User login
Bag-mask ventilation during intubation reduces severe hypoxemia
, according to data presented at the Critical Care Congress, sponsored by the Society of Critical Care Medicine.
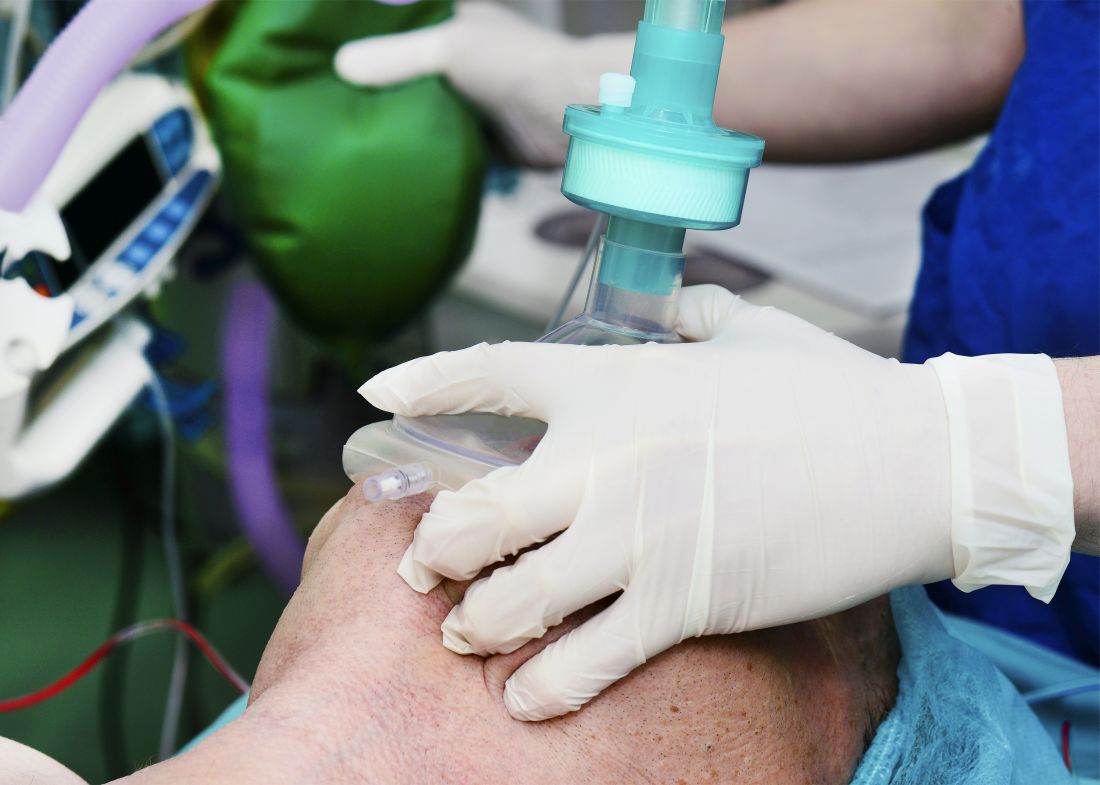
A multicenter study, published simultaneously in the Feb. 18 issue of the New England Journal of Medicine, randomized 401 critically-ill patients in the ICU who were undergoing tracheal intubation to receive either ventilation with a bag-mask device during induction for intubation or no ventilation.
The median lowest oxygen saturation between induction and 2 minutes after intubation was 96% in the bag-mask ventilated patients and 93% in the no-ventilation group, representing a 4.7% difference after adjusting for prespecified covariates (P = .01).
In a post-hoc analysis that adjusted for other factors such as the provision of preoxygenation, the preoxygenation device, pneumonia, and gastrointestinal bleeding, there was a 5.2% difference between the two groups in median lowest oxygen saturation, favoring the bag-mask group.
Bag-mask ventilation was also associated with almost a halving in the incidence of severe hypoxemia – defined as an oxygen saturation below 80% – compared with no-ventilation (10.9% vs. 22.8%; relative risk = 0.48). There was also a lower incidence of patients with an oxygen saturation below 90% and below 70% in the bag-mask ventilation group, compared with the no-ventilation group.
Overall, the median decrease in oxygen saturation from induction to the lowest point was 1% in the bag-mask group, and 5% in the no-ventilation group.
The study saw no effects of factors such as body-mass index, operator experience, or Acute Physiology and Chronic Health Evaluation (APACHE II) score. The patients had a median age of 60 years, about half had sepsis or septic shock, and close to 60% had hypoxemic respiratory failure as an indication for tracheal intubation.
