User login
In pain treatment, racial bias common among physician trainees
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
REPORTING FROM APS 2019
FDA approves generic naloxone spray for opioid overdose treatment
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
Short Takes
Short Takes
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Short Takes
Short Takes
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Ibrexafungerp effective against C. auris in two early case reports
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
FROM ECCMID 2019
Candida auris: Dangerous and here to stay
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.
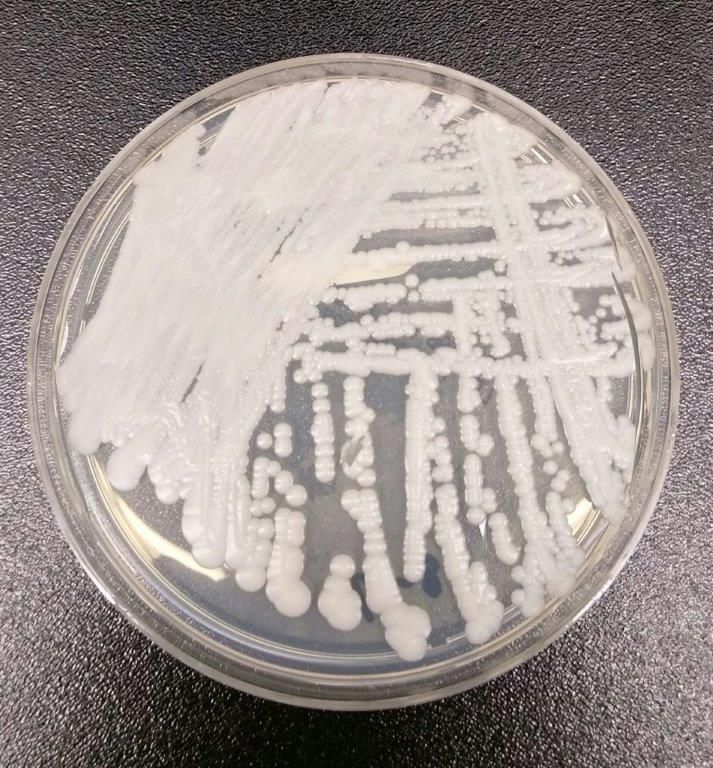
This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
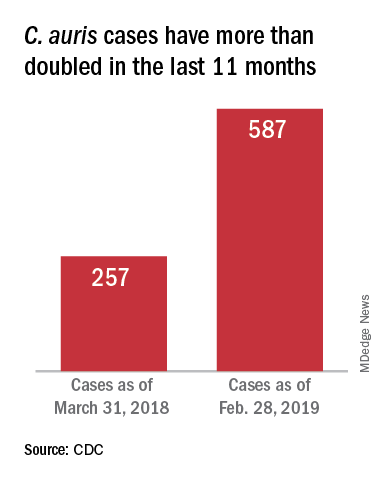
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”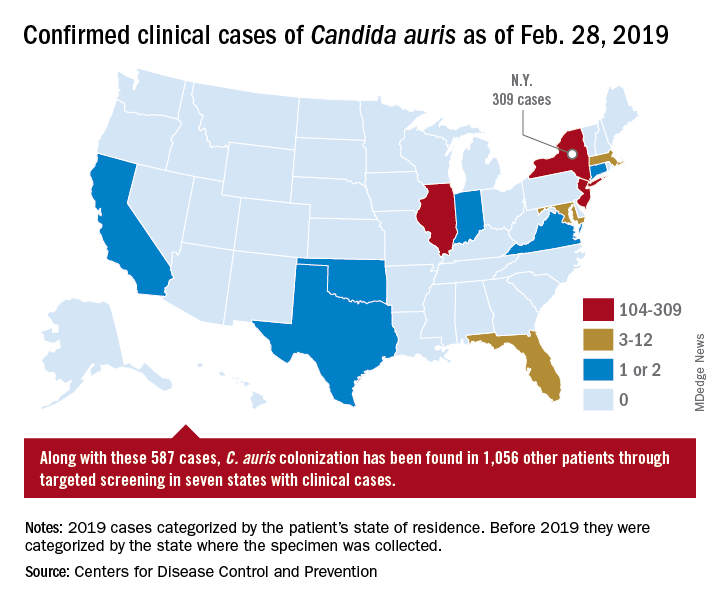
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.

This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.

This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Epinephrine linked with more refractory cardiogenic shock after acute MI
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Norepinephrine and epinephrine are the most commonly used vasopressors in clinical practice and in septic shock have been found to be equivalent in effectiveness. Their different physiological effects may influence their effectiveness in cardiogenic shock, and previous retrospective studies have suggested that epinephrine may have worse clinical outcomes in this setting.
