User login
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
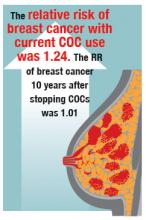
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.