Jonathan D. Casey, MD, of Vanderbilt University, Nashville, Tenn., and his coauthors wrote that their results suggested for every nine critically ill patients undergoing tracheal intubation, bag-mask ventilation would prevent severe hypoxemia in one patient.
“These findings are important because oxygen saturation is an established endpoint in airway management trials and is a contributing factor to periprocedural cardiac arrest and death,” they wrote.
They noted that there are conflicting guidelines on the use of bag-mask ventilation during tracheal intubation, with some recommending its use for all patients – even those who are not hypoxemic – and others advising their use only for patients with hypoxemia. This study excluded patients who were identified as hypoxemic or in whom bag-mask ventilation was contraindicated.
Despite concerns about bag-mask ventilation increasing the risk the aspiration, the study showed no significant difference between the two groups in the incidence of operator-reported aspiration or the presence of a new opacity on chest radiograph in the 48 hours after intubation.
The authors acknowledged that, given the low incidence of operator-reported aspiration during tracheal intubation, a much larger study would be needed to show whether bag-mask ventilation did increase the risk of aspiration.
“However, our trial provides some reassurance, since the incidence of operator-reported aspiration was numerically lower in the bag-mask ventilation group than in the no-ventilation group,” they wrote.
There were also no significant differences between the two groups in oxygen saturation, fraction of inspired oxygen or positive end-expiratory pressure in the 24 hours after intubation. Bag-mask ventilation was also associated with similar rates of in-hospital mortality, number of ventilator-free days, and days out of the ICU as no-ventilation.
The authors noted that their trial focused on critically-ill patients in the ICU, so the results may not be generalizable to patients in the emergency department or in a prehospital setting.
The study and some authors were supported by the National Institutes of Health. Two authors declared personal fees from the pharmaceutical industry unrelated to the study, and no other conflicts of interest were declared.
SOURCE: Casey J et al. N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMoa1812405
Debate around the question of whether to use bag-mask ventilation in critically-ill patients has been limited by the lack of high-quality evidence on the risk of aspiration or on the benefits of this approach. This study found no evidence of an increase in the incidence of aspiration, despite using multiple measures to detect it, which provide some reassurance that manual ventilation during tracheal intubation is not likely to cause significant harm.
One significant limitation of this trial, however, is that it did not standardize the preoxygenation strategy across the two groups, so significantly more patients in the bag-mask group received bag-mask ventilation before induction. Median oxygen saturation before induction was the same in the two groups, but this does not rule out the possibility of differences in the arterial pressure of oxygen.
This study may not settle the question of whether to use bag-mask ventilation during tracheal intubation, but it provides strong suggestion that the practice is not harmful.
Patricia A. Kritek, MD, and Andrew M. Luks, MD, are with the division of pulmonary, critical care, and sleep medicine at the University of Washington in Seattle. These comments are adapted from their editorial accompanying the paper by Casey et al. (N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMe1900708). Dr. Luks declared personal fees from private industry outside the submitted work. Dr. Kritek reported having nothing to disclose.
Debate around the question of whether to use bag-mask ventilation in critically-ill patients has been limited by the lack of high-quality evidence on the risk of aspiration or on the benefits of this approach. This study found no evidence of an increase in the incidence of aspiration, despite using multiple measures to detect it, which provide some reassurance that manual ventilation during tracheal intubation is not likely to cause significant harm.
One significant limitation of this trial, however, is that it did not standardize the preoxygenation strategy across the two groups, so significantly more patients in the bag-mask group received bag-mask ventilation before induction. Median oxygen saturation before induction was the same in the two groups, but this does not rule out the possibility of differences in the arterial pressure of oxygen.
This study may not settle the question of whether to use bag-mask ventilation during tracheal intubation, but it provides strong suggestion that the practice is not harmful.
Patricia A. Kritek, MD, and Andrew M. Luks, MD, are with the division of pulmonary, critical care, and sleep medicine at the University of Washington in Seattle. These comments are adapted from their editorial accompanying the paper by Casey et al. (N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMe1900708). Dr. Luks declared personal fees from private industry outside the submitted work. Dr. Kritek reported having nothing to disclose.
Debate around the question of whether to use bag-mask ventilation in critically-ill patients has been limited by the lack of high-quality evidence on the risk of aspiration or on the benefits of this approach. This study found no evidence of an increase in the incidence of aspiration, despite using multiple measures to detect it, which provide some reassurance that manual ventilation during tracheal intubation is not likely to cause significant harm.
