User login
Why do so many women aged 65 years and older die of cervical cancer?
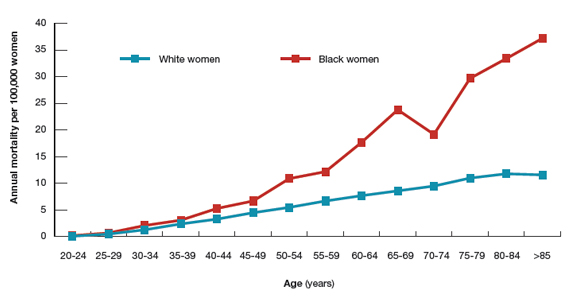
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1
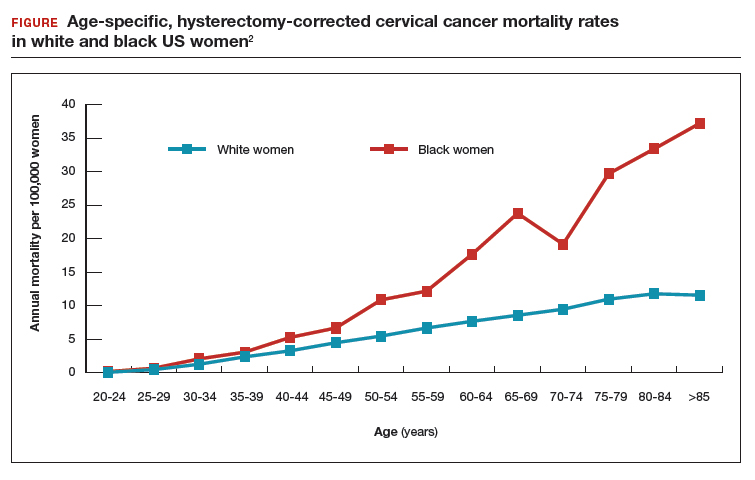
A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
Surprisingly, the cervical cancer death rate is greater among women aged >65 years than among younger women1,2 (FIGURE). Paradoxically, most of our screening programs focus on women <65 years of age. A nationwide study from Denmark estimated that the cervical cancer death rate per 100,000 women at ages 40 to 44 and 65 to 69 was 3.8 and 9.0, respectively.1 In other words, the cervical cancer death rate at age 65 to 69 years was 2.36 times higher than at age 40 to 44 years.1

A study from the United States estimated that the cervical cancer death rate per 100,000 white women at ages 40 to 44 and 65 to 69 was 3.3 and 8.6, respectively,2 very similar to the findings from Denmark. The same US study estimated that the cervical cancer death rate per 100,000 black women at ages 40 to 44 and 65 to 69 was 5.3 and 23.8, highlighting the fact that, in the United States, cervical cancer disease burden is disproportionately greater among black than among white women.2 In addition, the cervical cancer death rate among black women at age 65 to 69 was 4.49 times higher than at age 40 to 44 years.2
Given the high death rate from cervical cancer in women >65 years of age, it is paradoxical that most professional society guidelines recommend discontinuing cervical cancer screening at 65 years of age, if previous cervical cancer screening is normal.3,4 Is the problem due to an inability to implement the current guidelines? Or is the problem that the guidelines are not optimally designed to reduce cervical cancer risk in women >65 years of age?
The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) recommend against cervical cancer screening in women >65 years of age who have had adequate prior screening and are not otherwise at high risk for cervical cancer. However, ACOG and the USPSTF caution that there are many groups of women that may benefit from continued screening after 65 years of age, including women with HIV infection, a compromised immune system, or previous high-grade precancerous lesion or cervicalcancer; women with limited access to care; women from racial/ethnic minority groups; and migrant women.4 Many clinicians remember the guidance, “discontinue cervical cancer screening at 65 years” but do not recall all the clinical factors that might warrant continued screening past age 65. Of special concern is that black,2 Hispanic,5 and migrant women6 are at much higher risk for invasive cervical cancer than white or US-born women.
