User login
A link between A-fib and sleep apnea is no surprise, but why?
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Anathemas of psychiatric practice
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
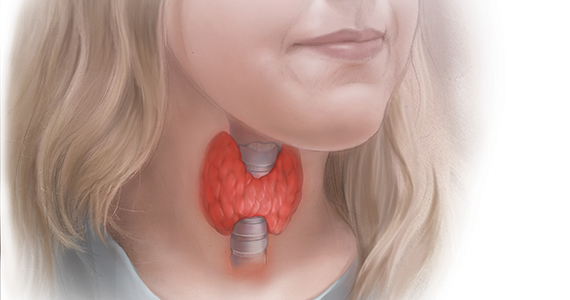
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
Treat or treat
In Charles M. Schulz’s “It’s the Great Pumpkin, Charlie Brown,” the Peanuts gang goes trick or treating door to door. While everyone else gets candy, chewing gum, and chocolate bars, Charlie Brown just gets a bag of rocks. Everyone got treats except Charlie Brown, who only got tricks. Sometimes it seems that my patients are trick or treating, too. Sadly, the tricks come way too often.

Linus tried to avoid tricks by staking out a sincere pumpkin patch in the hope that the Great Pumpkin would rise and deliver him candy and toys. Alas, our patients sometimes sincerely believe things like alkalinization, naturopathy, and antineoplastons will deliver the treats they need to cure their cancer. They will be similarly disappointed.
Most patients depend on us to skew the treat to trick ratio favorably. They trust us to know what to recommend to lengthen life and reduce suffering. Their faith is both a profound privilege and a daunting responsibility.
My patient was hospitalized with hypercalcemia, the latest complication deriving from a decade of progressive multiple myeloma. He was on his 11th line of therapy complicated by at least a grade 3 neuropathy resulting in an unstable gait, chronic pain requiring opioid analgesia, two hospitalizations in the last year for severe infections, and venous thromboembolism on anticoagulation, all resulting in an ECOG performance status no better than a 2. He stabilized and then we needed to talk about next steps.
A clinical trial would be ideal, but he would be excluded from any that we have open and travel isn’t really an option for him. I could choose to treat him with selinexor. It is approved by the Food and Drug Administration and has about a one-in-four chance of producing a short remission in a population of patients that would not include my patient. It also has a three-in-four chance of significant side effects. I could also create a combination regimen with drugs that he has already been exposed to, knowing that response is unlikely and side effects are certain.
This situation is not unique; in fact it is an all too frequent occurrence. The easiest path forward for me would be to recommend treatment. The patient expects treatment and would readily consent to whatever regimen I proposed. He would bear whatever side effects resulted as an expected consequence of therapy. On the surface, this easy path appears to be the proverbial “treat.” But really, further treatment is the “trick” because it is not known to prolong life and would certainly add side effects. The problem, of course, is knowing both when treats become tricks and how to let patients know this, too.
No one knows exactly when treats become tricks, least of all me. Every month I get a report updating me on the status of a former patient being treated elsewhere. This is someone who I thought had no more treatment options. I am humbled every time a colleague, or fellow, recommends a treatment I had never considered. I am not perfect; I do the best I can. My recommendation might be wrong.
Yet I have watched my patient steadily deteriorate and cognitively decline no matter what treatment I employed, whether or not the monoclonal spike decreased. There is no evidence that treatment under such circumstances benefits the patient at all. Moreover, I have sat through many morbidity and mortality conferences where the conclusion was that we should have consulted hospice sooner. Like so many hematologists and oncologists every day, I needed to have a goals-of-care conversation with my patient knowing that treatment could possibly help, but probably would not.
Crucial conversations like these are difficult for everybody. There are techniques to employ that my palliative care colleagues recommend. I tried to remember them as I started talking to my patient and his wife. He listened and clearly understood the gravity of the situation and the resulting poor prognosis regardless of treatment. I recommended hospice. He declined.
Getting to this point was uncomfortable enough, but then I came to a decision that I am still struggling with – acquiesce to his wishes and treat while feeling that I should not, or decline to treat further and transfer his care to someone more willing? This is not the kind of trick or treat I enjoy.
I look forward to the day when discussions of end of life are less awkward. Small movements have started to bring these conversations into the open. One such movement choreographs a dinner to encourage frank and open discussion of death (https://deathoverdinner.org/). Another reimagines the doula – a childbirth coach – as a coach at the end of life (https://www.agentlerparting.com/). Another provides a step-by-step approach to generating an end-of-life conversation (https://theconversationproject.org/). These, and many other efforts, did not occur in a vacuum. They emerged because of the growing recognition that the modern delivery of health care, and the culture it created, is inadequate for the end of life.