One significant limitation of this trial, however, is that it did not standardize the preoxygenation strategy across the two groups, so significantly more patients in the bag-mask group received bag-mask ventilation before induction. Median oxygen saturation before induction was the same in the two groups, but this does not rule out the possibility of differences in the arterial pressure of oxygen.
This study may not settle the question of whether to use bag-mask ventilation during tracheal intubation, but it provides strong suggestion that the practice is not harmful.
Patricia A. Kritek, MD, and Andrew M. Luks, MD, are with the division of pulmonary, critical care, and sleep medicine at the University of Washington in Seattle. These comments are adapted from their editorial accompanying the paper by Casey et al. (N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMe1900708). Dr. Luks declared personal fees from private industry outside the submitted work. Dr. Kritek reported having nothing to disclose.
, according to data presented at the Critical Care Congress, sponsored by the Society of Critical Care Medicine.

A multicenter study, published simultaneously in the Feb. 18 issue of the New England Journal of Medicine, randomized 401 critically-ill patients in the ICU who were undergoing tracheal intubation to receive either ventilation with a bag-mask device during induction for intubation or no ventilation.
The median lowest oxygen saturation between induction and 2 minutes after intubation was 96% in the bag-mask ventilated patients and 93% in the no-ventilation group, representing a 4.7% difference after adjusting for prespecified covariates (P = .01).
In a post-hoc analysis that adjusted for other factors such as the provision of preoxygenation, the preoxygenation device, pneumonia, and gastrointestinal bleeding, there was a 5.2% difference between the two groups in median lowest oxygen saturation, favoring the bag-mask group.
Bag-mask ventilation was also associated with almost a halving in the incidence of severe hypoxemia – defined as an oxygen saturation below 80% – compared with no-ventilation (10.9% vs. 22.8%; relative risk = 0.48). There was also a lower incidence of patients with an oxygen saturation below 90% and below 70% in the bag-mask ventilation group, compared with the no-ventilation group.
Overall, the median decrease in oxygen saturation from induction to the lowest point was 1% in the bag-mask group, and 5% in the no-ventilation group.
The study saw no effects of factors such as body-mass index, operator experience, or Acute Physiology and Chronic Health Evaluation (APACHE II) score. The patients had a median age of 60 years, about half had sepsis or septic shock, and close to 60% had hypoxemic respiratory failure as an indication for tracheal intubation.
Jonathan D. Casey, MD, of Vanderbilt University, Nashville, Tenn., and his coauthors wrote that their results suggested for every nine critically ill patients undergoing tracheal intubation, bag-mask ventilation would prevent severe hypoxemia in one patient.
“These findings are important because oxygen saturation is an established endpoint in airway management trials and is a contributing factor to periprocedural cardiac arrest and death,” they wrote.
They noted that there are conflicting guidelines on the use of bag-mask ventilation during tracheal intubation, with some recommending its use for all patients – even those who are not hypoxemic – and others advising their use only for patients with hypoxemia. This study excluded patients who were identified as hypoxemic or in whom bag-mask ventilation was contraindicated.
Despite concerns about bag-mask ventilation increasing the risk the aspiration, the study showed no significant difference between the two groups in the incidence of operator-reported aspiration or the presence of a new opacity on chest radiograph in the 48 hours after intubation.
The authors acknowledged that, given the low incidence of operator-reported aspiration during tracheal intubation, a much larger study would be needed to show whether bag-mask ventilation did increase the risk of aspiration.
“However, our trial provides some reassurance, since the incidence of operator-reported aspiration was numerically lower in the bag-mask ventilation group than in the no-ventilation group,” they wrote.
There were also no significant differences between the two groups in oxygen saturation, fraction of inspired oxygen or positive end-expiratory pressure in the 24 hours after intubation. Bag-mask ventilation was also associated with similar rates of in-hospital mortality, number of ventilator-free days, and days out of the ICU as no-ventilation.
The authors noted that their trial focused on critically-ill patients in the ICU, so the results may not be generalizable to patients in the emergency department or in a prehospital setting.
The study and some authors were supported by the National Institutes of Health. Two authors declared personal fees from the pharmaceutical industry unrelated to the study, and no other conflicts of interest were declared.
SOURCE: Casey J et al. N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMoa1812405
, according to data presented at the Critical Care Congress, sponsored by the Society of Critical Care Medicine.

A multicenter study, published simultaneously in the Feb. 18 issue of the New England Journal of Medicine, randomized 401 critically-ill patients in the ICU who were undergoing tracheal intubation to receive either ventilation with a bag-mask device during induction for intubation or no ventilation.
The median lowest oxygen saturation between induction and 2 minutes after intubation was 96% in the bag-mask ventilated patients and 93% in the no-ventilation group, representing a 4.7% difference after adjusting for prespecified covariates (P = .01).
In a post-hoc analysis that adjusted for other factors such as the provision of preoxygenation, the preoxygenation device, pneumonia, and gastrointestinal bleeding, there was a 5.2% difference between the two groups in median lowest oxygen saturation, favoring the bag-mask group.
Bag-mask ventilation was also associated with almost a halving in the incidence of severe hypoxemia – defined as an oxygen saturation below 80% – compared with no-ventilation (10.9% vs. 22.8%; relative risk = 0.48). There was also a lower incidence of patients with an oxygen saturation below 90% and below 70% in the bag-mask ventilation group, compared with the no-ventilation group.
Overall, the median decrease in oxygen saturation from induction to the lowest point was 1% in the bag-mask group, and 5% in the no-ventilation group.
The study saw no effects of factors such as body-mass index, operator experience, or Acute Physiology and Chronic Health Evaluation (APACHE II) score. The patients had a median age of 60 years, about half had sepsis or septic shock, and close to 60% had hypoxemic respiratory failure as an indication for tracheal intubation.
Jonathan D. Casey, MD, of Vanderbilt University, Nashville, Tenn., and his coauthors wrote that their results suggested for every nine critically ill patients undergoing tracheal intubation, bag-mask ventilation would prevent severe hypoxemia in one patient.
“These findings are important because oxygen saturation is an established endpoint in airway management trials and is a contributing factor to periprocedural cardiac arrest and death,” they wrote.
They noted that there are conflicting guidelines on the use of bag-mask ventilation during tracheal intubation, with some recommending its use for all patients – even those who are not hypoxemic – and others advising their use only for patients with hypoxemia. This study excluded patients who were identified as hypoxemic or in whom bag-mask ventilation was contraindicated.
Despite concerns about bag-mask ventilation increasing the risk the aspiration, the study showed no significant difference between the two groups in the incidence of operator-reported aspiration or the presence of a new opacity on chest radiograph in the 48 hours after intubation.
The authors acknowledged that, given the low incidence of operator-reported aspiration during tracheal intubation, a much larger study would be needed to show whether bag-mask ventilation did increase the risk of aspiration.
“However, our trial provides some reassurance, since the incidence of operator-reported aspiration was numerically lower in the bag-mask ventilation group than in the no-ventilation group,” they wrote.
There were also no significant differences between the two groups in oxygen saturation, fraction of inspired oxygen or positive end-expiratory pressure in the 24 hours after intubation. Bag-mask ventilation was also associated with similar rates of in-hospital mortality, number of ventilator-free days, and days out of the ICU as no-ventilation.
The authors noted that their trial focused on critically-ill patients in the ICU, so the results may not be generalizable to patients in the emergency department or in a prehospital setting.
The study and some authors were supported by the National Institutes of Health. Two authors declared personal fees from the pharmaceutical industry unrelated to the study, and no other conflicts of interest were declared.
SOURCE: Casey J et al. N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMoa1812405
FROM CCC48
Key clinical point: Bag-mask ventilation during tracheal intubation reduces the risk of severe hypoxemia.
Major finding: For every nine patients who receive bag-mask ventilation during tracheal intubation, one case of severe hypoxemia is avoided.
Study details: Randomized, controlled trial in 401 critically-ill patients undergoing tracheal intubation.
Disclosures: The study and some authors were supported by the National Institutes of Health. Two authors declared personal fees from the pharmaceutical industry unrelated to the study.
Source: Casey J et al. N Engl J Med. 2019 Feb 18. doi: 10.1056/NEJMoa1812405
Flu season showing its staying power
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.