The optimal implementation of the ACOG and USPSTF guidelines are undermined by a fractured health care system, where key pieces of information may be unavailable to the clinician tasked with making a decision about discontinuing cervical cancer screening. Imagine the case in which a 65-year-old woman pre‑sents to her primary care physician for cervical cancer screening. The clinician performs a cervical cytology test and obtains a report of “no intraepithelial lesion or malignancy.” The clinician then recommends that the patient discontinue cervical cancer screening. Unbeknownst to the clinician, the patient had a positive HPV 16/18/45 test within the past 10 years in another health system. In this case, it would be inappropriate to terminate the patient from cervical cancer screening.
Continue to: Testing for hrHPV is superior to cervical cytology in women >65 years...
Testing for hrHPV is superior to cervical cytology in women >65 years
In Sweden, about 30% of cervical cancer cases occur in women aged >60 years.7 To assess the prevalence of oncogenic high-risk HPV (hrHPV), women at ages 60, 65, 70, and 75 years were invited to send sequential self-collected vaginal samples for nucleic acid testing for hrHPV. The prevalence of hrHPV was found to be 4.4%. Women with a second positive, self-collected, hrHPV test were invited for colposcopy, cervical biopsy, and cytology testing. Among the women with two positive hrHPV tests, cervical biopsy revealed 7 cases of cervical intraepithelial neoplasia grade 2 (CIN2), 6 cases of CIN1, and 4 biopsies without CIN. In these women 94% of the cervical cytology samples returned, “no intraepithelial lesion or malignancy” and 6% revealed atypical squamous cells of undetermined significance. This study suggests that, in women aged >65 years, cervical cytology may have a high rate of false-negative results, possibly due to epithelial atrophy. An evolving clinical pearl is that, when using the current cervical cancer screening guidelines, the final screen for cervical cancer must include a nucleic acid test for hrHPV.
In women 65 to 90 years, the prevalence of hrHPV is approximately 5%
In a study of 40,382 women aged 14 to 95 years, the prevalence of hrHPV was 46% in 20- to 23-year-old women and 5.7% in women older than 65 years of age.8 In a study of more than 108,000 women aged 69 to >89 years the prevalence of hrHPV was 4.3%, and similar prevalence rates were seen across all ages from 69 to >89 years.9 The carcinogenic role of persistent hrHPV infection in women >65 years is an important area for future research.
Latent HPV virus infection
Following a primary varicella-zoster infection (chickenpox), the virus may remain in a latent state in sensory ganglia, reactivating later in life to cause shingles. Thirty percent of people who have a primary chickenpox infection eventually will develop a case of shingles. Immunocompromised populations are at an increased risk of developing shingles because of reduced T-cell mediated immunity.
A recent hypothesis is that in immunocompromised and older women, latent HPV can reactivate and cause clinically significant infection.10 Following renal transplantation investigators have reported a significant increase in the prevalence of genital HPV, without a change in sexual behavior.11 In cervical tissue from women with no evidence of active HPV infection, highly sensitive PCR-based assays detected HPV16 virus in a latent state in some women, possibly due to disruption of the viral E2 gene.12 If latent HPV infection is a valid biological concept, it suggests that there is no “safe age” at which to discontinue screening for HPV infection because the virus cannot be detected in screening samples while it is latent.
Options for cervical cancer screening in women >65 years
Three options might reduce the morbidity and mortality associated with cervical cancer in women >65 years.
Option 1: Double-down on trying to effectively implement current guidelines. The high rate of cervical cancer mortality in women >65 years of age indicates that the current guidelines, as implemented in real clinical practice, are not working. A problem with the current screening guidelines is that clinicians are expected to be capable of finding all relevant cervical cancer test results and properly interpreting the results. Clinicians are over-taxed and fallible, and the current approach is not likely to be successful unless additional information technology solutions are implemented.
Continue to: Health systems could use information...
Health systems could use information technology to mitigate these problems. For example, health systems could deploy software to assemble every cervical screening result on each woman and pre‑sent those results to clinicians in a single integrated view in the electronic record. Additionally, once all lifetime screening results are consolidated in one view, artificial intelligence systems could be used to analyze the totality of results and identify women who would benefit by continued screening past age 65 and women who could safely discontinue screening.