Until our culture changes, though, we are left with tough conversations and tougher decisions with our patients who are at the end of their cancer journey. I wish I could tell my junior colleagues that it gets easier with experience. In many ways it gets worse because of the long relationships we develop. As long as the rewards of treats are greater than the disappointments of tricks, though, I will continue trick or treating in my white coat costume.
Dr. Kalaycio is editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
In Charles M. Schulz’s “It’s the Great Pumpkin, Charlie Brown,” the Peanuts gang goes trick or treating door to door. While everyone else gets candy, chewing gum, and chocolate bars, Charlie Brown just gets a bag of rocks. Everyone got treats except Charlie Brown, who only got tricks. Sometimes it seems that my patients are trick or treating, too. Sadly, the tricks come way too often.

Linus tried to avoid tricks by staking out a sincere pumpkin patch in the hope that the Great Pumpkin would rise and deliver him candy and toys. Alas, our patients sometimes sincerely believe things like alkalinization, naturopathy, and antineoplastons will deliver the treats they need to cure their cancer. They will be similarly disappointed.
Most patients depend on us to skew the treat to trick ratio favorably. They trust us to know what to recommend to lengthen life and reduce suffering. Their faith is both a profound privilege and a daunting responsibility.
My patient was hospitalized with hypercalcemia, the latest complication deriving from a decade of progressive multiple myeloma. He was on his 11th line of therapy complicated by at least a grade 3 neuropathy resulting in an unstable gait, chronic pain requiring opioid analgesia, two hospitalizations in the last year for severe infections, and venous thromboembolism on anticoagulation, all resulting in an ECOG performance status no better than a 2. He stabilized and then we needed to talk about next steps.
A clinical trial would be ideal, but he would be excluded from any that we have open and travel isn’t really an option for him. I could choose to treat him with selinexor. It is approved by the Food and Drug Administration and has about a one-in-four chance of producing a short remission in a population of patients that would not include my patient. It also has a three-in-four chance of significant side effects. I could also create a combination regimen with drugs that he has already been exposed to, knowing that response is unlikely and side effects are certain.
This situation is not unique; in fact it is an all too frequent occurrence. The easiest path forward for me would be to recommend treatment. The patient expects treatment and would readily consent to whatever regimen I proposed. He would bear whatever side effects resulted as an expected consequence of therapy. On the surface, this easy path appears to be the proverbial “treat.” But really, further treatment is the “trick” because it is not known to prolong life and would certainly add side effects. The problem, of course, is knowing both when treats become tricks and how to let patients know this, too.
No one knows exactly when treats become tricks, least of all me. Every month I get a report updating me on the status of a former patient being treated elsewhere. This is someone who I thought had no more treatment options. I am humbled every time a colleague, or fellow, recommends a treatment I had never considered. I am not perfect; I do the best I can. My recommendation might be wrong.
Yet I have watched my patient steadily deteriorate and cognitively decline no matter what treatment I employed, whether or not the monoclonal spike decreased. There is no evidence that treatment under such circumstances benefits the patient at all. Moreover, I have sat through many morbidity and mortality conferences where the conclusion was that we should have consulted hospice sooner. Like so many hematologists and oncologists every day, I needed to have a goals-of-care conversation with my patient knowing that treatment could possibly help, but probably would not.
Crucial conversations like these are difficult for everybody. There are techniques to employ that my palliative care colleagues recommend. I tried to remember them as I started talking to my patient and his wife. He listened and clearly understood the gravity of the situation and the resulting poor prognosis regardless of treatment. I recommended hospice. He declined.
Getting to this point was uncomfortable enough, but then I came to a decision that I am still struggling with – acquiesce to his wishes and treat while feeling that I should not, or decline to treat further and transfer his care to someone more willing? This is not the kind of trick or treat I enjoy.
I look forward to the day when discussions of end of life are less awkward. Small movements have started to bring these conversations into the open. One such movement choreographs a dinner to encourage frank and open discussion of death (https://deathoverdinner.org/). Another reimagines the doula – a childbirth coach – as a coach at the end of life (https://www.agentlerparting.com/). Another provides a step-by-step approach to generating an end-of-life conversation (https://theconversationproject.org/). These, and many other efforts, did not occur in a vacuum. They emerged because of the growing recognition that the modern delivery of health care, and the culture it created, is inadequate for the end of life.