Study design: A multicenter, prospective, randomized, double-blind study.
Setting: ICUs in nine French hospitals.
Synopsis: Adults (older than 18 years old) who suffered cardiogenic shock following successful revascularization after AMI were enrolled. Fifty-seven patients were randomly assigned to receive either norepinephrine or epinephrine with patients, nurses, and physicians unaware of which study drug was being used. The primary outcome variable was change in cardiac index within the first 72 hours, and refractory cardiogenic shock served as the main safety endpoint. This study was stopped early because of the higher risk of refractory cardiogenic shock noted in the epinephrine group, compared with that seen in the norepinephrine group (10 of 27 vs. 2 of 30; P = .011). There was no difference in evolution of cardiac index (P = .43) between the two groups. Potentially harmful metabolic and physiologic changes were noted in the epinephrine group including greater lactic acidosis and increased heart rate.
This study was underpowered for clinical endpoints because of the study’s early termination. It also did not include patients in cardiogenic shock from other causes, such as myositis or postcardiopulmonary bypass.
Bottom line: For patients in cardiogenic shock after AMI with successful reperfusion, epinephrine use was associated with increased refractory cardiogenic shock, compared with norepinephrine use.
Citation: Levy B et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018 Jul 10;72(2):173-82.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Poor response to statins hikes risk of cardiovascular events
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
Guidelines always look good on paper, but they’re only as good as their implementation, Márcio S. Bittencourt, MD, wrote in an accompanying editorial.
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guideline pinned effective statin therapy as a lowering of LDL cholesterol by at least 40%. This target aligns well with data accumulated in randomized controlled studies, but it doesn’t benefit patients unless it can be put into practice.
“An important step after a guideline publication is the assessment of its uptake among health practitioners and patients in the real world, as well as of the impact of its adherence on clinical outcomes. These analyses may not only verify its appropriateness, providing feedback for continuous improvement of recommendations, but also identify targets to optimize delivery of health to the society.”
To understand suboptimal statin response, we must understand the many possible reasons behind it – on the part of both physicians and patients.
Physicians may prefer to prescribe low-potency statins for several reasons, including unawareness of guideline recommendations, doubtfulness of better outcomes with higher potent statins or when a lower LDL is attained, and fear of adverse reactions or drug interactions, Dr. Bittencourt noted. “Moreover, doctors may be reluctant to up-titrate drugs when the treatment goals are not achieved, the so-called therapeutic inertia.”
In this study, for example, optimal responders were more likely to initially receive moderately potent statins. Suboptimal responders, on the other hand, were more likely to receive low-potency statins.
“This probably explains why baseline LDL was higher in optimal responders, indicating that higher LDL motivates the physician to be more aggressive upfront.”
Patients bring their own issues to the treatment table.
“Although an inter-individual response to statins may occur according to the genetic background, most cases where LDL response is less than expected are probably due to lack of adherence or persistence to the treatment. ... Of note, poor adherence to lipid-lowering therapy, together with low-intensity therapy, as opposed to high-intensity treatment, is associated with higher cardiovascular risk.”
Effective implementation of guidelines “has been a challenge for a long time. Both physicians and patients should be targets for approaches aiming at improving adherence to guidelines.”
For clinicians, these could include continuing medical education and simplified treatment algorithms. Patients, too, would benefit from some teaching.
“Patients and society should be educated on the scientific evidence documenting the benefits of lipid-lowering therapy, and antistatin propaganda based on pseudoscience should be strongly disavowed and demystified by health authorities.”
Dr. Bittencourt is an internist at the University Hospital San Paolo, Brazil.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
About half of patients taking statins for hyperlipidemia don’t adequately respond, leaving them at a 22% increased risk of cardiovascular disease, compared with optimal responders.
Over 6 years, there were about 2,000 more cardiovascular events among those who failed to experience the national treatment target of at least a 40% reduction in LDL cholesterol, according to Stephen F. Weng, MD, and his colleagues. The report is in Heart.
Physicians’ choice of initial statin weighed heavily in the outcomes. Patients who ended up with an optimal response were more likely to get a more potent statin right off, while those with a poorer response were more likely to get a less-potent statin.
“This study provides ‘real world evidence’ that 50% of patients started on statins do not derive the intended therapeutic benefit from them, significantly increasing their risk of future cardiovascular disease,” wrote Dr. Weng of the University of Nottingham, England, and his colleagues. “These findings contribute to the debate on the effectiveness of statin therapy and highlight the need for personalized medicine in lipid management for patients.”
