User login
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Codes, Contracts, and Commitments: Who Defines What is a Profession?
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
A professional is someone who can do his best work when he doesn’t feel like it.
Alistair Cooke
When I was a young person with no idea about growing up to be something, my father used to tell me there were 4 learned professions: medicine to heal the body, law to protect the body politic, teaching to nurture the mind, and the clergy to care for the soul.1 That adage, or some version of it, is attributed to a variety of sources, likely because it captures something essential and timeless about the learned professions. I write this as a much older person, and it has been my privilege to have worked in some capacity in all 4 of these venerable vocations.
There are many more recognized professions now than in my father’s time with new ones still emerging as the world becomes more complicated and specialized. In November 2025, however, the growth of the professions was dealt a serious blow when the US Department of Education (DOE) redefined what constitutes a profession for the purpose of federal funding of graduate degrees.2 The internet is understandably abuzz with opinions across the political spectrum. What is missing from many of these discussions is an understanding of the criteria for a profession and, even more importantly, who has the authority to decide when an individual or a group has met that standard.
But first, what and why did the DOE make this change? The One Big Beautiful Bill Act charged the DOE with reducing what it claims is massive overspending on graduate education by limiting the programs that meet the definition of a “professional degree” eligible for higher funding. Of my father’s 4, medicine (including dentistry) and law made the cut with students in those professions able to borrow up to $200,000 in direct unsubsidized student loans while those in other programs would be limited to $100,000.2
As one of the oldest and most respected professions in America, nursing has received the most media attention, yet there are also other important and valued professions that are missing from the DOE list.3 The excluded professions also include: physician assistants, physical therapists, audiologists, architects, accountants, educators, and social workers. The proposed regulatory changes are not yet finalized and Congressional representatives, health care experts, and a myriad of professional associations have rightly objected the reclassification will only worsen the critical shortage of nurses, teachers, and other helping professions the country is already facing.4
There are thousands of federal health care professionals who worked long and hard to achieve their goals whom this Act undervalues. Moreover, the regulatory change leaves many students enrolled in education and training programs under federal practice auspices confused and overwhelmed. Perhaps they can take some hope and inspiration from the recognition that historically and philosophically, no agency or administration can unilaterally define what is a profession.
The literature on professionalism is voluminous, in large part because it has been surprisingly difficult to reach a consensus definition. A proposed definition from scholars captures most of the key aspects of a profession. While it is drawn from the medical literature, it applies to most of the caring professions the DOE disqualified. For pedagogic purposes, the definition is parsed into discrete criteria in the Table.5

Even this simple summary makes it obvious that a government agency alone could not possibly have the competence to determine who meets these complex technical and moral criteria. The members of the profession must assume a primary role in that determination. The complicated history of the professions shows that the locus of these decisions has resided in various combinations of educational institutions, such as nursing schools,6 professional societies (eg, National Association of Social Workers),7 and certifying boards (eg, National Commission on Certification of Physician Assistants).8 States, not the federal government, have long played a key part in defining professions in the US, through their authority to grant licenses to practice.9
In response to criticism, the DOE has stated that “the definition of a ‘professional degree’ is an internal definition used by the Department of Education to distinguish among programs that qualify for higher loan limits, not a value judgment about the importance of programs. It has no bearing on whether a program is professional in nature or not.”2 Given the ancient compact between society and the professions in which the government subsidizes the training of professionals dedicated to public service, it is hard to see how these changes can be dismissed as merely semantic and not a promissory breach.10
I recognize that this abstract editorial is little comfort to beleaguered and demoralized professionals and students. Still, it offers a voice of support for each federal practitioner or trainee who fulfills the epigraph’s description of a professional day after day. The nurse who works the extra shift without complaint or resentment so that veterans receive the care they deserve, the social worker who responds on a weekend night to an active duty family without food so they do not spend another night hungry, and the physician assistant who makes it into the isolated public health clinic despite the terrible weather so there is someone ready to take care for patients in need. The proposed policy shift cannot in any meaningful sense rob them of their identity as individuals committed to a code of caring. However, without an intact social compact, it may well remove their practical ability to remain and enter the helping professions to the detriment of us all.