Until our culture changes, though, we are left with tough conversations and tougher decisions with our patients who are at the end of their cancer journey. I wish I could tell my junior colleagues that it gets easier with experience. In many ways it gets worse because of the long relationships we develop. As long as the rewards of treats are greater than the disappointments of tricks, though, I will continue trick or treating in my white coat costume.
Dr. Kalaycio is editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
In Charles M. Schulz’s “It’s the Great Pumpkin, Charlie Brown,” the Peanuts gang goes trick or treating door to door. While everyone else gets candy, chewing gum, and chocolate bars, Charlie Brown just gets a bag of rocks. Everyone got treats except Charlie Brown, who only got tricks. Sometimes it seems that my patients are trick or treating, too. Sadly, the tricks come way too often.

Linus tried to avoid tricks by staking out a sincere pumpkin patch in the hope that the Great Pumpkin would rise and deliver him candy and toys. Alas, our patients sometimes sincerely believe things like alkalinization, naturopathy, and antineoplastons will deliver the treats they need to cure their cancer. They will be similarly disappointed.
Most patients depend on us to skew the treat to trick ratio favorably. They trust us to know what to recommend to lengthen life and reduce suffering. Their faith is both a profound privilege and a daunting responsibility.
My patient was hospitalized with hypercalcemia, the latest complication deriving from a decade of progressive multiple myeloma. He was on his 11th line of therapy complicated by at least a grade 3 neuropathy resulting in an unstable gait, chronic pain requiring opioid analgesia, two hospitalizations in the last year for severe infections, and venous thromboembolism on anticoagulation, all resulting in an ECOG performance status no better than a 2. He stabilized and then we needed to talk about next steps.
A clinical trial would be ideal, but he would be excluded from any that we have open and travel isn’t really an option for him. I could choose to treat him with selinexor. It is approved by the Food and Drug Administration and has about a one-in-four chance of producing a short remission in a population of patients that would not include my patient. It also has a three-in-four chance of significant side effects. I could also create a combination regimen with drugs that he has already been exposed to, knowing that response is unlikely and side effects are certain.
This situation is not unique; in fact it is an all too frequent occurrence. The easiest path forward for me would be to recommend treatment. The patient expects treatment and would readily consent to whatever regimen I proposed. He would bear whatever side effects resulted as an expected consequence of therapy. On the surface, this easy path appears to be the proverbial “treat.” But really, further treatment is the “trick” because it is not known to prolong life and would certainly add side effects. The problem, of course, is knowing both when treats become tricks and how to let patients know this, too.
No one knows exactly when treats become tricks, least of all me. Every month I get a report updating me on the status of a former patient being treated elsewhere. This is someone who I thought had no more treatment options. I am humbled every time a colleague, or fellow, recommends a treatment I had never considered. I am not perfect; I do the best I can. My recommendation might be wrong.
Yet I have watched my patient steadily deteriorate and cognitively decline no matter what treatment I employed, whether or not the monoclonal spike decreased. There is no evidence that treatment under such circumstances benefits the patient at all. Moreover, I have sat through many morbidity and mortality conferences where the conclusion was that we should have consulted hospice sooner. Like so many hematologists and oncologists every day, I needed to have a goals-of-care conversation with my patient knowing that treatment could possibly help, but probably would not.
Crucial conversations like these are difficult for everybody. There are techniques to employ that my palliative care colleagues recommend. I tried to remember them as I started talking to my patient and his wife. He listened and clearly understood the gravity of the situation and the resulting poor prognosis regardless of treatment. I recommended hospice. He declined.
Getting to this point was uncomfortable enough, but then I came to a decision that I am still struggling with – acquiesce to his wishes and treat while feeling that I should not, or decline to treat further and transfer his care to someone more willing? This is not the kind of trick or treat I enjoy.
I look forward to the day when discussions of end of life are less awkward. Small movements have started to bring these conversations into the open. One such movement choreographs a dinner to encourage frank and open discussion of death (https://deathoverdinner.org/). Another reimagines the doula – a childbirth coach – as a coach at the end of life (https://www.agentlerparting.com/). Another provides a step-by-step approach to generating an end-of-life conversation (https://theconversationproject.org/). These, and many other efforts, did not occur in a vacuum. They emerged because of the growing recognition that the modern delivery of health care, and the culture it created, is inadequate for the end of life.
Until our culture changes, though, we are left with tough conversations and tougher decisions with our patients who are at the end of their cancer journey. I wish I could tell my junior colleagues that it gets easier with experience. In many ways it gets worse because of the long relationships we develop. As long as the rewards of treats are greater than the disappointments of tricks, though, I will continue trick or treating in my white coat costume.
Dr. Kalaycio is editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
A few pearls can help prepare the mind
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.