User login
Reduce maternal morbidity by the expeditious and decisive treatment of severe hypertension in pregnancy
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Leadership hacks: structural tension
My leadership experience was limited when I became department chair 6 years ago. Recognizing the deficit immediately, I began reading self-help and leadership books, sought training in coaching techniques, and have attended innumerable leadership courses. I still have a lot to learn, but I am a lot more comfortable with my leadership skills than I was. Since I am often asked for advice with regard to advancing into administrative leadership positions, I want to share what I have learned with others.
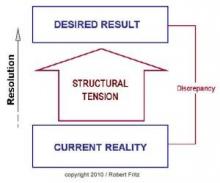
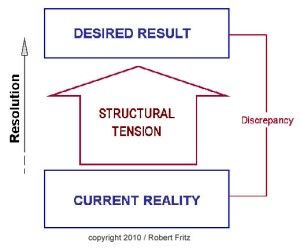
In one of my previous columns, I reviewed the concept of drama triangles and introduced structural tension as a model for addressing and breaking them. The structural tension model is attributed to Robert Fritz and is presented in Figure 1. I have found this model very helpful for coaching toward desired change.
Kelly, the supervisor/manager/director/chairman, expects all physicians to carry a heavy clinical load while also conducting research, writing papers, and securing as many grants as possible. Of course, the expected clinical load encroaches on the time required for academic pursuits. Tension increases among the faculty as the clinical load prohibits academic work resulting in unmet expectations for Kelly and dissatisfaction and disengagement for everyone else. A staff meeting is called to address the worsening workplace environment.
Since Kelly is at a loss, the administrator, Pat, offers to run the meeting in an attempt to address the problem. Pat asks the assembled team to describe the ideal state, or desired result, for the department. Eagerly, the participants begin to list the components of their ideal state including error-free scheduling, adequate staffing, an efficient electronic medical record, uninterrupted administrative time, sufficient research support, and several other important requirements to successfully meet department expectations.
Next, Pat asks the staff to list the current state. Once again, the participants are more than happy to call out the current situation as they see it, including patients arriving without records, slow rooming times, add-on appointments outside clinic schedules, poor statistical support, and several other impediments to optimal efficiency.
Critically, Pat resists the temptation to recount and defend all the efforts being made to address each of these difficult issues. Instead, Pat asks the team what they can do to begin moving from the current state to the ideal state. In contrast to the other questions, the team hesitates to answer this one. The administration is supposed to fix the problems, not them. Pat persists, though, and is willing to wait in awkward silence for someone to offer a suggestion. Finally, a junior faculty member speaks up and recommends that the physician staff meet with the schedulers to reconfigure their clinic templates to more realistically reflect their available time. As murmurs flow across the room, another physician offers that perhaps they could cross cover for each other to allow uninterrupted administrative time. More and more physicians then join in suggesting more and more opportunities to streamline processes to create efficiencies.
Reflecting on the meeting afterward, Kelly was astounded not only at the process, but at the engagement of the faculty. Kelly was under the impression that the faculty was too frustrated to effectively participate. Pat was thrilled to have so many good ideas to work on. Importantly, these ideas came from the staff (bottom-up) rather than from administration (top-down), which increases staff involvement in the projects already set up in addition to creating new ones. The staff, in turn, felt that their concerns were heard and were inspired to take on the new challenges they created for themselves.
The structural tension model is more nuanced than my illustrative example suggests, but it does provide a useful framework to address problems and create solutions. Different problems, though, lend themselves to different solutions and structural tension cannot address every problem a leader faces. It is just one more tool in the leadership toolbox.
For more reading: Fritz R, “The Path of Least Resistance” (New York: Random House, 1984).
My leadership experience was limited when I became department chair 6 years ago. Recognizing the deficit immediately, I began reading self-help and leadership books, sought training in coaching techniques, and have attended innumerable leadership courses. I still have a lot to learn, but I am a lot more comfortable with my leadership skills than I was. Since I am often asked for advice with regard to advancing into administrative leadership positions, I want to share what I have learned with others.