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
- Wade JW. Public responsibilities of the learned professions. Louisiana Law Rev. 1960;21:130-148
- US Department of Education. Myth vs. fact: the definition of professional degrees. Press Release. November 24, 2025. Accessed December 22, 2025. https://www.ed.gov/about/news/press-release/myth-vs-fact-definition-of-professional-degrees
- Laws J. Full list of degrees not classed as “professional” by Trump admin. Newsweek. Updated November 26, 2025. Accessed December 22, 2025. https://www.newsweek.com/full-list-degrees-professional-trump-administration-11085695
- New York Academy of Medicine. Response to stripping “professional status” as proposed by the Department of Education. New York Academy of Medicine. November 24, 2025. Accessed December 22, 2025. https://nyam.org/article/response-to-stripping-professional-status-as-proposed-by-the-department-of-education
- Cruess SR, Johnston S, Cruess RL. “Profession”: a working definition for medical educators. Teach Learn Med. 2004;16:74-76. doi:10.1207/s15328015tlm1601_15
- American Association of Colleges of Nursing. Nursing is a professional degree. American Association of Colleges of Nursing. Accessed December 20, 2025. https://www.aacnnursing.org/policy-advocacy/take-action/nursing-is-a-professional-degree
- National Association of Social Workers. Social work is a profession. Social Workers. Accessed December 20, 2025. https://www.socialworkers.org
- National Commission on Certification of Physician Assistants. Accessed December 20, 2025. https://www.nccpa.net/about-nccpa/#who-we-are
- The Federation of State Boards of Physical Therapy. Accessed December 20, 2025. https://www.fsbpt.org/About-Us/Staff-Home
- Cruess SR, Cruess RL. Professionalism and medicine’s contract with social contract with society. Virtual Mentor. 2004;6:185-188. doi:10.1001/virtualmentor.2004.6.4.msoc1-040
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Codes, Contracts, and Commitments: Who Defines What is a Profession?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
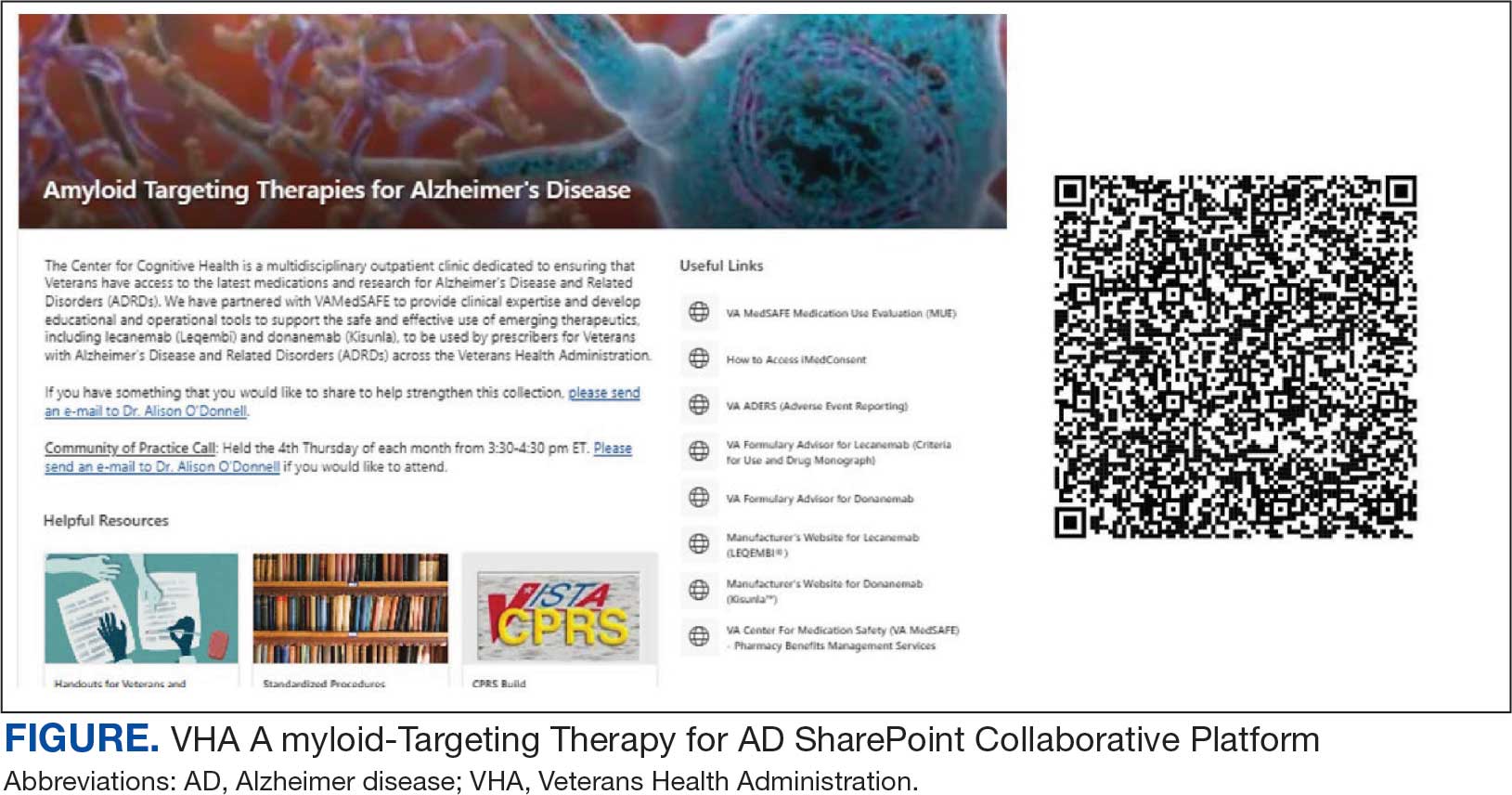
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.
Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.
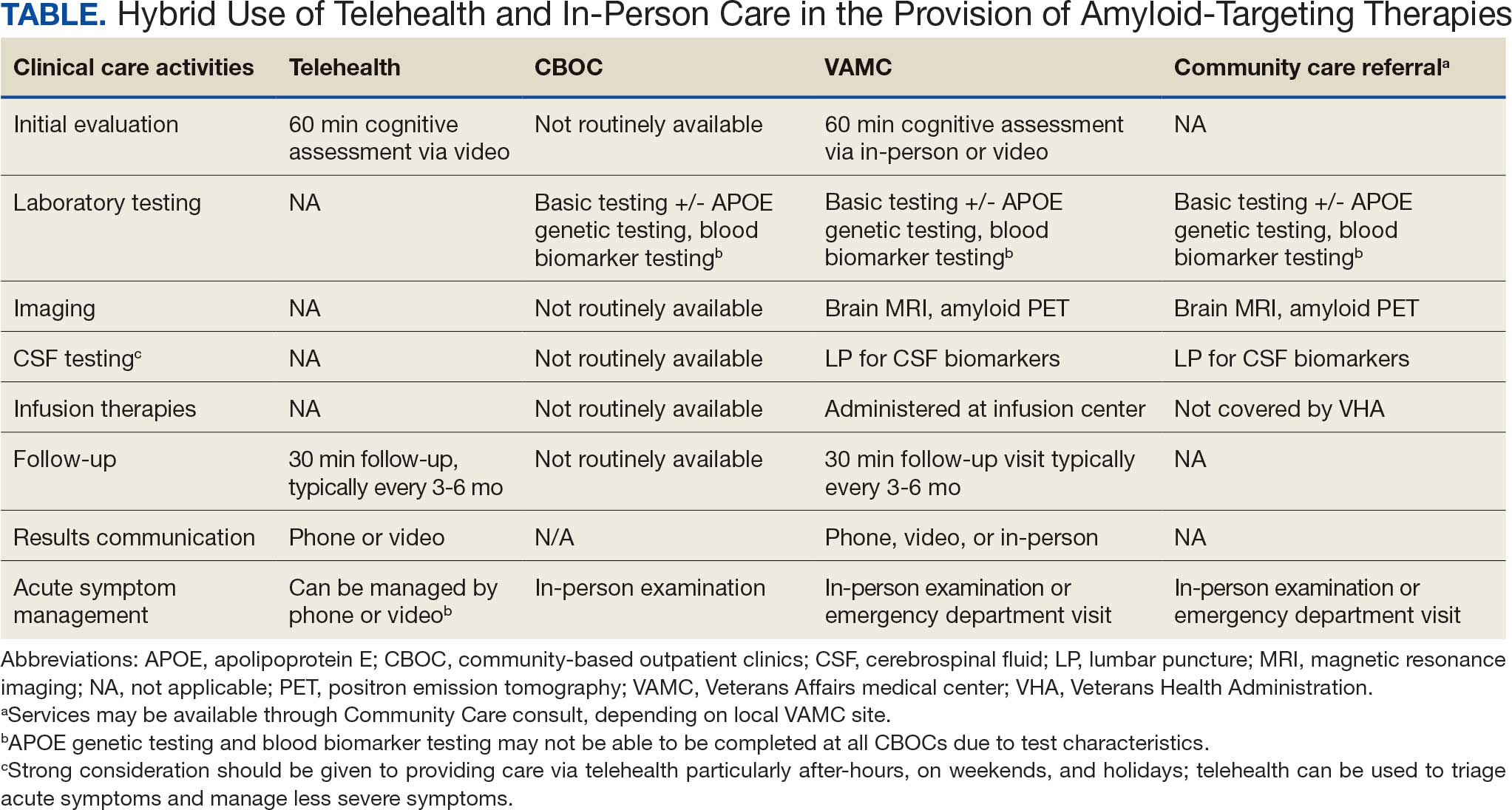
The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Once and Future Veterans Health Administration
The Once and Future Veterans Health Administration
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3
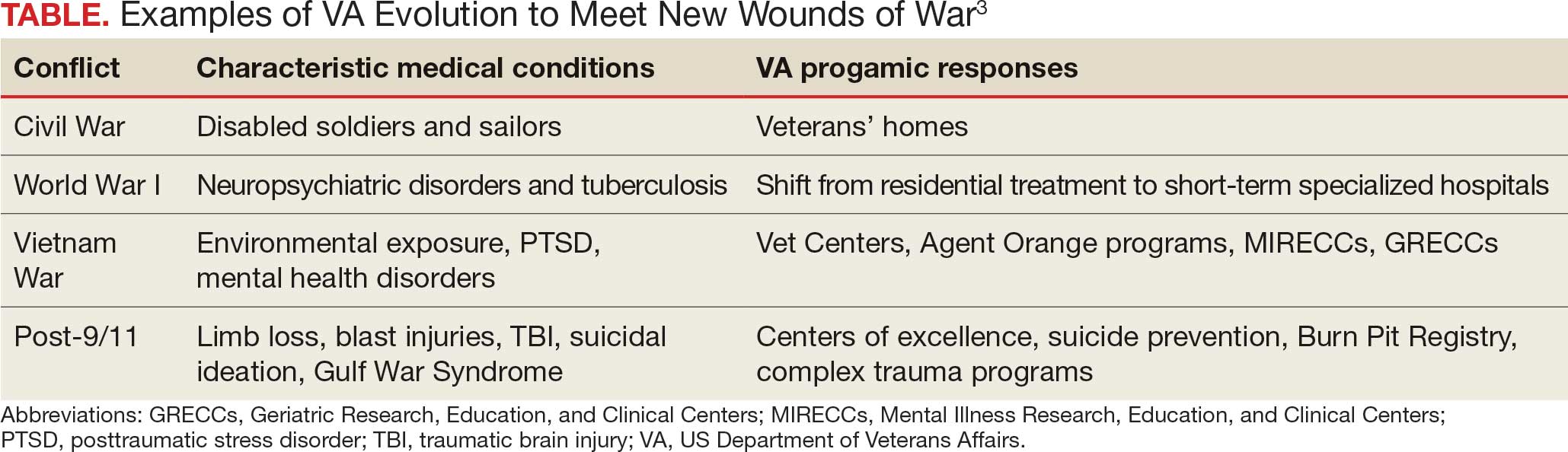
Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3

Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
He who thus considers things in their first growth and origin ... will obtain the clearest view of them. Politics, Book I, Part II by Aristotle
Many seasoned observers of federal practice have signaled that the future of US Department of Veterans Affairs (VA) health care is threatened as never before. Political forces and economic interests are siphoning Veterans Health Administration (VHA) capital and human resources into the community with an ineluctable push toward privatization.1
This Veterans Day, the vitality, if not the very viability of veteran health care, is in serious jeopardy, so it seems fitting to review the rationale for having institutions dedicated to the specialized medical treatment of veterans. Aristotle advises us on how to undertake this intellectual exercise in the epigraph. This column will revisit the historical origins of VA medicine to better appreciate the justification of an agency committed to this unique purpose and what may be sacrificed if it is decimated.
The provision of medical care focused on the injuries and illnesses of warriors is as old as war. The ancient Romans had among the first veterans’ hospital, named a valetudinarium. Sick and injured members of the Roman legions received state-of-the-art medical and surgical care from military doctors inside these facilities.2
In the United States, federal practice emerged almost simultaneously with the birth of a nation. Wounded troops and families of slain soldiers required rehabilitation and support from the fledgling federal government. This began a pattern of development in which each war generated novel injuries and disorders that required the VA to evolve (Table).3

Many arguments can be marshalled to demonstrate the importance of not just ensuring VA health care survives but also has the resources needed to thrive. I will highlight what I argue are the most important justifications for its existence.
