User login
Premature mortality across most psychiatric disorders
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
Clinical trials in sarcoma bring hope and promise
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
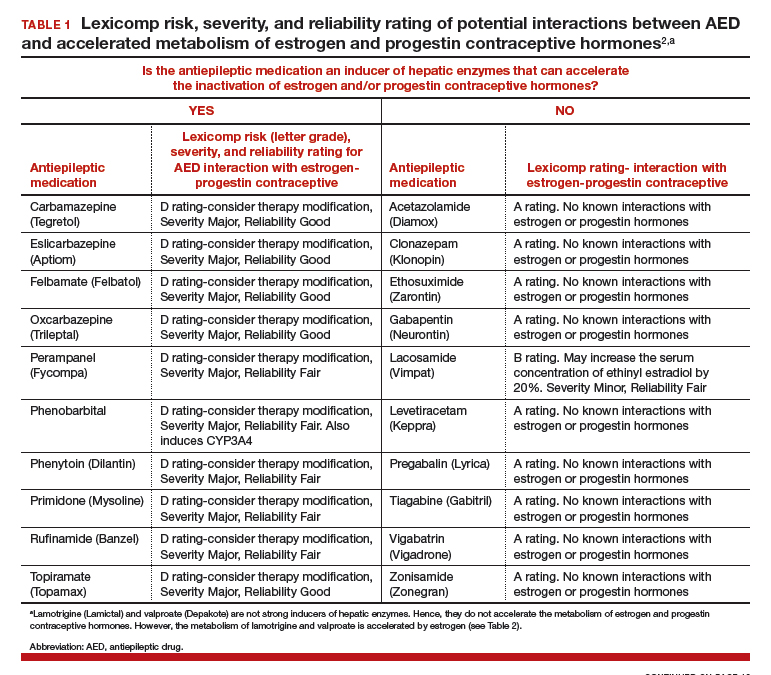
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Clinical outcomes in diabetes: It’s not just the glucose (and it’s not so simple)
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
Reunion
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Transformative advances are unfolding in psychiatry
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.