In one of my previous columns, I reviewed the concept of drama triangles and introduced structural tension as a model for addressing and breaking them. The structural tension model is attributed to Robert Fritz and is presented in Figure 1. I have found this model very helpful for coaching toward desired change.
Kelly, the supervisor/manager/director/chairman, expects all physicians to carry a heavy clinical load while also conducting research, writing papers, and securing as many grants as possible. Of course, the expected clinical load encroaches on the time required for academic pursuits. Tension increases among the faculty as the clinical load prohibits academic work resulting in unmet expectations for Kelly and dissatisfaction and disengagement for everyone else. A staff meeting is called to address the worsening workplace environment.
Since Kelly is at a loss, the administrator, Pat, offers to run the meeting in an attempt to address the problem. Pat asks the assembled team to describe the ideal state, or desired result, for the department. Eagerly, the participants begin to list the components of their ideal state including error-free scheduling, adequate staffing, an efficient electronic medical record, uninterrupted administrative time, sufficient research support, and several other important requirements to successfully meet department expectations.
Next, Pat asks the staff to list the current state. Once again, the participants are more than happy to call out the current situation as they see it, including patients arriving without records, slow rooming times, add-on appointments outside clinic schedules, poor statistical support, and several other impediments to optimal efficiency.
Critically, Pat resists the temptation to recount and defend all the efforts being made to address each of these difficult issues. Instead, Pat asks the team what they can do to begin moving from the current state to the ideal state. In contrast to the other questions, the team hesitates to answer this one. The administration is supposed to fix the problems, not them. Pat persists, though, and is willing to wait in awkward silence for someone to offer a suggestion. Finally, a junior faculty member speaks up and recommends that the physician staff meet with the schedulers to reconfigure their clinic templates to more realistically reflect their available time. As murmurs flow across the room, another physician offers that perhaps they could cross cover for each other to allow uninterrupted administrative time. More and more physicians then join in suggesting more and more opportunities to streamline processes to create efficiencies.
Reflecting on the meeting afterward, Kelly was astounded not only at the process, but at the engagement of the faculty. Kelly was under the impression that the faculty was too frustrated to effectively participate. Pat was thrilled to have so many good ideas to work on. Importantly, these ideas came from the staff (bottom-up) rather than from administration (top-down), which increases staff involvement in the projects already set up in addition to creating new ones. The staff, in turn, felt that their concerns were heard and were inspired to take on the new challenges they created for themselves.
The structural tension model is more nuanced than my illustrative example suggests, but it does provide a useful framework to address problems and create solutions. Different problems, though, lend themselves to different solutions and structural tension cannot address every problem a leader faces. It is just one more tool in the leadership toolbox.
For more reading: Fritz R, “The Path of Least Resistance” (New York: Random House, 1984).
My leadership experience was limited when I became department chair 6 years ago. Recognizing the deficit immediately, I began reading self-help and leadership books, sought training in coaching techniques, and have attended innumerable leadership courses. I still have a lot to learn, but I am a lot more comfortable with my leadership skills than I was. Since I am often asked for advice with regard to advancing into administrative leadership positions, I want to share what I have learned with others.
In one of my previous columns, I reviewed the concept of drama triangles and introduced structural tension as a model for addressing and breaking them. The structural tension model is attributed to Robert Fritz and is presented in Figure 1. I have found this model very helpful for coaching toward desired change.
Kelly, the supervisor/manager/director/chairman, expects all physicians to carry a heavy clinical load while also conducting research, writing papers, and securing as many grants as possible. Of course, the expected clinical load encroaches on the time required for academic pursuits. Tension increases among the faculty as the clinical load prohibits academic work resulting in unmet expectations for Kelly and dissatisfaction and disengagement for everyone else. A staff meeting is called to address the worsening workplace environment.