The study comprised 165,411 primary care patients who had hypercholesterolemia but were free of cardiovascular disease at baseline. Statins were prescribed with the goal of at least a 40% reduction in baseline LDL within 24 months of the start of therapy.
Patients had a mean age of 62 years, with a mean baseline LDL of 4.1 mmol/L (158 mg/dL). About 49% were women.
The primary endpoints were the number of patients who did not achieve the 40% or higher reduction in baseline LDL and the between-group risk differences in cardiovascular events (coronary heart disease, stroke or transient ischemic attack, peripheral vascular disease, cardiovascular death).
After 24 months, 51.2% of patients experienced a suboptimal LDL response, with a mean reduction of 2.1 mmol/L (81 mg/dL) compared with 3.1 mmol/L (120 mg/dL). Compared with optimal responders, these patients were significantly more likely to have received a low-potency statin (29% vs. 18%).
Incident cardiovascular events occurred in 14% of the overall group (coronary artery disease, 8%; stroke/TIA, 3%; peripheral vascular disease 1.9%; cardiovascular death, 1%). All of these outcomes were significantly more common among suboptimal responders than optimal responders.
During a mean of 6 years of follow-up, there were 22,798 cardiovascular disease events overall, with significantly more occurring in suboptimal than optimal responders (12,142 vs. 10,656). This translated to a cardiovascular disease rate of 22.6 and 19.7 per 1,000 person-years, respectively.
In a multivariate analysis controlling for age and baseline LDL level, suboptimal responders were 22% more likely to have a cardiovascular disease incident than were optimal responders.
Among suboptimal responders, every unit decrease of 1 mmol/L (39 mg/dL) conferred a significant 6% risk reduction in cardiovascular disease (odds ratio, 0.94).
The benefit was not universal, the authors pointed out. “In this group, the decreased risk remained significant for only stroke/TIA and was not significant for other constituent cardiovascular disease outcomes. However, in patients with an optimal response, an even greater protective effect of LDL reduction and future cardiovascular disease was seen [13%; OR, 0.87],” and this reduction was significant for all of the individual outcomes.
“The study also highlights the benefit of reducing LDL to optimal values, which would lead to better cardiovascular disease outcomes for patients currently on statins,” the authors concluded.
None of the authors had any relevant financial disclosures.
SOURCE: Weng S. et al. Heart 2019 Apr. doi: 10.1136/heartjnl-2018-314253.
FROM HEART
Prevalence and outcomes of incidental imaging findings
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
More Medicare beneficiaries receiving hospice care services than in previous years
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Statin exposure associated with idiopathic inflammatory myositis
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: What is the association between exposure to statin medications and histologically confirmed idiopathic inflammatory myositis?
Background: More than 200 million people worldwide use statin therapy, mostly for cardiovascular risk reduction. There is mounting evidence of an infrequent side effect known as idiopathic inflammatory myositis (IIM), that requires immunosuppressive therapy rather than just discontinuation of the medication. While there is a recently described association of statin use with an immune-mediated necrotizing myositis through the formation of an autoantibody against HMG-CoA Reductase, this epidemiological study aimed to look at the incidence of statin use against all confirmed cases of IIM.
Study design: Retrospective, population-based, case-control study.
Setting: Northwest Adelaide Health Study in Adelaide, Australia.
Synopsis: A retrospective, population-based, case-control study was conducted that compared the incidence of histologically confirmed IIM identified from the South Australian Myositis Database in patients 40 years or older with known statin exposure (n = 221) against population-based controls obtained from the North West Adelaide Health Study. The unadjusted and adjusted odds ratios and 95% confidence intervals were calculated using the conditional logistic regression analysis for the risk of statin exposure associated with IIM. There was an almost twofold (79%) increased likelihood of statin exposure in patients with IIM by comparison with controls (adjusted OR, 1.79; 95% CI, 1.23-2.60; P = .001). This study’s results indicate that patients with histologically confirmed IIM had a significantly increased likelihood of statin exposure, compared with population-based matched controls. Results were similar even when excluding necrotizing myositis, which already has a known association with statin use, which suggests that statin use could be associated with all types of IIM.
Bottom line: There was a statistically significant association between statin use and the incidence of idiopathic inflammatory myositis, which suggests that this condition is a potential serious side effect of statin therapy.
Citation: Caughey GE et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an Australian population. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2859.
Dr. Nave is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.