Option 2: Adopt the Australian approach to cervical cancer screening. The current Australian approach to cervical cancer screening is built on 3 pillars: 1) school-based vaccination of all children against hrHPV, 2) screening all women from 25 to 74 years of age every 5 years using nucleic acid testing for hrHPV, and 3) providing a system for the testing of samples self-collected by women who are reluctant to visit a clinician for screening.13 Australia has one of the lowest cervical cancer death rates in the world.
Option 3: Continue screening most women past age 65. Women >65 years of age are known to be infected with hrHPV genotypes. hrHPV infection causes cervical cancer. Cervical cancer causes many deaths in women aged >65 years. There is no strong rationale for ignoring these three facts. hrHPV screening every 5 years as long as the woman is healthy and has a reasonable life expectancy is an option that could be evaluated in randomized studies.
Given the high rate of cervical cancer death in women >65 years of age, I plan to be very cautious about discontinuing cervical cancer screening until I can personally ensure that my patient has no evidence of hrHPV infection.
In 2008, Harald zur Hausen, MD, received the Nobel Prize in Physiology or Medicine for discovering that human papilloma virus (HPV) caused cervical cancer. In a recent study, 74% of cervical cancers were associated with HPV 16 or 18 infections. A total of 89% of the cancers were associated with one of the high-risk HPV genotypes, including HPV 16/18/31/33/45/52/58.1
Recently, HPV has been shown to be a major cause of oropharyngeal cancer. The Centers for Disease Control and Prevention calculated that in CY2015 in the United States there were 18,917 cases of HPV-associated oropharyngeal squamous cell cancer and 11,788 cases of cervical cancer.2 Most cases of HPV-associated oropharyngeal cancer occur in men, and HPV vaccination of boys may help to prevent this cancer type. Oncogenic HPV produce two proteins (E6 and E7) that promote viral replication and squamous cell growth by inhibiting the function of p53 and retinoblastoma protein. The immortalized HeLa cell line, derived from Ms. Henrietta Lack's cervical cancer, contains integrated HPV18 nucleic acid sequences.3,4
The discovery that HPV causes cancer catalyzed the development of nucleic acid tests to identify high-risk oncogenic HPV and vaccines against high-risk oncogenic HPV genotypes that prevent cervical cancer. From a public health perspective, it is more effective to vaccinate the population against oncogenic HPV genotypes than to screen and treat cancer. In the United States, vaccination rates range from a high of 92% (District of Columbia) and 89% (Rhode Island) to a low of 47% (Wyoming) and 50% (Kentucky and Mississippi).5 To reduce HPV-associated cancer mortality, the gap in vaccination compliance must be closed.
References
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67:918-924.
- Rosl F, Westphal EM, zur Hausen H. Chromatin structure and transcriptional regulation of human papillomavirus type 18 DNA in HeLa cells. Mol Carcinog. 1989;2:72-80.
- Adey A, Burton JN, Kitzman, et al. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature. 2013;500:207-211.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
- Hammer A, Kahlert J, Gravitt PE, et al. Hysterectomy-corrected cervical cancer mortality rates in Denmark during 2002-2015: a registry-based cohort study. Acta Obstet Gynecol Scand. 2019;98:1063-1069.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111-30.
- Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Stang A, Hawk H, Knowlton R, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995-2010. Ann Epidemiol. 2014;24:849-854.
- Hallowell BD, Endeshaw M, McKenna MT, et al. Cervical cancer death rates among U.S.- and foreign-born women: U.S., 2005-2014. Am J Prev Med. 2019;56:869-874.
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One. 2018;13:e0207714.
- Kjaer SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ACSUC/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-189.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118-123.
- Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267.
- Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563-1573.
- Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847.
- Cervical cancer screening. Cancer Council website. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/cervical-cancer-screening.html. Updated March 15, 2019. Accessed July 23, 2019.
Deciding when a picture is worth a thousand words and several thousand dollars
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
Beyond ‘selfies’: An epidemic of acquired narcissism
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Clinical trials: More to learn than the results
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
Psychosis as a common thread across psychiatric disorders
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.