Since Kelly is at a loss, the administrator, Pat, offers to run the meeting in an attempt to address the problem. Pat asks the assembled team to describe the ideal state, or desired result, for the department. Eagerly, the participants begin to list the components of their ideal state including error-free scheduling, adequate staffing, an efficient electronic medical record, uninterrupted administrative time, sufficient research support, and several other important requirements to successfully meet department expectations.
Next, Pat asks the staff to list the current state. Once again, the participants are more than happy to call out the current situation as they see it, including patients arriving without records, slow rooming times, add-on appointments outside clinic schedules, poor statistical support, and several other impediments to optimal efficiency.
Critically, Pat resists the temptation to recount and defend all the efforts being made to address each of these difficult issues. Instead, Pat asks the team what they can do to begin moving from the current state to the ideal state. In contrast to the other questions, the team hesitates to answer this one. The administration is supposed to fix the problems, not them. Pat persists, though, and is willing to wait in awkward silence for someone to offer a suggestion. Finally, a junior faculty member speaks up and recommends that the physician staff meet with the schedulers to reconfigure their clinic templates to more realistically reflect their available time. As murmurs flow across the room, another physician offers that perhaps they could cross cover for each other to allow uninterrupted administrative time. More and more physicians then join in suggesting more and more opportunities to streamline processes to create efficiencies.
Reflecting on the meeting afterward, Kelly was astounded not only at the process, but at the engagement of the faculty. Kelly was under the impression that the faculty was too frustrated to effectively participate. Pat was thrilled to have so many good ideas to work on. Importantly, these ideas came from the staff (bottom-up) rather than from administration (top-down), which increases staff involvement in the projects already set up in addition to creating new ones. The staff, in turn, felt that their concerns were heard and were inspired to take on the new challenges they created for themselves.
The structural tension model is more nuanced than my illustrative example suggests, but it does provide a useful framework to address problems and create solutions. Different problems, though, lend themselves to different solutions and structural tension cannot address every problem a leader faces. It is just one more tool in the leadership toolbox.
For more reading: Fritz R, “The Path of Least Resistance” (New York: Random House, 1984).
From the Editors: Halsted, Holmes, and penguins
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
Is it not ironic that in a profession that is always seeking answers – What does this patient have? Is that mass malignant? What’s the best way to make a diagnosis? – too much information has become a major problem?
Unlike William Stewart Halsted or Theodor Billroth, who blazed surgical trails in an age when much was unknown, today’s surgeons face a jungle of information obscuring the trail ahead. Every morning we wake up to another 30 or 40 unread emails. Journals multiply on our desks. The books we need to read pile up and spill over onto our desks, bookshelves, and side tables. Sometimes, it makes one long for the old days when definitive answers might not be found in the literature. These days, we know it is likely that someone has published exactly what we need at any particular moment, and yet finding it in the jungle of information can be a great challenge.
Another outcome of too much information is the accumulation in our brains of unsorted bits of medical/surgical knowledge. Some of those bits are pearls, and others are just gum wrappers that take up space. It becomes an overwhelming task of ranking, sorting, prioritizing, and discarding.
A friend of mine years of ago called his brain an iceberg on which thousands of penguins stand. The penguins just kept coming and, finally, in order to learn anything new, he had to push some penguins off the iceberg. We have a lot of penguins on our icebergs these days.
This brings to mind many doctors’ favorite fictional character, Sherlock Holmes. That denizen of 221B Baker Street was a master at data management. He always had the right information available in his head relevant for the mystery at hand. How did he do it? Recall that Dr. Watson (a surgeon, I might add) was intermittently shocked by what Holmes didn’t know, to which the tobacco- and opiate-addicted hero would reply that he purposely forgot things that did not help him solve his cases.