The ethical argument: President Abraham Lincoln and a long line of government officials for more than 2 centuries have called the provision of high-quality health care focused on veterans a sacred trust. Failing to fulfill that promise is a violation of the deepest principles of veracity and fidelity that those who govern owe to the citizens who selflessly sacrificed time, health, and even in some cases life, for the safety and well-being of their country.4
The quality argument: Dozens of studies have found that compared to the community, many areas of veteran medical care are just plain better. Two surveys particularly salient in the aging veteran population illustrate this growing body of positive research. The most recent and largest survey of Medicare patients found that VHA hospitals surpassed community-based hospitals on all 10 metrics.5 A retrospective cohort study of mortality compared veterans transported by ambulance to VHA or community-based hospitals. The researchers found that those taken to VHA facilities had a 30-day all cause adjustment mortality 20 times lower than those taken to civilian hospitals, especially among minoritized populations who generally have higher mortality.6
The cultural argument: Glance at almost any form of communication from veterans or about their health care and you will apprehend common cultural themes. Even when frustrated that the system has not lived up to their expectations, and perhaps because of their sense of belonging, they voice ownership of VHA as their medical home. Surveys of veteran experiences have shown many feel more comfortable receiving care in the company of comrades in arms and from health care professionals with expertise and experience with veterans’ distinctive medical problems and the military values that inform their preferences for care.7
The complexity argument: Anyone who has worked even a short time in a VHA hospital or clinic knows the patients are in general more complicated than similar patients in the community. Multiple medical, geriatric, neuropsychiatric, substance use, and social comorbidities are the expectation, not the exception, as in some civilian systems. Many of the conditions common in the VHA such as traumatic brain injury, service-connected cancers, suicidal ideation, environmental exposures, and posttraumatic stress disorder would be encountered in community health care settings. The differences between VHA and community care led the RAND Corporation to caution that “Community care providers might not be equipped to handle the needs of veterans.”8
Let me bring this 1000-foot view of the crisis facing federal practice down to the literal level of my own home. For many years I have had a wonderful mechanic who has a mobile bike service. I was talking to him as he fixed my trike. I never knew he was a Vietnam era veteran, and he didn’t realize that I was a career VA health care professional at the very VHA hospital where he received care. He spontaneously told me that, “when I first got out, the VA was awful, but now it is wonderful and they are so good to me. I would not go anywhere else.” For the many veterans of that era who would echo his sentiments, we must not allow the VA to lose all it has gained since that painful time
Another philosopher, Søren Kierkegaard, wrote that “life must be understood backwards but lived forwards.”9 Our own brief back to the future journey in this editorial has, I hope, shown that VHA medical institutions and health professionals cannot be replaced with or replicated by civilian systems and clinicians. Continued attempts to do so betray the trust and risks the health and well-being of veterans. It also would deprive the country of research, innovation, and education that make unparalleled contributions to public health. Ultimately, these efforts to diminish VHA compromise the solidarity of service members with each other and with their federal practitioners. If this trend to dismantle an organization that originated with the sole purpose of caring for veterans continues, then the public expressions of respect and gratitude will sound shallower and more tentative with each passing Veterans Day.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
- Quil L. Hundreds of VA clinicians warn that cuts threaten vet’s health care. National Public Radio. October 1, 2025. Accessed October 27, 2025. https://www.npr.org/2025/10/01/nx-s1-5554394/hundreds-of-va-clinicians-warn-that-cuts-threaten-vets-health-care
- Nutton V. Ancient Medicine. 2nd ed. Routledge; 2012.
- US Department of Veterans Affairs. VA History Summary. Updated June 13, 2025. Accessed October 27, 2025. https://department.va.gov/history/history-overview/
- Geppert CMA. Learning from history: the ethical foundation of VA health care. Fed Pract. 2016;33:6-7.
- US Department of Veterans Affairs. Nationwide patient survey shows VA hospitals outperform non-VA hospitals. News release. June 14, 2023. Accessed October 27, 2025. https://news.va.gov/press-room/nationwide-patient-survey-shows-va-hospitals-outperform-non-va-hospitals
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Vigilante K, Batten SV, Shang Q, et al. Camaraderie among US veterans and their preferences for health care systems and practitioners. JAMA Netw Open. 2025;8(4):e255253. doi:10.1001/jamanetworkopen.2025.5253
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10:9.
- Kierkegaard S. Journalen JJ:167 (1843) in: Søren Kierkegaards Skrifter. Vol 18. Copenhagen; 1997:306.
The Once and Future Veterans Health Administration
The Once and Future Veterans Health Administration
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Transplantation palliative care: The time is ripe
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Managing Resistance to Change Along the Journey to High Reliability
Managing Resistance to Change Along the Journey to High Reliability
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.
CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.

CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.

CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
Managing Resistance to Change Along the Journey to High Reliability
Managing Resistance to Change Along the Journey to High Reliability
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Antibiotic Stewardship in Acne: Practical Tips From Dr. Lorraine L. Rosamilia
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology