User login
Facial Rejuvenation: Combining Cosmeceuticals With Cosmetic Procedures
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
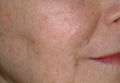
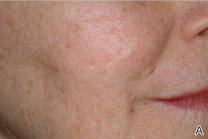
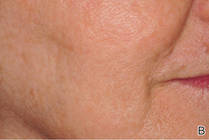
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.
|
|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.

|

|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.

|

|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Practice Points
- Copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs for postprocedure irritation and inflammation.
- Acetyl hexapeptide-3 is a topical variation of botulinum toxin to be used on its own or adjunctively with the injectable form.
- Topical hyaluronic acid can be used on its own or adjunctively with injectable fillers.
Interventions for the Treatment of Stretch Marks: A Systematic Review
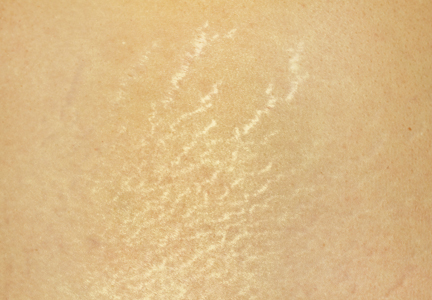
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
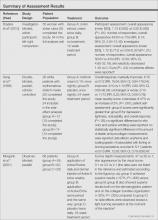
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
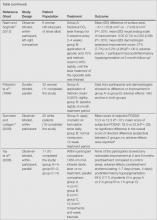
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Practice Points
- Given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could be considered for the treatment of stretch marks, though the evidence is insufficient.
- High-quality, randomized, placebo-controlled trials are needed in the future.
Devices and Topical Agents for Rosacea Management
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.
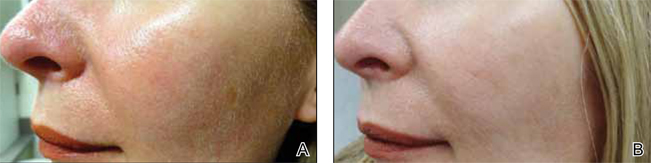
According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Practice Points
- Rosacea patients should be advised on appropriate skin care.
- Purpuric settings of the pulsed dye laser may be more effective in treating rosacea-associated erythema.
- Topical brimodine tartrate can control facial erythema, but patients should be warned of the potential risk for rebound erythema.