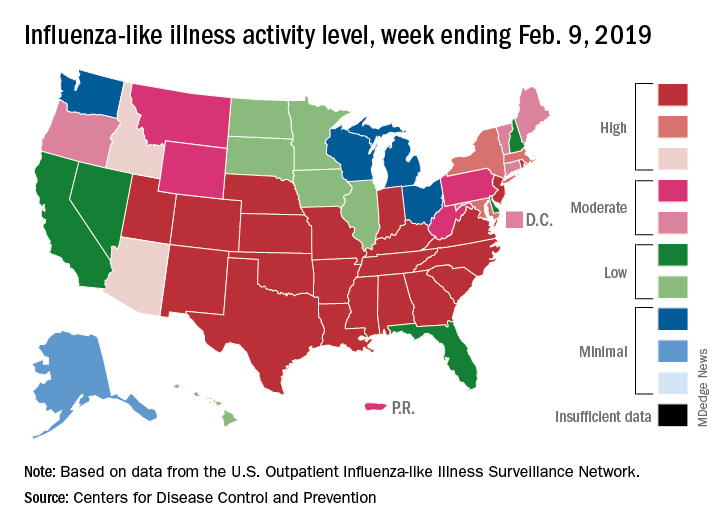
There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Deferoxamine does not improve 90-day outcomes after ICH
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
REPORTING FROM ISC 2019
Key clinical point: Deferoxamine does not improve disability at 90 days after intracranial hemorrhage.
Major finding: Approximately one-third of patients in both treatment groups had a good outcome.
Study details: A multicenter, randomized, double-blind study of 294 participants with intracranial hemorrhage.
Disclosures: The National Institutes of Health and National Institute of Neurological Disorders and Stroke supported this study.
Source: Selim MH et al. ISC 2019, Abstract LB22.
Fund projects, not people to address gender bias in research funding
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
FROM A LAUNCH EVENT HELD BY THE LANCET
Key clinical point: Funding bodies should focus on the science of a research project not on who is conducting the research.
Major finding: Between 2011 and 2016, 8.8% of projects proposed by female researchers and 12.7% of those proposed by male researchers were funded.
Study details: Analysis of nearly 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research during 2011-2016.
Disclosures: The research was unfunded. Dr. Witteman and all other commentators had no financial disclosures.
Source: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4.
In California, opioids most often prescribed in low-income, mostly white areas
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The most common users of opioids according to prescription drug records are residents of mostly low-income, white neighborhoods.
Major finding: Compared with 23.6% of all Californians, 44.2% of individuals in zip codes containing mostly low-income, white residents had at least one opioid prescription each year, compared with 16.1% of individuals in high-income zip codes with the lowest population of white residents.
Study details: An analysis of 29.7 million opioid prescription drug records by race and income in California during 2011-2015.
Disclosures: Dr. Schriger reported support from the Korein Foundation for his time working on the study by Friedman et al. The other authors from Friedman et al. reported no conflicts of interest.
Quick Byte: Needle-free injections
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
United States now over 100 measles cases for the year
according to the Centers for Disease Control and Prevention.
Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
Flu activity hits seasonal high
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
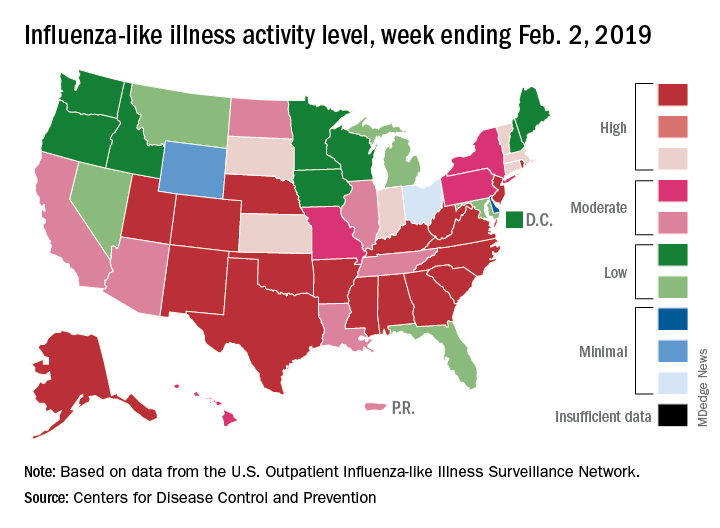
The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
SGLT2 inhibitors morph into HF drugs
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Cilostazol plus aspirin or clopidogrel reduces the risk of recurrent stroke
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
REPORTING FROM ISC
Key clinical point: Treating patients at high risk of recurrent stroke with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke more than either aspirin or clopidogrel alone and was just as safe.
Major finding: Dual therapy with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke by approximately half, compared with aspirin or clopidogrel alone.
Study details: A multicenter, randomized, open-label, parallel-group trial including 1,879 patients at high risk of recurrent stroke.
Disclosures: Otsuka Pharmaceutical funded the study. The presenter reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Source: Toyoda K et al. ISC 2019, Abstract LB3.