And so, what is the modern surgeon – who must keep in the forefront of his or her mind every best practice, algorithm, and guideline – to do in this age of too much information? Like Holmes, we need to sort what is critical from what is not and let go of those items that no longer are germane. We then need to triage the vast amount of information delivered to us yearly, weekly, monthly, daily, hourly. The stream of little notes flashing at you from your black mirror (the screen of your mobile device) needs to be controlled lest it control you.
So, might I suggest a few strategies I have used to triage the flow of information? For hourly and daily information, I tend to ignore everything except ACS NewsScope and the ACS Communities items I find most interesting. For monthly information, I tend to use ACS Surgery News (plug intended) because it is “news” – the stuff that just happened in the meeting sphere or has not yet hit print (sorry, e-publication). The Journal of the American College of Surgeons is another monthly source that is reliable.
You may see a theme here. I’ve used the American College of Surgeons as my main filter. What gets through the editors of these outlets generally is viable and useful information. That’s what I need to know for right now. Such knowledge allows me to push those penguins no longer needed off my iceberg and greet the new ones with joy. We often wonder what the benefit of membership in the College may be. For me, these filters are worth the price of admission.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
When cold-induced vasospasm is the tip of the iceberg
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
Beyond DSM-5: Clinical and biologic features shared by major psychiatric syndromes
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
It does not adequately inform psychiatric practitioners about the many clinical and biologic features shared across the various diagnostic categories. It does not do justice to the galloping advances in the neurobiology of psychiatric brain disorders and the wealth of potential biomarkers that will eventually endow psychiatry with an objective and ultimately more valid, not just reliable, diagnostic model that is compatible with a future of precision medicine.
The Research Domain Criteria (RDoC) Project1 is a valiant attempt to transcend the DSM’s “Chinese menu” approach to diagnosis. It was championed by the former director of the National Institute of Mental Health (NIMH), who used his authority to encourage investigators applying for federal grants to employ the RDoC principles in their research programs. Who does not recall the awkward moment, a few weeks before the official baptism of DSM-5 as psychiatry’s latest diagnostic Bible in May 2013? The NIMH director’s unflattering portrayal of the incipient DSM-5 was a well-publicized shot across the bow. The kerfuffle was later resolved, but its effects linger among clinical researchers who relentlessly hope for neuroscience advances to translate into a more objective diagnostic approach to psychiatric diagnoses. The neurobiologic foundations of psychopathology are bound to guide us to a more valid set of diagnostic categories, yet the pace remains painfully slow.
However, the copious advances in brain research are providing other dividends beyond a better diagnostic forest. Many intriguing insights are emerging about the connectedness among major psychiatric “trees,” including schizophrenia, bipolar disorders, and major depressive disorder. The following are examples of neurobiologic, clinical, and treatment commonalities across those psychotic and mood disorders.
Shared neurobiology
Progressive brain tissue loss/neurodegeneration. Numerous studies have established that abnormal neuroplasticity is a common theme during psychotic, manic, and depressive episodes. These findings have demonstrated that the more recurrent the episodes, the more prominent the atrophy in either overall brain volume or specific brain regions, especially in the hippocampus, prefrontal cortex, and cerebellum as measured on MRI.
White matter pathology. Multiple studies have reported loss of myelin integrity in psychotic and mood disorders. Abnormalities are detected by using diffusion tensor imaging and measuring anisotropy and diffusivity of water flow in white matter traits. White matter pathology can be associated with intra- and inter-hemispheric disconnectivity and impairment of brain functional integration that may contribute to positive, negative, and cognitive symptoms.
Neuroinflammation. Acute psychotic and mood episodes have been shown to be associated with significant elevation in inflammatory cytokines in CSF and serum, including interleukins (such as interleukin-6), tumor necrosis factor-alpha, interferon gamma, and C-reactive protein. Those inflammatory biomarkers subside when the acute episodes are treated. It is believed that activation of the microglia leads to release of proinflammatory cytokines.
