some of the general surgeons and all the radiologists and cardiologists. Accordingly, we had to train ourselves on these new devices and indications. Fortunately, training programs were by then producing endo-competent vascular surgeons and we were able to incorporate, and learn from, two of these younger surgeons, Michael Lepore, MD, and Deepak Nair, MD, who had joined our practice.
I suspect that many vascular surgeons who trained in the early eighties, and perhaps even nineties, were similarly self-taught. In fact, I suggest that some program directors, who now teach endovascular procedures, also had to learn on the job. This does not imply that we are all less skilled. Rather, that our generation of vascular surgeons come to the endo table with prejudices that favor open surgery and which may prevent us from fully embracing new technologies. Further, the host of new equipment alternatives makes it almost impossible to gain a global experience unless one has an extensive clinical practice or works within a large group or academic program. Thus, if we are not exposed to these devices and are not aware of their pluses and minuses, we might not be as good at them as we think we are.
An even more unfortunate repercussion of the endo-tsunami drowning open skills of young surgeons is that it may be having a similar effect on their more senior colleagues. Surgeons over the age of 50 are now in the majority and most have appropriately embraced endovascular procedures. However, in so doing their open volume falls and their expertise in this segment of their practice must diminish. I realize that there are many surgeons of my generation who are masters of all techniques, and I applaud their resilience. However, some may need to acknowledge that they may be just a little less proficient in the operating room. Accordingly, we need to be careful not to cast too many stones at our junior colleagues.
So, there are young vascular surgeons who may have lesser open skills, older surgeons who may have lesser endo skills, and some senior surgeons who may not be totally expert at either skill. I propose that it is now up to those of you, in the middle of your careers, to make sure that you keep up with changing paradigms, never lose your hard-earned skills, teach the new graduates all you can and, even more importantly, always remain aware of your inadequacies.
Russell Samson, MD, is a physician in the practice of Sarasota Vascular Specialists and clinical professor of surgery, Florida State University, Tallahassee. He is also the medical editor of Vascular Specialist.