Mitochondrial dysfunction. Many studies document various dysfunctions of the mitochondria in schizophrenia, bipolar disorders, and major depressive disorder. The consequences include oxidative stress due to a decrease in the antioxidant glutathione, produced in the mitochondria, which is vital for neutralizing the reactive oxygen and nitrogen species referred to as free radicals. There is a substantial increase of free radicals during acute psychotic and mood episodes, which contributes to neurodegeneration.
Glutamate pathway abnormalities. A large body of literature has focused on the glutamate N-methyl-
Gene/environment interaction. Neurogenetic advances have demonstrated some shared genes among schizophrenia, bipolar disorders, and major depressive disorder (such as the CACNA1C gene).2 Also, environmental factors, such as severe childhood maltreatment, lead to high rates of psychosis and mood disorders in adulthood. Risk genes in schizophrenia and mood disorders are likely to be overexpressed with adverse environmental factors and epigenetics.
Shortened telomeres. Patients with psychotic and mood disorders have been reported to have shorter telomeres—proteins that cap the end of chromosomes and shorten with repeated cycles of mitosis and aging—at a younger age, predicting early senescence and mortality. Telomere shortening is associated with multiple factors, including chronic stress, smoking, poor diet, obesity, infections, inflammation, and free radicals, all shared by major psychiatric disorders.
Genetic heterogeneity. Schizophrenia, bipolar disorders, and major depressive disorder are associated with complex genetics (eg, risk genes, mutations, and copy number variants) and various perinatal complications (eg, infections, gestational diabetes, vitamin D deficiency, hypoxia at delivery), which makes them highly heterogeneous syndromes, comprised of hundreds of biotypes. There are many established endophenotypes that a future diagnostic system should adopt.
Elevated cortisol levels. Increased serum cortisol levels are found in depression and schizophrenia related to HPA axis dysregulation as well as life stress. Hypercortisolemia can contribute to neurodegeneration as well as to multiple systemic medical disorders often encountered in mood and psychotic disorders.
Shared clinical features
Psychotic and mood disorders share several key clinical features, including:
- cognitive deficits
- substance use disorders (especially Cannabis and alcohol) as a common comorbidity
- increased suicide risk
- high prevalence of smoking
- premature mortality, by 10 to 20 years
- anxiety as a common comorbidity
- elevated cardiometabolic risk factors, even before pharmacotherapy
- recurrent relapses lead to treatment resistance
- various degrees of fixed false beliefs (delusions)
- perceptional aberrations (various types of hallucinations)
- response to dopamine-serotonin antagonists (atypical antipsychotics) as monotherapy or adjunctive therapy.
While it is fair to say that a diagnostic manual like DSM-5 should focus on the diagnosis of individual psychiatric diseases and syndromes, it is also reasonable to say that focusing primarily on clinical features does not do justice to the biologic complexities of psychiatric disorders and the importance of including biomarkers to increase the validity of psychopathological categories. The shared neurobiologic and clinical features across major psychiatric syndromes, such as schizophrenia, bipolar disorders, and depression, indicate how multifaceted psychiatric diagnosis can be. The same approach is applicable to other psychiatric syndromes, such as anxiety, personality disorders, attention-deficit/hyperactivity disorder, or dementia. Our field should move firmly and steadily toward a diagnostic schema that incorporates ongoing breakthroughs in psychiatric neuroscience as soon as they are replicated.
If psychopathology is a forest, then DSM-5 is a simplistic depiction of each tree’s structure as roots, a trunk, branches, and leaves. Psychiatry needs to move to a far more sophisticated perspective of each tree as an amazingly complex, dynamic, and evolving organism, designed genetically but continuously influenced by its environment. Psychiatry also should keep an eye on the entire forest and detect distinctive patterns as well as idiosyncratic or shared features among the trees. Major insights will ensue about the etiology, course, and management of each diagnostic tree or the mosaic of related trees.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
1. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395-397.
2. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
Stop using codeine, oxycodone, hydrocodone, tramadol, and aspirin in women who are breastfeeding
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.